Spatial Patterns and Species Distribution Model-Based Conservation Priorities for Scrophularia takesimensis on Ulleungdo
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Sources and Pre-Processing
2.3. Periodization and Definition of Unique Sites
2.4. Grid-Based Change Classification
2.5. Species Distribution Modeling
2.6. Statistical Analyses and Software
3. Results
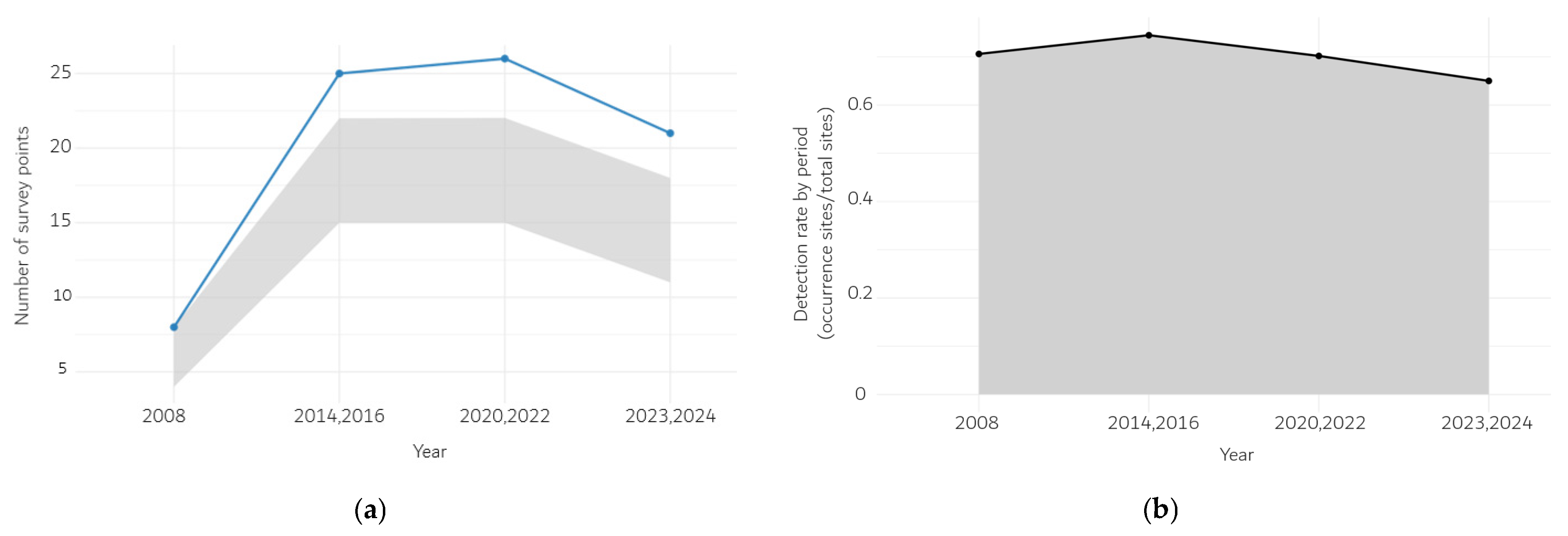
3.1. Number of Scrophularia takesimensis Occurrence Sites by Period
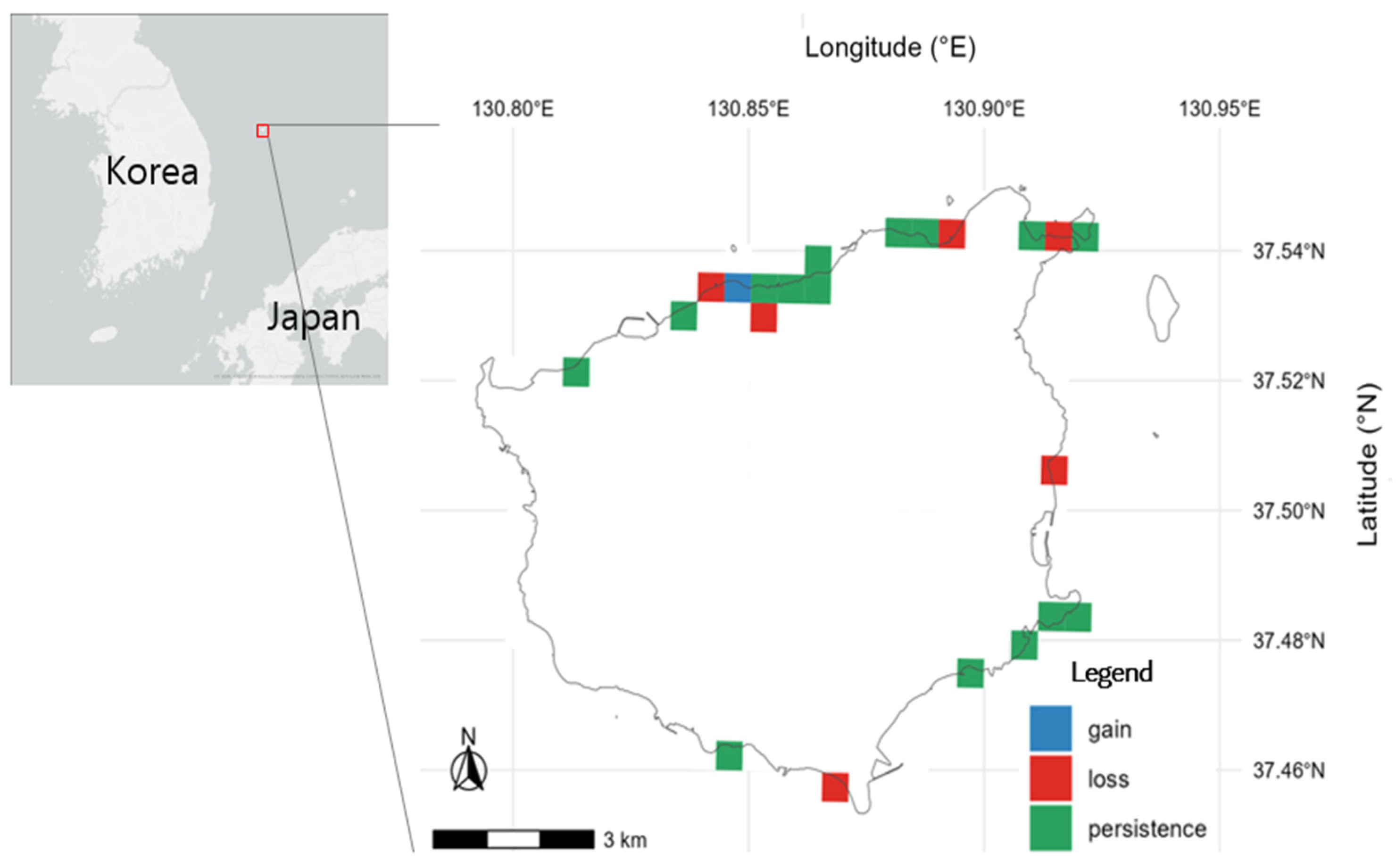
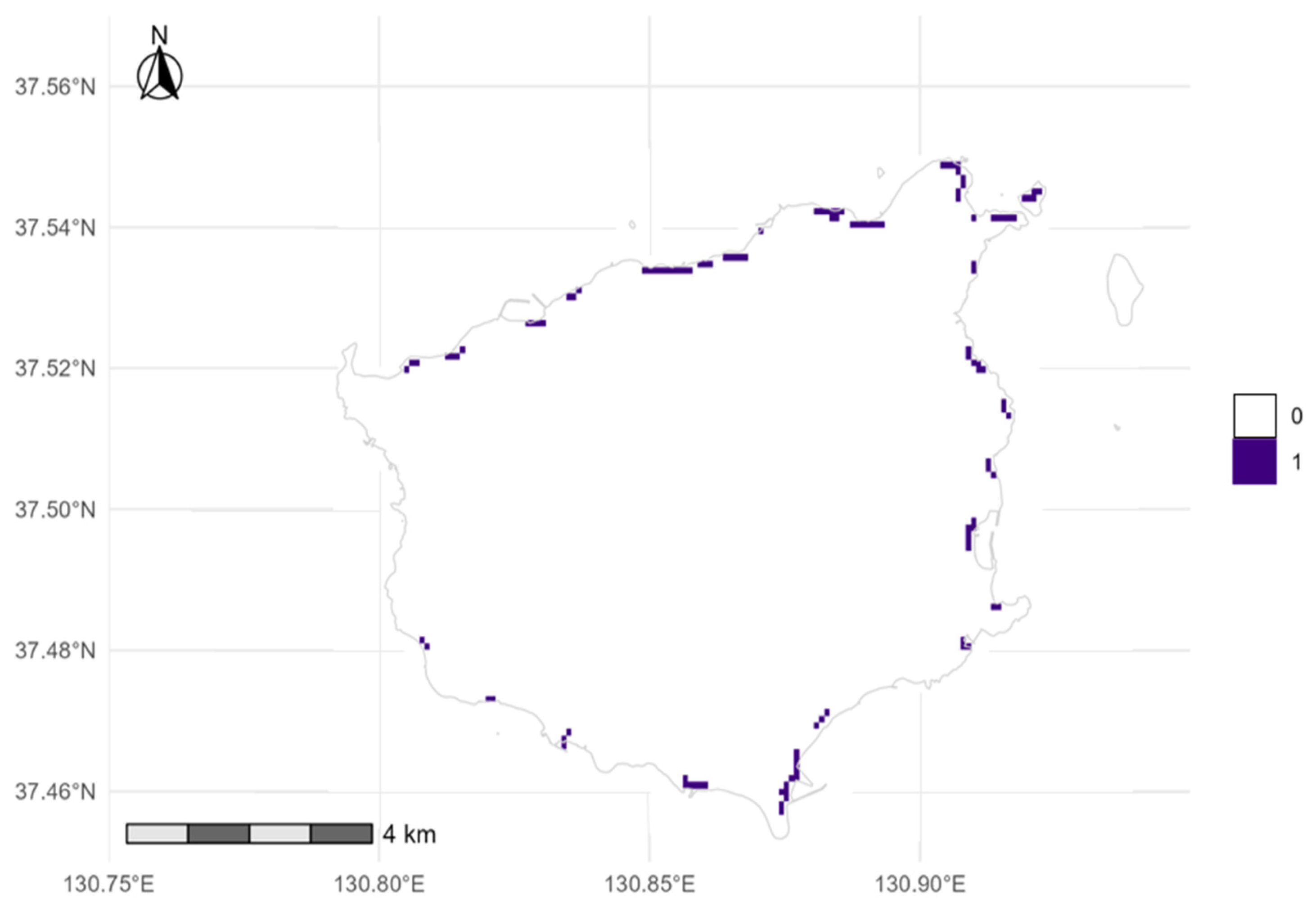
3.2. Distribution Patterns and Grid-Cell Change
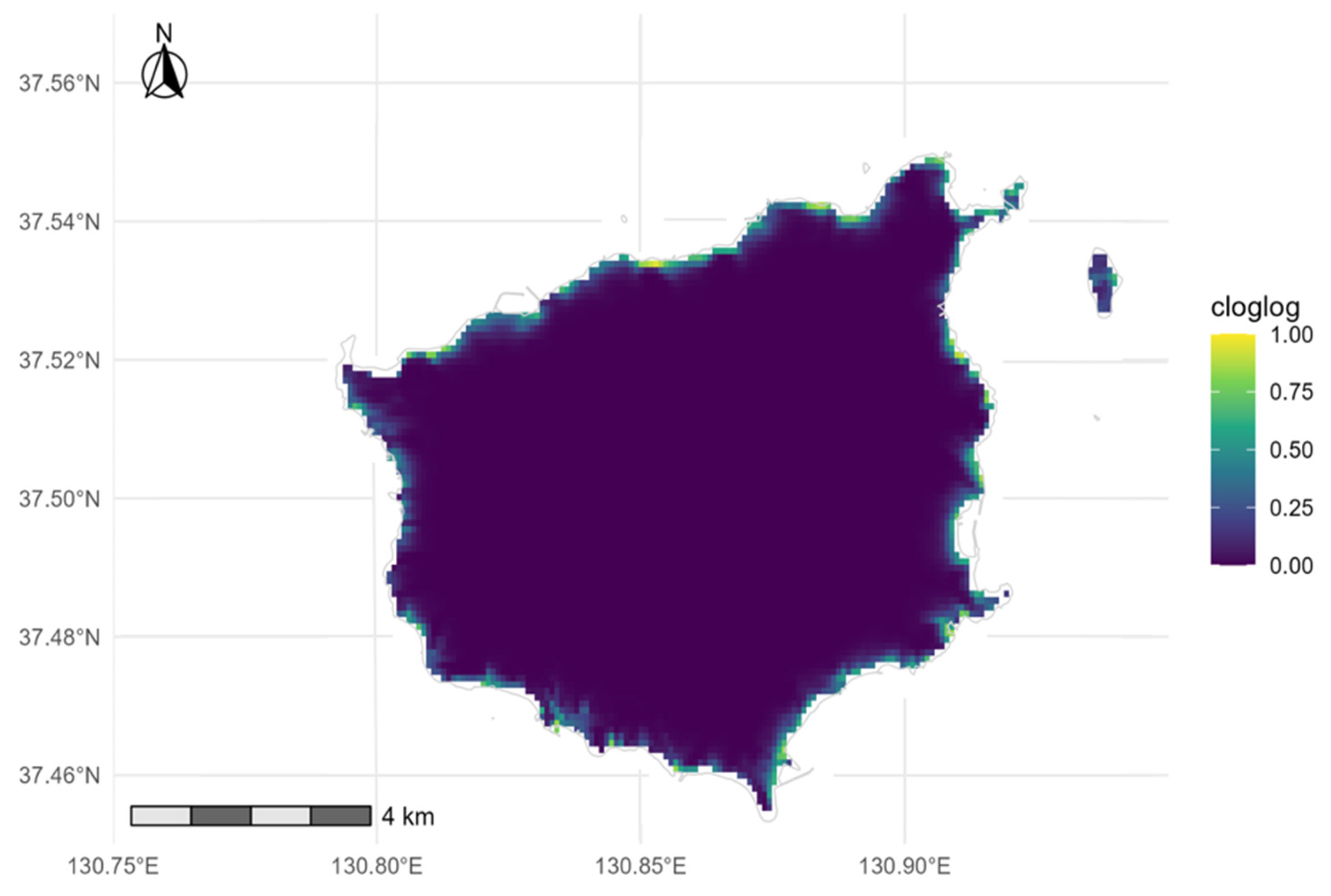
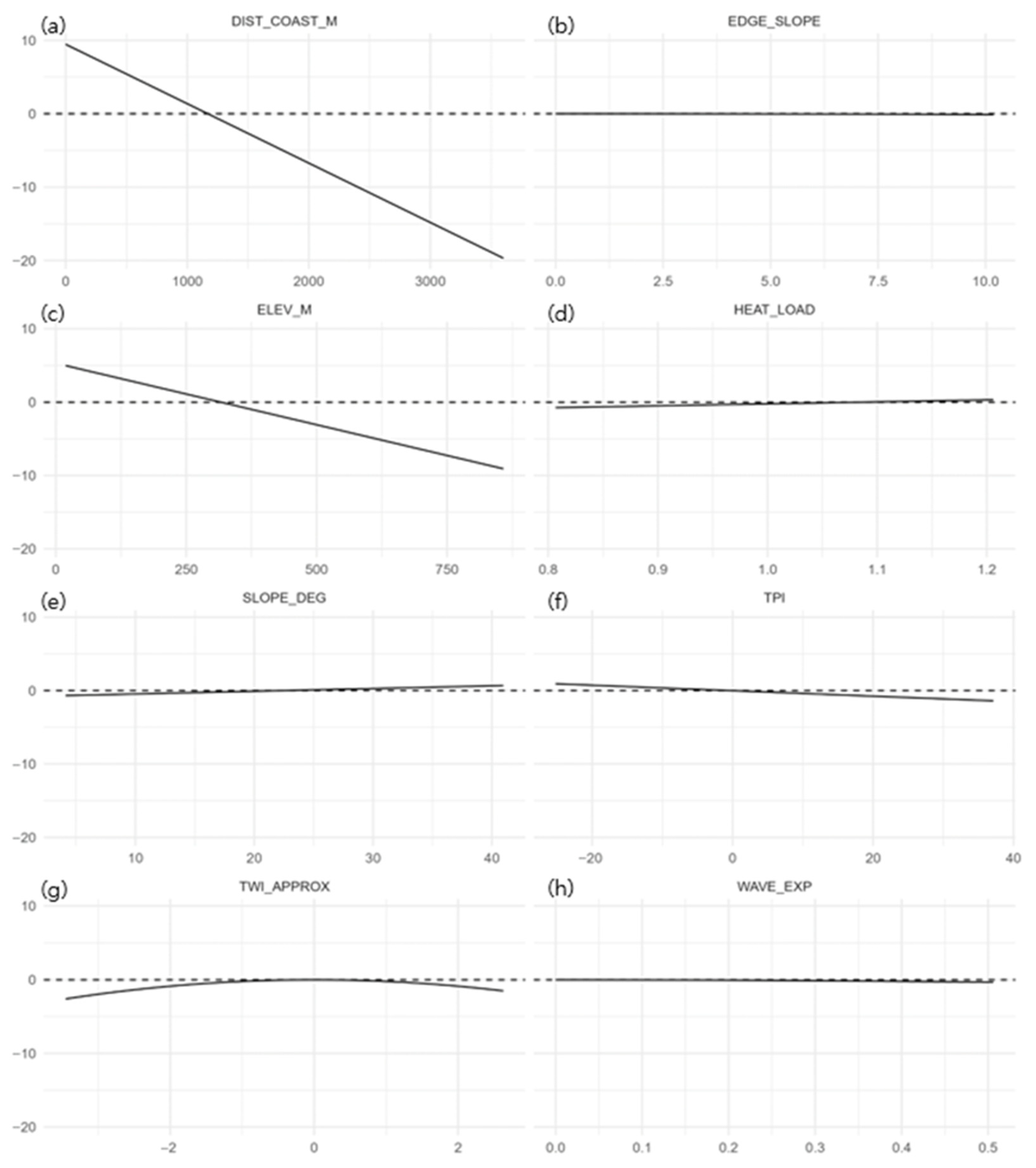
3.3. Species Distribution Model (SDM) Results
4. Discussion
4.1. Range Contraction and Underlying Threats
4.2. Spatial Asymmetry and Ecological Implications
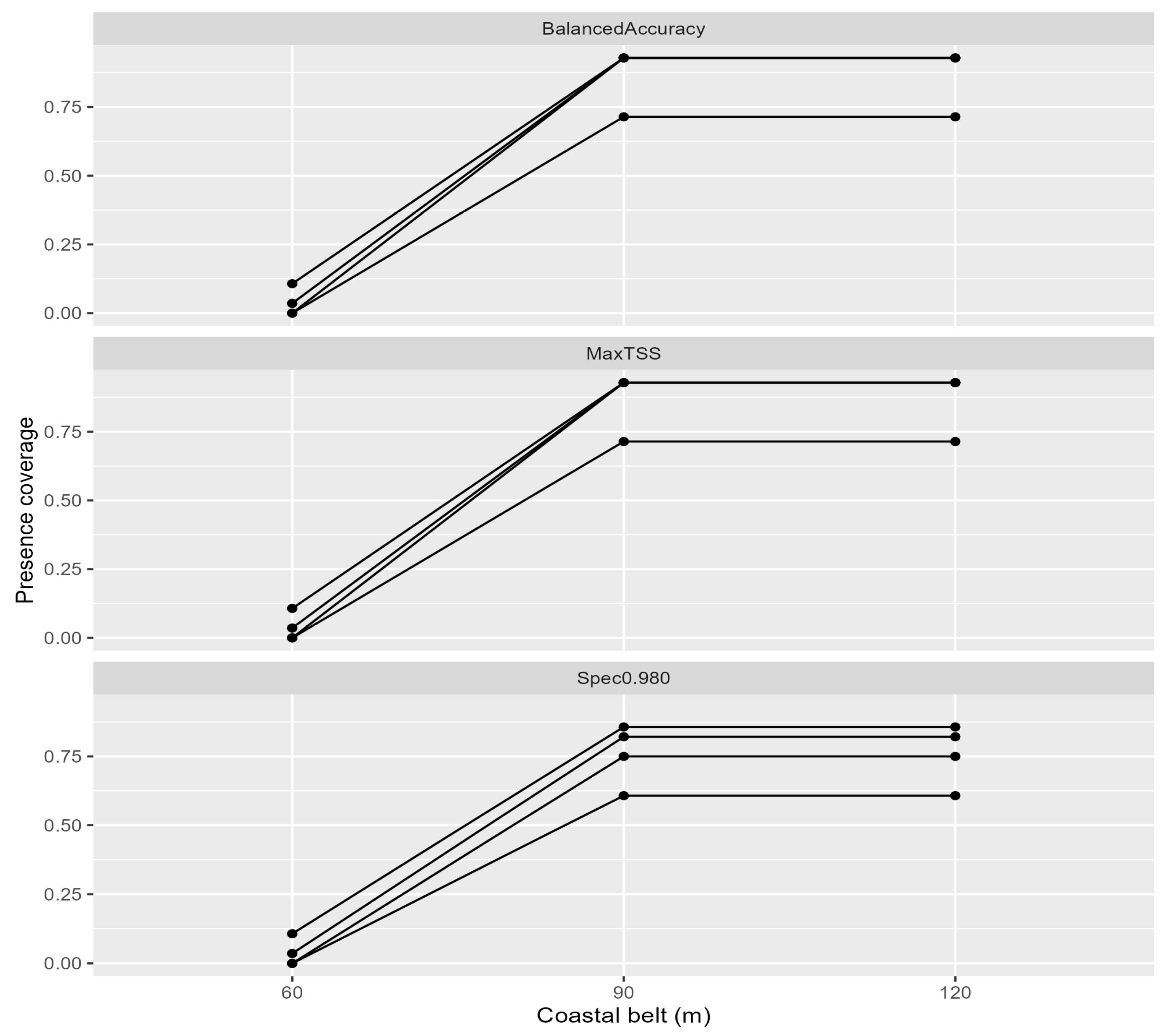
4.3. Interpretation of the SDM and Methodological Considerations
4.4. Data-Driven Spatial Conservation Strategy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| ε (m) | n_clusters (Folds) | AUC (Mean ± SD) | pAUC (Mean ± SD) |
|---|---|---|---|
| 150 | 19 | 0.988 ± 0.014 | 0.939 ± 0.071 |
| 200 | 17 | 0.987 ± 0.014 | 0.932 ± 0.073 |
| 250 | 15 | 0.984 ± 0.014 | 0.935 ± 0.074 |
| 300 | 14 | 0.987 ± 0.014 | 0.931 ± 0.076 |
| Ratio | n | (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) |
|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 40 | 0.925 | 0.010 | 0.810 | 0.319 | 0.933 | 0.019 | 0.020 | 0.019 | 0.057 |
| 15 | 40 | 0.925 | 0.007 | 0.808 | 0.294 | 0.950 | 0.013 | 0.023 | 0.013 | 0.070 |
| 20 | 40 | 0.924 | 0.006 | 0.805 | 0.304 | 0.957 | 0.009 | 0.018 | 0.012 | 0.054 |
| 25 | 40 | 0.925 | 0.005 | 0.807 | 0.302 | 0.966 | 0.007 | 0.023 | 0.014 | 0.068 |
| 30 | 40 | 0.923 | 0.004 | 0.802 | 0.286 | 0.971 | 0.007 | 0.024 | 0.008 | 0.073 |
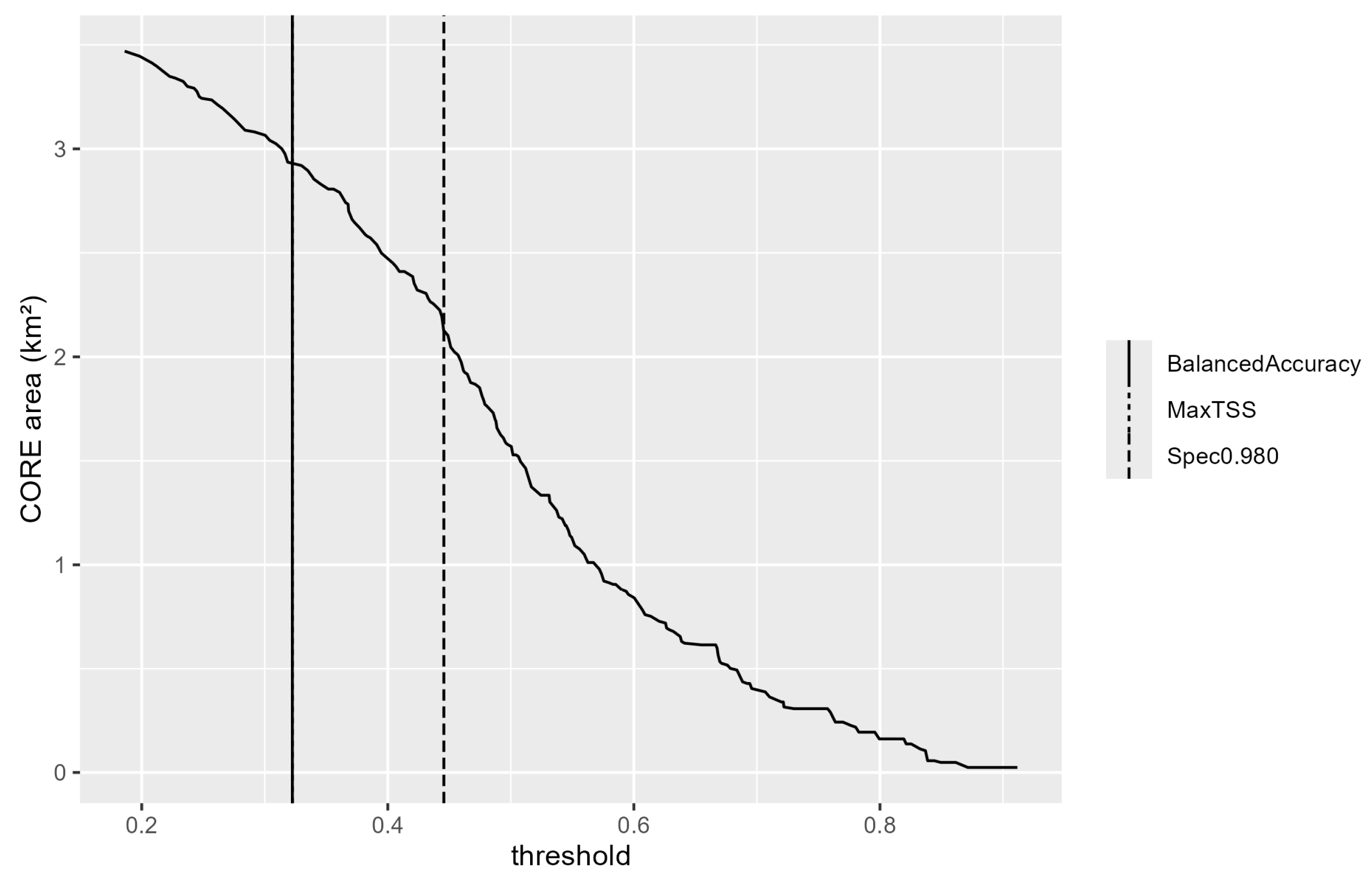
| Threshold | Thr | Pred. Area (km2) | Sens | Spec | PPV | NPV | Note |
|---|---|---|---|---|---|---|---|
| Balanced (apparent) | 0.239 | 3.728 | 1.000 | 0.946 | 0.412 | 1.000 | Pre-policy (median Youden across folds) |
| TP10 | 0.382 | 2.321 | 0.893 | 0.970 | 0.532 | 0.996 | Pre-policy |
| Coverage-target | 0.455 | 1.860 | 0.857 | 0.979 | 0.600 | 0.995 | Pre-policy (coverage at least 0.90) |
| Spec0.980 | 0.472 | 1.763 | 0.786 | 0.980 | 0.774 | 0.992 | Adopted (Pre-policy) |
Appendix B
References
- Xu, M.; Huang, X.; Li, J.; Qi, S.; Zhang, Y.; Zhang, X. Assessing the ecological risk and its driving forces on islands using the Pressure-State-Response model. Sci. Rep. 2025, 15, 23162. [Google Scholar] [CrossRef]
- Moustakas, A.; Shiri, Z.-S.; Mirela, T.; Savvas, Z.; Nazli, D.; Christos, Z.; Irene, C.; Turgay, D.; Tamer, A.; Cigdem, K.A.; et al. Climate land use and other drivers impacts on island ecosystem services: A global review. Sci. Total Environ. 2025, 973, 179147. [Google Scholar] [CrossRef]
- Caujapé-Castells, J.; Tye, A.; Crawford, D.J.; Santos-Guerra, A.; Sakai, A.; Beaver, K.; Lobin, W.; Florens, F.B.V.; Moura, M.; Jardim, R.; et al. Conservation of oceanic island floras: Present and future global challenges. Perspect. Plant Ecol. Evol. Syst. 2010, 12, 107–129. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, A.; Wang, Y. Scrophularia ningpoensis Hemsl: A review of its phytochemistry, pharmacology, quality control and pharmacokinetics. J. Pharm. Pharmacol. 2021, 73, 573–600. [Google Scholar] [CrossRef] [PubMed]
- Tamri, P. A mini-review on phytochemistry and pharmacological activities of Scrophularia striata. J. Herbmed Pharmacol. 2019, 8, 85–89. [Google Scholar] [CrossRef]
- Seo, Y.S.; Song, J.H.; Kim, H.S.; Nam, H.H.; Yang, S.; Choi, G.; Chae, S.W.; Lee, J.; Jung, B.; Kim, J.S.; et al. An Integrative Study of Scrophularia takesimensis Nakai in an Ovalbumin-Induced Murine Model of Asthma: The Effect on T Helper 2 Cell Activation. Pharmaceutics 2024, 16, 529. [Google Scholar] [CrossRef]
- Ministry of Environment (ME); National Institute of Ecology (NIE). Overview of Endangered Wildlife; ME; NIE: Yeongyang, Republic of Korea, 2023; pp. 520–521.
- Ministry of Environment (ME); National Institute of Biological Resources (NIBR). Red Data Book of Republic of Korea. Vascular Plants; ME; NIBR: Incheon, Republic of Korea, 2021; Volume 5, pp. 64–65.
- IUCN. IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 1 September 2025).
- Nakai, T. Notulae ad Plantas Asiae Orientalis (V). J. Jpn. Bot. 1932, 14, 631–637. [Google Scholar]
- Ahn, Y.H. Ecological characteristics and distribution of native Scrophularia takesimensis in Ulleung-do Island. Korean J. Environ. Sci. 2005, 14, 1087–1092. [Google Scholar] [CrossRef]
- Han, K.S.; Kim, M.Y.; Shu, G.U.; Kwon, H.J.; Song, H.G. Vegetation and soil properties of Scrophularia takesimensis population in Ulleung Island. J. Korean Environ. Res. Technol. 2010, 13, 24–31. [Google Scholar]
- Kim, S.S.; Kang, K.H.; Kang, S.G.; Shin, H.T.; Cho, G.H.; Kim, Y.Y.; Lee, M.S.; Yong, B.H. A discovery of new habitats and conservation strategy of Scrophularia takesimensis Nakai in Ulleung Island. J. Agric. Life Sci. 2013, 47, 83–94. [Google Scholar]
- Korea National Arboretum. The National Red List of Vascular Plants in Korea; Korea National Arboretum: Pocheon, Republic of Korea, 2021; p. 52. [Google Scholar]
- Gil, H.Y.; Maki, M.; Pimenova, E.A.; Taran, A.; Kim, S.C. Origin of the critically endangered endemic species Scrophularia takesimensis (Scrophulariaceae) on Ulleung Island, Korea: Implications for conservation. J. Plant Res. 2020, 133, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Na, S.T.; Lee, S.J.; Cho, K.H.; Shin, H.C. Spatial distribution patterns and implications for conservation of Scrophularia takesimensis (Scrophulariaceae), an endangered endemic species on Ulleung Island, Korea. J. Plant Biol. 2008, 51, 213–220. [Google Scholar] [CrossRef]
- Kim, S.S.; Gan, G.H. Conservation and status of distribution Scrophularia takesimensis Nakai Ulleung island, Korea. Proc. Korean Soc. Environ. Ecol. Conf. 2010, 20, 55–58. [Google Scholar]
- Choi, H.J.; Jang, H.D.; Isagi, Y.; Oh, B.U. Distribution and conservation status of the Critically Endangered Scrophularia takesimensis, a plant endemic to Ulleung Island, Republic of Korea. Oryx 2012, 46, 399–402. [Google Scholar] [CrossRef]
- Han, K.S.; So, S.K.; Lee, C.H.; Kim, M.Y. Taxonomy of the genus Scrophularia (Scrophulariaceae) in Korea. Korean J. Plant Taxon. 2009, 39, 237–246. [Google Scholar] [CrossRef]
- Lee, J.A.; Ahn, Y.H.; Park, K.W.; Kang, K.H. Study of artificial propagation about Scrophularia takesimensis. J. Korean Soc. Plant Environ. Des. 2007, 3, 19–26. [Google Scholar]
- Lee, J.H.; An, C.H.; Lee, Y.J.; Kim, S.C.; Sik, C.S.; Kim, S.M. Effect of Storage Condition on the Germination of Scrophularia buergeriana and Scrophularia takesimensis. Korean J. Med. Crop Sci. 2016, 24, 393–400. [Google Scholar] [CrossRef]
- Kim, M.I.; Hong, H.P.; Kwak, J.H.; Kim, M.S. Analysis of ground disasters in Ulleungdo Island caused by typhoons and heavy rains. Water Future 2016, 49, 38–45. [Google Scholar]
- Fois, M.; Bacchetta, G.; Cuena-Lombrana, A.; Donatella, C.; Maria, S.P.; Elena, S. Using extinctions in species distribution models to evaluate and predict threats: A contribution to plant conservation planning on the island of Sardinia. Environ. Conserv. 2018, 45, 11–19. [Google Scholar] [CrossRef]
- Gogol-Prokurat, M. Predicting habitat suitability for rare plants at local spatial scales using a species distribution model. Ecol. Appl. 2011, 21, 33–47. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A. A practical guide to MaxEnt for modeling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Shcheglovitova, M.; Anderson, R.P. Estimating optimal complexity for ecological niche models: A comparison of three approaches. Glob. Ecol. Biogeogr. 2013, 22, 921–931. [Google Scholar]
- Radosavljevic, A.; Anderson, R.P. Making better maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Ulleung-gun. Ulleungdo, Dokdo Geopark. Available online: https://www.ulleung.go.kr/geo/kr/page.do?mnu_uid=583& (accessed on 10 September 2025).
- Song, Y.S.; Park, K.H.; Park, M.E. Major, rare-earth and trace geochemistry of Ulleungdo volcanic rocks. J. Petrol. Soc. Korea 1999, 8, 70–75. [Google Scholar]
- Bae, G.B.; Choo, C.O. Geological heritage of the Ulleungdo·Dokdo National Geopark and its management system. J. Geol. Soc. Korea 2016, 52, 739–761. [Google Scholar] [CrossRef]
- Korea Meteorological Administration; Daegu Meteorological Office. Detailed Climate Change Analysis Report for Ulleung-gun, Gyeongsangbuk-do; Korea Meteorological Administration: Seoul, Republic of Korea, 2015; pp. 12–15.
- Kim, J.W.; Song, S.D.; Kim, S.H. A Syntaxonomical study of the vegetation of Ulleung-do and Tok-do, Korea. Rep. Surv. Nat. Environ. Korea 1996, 10, 137–202. [Google Scholar]
- Kim, H.H.; Kim, D.B.; Song, H.H.; Hwang, G.Y.; Kong, W.S. Phytogeographical characteristics of outermost islands in the Korean Peninsula. J. Korea Geogr. Soc. 2018, 53, 117–132. [Google Scholar]
- Ester, M.; Kriegel, H.-P.; Sander, J.; Xu, X. A Density-Based Algorithm for Discovering Clusters in Large Spatial Databases with Noise. In Proceedings of the Second International Conference on Knowledge Discovery and Data Mining (KDD-96), Portland, OR, USA, 2–4 August 1996; pp. 226–231. [Google Scholar]
- Phillips, S. Maxnet: Fitting ‘Maxent’ Species Distribution Models with glmnet. R Package Version 0.1.4. 2022. Available online: https://CRAN.R-project.org/package=maxnet/ (accessed on 4 June 2025).
- National Geographic Information Institute (NGII). DEM: Suwon, Republic of Korea. 2025. Available online: https://map.ngii.go.kr (accessed on 1 September 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2025; Available online: https://www.R-project.org/ (accessed on 5 September 2025).
- Hijmans, R. terra: Spatial Data Analysis. R Package Version 1.8-59. 2025. Available online: https://github.com/rspatial/terra (accessed on 4 June 2025).
- Pebesma, E. Simple features for R: Standardized support for spatial vector data. R J. 2018, 10, 439–446. [Google Scholar] [CrossRef]
- Pebesma, E.; Bivand, R. Spatial Data Science: With Applications in R, 1st ed.; Chapman and Hall/CRC: New York, NY, USA, 2023; p. 314. [Google Scholar]
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation. R Package Version 1.1.4. 2025. Available online: https://CRAN.R-project.org/package=dplyr/ (accessed on 4 June 2025).
- Wickham, H.; Vaughan, D.; Girlich, M. tidyr: Tidy Messy Data. R Package Version 1.3.1. 2025. Available online: https://CRAN.R-project.org/package=tidyr/ (accessed on 4 June 2025).
- Wickham, H.; Henry, L. purrr: Functional Programming Tools. R Package Version 1.1.0. 2025. Available online: https://CRAN.R-project.org/package=purrr/ (accessed on 6 June 2025).
- Wickham, H.; Bryan, J. readxl: Read Excel Files. R Package Version 1.4.5. 2025. Available online: https://CRAN.R-project.org/package=readxl/ (accessed on 4 June 2025).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef]
- Wickham, H.; Bryan, J. ggplot2: Elegant Graphics for Data Analysis. R Package Version 3.4.4. 2023. Available online: https://CRAN.R-project.org/package=ggplot2/ (accessed on 4 June 2025).
- Hernangómez, D.; Dunnington, D. tidyterra: Tidy Data Manipulation for Terra Objects. R Package Version 0.8.1. 2025. Available online: https://CRAN.R-project.org/package=tidyterra/ (accessed on 4 June 2025).
- Hamabata, T.; Kinoshita, G.; Kurita, K.; Cao, P.-L.; Ito, M.; Murata, J.; Komaki, Y.; Isagi, Y.; Makino, T. Endangered island endemic plants have vulnerable genomes. Commun. Biol. 2019, 2, 244. [Google Scholar] [CrossRef]
- Van Rossum, F.; Le Pajolec, S.; Raspé, O.; Godé, C. Assessing Population Genetic Status for Designing Plant Translocations. Front. Conserv. Sci. 2022, 3, 829332. [Google Scholar] [CrossRef]
- Jo, S.C. Genetic Diversity and Population Structure of Scrophularia takesimensis Nakai, an Endemic Species of Ulleung Island. Master’s Thesis, Mokpo National University, Mokpo, Republic of Korea, 2025; pp. 14–42. [Google Scholar]
- Bonanno, G.; Veneziano, V. Rise, Fall and hope for the Sicilian endemic plant Muscari gussonei: A story of survival in the face of narrow germination optimum, climate changes, desertification and habitat fragmentation. Sci. Total Environ. 2024, 912, 169208. [Google Scholar] [CrossRef]
- Kim, Y.D. Comprehensive Approaches on the Monitoring and Systematics of Plant Resources in Ulleung-Do; Korea Science and Engineering Foundation: Daegu, Republic of Korea, 2007; pp. 40–48. [Google Scholar]
- Caperta, A.D.; Espírito-Santo, M.D.; Silva, V.; Ferreira, A.; Paes, A.P.; Róis, A.S.; Costa, J.C.; Arsénio, P. Habitat specificity of a threatened and endemic, cliff-dwelling halophyte. AoB Plants 2014, 6, plu032. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H. A geomorphology on the Ulleungdo. J. Korean Geogr. Assoc. 2012, 19, 39–57. [Google Scholar]
- Ciccarese, G.; Tondo, M.; Mulas, M.; Bertolini, G.; Corsini, A. Rapid Assessment of Landslide Dynamics by UAV-RTK Repeated Surveys Using Ground Targets: The Ca’ Lita Landslide (Northern Apennines, Italy). Remote Sens. 2024, 16, 1032. [Google Scholar] [CrossRef]
- Matesanz, S.; Escudero, A.; Valladares, F. Impact of three global change drivers on a Mediterranean shrub. Ecology 2009, 90, 2609–2621. [Google Scholar] [CrossRef]
- Gonzalez-Varo, J.P.; Albaladejo, R.G.; Aparicio, A.; Arroyo, J. Linking genetic diversity, mating patterns and progeny performance in fragmented populations of a Mediterranean shrub. J. Appl. Ecol. 2010, 47, 1242–1252. [Google Scholar] [CrossRef]
| Background (m) | Spec0.980_thr | CORE_Area (km2) | Coverage_Recent |
|---|---|---|---|
| 300 | 0.494 | 1.108 | 0.714 |
| 450 | 0.380 | 1.593 | 0.789 |
| 600 | 0.522 | 0.930 | 0.607 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, G.-Y.; Kim, N.-Y.; Eom, T.-K.; Kim, D.; Lee, S.-E.; Ryu, T.-B. Spatial Patterns and Species Distribution Model-Based Conservation Priorities for Scrophularia takesimensis on Ulleungdo. Plants 2025, 14, 3498. https://doi.org/10.3390/plants14223498
Lee G-Y, Kim N-Y, Eom T-K, Kim D, Lee S-E, Ryu T-B. Spatial Patterns and Species Distribution Model-Based Conservation Priorities for Scrophularia takesimensis on Ulleungdo. Plants. 2025; 14(22):3498. https://doi.org/10.3390/plants14223498
Chicago/Turabian StyleLee, Gyeong-Yeon, Na-Yeong Kim, Tae-Kyung Eom, Deokki Kim, Seung-Eun Lee, and Tae-Bok Ryu. 2025. "Spatial Patterns and Species Distribution Model-Based Conservation Priorities for Scrophularia takesimensis on Ulleungdo" Plants 14, no. 22: 3498. https://doi.org/10.3390/plants14223498
APA StyleLee, G.-Y., Kim, N.-Y., Eom, T.-K., Kim, D., Lee, S.-E., & Ryu, T.-B. (2025). Spatial Patterns and Species Distribution Model-Based Conservation Priorities for Scrophularia takesimensis on Ulleungdo. Plants, 14(22), 3498. https://doi.org/10.3390/plants14223498






