Hemp Inflorescence as a Sustainable Biostimulant Tool to Boost Growth and Antioxidant Capacity in Oilseed Pumpkin
Abstract
1. Introduction
2. Results
2.1. One-Way ANOVA
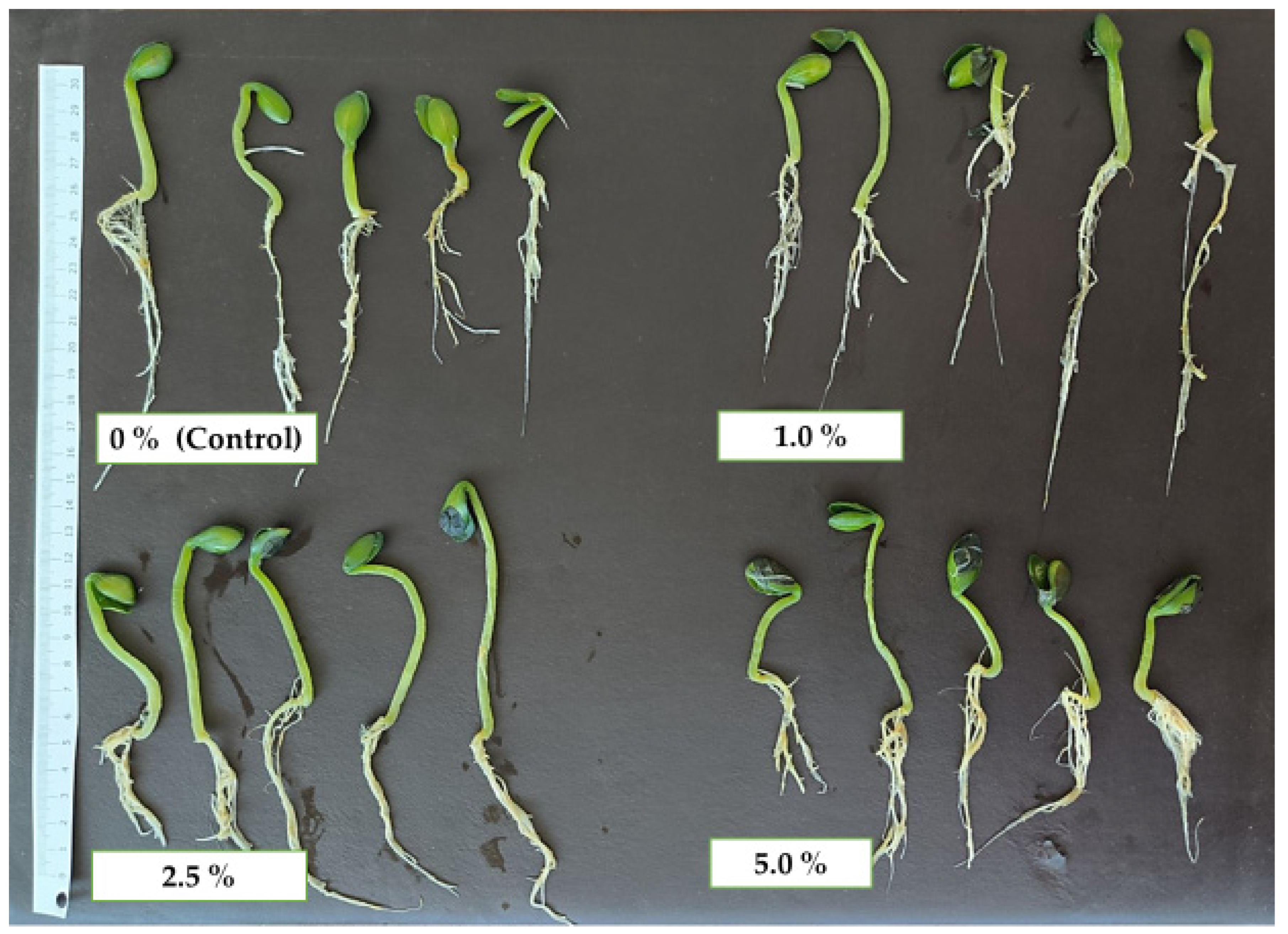
2.2. Morphological and Growth Parameters of the Hull-Less Oilseed Pumpkin Sprouts
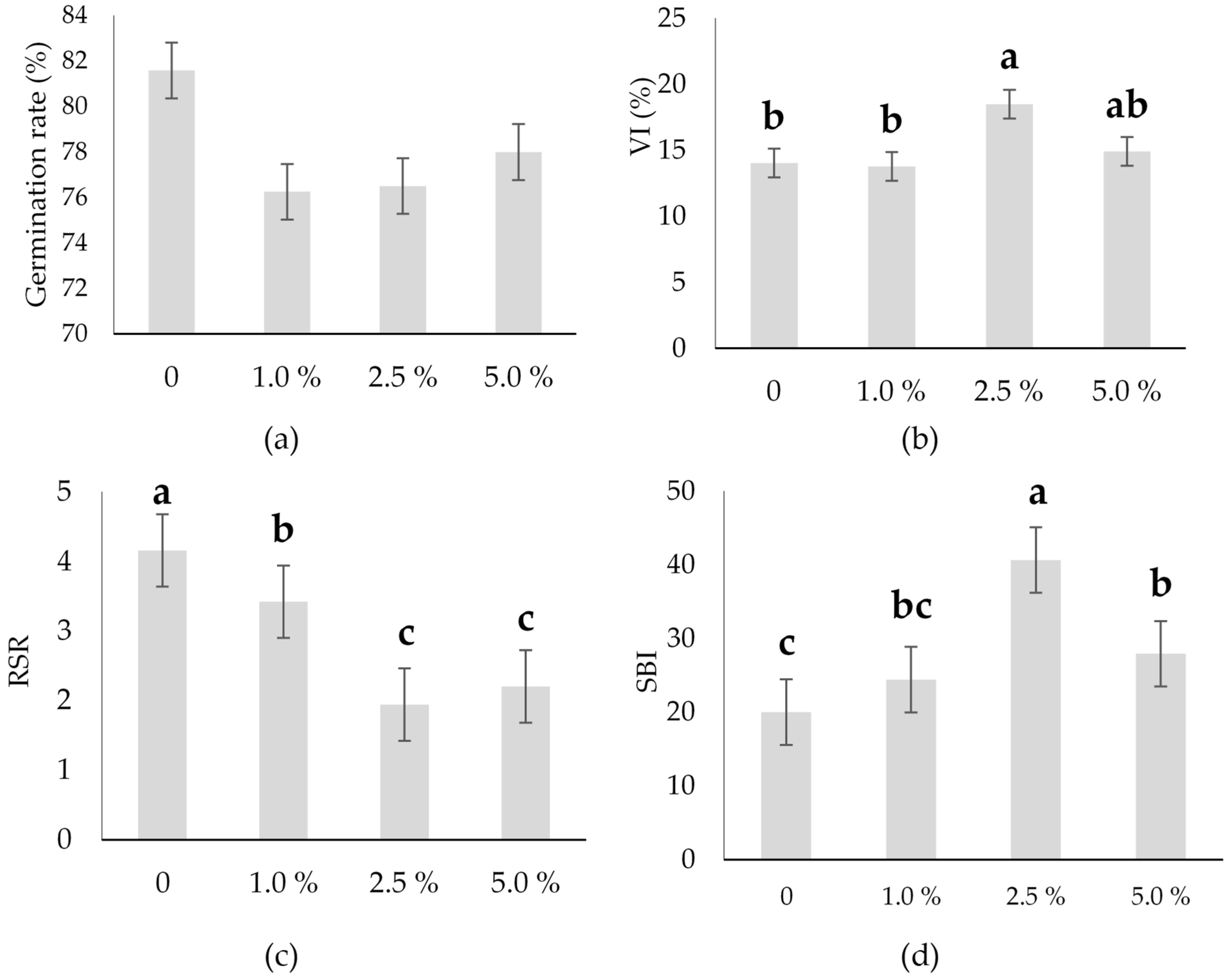
2.3. Total Germination Rate and Growth Parameters of the Hull-Less Oilseed Pumpkin Sprouts
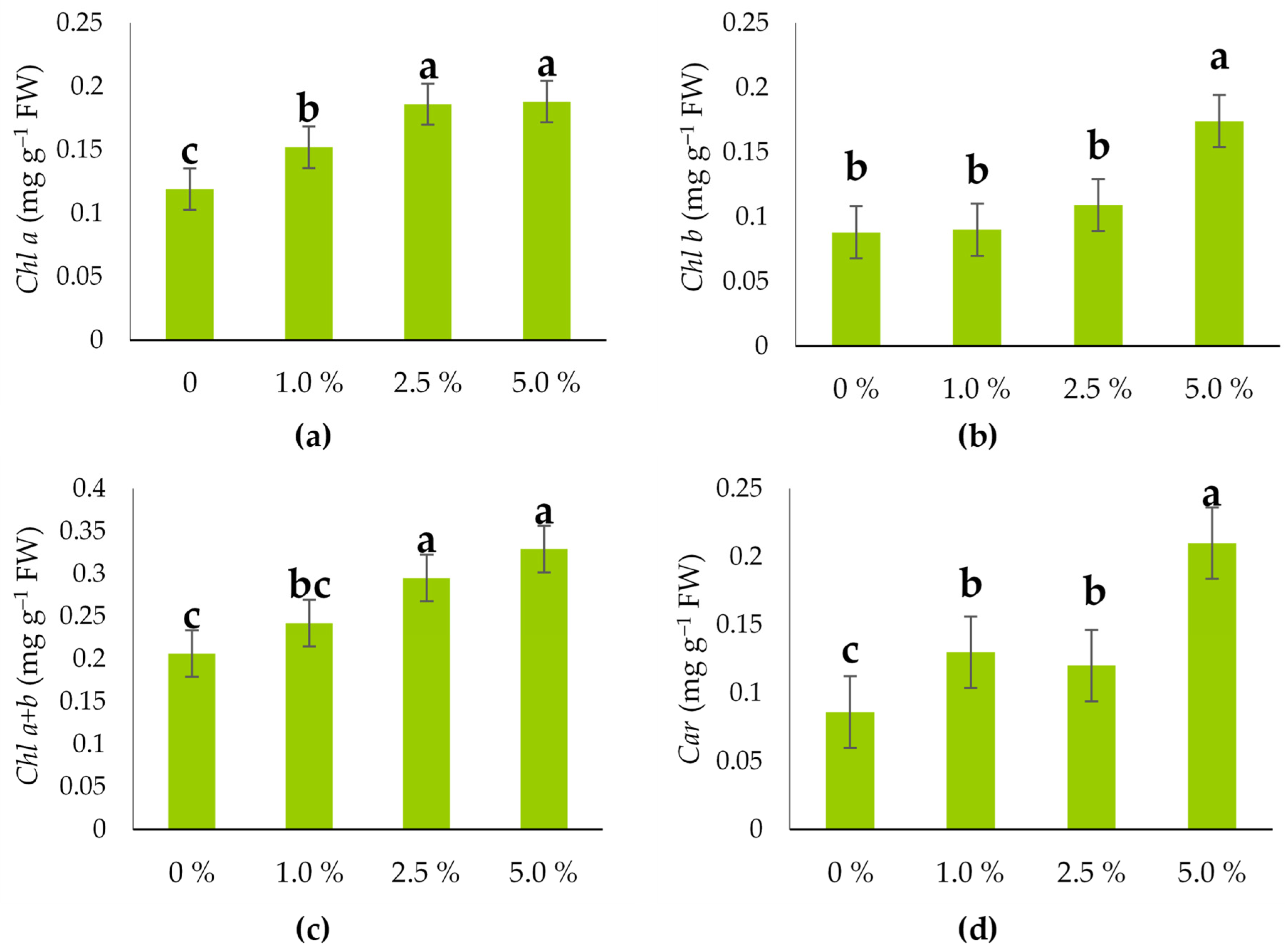
2.4. The Pigment Status of the Hull-Less Oilseed Pumpkin Sprouts
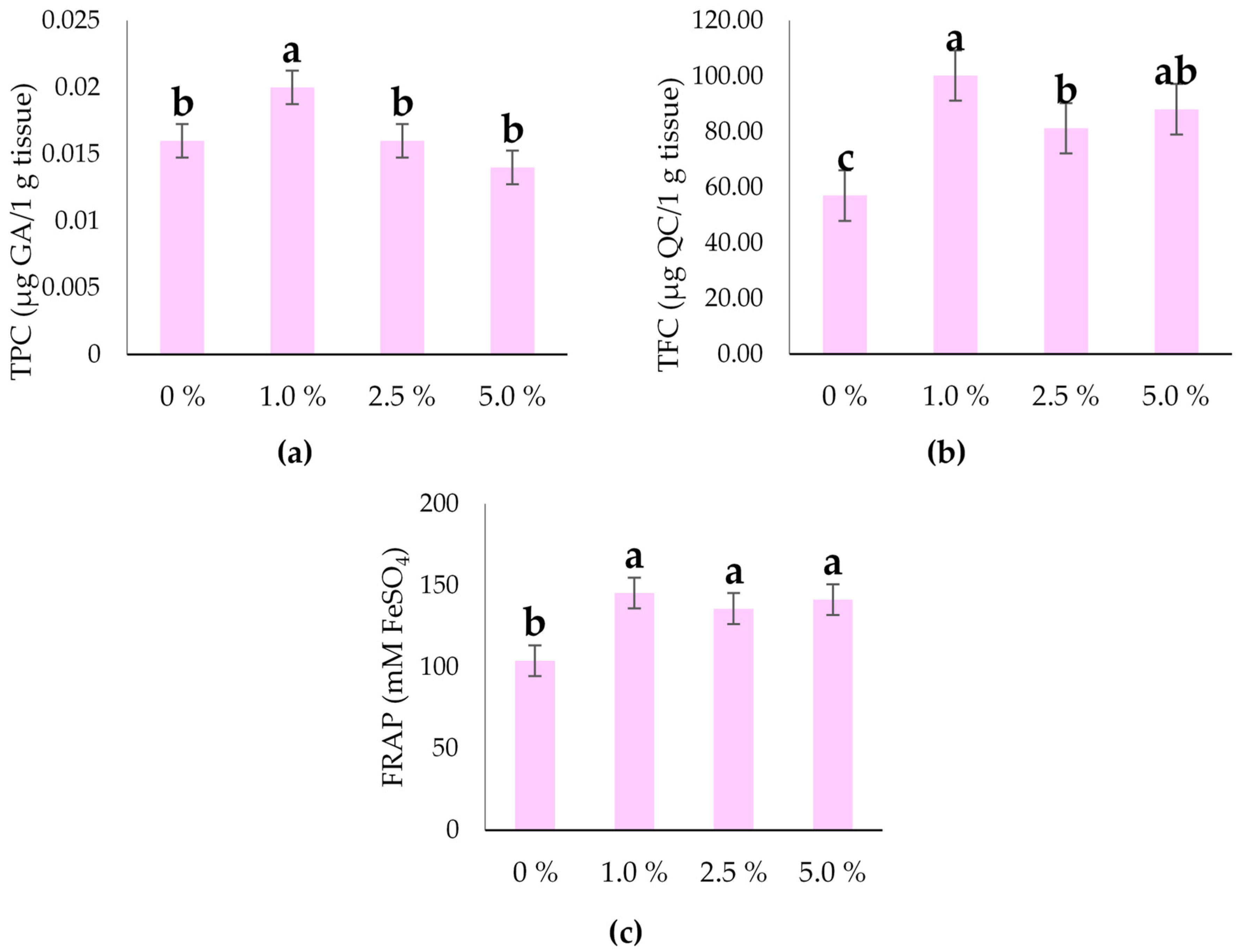
2.5. Total Phenols, Flavonoids, and Antioxidant Activity of the Hull-Less Oilseed Pumpkin Sprouts
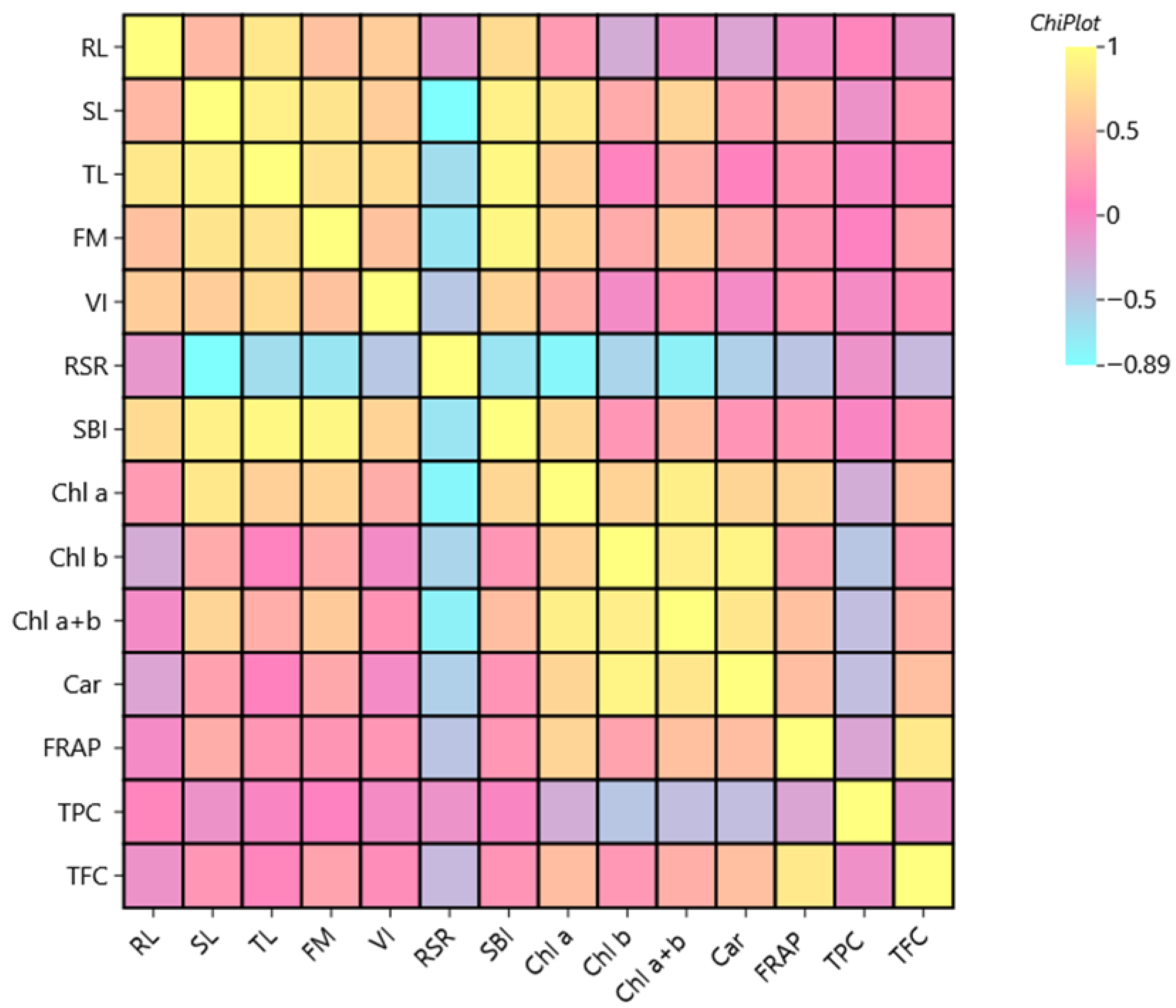
2.6. Correlation Analysis
3. Discussion
3.1. Morphological Parameters and Growth Analysis of the Hull-Less Oilseed Pumpkin Sprouts
3.2. Leaf Pigments, Phenols, Flavonoid Content, and Antioxidant Capacity of the Hull-Less Oilseed Pumpkin Sprouts
4. Materials and Methods
4.1. Preparation of Water Extracts
4.2. Seed Germination Bioassay in Petri Dishes
4.3. Total Germination and Morphological Parameters of Hull-Less Oilseed Pumpkin Sprouts
4.4. Analyses of the Pigment Content of the Hull-Less Oilseed Pumpkin Sprouts
4.5. Analyses of the Total Phenolic and Flavonoid Content of the Industrial Hemp Water Extract and Hull-Less Oilseed Pumpkin Sprouts
4.6. Antioxidant Activity of the Hull-Less Oilseed Pumpkin Sprouts
4.7. Biometric Approach
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| TG | total germination rate |
| RL | root length |
| SL | stem length |
| TL | total sprout length |
| FM | fresh mass |
| FW | Fresh weight |
| Chl a | Chlorophyll a |
| Chl b | Chlorophyll b |
| Car | Carotenoids |
| VI | Vigor index |
| RSR | Root to shoot ratio |
| SBI | Sprouts biomass index |
| SE | Standard Error |
| FRAP | ferric reducing antioxidant power |
| TPC | total phenol content |
| TFC | total flavonoid content |
References
- Đurić, M.; Mladenović, J.; Bošković-Rakočević, L.; Šekularac, G.; Brković, D.; Pavlović, N. Effect of Mineral Fertilizers on the Yield and Quality of Industrial Hemp (Cannabis sativa L.). Acta Agric. Serbica 2019, 24, 27–39. [Google Scholar] [CrossRef]
- Durán-Lara, E.F.; Valderrama, A.; Marican, A. Natural Organic Compounds for Application in Organic Farming. Agriculture 2020, 10, 41. [Google Scholar] [CrossRef]
- Suteu, D.; Rusu, L.; Zaharia, C.; Badeanu, M.; Daraban, G.M. Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products. Appl. Sci. 2020, 10, 8913. [Google Scholar] [CrossRef]
- Cenobio-Galindo, A.d.J.; Hernández-Fuentes, A.D.; González-Lemus, U.; Zaldívar-Ortega, A.K.; González-Montiel, L.; Madariaga-Navarrete, A.; Hernández-Soto, I. Biofungicides Based on Plant Extracts: On the Road to Organic Farming. Int. J. Mol. Sci. 2024, 25, 6879. [Google Scholar] [CrossRef]
- Ravlić, M.; Baličević, R.; Vinković, Ž.; Brozović, B.; Sarajić, A.; Kranjac, D. The Allelopathic Potential of Ruderal Plant Species on Tomato and Lettuce. Poljoprivreda 2024, 30, 3–9. [Google Scholar] [CrossRef]
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A Systematic Approach to Discover and Characterize Natural Plant Biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef]
- Perić, K.; Čupić, T.; Krizmanić, G.; Tokić, B.; Andrić, L.; Ravlić, M.; Meglič, V.; Tucak, M. The Role of Crop Wild Relatives and Landraces of Forage Legumes in Pre-Breeding as a Response to Climate Change. Agronomy 2024, 14, 1385. [Google Scholar] [CrossRef]
- Ravlić, M.; Baličević, R.; Lisjak, M.; Vinković, Ž.; Ravlić, J.; Županić, A.; Svitlica, B. Allelopathic Effect of Salvia pratensis L. on Germination and Growth of Crops. Crops 2025, 5, 45. [Google Scholar] [CrossRef]
- Godlewska, K.; Ronga, D.; Michalak, I. Plant Extracts—Importance in Sustainable Agriculture. Ital. J. Agron. 2021, 16, 1851. [Google Scholar] [CrossRef]
- Parađiković, N.; Vinković, T.; Vinković Vrček, I.; Žuntar, I.; Bojić, M.; Medić-Šarić, M. Effect of Natural Biostimulants on Yield and Nutritional Quality: An Example of Sweet Yellow Pepper (Capsicum annuum L.) Plants. J. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef]
- Brozović, B.; Jug, I.; Jug, D.; Stipešević, B.; Ravlić, M.; Đurđević, B. Biochar and Fertilization Effects on Weed Incidence in Winter Wheat. Agronomy 2021, 11, 2028. [Google Scholar] [CrossRef]
- Han, M.; Kasim, S.; Yang, Z.; Deng, X.; Saidi, N.; Uddin, M.; Shuib, E. Plant Extracts as Biostimulant Agents: A Promising Strategy for Managing Environmental Stress in Sustainable Agriculture. Phyton 2024, 93, 2149. [Google Scholar] [CrossRef]
- Szparaga, A. From biostimulant to possible plant bioprotectant agents. Agric. Eng. 2023, 27, 87–98. [Google Scholar] [CrossRef]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Akram, N.A.; Saleem, M.H.; Shafiq, S.; Naz, H.; Farid-ul-Haq, M.; Ali, B.; Shafiq, F.; Iqbal, M.; Jaremko, M.; Qureshi, K.A. Phytoextracts as Crop Biostimulants and Natural Protective Agents—A Critical Review. Sustainability 2022, 14, 14498. [Google Scholar] [CrossRef]
- Iljkić, D.; Vuković, M.; Dvojković, K.; Horvat, D.; Szpunar-Krok, E.; Jańczak-Pieniążek, M.; Rastija, M. Variety, Chemical Protection and Biostimulator Effect on Winter Wheat Status. Poljoprivreda 2024, 30, 28–35. [Google Scholar] [CrossRef]
- Gluhić, D. Primjena biostimulatora na bazi aminokiselina u poljoprivrednoj proizvodnji. Glas. Zašt. Bilja 2020, 43, 38–46. [Google Scholar] [CrossRef]
- Varga, I.; Kristić, M.; Lisjak, M.; Tkalec Kojić, M.; Iljkić, D.; Jović, J.; Kristek, S.; Markulj Kulundžić, A.; Antunović, M. Antioxidative Response and Phenolic Content of Young Industrial Hemp Leaves at Different Light and Mycorrhiza. Plants 2024, 13, 840. [Google Scholar] [CrossRef]
- Saa, S.; Olivos-Del Rio, A.; Castro, S.; Brown, P.H. Foliar Application of Microbial and Plant Based Biostimulants Increases Growth and Potassium Uptake in Almond (Prunus dulcis [Mill.] D.A. Webb). Front. Plant Sci. 2015, 6, 87. [Google Scholar] [CrossRef]
- Ravlić, M.; Markulj Kulundžić, A.; Baličević, R.; Marković, M.; Viljevac Vuletić, M.; Kranjac, D.; Sarajlić, A. Allelopathic Potential of Sunflower Genotypes at Different Growth Stages on Lettuce. Appl. Sci. 2022, 12, 12568. [Google Scholar] [CrossRef]
- Ali, Q.; Shehzad, F.; Waseem, M.; Shahid, S.; Hussain, A.I.; Haider, M.Z.; Habib, N.; Hussain, S.M.; Javed, M.T.; Perveen, R. Plant-Based Biostimulants and Plant Stress Responses. In Plant Ecophysiology and Adaptation Under Climate Change: Mechanisms and Perspectives I; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Graziani, G.; Cirillo, A.; Giannini, P.; Conti, S.; El-Nakhel, C.; Rouphael, Y.; Ritieni, A.; Di Vaio, C. Biostimulants Improve Plant Growth and Bioactive Compounds of Young Olive Trees under Abiotic Stress Conditions. Agriculture 2022, 12, 227. [Google Scholar] [CrossRef]
- Baličević, R.; Ravlić, M.; Lucić, K.; Tatarević, M.; Lucić, P.; Marković, M. Allelopathic Effect of Aloe vera (L.) Burm. F. on Seed Germination and Seedlings Growth of Cereals, Industrial Crops and Vegetables. Poljoprivreda 2018, 24, 13–19. [Google Scholar] [CrossRef]
- Varga, I.; Kraus, I.; Iljkić, D.; Jonjić, A.; Antunović, M. Tradicija proizvodnje industrijske konoplje u Hrvatskoj. Sjemenarstvo 2022, 33, 25–40. [Google Scholar] [CrossRef]
- Visković, J.; Dunđerski, D.; Adamović, B.; Jaćimović, G.; Latković, D.; Vojnović, Đ. Toward an Environmentally Friendly Future: An Overview of Biofuels from Corn and Potential Alternatives in Hemp and Cucurbits. Agronomy 2024, 14, 1195. [Google Scholar] [CrossRef]
- Klir, Ž.; Novoselec, J.; Antunović, Z. An Overview on the Use of Hemp (Cannabis sativa L.) in Animal Nutrition. Poljoprivreda 2019, 25, 52–61. [Google Scholar] [CrossRef]
- Matassa, S.; Esposito, G.; Pirozzi, F.; Papirio, S. Exploring the Biomethane Potential of Different Industrial Hemp (Cannabis sativa L.) Biomass Residues. Energies 2020, 13, 3361. [Google Scholar] [CrossRef]
- Varga, I.; Markulj Kulundžić, A.; Krolo, P.; Iljkić, D.; Tišma, M.; Kraus, I. Industrial Hemp Finola Variety Photosynthetic, Morphometric, Biomechanical, and Yield Responses to K Fertilization Across Different Growth Stages. Agronomy 2025, 15, 496. [Google Scholar] [CrossRef]
- Varga, I.; Iljkić, D.; Krolo, P.; Perić Fekete, A.; Kraus, I. The Source of K Fertilizer for Industrial Hemp (Cannabis sativa L.): Mechanical and Chemical Properties of Stem for Rammed Earth Walls. Agriculture 2024, 14, 2196. [Google Scholar] [CrossRef]
- Ranogajec, L.; Antunović, M.; Stipešević, B.; Varga, I. Stanje i potencijal proizvodnje industrijske konoplje u Hrvatskoj na osnovi SWOT analize. Poljoprivreda 2024, 30, 56–63. [Google Scholar] [CrossRef]
- Murkovic, M.; Hillebrand, A.; Winkler, J.; Leitner, E.; Pfannhauser, W. Variability of fatty acid content in pumpkin seeds (Cucurbita pepo L.). Z. Lebensm.-Unters. Forsch. 1996, 203, 216–219. [Google Scholar] [CrossRef]
- Sinkovič, L.; Verbič, J.; Kolmanič, A. Agronomical Traits of Oil Seed Pumpkin Cultivars (Cucurbita pepo subsp. pepo) and Nutritional Characteristics of Seeds, Oil Cakes and Pumpkin Oils. In ISHS Acta Horticulturae 1326, Proceedings of the VII South-Eastern Europe Symposium on Vegetables and Potatoes, Maribor, Slovenia, June 20–23 2017; International Society for Horticultural Science: Korbeek-Lo, Belgium, 2017; Volume 1326, pp. 215–222. [Google Scholar] [CrossRef]
- Klir, Z.; Castro-Montoya, J.M.; Novoselec, J.; Molkentin, J.; Domacinovic, M.; Mioc, B.; Antunovic, Z. Influence of Pumpkin Seed Cake and Extruded Linseed on Milk Production and Milk Fatty Acid Profile in Alpine Goats. Animals 2017, 11, 1772–1778. [Google Scholar] [CrossRef]
- Kaur, B.; Garcha, K.S.; Bhatia, D.; Khosa, J.S.; Sharma, M.; Mittal, A.; Dhatt, A.S. Identification of Single Major QTL and Candidate Gene(s) Governing Hull-Less Seed Trait in Pumpkin. Front. Plant Sci. 2022, 13, 948106. [Google Scholar] [CrossRef]
- Křístková, E.; Lebeda, A.; Sedláková, B. Species Spectra, Distribution and Host Range of Cucurbit Powdery Mildews in the Czech Republic, and in Some Other European and Middle Eastern Countries. Phytoparasitica 2009, 37, 337–350. [Google Scholar] [CrossRef]
- Jakop, M.; Grobelnik Mlakar, S.; Bavec, M.; Robačer, M.; Vukmanič, T.; Lisec, U.; Bavec, F. Yield Performance and Agronomic Efficiency in Oil Pumpkins (Cucurbita pepo L. Group Pepo) Depending on Production Systems and Varieties. Agricultura 2017, 14, 25–36. [Google Scholar] [CrossRef][Green Version]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. When Herbicides Don’t Really Matter: Weed Species Composition of Oil Pumpkin (Cucurbita pepo L.) Fields in Hungary. Crop Prot. 2018, 110, 236–244. [Google Scholar] [CrossRef]
- Godina Golija, M. Production and Consumption of Pumpkin Seed Oil in Goričko: Traditional and Contemporary Practices. Traditiones 2019, 48, 117–135. [Google Scholar] [CrossRef]
- Tomić, D.; Marjanović, M.; Radovanović, M.; Đurović, V.; Lazarević, Đ.; Stevović, V.; Pavlović, N. Preliminary Report: Interdependence of Seed Yield Components of Pumpkin (Cucurbita pepo L.) Genotypes. In AgroReS 2022, Proceedings of the XI International Symposium on Agricultural Sciences, Trebinje, Bosnia and Herzegovina, 26–28 May 2022; University of Banja Luka: Banja Luka, Bosnia and Herzegovina, 2022; p. 31. [Google Scholar]
- Tańska, M.; Ogrodowska, D.; Bartoszewski, G.; Korzeniewska, A.; Konopka, I. Seed Lipid Composition of New Hybrids of Styrian Oil Pumpkin Grown in Poland. Agronomy 2020, 10, 1104. [Google Scholar] [CrossRef]
- Csipkés, M. The Importance of Pumpkins and Oil Gourds in Romania. Ann. Univ. Oradea Econ. Sci. Ser. 2023, 32, 13. [Google Scholar] [CrossRef]
- Lelley, T.; Loy, B.; Murkovic, M. Hull-Less Oil Seed Pumpkin. In Oil Crops; Vollmann, J., Rajcan, I., Eds.; Springer: New York, NY, USA, 2009; pp. 469–492. [Google Scholar] [CrossRef]
- Boye, J.; Brault, D.; Couture, I.; Estevez, B.; Grenier, M.; Lefrançois, E.; Leblanc, M.; Lefebvre, M.; Moreau, G.; Ribéreau, S. Hulless Pumpkin Seed: A New Crop for Organic Production in Québec. Available online: https://irda.qc.ca/media/id5pdbxs/irda-ullesspumpkinseednewcroporganicproductioncultivars-fichessyntheses-2014.pdf (accessed on 4 August 2025).
- Meru, G.; Fu, Y.; Shrestha, S.; Michael, V.N.; Dorval, M.; Mainviel, R. Genomic Position and Markers Associated with the Hull-Less Seed Trait in Pumpkin. Plants 2022, 11, 1238. [Google Scholar] [CrossRef]
- Wargala, E.; Chrzanowska, A.; Bernatek-Samoraj, W.; Kot, I. Pumpkin (Cucurbita pepo L.) Seed Oil—Cosmetic, Food and Medical Raw Material. Herba Pol. 2023, 69, 7–14. [Google Scholar] [CrossRef]
- Salehi, B.; Capanoglu, E.; Adrar, N.; Catalkaya, G.; Shaheen, S.; Jaffer, M.; Giri, L.; Suyal, R.; Jugran, A.K.; Calina, D.; et al. Cucurbits Plants: A Key Emphasis to Its Pharmacological Potential. Molecules 2019, 24, 1854. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.; Dong, L.; Thorsen, T.H.; Raadam, M.H.; Khakimov, B.; Carreño-Quintero, N.; Madsen, C.K.; Borkhardt, B.; Larsen, F.H.; Motawia, M.S.; et al. Metabolic Engineering of Cucurbitacins in Cucurbita pepo Hairy Roots. Front. Plant Sci. 2022, 13, 1021907. [Google Scholar] [CrossRef] [PubMed]
- Šerá, B.; Hnilička, F. Genetic and Environmental Factors Affecting Seed Germination. Plants 2023, 12, 4106. [Google Scholar] [CrossRef]
- Hirano, H.; Watanabe, T.; Fukuda, M.; Fukao, T. The Impact of Carbohydrate Management on Coleoptile Elongation in Anaerobically Germinating Seeds of Rice (Oryza sativa L.) under Light and Dark Cycles. Plants 2023, 12, 1565. [Google Scholar] [CrossRef]
- Shah, S.S.H.; Latif, S.; Qureshi, R.; Ilyas, N.; Ahmad, M.S.; Rehman, S.; Khan, N.; Abdel-Maksoud, M.A.; El-Tayeb, M.A.; Saleh, I.A.; et al. Optimizing germination dynamics in seven key industrial and medicinal hemp varieties through seed priming techniques: An initial study for hemp cultivation in Pakistan. Ind. Crops Prod. 2024, 222, 119739. [Google Scholar] [CrossRef]
- Lisjak, M.; Ocvirk, D.; Špoljarević, M.; Teklić, T.; Liović, I.; Špoljarić Marković, S.; Volenik, M.; Mijić, A. Učinak primiranja sjemena sumporovodikom na klijanje i biokemijske pokazatelje sušnog stresa kod klijanaca suncokreta. Poljoprivreda 2025, 31, 1–12. [Google Scholar] [CrossRef]
- Jović, J.; Ivezić, V.; Popović, B.; Guberac, V.; Rastija, M.; Dujmović, L.; Orkić, V.; Kristek, S. Utjecaj primjene mikrobioloških preparata na odabrana svojstva kiselih tala. Poljoprivreda 2025, 31, 41–50. [Google Scholar] [CrossRef]
- Song, J.; Wang, H.; Chu, R.; Zhao, L.; Li, X.; An, S.; Qiang, M.; Du, W.; Li, Q. Differences in Physiological Characteristics, Seed Germination, and Seedling Establishment in Response to Salt Stress between Dimorphic Seeds in the Halophyte Suaeda liaotungensis. Plants 2023, 12, 1408. [Google Scholar] [CrossRef]
- Orkić, V.; Grubišić–Šestanj, S.; Ravnjak, B.; Rebekić, A.; Petrović, S.; Vila, S.; Kujundžić, S. Priježetveno Proklijavanje Kod Različitih Kultivara Pšenice. Poljoprivreda 2024, 30, 21–27. [Google Scholar] [CrossRef]
- Wazeer, H.; Shridhar Gaonkar, S.; Doria, E.; Pagano, A.; Balestrazzi, A.; Macovei, A. Plant-Based Biostimulants for Seeds in the Context of Circular Economy and Sustainability. Plants 2024, 13, 1004. [Google Scholar] [CrossRef]
- Petrović, E.; Vrandečić, K.; Ćosić, J.; Godena, S. Chemical Control of Olive Fungal Diseases: Strategies and Risks. Poljoprivreda 2024, 30, 44–53. [Google Scholar] [CrossRef]
- Pugelnik, I.; Rebekić, A.; Jelić Milković, S.; Lončarić, R. Stavovi Ekoloških Proizvođača o Ekološkoj Poljoprivredi u Republici Hrvatskoj. Poljoprivreda 2024, 30, 91–99. [Google Scholar] [CrossRef]
- Skowronek, P.; Wójcik, Ł.; Strachecka, A. Impressive Impact of Hemp Extract on Antioxidant System in Honey Bee (Apis mellifera) Organism. Antioxidants 2022, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Jolayemi, O.L.; Malik, A.H.; Ekblad, T.; Fredlund, K.; Olsson, M.E.; Johansson, E. Protein-Based Biostimulants to Enhance Plant Growth—State-of-the-Art and Future Direction with Sugar Beet as an Example. Agronomy 2022, 12, 3211. [Google Scholar] [CrossRef]
- Kovačić, Đ.; Radočaj, D.; Samac, D.; Jurišić, M. Influence of Thermal Pretreatment on Lignin Destabilization in Harvest Residues: An Ensemble Machine Learning Approach. AgriEngineering 2024, 6, 171–184. [Google Scholar] [CrossRef]
- Ravlić, M.; Baličević, R.; Svalina, T.; Posavac, D.; Ravlić, J. Herbicidal Potential of Meadow Sage (Salvia pratensis L.) against Velvetleaf (Abutilon theophrasti Med.) and Common Corn-Cockle (Agrostemma githago L.). Glas. Zaštite Bilja 2023, 67, 116–121. [Google Scholar] [CrossRef]
- Erhatić, R.; Horvat, D.; Zorić, Z.; Repajić, M.; Jović, T.; Herceg, M.; Habuš, M.; Srečec, S. Aqueous Extracts of Four Medicinal Plants and Their Allelopathic Effects on Germination and Seedlings: Their Morphometric Characteristics of Three Horticultural Plant Species. Appl. Sci. 2023, 13, 2258. [Google Scholar] [CrossRef]
- Janusauskaite, D. The Allelopathic Activity of Aqueous Extracts of Helianthus annuus L., Grown in Boreal Conditions, on Germination, Development, and Physiological Indices of Pisum sativum L. Plants 2023, 12, 1920. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Sobolev, A.P.; Giusti, A.M.; Vinci, G.; Cammarone, S.; Tortora, C.; Lamelza, L.; Prencipe, S.A.; et al. Industrial Hemp (Cannabis sativa L.) Inflorescences as Novel Food: The Effect of Different Agronomical Practices on Chemical Profile. Foods 2022, 11, 3658. [Google Scholar] [CrossRef]
- Visković, J.; Sikora, V.; Latković, D.; Zeremski, T.; Dunđerski, D.; Astatkie, T.; Noller, J.S.; Zheljazkov, V.D. Optimization of Hemp Production Technology for Fiber and Seed. Ind. Crops Prod. 2024, 219, 119127. [Google Scholar] [CrossRef]
- Habschied, K.; Jokić, S.; Aladić, K.; Šplajt, I.; Krstanović, V.; Mastanjević, K. Addition of Industrial Hemp (Cannabis sativa L.) Dry Inflorescence in Beer Production. Appl. Sci. 2025, 15, 624. [Google Scholar] [CrossRef]
- Bavec, F.; Gril, L.; Grobelnik-Mlakar, S.; Bavec, M. Seedlings of oil pumpkins as an alternative to seed sowing: Yield and production costs. Bodenkult.-Wien Munch. 2002, 53, 39–44. [Google Scholar]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Islam, M.M.; Rengel, Z.; Storer, P.; Siddique, K.H.M.; Solaiman, Z.M. Industrial Hemp (Cannabis sativa L.) Varieties and Seed Pre-Treatments Affect Seed Germination and Early Growth of Seedlings. Agronomy 2022, 12, 6. [Google Scholar] [CrossRef]
- Andrić, L. Ispitivanje vigora sjemena nekih domaćih kultivara soje (Glycine max (L.) Merrill). Poljoprivreda 2004, 10, 59–60. [Google Scholar]
- Varga, I.; Iljkić, D.; Šimunović, M.; Agić, D.; Herman, G.; Antunović, M. Morphological parameters of oil-pumpkin sprouts in different water-solution pH values. Sjemenarstvo 2024, 35, 27–38. [Google Scholar] [CrossRef]
- Lynch, J.; Marschner, P.; Rengel, Z. Effect of Internal and External Factors on Root Growth and Development. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: London, UK, 2012; pp. 331–346. [Google Scholar] [CrossRef]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The Use of a Plant-Based Biostimulant Improves Plant Performances and Fruit Quality in Tomato Plants Grown at Elevated Temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Mystkowska, I. The Effect of Biostimulants on the Chlorophyll Content and Height of Solanum tuberosum L. Plants. J. Ecol. Eng. 2022, 23, 72–77. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-Based Biostimulants Influence the Agronomical, Physiological, and Qualitative Responses of Baby Rocket Leaves under Diverse Nitrogen Conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef]
- Martínez-Lorente, S.E.; Martí-Guillén, J.M.; Pedreño, M.Á.; Almagro, L.; Sabater-Jara, A.B. Higher Plant-Derived Biostimulants: Mechanisms of Action and Their Role in Mitigating Plant Abiotic Stress. Antioxidants 2024, 13, 318. [Google Scholar] [CrossRef]
- Park, Y.R.; Kwon, S.-J.; Kim, J.H.; Duan, S.; Eom, S.H. Light-Induced Antioxidant Phenolic Changes among the Sprouts of Lentil Cultivar. Antioxidants 2024, 13, 399. [Google Scholar] [CrossRef] [PubMed]
- Kalinová, J.P.; Vrchotová, N.; Tříska, J. Flavonoids Profile in Pasta and Cookies Fortified with Common Buckwheat Sprouts or Microgreens Flour. J. Cereal Sci. 2025, 121, 104092. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Szafrańska, K. Biostimulators: A New Trend towards Solving an Old Problem. Front. Plant Sci. 2016, 7, 748. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, E.; Di Mola, I.; Ottaiano, L.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Foliar Application of Plant-Based Biostimulants Improves Yield and Upgrades Qualitative Characteristics of Processing Tomato. Ital. J. Agron. 2021, 16, 1825. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Charalambous, S.; Xylia, P.; Litskas, V.; Stavrinides, M.; Tzortzakis, N. Assessing the Biostimulant Effects of a Novel Plant-Based Formulation on Tomato Crop. Sustainability 2020, 12, 8432. [Google Scholar] [CrossRef]
- Teacă, C.-A.; Bodîrlău, R.; Oprea, A.; Tănase, C.; Colceru, S. Influence of Plant Extracts on Germination and Postgermination Development of Different Species. Cellulose Chem. Technol. 2008, 42, 47–53. [Google Scholar]
- Mutlu-Durak, H.; Yildiz Kutman, B. Seed Treatment with Biostimulants Extracted from Weeping Willow (Salix babylonica) Enhances Early Maize Growth. Plants 2021, 10, 1449. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, S.; Yadav, V. Screening Herbal Extracts as Biostimulant to Increase Germination, Plant Growth and Secondary Metabolite Production in Wheatgrass. Sci. Rep. 2024, 14, 607. [Google Scholar] [CrossRef]
- Norsworthy, J.K. Allelopathic potential of wild radish (Raphanus raphanistrum). Weed Technol. 2003, 17, 307–313. [Google Scholar] [CrossRef]
- Croatian Plant Genetic Resources Database, CPGRD Database. Available online: https://cpgrd.hapih.hr (accessed on 11 April 2025).
- Holm, G. Chlorophyll mutations in barley. Acta Agric. Scand. 1954, 4, 457–461. [Google Scholar] [CrossRef]
- Wettstein, D. Chlorophyll–letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–487. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagent. AJEV 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Keutgen, A.J.; Pawelzik, E. Quality and nutritional value of strawberry fruit under long term salt stress. Food Chem. 2008, 107, 1413–1420. [Google Scholar] [CrossRef]
- Available online: https://www.chiplot.online/correlation_heatmap.html (accessed on 30 July 2025).

| Parameter | Unit | Mean Square | F Value | p > F | LSD0.05 | Mean |
|---|---|---|---|---|---|---|
| Root length | 5.682 | 3.10 | * | 2.09 | 14.19 | |
| Stem length | cm | 18.376 | 23.27 | *** | 0.91 | 5.45 |
| Total length | 38.41 | 16.34 | *** | 2.36 | 19.64 | |
| Fresh mass | g per plant | 0.195 | 9.09 | *** | 0.23 | 14.10 |
| Germination rate | % | 24.759 | 0.21 | ns | - | 78.08 |
| VI | 18.994 | 2.99 | * | 3.88 | 15.30 | |
| RSR | 4.347 | 12.79 | *** | 0.898 | 2.93 | |
| SBI | 315.67 | 18.99 | *** | 6.282 | 28.21 | |
| Chl a | mg/g FW | 0.003 | 39.06 | *** | 0.017 | 0.161 |
| Chl b | 0.004 | 20.77 | *** | 0.029 | 0.115 | |
| Chl a + b | 0.009 | 8.36 | * | 0.062 | 0.268 | |
| Car | 0.008 | 28.38 | *** | 0.032 | 0.136 | |
| FRAP | mM FeSO4 | 1067.38 | 5.26 | * | 24.833 | 131.57 |
| TPC | µg GA/1 g tissue | 0.001 | 1.39 | * | 0.006 | 0.017 |
| TFC | µg QC/1 g tissue | 992.25 | 13.24 | ** | 16.297 | 81.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varga, I.; Antunović, M.; Kojić, M.T.; Markulj Kulundžić, A.; Iljkić, D.; Baličević, R.; Ravlić, M. Hemp Inflorescence as a Sustainable Biostimulant Tool to Boost Growth and Antioxidant Capacity in Oilseed Pumpkin. Plants 2025, 14, 3473. https://doi.org/10.3390/plants14223473
Varga I, Antunović M, Kojić MT, Markulj Kulundžić A, Iljkić D, Baličević R, Ravlić M. Hemp Inflorescence as a Sustainable Biostimulant Tool to Boost Growth and Antioxidant Capacity in Oilseed Pumpkin. Plants. 2025; 14(22):3473. https://doi.org/10.3390/plants14223473
Chicago/Turabian StyleVarga, Ivana, Manda Antunović, Monika Tkalec Kojić, Antonela Markulj Kulundžić, Dario Iljkić, Renata Baličević, and Marija Ravlić. 2025. "Hemp Inflorescence as a Sustainable Biostimulant Tool to Boost Growth and Antioxidant Capacity in Oilseed Pumpkin" Plants 14, no. 22: 3473. https://doi.org/10.3390/plants14223473
APA StyleVarga, I., Antunović, M., Kojić, M. T., Markulj Kulundžić, A., Iljkić, D., Baličević, R., & Ravlić, M. (2025). Hemp Inflorescence as a Sustainable Biostimulant Tool to Boost Growth and Antioxidant Capacity in Oilseed Pumpkin. Plants, 14(22), 3473. https://doi.org/10.3390/plants14223473










