Phloroglucinol α-Pyrones from Helichrysum: A Review on Structural Diversity, Plant Distribution and Isolation
Abstract
1. Introduction
2. Literature Search Methodology
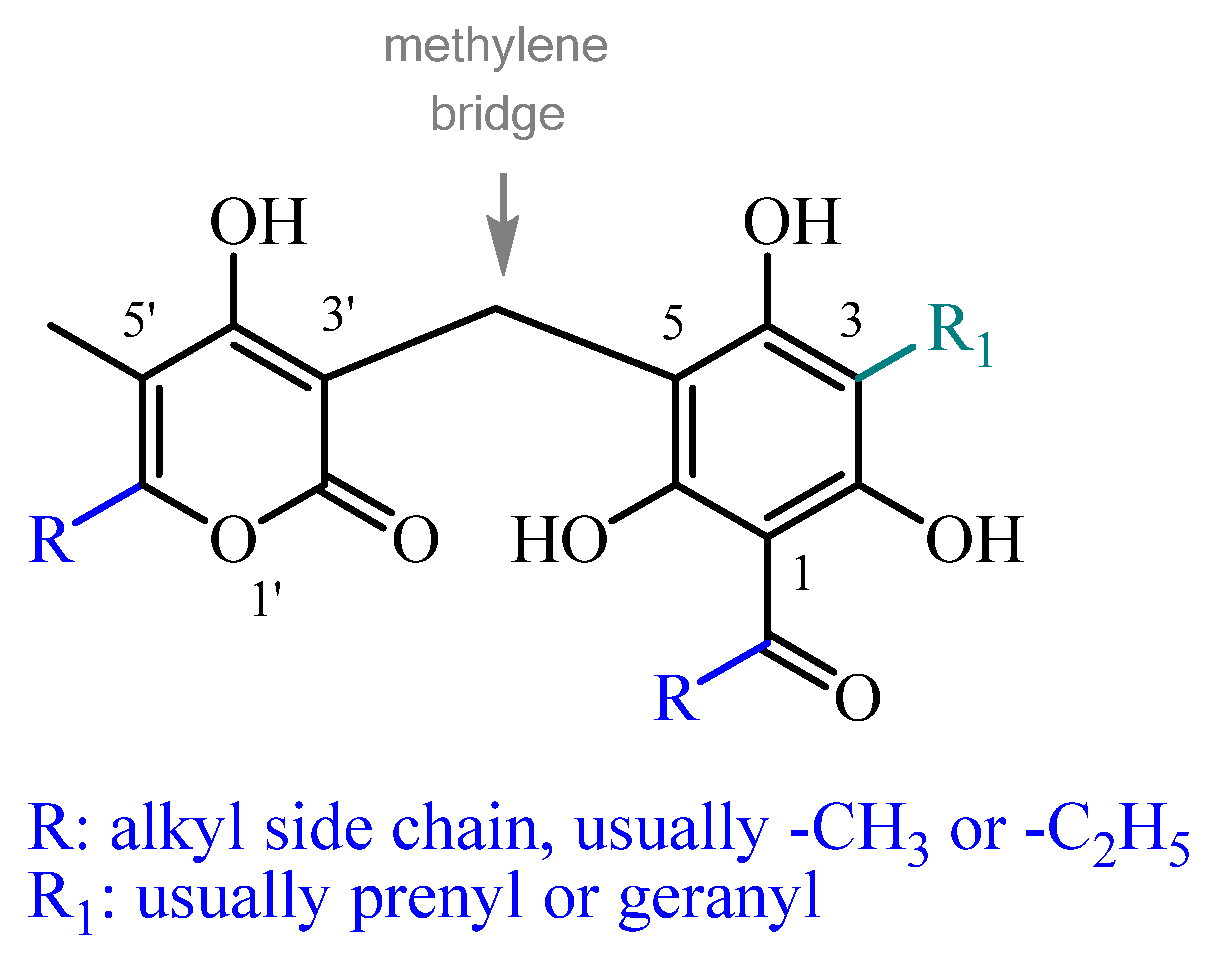
3. Phloroglucinol-α-Pyrones from Helichrysum
3.1. Alpha-Pyrone Ring Modifications
3.2. Phloroglucinol Ring Modifications
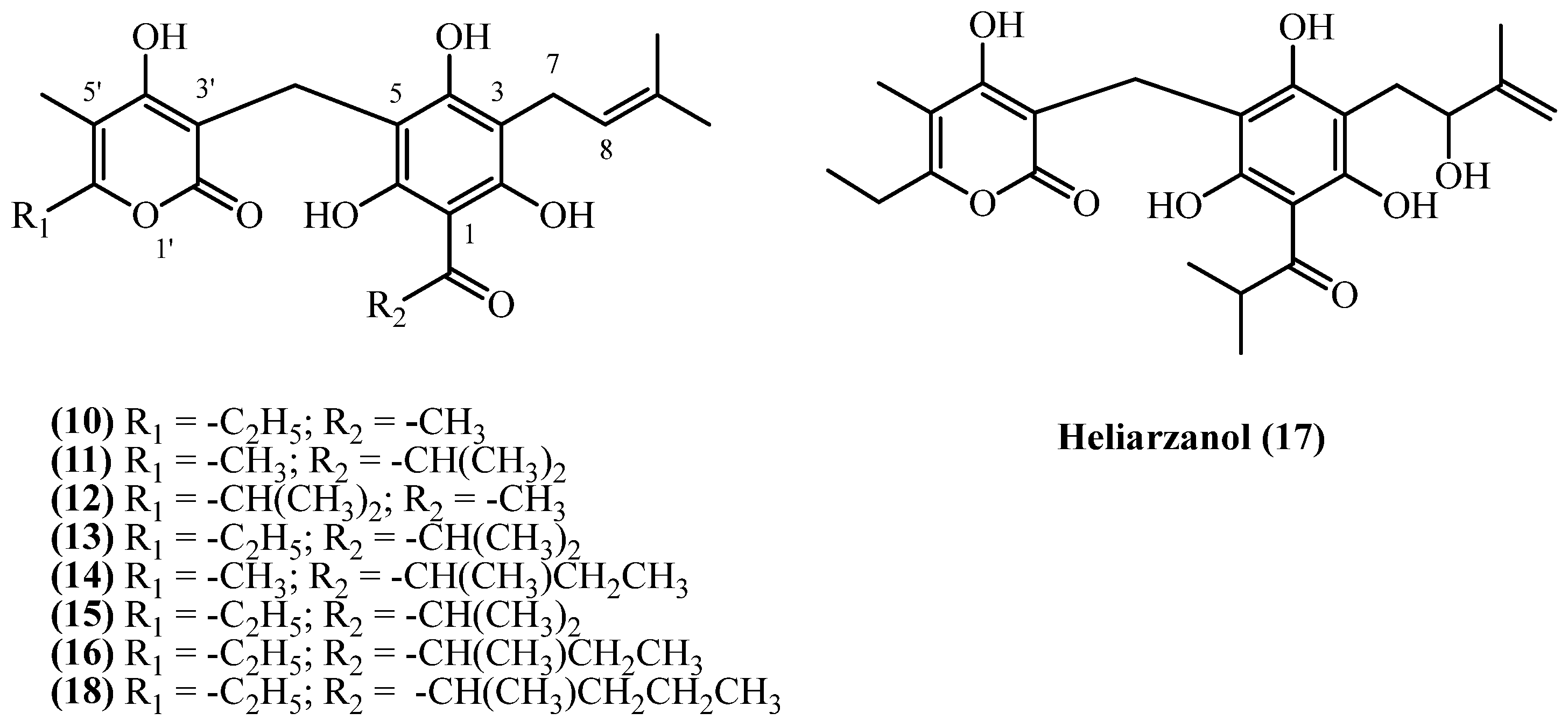
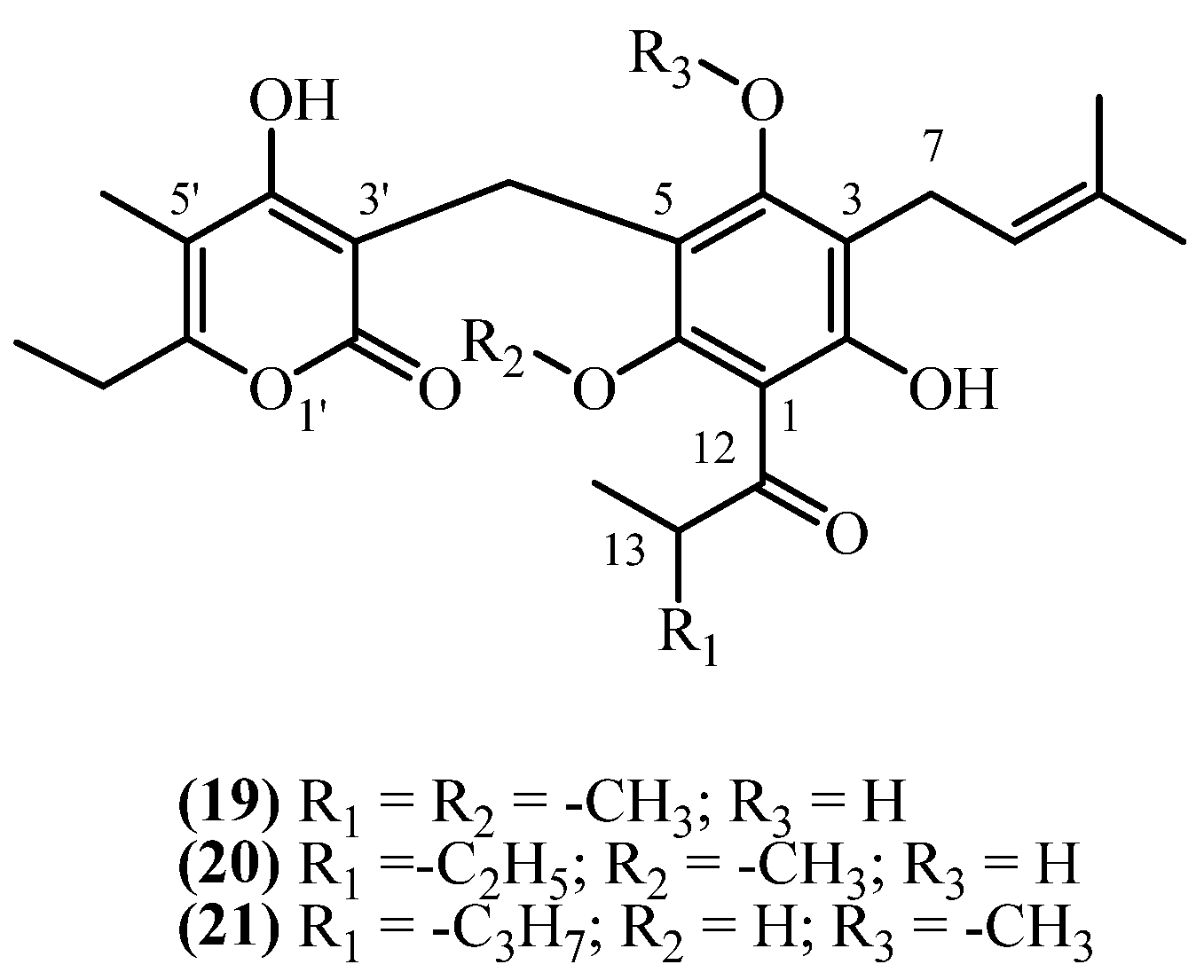
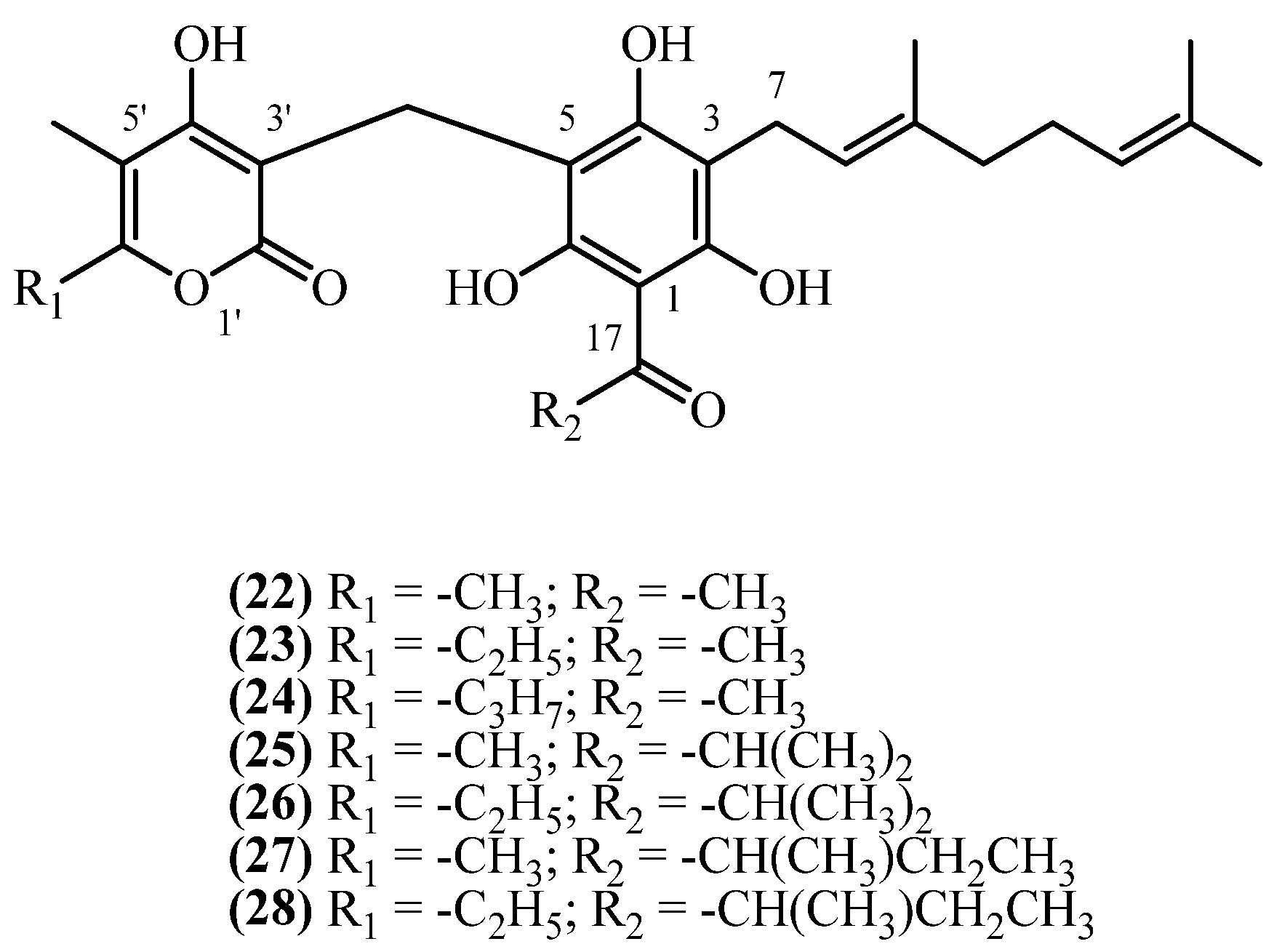
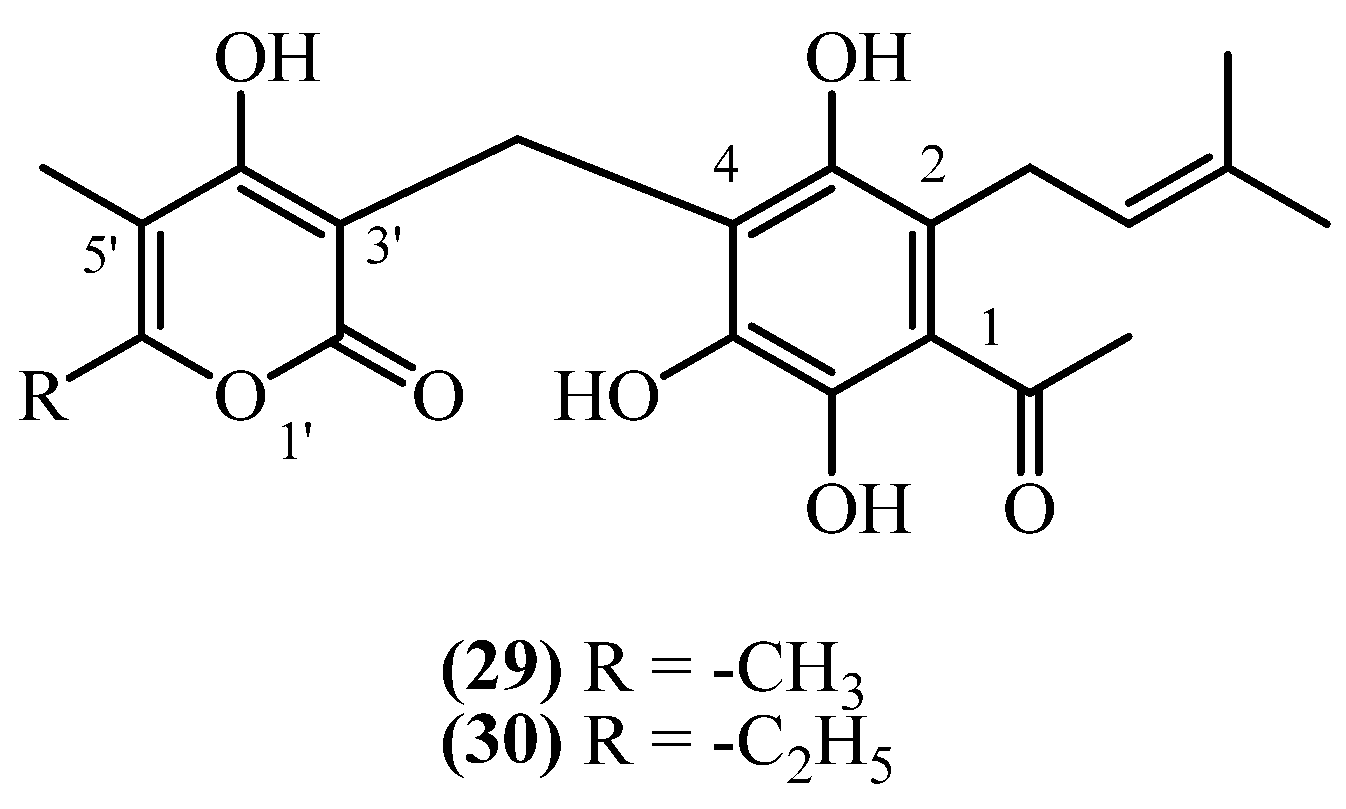
3.3. C3 Side Chain
3.4. C1 Acyl Group Diversity
| Plant Name | Compounds Discovered | Plant Parts | Ref. |
|---|---|---|---|
| Helichrysum arenarium (L.) Moench | Dipyrones: | ||
| Helipyrone A–C (4–6) | Roots | [5] | |
| 2-prenyl PGs: | |||
| Arenol A (29) | Aerial parts with flowers | [15] | |
| Helichrysum auriceps Hilliard | 3-prenyl methoxy PGs: | ||
| Auricepyrone (19), 23-Methylauricepyrone (20) | Roots | [6] | |
| Helichrysum cephaloideum DC. | 3-NA PGs: | ||
| Norauricepyrone (8), Methyl-norauricepyrone (9) | Roots | [16] | |
| 3-prenyl methoxy PGs: | |||
| Auricepyrone (19) | Roots | [6,7] | |
| Auricepyrone (19) | Aerial parts | [6] | |
| 23-Methylauricepyrone (20) | Roots | [6,7] | |
| 23-Methylauricepyrone (20) | Aerial parts | [6] | |
| 23-ethyl-6-O-desmethyl-4-O-methylauricepyrone (21) | Roots | [16] | |
| Benzofuranes: | |||
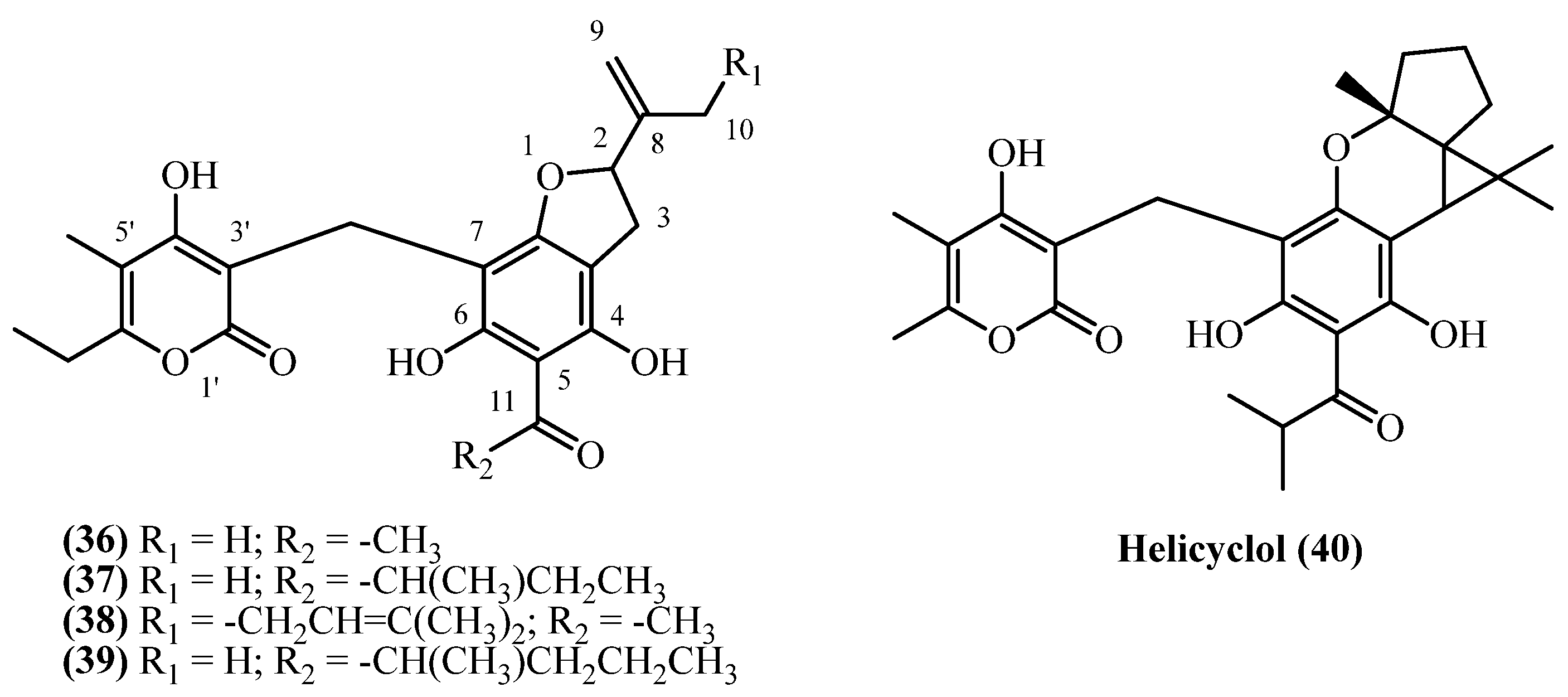
| 22-Methyl-22-ethyl-italipyrone (37) | Roots | [7,16] | |
| 22-Methyl-22-propyl-italipyrone (38) | Aerial parts | [7,16] | |
| Helichrysum italicum ssp. microphyllum | Monopyrones: | ||
| Micropyrone (3) | Aerial parts (non-woody) with flowers | [8,9,17] | |
| Dipyrones: | |||
| Helipyrone A (6) | Aerial parts (non-woody) with flowers | [8,9,17] | |
| 3-prenyl PGs: | |||
| Arzanol (10) | Aerial parts (non-woody) with flowers | [8,9,17] | |
| Heliarzanol (17) | Aerial parts (non-woody) with flowers | [9] | |
| Helichrysum italicum (Roth) G. Don | Monopyrones: | ||
| Micropyrone (3) | Aerial parts (non-woody) with flowers | [14] | |
| Dipyrones: | |||
| Helipyrone A (6) | Aerial parts with flowers | [7,12,18] | |
| Helitalone A (7) | non-woody Aerial parts with flowers | [14] | |
| 3-prenyl PGs: | |||
| Arzanol (10), Helitalone B (12) | non-woody Aerial parts with flowers | [14] | |
| Amino PGs: | |||
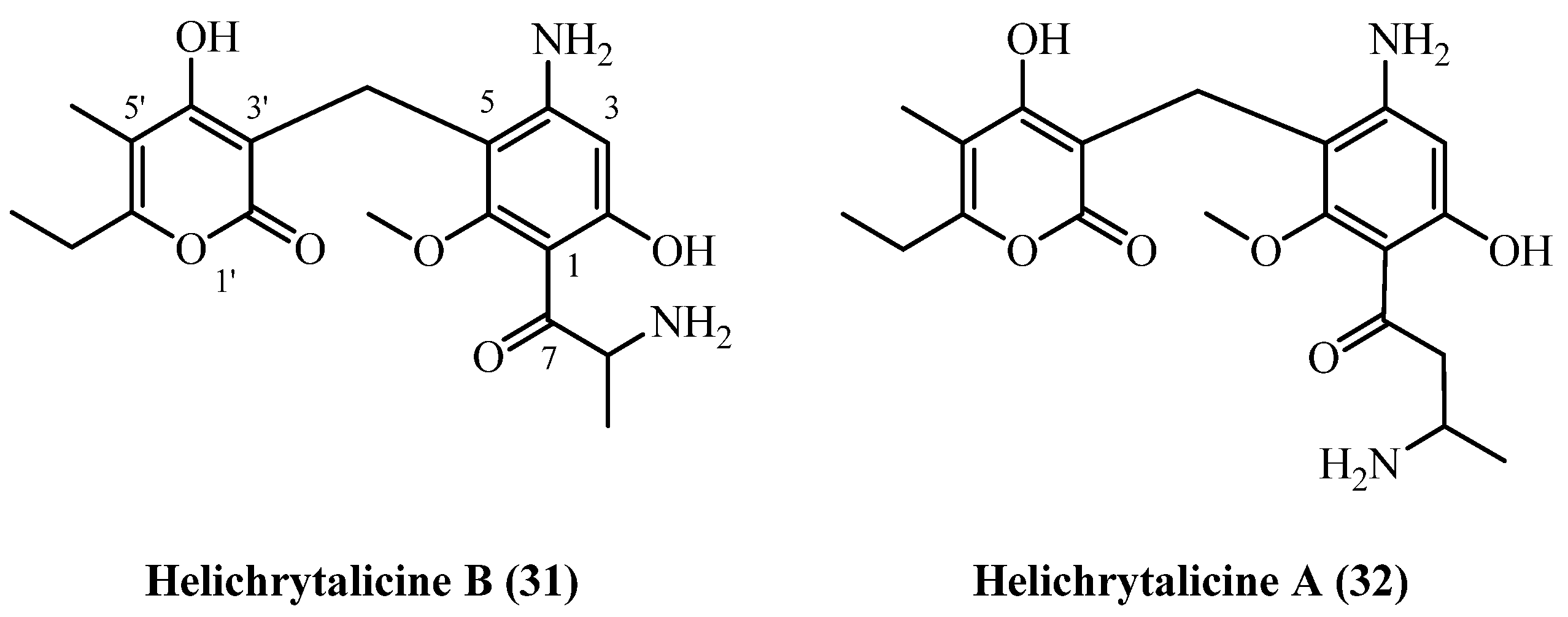
| Helichrytalicine B (31), Helichrytalicine A (32) | leaf material | [12] | |
| Benzofuranes: | |||
| Italipyrone (36) | Aerial parts with flowers | [7] | |
| Hetero-trimer PGs: | |||
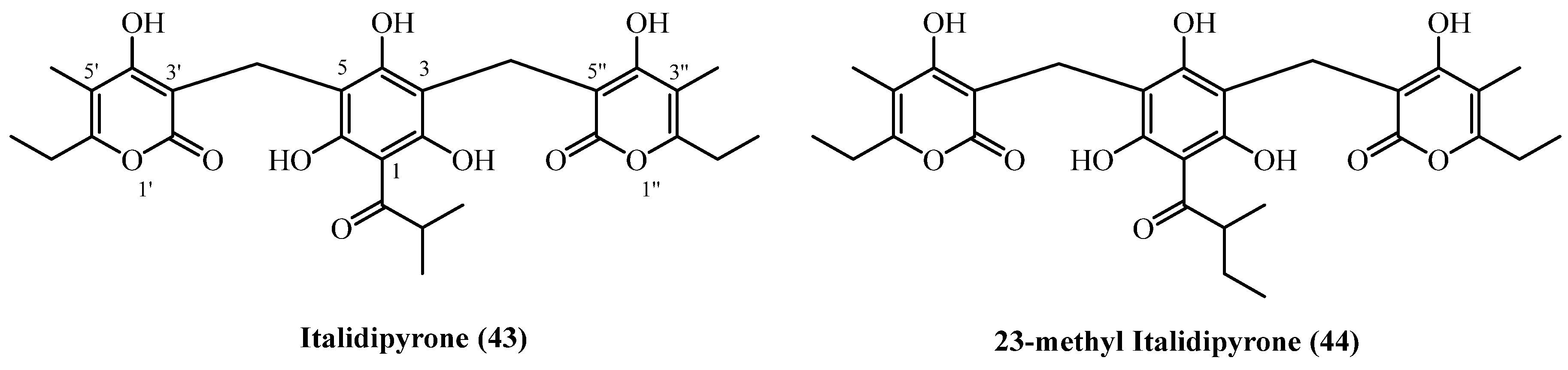
| Italidipyrone (43), 23-Methyl-italidipyrone (44) | Aerial parts with flowers | [7] | |
| Helichrysum mixtum (Kuntze) Moeser | 3-prenyl PGs: | ||
| 23-Methyl-6-O-desmethylauricepyrone (16), 23-ethyl-6-O-desmethyl-auricepyrone (18) | Roots | [16] | |
| Benzopyranes: | [16] | ||
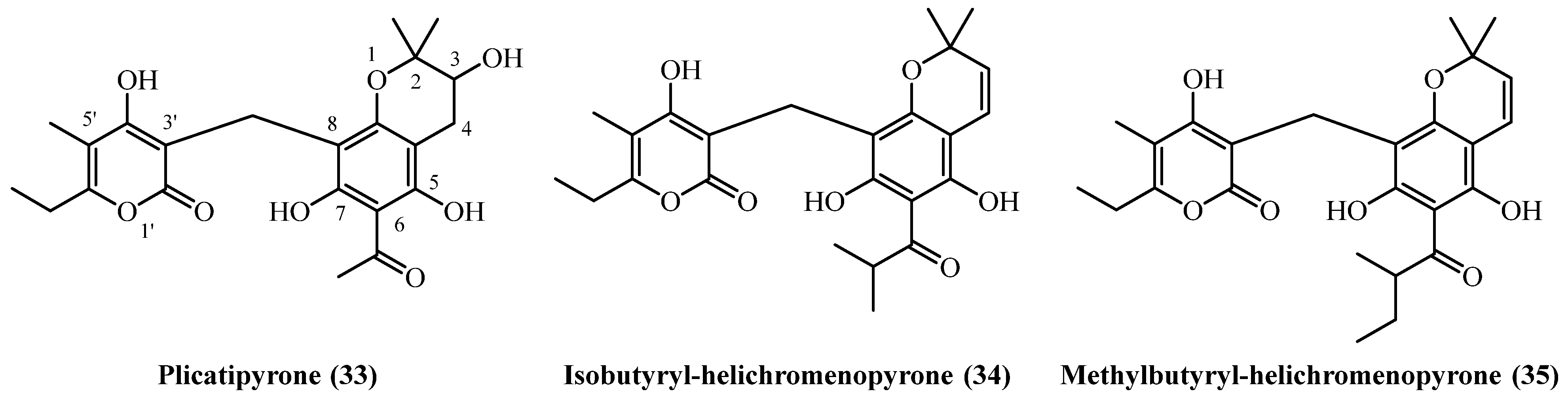
| Isobutyryl-helichromenopyrone (34) | Roots | [16] | |
| Methylbutyryl-helichromenopyrone (35) | Aerial parts | [16] | |
| 22-Methyl-22-ethyl-italipyrone (37), 22-Methyl-22-propyl-italipyrone (38) | Roots | [16] | |
| Helichrysum odoratissimum Sweet. | 3-prenyl PGs: | ||
| 6-O-Desmethylauricepyrone (13), 23-Methyl-6-O-desmethylauricepyrone (16) | Aerial parts | [7] | |
| Helichrysum oocephalum Boiss. | Dipyrones: | ||
| Helipyrone A–C (4–6) | Aerial parts | [11] | |
| 3-prenyl PGs: | |||
| Arenol B (11), Arenol C (14), 3-prenyl norauricepyrone (15), 23-Methyl-6-O-desmethylauricepyrone (16) | Aerial parts | [11] | |
| Achyroclinopyrone A (26, 28, 25, 27) | Aerial parts | [11] | |
| Benzofuranes: | |||
| Helicyclol (40) | Aerial parts | [11] | |
| Chromane PGs: | |||
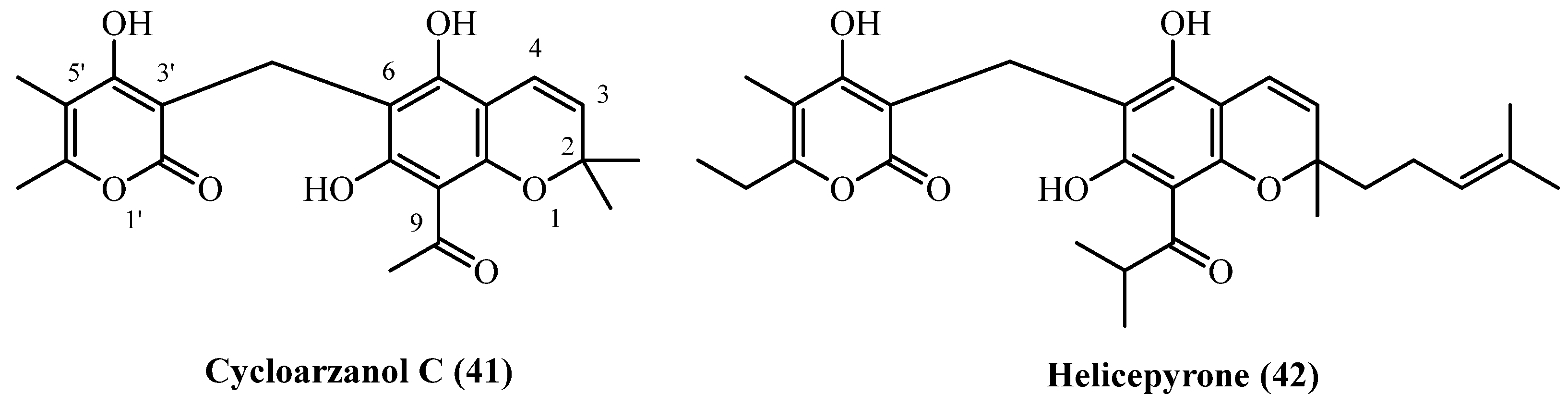
| Cycloarzanol C (41), Helicepyrone (42) | Aerial parts | [11] | |
| Hetero-trimer PGs: | |||
| Italidipyrone (43), 23-Methyl-italidipyrone (44) | Aerial parts | [11] | |
| Spiroketals: | |||
| Helispiroketals A-H (45, 46, 49, 50, 48, 47, 52, 51) | Aerial parts | [11] | |
| Helichrysum plicatum DC. ssp. plicatum | Benzopyranes: | ||
| Plicatipyrone (33) | Flower heads | [7] | |
| Helichrysum stenopterum DC. | 3-prenyl PGs: | ||
| 23-Methyl-6-O-desmethylauricepyrone (16), 23-ethyl-6-O-desmethyl-auricepyrone (18) | Aerial parts | [16] | |
| Helichrysum stoechas (L.) Moench | Dipyrones: | ||
| Helipyrone A (6) | Roots | [19] | |
| Helipyrone A (6) | Aerial parts with flowers | [13,20] | |
| Helipyrone B (Norhelipyrone) (5) | Roots | [19] | |
| Helipyrone B (Norhelipyrone) (5) | Aerial parts with flowers | [20] | |
| Helipyrone C (Bisnorhelipyrone) (4) | Roots | [19] | |
| Helipyrone C (Bisnorhelipyrone) (4) | Aerial parts with flowers | [20] | |
| 3-prenyl PGs: | |||
| Arzanol (10) | flowers | [13] | |
| 3-geranyl PGs: | |||
| 18,18-bis-desmethyl Achyroclinopyrone C (22) | Roots | [19] | |
| 18,18-bis-desmethyl Achyroclinopyrone C (22) | Aerial parts with flowers | [13] | |
| 18,18-bis-desmethyl Achyroclinopyrone A (23) | Roots | [19] | |
| 2-prenyl PGs: | |||
| Arenol A (29) | Aerial parts with flowers | [20] | |
| Homoarenol (30) | Aerial parts with flowers | [15,20] | |
| Benzopyranes: | |||
| Plicatipyrone (33), Italipyrone (36) | Aerial parts with flowers | [20] | |
| Helichrysum stoechas subsp. barrelieri (Ten.) Nym | Benzofuranes: | ||
| 20-(3,3′-Dimethylallyl)-italipyrone (39) | Flower heads | [7] | |
| Helichrysum zeyheri Less. | |||
| Monopyrones: | |||
| 3,5-dimethyl-4-hydroxy-6-isopropyl alphapyrone (1), 3,5-dimethyl-4-(methoxy)-6-isopropyl alphapyrone (2) | Aerial parts | [16] |
4. Monopyrones
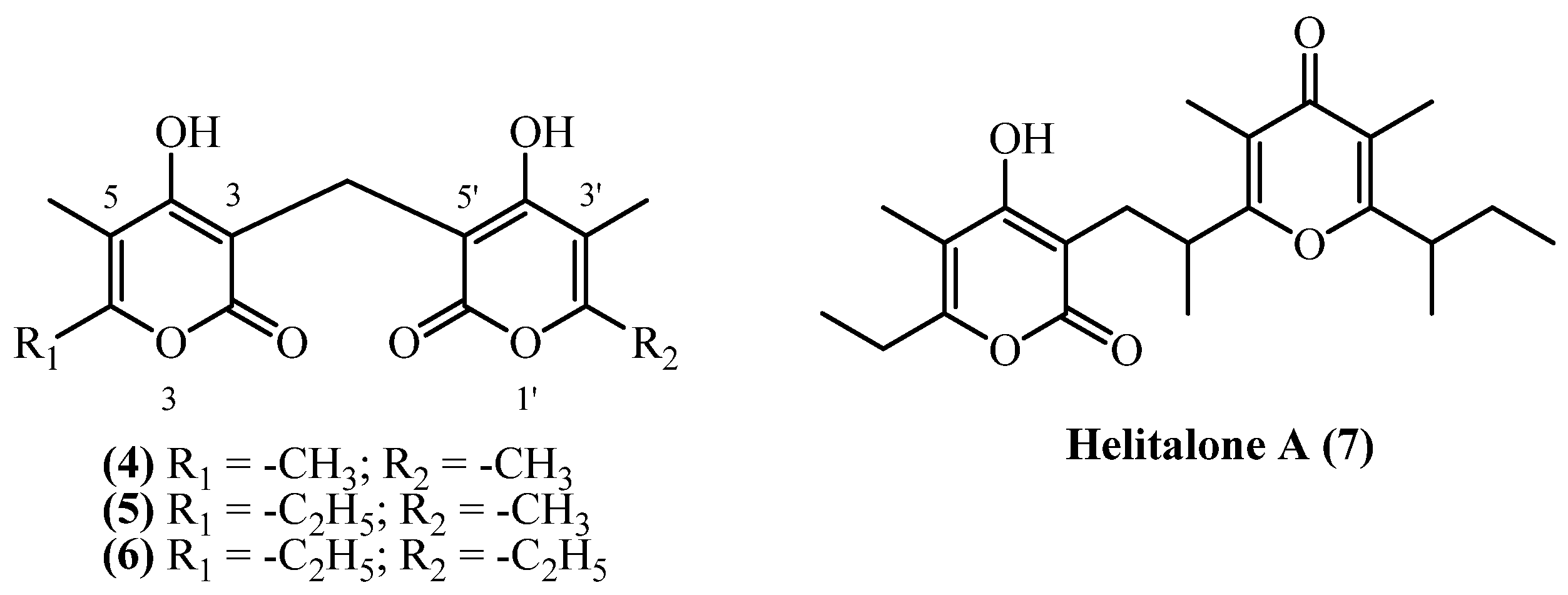
5. Dipyrones
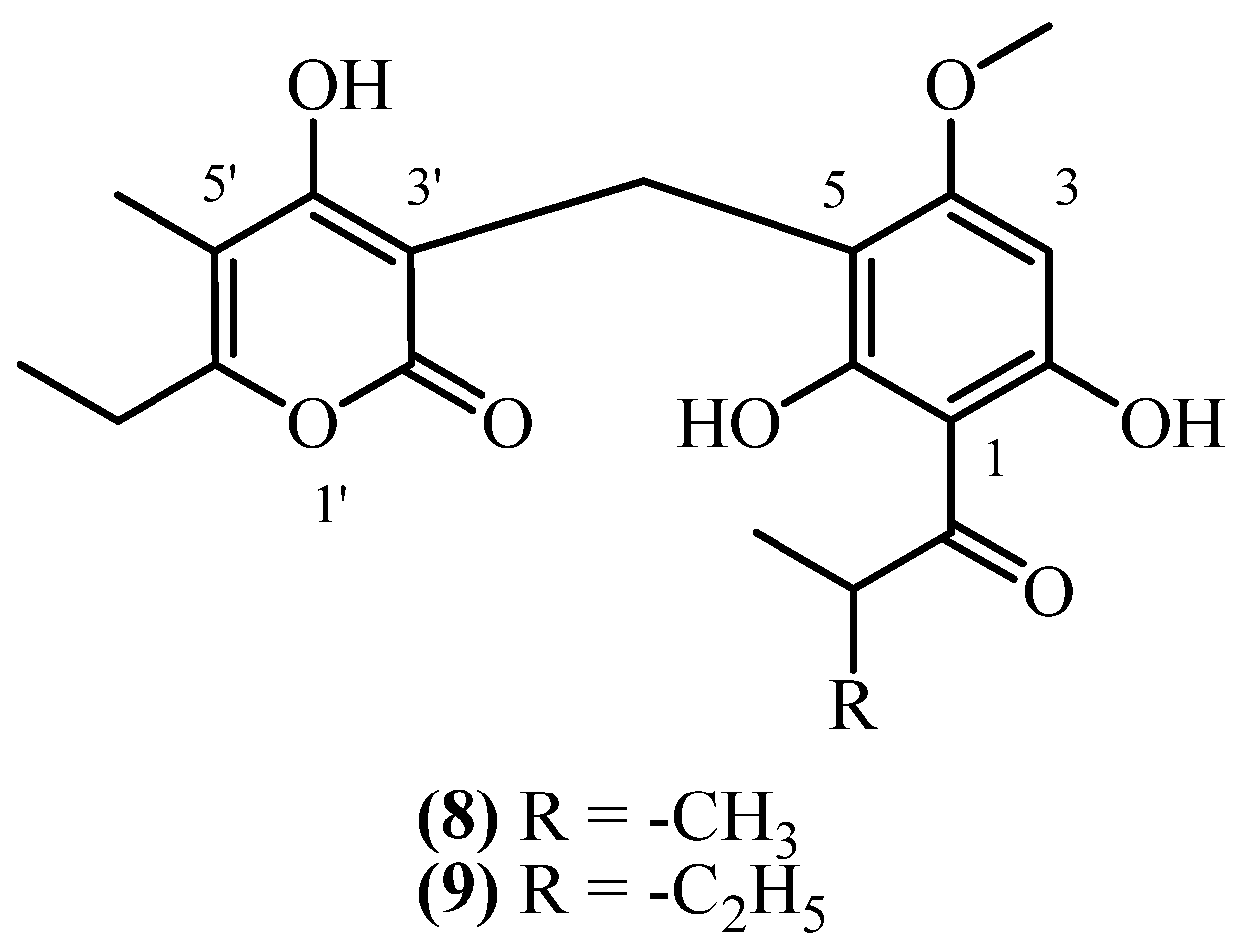
6. 3-NA PGs
7. 3-Prenyl PGs
8. 3-Prenyl Methoxy PGs
9. 3-Geranyl PGs
10. 2-Prenyl PGs
11. Amino PGs
12. Benzopyranes
13. Benzofuranes
14. Chromane PGs
15. Hetero-Trimer PGs
16. Spiroketals
17. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- Baričevič, D.; Stopar, K.; Bartol, T.; Luthar, Z. Genetics of Helichrysum spp. and Opportunities for Breeding. In Breeding of Ornamental Crops: Annuals and Cut Flowers; Springer: Berlin/Heidelberg, Germany, 2025; pp. 143–164. [Google Scholar]
- Lephatsi, M.M.; Choene, M.S.; Kappo, A.P.; Madala, N.E.; Tugizimana, F. An integrated molecular networking and docking approach to characterize the metabolome of Helichrysum splendidum and its pharmaceutical potentials. Metabolites 2023, 13, 1104. [Google Scholar] [CrossRef]
- Bojilov, D.; Manolov, S.; Ahmed, S.; Dagnon, S.; Ivanov, I.; Marc, G.; Oniga, S.; Oniga, O.; Nedialkov, P.; Mollova, S. HPLC Analysis and In Vitro and In Silico Evaluation of the Biological Activity of Polyphenolic Components Separated with Solvents of Various Polarities from Helichrysum italicum. Molecules 2023, 28, 6198. [Google Scholar] [CrossRef] [PubMed]
- Samanidis, I.; Krigas, N.; Athanasiadis, V.; Makrygiannis, I.; Mantiniotou, M.; Lalas, S.I. A Comparative Phytochemical Investigation of the Greek Members of the Genus Helichrysum Mill., with Emphasis on the Local Endemic Helichrysum amorginum Boiss and Orph. Plants 2025, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Vrkcoč, J.; Dolejš, L.; Buděšínský, M. Methylene-bis-2H-pyran-2-ones and phenolic constituents from the root of Helichrysum arenarium. Phytochemistry 1975, 14, 1383–1384. [Google Scholar] [CrossRef]
- Bohlmann, F.; Zdero, C. Neue phloroglucin-derivate aus Helichrysum-arten. Phytochemistry 1980, 19, 153–155. [Google Scholar] [CrossRef]
- Hänsel, R.; Cybulski, E.-M.; Çubukçu, B.; Meriçli, A.H.; Bohlmann, F.; Zdero, C. Neue pyron-derivate aus Helichrysum-arten. Phytochemistry 1980, 19, 639–644. [Google Scholar] [CrossRef]
- Appendino, G.; Ottino, M.; Marquez, N.; Bianchi, F.; Giana, A.; Ballero, M.; Sterner, O.; Fiebich, B.L.; Munoz, E. Arzanol, an anti-inflammatory and anti-HIV-1 phloroglucinol α-pyrone from Helichrysum italicum ssp. microphyllum. J. Nat. Prod. 2007, 70, 608–612. [Google Scholar] [CrossRef]
- Taglialatela-Scafati, O.; Pollastro, F.; Chianese, G.; Minassi, A.; Gibbons, S.; Arunotayanun, W.; Mabebie, B.; Ballero, M.; Appendino, G. Antimicrobial phenolics and unusual glycerides from Helichrysum italicum subsp. microphyllum. J. Nat. Prod. 2013, 76, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Atzeri, A.; Nieddu, M.; Appendino, G. New insights into the antioxidant activity and cytotoxicity of arzanol and effect of methylation on its biological properties. Chem. Phys. Lipids 2017, 205, 55–64. [Google Scholar] [CrossRef]
- Lavault, M.; Danton, O.; Tayarani-Najaran, Z.; Asili, J.; Iranshahi, M.; Emami, S.A.; Hamburger, M. HPLC-Based Activity Profiling for Antiprotozoal Compounds in the Endemic Iranian Medicinal Plant Helichrysum oocephalum. J. Nat. Prod. 2019, 82, 958–969. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Buommino, E.; Caputo, P.; Scognamiglio, M.; Chambery, A.; Donnarumma, G.; Fiorentino, A. Phytochemical study of Helichrysum italicum (Roth) G. Don: Spectroscopic elucidation of unusual amino-phlorogucinols and antimicrobial assessment of secondary metabolites from medium-polar extract. Phytochemistry 2016, 132, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Venditti, A.; Cásedas, G.; Frezza, C.; Guiso, M.; Sciubba, F.; Serafini, M.; Bianco, A.; Valero, M.S.; López, V. Everlasting flower (Helichrysum stoechas Moench) as a potential source of bioactive molecules with antiproliferative, antioxidant, antidiabetic and neuroprotective properties. Ind. Crops Prod. 2017, 108, 295–302. [Google Scholar] [CrossRef]
- Werner, J.; Ebrahim, W.; Özkaya, F.C.; Mándi, A.; Kurtán, T.; El-Neketi, M.; Liu, Z.; Proksch, P. Pyrone derivatives from Helichrysum italicum. Fitoterapia 2019, 133, 80–84. [Google Scholar] [CrossRef]
- Vrkoč, J.; Dolejš, L.; Sedmera, P.; Vašíčková, S.; Šorm, F. The structure of arenol and homoarenol, α-pyrone derivatives from Helichrysum arenarium (L.) moench. Tetrahedron Lett. 1971, 12, 247–250. [Google Scholar] [CrossRef]
- Jakupovic, J.; Kuhnke, J.; Schuster, A.; Metwally, M.; Bohlmann, F. Phloroglucinol derivatives and other constituents from South African Helichrysum species. Phytochemistry 1986, 25, 1133–1142. [Google Scholar] [CrossRef]
- Rosa, A.; Deiana, M.; Atzeri, A.; Corona, G.; Incani, A.; Melis, M.P.; Appendino, G.; Dessì, M.A. Evaluation of the antioxidant and cytotoxic activity of arzanol, a prenylated α-pyrone–phloroglucinol etherodimer from Helichrysum italicum subsp. microphyllum. Chem.-Biol. Interact. 2007, 165, 117–126. [Google Scholar] [CrossRef]
- Opitz, L.; Hänsel, R. Helipyron, ein methylen-bis-triacetsäurelacton aus Helichrysum italicum. Tetrahedron Lett. 1970, 11, 3369–3370. [Google Scholar] [CrossRef]
- Lavault, M.; Richomme, P. Constituents of Helichrysum stoechas variety olonnense. Chem. Nat. Compd. 2004, 40, 118–121. [Google Scholar] [CrossRef]
- Rios, J.; Recio, M.; Villar, A. Isolation and identification of the antibacterial compounds from Helichrysum stoechas. J. Ethnopharmacol. 1991, 33, 51–55. [Google Scholar] [CrossRef]
- Tomás-Lorente, F.; Iniesta-Sanmartín, E.; Tomás-Barberán, F.A.; Trowitzsch-Kienast, W.; Wray, V. Antifungal phloroglucinol derivatives and lipophilic flavonoids from Helichrysum decumbens. Phytochemistry 1989, 28, 1613–1615. [Google Scholar] [CrossRef]
- Akaberi, M.; Sahebkar, A.; Azizi, N.; Emami, S.A. Everlasting flowers: Phytochemistry and pharmacology of the genus Helichrysum. Ind. Crops Prod. 2019, 138, 111471. [Google Scholar] [CrossRef]
- Minassi, A.; Cicione, L.; Koeberle, A.; Bauer, J.; Laufer, S.; Werz, O.; Appendino, G. A Multicomponent Carba-Betti Strategy to Alkylidene Heterodimers–Total Synthesis and Structure–Activity Relationships of Arzanol. Eur. J. Org. Chem. 2012, 2012, 772–779. [Google Scholar] [CrossRef]
- Bauer, J.; Koeberle, A.; Dehm, F.; Pollastro, F.; Appendino, G.; Northoff, H.; Rossi, A.; Sautebin, L.; Werz, O. Arzanol, a prenylated heterodimeric phloroglucinyl pyrone, inhibits eicosanoid biosynthesis and exhibits anti-inflammatory efficacy in vivo. Biochem. Pharmacol. 2011, 81, 259–268. [Google Scholar] [CrossRef]
- Rosa, A.; Pollastro, F.; Atzeri, A.; Appendino, G.; Melis, M.P.; Deiana, M.; Incani, A.; Loru, D.; Dessì, M.A. Protective role of arzanol against lipid peroxidation in biological systems. Chem. Phys. Lipids 2011, 164, 24–32. [Google Scholar] [CrossRef]
- Piras, F.; Sogos, V.; Pollastro, F.; Appendino, G.; Rosa, A. Arzanol, a natural phloroglucinol α-pyrone, protects HaCaT keratinocytes against H2O2-induced oxidative stress, counteracting cytotoxicity, reactive oxygen species generation, apoptosis, and mitochondrial depolarization. J. Appl. Toxicol. 2024, 44, 720–732. [Google Scholar] [CrossRef]
- Piras, F.; Sogos, V.; Pollastro, F.; Rosa, A. Protective effect of arzanol against H2O2-induced oxidative stress damage in differentiated and undifferentiated SH-SY5Y Cells. Int. J. Mol. Sci. 2024, 25, 7386. [Google Scholar] [CrossRef]
- Borgonetti, V.; Caroli, C.; Governa, P.; Virginia, B.; Pollastro, F.; Franchini, S.; Manetti, F.; Les, F.; López, V.; Pellati, F.; et al. Helichrysum stoechas (L.) Moench reduces body weight gain and modulates mood disorders via inhibition of silent information regulator 1 (SIRT1) by arzanol. Phytother. Res. 2023, 37, 4304–4320. [Google Scholar] [CrossRef]
- Belekar, V.; Shah, A.; Garg, P. High-throughput virtual screening of phloroglucinol derivatives against HIV-reverse transcriptase. Mol. Divers. 2013, 17, 97–110. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.; Iniesta-Sanmartín, E.; Tomás-Lorente, F.; Rumbero, A. Antimicrobial phenolic compounds from three Spanish Helichrysum species. Phytochemistry 1990, 29, 1093–1095. [Google Scholar] [CrossRef]
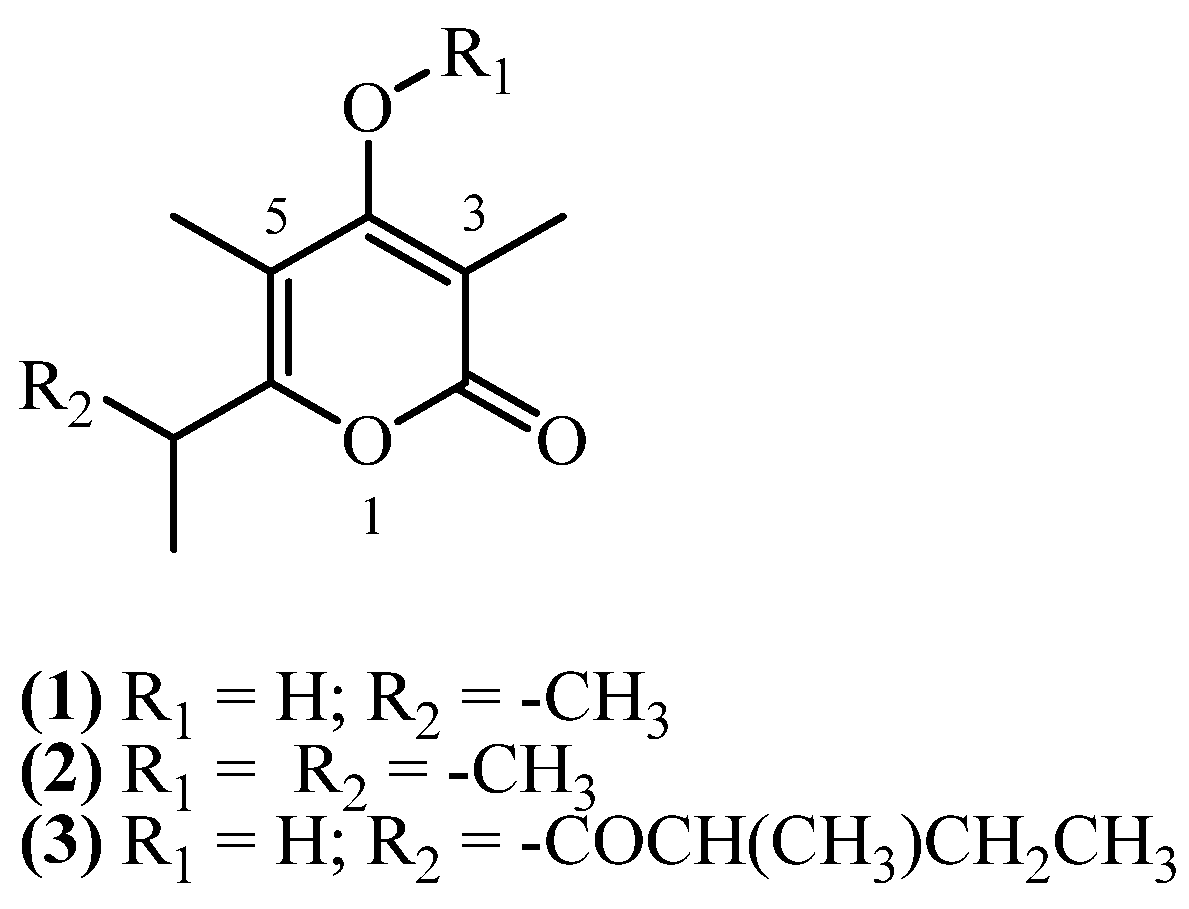
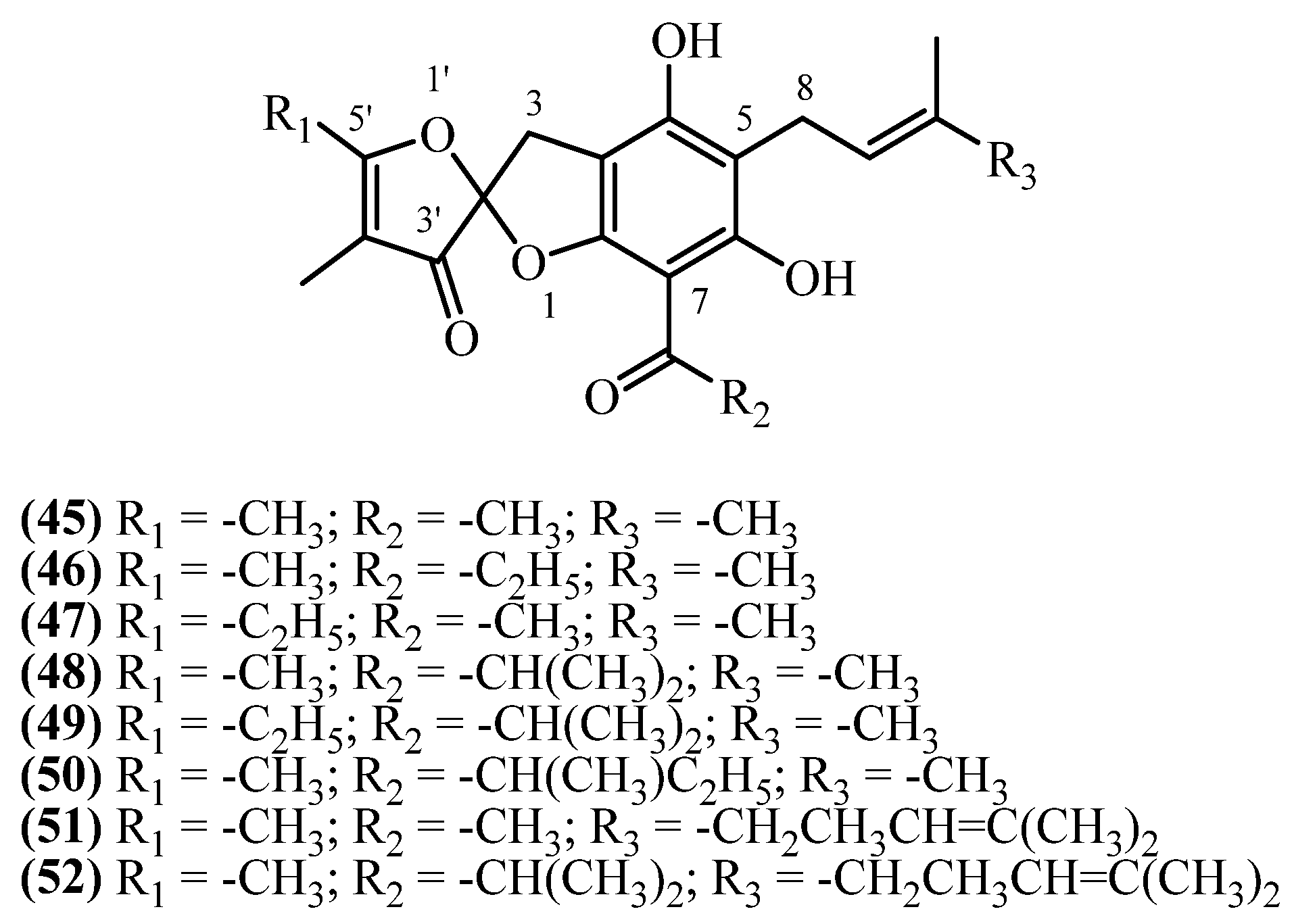
| Entry | Compound Name | Extraction Conditions | Extracted Amount | Plant Name, Location | Plant Parts | Subclass | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 3,5-dimethyl-4-hydroxy-6-isopropyl alphapyrone (1) | Extracted with Et2O-petrol (1:2) at rt. The extracts obtained after removal of long chain saturated carbons with MeOH, were first separated by CC (silica gel) and further by TLC (silica gel PF 254) | Plant material: 150 g Amount isolated: 20 mg Yield: 0.0133% | Plant name: Helichrysum zeyheri Less. Location: Transvaal, South Africa (wildgrown) | Aerial parts | Monopyrones | [16] |
| 2 | 3,5-dimethyl-4-(methoxy)-6-isopropyl alphapyrone (2) | The same as for entry 1, compound 1 | Plant material: 150 g Amount isolated: 50 mg Yield: 0.0333% | Plant name: Helichrysum zeyheri Less. Location: Transvaal, South Africa (wildgrown) | Aerial parts | Monopyrones | [16] |
| 3 | Micropyrone (3) | Maceration with acetone (3 × 4 L) at rt; residue (51 g) adsorbed on silica gel and fractionated by vacuum chromatography into petroleum-ether, EtOAc, and acetone fractions; the EtOAc eluate crystallized from EtOAc/petroleum ether | Plant material: 1000 g Amount isolated: 482 mg Yield: 0.0482% | Plant name: Helichrysum italicum ssp. Microphyllum Location: near Arzana (NU, Sardinia), Italy | Aerial parts (non-woody) + flowers | Monopyrones | [8] |
| 4 | Micropyrone (3) | Maceration with acetone (3 × 2 L, rt); crude residue (12 g) dissolved in EtOAc/hexane (1:1, 200 mL each) and left overnight; precipitate collected and recrystallised from toluene–acetone. Later silica-gel fractions (hexane/EtOAc 6:4) triturated with diethyl ether | Plant material: 540 g Amount isolated: 29 mg Yield: 0.0054% | Plant name: Helichrysum italicum ssp. Microphyllum Location: Sardinia, Italy (wildgrown) | Aerial parts + flowers | Monopyrones | [17] |
| 5 | Micropyrone (3) | Acetone extraction (2 × 15 L, rt); 50 g of the crude residue (from a total of 202 g gummy residue) was partitioned between petroleum ether and 90% MeOH (1 h, 30 °C); fractions of the aq MeOH phase chromatographed on silica gel (petroleum ether→EtOAc); finally—trituration with ether | Plant material: 5000 g Amount isolated: 162.5 mg Yield: 0.0129% | Plant name: Helichrysum italicum ssp. Microphyllum Location: Arzana (Sardinia), Italy (wildgrown) | Aerial parts + flowers | Monopyrones | [9] |
| 6 | Micropyrone (3) | Acetone extraction (3 × 5 L, rt); crude residue (20 g) fractionated by silica-gel vacuum liquid chromatography (hexane → EtOAc → CH2Cl2 → MeOH); chromatographed on Sephadex LH-20 and semi-prep HPLC | Plant material: 500 g Amount isolated: 7.5 mg Yield: 0.0015% | Plant name: Helichrysum italicum (Roth) G.Don Location: Sardinia, Italy (wildgrown) | non-woody Aerial parts + flowers | Monopyrones | [14] |
| 7 | Helipyrone C (Bisnorhelipyrone) (4) | Extracted with petroleum ether and ethanol, followed by solvent partitioning; CC on silica gel using chloroform–methanol (98:2), and after acetylation, the compounds were further purified by silica gel chromatography (hexane–ether, 9:1) and preparative TLC to yield diacetyl derivatives, which in turn, after deacetylation, provided helipyrone (6), bisnorhelipyrone (4), and norhelipyrone (5) | Plant material: 1500 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum arenarium (L.) Moench Location: near Malacky, Slovakia (wildgrown) | Roots | Dipyrones | [5] |
| 8 | Helipyrone C (Bisnorhelipyrone) (4) | Methanol percolation (20 L, 24 h, rt); crude MeOH extract (55 g dry extract) partitioned with petroleum ether, CH2Cl2, EtOAc, n-BuOH; CH2Cl2 fraction (12 g) silica-gel CC (n-hexane → CHCl3) then RP-HPLC (MeCN/TFA 55→100%) | Plant material: 4000 g Amount isolated: 1.9 mg Yield: 4.8 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Dipyrones | [11] |
| 9 | Helipyrone C (Bisnorhelipyrone) (4) | Successive extraction with hexane, CHCl3, EtOAc, MeOH at rt; silica-gel column chromatography (hexane–EtOAc); preparative TLC (CHCl3–MeOH 99:1) | Plant material: 400 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Dunes of the forest domain of Olonne, near Les Sables d’Olonne, Vendée, France | Roots | Dipyrones | [19] |
| 10 | Helipyrone C (Bisnorhelipyrone) (4) | Maceration of dried aerial parts with CH2Cl2 (3 × to exhaustion, RT); CH2Cl2 extract concentrated in vacuo, chromatographed on silica-gel 60 (CHCl3/MeOH gradient) then silica-gel 60 H; active fractions purified by preparative TLC | Plant material: 700 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Sierra Perenchiza, near Chiva, Valencia, Spain | Aerial parts + flowers | Dipyrones | [20] |
| 11 | Helipyrone B (Norhelipyrone) (5) | Same as for entry 7, compound 4 | Plant material: 1500 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum arenarium (L.) Moench Location: near Malacky, Slovakia (wildgrown) | Roots | Dipyrones | [5] |
| 12 | Helipyrone B (Norhelipyrone) (5) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 0.4 mg Yield: 1 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Dipyrones | [11] |
| 13 | Helipyrone B (Norhelipyrone) (5) | Procedure the same as entry 9, compound 4 | Plant material: 400 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Dunes of the forest domain of Olonne, near Les Sables d’Olonne, Vendée, France | Roots | Dipyrones | [19] |
| 14 | Helipyrone B (Norhelipyrone) (5) | Procedure the same as entry 10, compound 4 | Plant material: 700 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Sierra Perenchiza, near Chiva, Valencia, Spain | Aerial parts + flowers | Dipyrones | [20] |
| 15 | Helipyrone A (6) | Procedure the same as entry 11, compound 5 | Plant material: 1500 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum arenarium (L.) Moench Location: near Malacky, Slovakia (wildgrown) | Roots | Dipyrones | [5] |
| 16 | Helipyrone A (6) | Procedure the same as entry 3, compound 3 | Plant material: 1000 g Amount isolated: 245 mg Yield: 0.0245% | Plant name: Helichrysum italicum ssp. microphyllum Location: near Arzana (NU, Sardinia), Italy | Aerial parts (non-woody) + flowers | Dipyrones | [8] |
| 17 | Helipyrone A (6) | Maceration with acetone (3 × 2 L, RT); crude residue (12 g) dissolved in EtOAc/hexane (1:1, 200 mL each) and left overnight; precipitate collected and recrystallised from toluene–acetone. Fractionation on silica-gel column (hexane → EtOAc) | Plant material: 540 g Amount isolated: 120 mg Yield: 0.0219% | Plant name: Helichrysum italicum ssp. microphyllum Location: Sardinia, Italy (wildgrown) | Aerial parts + flowers | Dipyrones | [17] |
| 18 | Helipyrone A (6) | Same as entry 5, compound 3; Aqueous MeOH phase fraction crystallized from diethyl ether to give helipyrone | Plant material: 5000 g Amount isolated: 320 mg Yield: 0.0256% | Plant name: Helichrysum italicum ssp. microphyllum Location: Arzana (Sardinia), Italy (wildgrown) | Aerial parts + flowers | Dipyrones | [9] |
| 19 | Helipyrone A (6) | Methanol ultrasound extraction of dried leaves (3 × 1 h, RT); crude residue (38.7 g) partitioned between H2O and EtOAc; EtOAc layer (8.8 g) fractionated on Sephadex LH-20 (hexane/CHCl3/MeOH 3:1:1; EtOAc fractions from methanol extract subjected to silica-gel CC (2-butanone/hexane 1:4) and preparative TLC | Plant material: n.d. Amount isolated: 1.2 mg Yield: n.d. | Plant name: Helichrysum italicum (Roth) G.Don Location: Castel Volturno (Caserta, Italy) (wildgrown) | Leaf material | Dipyrones | [12] |
| 20 | Helipyrone A (6) | Methanol maceration; Celite adsorption; Soxhlet extraction with petroleum ether; The residue was purified by silica-gel chromatography (CH2Cl2–MeOH gradient) | Plant material: 37,000 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum italicum (Roth) G.Don Location: Italy (commerial source) | Aerial parts + flowers | Dipyrones | [7] |
| 21 | Helipyrone A (6) | Defatted by refluxing in petroleum ether (2 h); extracted three times with 80:20 ethanol–water (3 × 5 L, RT); combined hydroalcoholic extracts partitioned between H2O and CHCl3; CHCl3 phase concentrated to dryness; residue purified by silica-gel column chromatography (CHCl3→MeOH gradient) and preparative TLC | Plant material: 10,000 g Amount isolated: 250 mg Yield: 0.0025% | Plant name: Helichrysum italicum (Roth) G.Don Location: Italy (commerial source) | Aerial parts | Dipyrones | [18] |
| 22 | Helipyrone A (6) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 2.7 mg Yield: 6.79 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Dipyrones | [11] |
| 23 | Helipyrone A (6) | Procedure the same as entry 9, compound 4 | Plant material: 400 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Dunes of the forest domain of Olonne, near Les Sables d’Olonne, Vendée, France | Roots | Dipyrones | [19] |
| 24 | Helipyrone A (6) | Cold maceration (3 × 24 h, 4 °C) with MeOH (1 L each); combined extracts evaporated to dryness (3.5 g); after CHCl3/MeOH (95:5→60:40) silica-gel CC, purified on Sephadex LH-20 | Plant material: 35 g Amount isolated: 14.5 mg Yield: 0.041% | Plant name: Helichrysum stoechas (L.) Moench Location: Villanueva de Gállego (Zaragoza, Spain) | Aerial parts + flowers | Dipyrones | [13] |
| 25 | Helipyrone A (6) | Procedure the same as entry 10, compound 4 | Plant material: 700 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Sierra Perenchiza, near Chiva, Valencia, Spain | Aerial parts + flowers | Dipyrones | [20] |
| 26 | Helitalone A (7) | Procedure the same as entry 6, compound 3 | Plant material: 500 g Amount isolated: 5 mg Yield: 0.001% | Plant name: Helichrysum italicum (Roth) G.Don Location: Sardinia, Italy (wildgrown) | Non-woody Aerial parts + flowers | Dipyrones | [14] |
| 27 | Norauricepyrone (8) | Procedure the same as entry 1, compound 1 | Plant material: 190 g Amount isolated: 2 mg Yield: 0.001052% | Plant name: Helichrysum cephaloideum DC. Location: Transvaal, South Africa (wildgrown) | Roots | 3-NA PGs | [16] |
| 28 | Methyl-norauricepyrone (9) | Procedure the same as entry 1, compound 1 | Plant material: 190 g Amount isolated: 2 mg Yield: 0.001052% | Plant name: Helichrysum cephaloideum DC. Location: Transvaal, South Africa (wildgrown) | Roots | 3-NA PGs | [16] |
| 29 | Arzanol (10) | Procedure the same as entry 16, compound 6 | Plant material: 1000 g Amount isolated: 965 mg Yield: 0.0965% | Plant name: Helichrysum italicum ssp. microphyllum Location: near Arzana (NU, Sardinia), Italy | Aerial parts (non-woody) + flowers | 3-prenyl PGs | [8] |
| 30 | Arzanol (10) | Maceration with acetone (3 × 2 L, RT); crude residue (12 g) dissolved in EtOAc/hexane (1:1, 200 mL each) and left overnight; precipitate collected and recrystallised from toluene–acetone; mother liquors chromatographed on silica gel (hexane → EtOAc gradient) | Plant material: 540 g Amount isolated: 437 mg Yield: 0.081% | Plant name: Helichrysum italicum ssp. microphyllum Location: Sardinia, Italy (wildgrown) | Aerial parts + flowers | 3-prenyl PGs | [17] |
| 31 | Arzanol (10) | Same as entry 5, compound 3; aqueous MeOH phase fraction crystallized from ether, then silica-gel CC (petroleum ether/EtOAc) | Plant material: 5000 g Amount isolated: 3700 mg Yield: 0.296% | Plant name: Helichrysum italicum ssp. microphyllum Location: Arzana (Sardinia), Italy (wildgrown) | Aerial parts + flowers | 3-prenyl PGs | [9] |
| 32 | Arzanol (10) | Procedure the same as entry 6, compound 3 | Plant material: 500 g Amount isolated: 10 mg Yield: 0.002% | Plant name: Helichrysum italicum (Roth) G. Don Location: Sardinia, Italy (wildgrown) | Non-woody Aerial parts + flowers | 3-prenyl PGs | [14] |
| 33 | Arzanol (10) | Cold maceration (3 × 24 h, 4 °C) with MeOH (1 L each); combined extracts evaporated to dryness (3.5 g) and chromatographed on silica gel (n-BuOH:H2O 82:18 → 70:10:30) then Sephadex LH-20 | Plant material: 35 g Amount isolated: 170 mg Yield: 0.48% | Plant name: Helichrysum stoechas (L.) Moench Location: Villanueva de Gállego (Zaragoza, Spain) | Flowers | 3-prenyl PGs | [13] |
| 34 | Arenol B (11) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 0.4 mg Yield: 1 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | 3-prenyl PGs | [11] |
| 35 | Helitalone B (12) | Procedure the same as entry 6, compound 3 | Plant material: 500 g Amount isolated: 3.1 mg Yield: 0.00062% | Plant name: Helichrysum italicum (Roth) G. Don Location: Sardinia, Italy (wildgrown) | Non-woody Aerial parts + flowers | 3-prenyl PGs | [14] |
| 36 | 6-O-Desmethylauricepyrone (13) | Ether: petroleum-ether (3:1, RT) extraction of air-dried aerial parts; silica-gel column (Activity Stage II) followed by repeated preparative TLC (Si-gel GF254) | Plant material: 90 g Amount isolated: 120 mg Yield: 0.133% | Plant name: Helichrysum odoratissimum Sweet. Location: South Africa (wildgrown) | Aerial parts | 3-prenyl PGs | [7] |
| 37 | Arenol C (14) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 0.8 mg Yield: 2 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | 3-prenyl PGs | [11] |
| 38 | 3-prenyl norauricepyrone (15) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 0.5 mg Yield: 1.25 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | 3-prenyl PGs | [11] |
| 39 | 23-Methyl-6-O-desmethylauricepyrone (16) | Procedure the same as entry 1, compound 1 | Plant material: 10 g Amount isolated: 7 mg Yield: 0.0699% | Plant name: Helichrysum mixtum (Kuntze) Moeser Location: Transvaal, South Africa (wildgrown) | Roots | 3-prenyl PGs | [16] |
| 40 | 23-Methyl-6-O-desmethylauricepyrone (16) | Procedure the same as entry 36, compound 13 | Plant material: 90 g Amount isolated: 30 mg Yield: 0.033% | Plant name: Helichrysum odoratissimum Sweet. Location: South Africa (wildgrown) | Aerial parts | 3-prenyl PGs | [7] |
| 41 | 23-Methyl-6-O-desmethylauricepyrone (16) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 0.3 mg Yield: 7.5 × 10−6% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | 3-prenyl PGs | [11] |
| 42 | 23-Methyl-6-O-desmethylauricepyrone (16) | Procedure the same as entry 1, compound 1 | Plant material: 100 g Amount isolated: 90 mg Yield: 0.09% | Plant name: Helichrysum stenopterum DC. Location: Transvaal, South Africa (wildgrown) | Aerial parts | 3-prenyl PGs | [16] |
| 43 | Heliarzanol (17) | Same as entry 31, compound 10; Mother liquors from arzanol isolation chromatographed on silica gel (hexane/EtOAc 55:45) and preparative HPLC | Plant material: 5000 g Amount isolated: 4.5 mg Yield: 0.00036% | Plant name: Helichrysum italicum ssp. microphyllum Location: Arzana (Sardinia), Italy (wildgrown) | Aerial parts + flowers | 3-prenyl PGs | [9] |
| 44 | 23-ethyl-6-O-desmethyl-auricepyrone (18) | Procedure the same as entry 1, compound 1 | Plant material: 10 g Amount isolated: 7 mg Yield: 0.0699% | Plant name: Helichrysum mixtum (Kuntze) Moeser Location: Transvaal, South Africa (wildgrown) | Roots | 3-prenyl PGs | [16] |
| 45 | 23-ethyl-6-O-desmethyl-auricepyrone (18) | Procedure the same as entry 1, compound 1 | Plant material: 100 g Amount isolated: 90 mg Yield: 0.09% | Plant name: Helichrysum stenopterum DC. Location: Transvaal, South Africa (wildgrown) | Aerial parts | 3-prenyl PGs | [16] |
| 46 | Auricepyrone (19) | Extracted with diethyl ether/petroleum ether (1:2) at rt; combined extracts separated on silica gel column chromatography (activity grade II), further purified by repeated TLC (Si gel GF254) | Plant material: 50 g Amount isolated: 7.5 mg Yield: 0.01499% | Plant name: Helichrysum auriceps Hilliard Location: Natal (South Africa) | Roots | 3-prenyl methoxy PGs | [6] |
| 47 | Auricepyrone (19) | Same as entry 46, compound 19 | Plant material: 30 g Amount isolated: 3.75 mg Yield: 0.0125% | Plant name: Helichrysum cephaloideum DC. Location: Natal (South Africa) | Roots | 3-prenyl methoxy PGs | [6] |
| 48 | Auricepyrone (19) | Same as entry 46, compound 19 | Plant material: 50 g Amount isolated: 1.25 mg Yield: 0.0025% | Plant name: Helichrysum cephaloideum DC. Location: Natal (South Africa) | Aerial parts | 3-prenyl methoxy PGs | [6] |
| 49 | Auricepyrone (19) | Ether: petroleum-ether (1:7, RT) extraction of shredded roots; silica-gel column (Activity Stage II) followed by repeated preparative TLC (Si-gel GF254) | Plant material: 50 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum cephaloideum DC. Location: South Africa (wildgrown) | Roots | 3-prenyl methoxy PGs | [7] |
| 50 | Auricepyrone (19) | Procedure the same as entry 1, compound 1 | Plant material: 190 g Amount isolated: 15 mg Yield: 0.007894% | Plant name: Helichrysum cephaloideum DC. Location: Transvaal, South Africa (wildgrown) | Roots | 3-prenyl methoxy PGs | [16] |
| 51 | 23-Methylauricepyrone (20) | Procedure the same as entry 46, compound 19 | Plant material: 50 g Amount isolated: 22.5 mg Yield: 0.0449% | Plant name: Helichrysum auriceps Hilliard Location: Natal (South Africa) | Roots | 3-prenyl methoxy PGs | [6] |
| 52 | 23-Methylauricepyrone (20) | Procedure the same as entry 46, compound 19 | Plant material: 30 g Amount isolated: 11.25 mg Yield: 0.03749% | Plant name: Helichrysum cephaloideum DC. Location: Natal (South Africa) | Roots | 3-prenyl methoxy PGs | [6] |
| 53 | 23-Methylauricepyrone (20) | Procedure the same as entry 48, compound 19 | Plant material: 50 g Amount isolated: 3.75 mg Yield: 0.00749% | Plant name: Helichrysum cephaloideum DC. Location: Natal (South Africa) | Aerial parts | 3-prenyl methoxy PGs | [6] |
| 54 | 23-Methylauricepyrone (20) | Procedure the same as entry 49, compound 19 | Plant material: 50 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum cephaloideum DC. Location: South Africa (wildgrown) | Roots | 3-prenyl methoxy PGs | [7] |
| 55 | 23-ethyl-6-O-desmethyl-4-O-methylauricepyrone (21) | Procedure the same as entry 1, compound 1 | Plant material: 190 g Amount isolated: 15 mg Yield: 0.007894% | Plant name: Helichrysum cephaloideum DC. Location: Transvaal, South Africa (wildgrown) | Roots | 3-prenyl methoxy PGs | [16] |
| 56 | 18,18-bis-desmethyl Achyroclinopyrone C (22) | Chloroform extraction (plant dipped for 10 min at RT); silica-gel CC (n-hexane–EtOAc); preparative TLC (CHCl3); semipreparative reversed-phase HPLC (MeOH–phosphate buffer) | Plant material: 500 g Amount isolated: 16 mg Yield: 0.0032% | Plant name: Helichrysum decumbens Cambess. Location: La Manga del Mar Menor, Murcia, Spain (wildgrown) | Aerial parts + flowers | 3-geranyl PGs | [21] |
| 57 | 18,18-bis-desmethyl Achyroclinopyrone C (22) | Successive extraction with hexane, CHCl3, EtOAc, MeOH at rt; silica-gel CC (hexane–EtOAc); preparative TLC (CHCl3–MeOH 99:1) | Plant material: 400 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Dunes of the forest domain of Olonne, near Les Sables d’Olonne, Vendée, France | Roots | 3-geranyl PGs | [19] |
| 58 | 18,18-bis-desmethyl Achyroclinopyrone C (22) | Procedure the same as entry 24, compound 6 Obtained from silica-gel fractions after polarity-gradient CH2Cl2/MeOH chromatography, separated from arzanol by recrystallisation | Plant material: 35 g Amount isolated: 30 mg Yield: 0.08569% | Plant name: Helichrysum stoechas (L.) Moench Location: Villanueva de Gállego (Zaragoza, Spain) | Aerial parts + flowers | 3-geranyl PGs | [13] |
| 59 | 18,18-bis-desmethyl Achyroclinopyrone A (23) | Procedure the same as entry 56, compound 22 | Plant material: 500 g Amount isolated: 4 mg Yield: 0.0008% | Plant name: Helichrysum decumbens Cambess. Location: La Manga del Mar Menor, Murcia, Spain (wildgrown) | Aerial parts + flowers | 3-geranyl PGs | [21] |
| 60 | 18,18-bis-desmethyl Achyroclinopyrone A (23) | Procedure the same as entry 9, compound 4 | Plant material: 400 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Dunes of the forest domain of Olonne, near Les Sables d’Olonne, Vendée, France | Roots | 3-geranyl PGs | [19] |
| 61 | 8′-methyl-18,18-bis-desmethyl Achyroclinopyrone A (24) | Procedure the same as entry 56, compound 22 Detected as trace amounts by reversed-phase HPLC (MeOH–phosphate buffer) | Plant material: 500 g Amount isolated: traces Yield: n.d. | Plant name: Helichrysum decumbens Cambess. Location: La Manga del Mar Menor, Murcia, Spain (wildgrown) | Aerial parts + flowers | 3-geranyl PGs | [21] |
| 62 | Achyroclinopyrone C (25) | Same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 1.1 mg Yield: 2.8 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | 3-geranyl PGs | [11] |
| 63 | Achyroclinopyrone A (26) | Procedure the same as entry 62, compound 25 | Plant material: 4000 g Amount isolated: 1.5 mg Yield: 3.75 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | 3-geranyl PGs | [11] |
| 64 | Achyroclinopyrone D (27) | Procedure the same as entry 62, compound 25 | Plant material: 4000 g Amount isolated: 2.1 mg Yield: 5.3 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | 3-geranyl PGs | [11] |
| 65 | Achyroclinopyrone B (28) | Procedure the same as entry 62, compound 25 | Plant material: 4000 g Amount isolated: 1.2 mg Yield: 2.99 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | 3-geranyl PGs | [11] |
| 66 | Arenol A (29) | n.d. | Plant material: n.d. Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum arenarium (L.) Moench Location: n.d. | Non-woody Aerial parts + flowers | 2-prenyl PGs | [15] |
| 67 | Arenol A (29) | Procedure the same as entry 10, compound 4 | Plant material: 700 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Sierra Perenchiza, near Chiva, Valencia, Spain | Aerial parts + flowers | 2-prenyl PGs | [20] |
| 68 | Homoarenol (30) | Procedure the same as entry 10, compound 4 | Plant material: 700 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Sierra Perenchiza, near Chiva, Valencia, Spain | Aerial parts + flowers | 2-prenyl PGs | [20] |
| 69 | Homoarenol (30) | n.d. | Plant material: n.d. Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: n.d. | non-woody Aerial parts + flowers | 2-prenyl PGs | [15] |
| 70 | Helichrytalicine B (31) | Methanol ultrasound extraction of dried leaves (3 × 1 h, RT); crude residue (38.7 g) partitioned between H2O and EtOAc; EtOAc layer (8.8 g) fractionated on Sephadex LH-20 (hexane/CHCl3/MeOH 3:1:1), followed by silica-gel CC and preparative TLC | Plant material: n.d. Amount isolated: 2.3 mg Yield: n.d. | Plant name: Helichrysum italicum (Roth) G.Don Location: Castel Volturno (Caserta, Italy) (wildgrown) | Leaf material | Amino PGs | [12] |
| 71 | Helichrytalicine A (32) | Procedure the same as entry 70, compound 31 | Plant material: n.d. Amount isolated: 6 mg Yield: n.d. | Plant name: Helichrysum italicum (Roth) G.Don Location: Castel Volturno (Caserta, Italy) (wildgrown) | Leaf material | Amino PGs | [12] |
| 72 | Plicatipyrone (33) | Extracted with petroleum ether, followed by EtOH partition and chloroform extraction. The residue was purified by silica-gel CC using cyclohexane:EtOAc (8:2) | Plant material: 4500 g Amount isolated: 10 mg Yield: 0.000222% | Plant name: Helichrysum plicatum DC. ssp. plicatum Location: Yildiz Dagi and Camlibel (Sivas), Turkey (wildgrown) | Flower heads | Benzopyranes | [7] |
| 73 | Plicatipyrone (33) | Procedure the same as entry 10, compound 4 | Plant material: 700 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Sierra Perenchiza, near Chiva, Valencia, Spain | Aerial parts + flowers | Benzopyranes | [20] |
| 74 | Isobutyryl-helichromenopyrone (34) | Procedure the same as entry 1, compound 1 | Plant material: 10 g Amount isolated: 1 mg Yield: 0.01% | Plant name: Helichrysum mixtum (Kuntze) Moeser Location: Transvaal, South Africa (wildgrown) | Roots | Benzopyranes | [16] |
| 75 | methylbutyryl-helichromenopyrone (35) | Procedure the same as entry 1, compound 1 | Plant material: 100 g Amount isolated: 1 mg Yield: 0.001% | Plant name: Helichrysum mixtum (Kuntze) Moeser Location: Transvaal, South Africa (wildgrown) | Aerial parts | Benzopyranes | [16] |
| 76 | Italipyrone (36) | Procedure the same as entry 20, compound 6 | Plant material: 37,000 g Amount isolated: 25 mg Yield: 6.799 × 10−5% | Plant name: Helichrysum italicum (Roth) G.Don Location: Italy (commerial source) | Aerial parts + flowers | Benzofuranes | [7] |
| 77 | Italipyrone (36) | Procedure the same as entry 10, compound 4 | Plant material: 700 g Amount isolated: n.d. Yield: n.d. | Plant name: Helichrysum stoechas (L.) Moench Location: Sierra Perenchiza, near Chiva, Valencia, Spain | Aerial parts + flowers | Benzofuranes | [20] |
| 78 | 22-Methyl-22-ethyl-italipyrone (37) | Same as entry 49, compound 19 | Plant material: 50 g Amount isolated: 2.5 mg Yield: 0.005% | Plant name: Helichrysum cephaloideum DC. Location: South Africa (wildgrown) | Roots | Benzofuranes | [7] |
| 79 | 22-Methyl-22-ethyl-italipyrone (37) | Procedure the same as entry 1, compound 1 | Plant material: 190 g Amount isolated: 15 mg Yield: 0.007894% | Plant name: Helichrysum cephaloideum DC. Location: Transvaal, South Africa (wildgrown) | Roots | Benzofuranes | [16] |
| 80 | 22-Methyl-22-ethyl-italipyrone (37) | Procedure the same as entry 1, compound 1 | Plant material: 10 g Amount isolated: 5 mg Yield: 0.05% | Plant name: Helichrysum mixtum (Kuntze) Moeser Location: Transvaal, South Africa (wildgrown) | Roots | Benzofuranes | [16] |
| 81 | 22-Methyl-22-propyl-italipyrone (38) | Same as entry 49, compound 19; Isolated after de-acetylation | Plant material: 50 g Amount isolated: 2.5 mg Yield: 0.005% | Plant name: Helichrysum cephaloideum DC. Location: South Africa (wildgrown) | Roots | Benzofuranes | [7] |
| 82 | 22-Methyl-22-propyl-italipyrone (38) | Procedure the same as entry 1, compound 1 | Plant material: 200 g Amount isolated: 15 mg Yield: 0.007499% | Plant name: Helichrysum cephaloideum DC. Location: Transvaal, South Africa (wildgrown) | Aerial parts | Benzofuranes | [16] |
| 83 | 22-Methyl-22-propyl-italipyrone (38) | Procedure the same as entry 1, compound 1 | Plant material: 10 g Amount isolated: 5 mg Yield: 0.05% | Plant name: Helichrysum mixtum (Kuntze) Moeser Location: Transvaal, South Africa (wildgrown) | Roots | Benzofuranes | [16] |
| 84 | 20-(3,3′-Dimethylallyl)-italipyrone (39) | Extracted with petroleum ether; partitioned with EtOH; the dry residue extracted with chloroform. The extract (8.9 g) was purified by silica-gel CC, followed by rechromatography using cyclohexane:ethyl acetate (9:1) | Plant material: 1600 g Amount isolated: 80 mg Yield: 0.005% | Plant name: Helichrysum stoechas subsp. barrelieri (Ten.) Nym Location: Mudanya (Bursa), Turkey (wildgrown) | Flower heads | Benzofuranes | [7] |
| 85 | Helicyclol (40) | Procedure the same as entry 62, compound 25 | Plant material: 4000 g Amount isolated: 0.5 mg Yield: 1.2 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Benzofuranes | [11] |
| 86 | Cycloarzanol C (41) | Procedure the same as entry 62, compound 25 | Plant material: 4000 g Amount isolated: 0.5 mg Yield: 1.299 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Chromane PGs | [11] |
| 87 | Helicepyrone (42) | Procedure the same as entry 62, compound 25 | Plant material: 4000 g Amount isolated: 1 mg Yield: 2.5 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Chromane PGs | [11] |
| 88 | Italidipyrone (43) | Procedure the same as entry 20, compound 6 | Plant material: 37,000 g Amount isolated: 36 mg Yield: 9.729 × 10−5% | Plant name: Helichrysum italicum (Roth) G.Don Location: Italy (commerial source) | Aerial parts + flowers | Hetero-trimer PGs | [7] |
| 89 | Italidipyrone (43) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 0.8 mg Yield: 2 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Hetero-trimer PGs | [11] |
| 90 | 23-Methyl-italidipyrone (44) | Procedure the same as entry 20, compound 6 | Plant material: 37,000 g Amount isolated: 9 mg Yield: 2.43 × 10−5% | Plant name: Helichrysum italicum (Roth) G.Don Location: Italy (commerial source) | Aerial parts + flowers | Hetero-trimer PGs | [7] |
| 91 | 23-Methyl-italidipyrone (44) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 1.5 mg Yield: 3.8 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Hetero-trimer PGs | [11] |
| 92 | Helispiroketal A (45) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 1.9 mg Yield: 4.8 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Spiroketals | [11] |
| 93 | Helispiroketal B (46) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 0.9 mg Yield: 2.3 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Spiroketals | [11] |
| 94 | Helispiroketal F (47) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 1.2 mg Yield: 3 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Spiroketals | [11] |
| 95 | Helispiroketal E (48) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 1 mg Yield: 2.5 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Spiroketals | [11] |
| 96 | Helispiroketal C (49) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 0.7 mg Yield: 1.8 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Spiroketals | [11] |
| 97 | Helispiroketal D (50) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 1 mg Yield: 2.5 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Spiroketals | [11] |
| 98 | Helispiroketal H (51) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 1.1 mg Yield: 2.8 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Spiroketals | [11] |
| 99 | Helispiroketal G (52) | Procedure the same as entry 8, compound 4 | Plant material: 4000 g Amount isolated: 2.2 mg Yield: 5.5 × 10−5% | Plant name: Helichrysum oocephalum Boiss. Location: Mashhad, Razavi Khorasan, Iran | Aerial parts | Spiroketals | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voynikov, Y. Phloroglucinol α-Pyrones from Helichrysum: A Review on Structural Diversity, Plant Distribution and Isolation. Plants 2025, 14, 3460. https://doi.org/10.3390/plants14223460
Voynikov Y. Phloroglucinol α-Pyrones from Helichrysum: A Review on Structural Diversity, Plant Distribution and Isolation. Plants. 2025; 14(22):3460. https://doi.org/10.3390/plants14223460
Chicago/Turabian StyleVoynikov, Yulian. 2025. "Phloroglucinol α-Pyrones from Helichrysum: A Review on Structural Diversity, Plant Distribution and Isolation" Plants 14, no. 22: 3460. https://doi.org/10.3390/plants14223460
APA StyleVoynikov, Y. (2025). Phloroglucinol α-Pyrones from Helichrysum: A Review on Structural Diversity, Plant Distribution and Isolation. Plants, 14(22), 3460. https://doi.org/10.3390/plants14223460







