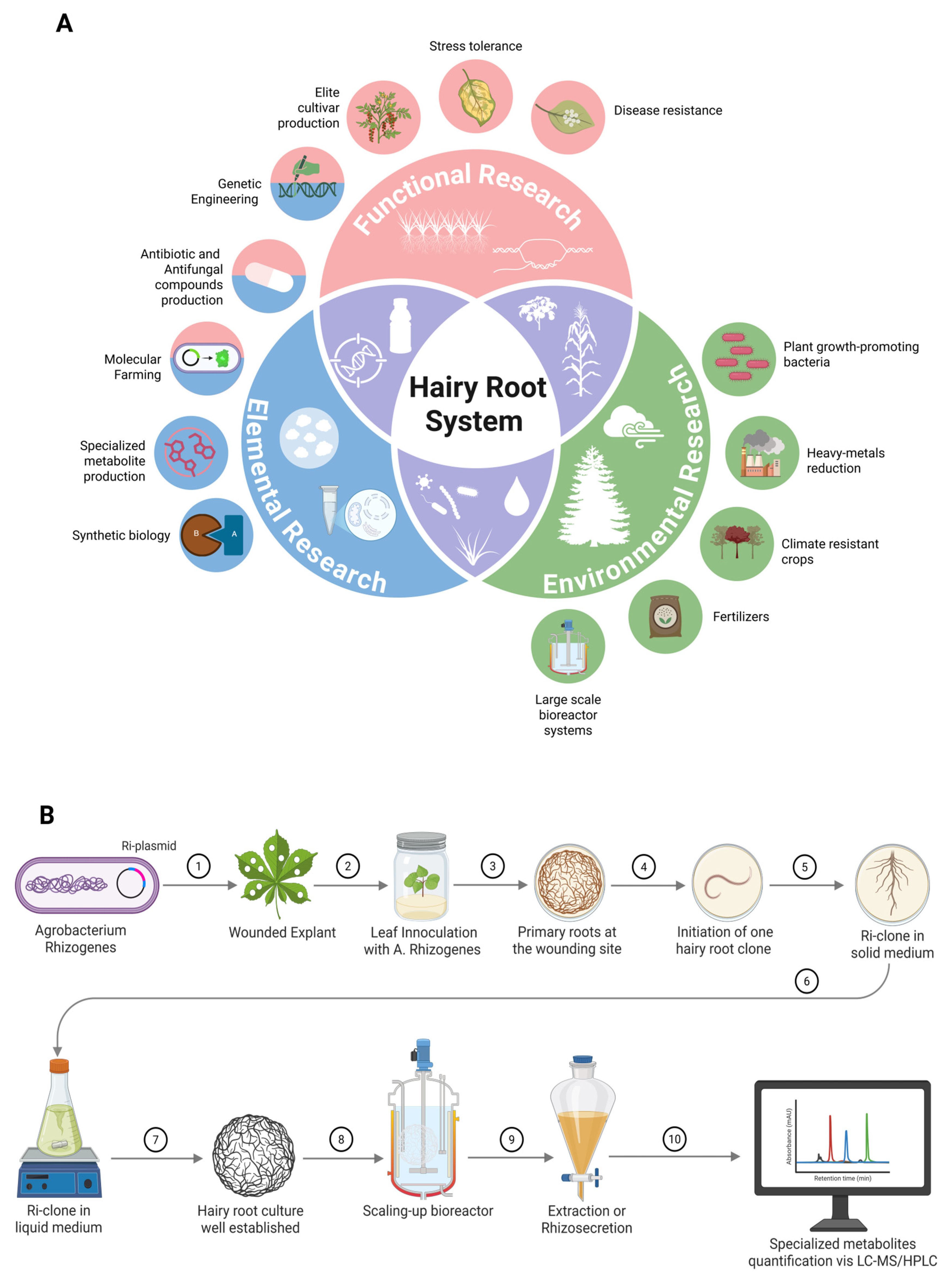
Reprogramming Hairy Root Cultures: A Synthetic Biology Framework for Precision Metabolite Biosynthesis
Abstract
1. Introduction
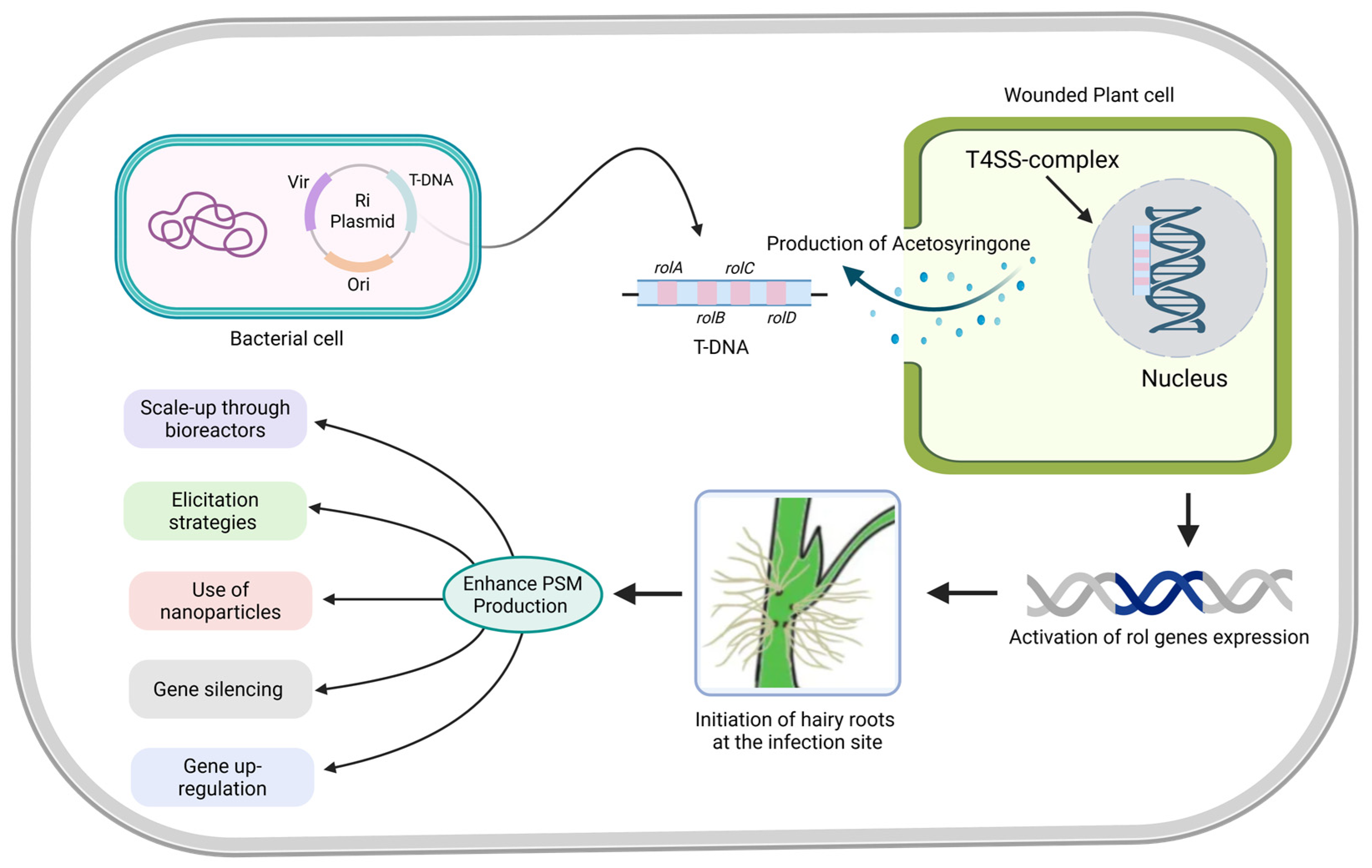
2. Induction Mechanism of Hairy Roots Cultures via T-DNA Insertion and Rol Genes Cluster
3. Major Factors Affecting Hairy Root Induction for the High-Yield Production of Specialized Metabolites
3.1. Plant Species and Explant Type
3.2. Agrobacterium Strains and Infection Methods
3.3. Pre-Culture and Co-Culture Conditions
3.4. Growth Regulators and Medium Composition
3.5. Environmental Factors
4. Metabolic Reprogramming in Hairy Root Cultures for High-Yield Specialized Metabolite Production
4.1. Metabolic Engineering for Pathways Optimization
4.2. Scaling Metabolite Production via Bioreactor Systems
4.3. Elicitation Strategies in Hairy Root Culture to Enhance Metabolite Biosynthesis
4.3.1. Organic and Biotic Elicitors
4.3.2. Abiotic Elicitors
- (a)
- Physical Elicitors
- (b)
- Chemical Elicitors
4.3.3. Inducible Metabolites Secreted into the Culture Medium of Hairy Roots
Alkaloids
Phenylpropanoids
Terpenoids
Anthraquinones
Recombinant Proteins
| Plant Species | Elicitor Used | Metabolite(s) | Compound Class | Fold Increase/Yield | Reference |
|---|---|---|---|---|---|
| Hyoscyamus muticus | AgNPs | Scopolamine | Tropane alkaloid | ~4-fold increase (media) | [102] |
| Rubia cordifolia | MeJA | Anthraquinones | Quinones | Significant | [140] |
| Plumbago rosea | Ag+, MeJA | Plumbagin | Naphthoquinone | ~3-fold (extracellular) | [144] |
| Coptis japonica | Yeast extract | Berberine | Isoquinoline alkaloid | Not quantified | [145] |
| Glycyrrhiza uralensis | UV-C, Chitosan | Glycyrrhizin | Triterpenoid saponin | Up to 10-fold (medium) | [146] |
| Salvia miltiorrhiza | MeJA | Tanshinones | Diterpenoids | Increased secretion | [147] |
| Panax ginseng | MeJA, CNCs | Ginsenosides | Triterpenoid saponins | 12-fold increase (total) | [148] |
| Beta vulgaris | AgNPs | Betalains | Alkaloids | 15-fold (culture media) | [117] |
| Catharanthus roseus | ZnO-NPs | Vinblastine | Indole alkaloid | 4.3-fold (media) | [149] |
| Silybum marianum | CuSO4 | Silymarin, Silybin | Flavonolignans | 7–10× (media) | [150] |
| Artemisia annua | NO3−/NH4+ | Artemisinin | Sesquiterpenoid | 2.5× (extracellular) | [151] |
5. Challenges and Limitations of Hairy Root Culture for Specialized Metabolite Production
5.1. Bioreactor Design and Scale-Up Constraints
5.2. Metabolite Specificity and Biosynthetic Limitations
5.3. Elicitation Variability and Culture Reproducibility
5.4. Genetic Stability and Long-Term Preservation Issues
6. Emerging Technologies and Future Directions
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tarraf, W.; De Carlo, A. In Vitro Biotechnology for Conservation and Sustainable Use of Plant Genetic Resources. Plants 2024, 13, 1897. [Google Scholar] [CrossRef] [PubMed]
- Solberg, S.O.; van Zonneveld, M.; Diederichsen, A. Editorial: Advances in conservation and utilization of plant genetic resources. Front. Plant Sci. 2024, 15, 1468904. [Google Scholar] [CrossRef] [PubMed]
- Doria, E.; Boncompagni, E.; Marra, A.; Dossena, M.; Verri, M.; Buonocore, D. Polyphenols extraction from vegetable wastes using a green and sustainable method. Front. Sustain. Food Syst. 2021, 5, 690399. [Google Scholar] [CrossRef]
- Halder, M.; Sarkar, S.; Jha, S. Elicitation: A biotechnological tool for enhanced production of secondary metabolites in hairy root cultures. Eng. Life Sci. 2019, 19, 880–895. [Google Scholar] [CrossRef]
- Biswas, D.; Chakraborty, A.; Mukherjee, S.; Ghosh, B. Hairy root culture: A potent method for improved secondary metabolite production of Solanaceous plants. Front. Plant Sci. 2023, 14, 1197555. [Google Scholar] [CrossRef]
- Chilton, M.-D.; Tepfer, D.A.; Petit, A.; David, C.; Casse-Delbart, F.; Tempé, J. Agrobacterium rhizogenes inserts T-DNA into the genomes of the host plant root cells. Nature 1982, 295, 432–434. [Google Scholar] [CrossRef]
- Gutierrez-Valdes, N.; Häkkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.-M.; Ritala, A.; Cardon, F. Hairy Root Cultures—A Versatile Tool With Multiple Applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef]
- Cao, X.; Xie, H.; Song, M.; Lu, J.; Ma, P.; Huang, B.; Wang, M.; Tian, Y.; Chen, F.; Peng, J.; et al. Cut–dip–budding delivery system enables genetic modifications in plants without tissue culture. Innovation 2023, 4, 100345. [Google Scholar] [CrossRef]
- Aneta, G.; Aneta, W.-S. Hairy root cultures as a multitask platform for green biotechnology. Plant Cell Tissue Organ Cult. 2022, 150, 493–509. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Hoang, T.T.; Le, N.T.; Tran, H.T.; Nguyen, C.X.; Moon, Y.H.; Chu, H.H.; Do, P.T. An Efficient Hairy Root System for Validation of Plant Transformation Vector and CRISPR/Cas Construct Activities in Cucumber (Cucumis sativus L.). Front. Plant Sci. 2021, 12, 770062. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, X.; He, X.; Xu, L.; Huang, Y.; Shao, H.; Zhang, D.; Tang, B.; Ma, H. The Soybean Basic Helix-Loop-Helix Transcription Factor ORG3-Like Enhances Cadmium Tolerance via Increased Iron and Reduced Cadmium Uptake and Transport from Roots to Shoots. Front. Plant Sci. 2017, 8, 1098. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.-L.; Liu, C.; Piao, C.-L.; Liu, C.-L. A Stable Agrobacterium rhizogenes-Mediated Transformation of Cotton (Gossypium hirsutum L.) and Plant Regeneration From Transformed Hairy Root via Embryogenesis. Front. Plant Sci. 2020, 11, 604255. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, S.M.; Acharjee, S.; Nomani, A.; Deka, M. Production of pharmaceutical active recombinant globular adiponectin as a secretory protein in Withania Somnifera hairy root culture. J. Biotechnol. 2020, 323, 302–312. [Google Scholar] [CrossRef]
- Uma, S.; Karthic, R.; Kalpana, S.; Backiyarani, S.; Saraswathi, M.S. A novel temporary immersion bioreactor system for large scale multiplication of banana (Rasthali AAB—Silk). Sci. Rep. 2021, 11, 20371. [Google Scholar] [CrossRef]
- Agarwal, A.; Jeevanandham, S.; Sangam, S.; Chakraborty, A.; Mukherjee, M. Exploring the role of carbon-based nanomaterials in microalgae for the sustainable production of bioactive compounds and beyond. ACS Omega 2022, 7, 22061–22072. [Google Scholar] [CrossRef]
- Silva, P.B.; Mendes, L.G.; Rehder, A.P.; Duarte, C.R.; Barrozo, M.A. Optimization of ultrasound-assisted extraction of bioactive compounds from acerola waste. J. Food Sci. Technol. 2020, 57, 4627–4636. [Google Scholar] [CrossRef]
- Ahmad, N.; Li, T.; Liu, Y.; Hoang, N.Q.V.; Ma, X.; Zhang, X.; Liu, J.; Yao, N.; Liu, X.; Li, H. Molecular and biochemical rhythms in dihydroflavonol 4-reductase-mediated regulation of leucoanthocyanidin biosynthesis in Carthamus tinctorius L. Ind. Crops Prod. 2020, 156, 112838. [Google Scholar] [CrossRef]
- Desborough, M.J.; Keeling, D.M. The aspirin story–from willow to wonder drug. Br. J. Haematol. 2017, 177, 674–683. [Google Scholar] [CrossRef]
- Athni, T.S.; Athni, S.S. The evolution of modern medicine: Garden to pill box. In Medicinal Plants: From Farm to Pharmacy; Springer: Cham, Switzerland, 2019; pp. 1–16. [Google Scholar]
- Herrero, M. Towards green extraction of bioactive natural compounds. Anal. Bioanal. Chem. 2024, 416, 2039–2047. [Google Scholar] [CrossRef]
- Li, C.; Wang, M. Application of Hairy Root Culture for Bioactive Compounds Production in Medicinal Plants. Curr. Pharm. Biotechnol. 2021, 22, 592–608. [Google Scholar] [CrossRef]
- Georgiev, M.I.; Pavlov, A.I.; Bley, T. Hairy root type plant in vitro systems as sources of bioactive substances. Appl. Microbiol. Biotechnol. 2007, 74, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Halder, M.; Roychowdhury, D.; Jha, S. A critical review on biotechnological interventions for production and yield enhancement of secondary metabolites in hairy root cultures. In Hairy Roots: An Effective Tool of Plant Biotechnology; Springer: Singapore, 2018; pp. 21–44. [Google Scholar]
- Gantait, S.; Mukherjee, E. Hairy root culture technology: Applications, constraints and prospect. Appl. Microbiol. Biotechnol. 2021, 105, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Gordon, J.E.; Christie, P.J. The agrobacterium Ti plasmids. In Plasmids: Biology and Impact in Biotechnology and Discovery; ASM Press: Washington, DC, USA, 2015; pp. 295–313. [Google Scholar]
- Hernández-Piedra, G.; Ruiz-Carrera, V.; Sánchez, A.J.; Azpeitia-Morales, A.; Calva-Calva, G. Induction of hairy roots on somatic embryos of rhizoclones from Typha domingensis seedlings. Plants 2020, 9, 1679. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Meng, X.; Xu, K.; Li, M.; Gmitter, F.G., Jr.; Liu, N.; Gai, Y.; Huang, S.; Wang, M.; Wang, M.; et al. Highly efficient hairy root genetic transformation and applications in citrus. Front. Plant Sci. 2022, 13, 1039094. [Google Scholar] [CrossRef]
- Mauro, M.L.; Bettini, P.P. Agrobacterium rhizogenes rolB oncogene: An intriguing player for many roles. Plant Physiol. Biochem. 2021, 165, 10–18. [Google Scholar] [CrossRef]
- van der Salm, T.P.; Hänisch ten Cate, C.H.; Dons, H.J. Prospects for applications of rol genes for crop improvement. Plant Mol. Biol. Report. 1996, 14, 207–228. [Google Scholar] [CrossRef]
- Mano, Y.; Nemoto, K. The pathway of auxin biosynthesis in plants. J. Exp. Bot. 2012, 63, 2853–2872. [Google Scholar] [CrossRef]
- Basak, S.; Parajulee, D.; Dhir, S.; Sangra, A.; Dhir, S.K. Improved Protocol for Efficient Agrobacterium-Mediated Transient Gene Expression in Medicago sativa L. Plants 2024, 13, 2992. Plants 2024, 13, 2992. [Google Scholar] [CrossRef]
- Anand, A.; Jones, T.J. Advancing Agrobacterium-based crop transformation and genome modification technology for agricultural biotechnology. In Agrobacterium Biology: From Basic Science to Biotechnology; Springer: Cham, Switzerland, 2018; pp. 489–507. [Google Scholar]
- Kiryushkin, A.S.; Ilina, E.L.; Guseva, E.D.; Pawlowski, K.; Demchenko, K.N. Hairy CRISPR: Genome editing in plants using hairy root transformation. Plants 2021, 11, 51. [Google Scholar] [CrossRef]
- Shu, H.; Luo, Z.; Peng, Z.; Wang, J. The application of CRISPR/Cas9 in hairy roots to explore the functions of AhNFR1 and AhNFR5 genes during peanut nodulation. BMC Plant Biol. 2020, 20, 417. [Google Scholar] [CrossRef]
- Jedličková, V.; Mácová, K.; Štefková, M.; Butula, J.; Staveníková, J.; Sedláček, M.; Robert, H.S. Hairy root transformation system as a tool for CRISPR/Cas9-directed genome editing in oilseed rape (Brassica napus). Front. Plant Sci. 2022, 13, 919290. [Google Scholar] [CrossRef] [PubMed]
- Hao, C.; Wang, S.; Wang, Y.H.; Hou, X.; Jiang, Y.; Jiang, B.; Zhang, X.; Hua, X.; Xue, Z. Technology and Application of Hairy Root Culture in Monocotyledons. Curr. Biochem. Eng. 2021, 7, 31–37. [Google Scholar] [CrossRef]
- Fan, Y.-L.; Zhang, X.-H.; Zhong, L.-J.; Wang, X.-Y.; Jin, L.-S.; Lyu, S.-H. One-step generation of composite soybean plants with transgenic roots by Agrobacterium rhizogenes-mediated transformation. BMC Plant Biol. 2020, 20, 208. [Google Scholar] [CrossRef]
- Makhzoum, A.B.; Sharma, P.; Bernards, M.A.; Trémouillaux-Guiller, J. Hairy roots: An ideal platform for transgenic plant production and other promising applications. In Phytochemicals, Plant Growth, and the Environment; Springer: New York, NY, USA, 2012; pp. 95–142. [Google Scholar]
- Aswati Nair, R.; Harsha, K.; Harshitha, K.; Shilpa, T.; Pillai, P. Secondary metabolite production from roots/rhizomes: Prospects and challenges in developing differentiated cultures using the plant’s hidden half. In Phytochemical Genomics: Plant Metabolomics and Medicinal Plant Genomics; Springer: Singapore, 2023; pp. 447–475. [Google Scholar]
- Gambino, G.; Gribaudo, I. Genetic transformation of fruit trees: Current status and remaining challenges. Transgenic Res. 2012, 21, 1163–1181. [Google Scholar] [CrossRef]
- Mikac, S.; Markulin, L.; Drouet, S.; Corbin, C.; Tungmunnithum, D.; Kiani, R.; Kabra, A.; Abbasi, B.H.; Renouard, S.; Bhambra, A. Bioproduction of Anticancer Podophyllotoxin and Related Aryltretralin-Lignans in Hairy Root Cultures of Linum flavum L. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Springer: Cham, Switzerland, 2021; pp. 503–540. [Google Scholar]
- Zhu, Y.; Zhu, X.; Wen, Y.; Wang, L.; Wang, Y.; Liao, C.; Zhao, M.; Li, T.; Liu, D.; Li, B. Plant hairy roots: Induction, applications, limitations and prospects. Ind. Crops Prod. 2024, 219, 119104. [Google Scholar] [CrossRef]
- Wang, H.; Wang, A.; Pu, H.; Yang, Y.; Ling, Z.; Xu, H.; Xu, J.; Yu, H.; Wu, X. Induction, Flavonoids Contents, and Bioactivities Analysis of Hairy Roots and True Roots of Tetrastigma hemsleyanum Diels et Gilg. Molecules 2023, 28, 2686. [Google Scholar] [CrossRef]
- Sathasivam, R.; Choi, M.; Radhakrishnan, R.; Kwon, H.; Yoon, J.; Yang, S.H.; Kim, J.K.; Chung, Y.S.; Park, S.U. Effects of various Agrobacterium rhizogenes strains on hairy root induction and analyses of primary and secondary metabolites in Ocimum basilicum. Front. Plant Sci. 2022, 13, 983776. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhou, Z.; Yin, Z.; Zhang, L.; Zhang, Q.; Xie, Y.; Chen, J. Effect of different Agrobacterium rhizogenes strains on hairy root induction and analysis of metabolites in Physalis peruviana L. J. Plant Physiol. 2025, 305, 154431. [Google Scholar] [CrossRef]
- Park, C.H.; Zhao, S.; Yeo, H.J.; Park, Y.E.; Baska, T.B.; Arasu, M.V.; Al-Dhabi, N.A.; Park, S.U. Comparison of different strains of Agrobacterium rhizogenes for hairy root induction and betulin and betulinic acid production in Morus alba. Nat. Prod. Commun. 2017, 12, 1934578X1701200403. [Google Scholar] [CrossRef]
- Mondéjar-López, M.; García-Simarro, M.P.; Navarro-Simarro, P.; Gómez-Gómez, L.; Ahrazem, O.; Niza, E. A review on the encapsulation of “eco-friendly” compounds in natural polymer-based nanoparticles as next generation nano-agrochemicals for sustainable agriculture and crop management. Int. J. Biol. Macromol. 2024, 280, 136030. [Google Scholar] [CrossRef]
- Bytton, A. High Yield Extraction Method for and Products of Corydalis Plants. U.S. Patent US11801279B2, 31 October 2023. [Google Scholar]
- Islam, K.; Rawoof, A.; Kumar, A.; Momo, J.; Ahmed, I.; Dubey, M.; Ramchiary, N. Genetic regulation, environmental cues, and extraction methods for higher yield of secondary metabolites in capsicum. J. Agric. Food Chem. 2023, 71, 9213–9242. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, B. Preliminary Assessment of Conditions Influencing Agrobacterium Rhizogenes-Mediated Transformation of Eurycoma Longifolia Jack. Somatic Embryos. Ph.D. Thesis, Universiti Sains Malaysia, Gelugor, Malaysia, 2013. [Google Scholar]
- Ebrahimi, M.A. Agrobacterium Rhizogenes-mediated Transformation of Peganum multisectum (Maxim) Bobrov and Harmine Production in Hairy Roots. J. Med. Plants By-Prod. 2017, 6, 201–212. [Google Scholar]
- D’Amelia, V.; Ruggiero, A.; Tranchida-Lombardo, V.; Leone, A.; Tucci, M.; Docimo, T. Biosynthesis of salvia specialized metabolites and biotechnological approaches to increase their production. In Salvia Biotechnology; Springer: Cham, Switzerland, 2017; pp. 241–270. [Google Scholar]
- Abbasi, B.H.; Stiles, A.R.; Saxena, P.K.; Liu, C.-Z. Gibberellic acid increases secondary metabolite production in Echinacea purpurea hairy roots. Appl. Biochem. Biotechnol. 2012, 168, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Murthy, H.N.; Hahn, E.-J.; Paek, K.-Y. Large-scale cultivation of adventitious roots of Echinacea purpurea in airlift bioreactors for the production of chichoric acid, chlorogenic acid and caftaric acid. Biotechnol. Lett. 2007, 29, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, L.; Zhang, D.; Li, X.; Yang, H.; Guan, K. Establishment of transient genetic transformation method for syntrichia caninervis mitt. Mol. Plant Breed 2019, 17, 7. [Google Scholar]
- Shams, S.; Naeem, B.; Ma, L.; Li, R.; Zhang, Z.; Cao, Y.; Yu, H.; Feng, X.; Qiu, Y.; Wu, H. Developing an Optimized Protocol for Regeneration and Transformation in Pepper. Genes 2024, 15, 1018. [Google Scholar] [CrossRef]
- Thilip, C.; Soundar Raju, C.; Varutharaju, K.; Aslam, A.; Shajahan, A. Improved Agrobacterium rhizogenes-mediated hairy root culture system of Withania somnifera (L.) Dunal using sonication and heat treatment. 3 Biotech 2015, 5, 949–956. [Google Scholar] [CrossRef]
- Abdelkawy, A.M.; Alshammari, S.O.; Hussein, H.-A.A.; Abou El-Enain, I.M.; Abdelkhalek, E.S.; Radwan, A.M.; Kenawy, S.K.; Maaty, D.A.; Abed, N.N.; Sabry, S. Effect of silver nanoparticles on tropane alkaloid production of transgenic hairy root cultures of Hyoscyamus muticus L. and their antimicrobial activity. Sci. Rep. 2023, 13, 10397. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhao, B.; Yuan, X. Improved growth of Artemisia annua L. hairy roots and artemisinin production under red light conditions. Biotechnol. Lett. 2001, 23, 1971–1973. [Google Scholar] [CrossRef]
- Vinodhini, J.; Karthikeyan, G.; Rajendran, L. Optimization of an efficient Agrobacterium mediated genetic transformation in chilli (Capsicum annuum). Vegetos 2024, 37, 717–724. [Google Scholar] [CrossRef]
- Isah, T.; Umar, S.; Mujib, A.; Sharma, M.P.; Rajasekharan, P.; Zafar, N.; Frukh, A. Secondary metabolism of pharmaceuticals in the plant in vitro cultures: Strategies, approaches, and limitations to achieving higher yield. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 132, 239–265. [Google Scholar] [CrossRef]
- Guan, S.; Kang, X.; Ge, J.; Fei, R.; Duan, S.; Sun, X. An efficient Agrobacterium-mediated transient transformation system and its application in gene function elucidation in Paeonia lactiflora Pall. Front. Plant Sci. 2022, 13, 999433. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, M.S.; Cao, Y.; Zhou, H.; Xia, Y. Root development enhanced by using indole-3-butyric acid and naphthalene acetic acid and associated biochemical changes of in vitro azalea microshoots. J. Plant Growth Regul. 2018, 37, 813–825. [Google Scholar] [CrossRef]
- Nakhooda, M.; Watt, M.P.; Mycock, D. The properties and interaction of auxins and cytokinins influence rooting of shoot cultures of Eucalyptus. Afr. J. Biotechnol. 2012, 11, 16568–16578. [Google Scholar]
- Alsoufi, A.S.; Staśkiewicz, K.; Markowski, M. Alterations in oleanolic acid and sterol content in marigold (Calendula officinalis) hairy root cultures in response to stimulation by selected phytohormones. Acta Physiol. Plant. 2021, 43, 44. [Google Scholar] [CrossRef]
- Castilho, C.V.; Leitão, S.G.; Silva, V.D.; Miranda, C.d.O.; Santos, M.C.d.S.; Bizzo, H.R.; da Silva, N.C. In vitro propagation of a carvacrol-producing type of Lippia origanoides Kunth: A promising oregano-like herb. Ind. Crops Prod. 2019, 130, 491–498. [Google Scholar] [CrossRef]
- Bahmani, H.; Maroufi, A.; Majdi, M.; Fakheri, B.A. Thymol production in hairy root culture of Sahendian savory (Satureja sahendica Bornm). Plant Biotechnol. Rep. 2021, 15, 177–186. [Google Scholar] [CrossRef]
- Xing, B.; Yang, D.; Guo, W.; Liang, Z.; Yan, X.; Zhu, Y.; Liu, Y. Ag+ as a more effective elicitor for production of tanshinones than phenolic acids in Salvia miltiorrhiza hairy roots. Molecules 2014, 20, 309–324. [Google Scholar] [CrossRef]
- Srivastava, M.; Singh, G.; Sharma, S.; Shukla, S.; Misra, P. Elicitation enhanced the yield of glycyrrhizin and antioxidant activities in hairy root cultures of Glycyrrhiza glabra L. J. Plant Growth Regul. 2019, 38, 373–384. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, J.Y. Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. In Biotechnology of Hairy Root Systems; Springer: Berlin/Heidelberg, Germany, 2013; pp. 55–89. [Google Scholar]
- Kaur, G.; Prakash, P.; Srivastava, R.; Verma, P.C. Enhanced secondary metabolite production in hairy root cultures through biotic and abiotic elicitors. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Springer: Cham, Switzerland, 2021; pp. 625–660. [Google Scholar]
- Yang, J.; Yang, X.; Li, B.; Lu, X.; Kang, J.; Cao, X. Establishment of in vitro culture system for Codonopsis pilosula transgenic hairy roots. 3 Biotech 2020, 10, 137. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K. Hairy root culture for mass-production of high-value secondary metabolites. Crit. Rev. Biotechnol. 2007, 27, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Faraz, R.; Gokhale, M.; Gothalwa, R. Hairy root culture through Agrobacterium rhizogenes for enhancement of secondary metabolites production in medicinal plants: A review. J. Adv. Microbiol. 2020, 4, 45–58. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Javed, S.B. From nature to lab: A review of secondary metabolite biosynthetic pathways, environmental influences, and in vitro approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Malarz, J.; Michalska, K.; Yudina, Y.V.; Stojakowska, A. Hairy root cultures as a source of polyphenolic antioxidants: Flavonoids, stilbenoids and hydrolyzable tannins. Plants 2022, 11, 1950. [Google Scholar] [CrossRef]
- Nakasha, J.J.; Shaharuddin, N.A.; Venkatachalam, V.; Sinniah, U.R. Enhancing hairy root proliferation: Optimization of auxin, carbon sources, and dark-light regimes in safed musli (Chlorophytum borivilianum). S. Afr. J. Bot. 2024, 165, 136–143. [Google Scholar] [CrossRef]
- Alpizar, E.; Dechamp, E.; Lapeyre-Montes, F.; Guilhaumon, C.; Bertrand, B.; Jourdan, C.; Lashermes, P.; Etienne, H. Agrobacterium rhizogenes-transformed roots of coffee (Coffea arabica): Conditions for long-term proliferation, and morphological and molecular characterization. Ann. Bot. 2008, 101, 929–940. [Google Scholar] [CrossRef]
- Liu, H.-C.; Chan, H.-S.; Nargotra, P.; Shih, H.-D.; Kuo, C.-H.; Liu, Y.-C. Development of Stephania tetrandra S. MOORE hairy root culture process for tetrandrine production. J. Biotechnol. 2024, 394, 11–23. [Google Scholar] [CrossRef]
- Rezazadehfar, P.; Rezayian, M.; Niknam, V.; Mirmasoumi, M. Elicitor-enhanced steroidal sapogenin accumulation in hairy root cultures of Trigonella foenum-graecum. Sci. Rep. 2024, 14, 19106. [Google Scholar] [CrossRef]
- Kamiński, J.; Bujak, P.; Długosz, M. Permeabilization of Calendula officinalis L. hairy root cultures for the release of accumulated triterpenoid saponins. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 159, 5. [Google Scholar] [CrossRef]
- Rahchamani, R.; Radjabian, T.; Abrishamchi, P. Ag+ ions are effective elicitors for enhancing the production of phenolic acids and tanshinones in Salvia aristata Aucher ex Benth. hairy roots. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 158, 44. [Google Scholar] [CrossRef]
- Karami, M.; Naghavi, M.R.; Nasiri, J.; Farzin, N.; Ignea, C. Enhanced production of withaferin A from the hairy root culture of Withania somnifera via synergistic effect of Methyl jasmonate and β-cyclodextrin. Plant Physiol. Biochem. 2024, 208, 108440. [Google Scholar] [CrossRef]
- Mahendran, G.; Vimolmangkang, S. Effect of carbon source and elicitors on biomass production and accumulation of friedelin and epifriedelanol in hairy roots of hemp (Cannabis sativa L.). Plant Cell Tissue Organ Cult. (PCTOC) 2024, 156, 61. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Lu, Y.; Jiao, J.; Fu, J.-X.; Xu, X.-J.; Yao, L.; Fu, Y.-J. Application of UV-B radiation for enhancing the accumulation of bioactive phenolic compounds in pigeon pea [Cajanus cajan (L.) Millsp.] hairy root cultures. J. Photochem. Photobiol. B Biol. 2022, 228, 112406. [Google Scholar] [CrossRef] [PubMed]
- Alok, A.; Jain, P.; Kumar, J.; Yajnik, K.; Bhalothia, P. Genome engineering in medicinally important plants using CRISPR/Cas9 tool. In Genome Engineering via CRISPR-Cas9 System; Elsevier: Amsterdam, The Netherlands, 2020; pp. 155–161. [Google Scholar]
- Balasubramani, S.; Chen, Q.; Zhou, Z.; Moola, A.K.; Duraisamy, S.M.; Prakash, P.; Gayathiri, E.; Satish, L.; Swamy, M.K. Transgenic medicinal plants for improved plant metabolites production. In Phytochemical Genomics: Plant Metabolomics and Medicinal Plant Genomics; Springer: Singapore, 2023; pp. 403–415. [Google Scholar]
- Das, S.; Kwon, M.; Kim, J.-Y. Enhancement of specialized metabolites using CRISPR/Cas gene editing technology in medicinal plants. Front. Plant Sci. 2024, 15, 1279738. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Pei, X.; Song, X.; Wang, S.; Yang, Z.; Zhu, J.; Lin, Q.; Zhu, Q.; Yang, X. Application and development of CRISPR technology in the secondary metabolic pathway of the active ingredients of phytopharmaceuticals. Front. Plant Sci. 2025, 15, 1477894. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, F.; Li, F.; Li, M.; Wang, Y.; Wang, G.; Sun, X.; Tang, K. Development of transgenic Artemisia annua (Chinese wormwood) plants with an enhanced content of artemisinin, an effective anti-malarial drug, by hairpin-RNA-mediated gene silencing. Biotechnol. Appl. Biochem. 2009, 52, 199–207. [Google Scholar] [CrossRef]
- Hao, X.; Pu, Z.; Cao, G.; You, D.; Zhou, Y.; Deng, C.; Shi, M.; Nile, S.H.; Wang, Y.; Zhou, W. Tanshinone and salvianolic acid biosynthesis are regulated by SmMYB98 in Salvia miltiorrhiza hairy roots. J. Adv. Res. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Sivanandhan, G.; Selvaraj, N.; Ganapathi, A.; Lim, Y.P. Up-regulation of Squalene synthase in hairy root culture of Withania somnifera (L.) Dunal yields higher quantities of withanolides. Ind. Crops Prod. 2020, 154, 112706. [Google Scholar] [CrossRef]
- Fu, R.; Shi, M.; Deng, C.; Zhang, Y.; Zhang, X.; Wang, Y.; Kai, G. Improved phenolic acid content and bioactivities of Salvia miltiorrhiza hairy roots by genetic manipulation of RAS and CYP98A14. Food Chem. 2020, 331, 127365. [Google Scholar] [CrossRef]
- Yin, Y.-C.; Hou, J.-M.; Tian, S.-K.; Yang, L.; Zhang, Z.-X.; Li, W.-D.; Liu, Y. Overexpressing chalcone synthase (CHS) gene enhanced flavonoids accumulation in Glycyrrhiza uralensis hairy roots. Bot. Lett. 2020, 167, 219–231. [Google Scholar] [CrossRef]
- Ricigliano, V.; Kumar, S.; Kinison, S.; Brooks, C.; Nybo, S.E.; Chappell, J.; Howarth, D.G. Regulation of sesquiterpenoid metabolism in recombinant and elicited Valeriana officinalis hairy roots. Phytochemistry 2016, 125, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Srivastava, V.; Rahman, L.U.; Kukreja, A. Overexpression of a Catharanthus tryptophan decarboxylase (tdc) gene leads to enhanced terpenoid indole alkaloid (TIA) production in transgenic hairy root lines of Rauwolfia serpentina. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 115, 377–384. [Google Scholar] [CrossRef]
- Sharafi, A.; Hashemi Sohi, H.; Mousavi, A.; Azadi, P.; Dehsara, B.; Hosseini Khalifani, B. Enhanced morphinan alkaloid production in hairy root cultures of Papaver bracteatum by over-expression of salutaridinol 7-o-acetyltransferase gene via Agrobacterium rhizogenes mediated transformation. World J. Microbiol. Biotechnol. 2013, 29, 2125–2131. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, A.; Sohi, H.H.; Mousavi, A.; Azadi, P.; Khalifani, B.H.; Razavi, K. Metabolic engineering of morphinan alkaloids by over-expression of codeinone reductase in transgenic hairy roots of Papaver bracteatum, the Iranian poppy. Biotechnol. Lett. 2013, 35, 445–453. [Google Scholar] [CrossRef]
- Vaccaro, M.; Bernal, V.O.; Malafronte, N.; De Tommasi, N.; Leone, A. High yield of bioactive abietane diterpenes in Salvia sclarea hairy roots by overexpressing cyanobacterial DXS or DXR genes. Planta Med. 2019, 85, 973–980. [Google Scholar] [CrossRef]
- Rahnama, H.; Razi, Z.; Dadgar, M.N.; Hasanloo, T. Enhanced production of flavonolignans in hairy root cultures of Silybum marianum by over-expression of chalcone synthase gene. J. Plant Biochem. Biotechnol. 2013, 22, 138–143. [Google Scholar] [CrossRef]
- Kim, J.A.; Baek, K.-H.; Son, Y.M.; Son, S.H.; Shin, H. Hairy root cultures of Taxus cuspidate for enhanced production of paclitaxel. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 144–150. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, P.; Mathur, A.; Mathur, A.K. Overexpression of tryptophan decarboxylase and strictosidine synthase enhanced terpenoid indole alkaloid pathway activity and antineoplastic vinblastine biosynthesis in Catharanthus roseus. Protoplasma 2018, 255, 1281–1294. [Google Scholar] [CrossRef]
- Mehrotra, S.; Mishra, S.; Srivastava, V. Hairy root cultures for monoterpene indole alkaloid pathway: Investigation and biotechnological production. In Hairy Roots: An Effective Tool of Plant Biotechnology; Springer: Singapore, 2018; pp. 95–121. [Google Scholar]
- Beyge, Ş.Ü. Design and Characterization of 5-L Autoclavable Batch-Culture Bioreactor. Doctoral Dissertation, Middle East Technical University, Ankara, Turkey, 2014. [Google Scholar]
- Jossen, V.; Eibl, R.; Eibl, D. Single-use bioreactors—An overview. In Single-Use Technology in Biopharmaceutical Manufacture; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 37–52. [Google Scholar]
- Khojasteh, A. Biotechnological Production of Rosmarinic Acid and Ruscogenins. Scaling Up the Optimized Processes to Benchtop Bioreactors. Doctoral Thesis, Universitat de Barcelona, Barcelona, Spain, 2019. [Google Scholar]
- Stiles, A.R.; Liu, C.-Z. Hairy root culture: Bioreactor design and process intensification. In Biotechnology of Hairy Root Systems; Springer: Berlin/Heidelberg, Germany, 2013; pp. 91–114. [Google Scholar]
- Mishra, B.N.; Ranjan, R. Growth of hairy-root cultures in various bioreactors for the production of secondary metabolites. Biotechnol. Appl. Biochem. 2008, 49, 1–10. [Google Scholar] [CrossRef]
- Hilton, M.; Rhodes, M. Growth and hyoscyamine production of ‘hairy root’cultures of Datura stramonium in a modified stirred tank reactor. Appl. Microbiol. Biotechnol. 1990, 33, 132–138. [Google Scholar] [CrossRef]
- Thakore, D.; Srivastava, A.K. Mass scale hairy root cultivation of Catharanthus roseus in bioreactor for indole alkaloid production. In Plant Cell and Tissue Differentiation and Secondary Metabolites: Fundamentals and Applications; Springer: Cham, Switzerland, 2021; pp. 487–502. [Google Scholar]
- Verdu-Navarro, F.; Moreno-Cid, J.A.; Weiss, J.; Egea-Cortines, M. The advent of plant cells in bioreactors. Front. Plant Sci. 2023, 14, 1310405. [Google Scholar] [CrossRef] [PubMed]
- Muniz, D.R.; Faria, R.O.; Benedito, V.A.; de Fátima, Â.; Modolo, L.V. Plant as Biofactories of Pharmaceuticals and Nutraceuticals. In Chemistry and Pharmacology of Naturally Occurring Bioactive Compounds; CRC Press: Boca Raton, FL, USA, 2013; pp. 551–573. [Google Scholar]
- Abo-Kadoum, M.; Abouelela, M.E.; Al Mousa, A.A.; Abo-Dahab, N.F.; Mosa, M.A.; Helmy, Y.A.; Hassane, A.M. Resveratrol biosynthesis, optimization, induction, bio-transformation and bio-degradation in mycoendophytes. Front. Microbiol. 2022, 13, 1010332. [Google Scholar] [CrossRef] [PubMed]
- Chu, M. Optimization of the Agrobacterium-Mediated Transformation Process of Grapevine Cell Cultures and Evaluation of Its Effect on Secondary Metabolism = Optimización del Proceso de Transformación Mediada por Agrobacterium de Cultivos Celulares de vid y Evaluación de su Efecto Sobre el Metabolismo Secundario. Doctoral Dissertation, University of Murcia, Murcia, Spain, 2017. [Google Scholar]
- Marchev, A.S.; Stoykova, I.D.; Georgiev, M.I. Large-scale production of Specialized metabolites In Vitro cultures. In Plant Cell Culture Protocols; Springer: Berlin/Heidelberg, Germany, 2024; pp. 303–322. [Google Scholar]
- Jeong, G.-T.; Park, D.-H.; Ryu, H.-W.; Hwang, B.; Woo, J.-C.; Kim, D.; Kim, S.-W. Production of antioxidant compounds by culture of Panax ginseng CA Meyer hairy roots: I. Enhanced production of secondary metabolite in hairy root cultures by elicitation. In Twenty-Sixth Symposium on Biotechnology for Fuels and Chemicals; Humana: Totowa, NJ, USA, 2005; pp. 1147–1157. [Google Scholar]
- Mawale, K.S.; Umashankar, K.; Darade, Y.R.; Shetty, N.P.; Parvatam, G. Nanoparticle-mediated elicitation of plant secondary metabolites, in vitro and in vivo. In Biotechnological Production of Bioactive Phytochemicals of Medicinal Value; Elsevier: Amsterdam, The Netherlands, 2024; pp. 195–220. [Google Scholar]
- Al-Khayri, J.M.; Narasimha, S.W.; Vennapusa, A.R.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q. Biotechnological approaches for the production of hypericin and other important metabolites from the genus Hypericum. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 156, 100. [Google Scholar] [CrossRef]
- Murthy, H.N.; Joseph, K.S.; Paek, K.Y.; Park, S.Y. Nanomaterials as novel elicitors of pharmacologically active plant specialized metabolites in cell and organ cultures: Current status and future outlooks. Plant Growth Regul. 2024, 104, 5–30. [Google Scholar] [CrossRef]
- Jeong, G.-T.; Park, D.-H.; Ryu, H.-W.; Lee, W.-T.; Park, K.; Kang, C.-H.; Hwang, B.; Woo, J.-C. Optimum conditions for transformed Panax ginseng hairy roots in flask culture. In Biotechnology for Fuels and Chemicals: The Twenty–Third Symposium; Humana Press Inc.: Totowa, NJ, USA, 2002; pp. 1129–1139. [Google Scholar]
- Wang, J.; Li, J.; Li, J.; Liu, S.; Wu, X.; Li, J.; Gao, W. Transcriptome profiling shows gene regulation patterns in ginsenoside pathway in response to methyl jasmonate in Panax Quinquefolium adventitious root. Sci. Rep. 2016, 6, 37263. [Google Scholar] [CrossRef]
- Wu, C.-H.; Murthy, H.N.; Hahn, E.-J.; Paek, K.-Y. Establishment of adventitious root co-culture of Ginseng and Echinacea for the production of secondary metabolites. Acta Physiol. Plant. 2008, 30, 891–896. [Google Scholar] [CrossRef]
- Awere, C.O.; Rakkammal, K.; Mwaura, M.M.; Anadebe, V.C.; Ramesh, M. Hairy-root technology: A metabolic engineering tool and specialized metabolite pathway elucidation and production of secondary metabolites. A review. Results Eng. 2024, 23, 102697. [Google Scholar] [CrossRef]
- Cheng, Q.; He, Y.; Li, G.; Liu, Y.; Gao, W.; Huang, L. Effects of combined elicitors on tanshinone metabolic profiling and SmCPS expression in Salvia miltiorrhiza hairy root cultures. Molecules 2013, 18, 7473–7485. [Google Scholar] [CrossRef]
- Yang, T.; Fang, L.; Nopo-Olazabal, C.; Condori, J.; Nopo-Olazabal, L.; Balmaceda, C.; Medina-Bolivar, F. Enhanced production of resveratrol, piceatannol, arachidin-1, and arachidin-3 in hairy root cultures of peanut co-treated with methyl jasmonate and cyclodextrin. J. Agric. Food Chem. 2015, 63, 3942–3950. [Google Scholar] [CrossRef]
- Moreno-Anzúrez, N.E.; Marquina, S.; Alvarez, L.; Zamilpa, A.; Castillo-España, P.; Perea-Arango, I.; Torres, P.N.; Herrera-Ruiz, M.; Díaz García, E.R.; García, J.T. A cytotoxic and anti-inflammatory campesterol derivative from genetically transformed hairy roots of Lopezia racemosa Cav.(Onagraceae). Molecules 2017, 22, 118. [Google Scholar] [CrossRef]
- Zhai, X.; Luo, D.; Li, X.; Han, T.; Kong, Z.; Ji, J.; Qin, L.; Zheng, C. Endophyte Chaetomium globosum D38 and its elicitors promote tanshinones accumulation of Salvia miltiorrhiza. bioRxiv 2017. [Google Scholar] [CrossRef]
- Patra, N.; Srivastava, A.K. Artemisinin production by plant hairy root cultures in gas-and liquid-phase bioreactors. Plant Cell Rep. 2016, 35, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.J.; Chang, H.N.; Liu, J.R.; Jung, K.H. Production and secretion of indole alkaloids in hairy root cultures of Catharanthus roseus: Effects of in situ adsorption, fungal elicitation and permeabilization. J. Ferment. Bioeng. 1994, 78, 229–234. [Google Scholar]
- Bhadra, R.; Vani, S.; Shanks, J.V. Production of indole alkaloids by selected hairy root lines of Catharanthus roseus. Biotechnol. Bioeng. 1993, 41, 581–592. [Google Scholar] [CrossRef]
- Shoji, T.; Ogawa, T.; Hashimoto, T. Jasmonate-induced nicotine formation in tobacco is mediated by tobacco COI1 and JAZ genes. Plant Cell Physiol. 2008, 49, 1003–1012. [Google Scholar] [CrossRef]
- Cardillo, A.B.; Perassolo, M.; Minoia, J.M.; Talou, J.R.; Giulietti, A.M. Tropane alkaloid production by the establishment of hairy root cultures of Brugmansia candida and elicitation. In Hairy Root Cultures Based Applications: Methods and Protocols; Springer: Singapore, 2020; pp. 123–132. [Google Scholar]
- Zhang, R.; Zhang, B.-L.; Li, G.-C.; Xie, T.; Hu, T.; Luo, Z.-Y. Enhancement of ginsenoside Rg 1 in Panax ginseng hairy root by overexpressing the α-l-rhamnosidase gene from Bifidobacterium breve. Biotechnol. Lett. 2015, 37, 2091–2096. [Google Scholar] [CrossRef]
- Du, S.; Xiang, T.; Song, Y.; Huang, L.; Sun, Y.; Han, Y. Transgenic hairy roots of Tetrastigma hemsleyanum: Induction, propagation, genetic characteristics and medicinal components. Plant Cell Tiss Organ Cult. 2015, 122, 373–382. [Google Scholar] [CrossRef]
- Chen, R.; Chen, X.; Zhu, T.; Liu, J.; Xiang, X.; Yu, J.; Tan, H.; Gao, S.; Li, Q.; Fang, Y. Integrated transcript and metabolite profiles reveal that Eb CHI plays an important role in scutellarin accumulation in Erigeron Breviscapus hairy roots. Front. Plant Sci. 2018, 9, 789. [Google Scholar]
- Zhang, B.; Zheng, L.P.; Yi Li, W.; Wen Wang, J. Stimulation of artemisinin production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr. Nanosci. 2013, 9, 363–370. [Google Scholar] [CrossRef]
- Komaraiah, P.; Reddy, G.; Reddy, P.S.; Raghavendra, A.; Ramakrishna, S.; Reddanna, P. Enhanced production of antimicrobial sesquiterpenes and lipoxygenase metabolites in elicitor-treated hairy root cultures of Solanum tuberosum. Biotechnol. Lett. 2003, 25, 593–597. [Google Scholar] [CrossRef]
- Pourianezhad, F.; Rahnama, H.; Mousavi, A.; Khosrowshahli, M.; Mafakheri, S. Effects of combined elicitors on parthenolide production and expression of parthenolide synthase (TpPTS) in Tanacetum parthenium hairy root culture. Plant Biotechnol. Rep. 2019, 13, 211–218. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Praveen, N.; Kim, E.-H.; Kim, S.-H.; Chung, I.-M. Production of anthraquinones, phenolic compounds and biological activities from hairy root cultures of Polygonum multiflorum Thunb. Protoplasma 2014, 251, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Perassolo, M.; Cardillo, A.B.; Mugas, M.L.; Montoya, S.C.N.; Giulietti, A.M.; Talou, J.R. Enhancement of anthraquinone production and release by combination of culture medium selection and methyl jasmonate elicitation in hairy root cultures of Rubia tinctorum. Ind. Crops Prod. 2017, 105, 124–132. [Google Scholar] [CrossRef]
- Pham, N.B.; Schäfer, H.; Wink, M. Production and secretion of recombinant thaumatin in tobacco hairy root cultures. Biotechnol. J. 2012, 7, 537–545. [Google Scholar] [CrossRef]
- Woods, R.R.; Geyer, B.C.; Mor, T.S. Hairy-root organ cultures for the production of human acetylcholinesterase. BMC Biotechnol. 2008, 8, 95. [Google Scholar] [CrossRef]
- Fischer, R.; Hoffmann, K.; Schillberg, S.; Emans, N. Antibody production by molecular farming in plants. J. Biol. Regul. Homeost. Agents 2000, 14, 83–92. [Google Scholar]
- Kumar, A.; Kumari, A.; Demiwal, P.; Roy, P.; Sircar, D. Enhanced production of bioactive plumbagin in hairy root cultures and adventitious root cultures of Plumbago zeylanica L. by a novel apocarotenoid elicitor, α-ionone. Ind. Crops Prod. 2023, 203, 117140. [Google Scholar] [CrossRef]
- Giri, A.; Narasu, M.L. Transgenic hairy roots: Recent trends and applications. Biotechnol. Adv. 2000, 18, 1–22. [Google Scholar] [CrossRef]
- Kojoma, M.; Seki, H.; Ohyama, K.; Kim, S.; Muranaka, T. Glycyrrhizin production in hairy root cultures of Glycyrrhiza uralensis induced triterpenoid biosynthetic gene. Planta Medica 2016, 82, P768. [Google Scholar] [CrossRef]
- Rahmani, N.; Radjabian, T. Integrative effects of phytohormones in the phenolic acids production in Salvia verticillata L. under multi-walled carbon nanotubes and methyl jasmonate elicitation. BMC Plant Biol. 2024, 24, 56. [Google Scholar] [CrossRef]
- Kim, O.T.; Bang, K.H.; Kim, Y.C.; Hyun, D.Y.; Kim, M.Y.; Cha, S.W. Upregulation of ginsenoside and gene expression related to triterpene biosynthesis in ginseng hairy root cultures elicited by methyl jasmonate. Plant Cell Tissue Organ Cult. (PCTOC) 2009, 98, 25–33. [Google Scholar] [CrossRef]
- Perveen, S.; Safdar, N.; Yasmin, A.; Bibi, Y. DAT and PRX1 gene expression modulates vincristine production in Catharanthus roseus L. propagates using Cu, Fe and Zn nano structures. Plant Sci. 2022, 320, 111264. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Y.; Farkhari, M. Silymarin production in inoculated Silybum marianum L. hairy roots culture with Piriformospora indica. Russ. J. Plant Physiol. 2023, 70, 145. [Google Scholar] [CrossRef]
- Wang, J.W.; Tan, R.X. Artemisinin production in Artemisia annua hairy root cultures with improved growth by altering the nitrogen source in the medium. Biotechnol. Lett. 2002, 24, 1153–1156. [Google Scholar] [CrossRef]
- Das, S.; Manna, D.; Mondal, T.; Mondal, P. Advanced Systems and Bioreactors for Large-Scale Secondary Metabolite Production in Medicinal Plants. In Biotechnology, Multiple Omics, and Precision Breeding in Medicinal Plants; CRC Press: Boca Raton, FL, USA, 2025; pp. 341–354. [Google Scholar]
- Khan, S.A.; Siddiqui, M.H.; Osama, K. Bioreactors for hairy roots culture: A review. Curr. Biotechnol. 2018, 7, 417–427. [Google Scholar] [CrossRef]
- Mehrotra, S.; Srivastava, V.; Rahman, L.U.; Kukreja, A. Hairy root biotechnology—Indicative timeline to understand missing links and future outlook. Protoplasma 2015, 252, 1189–1201. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Slavov, A.K.; Marchev, A.S. The Untapped Potential of Hairy Root Cultures and Their Multiple Applications. Int. J. Mol. Sci. 2024, 25, 12682. [Google Scholar] [CrossRef]
- Choi, Y.-E.; Kim, Y.-S.; Paek, K.-Y. Types and designs of bioreactors for hairy root culture. In Plant Tissue Culture Engineering; Springer: Dordrecht, Netherlands, 2008; pp. 161–172. [Google Scholar]
- Zhang, K.; Wang, N.; Gao, X.; Ma, Q. Integrated metabolite profiling and transcriptome analysis reveals tissue-specific regulation of terpenoid biosynthesis in Artemisia argyi. Genomics 2022, 114, 110388. [Google Scholar] [CrossRef]
- Kolewe, M.E.; Gaurav, V.; Roberts, S.C. Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol. Pharm. 2008, 5, 243–256. [Google Scholar] [CrossRef]
- Malik, S.; Cusidó, R.M.; Mirjalili, M.H.; Moyano, E.; Palazón, J.; Bonfill, M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: A review. Process Biochem. 2011, 46, 23–34. [Google Scholar] [CrossRef]
- Arya, A.; Gautam, S.; Goel, S.; Grewal, S.; Bhattacharyya, M. Metabolic engineering for high-value bioactive compounds from medicinal plants. In Phytochemical Genomics: Plant Metabolomics and Medicinal Plant Genomics; Springer: Singapore, 2023; pp. 521–544. [Google Scholar]
- Lane, A.; Boecklemann, A.; Woronuk, G.N.; Sarker, L.; Mahmoud, S.S. A genomics resource for investigating regulation of essential oil production in Lavandula angustifolia. Planta 2010, 231, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Bagal, D.; Chowdhary, A.A.; Mehrotra, S.; Mishra, S.; Rathore, S.; Srivastava, V. Metabolic engineering in hairy roots: An outlook on production of plant secondary metabolites. Plant Physiol. Biochem. 2023, 201, 107847. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, G.; Vimolmangkang, S. Elicitation boosts withaferin A and withanolide A production in Withania somnifera (L.) Dunal hairy root cultures. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 158, 29. [Google Scholar] [CrossRef]
- Häkkinen, S.T.; Moyano, E.; Cusidó, R.M.; Oksman-Caldentey, K.-M. Exploring the metabolic stability of engineered hairy roots after 16 years maintenance. Front. Plant Sci. 2016, 7, 1486. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; De la Fuente Jimenez, J.L.; Paul, S.; de Donato-Capote, M.; Castillo-Maldonado, I.; Betanzos-Cabrera, G.; Valiente-Banuet, J.I.; Sharma, A. Cell Cultures and Hairy Roots as Platform for Production of High-Value Metabolites: Current Approaches, Limitations, and Future Prospects. In Advances in Plant Transgenics: Methods and Applications; Springer: Singapore, 2019; pp. 23–57. [Google Scholar]
- Yoshimatsu, K.; Yamaguchi, H.; Shimomura, K. Cryopreservation of Panax ginseng hairy roots by vitrification method. Cryobiology 1994, 31, 580. [Google Scholar]
- Lawson, C.E.; Martí, J.M.; Radivojevic, T.; Jonnalagadda, S.V.R.; Gentz, R.; Hillson, N.J.; Peisert, S.; Kim, J.; Simmons, B.A.; Petzold, C.J. Machine learning for metabolic engineering: A review. Metab. Eng. 2021, 63, 34–60. [Google Scholar] [CrossRef]
- Patra, P.; Disha, B.; Kundu, P.; Das, M.; Ghosh, A. Recent advances in machine learning applications in metabolic engineering. Biotechnol. Adv. 2023, 62, 108069. [Google Scholar] [CrossRef]
- Kwon, M.S.; Lee, B.T.; Lee, S.Y.; Kim, H.U. Modeling regulatory networks using machine learning for systems metabolic engineering. Curr. Opin. Biotechnol. 2020, 65, 163–170. [Google Scholar] [CrossRef]
- Liu, Y.; Patra, B.; Singh, S.K.; Paul, P.; Zhou, Y.; Li, Y.; Wang, Y.; Pattanaik, S.; Yuan, L. Terpenoid indole alkaloid biosynthesis in Catharanthus roseus: Effects and prospects of environmental factors in metabolic engineering. Biotechnol. Lett. 2021, 43, 2085–2103. [Google Scholar] [CrossRef]
- Negi, S.; Singh, P.; Rawat, B.; Sharma, A.; Rawat, J.M.; Semwal, P. Unearthing the landscape: A bibliometric exploration of hairy root culture research over the past 41 years. In Vitro Cell. Dev. Biol.-Plant 2025, 61, 1–24. [Google Scholar] [CrossRef]
- Dhiman, N.; Patial, V.; Bhattacharya, A. The current status and future applications of hairy root cultures. In Biotechnological Approaches for Medicinal and Aromatic Plants: Conservation, Genetic Improvement and Utilization; Springer: Singapore, 2018; pp. 87–155. [Google Scholar]

| Plant Species | Metabolite | Business Value | Strategy | Yield | Reference |
|---|---|---|---|---|---|
| Tetrastigma hemsleyanum | Catechin | Anti-tumor, antioxidant | Hormones-induced co-culture time expansion | 692.63 ± 127.24 mg/g DW | [43] |
| Epicatechin | Antioxidant, lipid glucose-lowering | 163.34 ± 31.86 mg/g DW | [43] | ||
| Stephania tetrandra | Tetrandrine | Anti-rheumatic, anti-inflammatory | Use of WPM medium | 7.28 mg/L DW | [79] |
| Total phenolics | Antioxidant, anti-inflammatory | 7.28 mg/L DW | [79] | ||
| Trigonella foenum graecum | Total phenolics | Antioxidant, anti-inflammatory | Processing with SA | 15.082 ± 1.211 μg/DW | [80] |
| Flavanol | Antiallergic | 18.587 ± 2.564 μg/DW | |||
| Flavonoids | Antioxidant, anti-inflammatory | 15.082 ± 1.211 μg/DW | |||
| Anthocyanin | Antioxidant, anti-inflammatory | 2.727 ± 0.076 μg/DW | |||
| Calendula officinalis | Saponins | Antibacterial, antiviral | Triton X-100 addition | 1.2 mg/g DW | [81] |
| Aucher ex Benth. | Aalvianolic acid | Antibacterial | Processing with Ag+ | 31.49 ± 0.65 mg/L DW | [82] |
| Withania somnifera | Lactones | Anti-inflammatory, anti-tumor | Elicitor treatment with MeJA and β-CD | 17.45 mg/g DW | [83] |
| Cannabis sativa | Friedelin | Antidiabetic, hypolipidemic | Processing with SA | 5.018 ± 0.35 mg/g DW | [84] |
| Cannabis sativa | Epifriedelanol | Antidiabetic, hypolipidemic | Processing with SA | 5.018 ± 0.35 mg/g DW | [84] |
| pigeon pea | Flavonoids | Antiallergic | UV-B radiation | 414.95 ± 50.68 μg/g DW | [85] |
| Cajaninstilbenate | Antioxidant | UV-B radiation | 666.01 ± 702.14 μg/g DW | [85] |
| Application | Plant Species | Biological Impact | Reference |
|---|---|---|---|
| Metabolic engineering for pathway optimization | Artemisia annua | CRISPR-Cas9 knockout of squalene synthase (SQS) redirected precursor flux, leading to a 3.2-fold increase in artemisinin production. | [90] |
| Salvia miltiorrhiza | Overexpression of SmMYB1 activated diterpenoid biosynthetic genes, resulting in an 8.5-fold increase in tanshinone production. | [91] | |
| Catharanthus roseus | Introduction of strictosidine synthase enabled de novo production of monoterpene indole alkaloids. | [102] | |
| Withania somnifera | Co-overexpression of squalene epoxidase and cytochrome P450 genes significantly enhanced withanolide accumulation. | [92] | |
| Taxus chinensis | Fusion of biosynthetic enzymes facilitated efficient substrate channeling, increasing paclitaxel biosynthesis 5-fold. | [101] | |
| Elicitation-induced biosynthesis | Panax ginseng | Treatment with methyl jasmonate (MeJA) and cellulose nanocrystals resulted in a 12-fold increase in ginsenoside production. | [116] |
| Beta vulgaris | Silver nanoparticles (AgNP) stimulated betalain production 15-fold via ROS-mediated pathway activation. | [117] | |
| Hypericum perforatum | Blue light exposure triggered a 10-fold increase in hypericin accumulation by activating polyketide synthases. | [118] | |
| Glycyrrhiza glabra | UV-B irradiation enhanced flavonoid biosynthesis by inducing phenylpropanoid pathway genes. | [76] | |
| Catharanthus roseus | Zinc oxide nanoparticles (ZnO-NPs) enhanced vinblastine production by modulating oxidative stress responses. | [119] | |
| Scaling Production via Bioreactor Systems | Datura stramonium | Stirred tank reactor maintained 95% root viability and increased hyoscyamine production. | [109] |
| Catharanthus roseus | 3D-printed scaffold reactors improved vincristine production by 30%. | [110]. | |
| Glycyrrhiza uralensis | Perfusion-based bioreactor resulted in 10-fold increase in glycyrrhizin accumulation while preventing product inhibition. | [112] | |
| Polygonum cuspidatum | Co-culture with Saccharomyces cerevisiae enabled 25-fold increase in resveratrol production. | [113,114]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Ahmad, N.; Tao, Y.; Hussain, H.; Chang, Y.; Umar, A.W.; Liu, X. Reprogramming Hairy Root Cultures: A Synthetic Biology Framework for Precision Metabolite Biosynthesis. Plants 2025, 14, 1928. https://doi.org/10.3390/plants14131928
Liu C, Ahmad N, Tao Y, Hussain H, Chang Y, Umar AW, Liu X. Reprogramming Hairy Root Cultures: A Synthetic Biology Framework for Precision Metabolite Biosynthesis. Plants. 2025; 14(13):1928. https://doi.org/10.3390/plants14131928
Chicago/Turabian StyleLiu, Chang, Naveed Ahmad, Ye Tao, Hamad Hussain, Yue Chang, Abdul Wakeel Umar, and Xiuming Liu. 2025. "Reprogramming Hairy Root Cultures: A Synthetic Biology Framework for Precision Metabolite Biosynthesis" Plants 14, no. 13: 1928. https://doi.org/10.3390/plants14131928
APA StyleLiu, C., Ahmad, N., Tao, Y., Hussain, H., Chang, Y., Umar, A. W., & Liu, X. (2025). Reprogramming Hairy Root Cultures: A Synthetic Biology Framework for Precision Metabolite Biosynthesis. Plants, 14(13), 1928. https://doi.org/10.3390/plants14131928







