Emerging Mechanisms of Plant Responses to Abiotic Stress
Abstract
1. Introduction
2. Emerging Mechanisms Underlying Plant Responses to Major Abiotic Stresses
2.1. Drought Stress Responses
2.1.1. ABA Signaling: A Central Hormonal Regulator
2.1.2. Hormonal Crosstalk Among ABA, JA, and SA Under Drought Stress
2.1.3. Brassinosteroid Signaling and the Growth–Defense Trade-Off
2.1.4. Hydraulic and Electrical Signaling
2.1.5. Root System Plasticity and Water Acquisition
2.1.6. Transcriptional Regulation and Epigenetic Reprogramming
2.1.7. Drought Memory and Transgenerational Priming
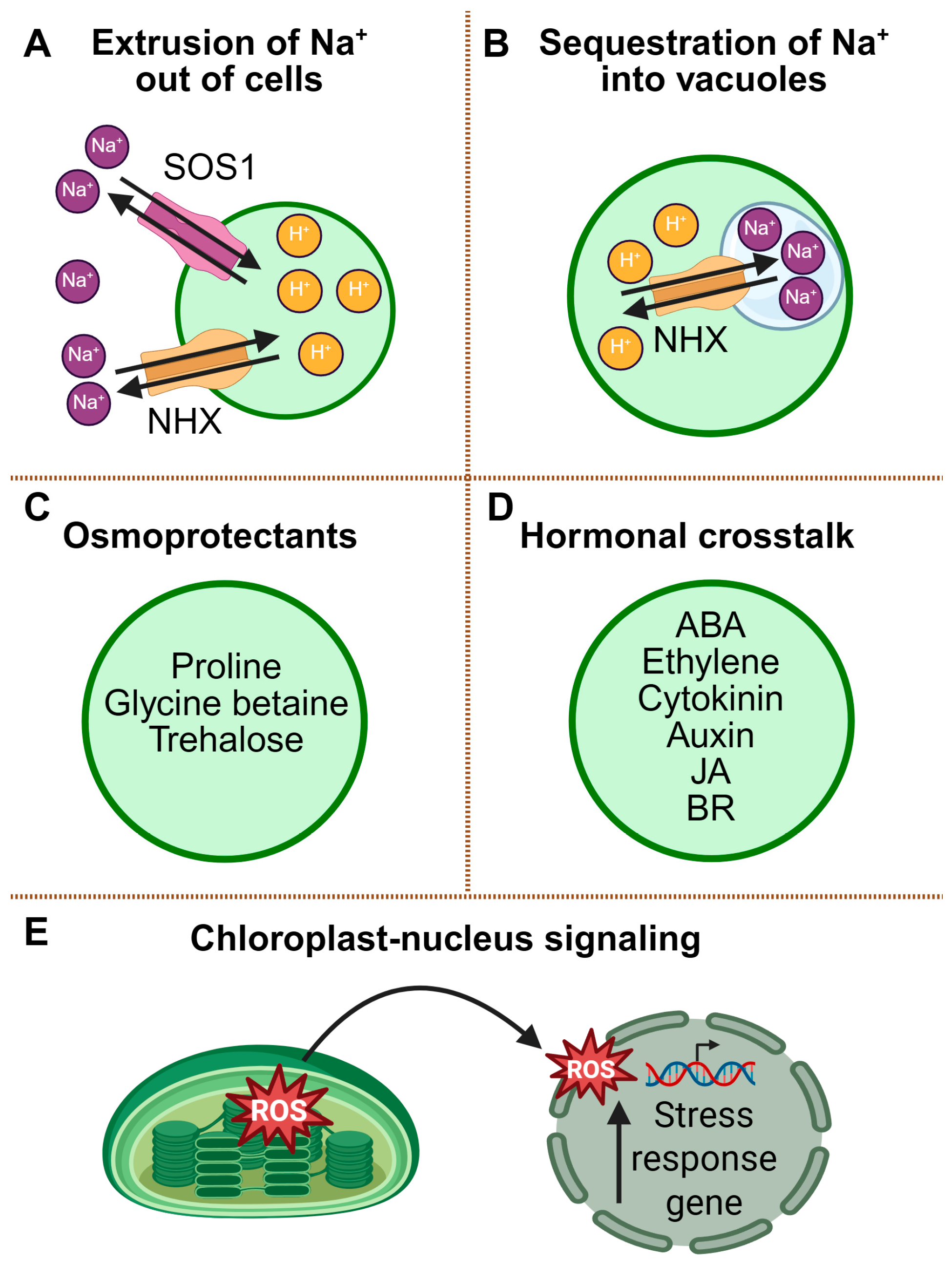
2.2. Salinity Stress Responses
2.2.1. Ionic Homeostasis and the SOS Pathway
2.2.2. Osmotic Adjustment and Compatible Solute Accumulation
2.2.3. Hormonal Crosstalk
2.2.4. ROS Signaling and Chloroplast-to-Nucleus Communication
2.2.5. Metabolic Reprogramming
2.2.6. Translational Protection
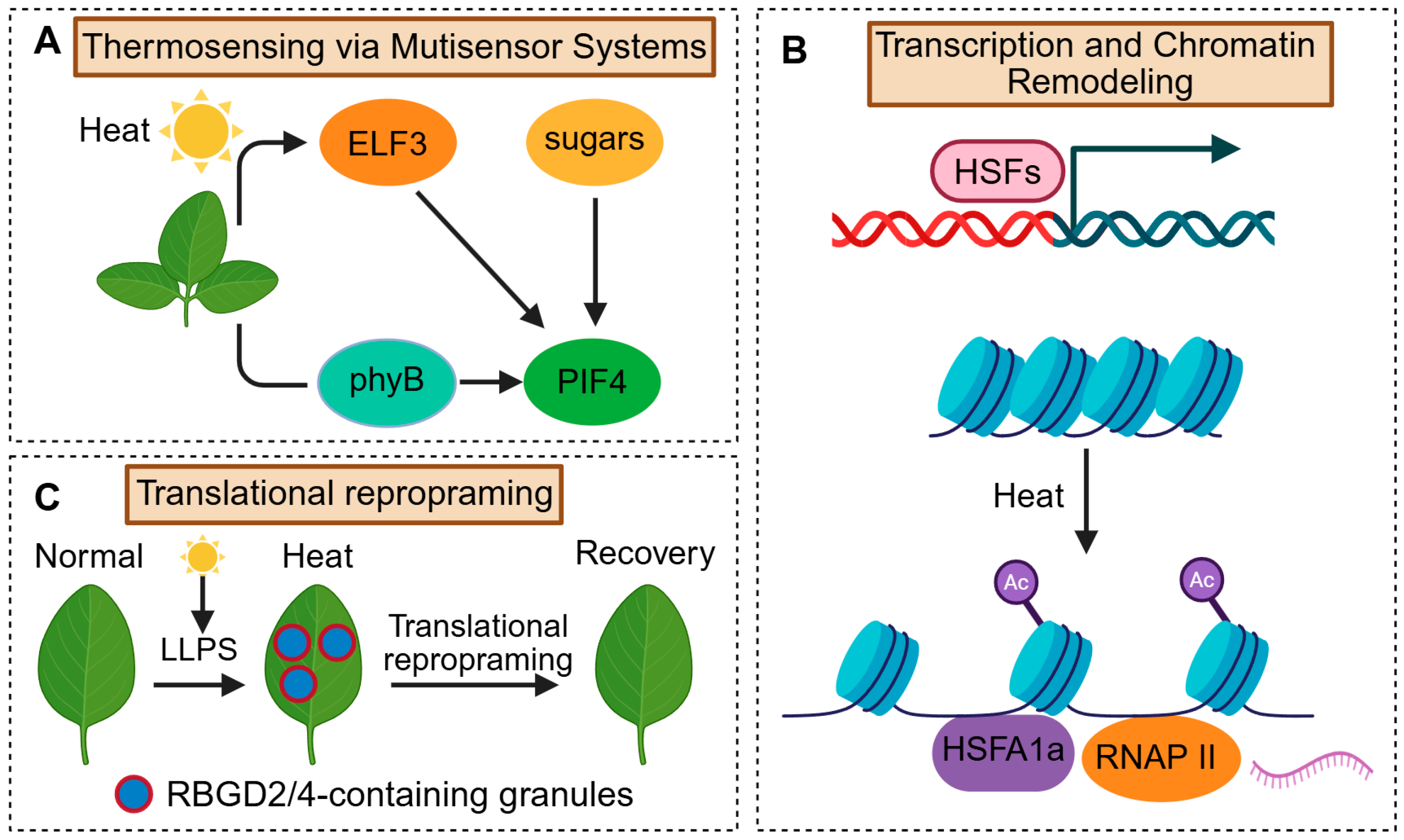
2.3. Heat Stress Responses
2.3.1. Thermosensing via Multisensor Systems
2.3.2. Additional Thermosensory Components and Signaling Pathways
2.3.3. Heat-Induced Transcriptional Regulation and Chromatin Remodeling
2.3.4. Translational Reprogramming and Post-Transcriptional Control
2.3.5. Heat Memory and Acclimation Mechanisms
2.4. Cold Stress Responses
2.4.1. Cold Perception and Calcium Signaling
2.4.2. The ICE1-CBF-COR Module
2.4.3. Redox Regulation of CBF Activity
2.4.4. Chromatin Remodeling and Cold Memory
2.4.5. Chloroplast Retrograde Signaling and the Malate Valve
2.4.6. Physiological and Metabolic Adjustments
2.5. Specific Regulatory Modules Under Combined Stresses
2.5.1. Drought–Heat Combination
2.5.2. Salinity–Cold Combination
2.6. Heavy Metal (HM) Stress Responses
2.6.1. Root Avoidance Responses and Rhizospheric Processes
2.6.2. Cell Wall Fixation, Intracellular Chelation, and Vacuolar Sequestration
2.6.3. Oxidative Stress and Activation of Antioxidant Defense
2.6.4. Hormonal Signaling and Epigenetic Regulation
2.6.5. Directed Allocation and Stress Memory
3. Future Prospects and Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | Abscisic Acid |
| Ca2+ | Calcium ions |
| ROS | Reactive oxygen species |
| SA | Salicylic Acid |
| JAs | Jasmonates |
| GAs | Gibberellins |
| BRs | Brassinosteroids |
| CRISPRa | CRISPR activation |
| SOS | Salt Overly Sensitive |
| CIPK24 | CBL-Interacting Protein Kinase 24 |
| CBL4 | Calcineurin B-like Protein 4 |
| HKT1 | high-affinity K+ transporters |
| RLK | receptor-like kinase |
| GIPC | glycosyl inositol phosphorylceramides |
| NF-Y | nuclear factor Y |
| TCA | tricarboxylic acid |
| GABA | γ-aminobutyric acid |
| HSR | heat shock response |
| HSP | heat shock protein |
| HSF | heat shock transcription factor |
| RBP | RNA-binding protein |
| phyB | phytochrome B |
| PIF4 | PHYTOCHROME INTERACTING FACTOR 4 |
| ICE1 | Inducer of CBF Expression 1 |
| COR | cold-responsive |
| HOS1 | High expression of osmotically responsive genes 1 |
| CAMKII | calmodulin kinase II |
| Trx-h2 | thioredoxin h2 |
| NLS | nuclear localization signal |
| ncRNA | non-coding RNA |
| HM | Heavy Metal |
| Cd | Cadmium |
| As | Arsenic |
| Pb | Lead |
| Hg | Mercury |
| PGPR | Plant Growth-Promoting Rhizobacteria |
| PCs | Phytochelatins |
| MTs | Metallothioneins |
| GSH | Glutathione |
| PCS | Phytochelatin Synthase |
| ABC transporters | ATP-Binding Cassette transporters |
| HMAs | Heavy Metal ATPases |
| ER | Endoplasmic Reticulum |
| PVC | PreVacuolar Compartment |
| TP | Tonoplast |
| Mel | Melatonin |
| IAA | Indole-3-Acetic Acid |
| LncRNA | Long non-coding RNA |
| siRNA | Small interfering RNA |
References
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.K. Abiotic stress responses in plants. Nat. Rev. Genet. 2022, 23, 104–119. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abrams. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Brandt, B.; Munemasa, S.; Wang, C.; Nguyen, D.; Yong, T.; Yang, P.G.; Poretsky, E.; Belknap, T.F.; Waadt, R.; Aleman, F.; et al. Calcium specificity signaling mechanisms in abscisic acid signal transduction in Arabidopsis guard cells. eLife 2015, 4, e03599. [Google Scholar] [CrossRef]
- Tripathy, B.C.; Oelmuller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef]
- Yadav, A.S.; Sureshkumar, S.; Sinha, A.K.; Balasubramanian, S. Dispersed Components Drive Temperature Sensing and Response in Plants. Science 2025, 388, 1161–1166. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Chong, L.; Jiao, Z.; Xu, R.; Niu, Q.; Zhu, Y. Salt stress activates the CDK8-AHL10-SUVH2/9 module to dynamically regulate salt tolerance in Arabidopsis. Nat. Commun. 2025, 16, 2454. [Google Scholar] [CrossRef]
- You, Z.; Guo, S.; Li, Q.; Fang, Y.; Huang, P.; Ju, C.; Wang, C. The CBL1/9-CIPK1 calcium sensor negatively regulates drought stress by phosphorylating the PYLs ABA receptor. Nat. Commun. 2023, 14, 5886. [Google Scholar] [CrossRef]
- Xiong, Y.; Song, X.; Mehra, P.; Yu, S.; Li, Q.; Tashenmaimaiti, D.; Bennett, M.; Kong, X.; Bhosale, R.; Huang, G. ABA-auxin cascade regulates crop root angle in response to drought. Curr. Biol. 2025, 35, 542–553.e4. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.J.; Choi, W.G.; Gilroy, S.; Morris, R.J. A ROS-assisted calcium wave dependent on the atrbohd nadph oxidase and TPC1 cation channel propagates the systemic response to salt stress. Plant Physiol. 2016, 171, 1771–1784. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.J.; Wang, P.; Zhang, B.; Li, M.; Chen, S.; Shi, R.; Tunlaya-Anukit, S.; Liu, X.; Wang, Z.; et al. The AREB1 transcription factor influences histone acetylation to regulate drought responses and tolerance in Populus Trichocarpa. Plant Cell 2019, 31, 663–686. [Google Scholar] [CrossRef]
- Ng, S.; Ivanova, A.; Duncan, O.; Law, S.R.; Van Aken, O.; De Clercq, I.; Wang, Y.; Carrie, C.; Xu, L.; Kmiec, B.; et al. A membrane-bound NAC transcription factor, ANAC017, mediates mitochondrial retrograde signaling in Arabidopsis. Plant Cell 2013, 25, 3450–3471. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tohge, T.; Ivakov, A.; Mueller-Roeber, B.; Fernie, A.R.; Mutwil, M.; Schippers, J.H.; Persson, S. Salt-related MYB1 coordinates abscisic acid biosynthesis and signaling during salt stress in Arabidopsis. Plant Physiol. 2015, 169, 1027–1041. [Google Scholar] [CrossRef] [PubMed]
- Barrero-Gil, J.; Salinas, J. Gene regulatory networks mediating cold acclimation: The CBF pathway. Adv. Exp. Med. Biol. 2018, 1081, 3–22. [Google Scholar] [CrossRef]
- Zheng, S.; Zhao, W.; Liu, Z.; Geng, Z.; Li, Q.; Liu, B.; Li, B.; Bai, J. Establishment and maintenance of heat-stress memory in plants. Int. J. Mol. Sci. 2024, 25, 8976. [Google Scholar] [CrossRef]
- Leuendorf, J.E.; Frank, M.; Schmulling, T. Acclimation, priming and memory in the response of Arabidopsis thaliana seedlings to cold stress. Sci. Rep. 2020, 10, 689. [Google Scholar] [CrossRef]
- Peng, Y.; Ming, Y.; Jiang, B.; Zhang, X.; Fu, D.; Lin, Q.; Zhang, X.; Wang, Y.; Shi, Y.; Gong, Z.; et al. Differential phosphorylation of Ca2+-permeable channel cyclic nucleotide-gated channel20 modulates calcium-mediated freezing tolerance in Arabidopsis. Plant Cell 2024, 36, 4356–4371. [Google Scholar] [CrossRef] [PubMed]
- Perrella, G.; Bäurle, I.; van Zanten, M. Epigenetic regulation of thermomorphogenesis and heat stress tolerance. New Phytol. 2022, 234, 1144–1160. [Google Scholar] [CrossRef]
- Alwutayd, K.M.; Rawat, A.A.; Sheikh, A.H.; Almeida-Trapp, M.; Veluchamy, A.; Jalal, R.; Karampelias, M.; Froehlich, K.; Alzaed, W.; Tabassum, N.; et al. Microbe-induced drought tolerance by ABA-mediated root architecture and epigenetic reprogramming. EMBO Rep. 2023, 24, e56754. [Google Scholar] [CrossRef]
- Zhuang, Y.; Wei, M.; Ling, C.; Liu, Y.; Amin, A.K.; Li, P.; Li, P.; Hu, X.; Bao, H.; Huo, H.; et al. EGY3 mediates chloroplastic ROS homeostasis and promotes retrograde signaling in response to salt stress in Arabidopsis. Cell Rep. 2021, 36, 109384. [Google Scholar] [CrossRef]
- Kuromori, T.; Fujita, M.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Inter-tissue and inter-organ signaling in drought stress response and phenotyping of drought tolerance. Plant J. 2022, 109, 342–358. [Google Scholar] [CrossRef]
- Casal, J.J.; Murcia, G.; Bianchimano, L. Plant thermosensors. Annu. Rev. Genet. 2024, 58, 135–158. [Google Scholar] [CrossRef]
- Li, M.; Kim, C. Chloroplast ROS and stress signaling. Plant Commun. 2022, 3, 100264. [Google Scholar] [CrossRef]
- Foyer, C.H.; Hanke, G. ROS production and signalling in chloroplasts: Cornerstones and evolving concepts. Plant J. 2022, 111, 642–661. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Liu, J.; Guo, M.; Ouyang, W.; Yan, J.; Xiong, L.; Li, X. Drought-responsive dynamics of H3K9ac-marked 3D chromatin interactions are integrated by OsbZIP23-associated super-enhancer-like promoter regions in rice. Genome Biol. 2024, 25, 262. [Google Scholar] [CrossRef]
- Huang, Y.; An, J.; Sircar, S.; Bergis, C.; Lopes, C.D.; He, X.; Da Costa, B.; Tan, F.Q.; Bazin, J.; Antunez-Sanchez, J.; et al. HSFA1a modulates plant heat stress responses and alters the 3D chromatin organization of enhancer-promoter interactions. Nat. Commun. 2023, 14, 469. [Google Scholar] [CrossRef] [PubMed]
- Roca Paixão, J.F.; Gillet, F.X.; Ribeiro, T.P.; Bournaud, C.; Lourenco-Tessutti, I.T.; Noriega, D.D.; Melo, B.P.; de Almeida-Engler, J.; Grossi-de-Sa, M.F. Improved drought stress tolerance in Arabidopsis by CRISPR/dCas9 fusion with a histone acetyltransferase. Sci. Rep. 2019, 9, 8080. [Google Scholar] [CrossRef]
- Fichman, Y.; Miller, G.; Mittler, R. Whole-plant live imaging of reactive oxygen species. Mol. Plant 2019, 12, 1203–1210. [Google Scholar] [CrossRef]
- Fàbregas, N.; Lozano-Elena, F.; Blasco-Escámez, D.; Tohge, T.; Martínez-Andújar, C.; Albacete, A.; Osorio, S.; Bustamante, M.; Riechmann, J.L.; Nomura, T.; et al. Overexpression of the vascular brassinosteroid receptor BRL3 confers drought resistance without penalizing plant growth. Nat. Commun. 2018, 9, 4680. [Google Scholar] [CrossRef]
- Yue, C.; Cao, H.; Zhang, S.; Shen, G.; Wu, Z.; Yuan, L.; Luo, L.; Zeng, L. Multilayer omics landscape analyses reveal the regulatory responses of tea plants to drought stress. Int. J. Biol. Macromol. 2023, 253, 126582. [Google Scholar] [CrossRef] [PubMed]
- Tricker, P.J.; ElHabti, A.; Schmidt, J.; Fleury, D. The physiological and genetic basis of combined drought and heat tolerance in Wheat. J. Exp. Bot. 2018, 69, 3195–3210. [Google Scholar] [CrossRef]
- Jiang, Z.; van Zanten, M.; Sasidharan, R. Mechanisms of plant acclimation to multiple abiotic stresses. Commun. Biol. 2025, 8, 655. [Google Scholar] [CrossRef]
- Foyer, C.H.; Kunert, K. The ascorbate-glutathione cycle coming of age. J. Exp. Bot. 2024, 75, 2682–2699. [Google Scholar] [CrossRef]
- Raghavendra, A.S.; Gonugunta, V.K.; Christmann, A.; Grill, E. ABA perception and signalling. Trends Plant Sci. 2010, 15, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Mogami, J.; Yamaguchi-Shinozaki, K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014, 21, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.R.; Jeon, B.W.; Zhang, W.; Assmann, S.M. Open stomata 1 (Ost1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol. 2013, 200, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, W.; Zhang, J.; Jiang, Z.; Xu, C.; Zhu, W.; Shi, B.; Yang, W.; Su, H.; Wang, X.; et al. NRT1.1B acts as an abscisic acid receptor in integrating compound environmental cues for plants. Cell 2025, 188, 5231–5248.e20. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Z.; Fiorentino, A.; Kuess, M.; Tharayil, N.; Kumar, R.; Leonard, E.; Noorai, R.; Hu, Q.; Luo, H. MicroRNA169 integrates multiple factors to modulate plant growth and abiotic stress responses. Plant Biotechnol. J. 2024, 22, 2541–2557. [Google Scholar] [CrossRef]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.; Pereira, A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef]
- Zavaliev, R.; Dong, X. NPR1, a key immune regulator for plant survival under biotic and abiotic stresses. Mol. Cell 2024, 84, 131–141. [Google Scholar] [CrossRef]
- Liu, Q.; Ma, W.; Chen, R.; Li, S.T.; Wang, Q.; Wei, C.; Hong, Y.; Sun, H.X.; Cheng, Q.; Zhao, J.; et al. Multiome in the same cell reveals the impact of osmotic stress on Arabidopsis root tip development at single-cell level. Adv. Sci. 2024, 11, e2308384. [Google Scholar] [CrossRef]
- Li, H.; Testerink, C.; Zhang, Y. How roots and shoots communicate through stressful times. Trends Plant Sci. 2021, 26, 940–952. [Google Scholar] [CrossRef]
- Kuromori, T.; Seo, M.; Shinozaki, K. ABA transport and plant water stress responses. Trends Plant Sci. 2018, 23, 513–522. [Google Scholar] [CrossRef]
- Christmann, A.; Weiler, E.W.; Steudle, E.; Grill, E. A hydraulic signal in root-to-shoot signalling of water shortage. Plant J. 2007, 52, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Fichman, Y.; Myers, R.J., Jr.; Grant, D.G.; Mittler, R. Plasmodesmata-localized proteins and ROS orchestrate light-induced rapid systemic signaling in Arabidopsis. Sci. Signal. 2021, 14, eabf0322. [Google Scholar] [CrossRef]
- Bogeat-Triboulot, M.B.; Brosché, M.; Renaut, J.; Jouve, L.; Le Thiec, D.; Fayyaz, P.; Vinocur, B.; Witters, E.; Laukens, K.; Teichmann, T.; et al. Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populus Euphratica, a poplar growing in arid regions. Plant Physiol. 2007, 143, 876–892. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Shahzad, Z.; Lonjon, F.; Loudet, O.; Vailleau, F.; Maurel, C. Natural variation at XND1 impacts root hydraulics and trade-off for stress responses in Arabidopsis. Nat. Commun. 2018, 9, 3884. [Google Scholar] [CrossRef]
- Postaire, O.; Tournaire-Roux, C.; Grondin, A.; Boursiac, Y.; Morillon, R.; Schäffner, A.R.; Maurel, C. PIP1 aquaporin contributes to hydrostatic pressure-induced water transport in both the root and rosette of Arabidopsis. Plant Physiol. 2010, 152, 1418–1430. [Google Scholar] [CrossRef]
- Shahzad, Z.; Canut, M.; Tournaire-Roux, C.; Martinière, A.; Boursiac, Y.; Loudet, O.; Maurel, C. A potassium-dependent oxygen sensing pathway regulates plant root hydraulics. Cell 2016, 167, 87–98.e14. [Google Scholar] [CrossRef] [PubMed]
- Boursiac, Y.; Chen, S.; Luu, D.T.; Sorieul, M.; van den Dries, N.; Maurel, C. Early effects of salinity on water transport in Arabidopsis roots. Plant Physiol. 2005, 139, 790–805. [Google Scholar] [CrossRef]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of climate change on crops adaptation and strategies to tackle its outcome: A review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Hatzig, S.V.; Nuppenau, J.N.; Snowdon, R.J.; Schießl, S.V. Drought stress has transgenerational effects on seeds and seedlings in winter oilseed rape (Brassica Napus L.). BMC Plant Biol. 2018, 18, 297. [Google Scholar] [CrossRef]
- Lafontaine, D.L.; Yang, L.; Dekker, J.; Gibcus, J.H. Hi-C 3.0: Improved protocol for genome-wide chromosome conformation capture. Curr. Protoc. 2021, 1, e198. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Q.; Ji, C.; Hu, G.; Zhang, P.; Wang, Y.; Yang, L.; Gu, X. Reorganization of the 3D chromatin architecture of rice genomes during heat stress. BMC Biol. 2021, 19, 53. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, N.; Teixeira da Silva, J.A.; Liu, X.; Deng, R.; Yao, Y.; Duan, J.; He, C. Physiological and transcriptomic analysis uncovers salinity stress mechanisms in a facultative crassulacean acid metabolism plant Dendrobium officinale. Front. Plant Sci. 2022, 13, 1028245. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.M.M.; Rais, U.; Sultan, H.; Tahir, A.; Bahadur, S.; Shah, A.; Iqbal, A.; Li, Y.; Khan, M.N.; Nie, L. Residual effect of microbial-inoculated biochar with nitrogen on rice growth and salinity reduction in paddy soil. Plants 2024, 13, 2804. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Tang, L.H.; Nie, J.W.; Zhang, C.R.; Han, X.; Li, Q.Y.; Qin, L.; Wang, M.H.; Huang, X.; Yu, F.; et al. Structure and activation mechanism of the rice Salt Overly Sensitive 1 (SOS1) Na+/H+ antiporter. Nat. Plants 2023, 9, 1924–1936. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, C.; Chen, Q.; Xie, Q.; Gao, Y.; He, L.; Li, Y.; Dong, Y.; Jiang, X.; Zhao, Y. Architecture and autoinhibitory mechanism of the plasma membrane Na+/H+ antiporter SOS1 in Arabidopsis. Nat. Commun. 2023, 14, 4487. [Google Scholar] [CrossRef]
- Salazar, O.R.; Chen, K.; Melino, V.J.; Reddy, M.P.; Hřibová, E.; Čížková, J.; Beránková, D.; Arciniegas Vega, J.P.; Cáceres Leal, L.M.; Aranda, M.; et al. SOS1 tonoplast neo-localization and the RGG protein SALTY are important in the extreme salinity tolerance of Salicornia bigelovii. Nat. Commun. 2024, 15, 4279. [Google Scholar] [CrossRef]
- Jiang, X.; Leidi, E.O.; Pardo, J.M. How do vacuolar NHX exchangers function in plant salt tolerance? Plant Signal. Behav. 2010, 5, 792–795. [Google Scholar] [CrossRef]
- El-Maarouf-Bouteau, H.; Sajjad, Y.; Bazin, J.; Langlade, N.; Cristescu, S.M.; Balzergue, S.; Baudouin, E.; Bailly, C. Reactive oxygen species, abscisic acid and ethylene interact to regulate sunflower seed germination. Plant Cell Environ. 2015, 38, 364–374. [Google Scholar] [CrossRef]
- Silva, P.O.; Medina, E.F.; Barros, R.S.; Ribeiro, D.M. Germination of salt-stressed seeds as related to the ethylene biosynthesis ability in three Stylosanthes species. J. Plant Physiol. 2014, 171, 14–22. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Shi, L.; Lv, G.; Li, X.; Liu, Y.; Jia, X.; Liu, J.; Chen, Y.; Zhu, L.; et al. Jasmonate signaling pathway confers salt tolerance through a NUCLEAR FACTOR-Y trimeric transcription factor complex in Arabidopsis. Cell Rep. 2024, 43, 113825. [Google Scholar] [CrossRef]
- Fu, H.; Yu, X.; Jiang, Y.; Wang, Y.; Yang, Y.; Chen, S.; Chen, Q.; Guo, Y. SALT OVERLY SENSITIVE 1 is inhibited by clade D Protein phosphatase 2C D6 and D7 in Arabidopsis thaliana. Plant Cell 2023, 35, 279–297. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, R.; Jiao, Y.; Jin, X.; Zhang, H.; Shi, L. Comparison of salt tolerance in Soja based on metabolomics of seedling roots. Front. Plant Sci. 2017, 8, 1101. [Google Scholar] [CrossRef] [PubMed]
- Richter, J.A.; Erban, A.; Kopka, J.; Zörb, C. Metabolic contribution to salt stress in two maize hybrids with contrasting resistance. Plant Sci. 2015, 233, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; De Lima, C.F.F.; De Smet, I. The heat is on: How crop growth, development and yield respond to high temperature. J. Exp. Bot. 2021, 72, 7359–7373. [Google Scholar] [CrossRef]
- Sajjad, M.; Bahadur, S.; Farooq, M.A.; Ren, M.X. Interactive impacts of heat stress and microplastics contamination on the growth and biochemical response of wheat (Triticum aestivum) and maize (Zea mays) plants. Ecotoxicology 2025, 34, 1036–1058. [Google Scholar] [CrossRef]
- Kerbler, S.M.; Wigge, P.A. Temperature Sensing in Plants. Annu. Rev. Plant Biol. 2023, 74, 341–366. [Google Scholar] [CrossRef]
- Delker, C.; Quint, M.; Wigge, P.A. Recent advances in understanding thermomorphogenesis signaling. Curr. Opin. Plant Biol. 2022, 68, 102231. [Google Scholar] [CrossRef]
- Casal, J.J.; Balasubramanian, S. Thermomorphogenesis. Annu. Rev. Plant Biol. 2019, 70, 321–346. [Google Scholar] [CrossRef]
- Qiu, Y.; Pasoreck, E.K.; Yoo, C.Y.; He, J.; Wang, H.; Bajracharya, A.; Li, M.; Larsen, H.D.; Cheung, S.; Chen, M. RCB initiates Arabidopsis thermomorphogenesis by stabilizing the thermoregulator PIF4 in the daytime. Nat. Commun. 2021, 12, 2042. [Google Scholar] [CrossRef]
- Huq, E.; Quail, P.H. PIF4, a phytochrome-interacting bHLH factor, functions as a negative regulator of phytochrome B signaling in Arabidopsis. EMBO J. 2002, 21, 2441–2450. [Google Scholar] [CrossRef]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.; Neme, A.; Hiltbrunner, A.; Wigge, P.A.; Schäfer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Hornitschek, P.; Kohnen, M.V.; Lorrain, S.; Rougemont, J.; Ljung, K.; López-Vidriero, I.; Franco-Zorrilla, J.M.; Solano, R.; Trevisan, M.; Pradervand, S.; et al. Phytochrome interacting factors 4 and 5 control seedling growth in changing light conditions by directly controlling auxin signaling. Plant J. 2012, 71, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Li, M.; Kim, R.J.; Moore, C.M.; Chen, M. Daytime temperature is sensed by phytochrome B in Arabidopsis through a transcriptional activator HEMERA. Nat. Commun. 2019, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; Hu, W.; Xu, N.; Seto, E.R.; Lagarias, J.C.; Chen, X.; Chen, M. A multisensor high-temperature signaling framework for triggering daytime thermomorphogenesis in Arabidopsis. Nat. Commun. 2025, 16, 5197. [Google Scholar] [CrossRef]
- Stief, A.; Altmann, S.; Hoffmann, K.; Pant, B.D.; Scheible, W.R.; Bäurle, I. Arabidopsis miR156 regulates tolerance to recurring environmental stress through SPL transcription factors. Plant Cell 2014, 26, 1792–1807. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Yang, J.; He, Y. Natural antisense transcripts of MIR398 genes suppress microR398 processing and attenuate plant thermotolerance. Nat. Commun. 2020, 11, 5351. [Google Scholar] [CrossRef]
- Farooq, M.; Tanveer, H.; Rehman, H.M.; Cheema, R.A.; Nawaz, S.; Ijaz, A.; Arif, M.; Lam, H.M. MicroRNAs-mediated heat stress regulations in plants: Mechanisms and targets. Plant Genome 2025, 18, e70112. [Google Scholar] [CrossRef]
- Silva, C.S.; Nayak, A.; Lai, X.; Hutin, S.; Hugouvieux, V.; Jung, J.H.; López-Vidriero, I.; Franco-Zorrilla, J.M.; Panigrahi, K.C.S.; Nanao, M.H.; et al. Molecular mechanisms of evening complex activity in Arabidopsis. Proc. Natl. Acad. Sci. USA 2020, 117, 6901–6909. [Google Scholar] [CrossRef]
- Jung, J.H.; Barbosa, A.D.; Hutin, S.; Kumita, J.R.; Gao, M.; Derwort, D.; Silva, C.S.; Lai, X.; Pierre, E.; Geng, F.; et al. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature 2020, 585, 256–260. [Google Scholar] [CrossRef]
- Bourguet, P.; Picard, C.L.; Yelagandula, R.; Pélissier, T.; Lorković, Z.J.; Feng, S.; Pouch-Pélissier, M.N.; Schmücker, A.; Jacobsen, S.E.; Berger, F.; et al. The histone variant H2A.W and linker histone H1 co-regulate heterochromatin accessibility and DNA methylation. Nat. Commun. 2021, 12, 2683. [Google Scholar] [CrossRef]
- Cortijo, S.; Charoensawan, V.; Brestovitsky, A.; Buning, R.; Ravarani, C.; Rhodes, D.; van Noort, J.; Jaeger, K.E.; Wigge, P.A. Transcriptional regulation of the ambient temperature response by H2A.Z nucleosomes and HSF1 transcription factors in Arabidopsis. Mol. Plant 2017, 10, 1258–1273. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.W.; Balcerowicz, M.; Di Antonio, M.; Jaeger, K.E.; Geng, F.; Franaszek, K.; Marriott, P.; Brierley, I.; Firth, A.E.; Wigge, P.A. An RNA thermoswitch regulates daytime growth in Arabidopsis. Nat. Plants 2020, 6, 522–532. [Google Scholar] [CrossRef]
- Catalan-Moreno, A.; Cela, M.; Menendez-Gil, P.; Irurzun, N.; Caballero, C.J.; Caldelari, I.; Toledo-Arana, A. RNA thermoswitches modulate staphylococcus aureus adaptation to ambient temperatures. Nucleic Acids Res. 2021, 49, 3409–3426. [Google Scholar] [CrossRef]
- Allen, J.R.; Strader, L.C. Beating the heat: Phase separation in plant stress granules. Dev. Cell 2022, 57, 563–565. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Gu, J.; Yao, J.; Li, Y.; Zhang, Z.; Xia, W.; Wang, Z.; Gui, X.; Li, L.; Li, D.; et al. Liquid-liquid phase separation of RBGD2/4 is required for heat stress resistance in Arabidopsis. Dev. Cell 2022, 57, 583–597.e6. [Google Scholar] [CrossRef] [PubMed]
- Dannfald, A.; Carpentier, M.C.; Merret, R.; Favory, J.J.; Deragon, J.M. Plant response to intermittent heat stress involves modulation of mRNA translation efficiency. Plant Physiol. 2025, 197, kiae648. [Google Scholar] [CrossRef]
- Crawford, T.; Siebler, L.; Sulkowska, A.; Nowack, B.; Jiang, L.; Pan, Y.; Lämke, J.; Kappel, C.; Bäurle, I. The Mediator kinase module enhances polymerase activity to regulate transcriptional memory after heat stress in Arabidopsis. EMBO J. 2024, 43, 437–461. [Google Scholar] [CrossRef]
- Staacke, T.; Mueller-Roeber, B.; Balazadeh, S. Stress resilience in plants: The complex interplay between heat stress memory and resetting. New Phytol. 2025, 245, 2402–2421. [Google Scholar] [CrossRef]
- Ritonga, F.N.; Chen, S. Physiological and molecular mechanism involved in cold stress tolerance in plants. Plants 2020, 9, 560. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Regulatory networks underlying plant responses and adaptation to cold stress. Annu. Rev. Genet. 2024, 58, 43–65. [Google Scholar] [CrossRef]
- Gusain, S.; Joshi, S.; Joshi, R. Sensing, signalling, and regulatory mechanism of cold-stress tolerance in plants. Plant Physiol. Biochem. 2023, 197, 107646. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dang, P.; Liu, L.; He, C. Cold acclimation by the CBF-COR pathway in a changing climate: Lessons from Arabidopsis thaliana. Plant Cell Rep. 2019, 38, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef]
- Wang, J.; Ren, Y.; Liu, X.; Luo, S.; Zhang, X.; Liu, X.; Lin, Q.; Zhu, S.; Wan, H.; Yang, Y.; et al. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant 2021, 14, 315–329. [Google Scholar] [CrossRef]
- Hwarari, D.; Guan, Y.; Ahmad, B.; Movahedi, A.; Min, T.; Hao, Z.; Lu, Y.; Chen, J.; Yang, L. ICE-CBF-COR signaling cascade and its regulation in plants responding to cold stress. Int. J. Mol. Sci. 2022, 23, 1549. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wei, F.; Chai, S.; Peng, S.; Huang, B.; Han, Q.; Zhao, T.; Zhang, P.; Tian, Y.; Xia, R.; et al. The OST1-HOS1-HAT1 module regulates cold response in Arabidopsis thaliana. New Phytol. 2025, 247, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, H.; Zhang, X.; Xie, Q.; Gong, Z.; Yang, S. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell 2015, 32, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Jin, J.B.; Lee, J.; Yoo, C.Y.; Stirm, V.; Miura, T.; Ashworth, E.N.; Bressan, R.A.; Yun, D.J.; Hasegawa, P.M. SIZ1-mediated sumoylation of ICE1 controls CBF3/DREB1a expression and freezing tolerance in Arabidopsis. Plant Cell 2007, 19, 1403–1414. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Song, C.P.; Gong, Z.; Yang, S.; Ding, Y. PUB25 and PUB26 dynamically modulate ICE1 stability via differential ubiquitination during cold stress in Arabidopsis. Plant Cell 2023, 35, 3585–3603. [Google Scholar] [CrossRef]
- Perez-Garcia, P.; Pucciariello, O.; Sanchez-Corrionero, A.; Cabrera, J.; Del Barrio, C.; Del Pozo, J.C.; Perales, M.; Wabnik, K.; Moreno-Risueno, M.A. The cold-induced factor CBF3 mediates root stem cell activity, regeneration, and developmental responses to cold. Plant Commun. 2023, 4, 100737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, M.; Zhang, X.; Zeng, R.; Peng, Y.; Shi, Y.; Wang, X.; Zhou, W.; Gong, Z.; Yang, S. A receptor-kinase cascade confers cold-induced root growth inhibition in Arabidopsis. Nat. Plants 2025, 11, 1441–1454. [Google Scholar] [CrossRef]
- Lee, E.S.; Park, J.H.; Wi, S.D.; Chae, H.B.; Paeng, S.K.; Bae, S.B.; Phan, K.A.T.; Kim, M.G.; Kwak, S.S.; Kim, W.Y.; et al. Demyristoylation of the cytoplasmic redox protein Trx-h2 is critical for inducing a rapid cold stress response in plants. Antioxidants 2022, 11, 2223. [Google Scholar] [CrossRef]
- Yang, H.; Berry, S.; Olsson, T.S.G.; Hartley, M.; Howard, M.; Dean, C. Distinct phases of polycomb silencing to hold epigenetic memory of cold in Arabidopsis. Science 2017, 357, 1142–1145. [Google Scholar] [CrossRef]
- Kim, D.H.; Xi, Y.; Sung, S. Modular function of long noncoding RNA, COLDAIR, in the vernalization response. PLoS Genet. 2017, 13, e1006939. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, P.; Cheema, J.; Bloomer, R.; Mikulski, P.; Liu, Q.; Zhang, Y.; Dean, C.; Ding, Y. In vivo single-molecule analysis reveals COOLAIR RNA structural diversity. Nature 2022, 609, 394–399. [Google Scholar] [CrossRef]
- Csorba, T.; Questa, J.I.; Sun, Q.; Dean, C. Antisense COOLAIR mediates the coordinated switching of chromatin states at FLC during vernalization. Proc. Natl. Acad. Sci. USA 2014, 111, 16160–16165. [Google Scholar] [CrossRef]
- Selinski, J.; Scheibe, R. Malate valves: Old shuttles with new perspectives. Plant Biol. 2019, 21, 21–30. [Google Scholar] [CrossRef]
- Krämer, M.; Kunz, H.H. Indirect export of reducing equivalents from the chloroplast to resupply NADP for C3 photosynthesis-growing importance for stromal NAD(H)? Front. Plant Sci. 2021, 12, 719003. [Google Scholar] [CrossRef]
- Exposito-Rodriguez, M.; Laissue, P.P.; Yvon-Durocher, G.; Smirnoff, N.; Mullineaux, P.M. Photosynthesis-dependent H2O2 transfer from chloroplasts to nuclei provides a high-light signalling mechanism. Nat. Commun. 2017, 8, 49. [Google Scholar] [CrossRef]
- Dietz, K.J.; Turkan, I.; Krieger-Liszkay, A. Redox- and Reactive Oxygen Species-Dependent Signaling into and out of the Photosynthesizing Chloroplast. Plant Physiol. 2016, 171, 1541–1550. [Google Scholar] [CrossRef]
- Zamani-Nour, S.; Lin, H.C.; Walker, B.J.; Mettler-Altmann, T.; Khoshravesh, R.; Karki, S.; Bagunu, E.; Sage, T.L.; Quick, W.P.; Weber, A.P.M. Overexpression of the chloroplastic 2-oxoglutarate/malate transporter disturbs carbon and nitrogen homeostasis in rice. J. Exp. Bot. 2021, 72, 137–152. [Google Scholar] [CrossRef]
- Breeze, E.; Mullineaux, P.M. The passage of H2O2 from chloroplasts to their associated nucleus during retrograde signalling: Reflections on the role of the nuclear envelope. Plants 2022, 11, 552. [Google Scholar] [CrossRef]
- Henschel, J.M.; Andrade, A.N.d.; dos Santos, J.B.L.; da Silva, R.R.; da Mata, D.A.; Souza, T.; Batista, D.S. Lipidomics in plants under abiotic stress conditions: An overview. Agronomy 2024, 14, 1670. [Google Scholar] [CrossRef]
- Dutta, S.; Islam, Z.; Das, S.; Barman, A.; Chowdhury, M.; Mondal, B.P.; Ajnabi, J.; Manna, D. Harmonizing plant resilience: Unveiling the symphony of membrane lipid dynamics in response to abiotic stresses: A review. Discover Plants 2025, 2, 61. [Google Scholar] [CrossRef]
- Elango, D.; Rajendran, K.; Van der Laan, L.; Sebastiar, S.; Raigne, J.; Thaiparambil, N.A.; El Haddad, N.; Raja, B.; Wang, W.; Ferela, A.; et al. Raffinose family oligosaccharides: Friend or foe for human and plant health? Front. Plant Sci. 2022, 13, 829118. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, S.; Grin, I.R.; Zharkov, D.O.; Yu, B.; Li, H. The dual role of methylglyoxal in plant stress response and regulation of DJ-1 protein. Physiol. Plant 2024, 176, e14608. [Google Scholar] [CrossRef] [PubMed]
- Alam, N.B.; Jain, M.; Mustafiz, A. Pyramiding D-lactate dehydrogenase with the glyoxalase pathway enhances abiotic stress tolerance in plants. Plant Physiol. Biochem. 2024, 207, 108391. [Google Scholar] [CrossRef]
- Winichayakul, S.; Xue, H.; Richardson, K.A.; Maher, D.; Reid, M.; Roberts, N. Lipid storage in green tissues alters redox homeostasis, malate metabolism, phospholipids, and nitrogen partitioning in plants. Plant Physiol. Biochem. 2025, 227, 110144. [Google Scholar] [CrossRef]
- Tan, J.W.; Shinde, H.; Tesfamicael, K.; Hu, Y.; Fruzangohar, M.; Tricker, P.; Baumann, U.; Edwards, E.J.; Rodríguez López, C.M. Global transcriptome and gene co-expression network analyses reveal regulatory and non-additive effects of drought and heat stress in grapevine. Front. Plant Sci. 2023, 14, 1096225. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, Z. Abscisic acid suppresses thermomorphogenesis in Arabidopsis thaliana. Plant Signal Behav. 2020, 15, 1746510. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Datta, R.; Hazra, S.; Sultana, A.; Mukhopadhyay, R.; Chattopadhyay, S. Transcriptomic profiling of Arabidopsis thaliana mutant pad2.1 in response to combined cold and osmotic stress. PLoS ONE 2015, 10, e0122690. [Google Scholar] [CrossRef]
- Moore, C.E.; Meacham-Hensold, K.; Lemonnier, P.; Slattery, R.A.; Benjamin, C.; Bernacchi, C.J.; Lawson, T.; Cavanagh, A.P. The effect of increasing temperature on crop photosynthesis: From enzymes to ecosystems. J. Exp. Bot. 2021, 72, 2822–2844. [Google Scholar] [CrossRef]
- Jiang, Z.; Verhoeven, A.; Li, Y.; Geertsma, R.; Sasidharan, R.; van Zanten, M. Deciphering acclimation to sublethal combined and sequential abiotic stresses in Arabidopsis thaliana. Plant Physiol. 2024, kiae581. [Google Scholar] [CrossRef]
- Rabbani, M.A.; Maruyama, K.; Abe, H.; Khan, M.A.; Katsura, K.; Ito, Y.; Yoshiwara, K.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 2003, 133, 1755–1767. [Google Scholar] [CrossRef]
- Liu, L.; Si, L.; Zhang, L.; Guo, R.; Wang, R.; Dong, H.; Guo, C. Metabolomics and transcriptomics analysis revealed the response mechanism of alfalfa to combined cold and saline-alkali stress. Plant J. 2024, 119, 1900–1919. [Google Scholar] [CrossRef]
- Zhao, F.J.; Tang, Z.; Song, J.J.; Huang, X.Y.; Wang, P. Toxic metals and metalloids: Uptake, transport, detoxification, phytoremediation, and crop improvement for safer food. Mol. Plant 2022, 15, 27–44. [Google Scholar] [CrossRef]
- Kosakivska, I.V.; Babenko, L.M.; Romanenko, K.O.; Korotka, I.Y.; Potters, G. Molecular mechanisms of plant adaptive responses to heavy metals stress. Cell Biol. Int. 2021, 45, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Panda, A.; Fatnani, D.; Parida, A.K. Uptake, impact, adaptive mechanisms, and phytoremediation of heavy metals by plants: Role of transporters in heavy metal sequestration. Plant Physiol. Biochem. 2025, 221, 109578. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Q.; Zhao, X.Y.; Xuan, W.; Wang, P.; Zhao, F.J. Rice roots avoid asymmetric heavy metal and salinity stress via an RBOH-ROS-auxin signaling cascade. Mol. Plant 2023, 16, 1678–1694. [Google Scholar] [CrossRef] [PubMed]
- Seregin, I.V.; Kozhevnikova, A.D. The role of Low-Molecular-Weight organic acids in metal homeostasis in plants. Int. J. Mol. Sci. 2024, 25, 9542. [Google Scholar] [CrossRef]
- Javed, M.T.; Saleem, M.H.; Aslam, S.; Rehman, M.; Iqbal, N.; Begum, R.; Ali, S.; Alsahli, A.A.; Alyemeni, M.N.; Wijaya, L. Elucidating silicon-mediated distinct morpho-physio-biochemical attributes and organic acid exudation patterns of cadmium stressed Ajwain (Trachyspermum ammi L.). Plant Physiol. Biochem. 2020, 157, 23–37. [Google Scholar] [CrossRef]
- Wu, R.; Sun, X.; Zhu, M.; Wang, Y.; Zhu, Y.; Fang, Z.; Liu, H.; Du, S. Abscisic acid-producing bacterium Azospirillum brasilense effectively reduces heavy metals (cadmium, nickel, lead, and zinc) accumulation in pak choi across various soil types. Ecotoxicol. Environ. Saf. 2025, 298, 118277. [Google Scholar] [CrossRef]
- Wang, S.; He, X.; Tian, J.; Wu, R.; Liu, H.; Fang, Z.; Du, S. NRT1.2 overexpression enhances the synergistic interplay between ABA-generating bacteria and biochars in reducing heavy metal accumulation in pak choi. Sci. Total Environ. 2024, 922, 171276. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, C.; Tang, F.; Zhou, Y.; Zhu, C.; Ding, Y. The cell wall functions in plant heavy metal response. Ecotoxicol. Environ. Saf. 2025, 299, 118326. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Annu. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef]
- Moenne, A.; González, A.; Sáez, C.A. Mechanisms of metal tolerance in marine macroalgae, with emphasis on copper tolerance in Chlorophyta and Rhodophyta. Aquat. Toxicol. 2016, 176, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shi, W.; Jie, Y. Overexpression of BnPCS1, a novel phytochelatin synthase gene from ramie (Boehmeria nivea), enhanced Cd tolerance, accumulation, and translocation in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 639189. [Google Scholar] [CrossRef]
- Gautam, N.; Tiwari, M.; Kidwai, M.; Dutta, P.; Chakrabarty, D. Functional characterization of rice metallothionein OsMT-I-Id: Insights into metal binding and heavy metal tolerance mechanisms. J. Hazard. Mater. 2023, 458, 131815. [Google Scholar] [CrossRef]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.Y.; Han, M.L.; Gao, Y.Q.; Zhang, C.Y.; Wang, Y.L.; Chao, Z.F.; Zhong, L.Y.; Chao, D.Y. Sec24C mediates a Golgi-independent trafficking pathway that is required for tonoplast localisation of ABCC1 and ABCC2. New Phytol. 2022, 235, 1486–1500. [Google Scholar] [CrossRef]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef]
- Liu, C.L.; Gao, Z.Y.; Shang, L.G.; Yang, C.H.; Ruan, B.P.; Zeng, D.L.; Guo, L.B.; Zhao, F.J.; Huang, C.F.; Qian, Q. Natural variation in the promoter of OsHMA3 contributes to differential grain cadmium accumulation between Indica and Japonica rice. J. Integr. Plant Biol. 2020, 62, 314–329. [Google Scholar] [CrossRef]
- Lei, C.Y.; Li, Y.N.; Liang, M.J.; Yang, Z.; Sun, Y.; Ji, C.L.; Zhang, C.H.; Li, R.Z.; Sun, X.P.; Cui, H.L. Effect and mechanism of Chlamydomonas reinhartii living cell agents in alleviating Cd stress on wheat seedlings. Huan Jing Ke Xue 2025, 46, 1795–1805. [Google Scholar] [CrossRef]
- Fu, Z.; Yao, Y.; Haq, M.Z.U.; Liu, Y.; Yang, D.; Yang, H.; Wu, Y. Glutathione’s role in mitigating cadmium stress in Pogostemon cablin: Insights from combined transcriptomic and metabolomic approaches. J. Hazard. Mater. 2025, 491, 137921. [Google Scholar] [CrossRef]
- Meyer, A.J. The integration of glutathione homeostasis and redox signaling. J. Plant Physiol. 2008, 165, 1390–1403. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Kang, Y.; Liu, A.; Li, P. Functional analysis and interaction networks of Rboh in poplar under abiotic stress. Front. Plant Sci. 2025, 16, 1553057. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, Z.; Zhou, X. WRKY transcription factors in response to metal stress in plants: A review. Int. J. Mol. Sci. 2024, 25, 10952. [Google Scholar] [CrossRef]
- Xu, X.; Dou, Y.; Zhao, S.; Zhao, C.; Chen, Y.; Jiang, M.; Shen, Z.; Chen, C. Rhizosphere microbes enhance plant resistance to cadmium through a root ROS-microbial IAA-root DNA methylation interkingdom signaling pathway. Cell Rep. 2025, 44, 116491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Jiang, L.; Chen, H.; Liu, H.; Xiong, M.; Niu, Y.; Xie, L.; Wang, L.; Mao, Z.; Guo, T.; et al. Gibberellin triggers ATG8-dependent autophagic degradation of DELLA proteins to promote seed germination and skotomorphogenesis under nutrient starvation in Arabidopsis. Mol. Plant 2025, S1674-2052(25)00361-2. [Google Scholar] [CrossRef]
- Miao, Z.; Ji, X.; Wei, L.; Miao, Z.; Xu, S. Melatonin modulates glucose metabolism reprogramming via targeting G6PD to alleviate lead-induced hepatocytes pyroptosis in common carp (Cyprinus carpio L.). Adv. Sci. 2025, 11, e01041. [Google Scholar] [CrossRef]
- Ziogas, A.; Novakovic, B.; Ventriglia, L.; Galang, N.; Tran, K.A.; Li, W.; Matzaraki, V.; van Unen, N.; Schlüter, T.; Ferreira, A.V.; et al. Long-term histone lactylation connects metabolic and epigenetic rewiring in innate immune memory. Cell 2025, 188, 2992–3012.e16. [Google Scholar] [CrossRef]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic regulation of gene expression by histone lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef]
- Rattan, P.; Gandotra, E.; Mittal, S. Long non-coding RNAs: Silent contributors to plant survival under abiotic stress. Biochem. Biophys. Res. Commun. 2025, 782, 152581. [Google Scholar] [CrossRef]
- Wen, X.; Ding, Y.; Tan, Z.; Wang, J.; Zhang, D.; Wang, Y. Identification and characterization of cadmium stress-related LncRNAs from Betula Platyphylla. Plant Sci. 2020, 299, 110601. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Yamaji, N.; Yokosho, K.; Ma, J.F. YSL16 is a phloem-localized transporter of the copper-nicotianamine complex that is responsible for copper distribution in rice. Plant Cell 2012, 24, 3767–3782. [Google Scholar] [CrossRef] [PubMed]
- Sintaha, M. Molecular mechanisms of plant stress memory: Roles of non-coding RNAs and alternative splicing. Plants 2025, 14, 2021. [Google Scholar] [CrossRef]
- Cong, W.; Miao, Y.; Xu, L.; Zhang, Y.; Yuan, C.; Wang, J.; Zhuang, T.; Lin, X.; Jiang, L.; Wang, N.; et al. Transgenerational memory of gene expression changes induced by heavy metal stress in rice (Oryza sativa L.). BMC Plant Biol. 2019, 19, 282. [Google Scholar] [CrossRef]
- Ou, X.; Zhang, Y.; Xu, C.; Lin, X.; Zang, Q.; Zhuang, T.; Jiang, L.; von Wettstein, D.; Liu, B. Transgenerational inheritance of modified DNA methylation patterns and enhanced tolerance induced by heavy metal stress in rice (Oryza sativa L.). PLoS ONE 2012, 7, e41143. [Google Scholar] [CrossRef]
- Pradhan, G.P.; Prasad, P.V.V.; Fritz, A.K.; Kirkham, M.B.; Gill, B.S. Effects of drought and high temperature stress on synthetic hexaploid wheat. Funct. Plant Biol. 2012, 39, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Luo, Y.; Zhong, B.; Xu, H.; Wang, F.; Li, W.; Lin, M.; Chen, J.; Chen, L.; Liang, M.; et al. The abscisic acid signaling negative regulator OsPP2C68 confers drought and salinity tolerance to rice. Sci. Rep. 2025, 15, 6730. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; Chen, X.; Wang, J.; Cheng, Z.; Ma, X.; Zheng, Q.; Xu, Z.; Zhang, F. Emerging Mechanisms of Plant Responses to Abiotic Stress. Plants 2025, 14, 3445. https://doi.org/10.3390/plants14223445
Zhao W, Chen X, Wang J, Cheng Z, Ma X, Zheng Q, Xu Z, Zhang F. Emerging Mechanisms of Plant Responses to Abiotic Stress. Plants. 2025; 14(22):3445. https://doi.org/10.3390/plants14223445
Chicago/Turabian StyleZhao, Wan, Xiaojie Chen, Jiahuan Wang, Zhongjie Cheng, Xuhui Ma, Qi Zheng, Zhaoshi Xu, and Fuyan Zhang. 2025. "Emerging Mechanisms of Plant Responses to Abiotic Stress" Plants 14, no. 22: 3445. https://doi.org/10.3390/plants14223445
APA StyleZhao, W., Chen, X., Wang, J., Cheng, Z., Ma, X., Zheng, Q., Xu, Z., & Zhang, F. (2025). Emerging Mechanisms of Plant Responses to Abiotic Stress. Plants, 14(22), 3445. https://doi.org/10.3390/plants14223445






