Woody Plant Transformation: Current Status, Challenges, and Future Perspectives †
Abstract
1. Introduction
2. Methodological Paradigms in Woody Plant Transformation
2.1. Tissue Culture-Based Platforms for Woody Plant Transformation
2.1.1. Advances in DNA Delivery for Tissue Culture-Based Transformation in Woody Plants
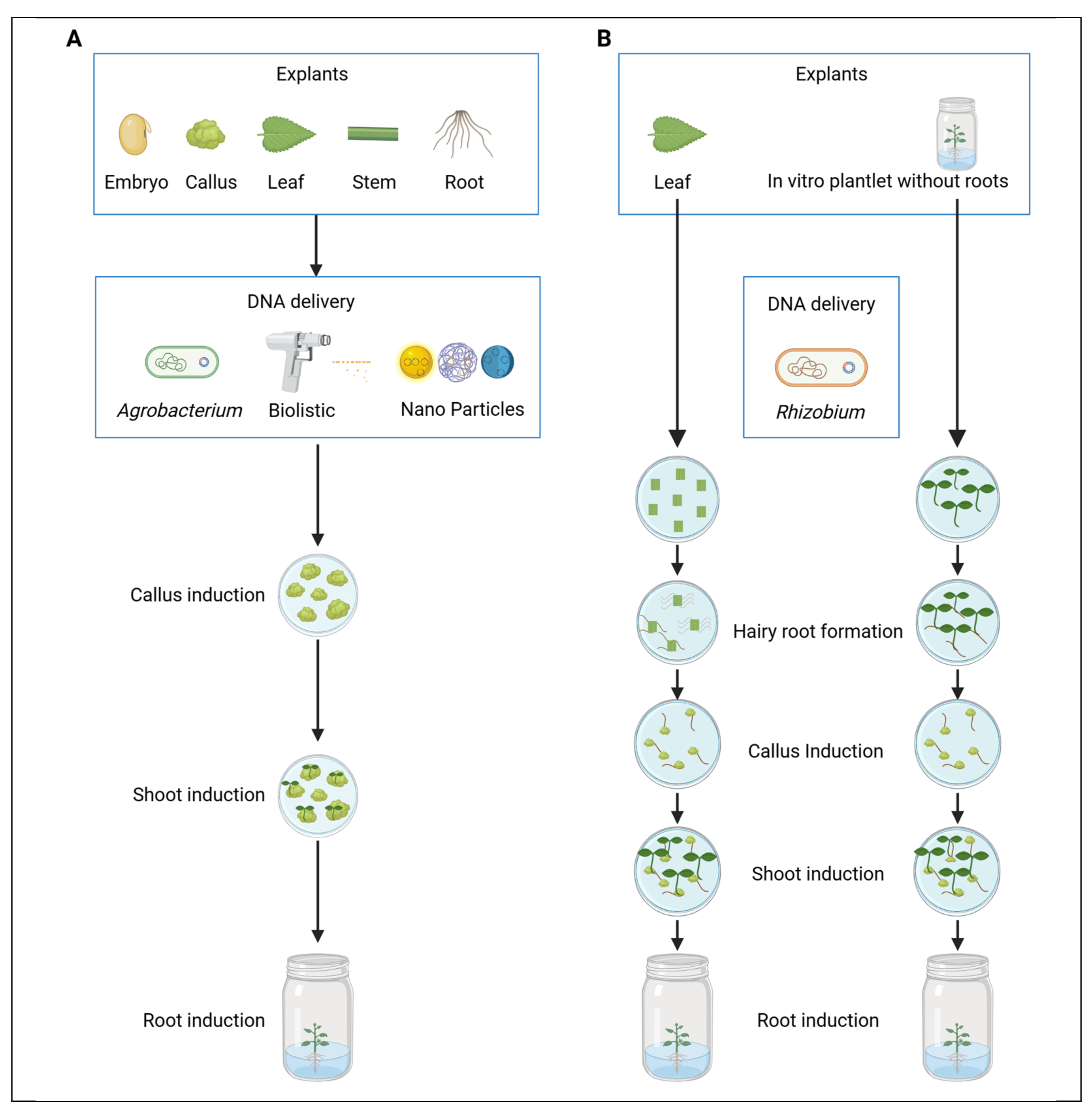
2.1.2. Advances in Plant Regeneration for Tissue Culture-Based Transformation in Woody Plants
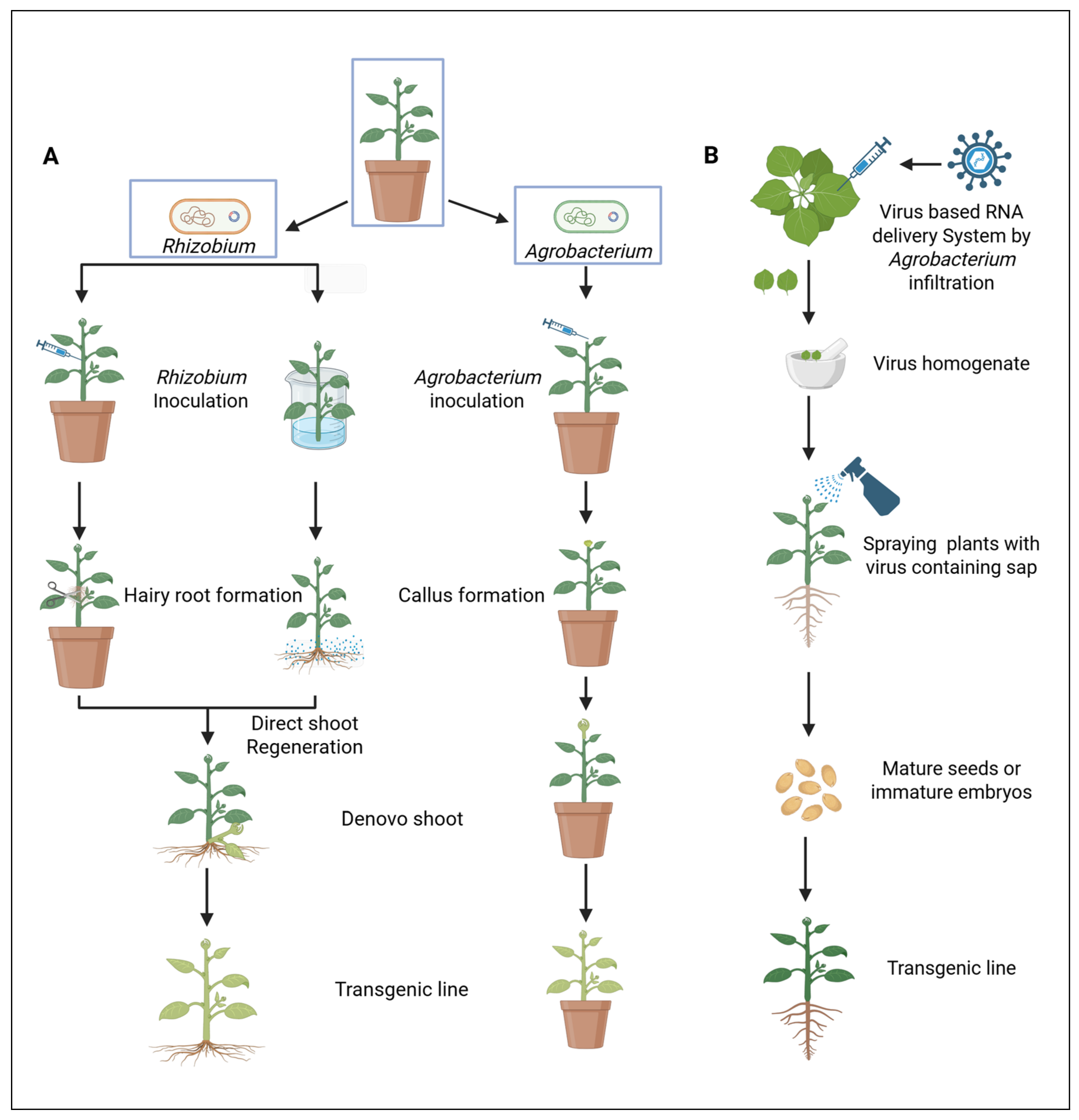
2.2. The Rise in Non-Tissue Culture-Based Platforms for Woody Plant Transformation
2.2.1. Advances in DNA Delivery for Non-Tissue Culture-Based Transformation in Woody Plants
2.2.2. Advances in Plant Regeneration for Non-Tissue Culture-Based Transformation in Woody Plants
3. Species-Specific Advances in Woody Plant Transformation
3.1. Citrus spp.
3.2. Eucalyptus spp.
3.3. Malus Domestica
3.4. Pinus spp.
3.5. Populus spp.
3.6. Prunus spp.
4. Challenges and Potential Solutions in Woody Plant Transformation
4.1. Adapting Innovations from Non-Woody Plant Systems
4.2. Elucidating the Molecular Basis of Recalcitrance in Woody Species
4.3. Challenges in Regulatory Approval and Commercialization
5. Conclusions and Future Opportunities
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| TALENS | Transcription activator like effector nuclease |
| RNPs | Ribonucleoproteins |
| MS | Murashige and Skoog medium |
| WPM | Woody plant basal medium |
| NAA | Naphthalene acetic acid |
| IAA | Indole-3-acetic acid |
| 2.4-D | 2,4-Dichlorophenoxyacetic acid |
| BA | 6-Benzylaminopurine |
| TDZ | Thidiazuron |
| GUS | β-glucuronidase |
| GFP | Green fluorescence protein |
References
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef]
- Fazan, L.; Song, Y.-G.; Kozlowski, G. The woody planet: From past triumph to manmade decline. Plants 2020, 9, 1593. [Google Scholar] [CrossRef]
- Ahmar, S.; Gill, R.A.; Jung, K.-H.; Faheem, A.; Qasim, M.U.; Mubeen, M.; Zhou, W. Conventional and molecular techniques from simple breeding to speed breeding in crop plants: Recent advances and future outlook. Int. J. Mol. Sci. 2020, 21, 2590. [Google Scholar] [CrossRef]
- Osakabe, Y.; Sugano, S.S.; Osakabe, K. Genome engineering of woody plants: Past, present and future. J. Wood Sci. 2016, 62, 217–225. [Google Scholar] [CrossRef]
- Thapliyal, G.; Bhandari, M.S.; Vemanna, R.S.; Pandey, S.; Meena, R.K.; Barthwal, S. Engineering traits through CRISPR/cas genome editing in woody species to improve forest diversity and yield. Crit. Rev. Biotechnol. 2023, 43, 884–903. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, Y.; Hu, Z.; Liu, S.; Zhang, X. Genetic transformation of forest trees and its research advances in stress tolerance. Forests 2024, 15, 441. [Google Scholar] [CrossRef]
- Wang, P.; Si, H.; Li, C.; Xu, Z.; Guo, H.; Jin, S.; Cheng, H. Plant genetic transformation: Achievements, current status and future prospects. Plant Biotechnol. J. 2025, 23, 2034–2058. [Google Scholar] [CrossRef]
- Petri, C.; Burgos, L. Transformation of fruit trees. Useful breeding tool or continued future prospect? Transgenic Res. 2005, 14, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kausch, A.P.; Nelson-Vasilchik, K.; Hague, J.; Mookkan, M.; Quemada, H.; Dellaporta, S.; Fragoso, C.; Zhang, Z.J. Edit at will: Genotype independent plant transformation in the era of advanced genomics and genome editing. Plant Sci. 2019, 281, 186–205. [Google Scholar] [CrossRef]
- Lee, K.; Wang, K. Strategies for genotype-flexible plant transformation. Curr. Opin. Biotechnol. 2023, 79, 102848. [Google Scholar] [CrossRef]
- Walden, R.; Wingender, R. Gene-transfer and plant-regeneration (techniques). Trends Biotechnol. 1995, 13, 324–331. [Google Scholar] [CrossRef]
- Lu, H.; Jawdy, S.; Chen, J.-G.; Yang, X.; Kalluri, U.C. Poplar transformation with variable explant sources to maximize transformation efficiency. Sci. Rep. 2025, 15, 1320. [Google Scholar] [CrossRef]
- Yuan, G.; Liu, Y.; Yao, T.; Muchero, W.; Chen, J.-G.; Tuskan, G.A.; Yang, X. eYGFPuv-assisted transgenic selection in Populus deltoides WV94 and multiplex genome editing in protoplasts of P. trichocarpa × P. deltoides Clone ‘52-225’. Plants 2023, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, M.; Dominguez, M.M.; Irigoyen, S.; Padilla, C.S.; Mandadi, K.K. Rhizobium rhizogenes-mediated hairy root induction and plant regeneration for bioengineering citrus. Plant Biotechnol. J. 2023, 21, 1728–1730. [Google Scholar] [CrossRef]
- Wu, H.; Acanda, Y.; Jia, H.; Wang, N.; Zale, J. Biolistic transformation of Carrizo citrange (Citrus sinensis Osb. × Poncirus trifoliata L. Raf.). Plant Cell Rep. 2016, 35, 1955–1962. [Google Scholar] [CrossRef]
- Wei, L.; Liu, J.; Jiang, G. Nanoparticle-specific transformations dictate nanoparticle effects associated with plants and implications for nanotechnology use in agriculture. Nat. Commun. 2024, 15, 7389. [Google Scholar] [CrossRef]
- Hoengenaert, L.; Anders, C.; Van Doorsselaere, J.; Vanholme, R.; Boerjan, W. Transgene-free genome editing in poplar. New Phytol. 2025, 247, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tomes, S.; Gleave, A.P.; Hall, W.; Luo, Z.; Xu, J.; Yao, J.L. Significant improvement of apple (Malus domestica Borkh.) transgenic plant production by pre-transformation with a Baby boom transcription factor. Hortic. Res. 2022, 9, uhab014. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, B.; Citovsky, V. Pathways of DNA transfer to plants from Agrobacterium tumefaciens and related bacterial species. Annu. Rev. Phytopathol. 2019, 57, 231–251. [Google Scholar] [CrossRef]
- Gelvin, S.B. Agrobacterium-mediated plant transformation: The biology behind the “gene-jockeying” tool. Microbiol. Mol. Biol. Rev. 2003, 67, 16–37. [Google Scholar] [CrossRef]
- Stachel, S.E.; Nester, E.W.; Zambryski, P.C. A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proc. Natl. Acad. Sci. USA 1986, 83, 379–383. [Google Scholar] [CrossRef]
- Stachel, S.E.; Zambryski, P.C. Agrobacterium tumefaciens and the susceptible plant cell: A novel adaptation of extracellular recognition and DNA conjugation. Cell 1986, 47, 155–157. [Google Scholar] [CrossRef]
- De Saeger, J.; Park, J.; Chung, H.S.; Hernalsteens, J.P.; Van Lijsebettens, M.; Inzé, D.; Van Montagu, M.; Depuydt, S. Agrobacterium strains and strain improvement: Present and outlook. Biotechnol. Adv. 2021, 53, 107677. [Google Scholar] [CrossRef]
- Atkinson, R.G.; Gunaseelan, K.; Wang, M.Y.; Luo, L.; Wang, T.; Norling, C.L.; Johnston, S.L.; Maddumage, R.; Schröder, R.; Schaffer, R.J. Dissecting the role of climacteric ethylene in kiwifruit (Actinidia chinensis) ripening using a 1-aminocyclopropane-1-carboxylic acid oxidase knockdown line. J. Exp. Bot. 2011, 62, 3821–3835. [Google Scholar] [CrossRef]
- Gutierrez-Valdes, N.; Häkkinen, S.T.; Lemasson, C.; Guillet, M.; Oksman-Caldentey, K.-M.; Ritala, A.; Cardon, F. Hairy root cultures—A versatile tool with multiple applications. Front. Plant Sci. 2020, 11, 33. [Google Scholar] [CrossRef] [PubMed]
- Spanò, L.; Mariotti, D.; Cardarelli, M.; Branca, C.; Costantino, P. Morphogenesis and auxin sensitivity of transgenic tobacco with different complements of Ri T-DNA 1. Plant Physiol. 1988, 87, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Vilaine, F.; Casse-Delbart, F. Independent induction of transformed roots by the TL and TR regions of the Ri plasmid of agropine type Agrobacterium rhizogenes. Mol. Gen. Genet. MGG 1987, 206, 17–23. [Google Scholar] [CrossRef]
- Nemoto, K.; Hara, M.; Suzuki, M.; Seki, H.; Oka, A.; Muranaka, T.; Mano, Y. Function of the aux and rol genes of the Ri plasmid in plant cell division in vitro. Plant Signal. Behav. 2009, 4, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- De Paolis, A.; Mauro, M.L.; Pompom, M.; Cardarelli, M.; Spanò, L.; Costantino, P. Localization of agropine-synthesizing functions in the TR region of the root-inducing plasmid of Agrobacterium rhizogenes 1855. Plasmid 1985, 13, 1–7. [Google Scholar] [CrossRef]
- Liu, L.; Qu, J.; Wang, C.; Liu, M.; Zhang, C.; Zhang, X.; Guo, C.; Wu, C.; Yang, G.; Huang, J.; et al. An efficient genetic transformation system mediated by Rhizobium rhizogenes in fruit trees based on the transgenic hairy root to shoot conversion. Plant Biotechnol. J. 2024, 22, 2093–2103. [Google Scholar] [CrossRef]
- Laffon, M.; Bruat, M.; Chefdor, F.; Colas, C.; Heng, S.; Sena-Velez, M.; Larcher, M.; Héricourt, F.; Depierreux, C.; Morabito, D.; et al. Hairy root induction in hybrid poplar (Populus tremula × Populus alba) for sustainable growth and specialized metabolites production with antioxidant activities. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 156, 2. [Google Scholar] [CrossRef]
- Plasencia, A.; Soler, M.; Dupas, A.; Ladouce, N.; Silva-Martins, G.; Martinez, Y.; Lapierre, C.; Franche, C.; Truchet, I.; Grima-Pettenati, J. Eucalyptus hairy roots, a fast, efficient and versatile tool to explore function and expression of genes involved in wood formation. Plant Biotechnol. J. 2016, 14, 1381–1393. [Google Scholar] [CrossRef]
- Klein, T.M.; Wolf, E.D.; Wu, R.; Sanford, J.C. High-velocity microprojectiles for delivering nucleic acids into living cells. Nature 1987, 327, 70–73. [Google Scholar] [CrossRef]
- Valjakka, M.; Aronen, T.; Kangasjärvi, J.; Vapaavuori, E.; Häggman, H. Genetic transformation of silver birch (Betula pendula) by particle bombardment. Tree Physiol. 2000, 20, 607–613. [Google Scholar] [CrossRef]
- Ye, X.; Brown, S.K.; Scorza, R.; Cordts, J.; Sanford, J.C. Genetic transformation of peach tissues by particle bombardment. J. Am. Soc. Hortic. Sci. 1994, 119, 367–373. [Google Scholar] [CrossRef]
- Fernando, D.D.; Richards, J.L.; Kikkert, J.R. In vitro germination and transient GFP expression of American chestnut (Castanea dentata) pollen. Plant Cell Rep. 2006, 25, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Tuo, D.; Ma, C.; Yan, P.; Kong, H.; Zhou, P.; Guo, A.; Zhao, H.; Shen, W. Genetic transformation and gene delivery strategies in Carica papaya L. Trop. Plants 2023, 2, 5. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, W.; Takechi, K.; Takio, S.; Ono, K.; Takano, H. Stable genetic transformation of Larix gmelinii L. by particle bombardment of zygotic embryos. Plant Cell Rep. 2005, 24, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.H.; Häggman, H.; Elfstrand, M.; Aronen, T.; von Arnold, S. Transformation of Norway spruce (Picea abies) by particle bombardment. In Genetic Transformation of Plants; Jackson, J.F., Linskens, H.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 127–146. [Google Scholar]
- Rey, M.; Humara, J.M.; Lopez, M.; Gonzalez, M.V.; Rodriguez, R.; Tavazza, R.; Ancora, G.; Ordas, R.J. Foreign gene expression in Pinus nigra, P. radiata and P. pinea following particle bombardment. In Somatic Cell Genetics and Molecular Genetics of Trees; Ahuja, M.R., Boerjan, W., Neale, D.B., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 113–117. [Google Scholar]
- Ozyigit, I.I.; Yucebilgili Kurtoglu, K. Particle bombardment technology and its applications in plants. Mol. Biol. Rep. 2020, 47, 9831–9847. [Google Scholar] [CrossRef]
- Rivera, A.L.; Gómez-Lim, M.; Fernández, F.; Loske, A.M. Physical methods for genetic plant transformation. Phys. Life Rev. 2012, 9, 308–345. [Google Scholar] [CrossRef]
- Lv, Z.; Jiang, R.; Chen, J.; Chen, W. Nanoparticle-mediated gene transformation strategies for plant genetic engineering. Plant J. 2020, 104, 880–891. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, Z.; Wang, Y.; Chen, W.; Sun, C.; Cui, B.; Cui, J.; Yu, M.; Zeng, Z.; Guo, S.; et al. Pollen magnetofection for genetic modification with magnetic nanoparticles as gene carriers. Nat. Plants 2017, 3, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Rani, N.; Kumari, K.; Hooda, V. The role of nanoparticles in transforming plant genetic engineering: Advancements, challenges and future prospects. Funct. Integr. Genom. 2025, 25, 23. [Google Scholar] [CrossRef]
- Rohatgi, V.; Challagulla, N.V.; Pudake, R.N. Chapter 23—Current status and future prospects of nanoparticles as plant genetic materials carrier. In Nano-Enabled Agrochemicals in Agriculture; Ghorbanpour, M., Shahid, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 407–424. [Google Scholar]
- Ahmar, S.; Mahmood, T.; Fiaz, S.; Mora-Poblete, F.; Shafique, M.S.; Chattha, M.S.; Jung, K.-H. Advantage of nanotechnology-based genome editing system and its application in crop improvement. Front. Plant Sci. 2021, 12, 663849. [Google Scholar] [CrossRef]
- Nageswara-Rao, M.; Soneji, J.R.; Kwit, C.; Stewart, C.N. Advances in biotechnology and genomics of switchgrass. Biotechnol. Biofuels 2013, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Hu, J.; Sun, Y.; Li, B.; Zhang, D.; Li, W.; Liu, J.; Li, D.; Gao, C.; Zhang, Y.; et al. Highly efficient heritable genome editing in wheat using an RNA virus and bypassing tissue culture. Mol. Plant 2021, 14, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.D.; Juan, M.; Dubcovsky, J.; Gallavotti, A. Recent advances in crop transformation technologies. Nat. Plants 2022, 8, 1343–1351. [Google Scholar] [CrossRef]
- Ying, W.; Wen, G.; Xu, W.; Liu, H.; Ding, W.; Zheng, L.; He, Y.; Yuan, H.; Yan, D.; Cui, F.; et al. Agrobacterium rhizogenes: Paving the road to research and breeding for woody plants. Front. Plant Sci. 2023, 14, 1196561. [Google Scholar] [CrossRef]
- Liu, L.; Sun, M.; Qi, X.; Zhang, J.; Jiang, M.; Cai, H.; Yang, M.; Wang, J. Establishment of a leaf regeneration system and its molecular basis in Poplar 741. BMC Plant Biol. 2025, 25, 1100. [Google Scholar] [CrossRef]
- Li, H.; Wang, H.; Guan, L.; Li, Z.; Wang, H.; Luo, J. Optimization of high-efficiency tissue culture regeneration systems in gray poplar. Life 2023, 13, 1896. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Li, X.; Shang, X. Regeneration and genetic transformation in Eucalyptus species, current research and future perspectives. Plants 2024, 13, 2843. [Google Scholar] [CrossRef]
- Ricci, A.; Capriotti, L.; Mezzetti, B.; Navacchi, O.; Sabbadini, S. Adventitious shoot regeneration from in vitro leaf explants of the peach rootstock Hansen 536. Plants 2020, 9, 755. [Google Scholar] [CrossRef]
- Boutilier, K.; Offringa, R.; Sharma, V.K.; Kieft, H.; Ouellet, T.; Zhang, L.; Hattori, J.; Liu, C.M.; van Lammeren, A.A.; Miki, B.L.; et al. Ectopic Expression of BABY BOOM Triggers a Conversion from Vegetative to Embryonic Growth. Plant Cell 2002, 14, 1737–1749. [Google Scholar] [CrossRef]
- Srinivasan, C.; Liu, Z.; Heidmann, I.; Supena, E.D.J.; Fukuoka, H.; Joosen, R.; Lambalk, J.; Angenent, G.; Scorza, R.; Custers, J.B.M.; et al. Heterologous expression of the BABY BOOM AP2/ERF transcription factor enhances the regeneration capacity of tobacco (Nicotiana tabacum L.). Planta 2007, 225, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.F.; Kou, Y.P.; Gao, B.; Soliman, T.M.A.; Xu, K.D.; Ma, N.; Cao, X.; Zhao, L.J. Identification and functional analysis of BABY BOOM genes from Rosa canina. Biol. Plant. 2014, 58, 427–435. [Google Scholar] [CrossRef]
- Zhang, J.; Hao, Z.; Ruan, X.; Weng, Y.; Chen, X.; Zhu, J.; Lu, L.; Lu, Y.; Ma, Y.; Chen, J.; et al. Role of BABY BOOM transcription factor in promoting somatic embryogenesis and genetic transformation in a woody Magnoliid Liriodendron. Plant Cell Environ. 2025, 48, 4859–4872. [Google Scholar] [CrossRef]
- Florez, S.L.; Erwin, R.L.; Maximova, S.N.; Guiltinan, M.J.; Curtis, W.R. Enhanced somatic embryogenesis in Theobroma cacao using the homologous BABY BOOM transcription factor. BMC Plant Biol. 2015, 15, 121. [Google Scholar] [CrossRef]
- Bueno, N.; Cuesta, C.; Centeno, M.L.; Ordás, R.J.; Alvarez, J.M. In vitro plant regeneration in conifers: The role of WOX and KNOX gene families. Genes 2021, 12, 438. [Google Scholar] [CrossRef]
- Hassani, S.B.; Trontin, J.-F.; Raschke, J.; Zoglauer, K.; Rupps, A. Constitutive overexpression of a conifer WOX2 homolog affects somatic embryo development in Pinus pinaster and promotes somatic embryogenesis and organogenesis in Arabidopsis seedlings. Front. Plant Sci. 2022, 13, 838421. [Google Scholar] [CrossRef] [PubMed]
- Klimaszewska, K.; Overton, C.; Stewart, D.; Rutledge, R.G. Initiation of somatic embryos and regeneration of plants from primordial shoots of 10-year-old somatic white spruce and expression profiles of 11 genes followed during the tissue culture process. Planta 2011, 233, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Bonga, J.M.; Klimaszewska, K.K.; von Aderkas, P. Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 100, 241–254. [Google Scholar] [CrossRef]
- Salaj, T.; Klubicová, K.; Matusova, R.; Salaj, J. Somatic Embryogenesis in Selected Conifer Trees Pinus nigra Arn. and Abies Hybrids. Front. Plant Sci. 2019, 10, 13. [Google Scholar] [CrossRef]
- Hu, W.; Li, W.; Xie, S.; Fagundez, S.; McAvoy, R.; Deng, Z.; Li, Y. Kn1 gene overexpression drastically improves genetic transformation efficiencies of citrus cultivars. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 125, 81–91. [Google Scholar] [CrossRef]
- Debernardi, J.M.; Tricoli, D.M.; Ercoli, M.F.; Hayta, S.; Ronald, P.; Palatnik, J.F.; Dubcovsky, J. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 2020, 38, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-P.; Wang, J.; Ye, J.-L.; Zhu, A.-D.; Guo, W.-W.; Deng, X.-X. Isolation and characterization of LEAFY COTYLEDON 1-LIKE gene related to embryogenic competence in Citrus sinensis. Plant Cell Tissue Organ Cult. (PCTOC) 2014, 119, 1–13. [Google Scholar] [CrossRef]
- Berenguer, E.; Carneros, E.; Pérez-Pérez, Y.; Gil, C.; Martínez, A.; Testillano, P.S. Small molecule inhibitors of mammalian GSK-3β promote in vitro plant cell reprogramming and somatic embryogenesis in crop and forest species. J. Exp. Bot. 2021, 72, 7808–7825. [Google Scholar] [CrossRef]
- Guo, T.; Bao, F.; Fan, Y.; Zhang, J.; Zhao, J. Small molecules, enormous functions: Potential approach for overcoming bottlenecks in embryogenic tissue induction and maintenance in conifers. Hortic. Res. 2024, 11, uhae180. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Noceda, C.; Pelletier, G.; Label, P.; Rodriguez, R.; Lelu-Walter, M.A. Biological characterization of young and aged embryogenic cultures of Pinus pinaster (Ait.). Vitr. Cell. Dev. Biol. Plant 2009, 45, 20–33. [Google Scholar] [CrossRef]
- Uddenberg, D.; Valladares, S.; Abrahamsson, M.; Sundström, J.F.; Sundås-Larsson, A.; von Arnold, S. Embryogenic potential and expression of embryogenesis-related genes in conifers are affected by treatment with a histone deacetylase inhibitor. Planta 2011, 234, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, M.; Valladares, S.; Merino, I.; Larsson, E.; von Arnold, S. Degeneration pattern in somatic embryos of Pinus sylvestris L. Vitr. Cell. Dev. Biol. Plant 2017, 53, 86–96. [Google Scholar] [CrossRef]
- Prasad, A.; Sidhic, J.; Sarbadhikary, P.; Narayanankutty, A.; George, S.; George, B.P.; Abrahamse, H. Role of metal nanoparticles in organogenesis, secondary metabolite production and genetic transformation of plants under in vitro condition: A comprehensive review. Plant Cell Tissue Organ Cult. (PCTOC) 2024, 158, 33. [Google Scholar] [CrossRef]
- Khadgi, A.; Sagawa, C.H.D.; Vernon, C.; Mermaz, B.; Irish, V.F. Optimization of in planta methodology for genome editing and transformation in Citrus. Front. Plant Sci. 2024, 15, 1438031. [Google Scholar] [CrossRef]
- Cosic, M.; Laurans, F.; Grand-Perret, C.; Laine-Prade, V.; Lesage Descauses, M.-C.; Pilate, G.; Dejardin, A. Development of an innovative in planta transformation method in poplar. In Proceedings of the IUFRO Tree Biotech 2017, Concepcion, Chile, 4–9 June 2017. [Google Scholar]
- Maher, M.F.; Nasti, R.A.; Vollbrecht, M.; Starker, C.G.; Clark, M.D.; Voytas, D.F. Plant gene editing through de novo induction of meristems. Nat. Biotechnol. 2020, 38, 84–89. [Google Scholar] [CrossRef]
- Ma, H.; Liu, N.; Sun, X.; Zhu, M.; Mao, T.; Huang, S.; Meng, X.; Li, H.; Wang, M.; Liang, H. Establishment of an efficient transformation system and its application in regulatory mechanism analysis of biological macromolecules in tea plants. Int. J. Biol. Macromol. 2023, 244, 125372. [Google Scholar] [CrossRef]
- Muchero, W.; Sondreli, K.L.; Chen, J.-G.; Urbanowicz, B.R.; Zhang, J.; Singan, V.; Yang, Y.; Brueggeman, R.S.; Franco-Coronado, J.; Abraham, N.; et al. Association mapping, transcriptomics, and transient expression identify candidate genes mediating plant–pathogen interactions in a tree. Proc. Natl. Acad. Sci. USA 2018, 115, 11573–11578. [Google Scholar] [CrossRef]
- Cao, X.; Xie, H.; Song, M.; Lu, J.; Ma, P.; Huang, B.; Wang, M.; Tian, Y.; Chen, F.; Peng, J.; et al. Cut-dip-budding delivery system enables genetic modifications in plants without tissue culture. Innovation 2023, 4, 100345. [Google Scholar] [CrossRef]
- Xie, X.; Agüero, C.B.; Wang, Y.; Walker, M.A. Genetic transformation of grape varieties and rootstocks via organogenesis. Plant Cell Tissue Organ Cult. (PCTOC) 2016, 126, 541–552. [Google Scholar] [CrossRef]
- Gleba, Y.; Klimyuk, V.; Marillonnet, S. Viral vectors for the expression of proteins in plants. Curr. Opin. Biotechnol. 2007, 18, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.H.; Ko, Y.; Lee, J.M. Enhancing virus-mediated genome editing for cultivated tomato through low temperature. Plant Cell Rep. 2025, 44, 22. [Google Scholar] [CrossRef] [PubMed]
- Faivre-Rampant, O.; Gilroy, E.M.; Hrubikova, K.; Hein, I.; Millam, S.; Loake, G.J.; Birch, P.; Taylor, M.; Lacomme, C. Potato virus X-induced gene silencing in leaves and tubers of potato. Plant Physiol. 2004, 134, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Kujur, S.; Senthil-Kumar, M.; Kumar, R. Plant viral vectors: Expanding the possibilities of precise gene editing in plant genomes. Plant Cell Rep. 2021, 40, 931–934. [Google Scholar] [CrossRef]
- Wang, X.; Shen, W.; Cui, H.; Dai, Z. Virus-induced gene silencing (VIGS) as a tool for functional genetic analysis in passion fruit plants. bioRxiv 2023. [Google Scholar] [CrossRef]
- Velázquez, K.; Agüero, J.; Vives, M.C.; Aleza, P.; Pina, J.A.; Moreno, P.; Navarro, L.; Guerri, J. Precocious flowering of juvenile citrus induced by a viral vector based on Citrus leaf blotch virus: A new tool for genetics and breeding. Plant Biotechnol. J. 2016, 14, 1976–1985. [Google Scholar] [CrossRef] [PubMed]
- Kelly, G.; Plesser, E.; Bdolach, E.; Arroyave, M.; Belausov, E.; Doron-Faigenboim, A.; Rozen, A.; Zemach, H.; Zach, Y.Y.; Goldenberg, L.; et al. In planta genome editing in citrus facilitated by co-expression of CRISPR/Cas and developmental regulators. Plant J. 2025, 122, e70155. [Google Scholar] [CrossRef] [PubMed]
- Mei, G.; Chen, A.; Wang, Y.; Li, S.; Wu, M.; Hu, Y.; Liu, X.; Hou, X. A simple and efficient in planta transformation method based on the active regeneration capacity of plants. Plant Commun. 2024, 5, 100822. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, H.; Zhang, S.; Gao, J.; Zeng, B.; Fan, C. Transgenic Eucalyptus urophylla × Eucalyptus grandis superior clone DH3229: Achieving glyphosate resistance through genetic modification. Plant Cell Tissue Organ Cult. (PCTOC) 2025, 160, 43. [Google Scholar] [CrossRef]
- Thanananta, N.; Vuttipongchaikij, S.; Apisitwanich, S. Agrobacterium-mediated transformation of a Eucalyptus camaldulensis × E. tereticornis hybrid using peeled nodal-stem segments with yeast HAL2 for improving salt tolerance. New For. 2018, 49, 311–327. [Google Scholar] [CrossRef]
- Maleki, S.S.; Mohammadi, K.; Ji, K.S. Study on factors influencing transformation efficiency in Pinus massoniana using Agrobacterium tumefaciens. Plant Cell Tissue Organ Cult. (PCTOC) 2018, 133, 437–445. [Google Scholar] [CrossRef]
- Song, J.; Lu, S.; Chen, Z.-Z.; Lourenco, R.; Chiang, V.L. Genetic transformation of Populus trichocarpa genotype Nisqually-1: A functional genomic tool for woody plants. Plant Cell Physiol. 2006, 47, 1582–1589. [Google Scholar] [CrossRef]
- Sulis, D.B.; Jiang, X.; Yang, C.; Marques, B.M.; Matthews, M.L.; Miller, Z.; Lan, K.; Cofre-Vega, C.; Liu, B.; Sun, R.; et al. Multiplex CRISPR editing of wood for sustainable fiber production. Science 2023, 381, 216–221. [Google Scholar] [CrossRef]
- Prieto, H.; Loyola, R.; Vergara, R.; Olivares, F.; Olmedo, B.; Toro, C.; Muñoz, M.; Mora, R.; Plantat, P.; Miccono, M.; et al. Gene editing in Prunus Spp.: The challenge of adapting regular gene transfer procedures for precision breeding. In Prunus—Recent Advances; Küden, A.B., Kuden, A., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Yang, J.; Yi, J.; Yang, C.; Li, C. Agrobacterium tumefaciens-mediated genetic transformation of Salix matsudana Koidz. using mature seeds. Tree Physiol. 2013, 33, 628–639. [Google Scholar] [CrossRef]
- Moore, G.; Khalaf, A.; Febres, V.; Fisher, L. Citrus transformation: Challenges and prospects. In Genetic Transformation; Alvarez, M.A., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Ma, H.; Meng, X.; Xu, K.; Li, M.; Gmitter, F.G.; Liu, N.; Gai, Y.; Huang, S.; Wang, M.; Wang, M.; et al. Highly efficient hairy root genetic transformation and applications in citrus. Front. Plant Sci. 2022, 13, 1039094. [Google Scholar] [CrossRef]
- Cervera, M.; Navarro, A.; Navarro, L.; Peña, L. Production of transgenic adult plants from clementine mandarin by enhancing cell competence for transformation and regeneration. Tree Physiol. 2008, 28, 55–66. [Google Scholar] [CrossRef]
- Cervera, M.; Juárez, J.; Navarro, L.; Peña, L. Genetic transformation of mature citrus plants. In Transgenic Plants: Methods and Protocols; Peña, L., Ed.; Humana Press: Totowa, NJ, USA, 2004; pp. 177–187. [Google Scholar]
- Conti, G.; Xoconostle-Cázares, B.; Marcelino-Pérez, G.; Hopp, H.E.; Reyes, C.A. Citrus genetic transformation: An overview of the current strategies and insights on the new emerging technologies. Front. Plant Sci. 2021, 12, 768197. [Google Scholar] [CrossRef]
- Serrano, L.; Rochange, F.; Semblat, J.P.; Marque, C.; Teulières, C.; Boudet, A.-M. Genetic transformation of Eucalyptus globulus through biolistics: Complementary development of procedures for organogenesis from zygotic embryos and stable transformation of corresponding proliferating tissue. J. Exp. Bot. 1996, 47, 285–290. [Google Scholar] [CrossRef][Green Version]
- Yao, J.-L.; Tomes, S.; Gleave, A.P. Transformation of apple (Malus × domestica) using mutants of apple acetolactate synthase as a selectable marker and analysis of the T-DNA integration sites. Plant Cell Rep. 2013, 32, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Wada, M.; Nishitani, C.; Komori, S. Stable and efficient transformation of apple. Plant Biotechnol. 2020, 37, 163–170. [Google Scholar] [CrossRef]
- Liu, S.; Ma, J.; Liu, H.; Guo, Y.; Li, W.; Niu, S. An efficient system for Agrobacterium-mediated transient transformation in Pinus tabuliformis. Plant Methods 2020, 16, 52. [Google Scholar] [CrossRef] [PubMed]
- Yibrah, H.S.; Grönroos, R.; Lindroth, A.; Franzén, H.; Clapham, D.; von Arnold, S. Agrobacterium rhizogenes-mediated induction of adventitious rooting from Pinus contorta hypocotyls and the effect of 5-azacytidine on transgene activity. Transgenic Res. 1996, 5, 75–85. [Google Scholar] [CrossRef]
- Xu, S.; Lai, E.; Zhao, L.; Cai, Y.; Ogutu, C.; Cherono, S.; Han, Y.; Zheng, B. Development of a fast and efficient root transgenic system for functional genomics and genetic engineering in peach. Sci. Rep. 2020, 10, 2836. [Google Scholar] [CrossRef]
- Liu, X.; Pijut, P.M. Agrobacterium-mediated transformation of mature Prunus serotina (black cherry) and regeneration of transgenic shoots. Plant Cell Tissue Organ Cult. (PCTOC) 2010, 101, 49–57. [Google Scholar] [CrossRef]
- Raman, V.; Rojas, C.M.; Vasudevan, B.; Dunning, K.; Kolape, J.; Oh, S.; Yun, J.; Yang, L.; Li, G.; Pant, B.D.; et al. Agrobacterium expressing a type III secretion system delivers Pseudomonas effectors into plant cells to enhance transformation. Nat. Commun. 2022, 13, 2581. [Google Scholar] [CrossRef]
- Kang, M.; Lee, K.; Finley, T.; Chappell, H.; Veena, V.; Wang, K. An improved Agrobacterium-mediated transformation and genome-editing method for maize inbred B104 using a ternary vector system and immature embryos. Front. Plant Sci. 2022, 13, 860971. [Google Scholar] [CrossRef]
- Yang, W.; Zhai, H.; Wu, F.; Deng, L.; Chao, Y.; Meng, X.; Chen, Q.; Liu, C.; Bie, X.; Sun, C.; et al. Peptide REF1 is a local wound signal promoting plant regeneration. Cell 2024, 187, 3024–3038.e14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ince, Y.Ç.; Kawamura, A.; Favero, D.S.; Suzuki, T.; Sugimoto, K. ELONGATED HYPOCOTYL5-mediated light signaling promotes shoot regeneration in Arabidopsis thaliana. Plant Physiol. 2024, 196, 2549–2564. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Tan, J.; Li, T.; Feng, Z.; Ding, Z.; Xie, X.; Chen, Y.; Chen, L.; Liu, Y.-G.; Zhu, Q.; et al. Overexpression of maize GOLDEN2 in rice and maize calli improves regeneration by activating chloroplast development. Sci. China Life Sci. 2023, 66, 340–349. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, H.; Peng, J.; Yao, W.J.; Wang, Y.P.; Zhang, F.L.; Wang, S.R.; Zhao, Y.; Zhao, X.Y.; Zhang, X.S.; et al. Enhancing wheat regeneration and genetic transformation through overexpression of TaLAX1. Plant Commun. 2024, 5, 100738. [Google Scholar] [CrossRef]
- Morończyk, J.; Brąszewska, A.; Wójcikowska, B.; Chwiałkowska, K.; Nowak, K.; Wójcik, A.M.; Kwaśniewski, M.; Gaj, M.D. Insights into the Histone Acetylation-Mediated Regulation of the Transcription Factor Genes That Control the Embryogenic Transition in the Somatic Cells of Arabidopsis. Cells 2022, 11, 863. [Google Scholar] [CrossRef]
- Martínez, Ó.; Arjones, V.; González, M.V.; Rey, M. Histone Deacetylase Inhibitors Increase the Embryogenic Potential and Alter the Expression of Embryogenesis-Related and HDAC-Encoding Genes in Grapevine (Vitis vinifera L., cv. Mencía). Plants 2021, 10, 1164. [Google Scholar] [CrossRef]
- Bie, X.M.; Dong, L.; Li, X.H.; Wang, H.; Gao, X.-Q.; Li, X.G. Trichostatin A and sodium butyrate promotes plant regeneration in common wheat. Plant Signal. Behav. 2020, 15, 1820681. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.-L.; Li, X.; Zhou, W.; Yan, J.-D.; Gao, Y.-R.; Li, X.-W.; Sun, J.-C.; Fang, K.-F.; Zhang, Q.; Xing, Y.; et al. Agrobacterium-mediated genetic transformation of Chinese chestnut (Castanea mollissima Blume). Plant Cell Tissue Organ Cult. (PCTOC) 2020, 140, 95–103. [Google Scholar] [CrossRef]
- Mathews, H.; Dewey, V.; Wagoner, W.; Bestwick, R.K. Molecular and cellular evidence of chimaeric tissues in primary transgenics and elimination of chimaerism through improved selection protocols. Transgenic Res. 1998, 7, 123–129. [Google Scholar] [CrossRef]
- Yang, X.; Maqbool, A.; Zang, J.; Niu, Y.; Liu, Z.; Wang, L.; Liu, M. Establishment of a simple Agrobacterium rhizogenes-mediated hairy root transformation system in sour jujube. Sci. Hortic. 2025, 341, 113984. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Su, P.; Yang, J.; Huang, L.; Gao, W. Genetic transformation system for woody plant Tripterygium wilfordii and its application to product natural celastrol. Front. Plant Sci. 2018, 8, 2221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Huo, Y.; Zhang, J.; Zhang, X.; Zhu, C. Agrobacterium rhizogenes-mediated RNAi of Tripterygium wilfordii and application for functional study of terpenoid biosynthesis pathway genes. Ind. Crops Prod. 2019, 139, 111509. [Google Scholar] [CrossRef]
- He, Y.; Zhang, T.; Sun, H.; Zhan, H.; Zhao, Y. A reporter for noninvasively monitoring gene expression and plant transformation. Hortic. Res. 2020, 7, 152. [Google Scholar] [CrossRef]
- Levengood, H.; Zhou, Y.; Zhang, C. Advancements in plant transformation: From traditional methods to cutting-edge techniques and emerging model species. Plant Cell Rep. 2024, 43, 273. [Google Scholar] [CrossRef]
- Tiwari, M.; Mishra, A.K.; Chakrabarty, D. Agrobacterium-mediated gene transfer: Recent advancements and layered immunity in plants. Planta 2022, 256, 37. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tannous, J.; Rush, T.A.; Del Valle, I.; Xiao, S.; Maharjan, B.; Liu, Y.; Weston, D.J.; De, K.; Tschaplinski, T.J.; et al. Utilizing plant synthetic biology to accelerate plant-microbe interactions research. BioDes. Res. 2025, 7, 100007. [Google Scholar] [CrossRef]
- Bigeard, J.; Colcombet, J.; Hirt, H. Signaling mechanisms in pattern-triggered immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, G.; Xu, H.; He, T.; Zong, Y.; Zhang, S.; Faheem, M.; Lu, R.; Zhou, L.; Liu, C. Comparative transcriptome analysis reveals compatible and recalcitrant genotypic response of barley microspore-derived embryogenic callus toward Agrobacterium infection. BMC Plant Biol. 2021, 21, 579. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Uppalapati, S.R.; Ryu, C.M.; Allen, S.N.; Kang, L.; Tang, Y.; Mysore, K.S. Salicylic acid and systemic acquired resistance play a role in attenuating crown gall disease caused by Agrobacterium tumefaciens. Plant Physiol. 2008, 146, 703–715. [Google Scholar] [CrossRef]
- Yuan, Z.C.; Edlind, M.P.; Liu, P.; Saenkham, P.; Banta, L.M.; Wise, A.A.; Ronzone, E.; Binns, A.N.; Kerr, K.; Nester, E.W. The plant signal salicylic acid shuts down expression of the vir regulon and activates quormone-quenching genes in Agrobacterium. Proc. Natl. Acad. Sci. USA 2007, 104, 11790–11795. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-h.; Jeon, E.-y.; Hwang, M.K.; Song, Y.J.; Kim, J.-Y. Development of super-infective ternary vector systems for enhancing the Agrobacterium-mediated plant transformation and genome editing efficiency. Hortic. Res. 2024, 11, uhae187. [Google Scholar] [CrossRef]
- Xu, M.; Du, Q.; Tian, C.; Wang, Y.; Jiao, Y. Stochastic gene expression drives mesophyll protoplast regeneration. Sci. Adv. 2021, 7, eabg8466. [Google Scholar] [CrossRef]
- Ogura, N.; Sasagawa, Y.; Ito, T.; Tameshige, T.; Kawai, S.; Sano, M.; Doll, Y.; Iwase, A.; Kawamura, A.; Suzuki, T.; et al. WUSCHEL-RELATED HOMEOBOX 13 suppresses de novo shoot regeneration via cell fate control of pluripotent callus. Sci. Adv. 2023, 9, eadg6983. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, Z.; Wang, G.; Cong, Y.; Yu, L.; Jia, R.; Qin, Y.; Zhang, G.; Li, B.; Yuan, D.; et al. Single-cell resolution analysis reveals the preparation for reprogramming the fate of stem cell niche in cotton lateral meristem. Genome Biol. 2023, 24, 194. [Google Scholar] [CrossRef]
- Song, X.; Guo, P.; Xia, K.; Wang, M.; Liu, Y.; Chen, L.; Zhang, J.; Xu, M.; Liu, N.; Yue, Z.; et al. Spatial transcriptomics reveals light-induced chlorenchyma cells involved in promoting shoot regeneration in tomato callus. Proc. Natl. Acad. Sci. USA 2023, 120, e2310163120. [Google Scholar] [CrossRef]
- Liu, X.; Bie, X.M.; Lin, X.; Li, M.; Wang, H.; Zhang, X.; Yang, Y.; Zhang, C.; Zhang, X.S.; Xiao, J. Uncovering the transcriptional regulatory network involved in boosting wheat regeneration and transformation. Nat. Plants 2023, 9, 908–925. [Google Scholar] [CrossRef]
- Nagle, M.F.; Yuan, J.; Kaur, D.; Ma, C.; Peremyslova, E.; Jiang, Y.; Goralogia, G.S.; Magnuson, A.; Li, J.Y.; Muchero, W.; et al. Genome-wide association study and network analysis of in vitro transformation in Populus trichocarpa support key roles of diverse phytohormone pathways and cross talk. New Phytol. 2024, 242, 2059–2076. [Google Scholar] [CrossRef] [PubMed]
- Tuskan, G.A.; Mewalal, R.; Gunter, L.E.; Palla, K.J.; Carter, K.; Jacobson, D.A.; Jones, P.C.; Garcia, B.J.; Weighill, D.A.; Hyatt, P.D.; et al. Defining the genetic components of callus formation: A GWAS approach. PLoS ONE 2018, 13, e0202519. [Google Scholar] [CrossRef]
- Nagle, M.F.; Yuan, J.; Kaur, D.; Ma, C.; Peremyslova, E.; Jiang, Y.; Niño de Rivera, A.; Jawdy, S.; Chen, J.-G.; Feng, K.; et al. GWAS supported by computer vision identifies large numbers of candidate regulators of in planta regeneration in Populus trichocarpa. G3 Genes|Genomes|Genet. 2024, 14, jkae026. [Google Scholar] [CrossRef]
- Hundleby, P.; Harwood, W. Regulatory constraints and differences of genome-edited crops around the globe. In Genome Editing: Current Technology Advances and Applications for Crop Improvement; Wani, S.H., Hensel, G., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 319–341. [Google Scholar]
- Van Der Meer, P.; Angenon, G.; Bergmans, H.; Buhk, H.J.; Callebaut, S.; Chamon, M.; Eriksson, D.; Gheysen, G.; Harwood, W.; Hundleby, P.; et al. The status under EU law of organisms developed through novel genomic techniques. Eur. J. Risk Regul. 2023, 14, 93–112. [Google Scholar] [CrossRef]
- Sprink, T.; Wilhelm, R.; Hartung, F. Genome editing around the globe: An update on policies and perceptions. Plant Physiol. 2022, 190, 1579–1587. [Google Scholar] [CrossRef]
- Fernández Ríos, D.; Quintana, S.A.; Gómez Paniagua, P.; Arrúa, A.A.; Brozón, G.R.; Bertoni Hicar, M.S.; Castro Alegría, A.; Goberna, M.F. Regulatory challenges and global trade implications of genome editing in agriculture. Front. Bioeng. Biotechnol. 2025, 13, 1609110. [Google Scholar] [CrossRef] [PubMed]
- Vora, Z.; Pandya, J.; Sangh, C.; Vaikuntapu, P.R. The evolving landscape of global regulations on genome-edited crops. J. Plant Biochem. Biotechnol. 2023, 32, 831–845. [Google Scholar] [CrossRef]
- Telem, R.S.; Wani, S.H.; Singh, N.B.; Nandini, R.; Sadhukhan, R.; Bhattacharya, S.; Mandal, N. Cisgenics—A sustainable approach for crop improvement. Curr. Genom. 2013, 14, 468–476. [Google Scholar] [CrossRef]
- Yue, J.J.; Yuan, J.L.; Wu, F.H.; Yuan, Y.H.; Cheng, Q.W.; Hsu, C.T.; Lin, C.S. Protoplasts: From isolation to CRISPR/Cas genome editing application. Front. Genome Ed. 2021, 3, 717017. [Google Scholar] [CrossRef]
- Waltz, E. Gene-edited CRISPR mushroom escapes US regulation. Nature 2016, 532, 293. [Google Scholar] [CrossRef]
- Waltz, E. With a free pass, CRISPR-edited plants reach market in record time. Nat. Biotechnol. 2018, 36, 6–7. [Google Scholar] [CrossRef]
- USDA. Japan Gives Green Light to Genome Edited Waxy Corn Product; United States Department of Agriculture: Washington, DC, USA, 2023. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Japan%20Gives%20Green%20Light%20to%20Genome%20Edited%20Waxy%20Corn%20Product_Tokyo_Japan_JA2023-0029.pdf (accessed on 6 November 2025).
- Yan, S.; Zhu, W.; Zhang, B.; Zhang, X.; Zhu, J.; Shi, J.; Wu, P.; Wu, F.; Li, X.; Zhang, Q.; et al. Pollen-mediated gene flow from transgenic cotton is constrained by physical isolation measures. Sci. Rep. 2018, 8, 2862. [Google Scholar] [CrossRef]
- Pons, E.; Navarro, A.; Ollitrault, P.; Peña, L. Pollen Competition as a Reproductive Isolation Barrier Represses Transgene Flow between Compatible and Co-Flowering Citrus Genotypes. PLoS ONE 2011, 6, e25810. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H. Molecular strategies for gene containment in transgenic crops. Nat. Biotechnol. 2002, 20, 581–586. [Google Scholar] [CrossRef]
- Lal, M.; Bhardwaj, E.; Chahar, N.; Dangwal, M.; Das, S. containment. In Reproductive Ecology of Flowering Plants: Patterns and Processes; Tandon, R., Shivanna, K.R., Koul, M., Eds.; Springer: Singapore, 2020; pp. 335–394. [Google Scholar]
- Schmidt, S.M.; Belisle, M.; Frommer, W.B. The evolving landscape around genome editing in agriculture. EMBO Rep. 2020, 21, e50680. [Google Scholar] [CrossRef]
- Van Vu, T.; Das, S.; Hensel, G.; Kim, J.-Y. Genome editing and beyond: What does it mean for the future of plant breeding? Planta 2022, 255, 130. [Google Scholar] [CrossRef] [PubMed]
- Lynas, M.; Adams, S.; Stockert, K. Gene editing achieves consistently higher favorability in social and traditional media than GMOs. GM Crops Food 2023, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]

| Species | Explant | DNA Delivery | Organ Regeneration | Transformation Time | Transformation Rate (%) | References |
|---|---|---|---|---|---|---|
| Citrus × poncirus | Seedlings | RR | Hairy root induction | 6 months | 28–75% | [14] |
| Eucalyptus urophylla × E. grandis DH3229 | Leaves | AT (Strain EHA105) | Callus-mediated organogenesis | >14 weeks | 4.07% | [90] |
| E. camaldulensis × E. tereticornis | Peeled nodal stem | AT (Strain EHA105) | Direct regeneration | >8 weeks | ~24% | [91] |
| Malus domestica | Leaves | RR (Strain K599) | Hairy root induction | 14 weeks | 3.3–20.6% | [30] |
| Malus domestica | Leaves | AT (Strain LBA4404) | Direct regeneration | 4 months | 3–30% | [18] |
| Pinus massoniana Lamb. | Embryo | AT (Strain EHA105) | Callus-mediated organogenesis | 15 weeks | 6–59.75% | [92] |
| Populus tremula × P. alba clone INRA 717-1B4 | Leaves, petioles, roots | AT (Strain GV3101) | Callus-mediated organogenesis, | >13 weeks | 15–25% | [12] |
| P. deltoides | Petioles and base of binary vein | AT (Strain EHA105) | Direct regeneration | 3–4 months | 8.7% | [13] |
| P. trichocarpa | Internodal stem | AT (Strain C58) | Callus-mediated organogenesis | 5–8 months | 13% | [93,94] |
| Prunus persica | Embryo, cotyledon | AT | Callus-mediated organogenesis | 8–12 months | 2% | [95] |
| Prunus avium | Epicotyl | AT (Strain GV3101) | Callus-mediated organogenesis | 6–8 months | 4% | [95] |
| Salix matsudana | Embryogenic shoots | AT (Strain LBA4404) | Callus-mediated organogenesis | ~5 months | 7.2% | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maharjan, B.K.; Islam, M.T.; Muzaffar, A.; Tschaplinski, T.J.; Tuskan, G.A.; Chen, J.-G.; Yang, X. Woody Plant Transformation: Current Status, Challenges, and Future Perspectives. Plants 2025, 14, 3420. https://doi.org/10.3390/plants14223420
Maharjan BK, Islam MT, Muzaffar A, Tschaplinski TJ, Tuskan GA, Chen J-G, Yang X. Woody Plant Transformation: Current Status, Challenges, and Future Perspectives. Plants. 2025; 14(22):3420. https://doi.org/10.3390/plants14223420
Chicago/Turabian StyleMaharjan, Bal Krishna, Md Torikul Islam, Adnan Muzaffar, Timothy J. Tschaplinski, Gerald A. Tuskan, Jin-Gui Chen, and Xiaohan Yang. 2025. "Woody Plant Transformation: Current Status, Challenges, and Future Perspectives" Plants 14, no. 22: 3420. https://doi.org/10.3390/plants14223420
APA StyleMaharjan, B. K., Islam, M. T., Muzaffar, A., Tschaplinski, T. J., Tuskan, G. A., Chen, J.-G., & Yang, X. (2025). Woody Plant Transformation: Current Status, Challenges, and Future Perspectives. Plants, 14(22), 3420. https://doi.org/10.3390/plants14223420