Secondary Somatic Embryogenesis in Plants: From Cellular Mechanisms to Biotechnological Potential
Abstract
1. Secondary Somatic Embryogenesis: Principles and Applications
2. Cellular Origin and Developmental Pathways
2.1. Direct vs. Indirect SE
2.2. Cellular Origin of SSE
2.3. Understanding and Managing Abnormal Morphology of SEs
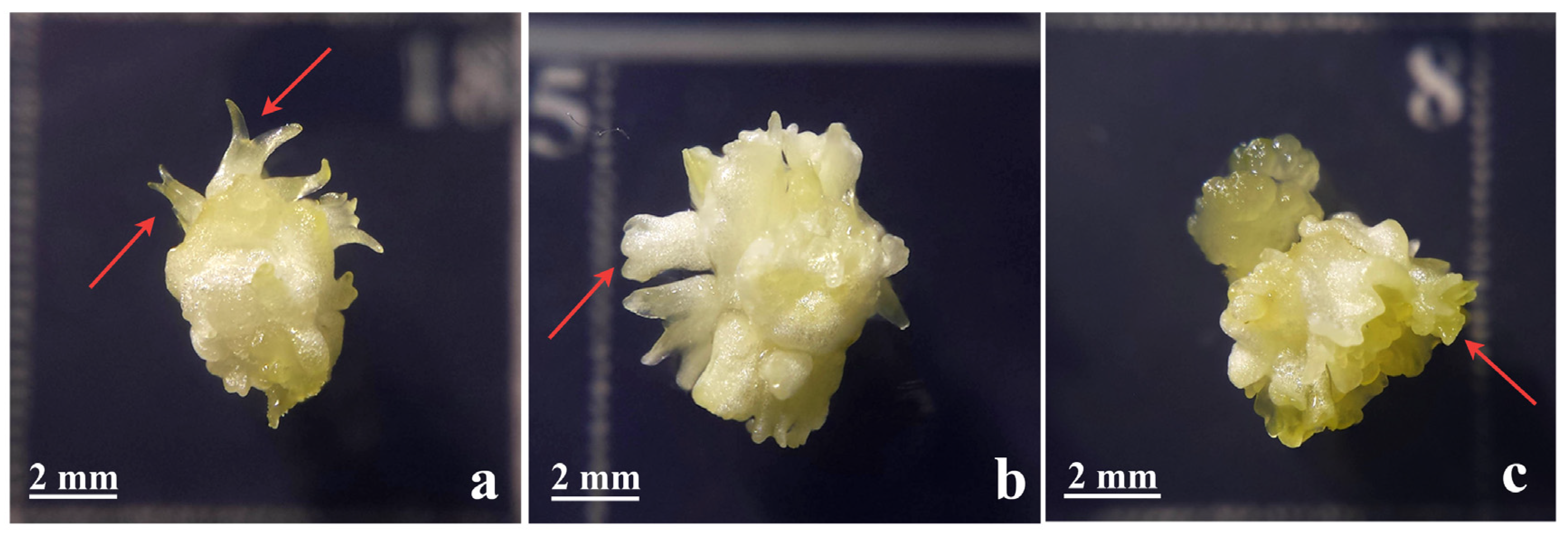
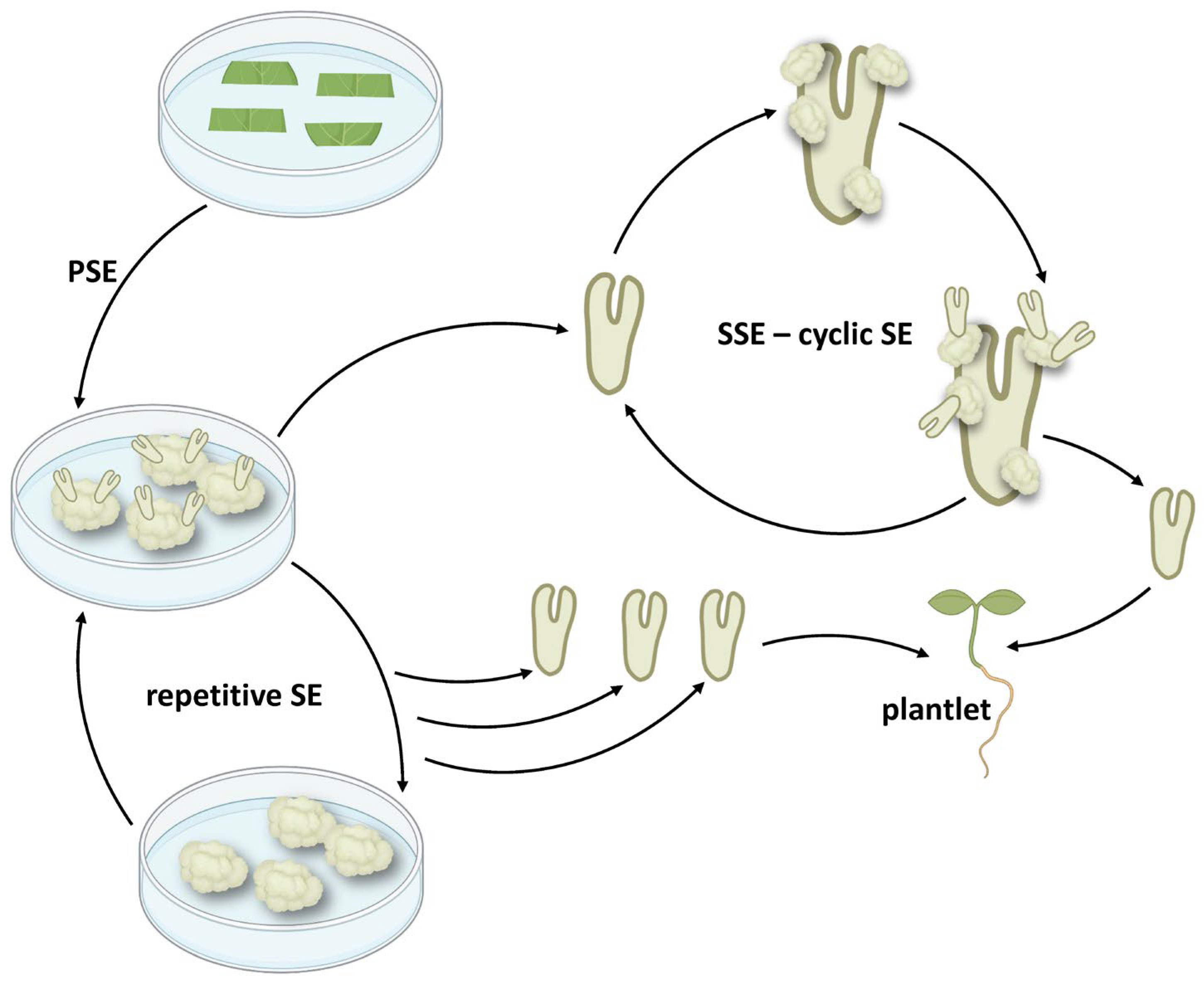
3. Cyclic Embryogenesis-Maintaining Embryogenic Potential
4. Genetic and Epigenetic Considerations: Stability and Variation
5. Applications in Plant Biotechnology
6. Challenges and Future Directions–Maximizing the Potential of Cyclic SE
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SE | Somatic embryogenesis |
| PSE | Primary somatic embryogenesis |
| SEs | Somatic embryos |
| PSEs | Primary somatic embryos |
| SSE | Secondary somatic embryogenesis |
| PGRs | Plant growth regulators |
| 2,4-D | 2,4-dichlorophenoxyacetic acid |
| SSEs | Secondary somatic embryos |
| MS | Murashige and Skoog medium |
| CIM | Callus induction medium |
| SH | Schenk and Hildebrandt medium |
| BA/BAP | Benzyladenine/Benzylaminopurine |
| NAA | 1-naphthaleneacetic acid |
| CPPU | N-2-Chloro-4-pyridyl-N’-phenylurea |
| TIS | Temporary immersion system |
References
- Schleiden, M.J. Beiträge zur phytogenesis. In Archiv für Anatomie, Physiologie und Wissenschaftliche Medicin, 1st ed.; Johannes, M., Ed.; Jahrgang: Berlin, Germany, 1838; Volume 608, pp. 5+137–176. [Google Scholar]
- Schwann, T. Mikroskopische Untersuchungen über die Uebereinstimmung in der Struktur und dem Wachsthum der Thiere und Pflanzen; Sander: Berlin, Germany, 1839. [Google Scholar]
- Martinez, M.; Corredoira, E. Recent Advances in Plant Somatic Embryogenesis: Where We Stand and Where to Go? Int. J. Mol. Sci. 2024, 25, 8912. [Google Scholar] [CrossRef] [PubMed]
- Feher, A.; Pasternak, T.P.; Dudit, D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult. 2003, 74, 201–228. [Google Scholar] [CrossRef]
- Bogdanović, M.D.; Ćuković, K.B.; Subotić, A.R.; Dragićević, M.B.; Simonović, A.D.; Filipović, B.K.; Todorović, S.I. Secondary somatic embryogenesis in Centaurium erythraea Rafn. Plants 2021, 10, 199. [Google Scholar] [CrossRef] [PubMed]
- Raemakers, C.J.J.M.; Jacobsen, E.; Visser, R.G.F. Secondary somatic embryogenesis and applications in plant breeding. Euphytica 1995, 81, 93–107. [Google Scholar] [CrossRef]
- Maximova, S.N.; Alemanno, L.; Young, A.; Ferriere, N.; Traore, A.; Guiltinan, M.J. Efficiency, genotypic variability, and cellular origin of primary and secondary somatic embryogenesis of Theobroma cacao L. Vitr. Cell. Dev. Biol. Plant 2002, 38, 252–259. [Google Scholar] [CrossRef]
- Polito, V.S.; McGranahan, G.; Pinney, K.; Leslie, C. Origin of somatic embryos from repetitively embryogenic cultures of walnut (Juglans regia L.): Implications for Agrobacterium-mediated transformation. Plant Cell Rep. 1989, 8, 219–221. [Google Scholar] [CrossRef]
- Murthy, H.N.; Hahn, E.J.; Paek, K.Y. Recurrent somatic embryogenesis and plant regeneration in Coriandrum sativum L. Sci. Hortic. 2008, 118, 168–171. [Google Scholar] [CrossRef]
- Li, X.; Krasnyanski, S.F.; Korban, S.S. Somatic embryogenesis, secondary somatic embryogenesis, and shoot organogenesis in Rosa. J. Plant Physiol. 2002, 159, 313–319. [Google Scholar] [CrossRef]
- Shi, X.; Dai, X.; Liu, G.; Zhang, J.; Ning, G.; Bao, M. Cyclic secondary somatic embryogenesis and efficient plant regeneration in camphor tree (Cinnamomum camphora L.). Vitr. Cell. Dev. Biol. Plant 2010, 46, 117–125. [Google Scholar] [CrossRef]
- Raemakers, K.J.; Jacobsen, E.; Visser, R.G. Direct cyclic somatic embryogenesis of cassava for mass production purposes. Methods Mol. Biol. 1999, 111, 61–70. [Google Scholar] [CrossRef]
- Panaia, M.; Bunn, E.; McComb, J. Primary and repetitive secondary somatic embryogenesis of Lepidosperma drummondii (Cyperaceae) and Baloskion tetraphyllum (Restionaceae) for land restoration and horticulture. Vitr. Cell. Dev. Biol. Plant 2011, 47, 379–386. [Google Scholar] [CrossRef]
- Pinto, G.; Park, Y.S.; Silva, S.; Neves, L.; Araújo, C.; Santos, C. Factors affecting maintenance, proliferation, and germination of secondary somatic embryos of Eucalyptus globulus Labill.: Basal medium and anti-browning agents. Plant Cell Tissue Organ Cult. 2008, 95, 69–78. [Google Scholar] [CrossRef]
- Bobadilla Landey, R.; Cenci, A.; Georget, F.; Bertrand, B.; Camayo, G.; Dechamp, E.; Herrera, J.C.; Santoni, S.; Lashermes, P.; Simpson, J.; et al. High Genetic and Epigenetic Stability in Coffea arabica Plants Derived from Embryogenic Suspensions and Secondary Embryogenesis as Revealed by AFLP, MSAP and the Phenotypic Variation Rate. PLoS ONE 2013, 8, e56372. [Google Scholar] [CrossRef] [PubMed]
- Kępczyńska, E.; Kępczyński, J. Hormonal regulation of somatic embryogenesis in Medicago spp. Plant Cell Tissue Organ Cult. 2023, 155, 613–625. [Google Scholar] [CrossRef]
- Olah, R.; Turcsan, M.; Olah, K.; Farkas, E.; Deak, T.; Jahnke, G.; Sardy, D.A.N. Somatic Embryogenesis: A Tool for Fast and Reliable Virus and Viroid Elimination for Grapevine and other Plant Species. Horticulturae 2022, 8, 508. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, O.R.; Kim, K.T.; Yang, D.C. High frequency of plant regeneration through cyclic secondary somatic embryogenesis in Panax ginseng. J. Ginseng Res. 2012, 36, 442–448. [Google Scholar] [CrossRef]
- Paisic-Ramirez, R.; Hernández-Amasifuen, A.D.; Sánchez-Aguilar, W.D.; Corazon-Guivin, M.A.; Bobadilla, L.G.; Mansilla-Córdova, P.J.; Caetano, A.C.; Zuta, M.Z.S.; Guerrero-Abad, J.C. Effect of osmoregulatory on the secondary somatic embryogenesis of cocoa (Theobroma cacao L.). J. Appl. Biol. Biotechnol. 2024, 12, 177–183. [Google Scholar] [CrossRef]
- Bustami, M.U.; Werbrouck, S.P.O. Cyclic Somatic Embryogenesis in Indonesian Elite Theobroma cacao L. Clones. Horticulturae 2024, 10, 24. [Google Scholar] [CrossRef]
- Heikrujam, M.; Kumar, D.; Kumar, S.; Gupta, S.C.; Agrawal, V. High efficiency cyclic production of secondary somatic embryos and ISSR based assessment of genetic fidelity among the emblings in Calliandra tweedii (Benth.). Sci. Hortic. 2014, 177, 63–70. [Google Scholar] [CrossRef]
- Eudes, F.; Acharya, S.; Laroche, A.; Selinger, L.B.; Cheng, K.J. A novel method to induce direct somatic embryogenesis, secondary embryogenesis and regeneration of fertile green cereal plants. Plant Cell Tissue Organ Cult. 2003, 73, 147–157. [Google Scholar] [CrossRef]
- Fernandez-Da Silva, R.; Menéndez-Yuffá, A. Transient gene expression in secondary somatic embryos from coffee tissues electroporated with the genes gus and bar. Electron. J. Biotechnol. 2003, 6, 26–35. [Google Scholar] [CrossRef]
- Karami, O.; Deljou, A.; Kordestani, G.K. Secondary somatic embryogenesis of carnation (Dianthus caryophyllus L.). Plant Cell Tissue Organ Cult. 2008, 92, 273–280. [Google Scholar] [CrossRef]
- Kumar, S.; Nadgauda, R. Control of Morphological Aberrations in Somatic Embryogenesis of Commiphora wightii (Arnott) Bhandari (Family: Bursaraceae) Through Secondary Somatic Embryogenesis. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 281–290. [Google Scholar] [CrossRef]
- Karami, O.; Deljou, A.; Pour, A.M. Repetitive somatic embryogenesis in carnation on picloram supplemented media. Plant Growth Regul. 2007, 51, 33–39. [Google Scholar] [CrossRef]
- Das Neves, L.O.; Duque, S.R.L.; De Almeida, J.S.; Fevereiro, P.S. Repetitive somatic embryogenesis in Medicago truncatula ssp. Narbonensis and M. truncatula Gaertn cv. Jemalong. Plant Cell Rep. 1999, 18, 398–405. [Google Scholar] [CrossRef]
- Zegzouti, R.; Arnould, M.F.; Favre, J.M. Histological investigation of the multiplication step in secondary somatic embryogenesis of Quercus robur L. Ann. For. Sci. 2001, 58, 681–690. [Google Scholar] [CrossRef]
- Joshi, S.; Paul, P.; Hartman, J.M.; Perry, S.E. AGL15 Promotion of Somatic Embryogenesis: Role and Molecular Mechanism. Front. Plant Sci. 2022, 13, 861556. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Bian, L.; Li, Y.; Shen, H. Cyclic secondary somatic embryogenesis and efficient plant regeneration in mountain ash (Sorbus pohuashanensis). Plant Cell Tissue Organ Cult. 2012, 111, 173–182. [Google Scholar] [CrossRef]
- Ćosić, T.; Vinterhalter, B.; Vinterhalter, D.; Mitić, N.; Cingel, A.; Savić, J.; Bohanec, B.; Ninković, S. In vitro plant regeneration from immature zygotic embryos and repetitive somatic embryogenesis in kohlrabi (Brassica oleracea var. gongylodes). Vitr. Cell. Dev. Biol. Plant 2013, 49, 294–303. [Google Scholar] [CrossRef]
- Da Silva, M.L.; Pinto, D.L.P.; de Campos, J.M.S.; de Carvalho, I.F.; Rocha, D.I.; Batista, D.S.; Otoni, W.C. Repetitive somatic embryogenesis from wild passion fruit (Passiflora cincinnata Mast.) anthers. Plant Cell Tissue Organ Cult. 2021, 146, 635–641. [Google Scholar] [CrossRef]
- Plata, E.; Ballester, A.; Vieitez, A.M. An anatomical study of secondary embryogenesis in Camellia reticulata. Vitr. Cell. Dev. Biol. Plant 1991, 27, 183–189. [Google Scholar] [CrossRef]
- Śliwińska, A.; Olszowska, O.; Furmanowa, M.; Nosov, A. Rapid multiplication of Polyscias filicifolia by secondary somatic embryogenesis. Vitr. Cell. Dev. Biol. Plant 2008, 44, 69–77. [Google Scholar] [CrossRef]
- Tokuji, Y.; Mizue, Y.; Masuda, H. Effects of Methyljasmonate and Concanavalin a on Embryogenesis and the Induction of Secondary Somatic Embryos of Carrot. Biosci. Biotechnol. Biochem. 1995, 59, 1675–1678. [Google Scholar] [CrossRef]
- Yadollahi, A.; Abdollahi, M.R.; Moieni, A.; Danaee, M. Effects of carbon source, polyethylene glycol and abscisic acid on secondary embryo induction and maturation in rapeseed (Brassica napus L.) microspore-derived embryos. Acta Physiol. Plant. 2011, 33, 1905–1912. [Google Scholar] [CrossRef]
- Yang, J.; Wu, S.; Li, C. High efficiency secondary somatic embryogenesis in Hovenia dulcis thunb. Through solid and liquid cultures. Sci. World J. 2013, 2013, 718754. [Google Scholar] [CrossRef]
- Zheng, Q.; Dessai, A.P.; Prakash, C.S. Rapid and repetitive plant regeneration in sweetpotato via somatic embryogenesis. Plant Cell Rep. 1996, 15, 381–385. [Google Scholar] [CrossRef]
- Anuradha, T.; Kumar, K.K.; Balasubramanian, P. Cyclic somatic embryogenesis of elite Indian cassava variety H-226. Indian J. Biotechnol. 2015, 14, 559–565. [Google Scholar]
- Daigny, G.; Paul, H.; Sangwan, R.S.; Sangwan-Norreel, B.S. Factors influencing secondary somatic embryogenesis in Malus × domestica Borkh. (cv ‘Gloster 69’). Plant Cell Rep. 1996, 16, 153–157. [Google Scholar] [CrossRef]
- Fang, J.Y.; Wetten, A.; Adu-Gyamfi, R.; Wilkinson, M.; Rodriguez-Lopez, C. Use of secondary somatic embryos promotes genetic fidelity in cryopreservation of cocoa (Theobroma cacao L.). Agric. Food Sci. 2009, 18, 152–159. [Google Scholar] [CrossRef]
- Raemakers, C.J.J.M.; Jacobsen, E.; Staritsky, G.; Amati, M.; Visser, R.G.F. Cyclic Somatic Embryogenesis and Plant Regeneration in Cassava. Ann. Bot. 1993, 71, 289–294. [Google Scholar] [CrossRef]
- Wang, T.D.; Huang, T.D.; Huang, H.S.; Hua, Y.W. Origin of secondary somatic embryos and genetic stability of the regenerated plants in Hevea brasiliensis. J. Rubber Res. 2017, 20, 101–116. [Google Scholar] [CrossRef]
- Hua, Y.W.; Huang, T.D.; Huang, H.S. Micropropagation of self-rooting juvenile clones by secondary somatic embryogenesis in Hevea brasiliensis. Plant Breed. 2010, 129, 202–207. [Google Scholar] [CrossRef]
- Junaid, A.; Mujib, A.; Sharma, M.P.; Tang, W. Growth regulators affect primary and secondary somatic embryogenesis in Madagaskar periwinkle (Catharanthus roseus (L.) G. Don) at morphological and biochemical levels. Plant Growth Regul. 2007, 51, 271–281. [Google Scholar] [CrossRef]
- Thengane, S.R.; Deodhar, S.R.; Bhosle, S.V.; Rawal, S.K. Repetitive somatic embryogenesis and plant regeneration in Garcinia indica Choiss. Vitr. Cell. Dev. Biol. Plant 2006, 42, 256–261. [Google Scholar] [CrossRef]
- Ramakrishnan Nair, R.; Dutta Gupta, S. High-frequency plant regeneration through cyclic secondary somatic embryogenesis in black pepper (Piper nigrum L.). Plant Cell Rep. 2006, 24, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Yao, X.; Zhong, C.; Li, D.; Wang, Z.; Huang, H. Recurrent somatic embryogenesis and development of somatic embryos in Akebia trifoliata (Thunb.) Koidz (Lardizabalaceae). Plant Cell Tissue Organ Cult. 2019, 139, 493–504. [Google Scholar] [CrossRef]
- Maheswaran, G.; Williams, E.G. Primary and Secondary Direct Somatic Embryogenesis from Immature Zygotic Embryos of Brassica campestris. J. Plant Physiol. 1986, 124, 455–463. [Google Scholar] [CrossRef]
- Tsolova, V.; Atanassov, A. Plant regeneration of vitis sp. via repetitive embryogenesis. Biotechnol. Biotechnol. Equip. 1996, 10, 32–36. [Google Scholar] [CrossRef]
- Mallón, R.; Covelo, P.; Vieitez, A.M. Improving secondary embryogenesis in Quercus robur: Application of temporary immersion for mass propagation. Trees Struct. Funct. 2012, 26, 731–741. [Google Scholar] [CrossRef]
- Ogata, Y.; Iizuka, M.; Nakayama, D.; Ikeda, M.; Kamada, H.; Koshiba, T. Possible involvement of abscisic acid in the induction of secondary somatic embryogenesis on seed-coat-derived carrot somatic embryos. Planta 2005, 221, 417–423. [Google Scholar] [CrossRef]
- Pavlović, S.; Vinterhalter, B.; Zdravković-Korać, S.; Vinterhalter, D.; Zdravković, J.; Cvikić, D.; Mitić, N. Recurrent somatic embryogenesis and plant regeneration from immature zygotic embryos of cabbage (Brassica oleracea var. capitata) and cauliflower (Brassica oleracea var. botrytis). Plant Cell Tissue Organ Cult. 2013, 113, 397–406. [Google Scholar] [CrossRef]
- Jariteh, M.; Ebrahimzadeh, H.; Niknam, V.; Vahdati, K.; Amiri, R. Antioxidant enzymes activities during secondary somatic embryogenesis in Persian walnut (Juglans regia L.). Afr. J. Biotechnol. 2011, 10, 4093–4099. [Google Scholar]
- Bhansali, R.R.; Driver, J.A.; Durzan, D.J. Rapid multiplication of adventitious somatic embryos in peach and nectarine by secondary embryogenesis. Plant Cell Rep. 1990, 9, 280–284. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.I.; Nogueira, F.C.S.; Pinheiro, C.B.; Brasil, J.N.; Jereissati, E.S.; Jucá, T.L.; Soares, A.A.; Santos, M.F.; Domont, G.B.; Campos, F.A.P. Proteome analysis of secondary somatic embryogenesis in cassava (Manihot esculenta). Plant Sci. 2008, 175, 717–723. [Google Scholar] [CrossRef]
- Wongtiem, P.; Courtois, D.; Florin, B.; Juchaux, M.; Peltier, D.; Broun, P.; Ducos, J.P. Effects of cytokinins on secondary somatic embryogenesis of selected clone Rayong 9 of Manihot esculenta Crantz for ethanol production. Afr. J. Biotechnol. 2011, 10, 1600–1608. [Google Scholar]
- Dai, J.L.; Tan, X.; Zhan, Y.G.; Zhang, Y.Q.; Xiao, S.; Gao, Y.; Xu, D.W.; Wang, T.; Wang, X.C.; You, X.L. Rapid and repetitive plant regeneration of Aralia elata Seem. via somatic embryogenesis. Plant Cell Tissue Organ Cult. 2011, 104, 125–130. [Google Scholar] [CrossRef]
- Steinmacher, D.A.; Guerra, M.P.; Saare-Surminski, K.; Lieberei, R. A temporary immersion system improves in vitro regeneration of peach palm through secondary somatic embryogenesis. Ann. Bot. 2011, 108, 1463–1475. [Google Scholar] [CrossRef]
- Lardet, L.; Dessailly, F.; Carron, M.P.; Rio, M.A.; Ferrière, N.; Montoro, P. Secondary somatic embryogenesis in Hevea brasiliensis (Müll. Arg.): An alternative process for long-term somatic embryogenesis. J. Rubber Res. 2009, 12, 215–228. [Google Scholar]
- Chen, J.T.; Hong, P.I. Cellular origin and development of secondary somatic embryos in Oncidium leaf cultures. Biol. Plant. 2012, 56, 215–220. [Google Scholar] [CrossRef]
- Saeed, T.; Shahzad, A. High frequency plant regeneration in Indian Siris via cyclic somatic embryogenesis with biochemical, histological and SEM investigations. Ind. Crops Prod. 2015, 76, 623–637. [Google Scholar] [CrossRef]
- Ćalić, D.; Zdravković-Korać, S.; Radojević, L. Secondary embryogenesis in androgenic embryo cultures of Aesculus hippocastanum L. Biol. Plant. 2005, 49, 435–438. [Google Scholar] [CrossRef]
- Vooková, B.; Kormuťák, A. Comparison of induction frequency, maturation capacity and germination of Abies numidica during secondary somatic embryogenesis. Biol. Plant. 2006, 50, 785–788. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, G.; Shi, X.; Xing, W.; Ning, G.; Liu, J.; Bao, M. Primary and repetitive secondary somatic embryogenesis in Rosa hybrida ‘Samantha’. Plant Cell Tissue Organ Cult. 2012, 109, 411–418. [Google Scholar] [CrossRef]
- Garcia, C.; Furtado de Almeida, A.-A.; Costa, M.; Britto, D.; Valle, R.; Royaert, S.; Marelli, J.-P. Abnormalities in somatic embryogenesis caused by 2,4-D: An overview. Plant Cell Tissue Organ Cult. 2019, 137, 193–212. [Google Scholar] [CrossRef]
- Ji, W.; Luo, Y.; Guo, R.; Li, X.; Zhou, Q.; Ma, X.; Wang, Y. Abnormal Somatic Embryo Reduction and Recycling in Grapevine Regeneration. J. Plant Growth Regul. 2017, 36, 912–918. [Google Scholar] [CrossRef]
- Smitha, P.D.; Kr, B.; Nair, A.S. Enhanced Secondary Somatic Embryogenesis in Suspension Culture of Four Diploid Banana Cultivars from Kerala. Int. J. Fruit Sci. 2020, 20, S617–S626. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.; Bach, A. High-yielding repetitive somatic embryogenesis in cultures of narcissus L. ‘carlton’. Acta Sci. Pol. Hortorum Cultus 2017, 16, 107–112. [Google Scholar]
- Li, L.; Qu, R. In vitro somatic embryogenesis in turf-type Bermudagrass: Roles of abscisic acid and gibberellic acid, and occurrence of secondary somatic embryogenesis. Plant Breed. 2002, 121, 155–158. [Google Scholar] [CrossRef]
- Grewal, S.; Rani, M. Repetitive somatic embryogenesis in aggregated liquid culture of Bunium persicum Boiss. Indian J. Exp. Biol. 1999, 37, 70–74. [Google Scholar]
- You, C.R.; Fan, T.J.; Gong, X.Q.; Bian, F.H.; Liang, L.K.; Qu, F.N. A high-frequency cyclic secondary somatic embryogenesis system for Cyclamen persicum Mill. Plant Cell Tissue Organ Cult. 2011, 107, 233–242. [Google Scholar] [CrossRef]
- Cantelmo, L.; Soares, B.O.; Rocha, L.P.; Pettinelli, J.A.; Callado, C.H.; Mansur, E.; Castellar, A.; Gagliardi, R.F. Repetitive somatic embryogenesis from leaves of the medicinal plant Petiveria alliacea L. Plant Cell Tissue Organ Cult. 2013, 115, 385–393. [Google Scholar] [CrossRef]
- Martinelli, L.; Candioli, E.; Costa, D.; Poletti, V.; Rascio, N. Morphogenic competence of Vitis rupestris S. secondary somatic embryos with a long culture history. Plant Cell Rep. 2001, 20, 279–284. [Google Scholar] [CrossRef]
- Pandey, M.; Dhar, U.; Samant, S.S.; Shirgurkar, M.V.; Thengane, S.R. Recurrent somatic embryogenesis and plant regeneration in Angelica glauca Edgew., a critically endangered medicinal plant of the Western Himalaya. J. Hortic. Sci. Biotechnol. 2011, 86, 493–498. [Google Scholar] [CrossRef]
- Parrott, W.A.; Bailey, M.A. Characterization of recurrent somatic embryogenesis of alfalfa on auxin-free medium. Plant Cell Tissue Organ Cult. 1993, 32, 69–76. [Google Scholar] [CrossRef]
- Durham, R.E.; Parrott, W.A. Repetitive somatic embryogenesis from peanut cultures in liquid medium. Plant Cell Rep. 1992, 11, 122–125. [Google Scholar] [CrossRef]
- Gautier, F.; Eliášová, K.; Leplé, J.-C.; Vondráková, Z.; Lomenech, A.-M.; Le Metté, C.; Label, P.; Costa, G.; Trontin, J.-F.; Teyssier, C.; et al. Repetitive somatic embryogenesis induced cytological and proteomic changes in embryogenic lines of Pseudotsuga menziesii [Mirb.]. BMC Plant Biol. 2018, 18, 164. [Google Scholar] [CrossRef]
- Vijaya Kumar, J.; Ranjitha Kumari, B.D.; Castaño, E. Cyclic somatic embryogenesis and efficient plant regeneration from callus of safflower. Biol. Plant. 2008, 52, 429–436. [Google Scholar] [CrossRef]
- Raemakers, C.J.J.M.; Schavemaker, C.M.; Jacobsen, E.; Visser, R.G.E. Improvements of cyclic somatic embryogenesis of cassava (Manihot esculenta Crantz). Plant Cell Rep. 1993, 12, 226–229. [Google Scholar] [CrossRef]
- Baker, C.M.; Wetzstein, H.Y. Repetitive somatic embryogenesis in peanut cotyledon cultures by continual exposure to 2,4-d. Plant Cell Tissue Organ Cult. 1995, 40, 249–254. [Google Scholar] [CrossRef]
- Schavemaker, C.M.; Jacobsen, E. Development of a cyclic somatic embryogenesis regeneration system for leek (Allium ampeloprasum L.) using zygotic embryos. Plant Cell Rep. 1995, 14, 227–231. [Google Scholar] [CrossRef]
- Martínez, M.T.; Vieitez, A.M.; Corredoira, E. Improved secondary embryo production in Quercus alba and Q. rubra by activated charcoal, silver thiosulphate and sucrose: Influence of embryogenic explant used for subculture. Plant Cell Tissue Organ Cult. 2015, 121, 531–546. [Google Scholar] [CrossRef]
- Mazri, M.A.; Naciri, R.; Belkoura, I. Maturation and conversion of somatic embryos derived from seeds of olive (Olea europaea L.) cv. Dahbia: Occurrence of secondary embryogenesis and adventitious bud formation. Plants 2020, 9, 1489. [Google Scholar] [CrossRef] [PubMed]
- Parra, R.; Amo-Marco, J.B. Secondary somatic embryogenesis and plant regeneration in myrtle (Myrtus communis L.). Plant Cell Rep. 1998, 18, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Opabode, J.T.; Ajibola, O.V.; Oyelakin, O.O.; Akinyemiju, O.A. Somatic embryogenesis and genetic uniformity of regenerated cassava plants from low-temperature preserved secondary somatic cotyledons. Biotechnologia 2015, 96, 246–258. [Google Scholar] [CrossRef]
- Remotti, P.C. Primary and secondary embryogenesis from cell suspension cultures of Gladiolus. Plant Sci. 1995, 107, 205–214. [Google Scholar] [CrossRef]
- Opabode, J.T.; Ajibola, O.V.; Oyelakin, O.O.; Akinyemiju, O.A. Somatic embryogenesis and genetic uniformity of cassava plants regenerated from secondary somatic cotyledons preserved in osmotic agents. Plant Tissue Cult. Biotechnol. 2016, 26, 47–54. [Google Scholar] [CrossRef]
- Vasic, D.; Alibert, G.; Skoric, D. Protocols for efficient repetitive and secondary somatic embryogenesis in Helianthus maximiliani “Schrader”. Plant Cell Rep. 2001, 20, 121–125. [Google Scholar] [CrossRef]
- Fernández-Guijarro, B.; Celestino, C.; Toribio, M. Influence of external factors on secondary embryogenesis and germination in somatic embryos from leaves of Quercus suber. Plant Cell Tissue Organ Cult. 1995, 41, 99–106. [Google Scholar] [CrossRef]
- Abdollahi, M.R.; Ghazanfari, P.; Corral-Martínez, P.; Moieni, A.; Seguí-Simarro, J.M. Enhancing secondary embryogenesis in Brassica napus by selecting hypocotyl-derived embryos and using plant-derived smoke extract in culture medium. Plant Cell Tissue Organ Cult. 2012, 110, 307–315. [Google Scholar] [CrossRef]
- Agarwal, S.; Kanwar, K.; Sharma, D.R. Factors affecting secondary somatic embryogenesis and embryo maturation in Morus alba L. Sci. Hortic. 2004, 102, 359–368. [Google Scholar] [CrossRef]
- Stamp, J.A.; Henshaw, G.G. Secondary somatic embryogenesis and plant regeneration in cassava. Plant Cell Tissue Organ Cult. 1987, 10, 227–233. [Google Scholar] [CrossRef]
- Lelu-Walter, M.-A.; Gautier, F.; Eliášová, K.; Sanchez, L.; Teyssier, C.; Lomenech, A.-M.; Le Metté, C.; Hargreaves, C.; Trontin, J.-F.; Reeves, C. High gellan gum concentration and secondary somatic embryogenesis: Two key factors to improve somatic embryo development in Pseudotsuga menziesii [Mirb.]. Plant Cell Tissue Organ Cult. 2018, 132, 137–155. [Google Scholar] [CrossRef]
- Ben Ali, N.; Benkaddour, R.; Rahmouni, S.; Boussaoudi, I.; Hamdoun, O.; Hassoun, M.; Azaroual, L.; Badoc, A.; Martin, P.; Lamarti, A. Secondary somatic embryogenesis in Cork oak: Influence of plant growth regulators. For. Sci. Technol. 2023, 19, 78–88. [Google Scholar] [CrossRef]
- Ben Ali, N.; Benkaddour, R.; Rahmouni, S.; Hamdoun, O.; Boussaoudi, I.; Hassoun, M.; Azaroual, L.; Badoc, A.; Martin, P.; Lamarti, A. Influence of exogenous polyamines on the secondary somatic embryogenesis of cork oak (Quercus suber L.). Bioengineered 2023, 14, 2288354. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liang, Z.; Shan, C.; Marsolais, F.; Tian, L. Genetic transformation and full recovery of alfalfa plants via secondary somatic embryogenesis. Vitr. Cell. Dev. Biol. Plant 2013, 49, 17–23. [Google Scholar] [CrossRef]
- Ninković, S.; Miljuš-Djukić, J.; Nešković, M. Genetic transformation of alfalfa somatic embryos and their clonal propagation through repetitive somatic embryogenesis. Plant Cell Tissue Organ Cult. 1995, 42, 255–260. [Google Scholar] [CrossRef]
- Borissova, A.; Hvarleva, T.; Bedzhov, I.; Kondakova, V.; Atanassov, A.; Atanassov, I. Agrobacterium—Mediated transformation of secondary somatic embryos from Rosa hybrida L. and recovery of transgenic plants. Biotechnol. Biotechnol. Equip. 2005, 19, 70–74. [Google Scholar] [CrossRef]
- Pavlović, S.; Savić, J.; Milojević, J.; Vinterhalter, B.; Girek, Z.; Adžić, S.; Zečević, B.; Banjac, N. Introduction of the Nicotiana protein kinase (NPK1) gene by combining Agrobacterium-mediated transformation and recurrent somatic embryogenesis to enhance salt tolerance in cauliflower. Plant Cell Tissue Organ Cult. 2020, 143, 635–651. [Google Scholar] [CrossRef]
- Xing, W.; Bao, Y.; Luo, P.; Bao, M.; Ning, G. An efficient system to produce transgenic plants via cyclic leave-originated secondary somatic embryogenesis in Rosa rugosa. Acta Physiol. Plant. 2014, 36, 2013–2023. [Google Scholar] [CrossRef]
- Groll, J.; Mycock, D.J.; Gray, V.M.; Laminski, S. Secondary somatic embryogenesis of cassava on picloram supplemented media. Plant Cell Tissue Organ Cult. 2001, 65, 201–210. [Google Scholar] [CrossRef]
- Lai, F.M.; McKersie, B.D. Scale-up of somatic embryogenesis in alfalfa (Medicago sativa L.) I subculture and indirect secondary somatic embryogenisis. Plant Cell Tissue Organ Cult. 1994, 37, 151–158. [Google Scholar] [CrossRef]
- Ding, X.; Yu, L.; Chen, L.; Li, Y.; Zhang, J.; Sheng, H.; Ren, Z.; Li, Y.; Yu, X.; Jin, S.; et al. Recent Progress and Future Prospect of CRISPR/Cas-Derived Transcription Activation (CRISPRa) System in Plants. Cells 2022, 11, 3045. [Google Scholar] [CrossRef]
- Salvagnin, U.; Unkel, K.; Sprink, T.; Bundock, P.; Sevenier, R.; Bogdanović, M.; Todorović, S.; Cankar, K.; Hakkert, J.C.; Schijlen, E.; et al. A comparison of three different delivery methods for achieving CRISPR/Cas9 mediated genome editing in Cichorium intybus L. Front. Plant Sci. 2023, 14, 1111110. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogdanović, M.D.; Ćuković, K.B. Secondary Somatic Embryogenesis in Plants: From Cellular Mechanisms to Biotechnological Potential. Plants 2025, 14, 3413. https://doi.org/10.3390/plants14223413
Bogdanović MD, Ćuković KB. Secondary Somatic Embryogenesis in Plants: From Cellular Mechanisms to Biotechnological Potential. Plants. 2025; 14(22):3413. https://doi.org/10.3390/plants14223413
Chicago/Turabian StyleBogdanović, Milica D., and Katarina B. Ćuković. 2025. "Secondary Somatic Embryogenesis in Plants: From Cellular Mechanisms to Biotechnological Potential" Plants 14, no. 22: 3413. https://doi.org/10.3390/plants14223413
APA StyleBogdanović, M. D., & Ćuković, K. B. (2025). Secondary Somatic Embryogenesis in Plants: From Cellular Mechanisms to Biotechnological Potential. Plants, 14(22), 3413. https://doi.org/10.3390/plants14223413






