Plant Growth Regulators Use in the In Vitro Culture of Agave Species
Abstract
1. Introduction
2. Historical, Economic, and Biotechnological Relevance of the Agave Species
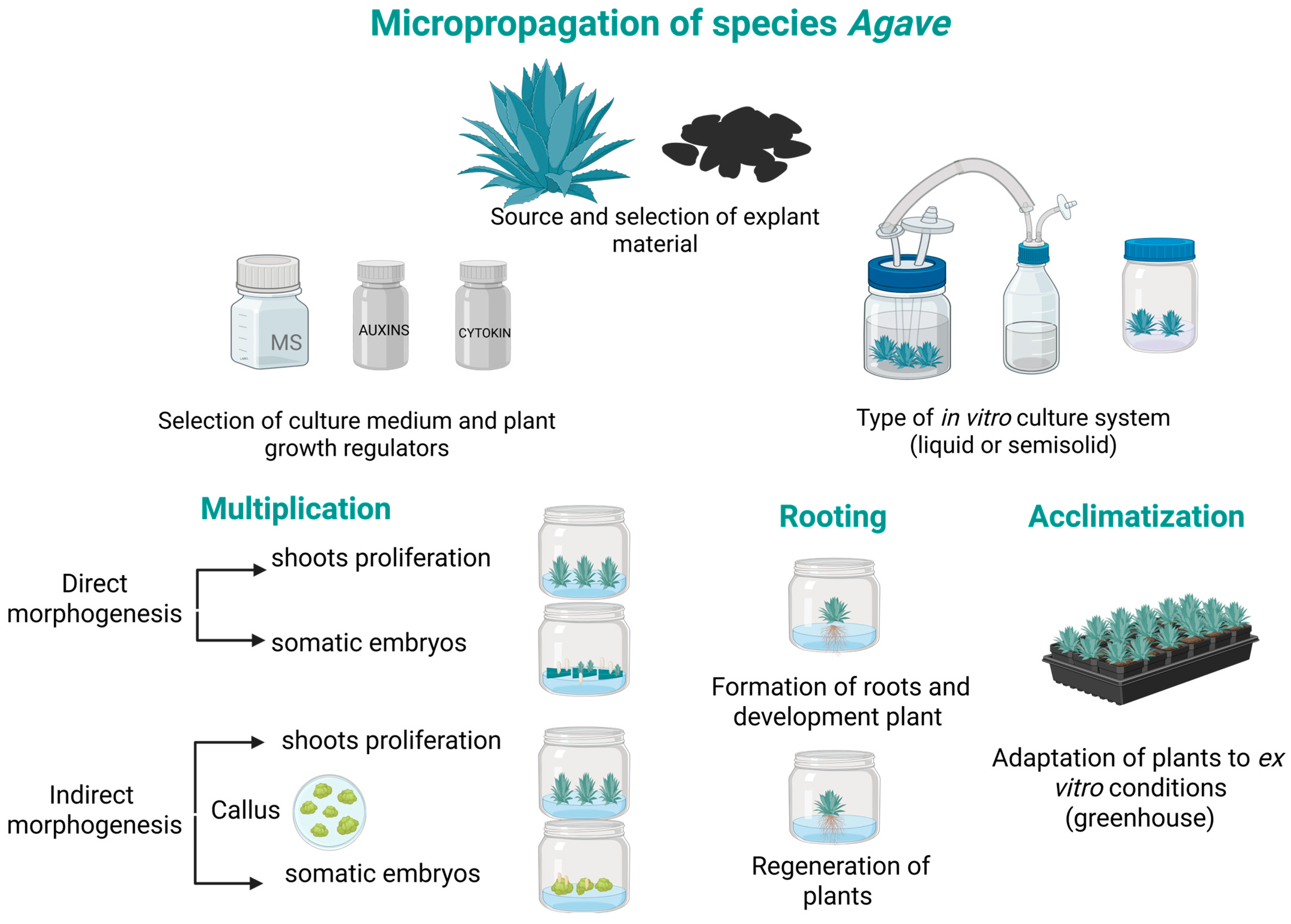
3. Plant Tissue Culture in Agave Species
3.1. Applications of PGRs in the In Vitro Culture of Agave Species
3.2. Comparative Overview of Agave In Vitro Protocols
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PGRs | Plant growth regulators |
| IAA | Indole acid acetic |
| IBA | Indole-3-Butyric acid |
| NAA | Naphthaleneacetic acid |
| 2,4-D | 2,4-Dichlorophenoxyacetic acid |
| BA | Benzyladenine |
| BAP | 6-Benzylaminopurine |
| KIN | Kinetin |
| TDZ | Thidiazuron |
| GA | Gibberellins |
| GA3 | Gibberellic acid |
| ABA | Abscisic acid |
| PBZ | Paclobutrazol |
| SA | Salicylic acid |
| JA | Jasmonic acid |
| MeJA | Methyl jasmonate |
| BRs | Brassinosteroids |
| NO | Nitric Oxide |
| SNP | Sodium nitroprusside |
| SLs | Strigolactones |
| SE | Somatic embryogenesis |
| MS | Murashige and Skoog medium |
| PEG | Polyethylene glycol |
| FOS | Fructooligosaccharides |
| ARR | Arabidopsis Response Factors |
| ARF | Auxin Response Factors |
| TIS | Temporary Immersion System |
| RITA® | Automated Temporary Immersion Bioreactor |
| SETIS® | Static Temporary Immersion Bioreactor |
References
- García-Mendoza, A.J. Los agaves de México. Ciencias 2007, 87, 14–23. [Google Scholar]
- Eguiarte, L.E.; Jiménez-Barrón, O.A.; Aguirre-Planter, E.; Scheinvar, E.; Gámez, N.; Gasca-Pineda, J.; Castellanos-Morales, G.; Moreno-Letelier, A.; Souza, V. Evolutionary ecology of Agave: Distribution patterns, phylogeny, and coevolution (an homage to Howard S. Gentry). Am. J. Bot. 2021, 108, 216–235. [Google Scholar] [CrossRef]
- Reyes-Samilpa, A.; Reyes-Agüero, J.A.; van‘t Hooft, A.; Álvarez-Fuentes, G.; Rössel Kipping, E.D. Fibers of Agave salmiana cultivar Xamni: Physical characterization and comparison between leaf maturity and growth environment. Emergent Mater. 2023, 6, 543–549. [Google Scholar] [CrossRef]
- Bautista-Justo, M.; García-Oropeza, L.; Barboza-Corona, J.E.; Parra-Negrete, L.A. El Agave tequilana Weber y la producción de tequila. Acta Univ. 2001, 11, 26–34. [Google Scholar] [CrossRef]
- Zizumbo-Villarreal, D.; Flores-Silva, A.; Colunga-García Marín, P. The archaic diet in Mesoamerica: Incentive for milpa development and species domestication. Econ. Bot. 2012, 66, 328–343. [Google Scholar] [CrossRef]
- Mancilla-Margalli, N.A.; López, M.G. Water-soluble carbohydrates and fructan structure patterns from Agave and Dasylirion species. J. Agric. Food Chem. 2006, 54, 7832–7839. [Google Scholar] [CrossRef] [PubMed]
- Mellado-Mojica, E.; López, M.G. Identification, classification, and discrimination of agave syrups from natural sweeteners by infrared spectroscopy and HPAEC-PAD. Food Chem. 2015, 167, 349–357. [Google Scholar] [CrossRef]
- Santiago-Martínez, A.; Pérez-Herrera, A.; Martínez-Gutiérrez, G.A.; Meneses, M.E. Contributions of agaves to human health and nutrition. Food Biosci. 2023, 53, 102753. [Google Scholar] [CrossRef]
- Raya, F.T.; de Carvalho, L.M.; José, J.; da Cruz, L.P.; Almeida, R.L.; Delevatti, H.A.A.; Silveira, N.M.; da Silva, S.F.; Pissolato, M.D.; de Oliveira, A.B.; et al. Artisanal refermentation of Agave cupreata bagasse with Saccharomyces cerevisiae MG5 as a valorization alternative. Agro Product. 2025. [Google Scholar] [CrossRef]
- Pérez-Zavala, M.L.; Hernández-Arzaba, J.C.; Bideshi, D.K.; Barboza-Corona, J.E. Agave: A natural renewable resource with multiple applications. J. Sci. Food Agric. 2020, 100, 5324–5333. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, T.A. History of plant tissue culture. Mol. Biotechnol. 2007, 37, 169–180. [Google Scholar] [CrossRef]
- Pasternak, T.P.; Steinmacher, D. Plant growth regulation in cell and tissue culture in vitro. Plants 2024, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Montes, E.; Hernández-Soriano, L.; Simpson, J. Advances in the micropropagation and genetic transformation of Agave species. Plants 2022, 11, 1757. [Google Scholar] [CrossRef]
- Abdalla, N.; El-Ramady, H.; Seliem, M.K.; El-Mahrouk, M.E.; Taha, N.; Bayoumi, Y.; Shalaby, T.A.; Dobránszki, J. An academic and technical overview on plant micropropagation challenges. Horticulturae 2022, 8, 677. [Google Scholar] [CrossRef]
- Hasnain, A.; Naqvi, S.; Ayesha, S.I.; Khalid, F.; Ellahi, M.; Iqbal, S.; Hassan, M.Z.; Abbas, A.; Adamski, R.; Markowska, D.; et al. Plants in vitro propagation with its applications in food, pharmaceuticals and cosmetic industries; current scenario and future approaches. Front. Plant Sci. 2022, 13, 1009395. [Google Scholar] [CrossRef]
- Wawrosch, C.; Zotchev, S.B. Production of bioactive plant secondary metabolites through in vitro technologies-status and outlook. Appl. Microbiol. Biotechno. 2021, 105, 6649–6668. [Google Scholar] [CrossRef]
- Domínguez Rosales, M.; González Jiménez, M.D.L.L.; Rosales Gómez, C.; Quiñones Valles, C.; Delgadillo Díaz de León, S.; Mireles Ordaz, S.J.; Pérez Molphe Balch, E. El cultivo in vitro como herramienta para el aprovechamiento, mejoramiento y conservación de especies del género agave. Investigación y Ciencia 2008, 16, 53–62. [Google Scholar]
- Monja-Mio, K.M.; Herrera-Alamillo, M.A.; Sánchez-Teyer, L.F.; Robert, M.L. Breeding strategies to improve production of agave (Agave spp.). In Advances in Plant Breeding Strategies: Industrial and Food Crops; Al-Khayri, J., Mohan Jain, S., Johnson, D., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 319–362. [Google Scholar]
- Lechuga-Campuzano, J.L.; Arzate-Fernández, A.M.; García-Núñez, H.G.; Mariezcurrena-Berasaín, M.D.; Reyes-Díaz, J.I. Viability and morphometry in agave seeds as an initial study for sustainable management and genetic preservation. Trop. Subtrop. Agroecosystems 2025, 28, 2. [Google Scholar] [CrossRef]
- Lopes, A.N.L.; dos Santos, E.A. Life cycle assessment in agave cultivation and processing: A review. J. Bioeng. Technol. Health 2025, 8, 229–234. [Google Scholar] [CrossRef]
- Ortiz-Hernández, Y.D.; Gutiérrez-Hernández, G.F.; Corzo-Ríos, L.J.; García-Ramírez, E.; Martínez-Tomás, S.H. Varietal and germinative characterization of Agave potatorum (Asparagaceae) seeds with different origins. Bot. Sci. 2018, 96, 628–639. [Google Scholar] [CrossRef]
- Sehgal, H.; Joshi, M. The journey and new breakthroughs of plant growth regulators in tissue culture. In Advances in Plant Tissue Culture; Rai, A.C., Kumar, A., Modi, A., Singh, M., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: San Diego, CA, USA, 2022; pp. 85–108. [Google Scholar] [CrossRef]
- Ochatt, S.J. Less frequently used growth regulators in plant tissue culture. In Plant Cell Culture Protocols, Methods in Molecular Biology; Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Humana Press: New York, NY, USA, 2024; Volume 2827, pp. 109–143. [Google Scholar] [CrossRef]
- Cooke, A.; Smith, D.; Booth, A. Beyond PICO: The SPIDER tool for qualitative evidence synthesis. Qual. Health Res. 2012, 22, 1435–1443. [Google Scholar] [CrossRef]
- Pardal-Refoyo, J.L.; Pardal-Peláez, B. Anotaciones para estructurar una revisión sistemática. Rev. ORL 2020, 11, 155–160. [Google Scholar] [CrossRef]
- APG IV. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG IV. Bot. J. Linn. Soc. 2016, 181, 1–20. [Google Scholar] [CrossRef]
- García-Mendoza, A.J.; Franco-Martínez, I.S. Los agaves, recursos fitogenéticos de los agroecosistemas tradicionales mexicanos de importancia para la agrobiodiversidad mundial. In Informe Final Proyecto CONABIO No. RG019/Proyecto Agrobiodiversidad Mexicana; Universidad Nacional Autónoma de México, Instituto de Biología: Ciudad de México, México, 2022. Available online: http://www.conabio.gob.mx/institucion/proyectos/resultados/InfRG019.pdf (accessed on 16 April 2025).
- Kablan, R.J.-F.; Sylvestre, M.; Onesippe-Potiron, C.; Bilba, K.; Kablan, A.L.C.; Arsène, M.-A.; Rousteau, A.; Cebrian-Torrejon, G. Agave species: A comprehensive review of taxonomy, chemistry, ethnobotany, and ethnopharmacology. In Studies in Natural Products Chemistry; Rahman, A.-U., Ed.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 187–225. [Google Scholar] [CrossRef]
- Davis, S.C.; Ortiz-Cano, H.G. Lessons from the history of Agave: Ecological and cultural context for valuation of CAM. Ann. Bot. 2023, 132, 819–833. [Google Scholar] [CrossRef]
- Lappe-Oliveras, P.; Arredondo-Fernández, R.; Valadez-Blanco, R.; Martínez-Monterrosa, Á.; Ojeda-Linares, C.I.; Moreno-Terrazas-Casildo, R.; Huerta-Beristain, G.; Astudillo-Melgar, F.; Flores-Montesinos, M.S.; Giles-Gómez, M.; et al. Mexican traditional alcoholic beverages: Production process, history, economy, social, and scientific importance. In Microbiology and Health Benefits of Traditional Alcoholic Beverages; Prakash, T.J., Ed.; Sikkim University: Gangtok, India, 2025; pp. 187–225. [Google Scholar]
- Pérez-Zavala, M.L.; Barboza-Pérez, U.E.; Barboza-Corona, J.E. Colecta y ensilaje de hojas de agave en dos comunidades de Guanajuato, México, como una alternativa de negocio. Cienc. Ergo-Sum 2024, 32. [Google Scholar] [CrossRef]
- Alducin-Martínez, C.; Ruiz-Mondragón, K.Y.; Jiménez-Barrón, O.; Aguirre-Planter, E.; Gasca-Pineda, J.; Eguiarte, L.E.; Medellín, R.A. Uses, knowledge and extinction risk faced by Agave species in Mexico. Plants 2022, 12, 124. [Google Scholar] [CrossRef]
- Álvarez-Ríos, G.D.; Figueredo-Urbina, C.J.; Casas, A. Management systems of agave for pulque production in Mexico. Etnobiología 2020, 18, 3–23. [Google Scholar]
- Trejo, L.; Soriano, D.; Romano-Grande, E.; Sánchez-Carmona, B.; Dávila-Navarro, D.E. Diversity of reproductive characters, seed set, and viability of Agave seeds used for pulque production and their wild relatives in Tlaxcala, Mexico. Genet. Resour. Crop Evol. 2024, 71, 2877–2903. [Google Scholar] [CrossRef]
- McAlvay, A.C. Agave spirits: The past, present, and future of mezcals. By Gary Paul Nabhan and David Suro Piñera. 2023. W.W. Norton and Company: New York, USA, 320 pp. Book Review. Ethnobiol. Lett. 2025, 16, 10–11. [Google Scholar] [CrossRef]
- Servicio de Información Agroalimentaria y Pesquera (SIAP). Cierre de la Producción Agrícola Por Estado, México; SIAP: Ciudad de México, México, 2022.
- Lozano-Arias, C.J. El tequila como fuente económica en México. Rev. Investig. Acad. Sin Front. 2024, 41. [Google Scholar] [CrossRef]
- Álvarez-Chávez, J.; Villamiel, M.; Santos-Zea, L.; Ramírez-Jiménez, A.K. Agave by-products: An overview of their nutraceutical value, current applications, and processing methods. Polysaccharides 2021, 2, 720–743. [Google Scholar] [CrossRef]
- Martínez-Herrera, R.E.; Rutiaga-Quiñones, O.M.; Alemán-Huerta, M.E. Integration of Agave plants into the polyhydroxybutyrate (PHB) production: A gift of the ancient Aztecs to the current bioworld. Ind. Crops Prod. 2021, 174, 114188. [Google Scholar] [CrossRef]
- Sarwar, M.B.; Ahmad, Z.; Rashid, B.; Hassan, S.; Gregersen, P.L.; Leyva, M.D.; Nagy, I.; Asp, T.; Husnain, T. De novo assembly of Agave sisalana transcriptome in response to drought stress provides insight into the tolerance mechanisms. Sci. Rep. 2019, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Vuelvas, O.F.; Chávez-Camacho, F.A.; Meza-Velázquez, J.A.; Mendez-Merino, E.; Ríos-Licea, M.M.; Contreras-Esquivel, J.C. A comparative FTIR study for supplemented agavin as functional food. Food Hydrocoll. 2020, 103, 105642. [Google Scholar] [CrossRef]
- Mellado-Mojica, E.; López, M.G. Fructan metabolism in Agave tequilana Weber Blue variety along its developmental cycle in the field. J. Agric. Food Chem. 2012, 60, 11704–11713. [Google Scholar] [CrossRef]
- Fernández-Lainez, C.; López-Velázquez, G.; de Vos, P. Health benefits of inulin and agavin-type fructans in food: Impact on microbiota, immune and gut barrier function. In The Book of Fructans; Van den Ende, W., Toksoy, O.E., Eds.; Elsevier: Cambridge, MA, USA, 2023; pp. 211–234. [Google Scholar] [CrossRef]
- Hernández-Vázquez, A.; Hernández, S.; Ortíz, I. Hydrothermal pretreatment of agave bagasse for biomethane production: Operating conditions and energy balance. Biomass Bioenergy 2020, 142, 105753. [Google Scholar] [CrossRef]
- Flores-Ríos, P.A.; Robles-Celerino, E.; Castañeda-Hidalgo, E. Generación y caracterización básica de bagazos de la agroindustria del mezcal en Oaxaca. Rev. Mex. Cienc. Agríc. 2020, 11, 1437–1445. [Google Scholar] [CrossRef]
- Avendaño-Rito, M.C.; Aquino-González, L.V.; Ríos, P.A.F.; Martínez-Vargas, A.; Sarubbi-Baltazar, F.A. Use of Agave angustifolia Haw. bagasse pulp as an additive powder for baking flour. Rev. Gestão Soc. Ambient. 2024, 18, e07057. [Google Scholar] [CrossRef]
- Phillips, A.; Schultz, C.J.; Burton, R.A. New crops on the block: Effective strategies to broaden our food, fibre, and fuel repertoire in the face of increasingly volatile agricultural systems. J. Exp. Bot. 2025, 76, 2043–2063. [Google Scholar] [CrossRef]
- Yan, X.; Corbin, K.R.; Burton, R.A.; Tan, D.K.Y. Agave: A promising feedstock for biofuels in the water-energy-food-environment (WEFE) nexus. J. Clean. Prod. 2020, 261, 121283. [Google Scholar] [CrossRef]
- de Paula, M.S.; Adarme, O.F.H.; Volpi, M.P.C.; Flores-Rodriguez, C.I.; Carazzolle, M.F.; Mockaitis, G.; Pereira, G.A.G. Unveiling the biogas potential of raw Agave leaf juice: Exploring a novel biomass source. Biomass Bioenergy 2025, 193, 107522. [Google Scholar] [CrossRef]
- Toqueiro, J.C.A.; Oliveira, O.B.; Adarme, O.F.H.; Mokaitis, G. Anaerobic digestion of Agave sisalana: Existing data, trends, and potential applications. J. Bioeng. Technol. Appl. Health 2025, 7, 380–385. [Google Scholar] [CrossRef]
- Coriolis. New Opportunities 2020: The Next Wave in New & Emerging Agricultural Industries in AUSTRALIA (Stage II); AgriFutures Australia: Wagga Wagga, NSW, Australia, 2020; Available online: https://www.agrifutures.com.au/wp-content/uploads/2021/05/21-052b.pdf (accessed on 18 June 2025).
- Top Shelf International. The New Australian Spirit: TSI’s Australian Agave Project. 2021. Available online: https://drive.google.com/file/d/1xbAXRQx6f7smgOzL4ltKSjri_NHJ9Oqh/view (accessed on 19 April 2025).
- Hoban, S. Agave: A Compact Business Case; GHD Pty Ltd., AgriFutures® Emerging Industries: Wagga Wagga, Australia, 2022; pp. 1–12. ISBN 978-1-76053-329-8. Available online: https://agrifutures.com.au/wp-content/uploads/2022/12/22-134.pdf (accessed on 20 May 2025).
- Abraham-Juárez, M.J.; Ramírez-Malagón, R.; Gil-Vega, K.C.; Simpson, J. AFLP analysis of genetic variability in three reproductive forms of Agave tequilana. Rev. Fitotec. Mex. 2009, 32, 171–175. [Google Scholar] [CrossRef]
- Chávez-Ortiz, L.; Morales-Domínguez, J.; Rodríguez-Sahagún, A.; Pérez-Molphe-Balch, E. In vitro propagation of Agave guiengola Gentry using semisolid medium and temporary immersion bioreactors. Phyton 2021, 90, 1003–1013. [Google Scholar] [CrossRef]
- Dévora-Rodríguez, D.G.; Pulido-Díaz, C.; Chávez-Simental, J.A.; Ortiz-Sánchez, I.A.; Loera-Gallegos, H.M.; Prieto-Ruiz, J.Á. Reguladores de crecimiento en el desarrollo vegetativo de vitroplantas de Agave durangensis Gentry. Ecosistemas 2021, 8, 1. [Google Scholar] [CrossRef]
- Vázquez-Martínez, O.; Gordon Núñez-Palenius, H.; Pérez-Molphe Balch, E.M.; Valencia-Posadas, M.; Pérez-Moreno, L.; Ruiz-Aguilar, G.M.L.; Gómez-Lim, M. In vitro-propagation of Agave tequilana Weber cv. azul in a temporary immersion system. Phyton 2022, 91, 83–96. [Google Scholar] [CrossRef]
- Twaij, B.M.; Jazar, Z.; Hasan, M.N. Trends in the use of tissue culture, applications and future aspects. Int. J. Plant Biol. 2020, 11, 8385. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. An introduction to plant cell, tissue, and organ culture: Current status and perspectives. In Plant Cell Culture Protocols; Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Methods in Molecular Biology; Humana: New York, NY, USA, 2024; Volume 2827. [Google Scholar] [CrossRef]
- Rodríguez-Garay, B.; Rodríguez-Domínguez, J.M. Micropropagation of Agave species. In Plant Cell Culture Protocols; Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1815. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Sheng, L.T.G.; Teixeira da Silva, J.A. Micropropagation in the Twenty-First Century. In Plant Cell Culture Protocols; Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1815. [Google Scholar] [CrossRef]
- Moreno-Hernández, M.R.; Mancilla-Álvarez, E.; Aguilar-Jiménez, D.; Bello-Bello, J.J. In Vitro Multiplication of Agave (A. marmorata and A. potatorum) by Temporary Immersion in SETIS™ Bioreactor. Methods Mol. Biol. 2024, 2759, 69–76. [Google Scholar] [CrossRef]
- Elhiti, M.; Stasolla, C. Transduction of signals during somatic embryogenesis. Plants 2022, 11, 178. [Google Scholar] [CrossRef]
- Bhatla, S.C.; Lal, M.A. Plant Growth Regulators: An Overview. In Plant Physiology, Development and Metabolism; Springer: Singapore, 2023. [Google Scholar] [CrossRef]
- Téllez Torres, A.G.; Jiménez Rodríguez, J.Á.; González Caballero, O.; Juárez Pérez, W.R.; Martínez Martínez, S.; Chávez Avila, V.M. Cultivo in vitro de Agave potatorum, especie amenazada endémica de México. Bot. Sci. 2023, 101, 883–894. [Google Scholar] [CrossRef]
- Mancilla-Álvarez, E.; Spinoso-Castillo, J.L.; Muñoz-Márquez Trujillo, R.A.; Palacios-Pardo, K.F.; Bello-Bello, J.J. Temporary immersion bioreactor as an efficient method for in vitro propagation of Agave marmorata. S. Afr. J. Bot. 2024, 169, 6–11. [Google Scholar] [CrossRef]
- Castillo-Martínez, C.R.; Velasco-Bautista, E.; Betazos-Jiménez, R.P.; Aragón-Cuevas, F. Mass propagation of tobala mezcal maguey (Agave potatorum Zucc.) in a temporary immersion system compared with a solid medium. Agro Product. 2023, 16, 2694. [Google Scholar] [CrossRef]
- Duarte-Aké, F.; De-la-Peña, C. High cytokinin concentration and nutrient starvation trigger DNA methylation changes in somaclonal variants of Agave angustifolia Haw. Ind. Crops Prod. 2021, 172, 114046. [Google Scholar] [CrossRef]
- Delgado-Aceves, L.; Portillo, L.; Folgado, R.; de Jesús Romo-Paz, F.; González-Arnao, M.T. New approaches for micropropagation and cryopreservation of Agave peacockii, an endangered species. Plant Cell Tissue Organ Cult. 2022, 150, 85–95. [Google Scholar] [CrossRef]
- Cancino-García, V.J.; Ramírez-Prado, J.H.; De-la-Peña, C. Auxin perception in Agave is dependent on the species’ Auxin Response Factors. Sci. Rep. 2020, 10, 3860. [Google Scholar] [CrossRef]
- Campos-Angeles, G.-V.; Alcara Vazques, S.; Enriquez del Valle, J.R.; Rodríguez-Ortiz, G.; Velasco Velasco, V.A. Acclimation of Agave potatorum Zucc. micropropagated plants. Agro Product. 2024, 17, 3. [Google Scholar] [CrossRef]
- Enríquez-Valle, J.R.; Chávez-Cruz, I.L.; Rodríguez-Ortiz, G.; Campos-Ángeles, G.V. Brotes enraizados de Agave nussaviorum García-Mend. variando sales inorgánicas, AIB e incubación. Rev. Mex. Cienc. Agríc. 2025, 15, e3205. [Google Scholar] [CrossRef]
- Hernández-Solis, M.; Arzate-Fernández, A.M.; Martínez-Martínez, S.Y.; Acosta-Villagrán, L. Effect of promoting compounds of indirect somatic embryogenesis in three Agave species. Agrociencia 2023, 56, 171–182. [Google Scholar] [CrossRef]
- Pourkhaloee, A.; Khosh-Khui, M.; Barba-Gonzalez, R. Somatic embryogenesis of tuberose (Agave amica L.) was improved by milk as a potential biostimulant in plant tissue culture. Int. J. Hortic. Sci. Technol. 2023, 10, 257–268. [Google Scholar] [CrossRef]
- Spinoso-Castillo, J.L.; Pérez-Sato, J.A.; Schettino-Salomón, S.S.; Bello-Bello, J.J. An alternative method for medium-term in vitro conservation of different plant species through gibberellin inhibitors. Vitr. Cell. Dev. Biol.-Plant 2022, 58, 606–614. [Google Scholar] [CrossRef]
- Arzate-Fernández, A.; Martínez Velasco, I.; Alvareza Aragón, C.; Martinez-Martinez, S.; Norman-Mondragon, T. Morphogenetic response of two Agave species regenerated in vitro. Trop. Subtrop. Agroecosyst. 2020, 23, 2. [Google Scholar] [CrossRef]
- Pradhan, N.; Singh, P.; Dwivedi, P.; Pandey, D.K. Evaluation of sodium nitroprusside and putrescine on polyethylene glycol induced drought stress in Stevia rebaudiana Bertoni under in vitro condition. Ind. Crops Prod. 2020, 154, 112754. [Google Scholar] [CrossRef]
- Romo-Paz, F.J.; Rodríguez-Garay, B. Effects of sodium nitroprusside (SNP) on in vitro shoot multiplication of Agave angustifolia Haw. In Proceedings of the Poster Presented at 3rd International Symposium on Agave, CIATEJ, Guadalajara, Mexico, 3–5 November 2016; Available online: https://ciatej.repositorioinstitucional.mx/jspui/bitstream/1023/357/1/Benjamin%208%20Poster.pdf (accessed on 20 May 2025).
- Suárez-González, E.M.; López, M.G.; Délano-Frier, J.P.; Gómez-Leyva, J.F. Expression of the 1-SST and 1-FFT genes and consequent fructan accumulation in Agave tequilana and A. inaequidens is differentially induced by diverse (a)biotic-stress related elicitors. J. Plant Physiol. 2014, 171, 359–372. [Google Scholar] [CrossRef]
- Martínez-Martínez, S.; Arzate-Fernández, A.; Hernández-Solis, M.; Acosta-Villagrán, L. Evaluation of the effect of exogenous putrescine during the maturation and germination in vitro of somatic embryos in two Agave genotypes. Trop. Subtrop. Agroecosyst. 2023, 26, 3. [Google Scholar] [CrossRef]
- Kıymaz, G.; Acemi, A. Effects of polyamines and optimized culture medium on in vitro organ development and accumulation of root phenolics in endangered Verbascum bugulifolium. Plant Cell Tissue Organ Cult. 2025, 162, 33. [Google Scholar] [CrossRef]
- Alam, N.; Ahmad, A.; Ahmad, N.; Anis, M. Polyamines mediated in vitro morphogenesis in cotyledonary node explants of Mucuna pruriens (L.) DC.: A natural source of L-dopa. J. Plant Growth Regul. 2023, 42, 5203–5215. [Google Scholar] [CrossRef]
- Ben Ali, N.; Benkaddour, R.; Rahmouni, S.; Hamdoun, O.; Boussaoudi, I.; Hassoun, M.; Azaroual, L.; Badoc, A.; Martin, P.; Lamarti, A. Influence of exogenous polyamines on the secondary somatic embryogenesis of cork oak (Quercus suber L.). Bioengineered 2023, 14, 2288354. [Google Scholar] [CrossRef]
- Azpeitia, A.; Chan, J.L.; Sáenz, L.; Oropeza, C. Effect of 22(S),23(S)-homobrassinolide on somatic embryogenesis in plumule explants of Cocos nucifera (L.) cultured in vitro. J. Hortic. Sci. Biotechnol. 2003, 78, 591–596. [Google Scholar] [CrossRef]
- Taha, H.S.; Nafie, E.M.; El-Bahr, M.K.; Mansur, R.M. Influence of 24-epibrassinolide on in vitro shootlet regeneration via direct organogenesis of Phaseolus vulgaris L. Afr. J. Biotechnol. 2014, 13, 28. [Google Scholar] [CrossRef]
- Elhiti, M.; Mira, M.M.; So, K.K.Y.; Stasolla, C.; Hebelstrup, K.H. Synthetic strigolactone GR24 improves Arabidopsis somatic embryogenesis through changes in auxin responses. Plants 2021, 10, 2720. [Google Scholar] [CrossRef]
- Singh, B. A review on the effects of jasmonates on plants grown under in vitro conditions. Afr. J. Biomed. Res. 2024, 27, 1723–1731. [Google Scholar] [CrossRef]
- Naziri, M.; Sadat, S.; Howyzeh, M.S. The effect of different hormone combinations on direct and indirect somatic embryogenesis in Agave americana. Iran. J. Plant Physiol. 2018, 9, 2. Available online: https://sanad.iau.ir/Journal/ijpp/Article/1025519 (accessed on 19 February 2025 ).
- Ríos-Ramírez, S.C.; Enríquez-del Valle, J.R.; Rodríguez-Ortiz, G.; Ruiz-Luna, J. Benzylaminopurine and indol-3-acetic acid concentrations in in vitro proliferation of Agave angustifolia adventitious shoots. Cien. Inv. Agr. 2017, 44, 285–294. [Google Scholar] [CrossRef]
- López Acevedo, L.; Merino Pérez, Y.E.; Enríquez del Valle, J.R.; Rodríguez Ortiz, G.; Lagunas Sánchez, Z.C. Organogénesis in vitro en tejidos de tallo de Agave marmorata y Agave angustifolia. Rev. Mex. Agroecosist. 2023, 5, 98–105. Available online: https://revistaremaeitvo.mx/index.php/remae/article/view/165 (accessed on 17 February 2025).
- Monja-Mio, K.M.; Olvera-Casanova, D.; Herrera-Alamillo, M.Á.; Sánchez-Teyer, F.L.; Robert, M.L. Comparison of conventional and temporary immersion systems on micropropagation (multiplication phase) of Agave angustifolia Haw. “Bacanora”. 3 Biotech 2021, 11, 77. [Google Scholar] [CrossRef]
- Acosta Villagran, L.; Arzate Fernandez, A.M.; García Núñez, H.G.; Martínez Martínez, S.Y.; Hernández Solís, M.; Reyes Díaz, J.I. Expresión embriogénica directa en Agave cupreata. Trop. Subtrop. Agroecosyst. 2024, 27, 3. [Google Scholar] [CrossRef]
- Moreno-Hernández, M.R.; López-Buenfil, J.A.; Serrano-Fuentes, M.K.; Contreras-Oliva, A.; Bello-Bello, J.J. Arbuscular mycorrhizal fungi improve the growth, nutrient uptake and survival of micropropagated agave (Agave marmorata Roezl) plantlets during acclimatization. J. Arid Environ. 2025, 228, 105330. [Google Scholar] [CrossRef]
- Aguilar Jiménez, D.; Rodríguez De la O, J.L. Micropropagación y aclimatación de Maguey Pitzometl (Agave marmorata Roezl) en la Mixteca Poblana. Rev. Colomb. Biotecnol. 2018, 20, 124–131. [Google Scholar] [CrossRef]
- Álvarez-Aragón, C.; Arzate-Fernández, A.M.; Martínez-Martínez, S.Y.; Martínez-Velasco, I. Regeneración de plantas de Agave marmorata Roezl vía embriogénesis somática. Trop. Subtrop. Agroecosyst. 2020, 23, 36. [Google Scholar] [CrossRef]
- Santacruz-Ruvalcaba, F.; Castañeda-Nava, J.J.; Villanueva-Gónzalez, J.P.; García-Sahagún, M.L.; Portillo, L.; Contreras-Pacheco, M.L. Micropropagación de Agave maximiliana Baker por proliferación de yemas axilares. Polibotánica 2022, 54, 139–151. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Cárcamo-Corona, R.G.; Aguilar-Jiménez, D.; Bello-Bello, J.J. Micropropagation of Agave (Agave potatorum Zucc.) through direct organogenesis. Agrociencia 2022, 56, 6. [Google Scholar] [CrossRef]
- Bautista-Castellanos, A.I.; Enríquez-del Valle, J.R.; Velasco-Velasco, V.A.; Rodríguez-Ortiz, G. Enraizado de brotes in vitro y aclimatación de plantas de Agave potatorum Zucc. Ecosist. Recur. Agropec. 2020, 7, e2618. [Google Scholar]
- Miranda, E.; Portillo, L. Regeneración de Agave spp. con harina de Opuntia como elicitor. Bol. Nakari 2019, 30, 23–32. [Google Scholar]
- Angeles-Vázquez, B.V.; Alvarez-Cervantes, J.; Tovar-Jiménez, X.; Rodríguez-Garay, B. Plant regeneration from indirect somatic embryogenesis of Agave salmiana Otto ex Salm-Dyck subsp. salmiana using zygotic embryo obtained by in-casa pollination as explants. Polibotánica 2023, 56, 171–182. [Google Scholar] [CrossRef]
- Canales-Mendoza, A.I.; Villanueva-Ibáñez, M.; Tovar-Jiménez, X.; Álvarez-Cervantes, J. Effect of the addition of fungal extracellular biogenic zinc oxide nanoparticles on the in vitro multiplication of Agave salmiana shoots. Plant Cell Tissue Organ Cult. 2023, 155, 479–491. [Google Scholar] [CrossRef]
- Seliem, M.K.; Abdalla, N.; El-Mahrouk, M.E. Cytokinin potentials on in vitro shoot proliferation and subsequent rooting of Agave sisalana Perr. Horticulturae 2025, 11, 929. [Google Scholar] [CrossRef]
- Delgado-Aceves, L.; González-Arnao, M.T.; Santacruz-Ruvalcaba, F.; Folgado, R.; Portillo, L. Indirect somatic embryogenesis and cryopreservation of Agave tequilana Weber cultivar ‘Chato’. Plants 2021, 10, 249. [Google Scholar] [CrossRef]
- Reyes-Silva, A.I.; Nuñez-Palenius, H.G.; Ocampo, G.; Pérez-Molphe-Balch, E. Regeneración in vitro de Agave wocomahi Gentry (Asparagaceae). Rev. Fitotec. Mex. 2022, 45, 493–501. [Google Scholar] [CrossRef]
| Species | Plant Tissue Culture | PGRs | Concentration (µM) | Culture Conditions | Effect | Reference |
|---|---|---|---|---|---|---|
| A. americana L. | Shoot induction | 2,4-D BA | 0.09 44.0 | Semisolid MS medium reduced in KNO3 and NH4NO3 | Although shoot formation occurred on the explant surface, the protocol appeared inadequate for supporting further growth and multiplication. | [88] |
| Multiplication | BA NAA | 13.2 0.54 | Semisolid MS medium with vitamins L2 | A significantly higher multiplication rate was achieved from a single explant. | ||
| Direct somatic embryogenesis | PIC | 10.25 | Semisolid MS medium with vitamins L2 | Somatic embryo formation was induced in 66.7% of the explants. | ||
| Indirect somatic embryogenesis | Dicamba | 6.75 | Semisolid MS medium with vitamins L2 | The observed embryogenic callus was friable, consisting of small, creamy, globular, and elongated cells that subsequently developed into somatic embryos. | ||
| A. amica L. | Callus induction | 2,4-D NAA | 2.25 2.7 | Semisolid MS medium | The treatment led to a 100% callus-induction rate, the highest observed. | [74] |
| Callus multiplication | BAP 2,4-D | 4.4 4.5 | Semisolid MS medium | The callus diameter doubled after two subcultures and increased fivefold by approximately 120 days. | ||
| Initiation of embryogenic callus | 2,4-D | 4.5 | Semisolid MS medium | The treatment resulted in the formation of nodular, friable, and semi-compact embryogenic callus after 90 days. | ||
| Development of somatic embryos | ABA | 3.8 | Semisolid MS medium 45 g L−1 maltose | Globular embryoids with proper formation initiated greening and root–shoot axis differentiation. | ||
| Rooting and bulb formation | IBA | 3.67 | Semisolid MS medium | Bulb formation occurred at the shoot base, culminating in the development of roots. | ||
| A. angustifolia Haw. | Organogenesis and assessment of somaclonal variation | BA | 88.8 | High BA concentration in culture media (5 months) | Enhanced organogenic capability, increased phenotypic variation, and changes in DNA methylation levels. | [68] |
| Indirect organogenesis and evaluation of explant origin (mother plant) | BAP IAA | 17.7 5.7 | Semisolid MS medium supplemented with coconut water Explants derived from mother plants subjected to fertigation treatments | The combination of growth regulators correlated with the nutritional status of the mother plant under 100% fertigation, resulting in up to 32.7 shoots per explant in vitro culture. | [89] | |
| Callus induction from embryonic axes | 2,4-D BAP | 23.0 13.0 | Semisolid MS medium with vitamins L2 | Callus with an average weight of 0.2 g, suitable for somatic embryo maturation. | [80] | |
| Somatic embryo maturation | Put | 1700 | Semisolid MS medium (60 days of incubation) | Exogenous putrescine increased the number of somatic embryos to 21.2 per explant. | ||
| Somatic embryo maturation | ABA | 34.2 | Semisolid MS medium (50%) | ABA-induced stress promoted the maturation of somatic embryos, enabling them to regenerate plantlets. | [73] | |
| Callus induction from embryonic axes | 2,4-D BA | 22.6 13.3 | Semisolid MS medium with L2 vitamins | Dedifferentiation into callus with embryogenic features, friable and beige. | ||
| Organogenesis stem tissue | BA IAA | 4.44 1.71 | Semisolid MS medium | In 4 explants, organogenesis response: adventitious buds formed. | [90] | |
| A. angustifolia Haw. “Bacanora” | Inoculum evaluation and multiplication | BA 2,4-D | 44.4 0.10 | Modified MS medium, Temporary Immersion System Frequency: 1 min/6 h | Growth regulators and inoculum density of 20 explants promoted the highest shoot formation (3.05 vigorous shoots) in TIS. | [91] |
| A. cupreata Trel. & A. Berger | Induction of embryogenic callus | 2,4-D BA | 23.0 13.0 | Semisolid MS medium with vitamins L2 | The percentage of embryogenic calluses was 66.67 ± 0.48%. | [80] |
| Maturation of somatic embryos | Put | 1700 | Semisolid MS medium | The mean number of somatic embryos per explant was 38.80 ± 9.88. | ||
| Induction of embryogenic callus | 2,4-D BA | 22.5 13.2 | Semisolid MS medium (75%) | Both non-embryogenic calluses (compact, whitish, and smooth) and embryogenic calluses (friable, beige) were produced, with embryogenic callus accounting for 33.30 ± 14.64%. | [73] | |
| Maturation of somatic embryos | ABA | 34.2 | Semisolid MS medium (50%) | Somatic embryo formation was observed at 90 days, with an average of 4.80 ± 3.62. | ||
| Induction of pro-embryogenic calluses | 2,4-D | 4.05 | Semisolid MS medium (25%) with vitamins L2 | Efficient formation of proembryogenic masses by 90%. | [92] | |
| Expression of direct somatic embryos | IAA | 2.9 | Semisolid MS medium (50%) | The concentration promoted the efficient formation of 7 direct somatic embryos per explant. | ||
| A. duranguensis Gentry | Multiplication | BA | 17.4 | Semisolid MS medium | A higher average number of shoots, 1.40 per explant, and a higher number of leaves per shoot, 2.70, were obtained. | [56] |
| A. guenguiola Gentry | Multiplication | BA | 8.8 | Semisolid MS medium | An average of 3.70 shoots per explant was obtained and shoot clusters were successfully generated. | [55] |
| Multiplication | BA | 4.4 | MS medium, Temporary immersion system | A propagation rate of 43 shoots per shoot cluster was achieved. | ||
| A. marmorata Roelz | In vitro establishment | BAP | 13.2 | Semisolid MS medium with ascorbic acid and cysteine | After 30 days of cultivation, the tips were transferred to the multiplication phase. | [66] |
| Shoot Multiplication | BAP IAA | 13.2 17.2 | Temporary immersion system (2 min / 8 h) | For the variable number of shoots per explant, the highest multiplication rate was observed in temporary immersion, with 19.60 shoots and an average size of 1.74 cm, and the lowest percentage of hyperhydricity (3.33%). | ||
| Multiplication | BAP IAA | 13.2 11.4 | Semisolid and liquid MS medium in the SETIS™ Frequency of 2 min/8 h for 45 to 60 days | Efficient multiplication of shoot clusters was obtained. | [62] | |
| Acclimatation | BAP IAA | 13.2 5.7 | Semisolid MS medium | Efficient shoot multiplication was obtained. Plants were obtained for mycorrhizal fungi treatments. | [93] | |
| Direct organogenesis | BAP IAA | 44.4 57.0 | Semisolid MS medium | IAA increased shoot and root length; BA + IAA promoted shoot proliferation, yielding up to 41 shoots per explant and 100% survival rate during acclimatization. | [94] | |
| Induction of callus | BA 2,4-D | 13.3 22.6 | Semisolid MS medium (25%) with vitamins L2 | Formation of callus masses that had a diameter of 10 and 20 mm with a weight of 0.5 g. | [95] | |
| Maturation of somatic embryos | 2,4-D BA (Pretreatment) | 0.45 44.4 | Semisolid MS medium (50%) | 19.4 somatic embryos were obtained per explant. | ||
| Maturation of somatic embryos | GA3 BA | 8.6 44.4 | Semisolid MS medium (50%) | Pretreatment with 44.40µM of BA formed 15.2 somatic embryos. | ||
| Callus induction | BA 2,4-D | 0.44 0.45 | Semisolid MS medium (25%) with vitamins L2 and MS | Obtaining compact and yellowish calluses, it was observed that the higher the concentration of auxin, the greater the weight of the callus. | [76] | |
| Shoot induction via indirect organogenesis. | BA | 22.2 | Semisolid MS medium (25%) with activated carbon | The highest number of shoots regenerated was 24.7 per explant. | ||
| Shoot induction via direct organogénesis | BA | 22.2 | Semisolid MS medium (25%) with activated carbon | 22.3 shoots were obtained per explant from the meristematic zone explant. | ||
| Organogenesis stem tissue | BA IAA | 4.4 1.7 | Semisolid MS medium | Organogenesis response, in 8 explants, the formation of adventitious buds occurred. | ||
| A. maximiliana Baker | Axillary multiplication | BA 2,4-D | 8.8 0.09 | Semisolid MS medium | A total of 26.93 new shoots were produced, exhibiting typical morphological quality. | [96] |
| A. nussaviorum García-Mendoza | Rooting | IBA | 2.5 | Semisolid MS medium (60%) | The highest percentage of shoots with roots was 83%. | [72] |
| A. peacockii Croucher | Multiplication | BA KIN | 26.6 27.8 | Semisolid MS medium | The combination significantly favored the morphogenetic response and produced the highest shoot generation with 87 shoots on average. | [62] |
| A. potatorum Zucc. | Rooting | IBA | 29.2 | Semisolid MS medium reduced in NH4NO3 | A significantly higher number (8.60 ± 1.01) of formed roots was observed compared with the control treatments. | [75] |
| Multiplication | BA | 8.8 | Semisolid MS medium | The significant treatment produced 6.60 shoots on average, with an average length of 4.53 cm. | [67] | |
| Multiplication | BA | 8.8 | Medium MS in System temporary immersion RITA® | An average of 14.4 shoots was obtained with an average of 2.3 cm. | ||
| Direct organogenesis | BAP IAA | 13.3 17.2 | Semisolid MS medium | Regeneration of adventitious shoots 9.73 shoots per explant. | [97] | |
| Rooting | IAA | 17.1 | Semisolid MS medium | Improved shoot development was achieved, with an average length of 5.77 cm. | ||
| Rooting | IBA | 2.85 | Semisolid MS medium (75%) | 96% of shoots developed roots and stems (6.4 mm diameter). | [98] | |
| Rooting Acclimatization | IBA | 2.85 or 5.70 | Semisolid MS medium | The addition of IBA to the culture medium enhanced plant growth, resulting in wider leaves, greater stem diameter, higher dry biomass, and overall larger plant size compared with shoots cultured without IBA. | [71] | |
| A. potatorum var. “Tóbala” | Direct Organogenesis | BAP 2,4-D | 6.6 2.2 | Semisolid MS medium (75%) Citric/ascorbic acid | 12.5 shoots/stem and leaf explant. More than 70% of the plants survived in the greenhouse after two months of cultivation. | [65] |
| Indirect Organogenesis | BAP 2,4-D | 8.8 2.2 | Semisolid MS medium (50%) Citric/ascorbic acid | Stem explants yielded up to 81 shoots, demonstrating high propagation efficiency. | ||
| A. rzedowskiana Gentry | Regeneration by somatic embryogenesis | 2,4-D BA | 9.0 2.6 | Reduced semisolid MS medium in NH4NO3 Nopal flour 2 g/L−1 | Direct formation of embryogenic structures at the early scutellar, initial multicellular, and coleoptilar stages. | [99] |
| A. salmiana ex Salm-Dyck | Expression and maturation of somatic embryos | ABA | 34.2 | Semisolid MS medium (50%) | Mean embryos per explant: 15.4 ± 3.62. | [73] |
| Induction of embryogenic callus | 2,4-D BA | 0.45 0.44 | Semisolid MS medium (25%) | It promoted callus formation, resulting in mucilaginous callus. | [75] | |
| Shoot induction via indirect organogenesis | BA | 44.0 | Semisolid MS medium (25%) with activated carbon | The highest number of shoots obtained was 23.80 per explant. | ||
| A. salmiana Otto ex Salm-Dyck subsp. salmiana | Induction of embryogenic callus | 2,4-D BAP | 9.0 1.3 | Semisolid MS medium with vitamins L2 | Statistically significant treatment in quantifying the number of calluses expressing somatic embryogenesis. | [100] |
| Expression of somatic embryos | BAP 2,4-D | 0.40 4.50 | Semisolid MS medium | A maximum of 42.41 ± 5.85 somatic embryos were generated. | ||
| A. salmiana Otto ex Salm-Dyck var. “ayoteco” | Axillary multiplication | 2,4-D BAP | 0.078 57.46 | Semisolid MS medium 2250 mg/L zinc nanoparticles | Differentiated shoot development, including leaf formation, was observed at 60 days. | [101] |
| A. sisalana Perr. | Axillary shoot proliferation | TDZ | 4.5 | Semisolid MS medium | The optimum significant shoot proliferation (14.67 shoots/explant). | [102] |
| A. tequilana Weber | Regeneration by somatic embryogenesis | PIC | 2.1 | Reduced semisolid MS medium in NH4NO3 Nopal flour 2 g/L | The treatment was effective in generating good-quality calluses and proembryogenic structures at all stages in leaf explants. | [99] |
| A. tequilana Weber cv. “chato” | Indirect somatic embryogenesis | PIC BAP | 49.6 3.32 | Semisolid MS medium | The highest average number of somatic embryos was produced (52.43 ± 5.74) | [103] |
| A. tequila Weber var. “azul” | Axillary multiplication Segmented stem explants | BA KIN | 13.2 18.8 | Semisolid MS medium | BA and KIN increased shoot number per explant, up to 18 (BA) and 26 (KIN); sagittal segmentation also increased axillary budding. | [57] |
| Axillary multiplication Segmented stem explants | BA IAA | 13.2 5.7 | MS liquid medium System temporary immersion RITA® Frequency of 5 min/4 h | The highest IAA concentration resulted in 20 shoots per explant. | ||
| A. wocomahi Gentry | Multiplication | BA | 4.4 | Semisolid MS medium | Resulted in the generation of 11.70 ± 4.8 shoots per explant. | [104] |
| Callus tissue induction | PIC BA | 6.1 17.6 | Semisolid MS medium | A greater callus induction rate (99.16%) was achieved in stem explants, with nodular callus tissue prevailing. | ||
| Somatic embryo induction | BA | 13.2 | Semisolid MS medium | A greater number of somatic embryolike structures were obtained. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Mendoza, E.A.; Pérez-Molphe-Balch, E.; Guzmán-Mendoza, R.; Ruiz-Aguilar, G.; García-Munguía, A.M.; Costilla-Salazar, R.; Núñez-Palenius, H.G. Plant Growth Regulators Use in the In Vitro Culture of Agave Species. Plants 2025, 14, 3402. https://doi.org/10.3390/plants14213402
Sánchez-Mendoza EA, Pérez-Molphe-Balch E, Guzmán-Mendoza R, Ruiz-Aguilar G, García-Munguía AM, Costilla-Salazar R, Núñez-Palenius HG. Plant Growth Regulators Use in the In Vitro Culture of Agave Species. Plants. 2025; 14(21):3402. https://doi.org/10.3390/plants14213402
Chicago/Turabian StyleSánchez-Mendoza, Estefany Alejandra, Eugenio Pérez-Molphe-Balch, Rafael Guzmán-Mendoza, Graciela Ruiz-Aguilar, Alberto M. García-Munguía, Rogelio Costilla-Salazar, and Héctor Gordon Núñez-Palenius. 2025. "Plant Growth Regulators Use in the In Vitro Culture of Agave Species" Plants 14, no. 21: 3402. https://doi.org/10.3390/plants14213402
APA StyleSánchez-Mendoza, E. A., Pérez-Molphe-Balch, E., Guzmán-Mendoza, R., Ruiz-Aguilar, G., García-Munguía, A. M., Costilla-Salazar, R., & Núñez-Palenius, H. G. (2025). Plant Growth Regulators Use in the In Vitro Culture of Agave Species. Plants, 14(21), 3402. https://doi.org/10.3390/plants14213402







