Combining in vitro and Field Studies to Predict Drought Tolerance in Vicia sativa L. Genotypes
Abstract
1. Introduction
2. Results
2.1. Genotypic Variation in Root and Shoot Biomass Under Osmotic Stress
2.2. Seed Yield Decreased Under Drought, with Strong Genotypic Variation
2.3. Significant Genotype and Environmental Effects on the Analysed Traits
2.4. Correlations Between in vitro and Field Traits
2.5. PCA Grouped Vetch Genotypes by Contrasting Biomass Production and Drought Tolerance
3. Discussion
3.1. In vitro Studies
3.2. Field Results
3.3. Proline Analysis
3.4. Principal Component Analysis
4. Materials and Methods
4.1. Plant Material
4.2. In vitro Experiments
4.2.1. Culture Medium
4.2.2. Roots and Shoots Characterisation
4.2.3. Proline Determination
4.3. Vetch Seed Production in Field Experiments
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bongaarts, J. United Nations Department of Economic and Social Affairs, Population Division World Family Planning 2020: Highlights, United Nations Publications, 2020. Popul. Dev. Rev. 2020, 46, 857–858. [Google Scholar] [CrossRef]
- Arsenović, D. Population of the World. In Demographic Challenges in Central Europe: Legal and Family Policy Response; Barzó, T., Ed.; Central European Academic Publishing: Miskolc-Budapest, Hungary, 2024; pp. 23–46. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Al-Amri, S.M.; El-Enany, A.E. Enhancing Rhizobium–Legume Symbiosis and Reducing Nitrogen Fertilizer Use Are Potential Options for Mitigating Climate Change. Agriculture 2023, 13, 2092. [Google Scholar] [CrossRef]
- Nirmal, N.; Anyimadu, C.F.; Khanashyam, A.C.; Bekhit, A.E.A.; Dhar, B.K. Alternative Protein Sources: Addressing Global Food Security and Environmental Sustainability. Sustain. Dev. 2024, 33, 3958–3969. [Google Scholar] [CrossRef]
- Khatun, M.; Sarkar, S.; Era, F.M.; Islam, A.K.M.M.; Anwar, M.P.; Fahad, S.; Datta, R.; Islam, A.K.M.A. Drought Stress in Grain Legumes: Effects, Tolerance Mechanisms and Management. Agronomy 2021, 11, 2374. [Google Scholar] [CrossRef]
- Rajput, A.; Ali Panhwar, Q.; Babar, H. Role of Leguminous Crops by Enhancing Soil Fertility and Plant Nutrition. In Legume Crops for Food Security—Cultivation and Benefits; Jimenez-Lopez, J.C., Escudero-Feliu, J., Eds.; IntechOpen: London, UK, 2024. [Google Scholar] [CrossRef]
- Mafakheri, A.; Siosemardeh, A.; Bahramnejad, B.; Struik, P.C.; Sohrabi, Y. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop Production under Drought and Heat Stress: Plant Responses and Management Options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef]
- Kopecká, R.; Kameniarová, M.; Černý, M.; Brzobohatý, B.; Novák, J. Abiotic Stress in Crop Production. Int. J. Mol. Sci. 2023, 24, 6603. [Google Scholar] [CrossRef]
- Yadollahi, P.; Eshghizadeh, H.R.; Razmjoo, J.; Zahedi, M.; Majidi, M.M.; Gheysari, M. Drought stress tolerance in vetch plants (Vicia sp.): Agronomic evidence and physiological signatures. J. Agric. Sci. 2024, 163, 13–26. [Google Scholar] [CrossRef]
- Renzetti, M.; Funck, D.; Trovato, M. Proline and ROS: A Unified Mechanism in Plant Development and Stress Response? Plants 2024, 14, 2. [Google Scholar] [CrossRef]
- Kaur, D.; Grewal, S.K.; Kaur, J.; Singh, S. Differential proline metabolism in vegetative and reproductive tissues determine drought tolerance in chickpea. Biol. Plant. 2017, 61, 359–366. [Google Scholar] [CrossRef]
- Arteaga, S.; Yabor, L.; Díez, M.J.; Prohens, J.; Boscaiu, M.; Vicente, O. The Use of Proline in Screening for Tolerance to Drought and Salinity in Common Bean (Phaseolus vulgaris L.) Genotypes. Agronomy 2020, 10, 817. [Google Scholar] [CrossRef]
- Tahir, N.A.; Rasul, K.S.; Lateef, D.D.; Aziz, R.R.; Ahmed, J.O. in vitro Evaluation of Iraqi Kurdistan Tomato Accessions Under Drought Stress Conditions Using Polyethylene Glycol-6000. Life 2024, 14, 1502. [Google Scholar] [CrossRef]
- Hirapara, K.M.; Gajera, H.P.; Savaliya, D.D.; Hirpara, D.G. Biochemical and Physiological Changes Influenced by Drought Stress in Chickpea (Cicer arietinum L.). Indian J. Agric. Biochem. 2022, 35, 79–86. [Google Scholar] [CrossRef]
- Eesha, A.; Sharma, R.; Chaudhary, N.S. Effect of PEG-6000 Osmoticum on Seed Germination and Seedling Growth of Lentil (Lens culinaris Medik.) Genotypes. Indian J. Agric. Res. 2024, 58, 456–461. [Google Scholar] [CrossRef]
- Sari, D. Effects of PEG-Induced Drought Stress and Different Boron Levels on Seed Germination and Seedling Growth Characteristics in Chickpea (Cicer arietinum L.) and Lentil (Lens culinaris Medik.). Turk. J. Agric. Res. 2023, 10, 154–161. [Google Scholar] [CrossRef]
- Abderemane, B.A.; Houasli, C.; Mitache, M.; Idrissi, O.; Fakiri, M. Physiological, agro-morphological, and germination responses of a worldwide chickpea (Cicer arietinum) collection subjected to drought stress by applying polyethylene glycol (PEG) on germinating seeds and by exposure plants to water restriction at the vegetative stage. Biocatal. Agric. Biotechnol. 2024, 56, 103011. [Google Scholar] [CrossRef]
- Beyaz, R. Morphological and biochemical changes in shoot and root organs of common vetch (Vicia sativa L.) after exposure to drought stress. Sci. Asia 2022, 48, 51. [Google Scholar] [CrossRef]
- Bukan, M.; Kereša, S.; Pejić, I.; Sudarić, A.; Lovrić, A.; Šarčević, H. Variability of Root and Shoot Traits under PEG-Induced Drought Stress at an Early Vegetative Growth Stage of Soybean. Agronomy 2024, 14, 1188. [Google Scholar] [CrossRef]
- Cabeza, A.; Casas, A.M.; Larruy, B.; Costar, M.A.; Martínez, V.; Contreras-Moreira, B.; Igartua, E. Genetic control of root/shoot biomass partitioning in barley seedlings. Front. Plant Sci. 2024, 15, 1408043. [Google Scholar] [CrossRef]
- Nivethitha, T.; Babu, C.; Jayamani, P.; Vijayalakshmi, D. Impact of drought stress on seedling vigor and physio-biochemical attributes of diverse mungbean genotypes for drought tolerance through in vitro and field evaluation. Euphytica 2024, 220, 182. [Google Scholar] [CrossRef]
- Afonso, P.; Castro, I.; Couto, P.; Leal, F.; Carnide, V.; Rosa, E.; Carvalho, M. Root Phenotyping: A Contribution to Understanding Drought Stress Resilience in Grain Legumes. Agronomy 2025, 15, 798. [Google Scholar] [CrossRef]
- Leht, M. Phylogenetics of Vicia (Fabaceae) based on morphological data. Feddes Repert. 2009, 120, 379–393. [Google Scholar] [CrossRef]
- Nguyen, V.; Riley, S.; Nagel, S.; Fisk, I.; Searle, I.R. Common Vetch: A Drought Tolerant, High Protein Neglected Leguminous Crop With Potential as a Sustainable Food Source. Front. Plant Sci. 2020, 11, 818. [Google Scholar] [CrossRef] [PubMed]
- Myrtsi, E.D.; Vlachostergios, D.N.; Petsoulas, C.; Evergetis, E.; Koulocheri, S.D.; Haroutounian, S.A. An Interdisciplinary Assessment of Biochemical and Antioxidant Attributes of Six Greek Vicia sativa L. Varieties. Plants 2023, 12, 2807. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Parra, E.; De La Rosa, L. Designing Novel Strategies for Improving Old Legumes: An Overview from Common Vetch. Plants 2023, 12, 1275. [Google Scholar] [CrossRef]
- López-Román, M.I.; Castaño-Herrero, C.; De La Rosa, L.; Ramírez-Parra, E. Optimizing Nitrogen Fixation in Vicia sativa: The Role of Host Genetic Diversity. Agronomy 2025, 15, 1479. [Google Scholar] [CrossRef]
- De La Rosa, L.; López-Román, M.I.; González, J.M.; Zambrana, E.; Marcos-Prado, T.; Ramírez-Parra, E. Common Vetch, Valuable Germplasm for Resilient Agriculture: Genetic Characterization and Spanish Core Collection Development. Front. Plant Sci. 2021, 12, 617873. [Google Scholar] [CrossRef]
- Xi, H.; Nguyen, V.; Ward, C.; Liu, Z.; Searle, I.R. Chromosome-level assembly of the common vetch (Vicia sativa) reference genome. GigaByte 2022, 2022, gigabyte38. [Google Scholar] [CrossRef]
- De la Rosa, L.; Zambrana, E.; Ramirez-Parra, E. Molecular bases for drought tolerance in common vetch: Designing new molecular breeding tools. BMC Plant Biol. 2020, 20, 71. [Google Scholar] [CrossRef]
- Aribi, M.M. Plant Gene Banks: Conservation of Genetic Resources. In Sustainable Utilization and Conservation of Plant Genetic Diversity; Al-Khayri, J.M., Jain, S.M., Penna, S., Eds.; Springer Nature: Singapore, 2024; Volume 35, pp. 753–775. [Google Scholar] [CrossRef]
- Damania, A.B.; Nagella, P. Evaluation and Documentation of Genetic Resources Collections. In Sustainable Utilization and Conservation of Plant Genetic Diversity; Al-Khayri, J.M., Jain, S.M., Penna, S., Eds.; Springer Nature: Singapore, 2024; Volume 35, pp. 777–794. [Google Scholar] [CrossRef]
- Anglin, N.L.; Wenzl, P.; Azevedo, V.; Lusty, C.; Ellis, D.; Gao, D. Genotyping Genebank Collections: Strategic Approaches and Considerations for Optimal Collection Management. Plants 2025, 14, 252. [Google Scholar] [CrossRef]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef]
- Herrera Flores, T.S.; Ortíz Cereceres, J.; Delgado Alvarado, A.; Acosta Galleros, J.A. Growth and, proline and carbohydrate content of bean seedlings subjected to drought stress. Rev. Mexicana Cienc. Agric. 2012, 3, 713–725. [Google Scholar]
- Jahan, M.S.L.; Kutty, M.S.; Pradeepkumar, T.; Santhoshkumar, A.V.; Suma, A. Characterisation of wild and cultivated cucurbit species and their response to water deficit stress. Genet. Resour. Crop Evol. 2025, 72, 343–358. [Google Scholar] [CrossRef]
- Geilfus, C.; Zörb, C.; Jones, J.J.; Wimmer, M.A.; Schmöckel, S.M. Water for agriculture: More crop per drop. Plant. Biol. 2024, 26, 499–507. [Google Scholar] [CrossRef]
- Iseki, K.; Takahashi, Y.; Muto, C.; Naito, K.; Tomooka, N. Diversity of Drought Tolerance in the Genus Vigna. Front. Plant Sci. 2018, 9, 729. [Google Scholar] [CrossRef]
- Verheyen, J.; Dhondt, S.; Abbeloos, R.; Eeckhout, J.; Janssens, S.; Leyns, F.; Scheldeman, X.; Storme, V.; Vandelook, F. High-throughput phenotyping reveals multiple drought responses of wild and cultivated Phaseolinae beans. Front. Plant Sci. 2024, 15, 1385985. [Google Scholar] [CrossRef]
- Iyem, E.; Yildirim, M.; Kizilgeci, F. Gemination, seedling growth and physio-biochemical indices of bread wheat (Triticum aestivum L.) genotypes under PEG induced drought stress. Agric. For. 2021, 67, 163–180. [Google Scholar] [CrossRef]
- Min, X.; Lin, X.; Ndayambaza, B.; Wang, Y.; Liu, W. Coordinated mechanisms of leaves and roots in response to drought stress underlying full-length transcriptome profiling in Vicia sativa L. BMC Plant. Biol. 2020, 20, 165. [Google Scholar] [CrossRef]
- Alicandri, E.; Badiani, E.; Paolacci, A.R.; Lo Presti, E.; Caridi, R.; Rea, R.; Pati, F.; Badiani, M.; Ciaffi, M.; Sorgonà, A. Screening for Drought Tolerance Within a Common Bean (Phaseolus vulgaris L.) Landrace Accessions Core Collection from the Lazio Region of Italy. Plants 2024, 13, 3132. [Google Scholar] [CrossRef] [PubMed]
- Riyaz, I.; Zaffar, A.; Fatima, S.; Shafi, S.; Bhat, R.; Showkat, S.; Khan, T.; Wani, F.J.; Zargar, S.M.; Prasad, P.V.V.; et al. Identification of water deficit stress tolerant genotypes of common bean using adaptive root and shoot traits under different screening systems. Sci. Rep. 2025, 15, 19888. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.H.K.; Ghosh, S.; Shahed, M.A. PEG-Induced osmotic stress alters root morphology and root hair traits in wheat genotypes. Plants 2021, 10, 1042. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, W.; Chen, C.; Chen, L. Plant root-shoot biomass allocation over diverse biomes: A global synthesis. Glob. Ecol. Conserv. 2019, 18, e00606. [Google Scholar] [CrossRef]
- Siddiqui, M.N.; Léon, J.; Naz, A.A.; Ballvora, A. Genetics and genomics of root system variation in adaptation to drought stress in cereal crops. J. Exp. Bot. 2020, 72, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Sofi, P.A.; Djanaguiraman, M.; Siddique, K.H.M.; Prasad, P.V.V. Reproductive fitness in common bean (Phaseolus vulgaris L.) under drought stress is associated with root length and volume. Ind. J. Plant Physiol. 2018, 23, 796–809. [Google Scholar] [CrossRef]
- Ikram, S.; Bhattarai, S.; Walsh, K.B. Screening New Mungbean Varieties for Terminal Drought Tolerance. Agriculture 2024, 14, 1328. [Google Scholar] [CrossRef]
- Cakmakci, S.; Aydinoglu, B.; Karaca, M.; Bilgen, M. Heritability of yield components in common vetch (Vicia sativa L.). Acta Agric. Scand. B Soil Plant Sci. 2006, 56, 54–59. [Google Scholar] [CrossRef]
- Gupta, S.K.; Dwivedi, V.; Kute, N.S.; Francis, P.; Parida, S.K.; Chattopadhyay, D. Identification of a Stable Drought-Tolerant High-Yielding Line for Chickpea Crop Improvement. Plant. Mol. Biol. Rep. 2025, 43, 262–268. [Google Scholar] [CrossRef]
- Rana, V.; Ram, S.; Nehra, K. Proline biosynthesis and its role in abiotic stress. Int. J. Agric. Innov. Res. 2017, 6, 473–478. [Google Scholar]
- Muktadir, M.A.; Adhikari, K.N.; Merchant, A.; Belachew, K.Y.; Vandenberg, A.; Stoddard, F.L.; Khazaei, H. Physiological and Biochemical Basis of Faba Bean Breeding for Drought Adaptation—A Review. Agronomy 2020, 10, 1345. [Google Scholar] [CrossRef]
- Signorelli, S.; Imparatta, C.; Rodríguez-Ruiz, M.; Borsani, O.; Corpas, F.J.; Monza, J. In vivo and in vitro approaches demonstrate proline is not directly involved in the protection against superoxide, nitric oxide, nitrogen dioxide and peroxynitrite. Funct. Plant Biol. 2016, 43, 870–879. [Google Scholar] [CrossRef]
- Oguz, M.C.; Aycan, M.; Oguz, E.; Poyraz, I.; Yildiz, M. Drought Stress Tolerance in Plants: Interplay of Molecular, Biochemical and Physiological Responses in Important Development Stages. Physiologia 2022, 2, 180–197. [Google Scholar] [CrossRef]
- Kusvuran, S.; Dasgan, H.Y. Effects of drought stress on physiological and biochemicalchanges in Phaseolus vulgaris L. Legume Res. 2017, 40, 55–62. [Google Scholar] [CrossRef]
- Morosan, M.; Hassan, M.A.; Naranjo, M.A.; López-Gresa, M.P.; Boscaiu, M.; Vicente, O. Comparative analysis of drought responses in Phaseolus vulgaris (common bean) and P. coccineus (runner bean) cultivars. EuroBiotech J. 2017, 1, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.R.; Sarvestani, R.; Mohammadi, B.; Baghery, A. Drought Stress-Induced Changes at Physiological and Biochemical Levels in Some Common Vetch (Vicia sativa L.) Genotypes. J. Agric. Sci. Technol. 2014, 16, 505–516. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water culture method for growing plants without soil. Agric. Exp. Stn. 1938, 347, 1–32. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
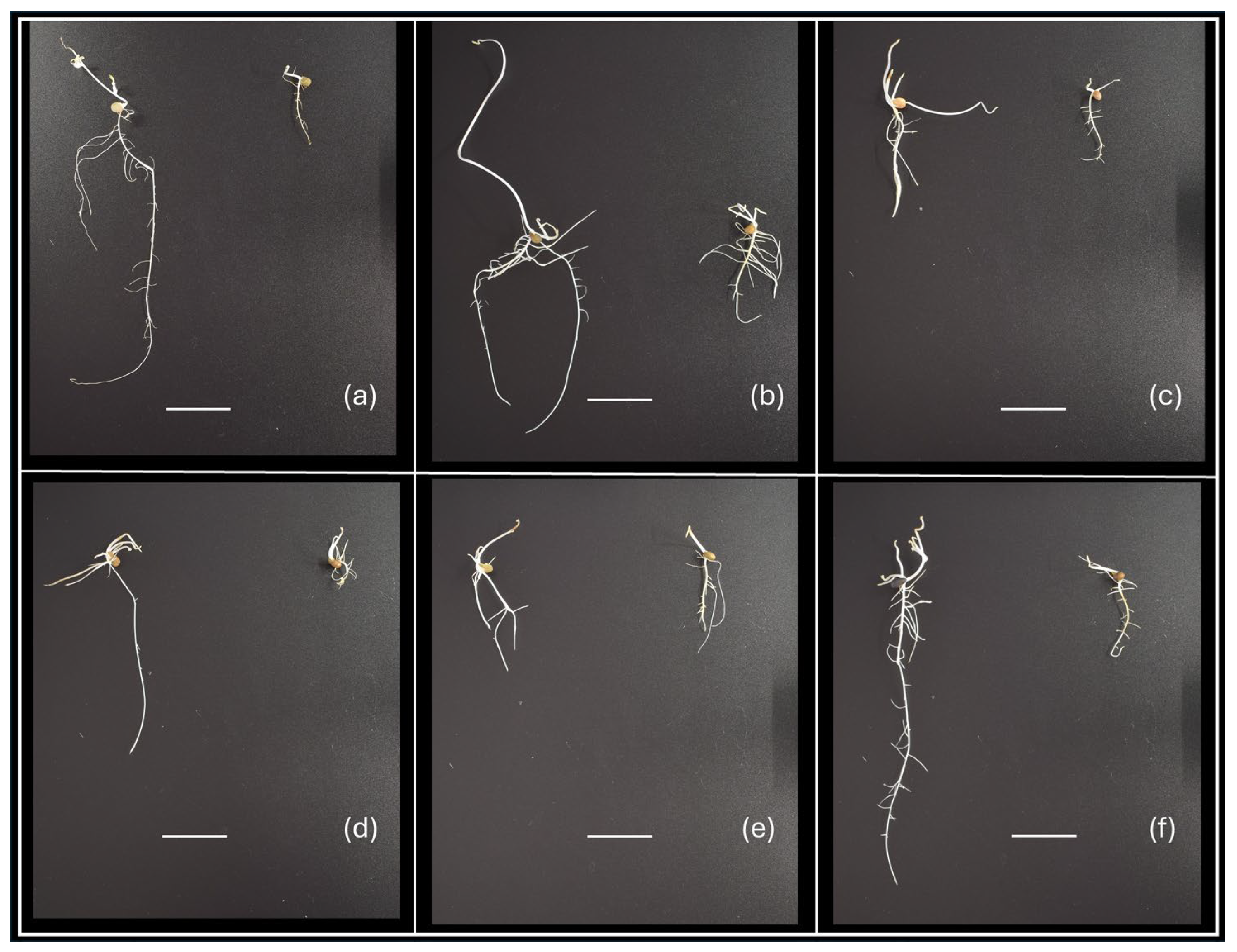


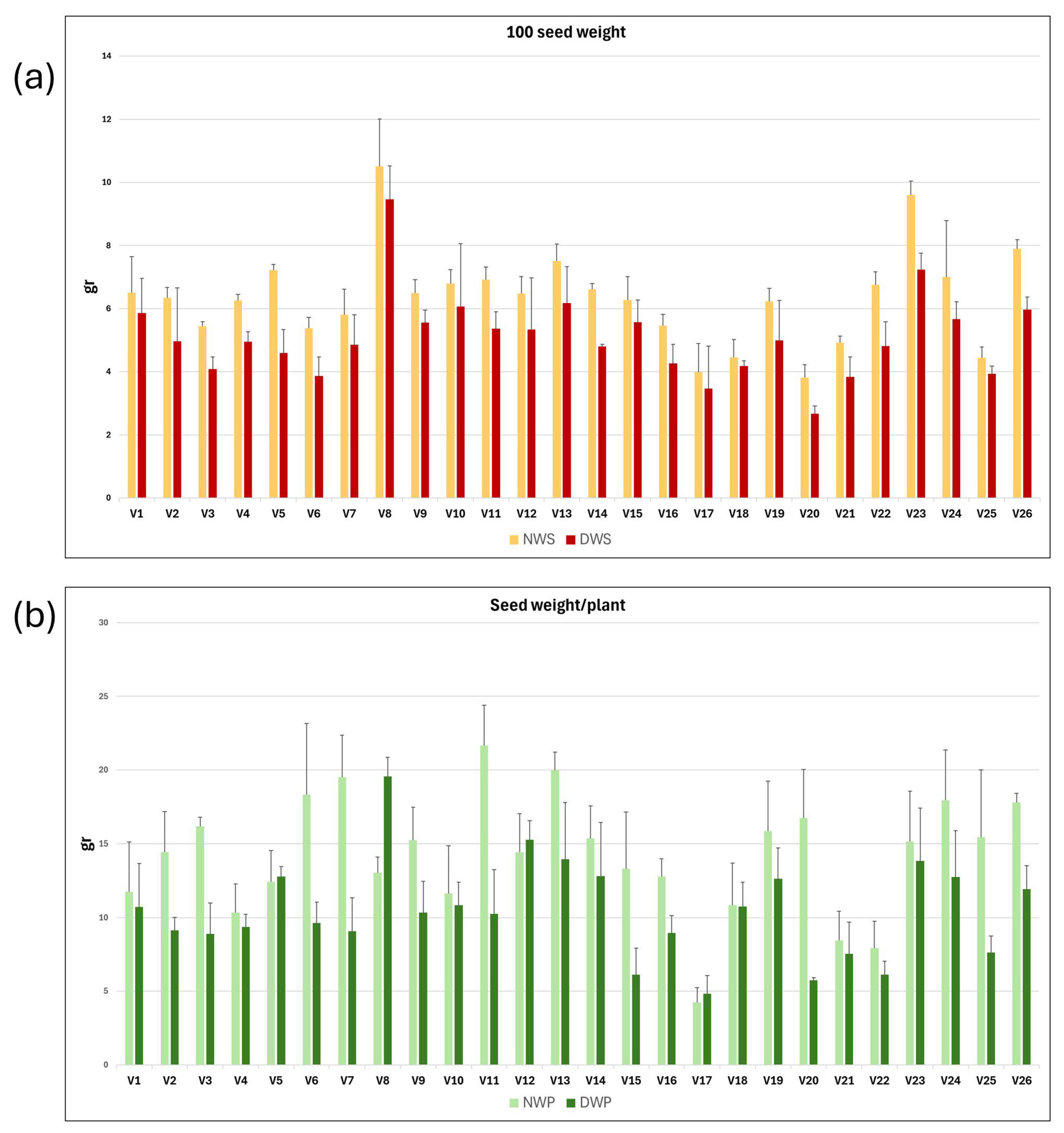
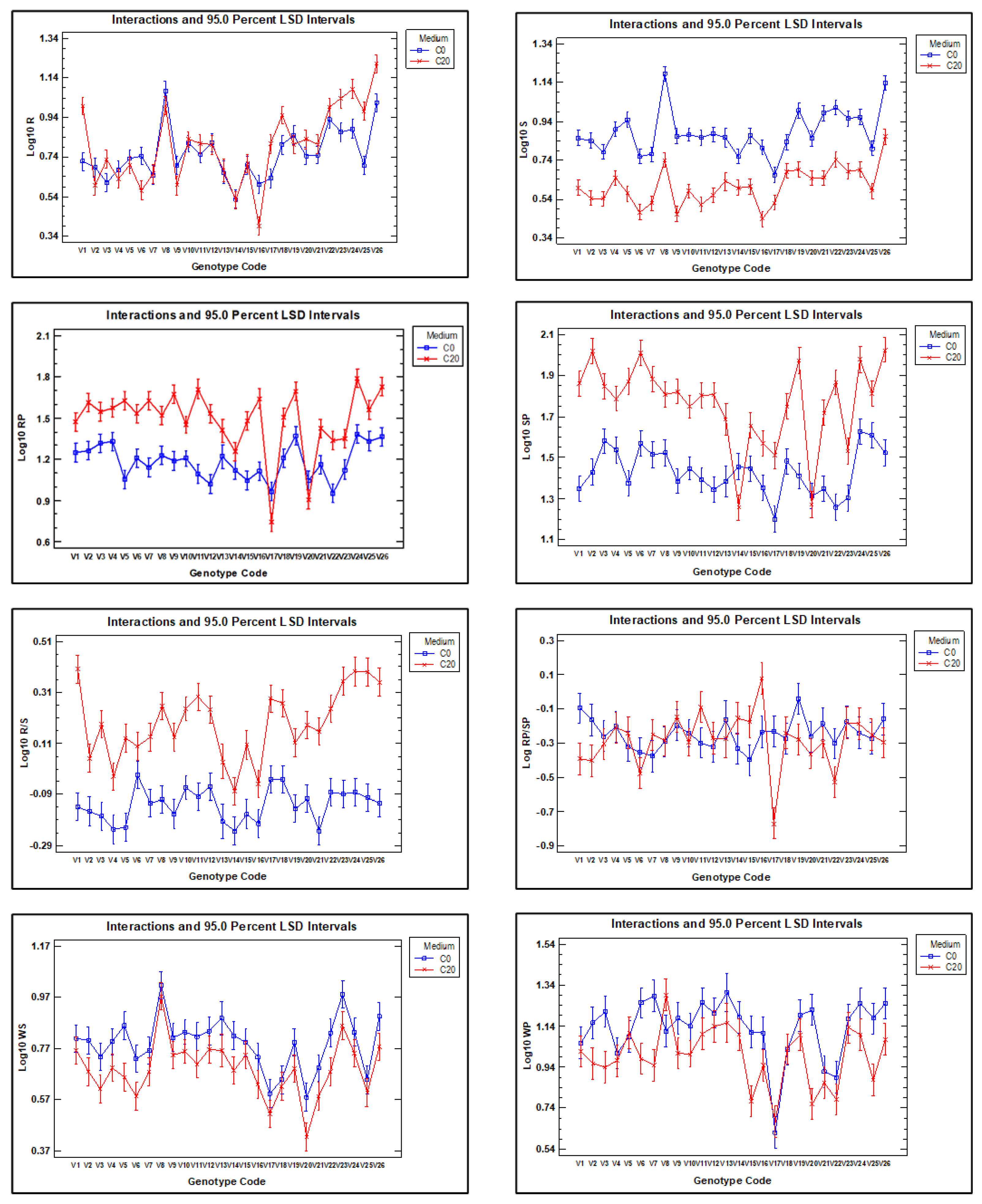
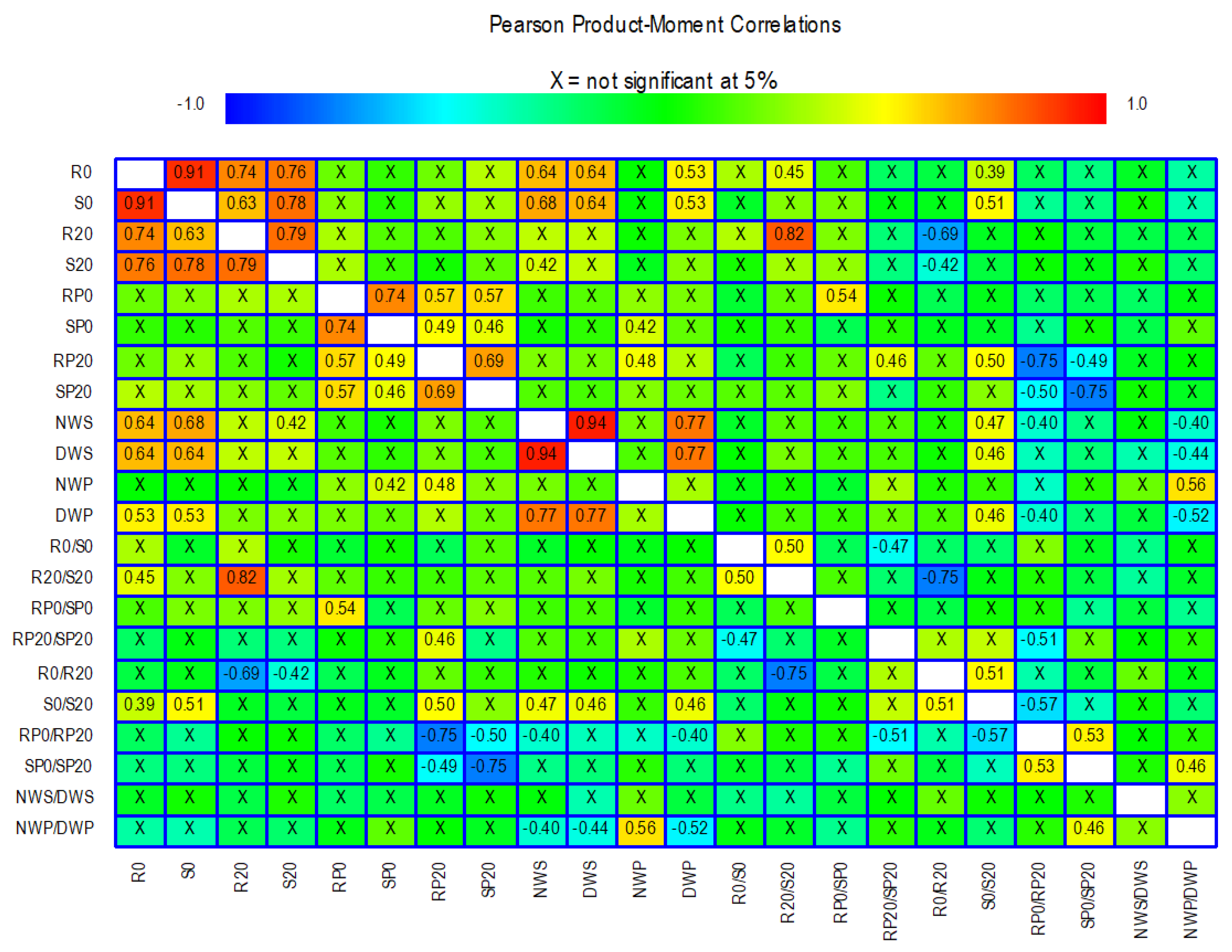

| Trait | Description |
|---|---|
| R0 | Root dry weight of seedlings grown in C0 medium (mg) |
| S0 | Shoot dry weight of seedlings grown in C0 medium (mg) |
| R20 | Root dry weight of seedlings grown in C20 medium (mg) |
| S20 | Shoot dry weight of seedlings grown in C20 medium (mg) |
| RP0 | Proline concentration in roots of seedlings grown in C0 medium (µg/100 mg tissue) |
| SP0 | Proline concentration in shoots of seedlings grown in C0 medium (µg/100 mg tissue) |
| RP20 | Proline concentration in roots of seedlings grown in C20 medium (µg/100 mg tissue) |
| SP20 | Proline concentration in shoots of seedlings grown in C20 medium (µg/100 mg tissue) |
| NWS | Weight of 100 seeds of plants grown under rainfed conditions (gr) |
| DWS | Weight of 100 seeds of plants grown under drought conditions (gr) |
| NWP | Seed weight per plant grown under rainfed conditions (gr) |
| DWP | Seed weight per plant grown under drought conditions (gr) |
| R0/S0 | Relationship between root dry weight and shoot dry weight of seedlings grown in C0 medium |
| R20/S20 | Relationship between root dry weight and shoot dry weight of seedlings grown in C20 medium |
| R0/R20 | Ratio between the dry weight of roots of seedlings grown in C0 medium and in C20 medium |
| S0/S20 | Ratio between the dry weight of shoots of seedlings grown in C0 medium and in C20 medium |
| RP0/SP0 | Ratio between root proline concentration and shoot proline concentration in seedlings grown in C0 medium |
| RP20/SP20 | Ratio between root proline concentration and shoot proline concentration in seedlings grown in C20 medium |
| RP0/RP20 | Ratio between the proline concentration of roots of seedlings grown in C0 medium and in C20 medium |
| SP0/SP20 | Ratio between the proline concentration of shoots of seedlings grown in C0 medium and in C20 medium |
| NWS/DWS | Ratio between the weight of 100 seeds of plants grown under rainfed and drought conditions, respectively |
| NWP/DWP | Ratio between the seed weight per plants grown under rainfed and drought conditions, respectively |
| Traits | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R0 (mg) | S0 (mg) | R20 (mg) | S20 (mg) | RP0 (µg/100 mg Tissue) | SP0 (µg/100 mg Tissue) | RP20 (µg/100 mg Tissue) | SP20 (µg/100 mg Tissue) | NWS (gr) | DWS (gr) | NWP (gr) | DWP (gr) | R0/ S0 | R20/ S20 | RP0/ SP0 | RP20/ SP20 | R0/ R20 | S0/ S20 | RP0/ RP20 | SP0/ SP20 | NWS/ DWS | NWP/ DWP | |
| Mean | 5.99 | 7.99 | 6.97 | 4.16 | 15.96 | 27.88 | 34.80 | 64.44 | 6.32 | 5.10 | 14.26 | 10.44 | 0.75 | 1.67 | 0.58 | 0.56 | 0.86 | 1.92 | 0.55 | 0.49 | 1.25 | 1.45 |
| Min | 3.39 | 4.62 | 2.46 | 2.74 | 9.07 | 16.49 | 5.58 | 18.36 | 3.81 | 2.67 | 3.98 | 4.82 | 0.58 | 0.84 | 0.40 | 0.17 | 0.52 | 1.39 | 0.26 | 0.24 | 1.06 | 0.67 |
| Max | 11.90 | 15.37 | 16.32 | 7.28 | 24.32 | 42.52 | 62.53 | 107.3 | 10.51 | 9.47 | 21.64 | 19.56 | 0.97 | 2.55 | 0.91 | 1.21 | 1.63 | 2.78 | 1.68 | 1.15 | 1.43 | 2.91 |
| SD | 1.96 | 2.40 | 3.23 | 1.01 | 4.60 | 6.97 | 13.41 | 24.70 | 1.51 | 1.33 | 3.98 | 3.30 | 0.11 | 0.51 | 0.12 | 0.20 | 0.28 | 0.36 | 0.32 | 0.21 | 0.11 | 0.52 |
| CV | 32.79 | 30.10 | 46.31 | 24.29 | 28.85 | 25.01 | 38.53 | 38.34 | 23.39 | 26.04 | 26.04 | 31.57 | 14.90 | 31.09 | 20.15 | 35.61 | 29.75 | 18.40 | 58.27 | 43.84 | 8.61 | 35.62 |
| h2 (%) | 90.3 | 87.7 | 87.7 | 87.8 | 65.9 | 78.6 | 73.1 | 77.4 | 82.8 | 63.8 | 63.9 | 68.2 | 53.7 | 74.5 | 30.70 | 41.50 | 70.6 | 61.9 | 78.6 | 63.6 | 50.4 | 41.5 |
| Trait | Source of Variation | d.f. | Sum Square | Mean Square | F-Ratio |
|---|---|---|---|---|---|
| R | Genotype (G) | 25 | 3.4161 | 0.1366 | 41.99 *** |
| Medium (M) | 1 | 0.0675 | 0.0675 | 20.75 *** | |
| GxM | 25 | 0.6216 | 0.0248 | 7.64 *** | |
| Residual | 104 | 0.3384 | 0.0032 | ||
| Total | 155 | 4.4436 | |||
| S | Genotype (G) | 25 | 1.5246 | 0.0610 | 30.94 *** |
| Medium (M) | 1 | 3.0190 | 3.0190 | 1531.6 *** | |
| GxM | 25 | 0.2254 | 0.0090 | 4.57 *** | |
| Residual | 104 | 0.2050 | 0.0019 | ||
| Total | 155 | 4.9741 | |||
| R/S | Genotype (G) | 25 | 1.2561 | 0.0502 | 12.60 *** |
| Medium (M) | 1 | 3.9894 | 3.9894 | 1000.30 *** | |
| GxM | 25 | 0.4785 | 0.0191 | 4.80 *** | |
| Residual | 104 | 0.4148 | 0.0039 | ||
| Total | 155 | 6.1387 | |||
| RP | Genotype (G) | 25 | 3.9027 | 0.1561 | 23.18 *** |
| Medium (M) | 1 | 3.7016 | 3.7016 | 549.75 *** | |
| GxM | 25 | 1.4189 | 0.0567 | 8.43 *** | |
| Residual | 104 | 0.7002 | 0.0067 | ||
| Total | 155 | 9.7235 | |||
| SP | Genotype (G) | 25 | 2.7394 | 0.1095 | 19.88 *** |
| Medium (M) | 1 | 4.3797 | 4.3797 | 794.56 *** | |
| GxM | 25 | 1.2693 | 0.0507 | 9.21 *** | |
| Residual | 104 | 0.5732 | 0.0055 | ||
| Total | 155 | 8.9618 | |||
| RP/SP | Genotype (G) | 25 | 1.1339 | 0.0436 | 3.78 *** |
| Medium (M) | 1 | 0.0425 | 0.0425 | 3.54 - | |
| GxM | 25 | 1.2801 | 0.0512 | 4.27 *** | |
| Residual | 104 | 3.7031 | 0.0120 | ||
| Total | 155 | ||||
| WS | Genotype (G) | 25 | 1.6358 | 0.0654 | 14.88 *** |
| Field growth (M) | 1 | 0.3914 | 0.3914 | 89.03 *** | |
| GxM | 25 | 0.0624 | 0.0024 | 0.57 - | |
| Residual | 104 | 0.4572 | 0.0044 | ||
| Total | 155 | 2.5468 | |||
| WP | Genotype (G) | 25 | 2.3932 | 0.0957 | 12.69 *** |
| Field growth (M) | 1 | 0.7181 | 0.7181 | 95.17 *** | |
| GxM | 25 | 0.8242 | 0.0329 | 4.37 *** | |
| Residual | 104 | 0.7847 | 0.0075 | ||
| Total | 155 | 4.7202 |
| Trait | PC1 | PC2 |
|---|---|---|
| R0 | 0.3956 | −0.0320 |
| S0 | 0.3804 | 0.0880 |
| R20 | 0.3300 | −0.3467 |
| S20 | 0.3125 | −0.1813 |
| NWS | 0.3524 | 0.2346 |
| DWS | 0.3578 | 0.1961 |
| NWP | 0.0254 | 0.1025 |
| DWP | 0.3070 | 0.2593 |
| R0/S0 | 0.0755 | −0.2930 |
| R20/S20 | 0.2262 | −0.3957 |
| R0/R20 | −0.1051 | 0.4938 |
| S0/S20 | 0.1675 | 0.3917 |
| NWS/DWS | −0.0825 | 0.1198 |
| NWP/DWP | −0.2051 | −0.1123 |
| Group Name | Trait | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R0 | S0 | R20 | S20 | NWS | DWS | NWP | DWP | R0/ S0 | R20/S20 | R0/ R20 | S0/ S20 | NWS/ DWS | NWP/ DWP | ||
| A | Mean | 4.00 | 6.43 | 2.46 | 2.74 | 5.46 | 4.27 | 12.78 | 8.97 | 0.63 | 0.90 | 1.63 | 2.34 | 0.71 | 1.45 |
| SD | 0.35 | 1.04 | 0.20 | 0.13 | 0.36 | 0.60 | 1.20 | 1.18 | 0.11 | 0.03 | 0.04 | 0.34 | 0.12 | 0.32 | |
| CV | 0.09 | 5.61 | 0.08 | 0.05 | 0.07 | 0.14 | 0.09 | 0.13 | 0.18 | 0.04 | 0.02 | 0.14 | 0.17 | 0.22 | |
| Min | 3.61 | 7.60 | 2.23 | 2.60 | 5.12 | 3.75 | 12.07 | 7.80 | 0.54 | 0.86 | 1.59 | 2.00 | 0.59 | 1.19 | |
| Max | 4.27 | 2.00 | 2.59 | 2.84 | 5.83 | 4.92 | 14.17 | 10.15 | 0.76 | 0.91 | 1.67 | 2.68 | 0.83 | 1.82 | |
| Homogeneous group | a | ab | a | a | a | abc | ab | abc | a | a | a | cd | d | b | |
| B | Mean | 11.90 | 15.37 | 10.03 | 5.53 | 10.51 | 9.47 | 13.04 | 19.56 | 0.78 | 1.82 | 1.20 | 2.78 | 0.44 | 0.67 |
| SD | 0.83 | 0.88 | 1.18 | 0.10 | 1.50 | 1.06 | 1.08 | 1.29 | 0.07 | 0.25 | 0.22 | 0.18 | 0.10 | 0.09 | |
| CV | 0.07 | 14.71 | 0.12 | 0.02 | 0.14 | 0.11 | 0.08 | 0.07 | 0.09 | 0.14 | 0.18 | 0.06 | 0.22 | 0.14 | |
| Min | 10.97 | 16.36 | 8.88 | 5.43 | 8.78 | 8.44 | 11.80 | 18.10 | 0.73 | 1.58 | 0.98 | 2.61 | 0.35 | 0.57 | |
| Max | 12.58 | 1.66 | 11.25 | 5.64 | 11.41 | 10.55 | 13.76 | 20.57 | 0.86 | 2.07 | 1.42 | 2.96 | 0.54 | 0.76 | |
| Homogeneous group | e | d | de | c | d | e | ab | d | ab | cd | bc | d | abc | a | |
| C | Mean | 4.82 | 7.19 | 4.58 | 3.68 | 6.08 | 4.71 | 14.36 | 9.57 | 0.69 | 1.25 | 1.09 | 1.97 | 0.57 | 1.59 |
| SD | 0.82 | 1.52 | 0.99 | 0.57 | 0.75 | 0.88 | 3.99 | 2.58 | 0.15 | 0.24 | 0.27 | 0.39 | 0.14 | 0.59 | |
| CV | 0.17 | 4.73 | 0.22 | 0.16 | 0.12 | 0.19 | 0.28 | 0.27 | 0.21 | 0.19 | 0.24 | 0.20 | 0.25 | 0.37 | |
| Min | 2.85 | 11.04 | 2.93 | 2.71 | 4.69 | 3.17 | 6.30 | 4.87 | 0.40 | 0.78 | 0.69 | 1.24 | 0.31 | 0.68 | |
| Max | 6.03 | 6.30 | 6.62 | 4.82 | 7.41 | 6.79 | 23.27 | 16.97 | 1.02 | 1.71 | 1.70 | 2.93 | 0.82 | 3.13 | |
| Homogeneous group | ab | b | b | b | b | b | b | b | a | b | b | bc | cd | b | |
| D | Mean | 6.23 | 7.89 | 6.29 | 3.99 | 6.79 | 5.59 | 16.71 | 12.60 | 0.79 | 1.64 | 1.00 | 2.03 | 0.51 | 1.42 |
| SD | 1.20 | 1.26 | 1.03 | 0.75 | 0.60 | 1.28 | 4.44 | 2.89 | 0.12 | 0.45 | 0.18 | 0.38 | 0.12 | 0.61 | |
| CV | 0.19 | 5.94 | 0.16 | 0.19 | 0.09 | 0.23 | 0.27 | 0.23 | 0.15 | 0.27 | 0.18 | 0.19 | 0.24 | 0.43 | |
| Min | 4.43 | 10.44 | 4.00 | 2.77 | 5.75 | 3.68 | 8.10 | 8.30 | 0.62 | 0.97 | 0.78 | 1.35 | 0.31 | 0.64 | |
| Max | 8.93 | 4.50 | 8.48 | 5.16 | 8.10 | 8.19 | 24.50 | 18.33 | 0.99 | 2.56 | 1.47 | 2.62 | 0.71 | 2.95 | |
| Homogeneous group | c | b | c | b | c | cd | b | c | b | c | b | bc | bc | b | |
| E | Mean | 5.31 | 6.24 | 7.96 | 4.14 | 4.18 | 3.57 | 11.82 | 7.24 | 0.86 | 1.98 | 0.69 | 1.53 | 0.46 | 1.71 |
| SD | 0.89 | 1.16 | 1.81 | 0.83 | 0.59 | 0.85 | 5.79 | 2.57 | 0.09 | 0.50 | 0.19 | 0.24 | 0.14 | 0.90 | |
| CV | 0.17 | 4.41 | 0.23 | 0.20 | 0.14 | 0.24 | 0.49 | 0.36 | 0.10 | 0.25 | 0.27 | 0.16 | 0.30 | 0.53 | |
| Min | 3.92 | 7.73 | 5.63 | 3.10 | 3.35 | 1.92 | 3.37 | 3.77 | 0.72 | 1.05 | 0.49 | 1.23 | 0.31 | 0.62 | |
| Max | 6.56 | 3.32 | 10.74 | 5.37 | 5.03 | 4.37 | 20.71 | 12.60 | 1.02 | 2.54 | 1.11 | 2.09 | 0.77 | 3.53 | |
| Homogeneous group | b | a | d | b | a | a | a | a | b | d | a | a | b | b | |
| F | Mean | 7.83 | 9.92 | 11.92 | 5.32 | 7.55 | 5.91 | 14.11 | 11.07 | 0.79 | 2.27 | 0.67 | 1.86 | 0.36 | 1.31 |
| SD | 1.80 | 2.43 | 2.98 | 1.19 | 1.44 | 1.01 | 4.60 | 3.55 | 0.07 | 0.42 | 0.15 | 0.11 | 0.08 | 0.34 | |
| CV | 0.23 | 6.41 | 0.25 | 0.22 | 0.19 | 0.17 | 0.33 | 0.32 | 0.09 | 0.19 | 0.22 | 0.06 | 0.22 | 0.26 | |
| Min | 4.81 | 14.73 | 7.90 | 3.65 | 5.08 | 3.96 | 6.39 | 5.08 | 0.68 | 1.62 | 0.48 | 1.68 | 0.25 | 0.66 | |
| Max | 10.94 | 8.33 | 17.21 | 7.42 | 10.10 | 7.60 | 21.85 | 17.82 | 0.95 | 2.97 | 0.93 | 2.08 | 0.51 | 1.96 | |
| Homogeneous group | d | c | e | c | c | d | ab | bc | b | d | a | b | a | b | |
| Working Code | Variety Name or Spanish Genebank Number | Country | Local Origin | Type of Plant Material |
|---|---|---|---|---|
| V1 | AITANA | SPA | commercial variety | |
| V2 | BGE000529 | GRC | Vromovrisi (Peloponissos) | landrace |
| V3 | BGE000587 | IRN | Isfahan_Arak | landrace |
| V4 | BGE000600 | IRN | Firuz Kuh (Teheran) | landrace |
| V5 | BGE001163 | SPA | Guareña (Badajoz) | landrace |
| V6 | BGE004356 | SPA | Tolox (Málaga) | landrace |
| V7 | BGE004375 | SPA | Mala (Granada) | landrace |
| V8 | BGE005449 | SPA | Andujar (Jaen) | landrace |
| V9 | BGE007269 | SPA | Socuellamos (Ciudad Real) | landrace |
| V10 | BGE014945 | SPA | Valdeganga (Albacete) | landrace |
| V11 | BGE014946 | SPA | Iniesta (Cuenca) | landrace |
| V12 | BGE022207 | SPA | Mao-Mahon (Islas Baleares) | landrace |
| V13 | BGE022757 | ITA | Caltavuturo (Palermo) | landrace |
| V14 | BGE025608 | SPA | Valdelacasa de Tajo (Cáceres) | landrace |
| V15 | BGE022210 | SPA | Benilloba (Alicante) | landrace |
| V16 | BGE026275 | SPA | Cazorla (Jaen) | landrace |
| V17 | BGE000418 | IRN | Borujerd_Korramabad (Lorestan) | landrace |
| V18 | BGE000528 | TUR | Kurtkoy (Istambul) | landrace |
| V19 | BGE014901 | SPA | Guadarrama (Madrid) | wild population |
| V20 | BGE016970 | SPA | Madrid | wild population |
| V21 | BGE029065 | SPA | Sevilla | landrace |
| V22 | SENDA | SPA | commercial variety | |
| V23 | BGE004289 | SPA | Pinos Puente (Granada) | landrace |
| V24 | BGE004419 | SPA | Cadiar (Granada) | landrace |
| V25 | BGE027063 | SPA | Torvizcon (Granada) | landrace |
| V26 | VERDOR | SPA | commercial variety |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, J.M.; Loarce, Y.; Sánchez-Gordo, N.; De la Rosa, L.; Ramírez-Parra, E. Combining in vitro and Field Studies to Predict Drought Tolerance in Vicia sativa L. Genotypes. Plants 2025, 14, 3376. https://doi.org/10.3390/plants14213376
González JM, Loarce Y, Sánchez-Gordo N, De la Rosa L, Ramírez-Parra E. Combining in vitro and Field Studies to Predict Drought Tolerance in Vicia sativa L. Genotypes. Plants. 2025; 14(21):3376. https://doi.org/10.3390/plants14213376
Chicago/Turabian StyleGonzález, Juan M., Yolanda Loarce, Noa Sánchez-Gordo, Lucía De la Rosa, and Elena Ramírez-Parra. 2025. "Combining in vitro and Field Studies to Predict Drought Tolerance in Vicia sativa L. Genotypes" Plants 14, no. 21: 3376. https://doi.org/10.3390/plants14213376
APA StyleGonzález, J. M., Loarce, Y., Sánchez-Gordo, N., De la Rosa, L., & Ramírez-Parra, E. (2025). Combining in vitro and Field Studies to Predict Drought Tolerance in Vicia sativa L. Genotypes. Plants, 14(21), 3376. https://doi.org/10.3390/plants14213376






