1. Introduction
Tomato (
Solanum lycopersicum L.) is a globally important horticultural crop with substantial economic significance in both fresh markets and processing industries [
1,
2,
3]. In China, its production is increasingly concentrated in greenhouse systems, where advanced solar greenhouse technology allows precise control of light, temperature, and humidity, thereby improving yield and fruit quality [
4,
5]. However, intensive greenhouse-based farming still faces persistent challenges, including land-use constraints, disease pressure, and declining resource-use efficiency [
6,
7,
8]. Overreliance on chemical fertilizers further exacerbates soil degradation, nutrient imbalances, and reduced microbial activity, ultimately threatening the sustainability of high-input agricultural systems [
9]. These issues underscore the urgent need to develop fertilization strategies that can sustain soil fertility, enhance microbial function, and support stable crop productivity under intensive greenhouse conditions.
Organic fertilizers (OFs), derived from renewable biological materials, have emerged as pivotal tools in the transition toward sustainable agriculture. Their multifunctional benefits—enhancing soil fertility, improving structure, increasing organic matter content, and stimulating microbial activity—position them as ecologically sound alternatives to chemical fertilizers [
10,
11]. Judicious application of OFs can facilitate long-term nutrient release, promote microbial-mediated nutrient cycling, and enhance crop productivity and resilience. However, limitations such as variable quality, relatively high costs, and inconsistent field performance hinder their broader adoption in high-efficiency protected cultivation systems. Furthermore, current studies often focus on single fertilizer types or specific application rates, lacking a systematic comparison of different organic fertilizers and their interactive effects on the soil–microbe–plant continuum [
12,
13].
Soil microbial communities are integral to nutrient dynamics, soil health, and plant performance. Fertilization influences not only soil physicochemical properties but also microbial structure and function, mediating nutrient availability and plant growth through belowground–aboveground feedbacks. In turn, plant roots exude metabolites that shape microbial community composition, forming a tightly coupled tripartite system of soil–microbe–plant interactions [
14,
15,
16,
17]. Understanding the mechanisms by which organic fertilizers modulate this complex system is essential for designing optimized nutrient management strategies.
Despite growing interest in organic fertilization, how fertilizer type and application rate jointly shape the soil–microbe–plant system of greenhouse tomato remains insufficiently resolved, particularly with respect to integrative links among soil properties, rhizosphere microbiota, and crop performance. Here, we set out to determine how three organic fertilizer types—bio-organic fertilizer (BOF), bone meal fertilizer (BMF), and bone calcium fertilizer (BCF)—applied at 7500, 15,000, 30,000, and 45,000 kg·ha−1 affect plant performance (growth, yield, fruit quality) together with concurrent changes in soil physicochemical properties and the rhizosphere microbiome (alpha diversity and community composition). We further examined soil–microbe linkages using co-occurrence network analysis and Mantel tests and evaluated indirect pathways connecting fertilization, soil and microbial attributes, and crop performance via partial least squares path modeling (PLS-PM) to identify agronomically suitable application ranges under greenhouse conditions. Based on prior evidence, we hypothesized that fertilizer type and rate would be associated with shifts in soil resource status and rhizosphere microbial diversity/structure, which would, in turn, be linked to variation in photosynthetic traits and yield.
2. Results
2.1. Growth Characteristics Analysis
2.1.1. Response of Tomato Photosynthetic Physiology to Different Fertilization Regimes
Net photosynthetic rate (Pn), stomatal conductance (gs), and transpiration rate (Tr) were measured under three organic fertilizer treatments—bone calcium fertilizer (BCF), bone mud fertilizer (BMF), and bio-organic fertilizer (BOF)—each applied at four dosage levels, along with an unfertilized control. Across all developmental stages, fertilization significantly increased Pn, gs, and Tr relative to the control (
p < 0.01;
Table S1). Percent changes reported below are defined relative to the concurrent control at the same sampling stage.
During the flowering and fruit-setting stages, net photosynthetic rate (Pn) under BOF4 reached 34.9 μmol CO
2·m
−2·s
−1 (+29.5% vs. control at the same stage), exceeding BCF4 (+25.5%) and BMF4 (+18.8%). A similar pattern was observed at the fruit-expansion stage, where CO
2 assimilation under BOF4 consistently surpassed the other treatments. By contrast, low-dose treatments (BCF1 and BMF1) produced only modest gains in Pn (+12.0% and +7.4%, respectively), indicating that higher-rate BOF most strongly enhances carbon-assimilation capacity under greenhouse conditions (
Table S1).
Stomatal conductance (gs) likewise peaked under BOF4, reaching 0.50 mol H
2O·m
−2·s
−1 (+50.0% vs. control at the same stage), with BCF4 and BMF4 increasing by +42.0% and +37.0%, respectively. The elevated gs under BOF4 suggests enhanced stomatal opening and CO
2 diffusion into the mesophyll, thereby supporting sustained photosynthetic activity (
Table S1).
Transpiration rate (Tr) was also enhanced by fertilization; at flowering, BOF4 recorded 19.2 mmol H
2O·m
−2·s
−1 (+24.5% vs. control at the same stage), higher than BCF4 (+17.2%) and BMF4 (+10.4%). The higher Tr under BOF4 likely contributed to thermal regulation and improved gas exchange within the greenhouse microclimate, supporting overall photosynthetic performance and stress tolerance (
Table S1).
Taken together, organic fertilization markedly alters leaf gas-exchange dynamics in greenhouse tomatoes. Among all treatments, high-dose BOF (BOF4) produced the most pronounced improvements in photosynthetic capacity, underscoring its superior efficacy in optimizing physiological processes that underpin growth and productivity.
2.1.2. Effect of Different Fertilization Treatments on Plant Height and Stem Diameter of Greenhouse Tomatoes
Organic fertilization significantly enhanced tomato vegetative growth, with both plant height and stem diameter showing consistent, dose-dependent increases across treatments (
Figure S1a,b). High-rate bio-organic fertilizer (BOF4) exhibited the greatest effect, with final plant height and stem diameter at 70 days post-transplanting reaching 136.9 cm and 14.5 mm, respectively, both markedly higher than other treatments and the unfertilized control (
p < 0.01). Lower application rates produced only minor improvements relative to the control. These findings indicate that BOF, particularly at higher rates, can sustain structural growth and vigor throughout the cultivation period.
2.2. Tomato Yield and Quality Analysis
At the onset of the harvest stage, single-plant tomato yields were measured across treatments. Fertilization significantly increased individual plant yield, with a dose-dependent trend observed. Among the fertilizer types, the yield-promoting effects followed the order: BOF > BCF > BMF. Compared with the control, organic fertilization led to yield increases ranging from 4.3% to 40.8%. The BOF4 treatment recorded the highest single-plant yield at 8.2 kg·plant
−1, representing a 40.8% improvement over the control. BCF4 and BMF4 achieved yields of 8.10 kg and 8.1 kg per plant, respectively, showing increases of 38.9% and 38.4%, and were significantly higher than most other treatments, indicating the yield-enhancing potential of high-dose organic fertilizer applications (
Table S2).
Subsequent total yield estimations were extrapolated to a per-hectare basis. The highest yield was observed in the BOF4 treatment, forming a high-yield cluster alongside BMF4, BCF3, and BCF4, with no statistically significant differences among them. Treatments such as BCF2, BOF2, and BOF3 constituted the medium-yield group, while BCF1, BOF1, BMF2, and BMF3 comprised the low-yield group. BMF1 and the control consistently showed the lowest yields. These results were consistent with single-plant data, further reinforcing the superiority of BOF—especially BOF4—as the most effective in yield enhancement, followed by BCF, with BMF exhibiting relatively modest effects (
Table S2).
In terms of fruit quality, as assessed by soluble sugar content (SSC, °Brix), fertilization treatments significantly influenced sugar accumulation. Treatments achieving > 7.0 °Brix sugar content included BOF4, BCF4, and BMF4, representing 23% of total treatments and meeting the criteria for high-sweetness classification. Treatments with sugar contents between 6.5 °Brix and 7.0 °Brix—considered “good sweetness”—included BCF2, BCF3, BMF1, BMF3, and BOF3, accounting for 38.5%. Treatments such as BCF1, BMF2, BOF1, and BOF2 had sugar contents between 6.0 °Brix and 6.5 °Brix, while the control group fell below 6.0 °Brix, classifying as low sweetness. Notably, BCF4 ranked second only to BOF4 in sugar content and was significantly higher than most other treatments, underscoring its quality-enhancing capacity. BOF3 ranked third and was also significantly superior to the majority of treatments. No significant differences were observed among BCF2, BCF3, BMF1, and BMF3, nor among BCF1, BMF2, BOF1, and BOF2. Overall, BOF demonstrated superior efficacy in improving both yield and fruit quality, followed by BCF, while BMF showed relatively weaker performance (
Table S2).
2.3. Soil Physicochemical Properties Analysis
2.3.1. Changes in Soil Moisture Content
Fertilization treatments significantly influenced soil moisture content (
Table S3). At 56 days post-planting, BOF1 treatment showed the highest moisture (48.1%,
p < 0.01), while BOF4 had the lowest (41.4%). By day 70, BCF2 and BMF3 treatments had significantly higher moisture (32.8% and 32.2%) than others, while BOF4 remained lowest (30.8%). These results suggest that moderate doses of BCF and BMF contribute to soil moisture retention, while higher BOF doses may disrupt water retention.
2.3.2. Changes in Soil pH
Soil pH exhibited a general decline with increasing fertilizer application across all treatments, reflecting the acidifying effects of organic fertilizers (
Table S4, 70 DAP). The pH values in BCF1, BMF2, and BOF1 were significantly higher than those in high-dose treatments such as BCF4 and BOF4, yet still lower than the unfertilized control (pH = 7.15). These results indicate that high-dose organic fertilization may lead to notable soil acidification, which could potentially alter nutrient availability and microbial activity.
2.3.3. Changes in Soil Nutrients
Organic fertilizer treatments markedly increased the contents of soil organic matter (OM), total nitrogen (TN), available phosphorus (AP), and available potassium (AK), with concentrations rising proportionally to fertilizer dosage. The BOF4 treatment group showed the highest OM content (30.9 g·kg
−1), representing a 42.6% increase over the control group, highlighting the superior organic matter enrichment capacity of BOF. BCF and BMF treatments also enhanced OM levels, albeit to a lesser extent (
Table S4).
Total nitrogen content was significantly elevated under all fertilization treatments. BOF4 achieved the highest TN level (0.3 mg·kg−1), 82% higher than the control, indicating BOF’s superior nitrogen-supplying capacity, likely attributed to its stable N-release formulation.
Available phosphorus also exhibited a clear upward trend with increasing fertilizer levels. High-dose treatments (BCF4, BMF4, BOF3, BOF4) all exceeded 150 mg·kg−1 in AP, a range considered agronomically optimal for tomato root uptake and stress resistance enhancement.
Available potassium was elevated across all treatments, with levels ranging from 200 to 400 mg·kg
−1. BOF4 recorded the highest AK (287.7 mg·kg
−1), slightly ahead of BCF4 (285.6 mg·kg
−1), indicating that high-dose fertilization effectively met potassium demand during critical growth periods (
Table S4).
2.4. Soil Microbial Community Analysis
2.4.1. Alpha Diversity
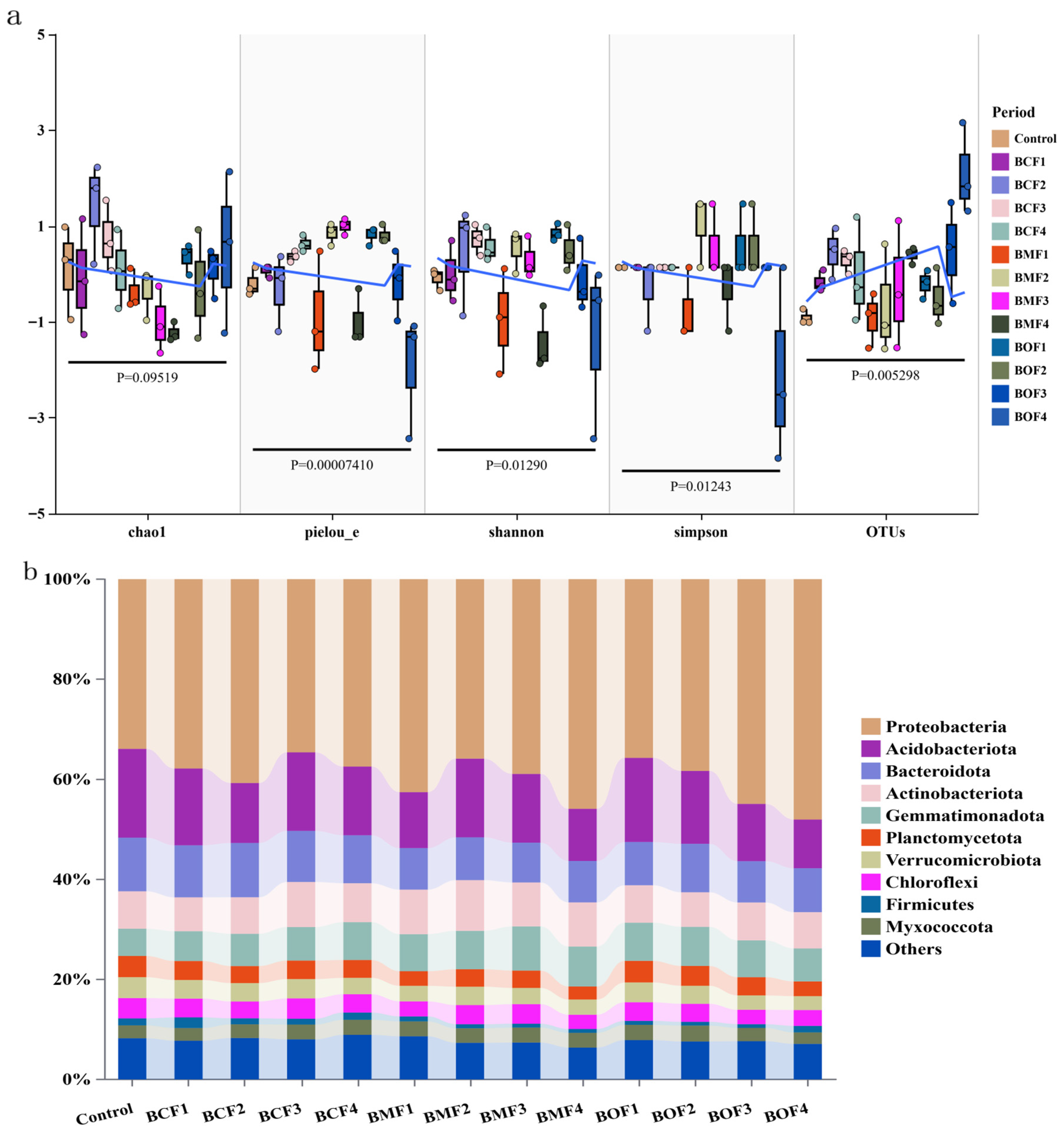
To aid interpretation, we briefly define the diversity metrics used here: OTU richness/Chao1 index represents species richness (number of taxa; Chao1 is a richness estimator accounting for rare taxa), while Shannon and Simpson indices describe alpha diversity, with Shannon emphasizing both richness and evenness and Simpson emphasizing evenness/dominance (higher Simpson = more even). Illumina NovaSeq sequencing produced a total of 5,218,818 raw reads from 39 soil samples, and after stringent quality control, 4,818,485 high-quality sequences were retained. The Q20 values ranged from 99.3% to 99.5%, indicating high sequencing accuracy and reliability. The rarefaction curves reached a plateau, suggesting that the sequencing depth was sufficient to capture the majority of microbial diversity and that the data were adequate for downstream analyses (
Table S4).
Alpha diversity analysis revealed that the application of BOF, particularly under the BOF4 treatment, significantly enhanced the operational taxonomic unit (OTU) richness and Chao1 index of the soil microbial community compared to the control. This indicates a marked improvement in microbial species richness. BCF4 ranked second in terms of richness indices. Furthermore, the Shannon index demonstrated that BCF3 and BCF4 treatments notably increased microbial diversity, with improvements of 2.58–2.77% relative to the control. In contrast, the Simpson index showed that only the BOF1 treatment significantly improved community evenness, while other treatments exhibited no significant differences compared to the control. These results suggest that organic fertilizer application primarily enhanced microbial richness while exerting limited effects on community evenness (
Figure 1a).
2.4.2. Major Species Composition and Abundance Analysis
At the phylum level, the dominant bacterial taxa across all treatment groups were Proteobacteria, Acidobacteriota, Bacteroidota, and Actinobacteriota, which are widely recognized as core microbial constituents involved in soil nutrient cycling and organic matter turnover. Notably, the application of high-dose BOF significantly enriched the abundance of Proteobacteria, with its relative abundance reaching 48.0% in the BOF4 treatment, compared to 33.9% in the unfertilized control. This represents a substantial increase, suggesting that BOF promotes the proliferation of copiotrophic and functionally beneficial taxa. Similarly, BMF4 and BOF3 treatments also led to relatively higher abundances of Proteobacteria, reaching 45.9% and 44.9%, respectively, further underscoring the fertilization-dependent shifts in microbial community composition (
Figure 1b).
2.4.3. NMDS + PERMANOVA Analysis
To interpret beta-diversity patterns mechanistically and test them statistically, we combined non-metric multidimensional scaling (NMDS)—which visualizes multivariate differences in community composition—with permutational multivariate analysis of variance (PERMANOVA)—which formally tests whether between-group differences are significant on the same dissimilarity matrix (Bray–Curtis). In this framework, NMDS stress quantifies the goodness-of-fit of the ordination (lower values = better), whereas PERMANOVA R2 indicates the variance explained by treatment (effect size) and p provides permutation-based significance.
The NMDS ordination revealed distinct separations among treatment groups, indicating that both fertilizer type (BCF, BMF, BOF) and application rate exerted substantial impacts on microbial assemblages. Spatial proximity of sample points reflected compositional similarity, with the BOF4 treatment (blue diamonds) forming a tightly clustered group that was clearly segregated from others along the NMDS axes, suggesting a pronounced restructuring of the microbiome under high BOF input (
Figure 1c). Conversely, treatments such as BMF1 exhibited dispersed sample distributions, implying elevated intra-treatment heterogeneity and a less deterministic response of microbial communities.
The PERMANOVA analysis statistically validated the observed patterns, revealing a significant effect of fertilization treatments on community structure (R2 = 0.5127, p = 0.001), with treatments explaining 51.3% of the observed compositional variance. Furthermore, the low NMDS stress value (0.0895 < 0.1) indicates high fidelity of dimensional reduction, substantiating the reliability of the ordination.
Collectively, these results demonstrate that fertilization significantly modulated the soil microbial community composition, with BOF4 exerting the most substantial influence. Differential community assembly across treatments is likely driven by alterations in soil physicochemical conditions, which mediate microbial niche selection and functional processes, ultimately influencing nutrient cycling and plant performance.
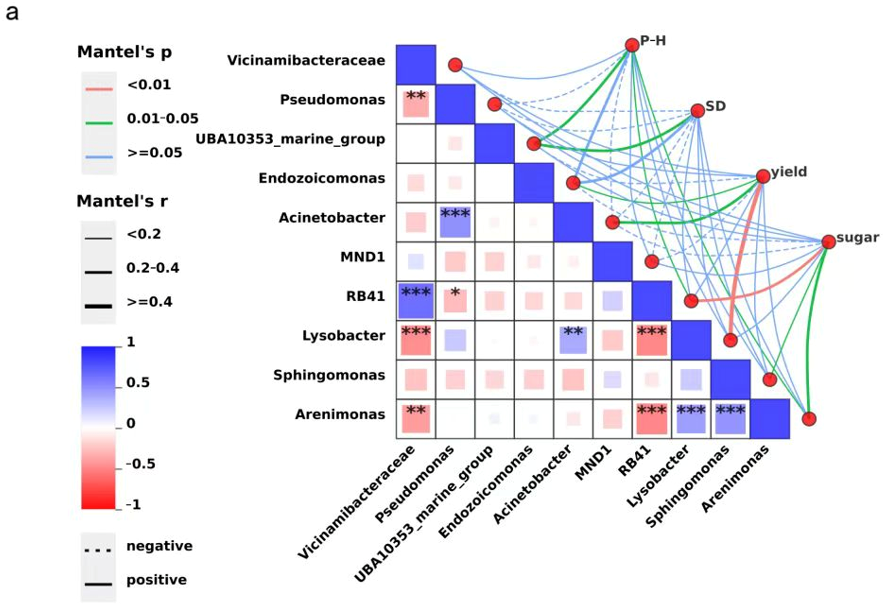
2.5. Mantel Test Analysis
The Mantel test further validated the intricate associations among soil microbial communities, physicochemical soil parameters, and plant phenotypic traits. A significant negative correlation between Vicinamibacteraceae and Pseudomonas (r = −0.426, p = 0.0069) suggests potential antagonism in resource acquisition or ecological niche overlap, which could limit functional redundancy within the rhizosphere. Conversely, a strong positive correlation between Acinetobacter and Pseudomonas (r = 0.606, p < 0.001) indicates possible functional effects, as both genera are known to participate in organic matter degradation and nutrient cycling, thereby supporting plant nutrient acquisition. Additionally, Vicinamibacteraceae exhibited a highly significant positive association with RB41 (r = 0.798, p < 0.001), implying shared ecological strategies that may stabilize soil microbial communities under similar environmental conditions. The significant positive correlation between Lysobacter and Acinetobacter (r = 0.472, p = 0.0024) points toward potential cooperative roles in suppressing pathogens and enhancing soil enzymatic activity.
Microbe–plant trait correlations revealed functionally relevant associations. Plant height was significantly and positively correlated with UBA10353_marine_group,
RB41, and
Acinetobacter (
p < 0.05), all of which are potentially involved in nutrient mobilization and phytohormone production. Stem diameter was significantly associated only with UBA10353_marine_group (
p < 0.05), suggesting its role in enhancing structural growth via improved nutrient uptake efficiency. Yield displayed significant positive correlations with
Endozoicomonas and Acinetobacter and a very strong correlation with
Lysobacter (
p < 0.01), taxa that may contribute to improved nutrient assimilation and biocontrol functions. Soluble sugar content was significantly positively associated with
Sphingomonas and
Acinetobacter (
p < 0.05) and highly significantly with RB41 (
p < 0.01), suggesting that these taxa could facilitate carbohydrate biosynthesis through metabolic modulation of carbon fluxes (
Figure 2a).
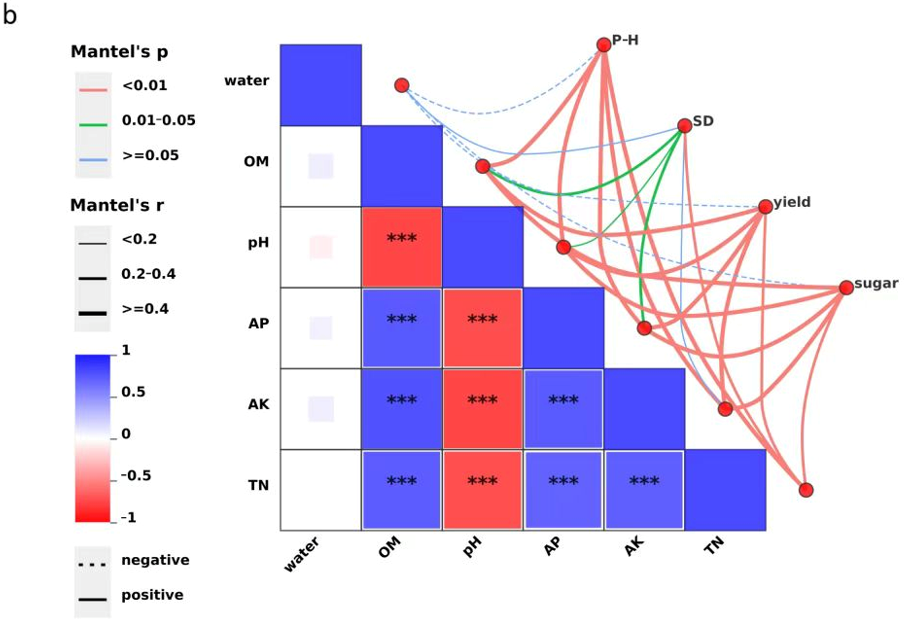
Similarly, soil physicochemical variables were closely linked to plant growth and fruit quality. Plant height was very significantly positively correlated with soil organic matter (OM), pH, available phosphorus (AP), available potassium (AK), and total nitrogen (TN) (
p < 0.01). Stem diameter was also very significantly correlated with TN (
p < 0.01) and significantly with OM, pH, and AP (
p < 0.05). Both yield and sugar content exhibited very strong positive associations with OM, pH, AP, AK, and TN (
p < 0.01) (
Figure 2b). OM, as a core indicator of soil health, likely enhances plant productivity by improving nutrient availability, enriching beneficial microbial populations, and buffering soil acidity. In contrast, the observed negative correlation between pH and AP availability suggests that elevated pH may reduce phosphorus solubility, thereby constraining crop development. Soil moisture content exhibited a weak negative correlation with plant height, yield, and sugar content, potentially reflecting the complex interactions of water with other abiotic and biotic factors in the greenhouse environment.
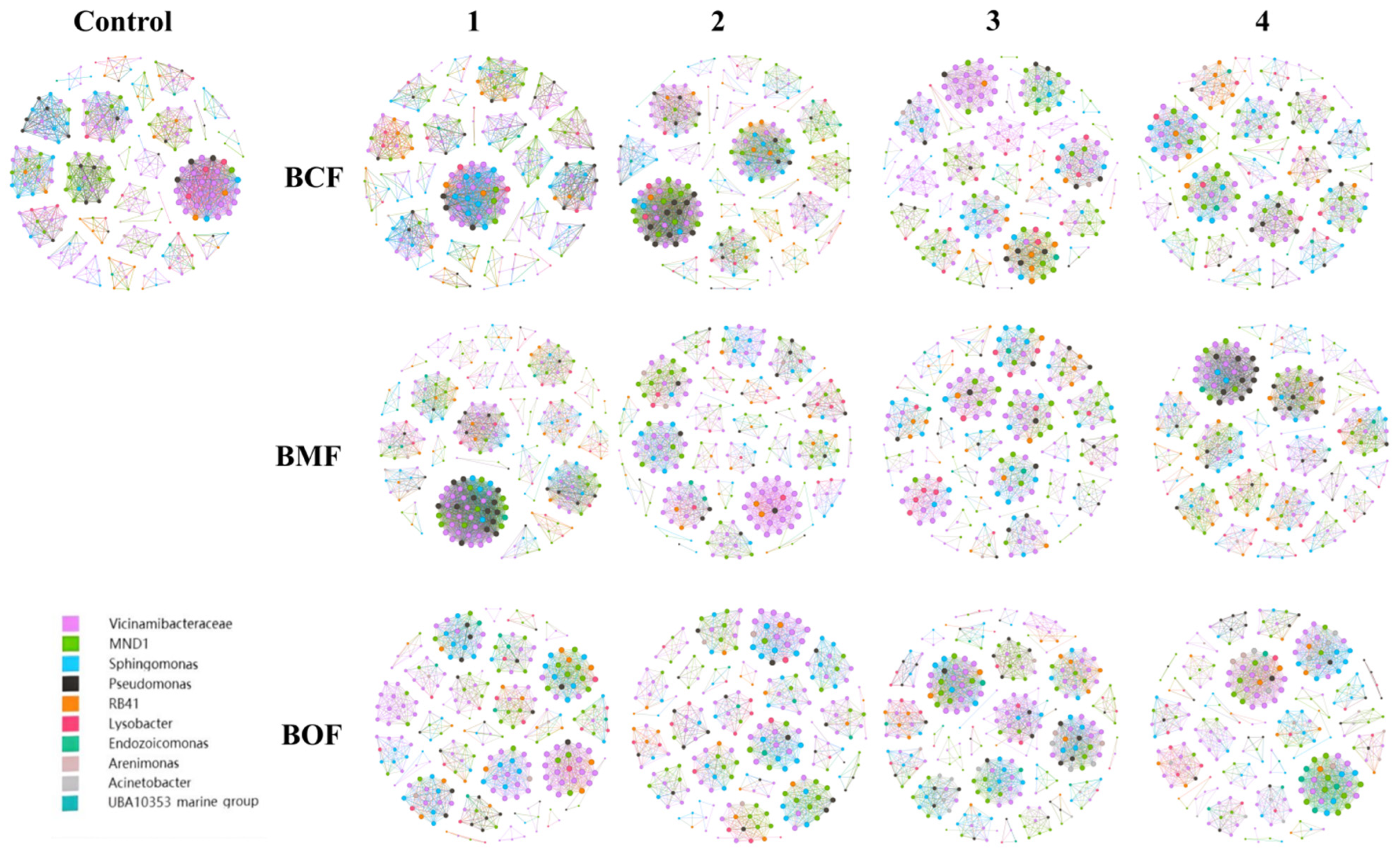
2.6. Co-Occurrence Network Analysis
The co-occurrence network analysis revealed that both fertilizer type and application concentration exerted significant impacts on the topological structure, connectivity, and modular organization of the soil microbial community (
Figure 3). Compared with the untreated control (Control), fertilized treatments exhibited pronounced differences in network density, node and edge numbers, and clustering coefficients, suggesting that fertilization substantially modulates microbial community interactions (
Table S6).
Low-concentration treatments (BCF1, BMF1, BOF1) tended to maintain higher network stability and promote cooperative interactions within the microbial community, as indicated by elevated clustering coefficients (e.g., BOF1: 0.92). In contrast, high-concentration treatments (BCF4, BMF4, BOF4) resulted in increased network complexity, exemplified by a notable rise in node number under BMF4 treatment, potentially reflecting intensified microbial competition and niche differentiation.
Fertilizer type also differentially influenced network properties. BCF series fertilizers, particularly BCF2, significantly increased network density (0.0605) and average degree (16.34), indicating an enhancement in inter-microbial connectivity. Interestingly, BMF1 exhibited the highest overall network density (0.0612), suggesting that low concentrations of BMF strongly promote microbial interaction networks. However, the clustering coefficient declined under high-concentration BMF4 treatment, implying potential disruption of cooperative structures due to competitive stress.
The BOF series fertilizers demonstrated a consistent positive effect on network cohesion. Specifically, BOF2 exhibited the highest clustering coefficient (0.92), reflecting enhanced cooperative interactions and potential community stabilization under this treatment.
Modularity analysis further highlighted the effects of fertilization on microbial functional organization. BMF1 treatment yielded a relatively low modularity index (0.74), suggesting limited compartmentalization and functional differentiation. In contrast, BOF2 exhibited the highest modularity (0.92), indicating enhanced functional partitioning likely driven by the selective enrichment of specific microbial guilds.
Collectively, these findings demonstrate that fertilization profoundly alters microbial network architecture, thereby influencing ecological functionality and stability. Low-concentration fertilization tends to sustain cooperative interactions and network stability, whereas high-concentration inputs may intensify competitive dynamics and restructure microbial niche distribution. Among the tested fertilizers, BOF series treatments exerted the most substantial influence in promoting microbial network stability and modular complexity, underscoring their potential to enhance soil microbial ecosystem resilience through selective facilitation of functional microbial groups.
2.7. PLS-PM Analysis
Partial Least Squares Path Modeling (PLS-PM) was employed to unravel the complex interactions among microbial diversity, soil physicochemical properties, plant growth, photosynthetic activity, and yield quality, thereby elucidating potential mechanisms underlying soil–microbe–plant interactions. The model demonstrated strong overall explanatory power, with a goodness-of-fit (GoF) value of 0.6503.
Path analysis revealed that microbial diversity exerted the strongest direct effect on soil properties (path coefficient β = 0.7287, p < 0.0001), emphasizing the foundational role of microbial communities in regulating soil nutrient availability, structure, and overall environmental quality. In turn, soil properties had significant positive effects on both plant growth (β = 0.4717, p = 0.0089) and photosynthetic efficiency (β = 0.7319, p < 0.0001), indicating that improved soil quality facilitates not only vegetative development but also physiological performance. Additionally, plant growth positively influenced photosynthesis (β = 0.3869, p = 1.58 × 10−4), suggesting a reinforcing relationship between biomass accumulation and resource assimilation capacity.
In terms of yield formation, soil properties had the most pronounced direct impact on yield quality (β = 0.5205, p = 0.0109), followed by microbial diversity (β = 0.2783, p = 0.0275), underscoring the central role of soil–microbe interactions in determining crop productivity. Furthermore, microbial diversity also had a strong indirect effect on yield quality through soil improvement pathways (indirect β = 0.4813), highlighting its multifaceted contribution to agroecosystem function. Conversely, photosynthesis exhibited a weak and statistically non-significant direct effect on yield quality (β = 0.1842, p = 0.3706), and plant growth showed no significant direct association (β = −0.0207, p = 0.8787), indicating that aboveground traits alone are insufficient to explain yield variability under the tested conditions.
The model’s coefficients of determination (R
2) confirmed its strong explanatory capacity across system components. Microbial diversity explained 53.1% of the variance in soil properties (R
2 = 0.531); soil conditions accounted for 51.0% of the variance in plant growth (R
2 = 0.510); photosynthesis was largely driven by soil and plant variables, with R
2 = 0.857; and yield quality exhibited R
2= 0.799, indicating that microbial diversity, soil quality, and photosynthesis collectively explained nearly 80% of its variation (
Figure 4).
These findings collectively underscore the ecological and functional centrality of microbial communities in shaping soil quality and crop productivity. While photosynthetic rate and vegetative growth are necessary physiological processes, they were not sufficient predictors of yield under the tested fertilization regimes. Instead, belowground biotic and abiotic interactions—especially those modulated by microbial diversity—play a dominant role in driving agricultural output and system resilience.
3. Discussion
This study provided a comprehensive evaluation of the impact of different types and application rates of organic fertilizers on soil physicochemical properties, microbial community structure, and tomato growth in a controlled greenhouse environment. The results highlighted the complex interactions between soil quality, rhizosphere microbial dynamics, and plant performance, emphasizing that fertilization practices can significantly influence agroecosystem functioning by altering soil nutrient availability and the rhizosphere microbiome.
To link nutrient inputs with plant performance, we compared the labeled dry-basis compositions with the as-applied (fresh-weight) rates. The BOF treatments supplied more nitrogen per hectare than BCF or BMF, and the largest increases in photosynthetic traits and yield were observed under the higher-rate BOF treatments. This pattern suggests that N availability was the predominant agronomic driver. Concurrent changes in soil properties (total N, Olsen-P, available K, and organic matter) reflect how inputs moved through and were processed by the soil. Within our conceptual framework, fertilizer type × rate determines nutrient delivery, and the soil mediates downstream effects on rhizosphere microbial structure and plant performance.
The application of high-dose bio-organic fertilizer (BOF4) resulted in a significant increase in microbial species richness, as indicated by elevated OTU and Chao1 indices. This suggested that nutrient-rich organic inputs fostered microbial biodiversity, a key factor in sustaining soil fertility and enhancing plant resilience. Notably, the Simpson index revealed that higher fertilization levels led to microbial dominance, potentially compromising community evenness. This finding was consistent with previous studies, which reported that organic amendments selectively enrich certain microbial taxa, often at the expense of others, thereby disrupting microbial equilibrium [
18,
19].
A particularly significant observation was the marked increase in the relative abundance of Proteobacteria under the BOF4 treatment. This phylum is well known for its essential roles in organic matter decomposition, nitrogen fixation, and plant pathogen suppression [
19]. The selective enrichment of Proteobacteria at higher fertilization levels suggested that organic fertilizers promote the proliferation of functionally beneficial microbial taxa, thereby facilitating nutrient cycling and enhancing crop health. These results aligned with previous studies reporting that organic amendments stimulate the abundance of key microbial groups crucial for soil biogeochemical processes [
20].
In addition to shifts in microbial communities, high-dose organic fertilization significantly increased soil organic matter (OM), total nitrogen (TN), available phosphorus (AP), and available potassium (AK), all of which were positively correlated with the abundance of Proteobacteria. These relationships suggested that microbial communities not only responded to nutrient enrichment but also actively mediated soil nutrient retention and transformation, reinforcing the critical role of microbial diversity in driving soil fertility and crop yield formation.
Beyond taxonomic composition, fertilization also altered the topology of microbial co-occurrence networks. Low-dose treatments were associated with higher clustering coefficients, indicating enhanced microbial cooperation and community stability. This supports the view that moderate nutrient availability fosters cooperative microbial associations and community stability, which are important for maintaining soil health. In contrast, high-dose treatments increased network complexity, as evidenced by a greater number of nodes, but concurrently reduced clustering coefficients. These changes suggested that while increased nutrient levels promoted microbial diversity, they also intensified interspecies competition, thereby reducing the structural cohesion of the microbial network. This pattern aligned with previous studies indicating that nutrient over-enrichment disrupts microbial interaction networks and weakens ecological stability [
18,
19]. Our findings implied that moderate application rates—particularly BOF2—optimized the balance between diversity and stability, enhancing microbial functionality without destabilizing community structure.
The model revealed that soil nutrient status (notably total N) and microbial diversity together structured the downstream responses, with the soil block serving as the proximal mediator between fertilization and plant traits. To further elucidate the mechanisms linking microbial diversity, soil function, and crop outcomes, we employed Partial Least Squares Path Modeling (PLS-PM). The model revealed that microbial diversity exerted the strongest direct effect on soil properties, reinforcing its central role in regulating soil nutrient availability and structure [
20,
21]. Improved soil properties, in turn, significantly enhanced plant growth and photosynthetic efficiency, highlighting the indirect pathways through which microbial diversity influences aboveground physiological processes. Accordingly, the stronger BOF responses are consistent with its higher N input per hectare, while paths are interpreted as associations rather than proof of direct causation.
Importantly, physiological parameters such as net photosynthetic rate (Pn), stomatal conductance (gs), and transpiration rate (Tr) showed positive associations with tomato yield and fruit quality. However, the PLS-PM results indicated that their direct effects on yield were weak and statistically insignificant. This discrepancy suggested that while photosynthetic efficiency contributed to plant growth, it was not the sole determinant of yield formation under organic fertilization. Instead, belowground interactions—particularly those mediated by microbial diversity and soil nutrient status—emerged as the dominant drivers of crop productivity. These findings underscored the quantitative relationship between physiological parameters and yield, while also explaining why their direct effects appeared limited when analyzed in the structural model.
This interpretation was further supported by Mantel’s test, which revealed strong positive correlations of OM, AP, AK, and TN with both yield and sugar content. In contrast, soil pH showed a significant negative correlation with AP and yield, indicating that soil acidification might have hindered phosphorus availability and reduced yield potential [
22,
23].
Future equal-N comparisons across fertilizer types would further disentangle type-specific effects from nutrient supply, complementing the system-level evidence reported here. While this study provided valuable insights into the short-term effects of organic fertilization, future research should focus on long-term field trials to assess the cumulative effects of organic fertilizers on soil health, microbial succession, and crop productivity. Moreover, integrating molecular approaches such as metagenomics and metabolomics will be crucial in uncovering the root–microbe feedback mechanisms that govern sustainable soil management.