Abstract
Grain legume crops are rich in nutritional value and play a crucial role in global food sustainability. Like many other crops, they are affected by various abiotic stresses that reduce yield and seed quality, thereby threatening food security. Several strategies have been proposed to mitigate these effects and enhance yield. Among them, the use of biostimulants offers a sustainable and efficient approach to improving stress tolerance in the short term. However, the molecular mechanisms underlying the effects of individual or combined molecules remain poorly understood and could significantly influence the development of edited crops with enhanced stress tolerance in the long term. Melatonin (MT) has emerged as a versatile biostimulant, providing multiple benefits across different crop species. Given its key role in plant physiological processes, along with endogenous production, receptor identification, and signaling functions, it has been suggested to act as a hormone-like molecule. Nonetheless, the molecular response triggered by its application remains under investigation, particularly in grain legume species. This review explores the current state of MT applications for alleviating abiotic stress in grain legume crops, with emphasis on drought, salinity, metals/metalloids, and heat stress. We integrate biochemical, molecular, and physiological evidence to highlight the main scientific gaps regarding MT function in grain legumes. Finally, we discuss the biotechnological prospects of combining MT with modern breeding tools, as well as strategies for its delivery and sustainable production.
1. Introduction
Grain legume crops are an important source of nutrients in the human diet, particularly in low-income and developing countries. In 2023, the harvested area of pulses reached 96 million hectares, with a production of 94 million tons [1]. These staple foods, together with cereals, will be essential to meet human food requirements in the coming years, when significant population growth is expected [2]. Legumes are rich in proteins, minerals, essential amino acids, dietary fiber, vitamins, and bioactive compounds, making them highly nutritious foods [3], with the potential to prevent chronic diseases such as obesity, type II diabetes, and cardiovascular disorders [4]. However, as in many other crops, the yield and sustainability of grain legumes are threatened by multiple abiotic stressors, such as drought, salinity, metal and metalloid soil contamination, and heat, among other increasingly recurrent factors. Indeed, these stressors can occur simultaneously or sequentially, producing devastating effects on smallholder and family farming systems [5]. To address this scenario, multiple strategies have been implemented in grain legume crops to select and release varieties more adapted to fluctuating climatic conditions or to protect elite varieties from stressful environments. These strategies include classical and marker-assisted breeding platforms [6], transgenic approaches, varieties developed through genome-editing tools such as CRISPR/Cas [7], and the use of biostimulants to improve agronomic performance and seed quality under stress [8]. Additionally, efficient agronomic management practices, such as irrigation [9] and crop rotation systems [10], are fundamental to improving grain legume crop production worldwide.
Currently, the use of biostimulants in agriculture as a sustainable strategy to mitigate the detrimental effects of abiotic stressors on crop yield is under continuous investigation. The diversification of biostimulants includes plant growth-promoting microorganisms, humic substances, phenolic compounds, amino acids, seaweed polysaccharides, and plant hormones [11,12,13]. Among the most studied elicitor molecules, plant hormones such as absicic acid (ABA), salicylic acid (SA), jasmonates (JA), gibberellins (GA), cytokinins (CK), brassinosteroids (BR), and strigolactones (SL) have shown great potential to increase abiotic stress tolerance by modulating central physiological and cellular processes in different plant species [14].
In recent years, scientific evidence has suggested that melatonin (N-Acetyl-5-methoxytryptamine), a biogenic indole amine, plays a role in maintaining plant cellular homeostasis. Melatonin (MT) is a naturally occurring molecule identified in eukaryotic organisms (animals, plants, algae) and in prokaryotes (bacteria). In plants, MT has been reported to participate in several physiological processes, including germination [15], root growth [16], stomatal regulation [17], fruit ripening [18], and stress responses [19]. In this context, MT has been described as a novel plant hormone with pleiotropic effects. Under abiotic stress, this molecule acts as a hormonal compound and/or regulator of several cellular processes in plants, such as protecting and stimulating photosynthesis [20,21], inducing enzymatic antioxidant responses, and promoting the synthesis of osmoprotectant compounds [22]. The discovery of the MT receptor PMTR1 in guard cells of Arabidopsis and Vicia faba opened new avenues to understanding its molecular role in stomatal regulation [23]. Furthermore, MT applications in Malus prunifolia, Malus hupehensis, and Zea mays under drought conditions have been shown to downregulate ABA biosynthetic components, thereby inducing stomatal opening and restoring photosynthesis [24,25,26]. Otherwise, contradictory results have been reported in Arachis hypogaea, through the assessment of the same ABA-related genes [27], while in fodder soybean (Glycine max L.), MT triggered ABA-independent drought resistance mechanisms [28]. Moreover, interactions with other cellular components or cis-regulatory elements that explain its pleiotropic effects on physiological processes remain elusive. Moreover, despite growing evidence on the benefits of MT applications in alleviating abiotic stress in different species [20,29], the regulation of endogenous MT production, its interaction with other hormones, and its molecular mechanisms of action still need to be elucidated. This review aims to summarize the current knowledge on the use of MT in grain legume crops to alleviate abiotic stress, highlight the main insights into its molecular mechanisms, and discuss biotechnological prospects for future research on this versatile molecule.
2. Literature Search Methodology
We performed a literature search using databases such as PubMed, Web of Science, Scopus, ScienceDirect, and Google Scholar, with key terms including “melatonin”, “legumes”, “grain legumes”, “abiotic stress”, and “biostimulants”. The key terms were used alone or in combination to filter studies specifically related to grain legume species. To construct the tables, additional key terms were included for selected abiotic stress factors, such as “drought”, “salinity”, “metal”, “metalloids”, and “heat”. The methodology described in all selected articles was analyzed, and information regarding each stress treatment, type of melatonin application, concentration of melatonin solution, and the main physiological, biochemical, and molecular effects was extracted. We included only studies published in English. No restriction on publication date was applied.
3. Abiotic Stress Alleviation in Grain Legumes Through MT Applications
3.1. Drought Stress
Drought is one of the major abiotic stresses affecting plant metabolism, with significant impacts on the growth, development, and productivity of different species [30]. In addition to its ecophysiological consequences on forests and native species, from an agricultural perspective, its importance lies in the reduction of crop yields, thereby threatening global agrifood security [31]. Thus, it is crucial to establish strategies to maintain or increase yields in drought-affected regions to meet future food demands, considering a projected world population of 10 billion by 2050 [2]. Drought-affected areas are distributed across all five continents, and no improvement in this situation is expected in the coming years due to fluctuations in precipitation patterns exacerbated by climate change. Indeed, projected climate change models show increasingly severe drought scenarios regardless of whether greenhouse gas emissions increase or decrease, across much of the world [32]. Experiments performed in grain legume crops have shown a positive relationship between drought intensity and yield reduction; however, interspecific and varietal differences exist [33]. This broad genotypic and phenotypic variability has enabled the selection of more drought-tolerant varieties, which display differentiated metabolic responses under drought conditions. Some studies have reported that grain legume species respond to drought using at least two resistance mechanisms: avoidance and tolerance. Avoidance mechanisms are mediated by the efficient regulation of stomatal opening [34], whereas tolerance mechanisms involve osmotic adjustment, which maintains turgor and root growth, enabling water uptake under low soil water potential [35]. These mechanisms have been observed in both classical grain legume crops and woody legume species [36]. However, while stomatal regulation decreases CO2 fixation, negatively affecting photosynthesis and yield, osmotic adjustment mechanisms may be more energetically favorable [35]. In addition, drought induces profound changes in the primary and secondary metabolism of grain legumes [37,38]. Activation of metabolic pathways leading to the synthesis of osmoprotectant molecules can alter the allocation of photo-assimilates and other compounds to the developing grain, ultimately affecting its nutritional quality [39]. Another metabolic consequence of drought is the overproduction of reactive oxygen species (ROS), which generates redox imbalance at the cellular level, leading to thylakoid membrane damage through the oxidation of lipids and proteins, and impairing photosynthesis and other physiological functions [40].
One strategy to manage drought stress is the use of biostimulant formulations that enhance plant metabolic stress responses, such as the activation of enzymatic and non-enzymatic antioxidant machinery, and the induction of osmotic adjustment mechanisms [41]. Commonly used biostimulants include amino acids, peptides, seaweed polysaccharides, and silicon, among others [12,42]. Over the last decade, increasing evidence has supported the use of MT as a biostimulant to improve plant physiological functions under abiotic stress [21,43]. In grain legume species, MT has been applied to mitigate the detrimental effects of drought stress on plant physiology in soybean [28,44,45,46,47,48,49,50], mung bean [51], lentil [52], chickpea [53], and common bean [54,55]. Table 1 summarizes the main studies reporting the ameliorative effects of MT on drought-stressed grain legumes. A common response observed after MT application under drought conditions is an increase in enzymatic antioxidant activity, which decreases oxidative stress triggered by drought and improves the functionality of the photosynthetic machinery, ultimately enhancing biomass and yield (Table 1). In soybean, MT application has also been shown to affect the expression of genes involved in nitrogen metabolism, thereby influencing nitrogen accumulation and photosynthetic capacity [48]. In addition, the synthesis of osmoprotectant molecules, such as amino acids and soluble sugars, has been reported in several grain legume crops following MT treatment under drought stress.

Table 1.
Studies on MT applications in grain legumes for alleviating drought stress.
3.2. Salinity Stress
Salinity stress is a major abiotic factor limiting crop yield, particularly in arid and semi-arid regions where salt concentrations are naturally high. Soil salinization can result from several factors, including the conversion of coastal habitats into agricultural lands, flooding, sea-level rise, and geological conditions, among others [56,57,58]. Salinity stress disrupts various physiological processes in grain legume crops, such as germination, growth, development, and nitrogen fixation [59]. In plants, two main stages of salt stress have been identified: the osmotic phase and the ionic phase. The osmotic and ionic stress imposed by salinity reduces cell expansion and, consequently, tissue growth, thereby affecting the yield and quality of grain legumes [60]. The first, early phase is associated with the effects of high salt concentrations outside the root zone, while the second refers to the toxic effects of salt accumulation within plant tissues [61]. Therefore, the molecular mechanisms of salt stress tolerance in plants are temporally and spatially separated. Elevated Na+ and Cl− concentrations interfere with the assimilation of other nutrients such as N, P, B, K, Ca, Mg, Zn, Cu, and Fe, among others, inducing a nutritional ionic imbalance that impairs photosynthesis and other biochemical processes [59,62]. At the cellular level, salinity stress leads to an accumulation of toxic ions in the cytoplasm, disturbing ionic equilibrium and increasing ROS concentrations, which in turn causes the disintegration of cellular and organelle membranes. Such damage compromises photosystem functionality and reduces chlorophyll and carotenoid content, thereby impairing photosynthesis and productivity [63]. Most grain legumes are sensitive to salinity stress, which disrupts essential physiological functions throughout their phenology. Common responses to salinity stress in grain legumes include Na+ sequestration and exclusion from the cytoplasm, accumulation of osmoprotectant molecules (Pro, GB, GABA, PAs), enhanced antioxidant enzyme activity (CAT, POD, SOD, APX, GR, DHAR, and MDHAR), and hormonal regulation, all of which contribute to restoring ionic and oxidative homeostasis [60,64]. Various strategies have been used to alleviate salt stress in legume species, including chemical priming, inoculation with nitrogen-fixing bacteria, plant growth-promoting rhizobacteria, arbuscular mycorrhizal fungi, genetic manipulation, and the identification of salinity-adapted cultivars [65]. Most of these approaches focus on improving nutrient uptake, enhancing antioxidant machinery, and stimulating the osmoprotectant synthesis. In chemical priming, one of the most commonly used molecules is SA, which enhances photosynthesis and antioxidant responses, thereby reducing the effects of salinity in Pisum sativum [66] and Vicia faba [67]. By contrast, the use of MT to alleviate salinity stress has recently emerged as an alternative in several crops, including grain legumes. For instance, in soybean, exogenous MT applications induce the expression of genes involved in cell division, carbohydrate metabolism, fatty acid biosynthesis, and ascorbate metabolism [44], thereby counteracting the inhibitory effects of salt stress on gene expression. Similarly, in common beans, MT application to salt-stressed sprouts alleviated growth inhibition, increased antioxidant enzyme activity, and upregulated the expression of genes involved in the phenylpropanoid pathway [68]. Table 2 summarizes recent reports on MT use in grain legumes, highlighting their biochemical, molecular, and physiological effects under salinity stress.

Table 2.
Studies on MT applications in grain legumes for alleviating salinity stress.
3.3. Metal and Metalloid Stress
Due to their sessile nature, plants are often exposed to high concentrations of metals and metalloids in the soil, leading to a range of anatomical, physiological, and molecular responses. This exposure results from the widespread accumulation of these elements in soils and is largely associated with anthropogenic activities such as industrialization, excessive fertilizer use, and mining [79]. In addition to their toxic effects on crop physiology and implications for yield, the accumulation of metals and metalloids in plant tissues poses a threat to food security and human health due to their entry into the food chain [80,81]. While some of these elements are essential for plant metabolism, acting as cofactors in various enzymatic reactions, they become hazardous at high concentrations, inducing oxidative stress and cellular damage. Significant impacts on physiological processes such as transpiration, stomatal conductance, photosynthesis, and chlorophyll content, along with growth inhibition, have been associated with metal exposure in plants [82]. The toxicity of metals and metalloids has been reported in several species, with cadmium (Cd) being one of the most widely studied. The primary effects of Cd in plants include growth inhibition, chlorosis, impaired stomatal opening, disturbed water balance, reduced nutrient uptake, and decreased photosynthetic rates [83]. At the cellular level, Cd induces oxidative stress and ROS production, impairing enzymatic antioxidant machinery, with effects that vary depending on Cd concentration and plant species [84]. In legumes specifically, Cd exposure negatively affects germination rate, stem and primary root length, leaf number, lateral root development, and seedling dry weight in common bean [85], while reductions in roots and shoots’ dry weight have also been observed in soybean and lupine [86]. Likewise, reduced root biomass has been reported in peanut plants exposed to high concentrations of Fe [87]. Arsenic (As) toxicity has been described in soybean, where exposure leads to reduced seedling growth and root tip necrosis [88], while in Cajanus cajan, significant alterations in root growth and anatomy have been observed under As stress [89]. Some plant species exhibit mechanisms to cope with metals and metalloid stress by producing chelating molecules such as metallothioneins, phytochelatins, amino acids, nicotinamides, glutathione, and defensins [90,91].
The use of biostimulants to mitigate the detrimental effects of metal and metalloids on plant metabolism has been previously reviewed [92]. Among the most commonly used are microbial consortia, plant extracts, seaweed extracts, humic substances, and protein hydrolysates. These compounds enhance antioxidant responses in plants under metal/metalloid stress, thereby helping to restore oxidative homeostasis. In the case of MT, exogenous applications in tomato have been shown to reduce Cd root-to-shoot translocation and enhance sulfur (S) metabolism [93]. In wheat, MT alleviates Cd stress by increasing nitric oxide (NO) levels and decreasing oxidative stress [94]. In addition, reports describing the effects of MT under metal and metalloid stress in peanut, soybean, faba bean, and mung bean are summarized in Table 3, highlighting the main biochemical, molecular, and physiological responses. Overall, MT applications in grain legumes exposed to metal/metalloid stress have shown metal/metalloid-dependent responses, with multiple effects in ameliorating oxidative stress.

Table 3.
Studies on MT applications in grain legumes for alleviating metal and metalloid stress.
3.4. Heat Stress
It is well-known that heat stress events have increased due to climate change, leading to reduced plant growth and crop yields [100]. According to some studies, the average temperature on Earth’s surface increased by 1 °C between 1951 and 2012, with projections indicating a further increase of 1.5 °C between 2030 and 2052, between 2.5 and 4.8 °C by 2100 [101]. Therefore, heat stress is expected to become a critical factor for plants in the coming years. Heat stress negatively affects root and shoot growth, seed filling, photosynthesis, and protein synthesis, and induces the production of reactive oxygen species (ROS), which damage molecules and cellular organelles in plants [102,103,104,105]. In grain legume crops, negative impacts have also been reported under heat stress. Flowering has been identified as a particularly sensitive stage, with heat causing abnormal pollen grains and reducing pollen viability, pollen tube growth, and pollen germination [106,107]. Additionally, heat stress has a detrimental impact on pollen traits, thereby reducing yield components. For example, Wang et al. [102] reported that Cicer arietinum plants exposed to heat stress early in the flowering stage showed reductions in seed number per plant and seed size. Similarly, Sita et al. [108] found that heat stress reduced pollen tube elongation in Lens culinaris, leading to decreased grain yield. Conversely, Barros et al. [109] observed that heat stress induced flower abortion, reduced pollen viability and seed number, and decreased yield in Vigna unguiculata.
It has also been reported that grain legume crops respond to heat stress through tolerance and avoidance mechanisms, such as increased transpiration rates, development of waxy protective layers, synthesis of osmoprotectant molecules, activation of enzymatic and non-enzymatic antioxidants, and regulation of plant hormone levels [110,111,112]. In this context, positive responses have been observed following the exogenous application of certain biostimulants in grain legume crops exposed to heat stress. For instance, the exogenous application of proline, glycine betaine, salicylic acid, and abscisic acid enhanced plant growth and yield in Cicer arietinum [113,114,115]. Similarly, exogenous application of MT has been suggested to improve physiological processes in grain legume crops, thereby increasing growth and yield [116]. Table 4 summarizes studies on MT applications in grain legumes under heat stress, highlighting their main biochemical and physiological effects.

Table 4.
Studies on MT applications in grain legumes for alleviating heat stress.
4. Integrating Molecular and Biochemical Insights into MT Functioning in Grain Legumes
The stress-mitigating effects of MT treatments have been studied in several grain legume species (Table 1, Table 2, Table 3 and Table 4), yielding results similar to those observed in other crops [121,122]. The favorable physiological effects of MT-treated plants appear to include both direct and indirect responses, involving enhanced antioxidant capacity triggered by MT. This enhancement is characterized by increased activity of antioxidant enzymes and higher levels of antioxidant compounds, such as flavonoids, phenolic compounds, proline, and melatonin itself (Table 1, Table 2, Table 3 and Table 4). MT is an intrinsic antioxidant molecule with a high capacity to scavenge ROS [123]. Owing to its amphipathic properties, it can cross biological membranes to broaden its targets [124]. Environmental stresses lead to an imbalance of reactive oxygen species (ROS), exposing plant membranes, organelles, and biomolecules to oxidative damage, thereby disrupting cellular homeostasis [125]. Superoxide (O2−) is one of the main ROS produced under abiotic stress, as a consequence of electron leakage in the electron transport chain (ETC) of both chloroplasts and mitochondria [126]. Additionally, hydrogen peroxide (H2O2) and hydroxyl radicals (OH) can form as a result of superoxide reactions [127]. High levels of ROS induce the expression of antioxidant enzymes (SOD, APX, CAT, POD, among others), along with the production of molecules derived from the phenylpropanoid pathway, such as phenolic compounds and flavonoids, to cope with cellular oxidative stress [128]. This activation of antioxidant machinery involves signal transduction and the expression of regulatory genes, which have been extensively studied in model plant species [129] but less explored in legumes. Thus, the fine-tuned regulatory role of MT in antioxidant machinery activation remains elusive. Two main questions therefore need to be addressed: (1) To what extent is the boost in antioxidant capacity a direct result of MT’s ROS scavenging? (2) Is MT directly involved in activating signaling pathways that regulate the transcription of antioxidant enzymes and molecules? The first question is difficult to answer due to experimental limitations, such as the inability to fully silence endogenous MT production in order to quantify ROS scavenging attributable solely to MT applications. For the second question, some insights have been reported in legumes. Interestingly, the effects of MT depend on its dose and mode of application, showing an active role in plant physiology at low concentrations across different organs [130]. Integrated transcriptomics and metabolomics studies have explored global responses and mechanisms triggered after MT applications under abiotic stress in common bean [68,72,73]. MT has been shown to increase the expression of genes related to cell wall metabolism in bean sprouts under salinity stress [73] and to enhance the expression of phenylpropanoid biosynthesis genes such as 4-coumarate-CoA ligase (4CL) and peroxidase (POD), along with lignin accumulation [68], suggesting a link between MT and cell wall reinforcement. In addition, under salt stress, MT treatment enhanced tryptophan metabolism and upregulated the expression of a putative transmembrane salt transport gene [72]. In soybean, available evidence under salt stress suggests that MT upregulates the expression of genes involved in cell division, photosynthesis, carbohydrate metabolism, fatty acid biosynthesis, and ascorbate metabolism, although no clear specific mechanism of action has been established [44]. In alfalfa (Medicago sativa L.), exogenous MT induces the transcription of genes involved in Ca2+ signaling, starch and sucrose metabolism, plant hormone signal transduction, and key transcription factors (C3Hs, MYBs, ERFs, and WRKYs), suggesting that MT plays an active role in starch and sugar metabolism and hormone-regulated signal transduction during salt stress [131]. Additionally, changes in the expression of calmodulin-like proteins (CLPs) in common bean roots after MT treatment confirm the interaction between the Ca2+ signaling pathways and MT in legumes [132]. The interaction between MT and calmodulin proteins has also been reported in other plant species [133,134], suggesting an indirect role of MT in regulating intracellular Ca2+ levels [135]. In Vicia faba, MT binds to calmodulin, altering its subcellular distribution. Furthermore, Ca2+ and melatonin treatments protect against As toxicity by enhancing antioxidant enzyme activity, regulating the AsA-GSH pathway, and activating plasma membrane H+-ATPase and Ca2+-dependent protein kinases [98].
On the other hand, the endogenous biosynthesis and regulation of MT production remain under active investigation. Although MT biosynthetic pathways have been more thoroughly characterized in model species, they are less understood in legume crops [136]. The main substrate for MT biosynthesis is tryptophan, which is first converted to tryptamine by tryptophan decarboxylase (TDC) and subsequently to serotonin by tryptamine 5-hydroxylase (T5H). Finally, serotonin undergoes acetylation and methylation, catalyzed by serotonin N-acetyltransferase (SNAT) and N-acetylserotonin methyltransferase (ASMT), respectively, to form melatonin [129]. Alternatively, this last step can also be catalyzed by caffeic acid O-methyltransferase (COMT). Interestingly, the expression of genes encoding these enzymes (SNAT, ASMT, T5H, TDC) increased in pepper after MT treatment, under cold stress [137]. Similarly, the expression of SNAT and ASMT genes increased following MT treatment during temperature stress in soybean [119], suggesting that exogenous MT may induce endogenous MT biosynthesis under stress conditions. Additionally, MT biosynthetic enzymes have been implicated in autophagy, antioxidant signaling, and stress responses in cassava [138]. Although some enzymatic steps are shared among species, the specific genes, gene copy numbers, gene compartmentalization, or possible alternative enzymes involved in MT biosynthesis in legumes have not been fully identified. The MT biosynthetic pathway in plants can vary among species and environmental conditions, and it is localized in multiple cellular compartments, including the chloroplast, mitochondria, and cytoplasm [138,139,140]. This compartmentalization likely contributes to the pleiotropic effects of MT in plants. Figure 1 presents a simplified schematic representation of MT functioning, highlighting the most studied steps, main effects, and critical gaps that remain in grain legumes.
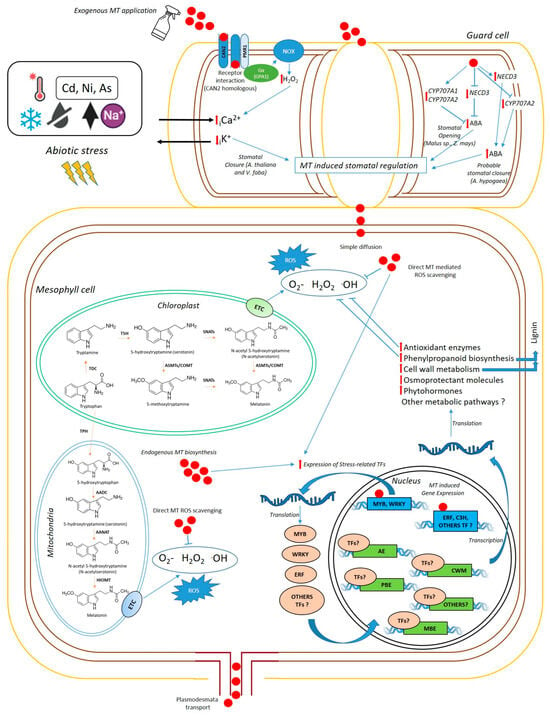
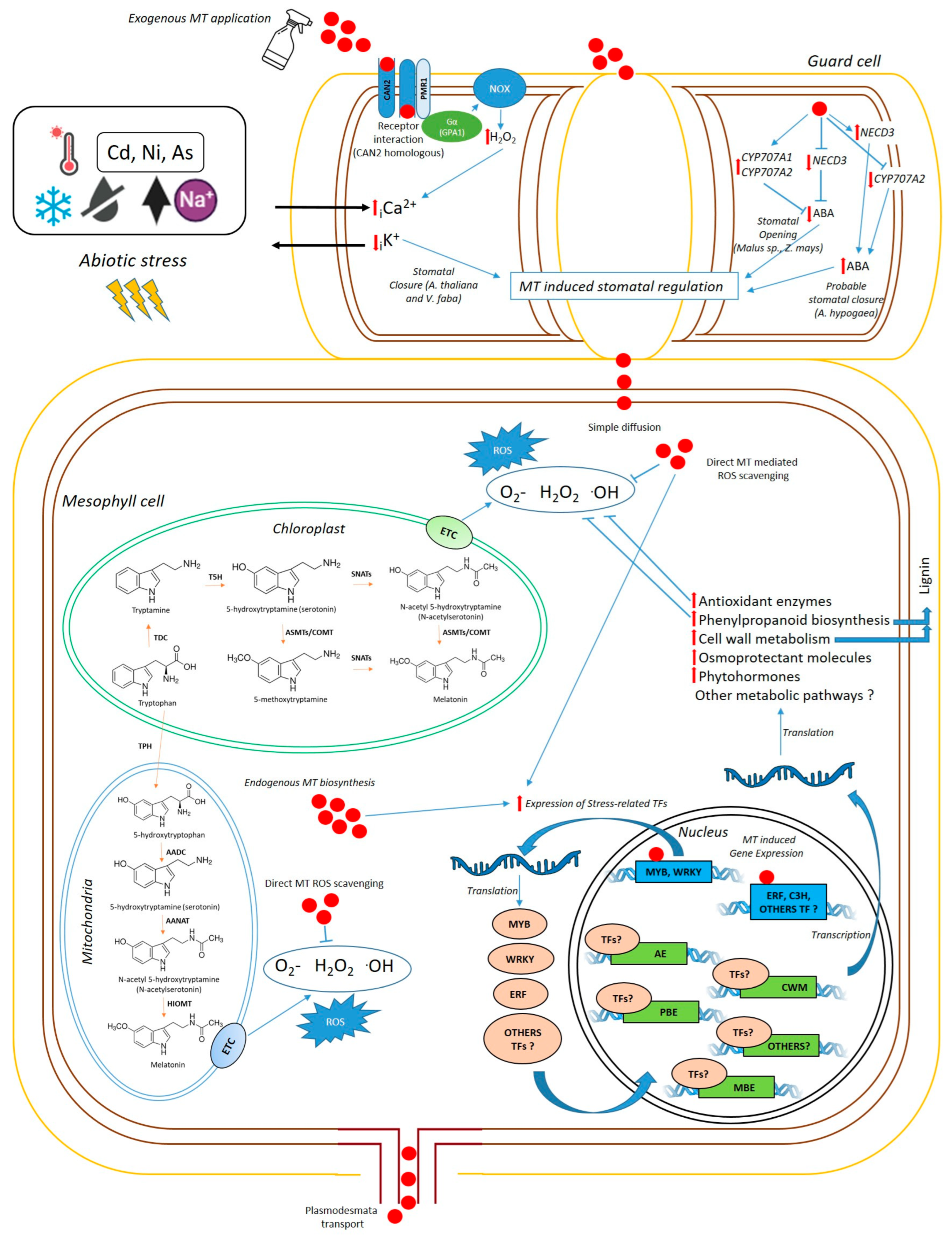
Figure 1.
Simplified schematic representation of MT functioning in plant cells of grain legumes at the leaf level (Original figure). In A. thaliana and V. faba, MT induces stomatal closure in guard cells through interaction with the CAN2/PMR1 receptor. CAN2/PMR1 activates H2O2 production via NADPH oxidase (NOX) through Gα(GPA1), inducing Ca2+ influx and K+ efflux, thereby triggering stomatal closure. In other species (Malus prunifolia, Malus hupehensis, and Zea mays), MT induces downregulation of NCED3 gene expression, decreasing ABA levels and promoting stomatal opening under drought. MT also upregulates ABA catabolism, inducing the expression of CYP707A1 and CYP707A2. Otherwise, in Araquis hypogaea, MT induces an upregulation of NCED3, and a downregulation of CYP707A2, increasing ABA levels. MT can enter mesophyll cells by simple diffusion or be transported across plasmodesmata. MT can reduce oxidative stress either by directly scavenging reactive oxygen species (ROS) or by activating the expression of stress-related transcription factors (TFs) which enhance the expression of proteins involved in multiple metabolic pathways to ameliorate abiotic stress, such as antioxidant enzymes (AE), phenylpropanoid biosynthetic enzymes (PBE), and enzymes involved in cell wall metabolism (CWM). Additionally, exogenous MT induces the expression of melatonin biosynthetic enzymes (MBE), enhancing endogenous MT synthesis.
5. Biotechnological Prospects for MT Research in Grain Legumes
The available information on the genetic control of metabolic responses induced under abiotic stress conditions, together with access to molecular editing tools, opens opportunities to manipulate key metabolic pathways to enhance stress tolerance in crops [141]. However, in grain legume crops, the implementation of editing protocols is particularly challenging due to the recalcitrant nature of transformation efficiency and in vitro regeneration of legume species [142]. Nevertheless, advances using CRISPR/Cas9 editing have been reported mainly for soybean to alleviate abiotic stress such as drought [143,144,145] and salinity [146,147]. Additionally, editing or transformation/regeneration protocols have been implemented for chickpea [148], common bean [149], faba beans [150], and other legume species [151], expanding the possibilities for genome editing in legumes. Because of the pleiotropic nature of MT action, identifying specific genetic targets to enhance MT-mediated abiotic stress tolerance without affecting other pivotal cellular processes could be challenging. However, recent advances have opened the possibility of modifying target genes involved in endogenous melatonin biosynthesis or the regulatory control of tissue-specific melatonin production [152]. Likewise, modifying MT receptors in cellular organelles susceptible to oxidative damage could enhance MT efficiency or tissue-specific translocation [153]. Recently, in soybean, a 31-fold increase in melatonin content was achieved using a synthetic transcriptional regulator, showing the potential for melatonin bio-production and enhanced stress resilience in plants [154]. In this context, further efforts are needed to identify species-specific biosynthetic pathways, gene copy numbers and sequence variants, their cellular compartmentalization, as well as additional melatonin receptors in legume crop species.
Current breeding approaches, such as candidate-gene and genome-wide association studies (GWAS) and genomic selection, will play a major role in identifying and selecting genetic variants and, consequently, cultivars more responsive to melatonin applications, allowing us to explore the broad genetic diversity present in grain legume crops [132]. For instance, the analysis of 111 CLPs in common bean identified nine MT-responsive genes expressed in roots, providing valuable information about the tissue-specific expression of CLP in the context of MT signaling pathways [132]. In Dianthus caryophyllus L., an ornamental species, ten genes involved in MT biosynthetic pathways were identified by in-silico genome-wide analysis, with some of them showing a MT dose-dependent expression, opening opportunities for functional editing [155]. In maize, alleles associated with root traits were identified by exploring the natural variation of melatonin biosynthetic genes [156], thus providing key loci for breeding maize varieties with superior root systems. Additionally, a valuable trait for human health applications is the search for plant species with higher MT content. A recent study showed the potential of GWAS to identify genes associated with MT content in Sesamum indicum L., an oilseed species with high MT content, exposing its biotechnological potential [157]. Moreover, a negative regulator of melatonin content and signaling was identified using GWAS in a natural population of 228 cassava accessions (Manihot esculenta). Melatonin accumulation 1 (MA1) encodes type 2C protein phosphatase 1 (PP2C1), which catalyzes the dephosphorylation of MeRAV1/2 and MeWRKY20, two transcriptional regulators of the melatonin biosynthetic pathway. In addition, MePP2C1 dephosphorylates the phyto-melatonin receptor MePMTR1, repressing its binding to melatonin [158]. These studies highlight the importance of modern breeding approaches in MT research.
Complementary to the aforementioned approaches, another major technology gap in MT research is the development of efficient MT delivery systems. MT is light-sensitive [159] and can degrade if applied under high-light conditions. Therefore, developing nanoparticle-based delivery protocols to protect MT during field application is key to ensuring its functionality under abiotic stress conditions. Some advances have been made in this area for biomedical uses of MT, such as selenium-based nanoparticles, chitosan-based nanoparticles, solid lipid nanoparticles, and nanostructured lipid carriers [160]. For crop applications, an efficient delivery system based on mesoporous silica nanoparticles with or without chitosan improved Cd tolerance in rice, reducing Cd concentrations in rice leaves by 43% [161]. Other candidate delivery systems for MT applications in crops, including chitosan and metallic nanoparticles, have been previously reviewed [162]. Despite these advances, challenges such as cost, stability, and delivery specificity still need to be addressed. Moreover, the use of microorganisms such as endophytic bacteria and rhizobacteria as a low-cost and sustainable MT source for production and delivery has also been explored with promising applications [163,164]. Combined with advances in microbial editing tools and synthetic biology, this approach could revolutionize the biomanufacturing of MT, lowering production costs and improving shelf life and stability (Figure 2).
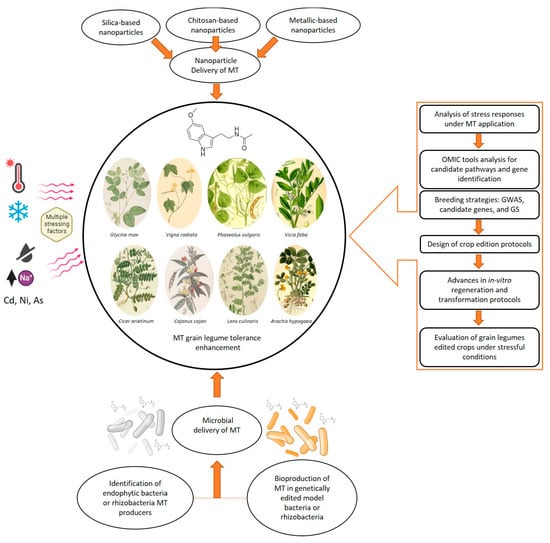
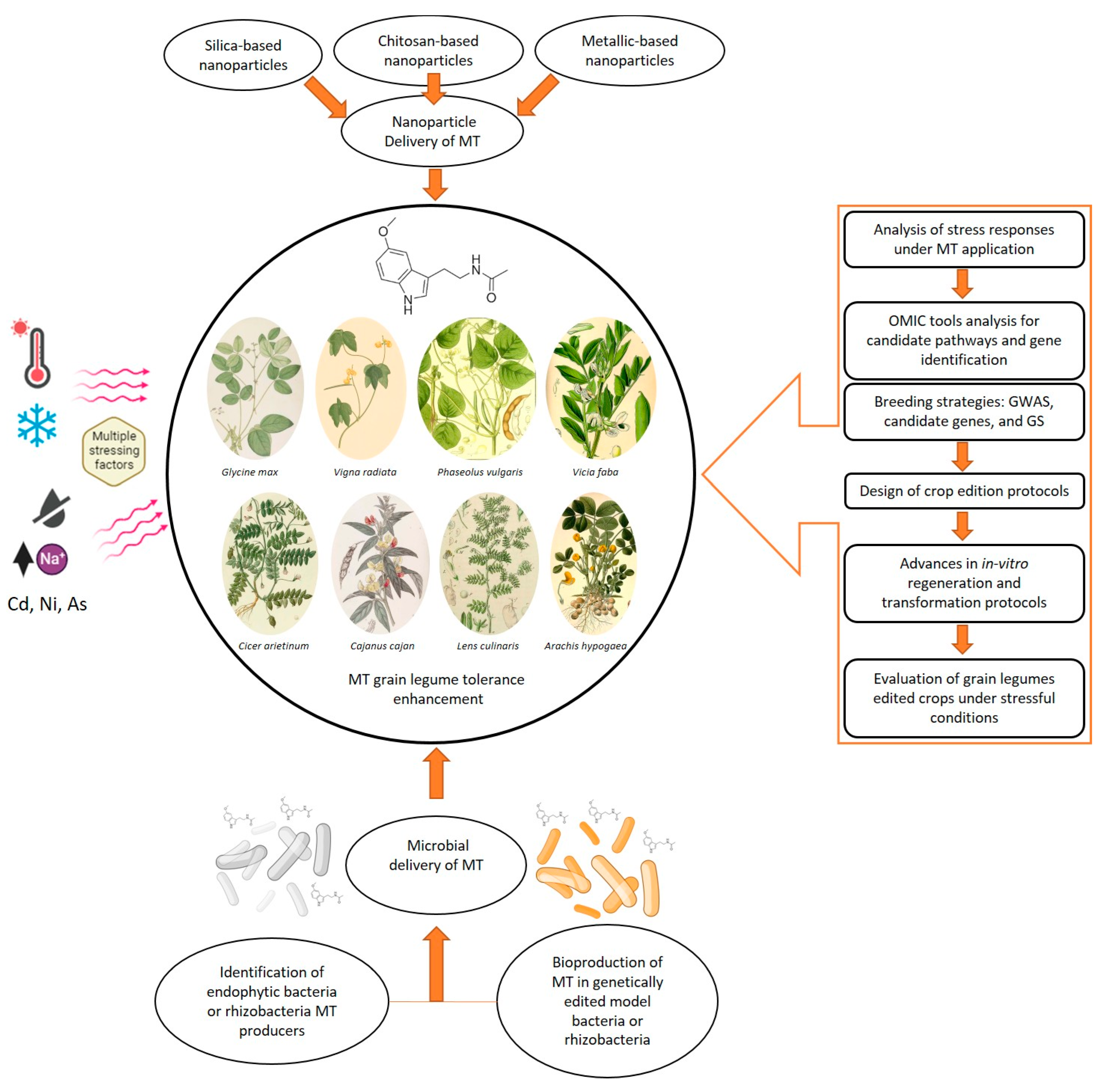
Figure 2.
Schematic representation of a methodological workflow based on melatonin applications in grain legumes under stress, highlighting how modern breeding strategies, biotechnology tools, improved delivery protocols, and microbial MT production can support MT research for sustainable agriculture (Original figure). Grain legume illustrations were obtained from www.plantillustrations.org. GWAS: genome-wide association studies; GS: genome selection.
6. Conclusions
In this review, we have highlighted recent advances in MT research for grain legume crops, detailing key biochemical and molecular insights into the effects of MT under major abiotic stress conditions. Common responses were observed in grain legumes, regardless of species or stress type, including the enhancement of enzymatic antioxidant machinery and nitrogen metabolism, together with the induction of antioxidant and osmoprotectant molecules. These responses boost photosynthetic capacity, growth, and ultimately yield in several grain legume species. Despite numerous reports assessing the effects of MT on grain legumes under abiotic stress, significant scientific gaps remain in understanding MT mechanisms and the regulation of endogenous MT, which must be addressed in the future. Additionally, the application of OMICs, genome editing tools, and breeding strategies will facilitate the selection of cultivars more responsive to MT and the development of edited varieties to enhance grain legume tolerance to abiotic stress. Finally, ongoing efforts to improve MT delivery and stability—through nanoparticles and microbial production—are expected to ensure the sustainability of MT applications.
Author Contributions
Conceptualization, H.A.G.; Investigation, H.A.G. and J.G.-V.; Writing—original draft, H.A.G., J.G.-V. and P.A.-J.; Writing—review and editing, H.A.G., J.G.-V. and P.A.-J.; Funding acquisition, H.A.G. and P.A.-J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANID Fondecyt Postdoctorado 3250436 and ANID Anillo ATE230007.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
H.A.G. thanks ANID Fondecyt Postdoctorado 3250436 grant. P.A.J. thanks ANID Anillo ATE230007 grant. We thank to Dirección de Investigación, Vicerrectoría de Investigación y Doctorados, Universidad Autónoma de Chile, for supporting the English language revision.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
MT: melatonin, CRISPR: clustered regularly interspaced short palindromic repeats, ABA: abscisic acid, SA: salicylic acid, JA: jasmonates, GA: gibberellins, CK: cytokinins, BR: brassinosteroids, SL: strigolactones, ROS: reactive oxygen species, SOD: superoxide dismutase, POD: peroxidase, CAT: catalase, APX: ascorbate peroxidase, GPX: glutathione peroxidase, PPO: polyphenol oxidase, MDHAR: monodehydroascorbate reductase, DHAR: dehydroascorbate reductase, GR: glutathione reductase, PEG: polyethylene glycol, MDA: malondialdehyde, Pro: proline, GB: glycine betaine, GABA: γ-aminobutyric acid, PAs: polyamines, GOGAT: glutamine oxoglutarate aminotransferase, NR: nitrate reductase, GS: glutamine synthetase, GDH: glutamate dehydrogenase, DPPH: 2,2-diphenyl-1-picrylhydrazil, AsA: ascorbate, GSH: glutathione, GA4: gibberellin, UE: urease, DAS: days after sowing, ETC: electron transport chain, CLPs: calmodulin-like proteins, TDC: tryptophan decarboxylase, T5H: tryptamine 5-hydroxylase, SNAT: serotonin N-acetyltransferase, ASMT: N-acetylserotonin methyltransferase, COMT: caffeic acid O-methyltransferase, GWAS: genome-wide association studies.
References
- FAO FAOSTAT, Statistical Database. 2022. Available online: https://www.fao.org/faostat/en/#home. (accessed on 1 July 2025).
- Hickey, L.T.; Hafeez, A.N.; Robinson, H.; Jackson, S.A.; Leal-Bertioli, S.C.M.; Tester, M.; Gao, C.; Godwin, I.D.; Hayes, B.J.; Wulff, B.B.H. Breeding Crops to Feed 10 Billion. Nat. Biotechnol. 2019, 37, 744–754. [Google Scholar] [CrossRef]
- Márquez, K.; Arriagada, O.; Pérez-Díaz, R.; Cabeza, R.A.; Plaza, A.; Arévalo, B.; Meisel, L.A.; Ojeda, D.; Silva, H.; Schwember, A.R.; et al. Nutritional Characterization of Chilean Landraces of Common Bean. Plants 2024, 13, 817. [Google Scholar] [CrossRef]
- Didinger, C.; Thompson, H.J. The Role of Pulses in Improving Human Health: A Review. Legume Sci. 2022, 4, e147. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. Plant Responses to Multifactorial Stress Combination. New Phytol. 2022, 234, 1161–1167. [Google Scholar] [CrossRef]
- Rubiales, D.; Annicchiarico, P.; Vaz Patto, M.C.; Julier, B. Legume Breeding for the Agroecological Transition of Global Agri-Food Systems: A European Perspective. Front. Plant Sci. 2021, 12, 782574. [Google Scholar] [CrossRef]
- Détain, A.; Bhowmik, P.; Leborgne-Castel, N.; Ochatt, S. Latest Biotechnology Tools and Targets for Improving Abiotic Stress Tolerance in Protein Legumes. Environ. Exp. Bot. 2022, 197, 104824. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, P.; Patidar, J.K. Chapter 13—Biostimulants for Improving Nutritional Quality in Legumes. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Vaishnav, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 261–275. ISBN 978-0-323-85579-2. [Google Scholar]
- Blessing, C.H.; Mariette, A.; Kaloki, P.; Bramley, H. Profligate and Conservative: Water Use Strategies in Grain Legumes. J. Exp. Bot. 2018, 69, 349–369. [Google Scholar] [CrossRef]
- Li, J.; Liu, K.; Zhang, J.; Huang, L.; Coulter, J.A.; Woodburn, T.; Li, L.; Gan, Y. Soil–Plant Indices Help Explain Legume Response to Crop Rotation in a Semiarid Environment. Front. Plant Sci. 2018, 9, 1488. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Fernandes, Â.; Plexida, S.; Chrysargyris, A.; Tzortzakis, N.; Barreira, J.C.M.; Barros, L.; Ferreira, I.C.F.R. Biostimulants Application Alleviates Water Stress Effects on Yield and Chemical Composition of Greenhouse Green Bean (Phaseolus vulgaris L.). Agronomy 2020, 10, 181. [Google Scholar] [CrossRef]
- Mandal, S.; Anand, U.; López-Bucio, J.; Radha; Kumar, M.; Lal, M.K.; Tiwari, R.K.; Dey, A. Biostimulants and Environmental Stress Mitigation in Crops: A Novel and Emerging Approach for Agricultural Sustainability under Climate Change. Environ. Res. 2023, 233, 116357. [Google Scholar] [CrossRef]
- EL Sabagh, A.; Islam, M.S.; Hossain, A.; Iqbal, M.A.; Mubeen, M.; Waleed, M.; Reginato, M.; Battaglia, M.; Ahmed, S.; Rehman, A.; et al. Phytohormones as Growth Regulators During Abiotic Stress Tolerance in Plants. Front. Agron. 2022, 4, 765068. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Zhong, C.; Zhang, Y.; Wang-Pruski, G.; Zhang, Z.; Wu, J. Alleviating Effect of Melatonin on Melon Seed Germination Under Autotoxicity and Saline-Alkali Combined Stress. J. Plant Growth Regul. 2023, 42, 2474–2485. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Yang, L.; Chan, Z. Melatonin Antagonizes Cytokinin Responses to Stimulate Root Growth in Arabidopsis. J. Plant Growth Regul. 2022, 42, 1833–1845. [Google Scholar] [CrossRef]
- Jensen, N.B.; Ottosen, C.-O.; Zhou, R. Exogenous Melatonin Alters Stomatal Regulation in Tomato Seedlings Subjected to Combined Heat and Drought Stress through Mechanisms Distinct from ABA Signaling. Plants 2023, 12, 1156. [Google Scholar] [CrossRef]
- Tijero, V.; Muñoz, P.; Munné-Bosch, S. Melatonin as an Inhibitor of Sweet Cherries Ripening in Orchard Trees. Plant. Physiol. Biochem. 2019, 140, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Functions of Melatonin in Plants: A Review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Reiter, R.J.; Chan, Z. Phytomelatonin: A Universal Abiotic Stress Regulator. J. Exp. Bot. 2018, 69, 963–974. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Jiang, D.; Li, J.; Zhang, K.; Sun, H.; Zhang, Y.; Bai, Z.; et al. Exogenous Melatonin Promotes Seed Germination and Osmotic Regulation under Salt Stress in Cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.; Zhang, J.; Shan, C.; Rengel, Z.; Song, Z.; Chen, Q. Phytomelatonin Receptor PMTR 1-mediated Signaling Regulates Stomatal Closure in Arabidopsis Thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.-X.; Liang, D.; Chang, C.; Jia, D.; Ma, F. Melatonin Mediates the Regulation of ABA Metabolism, Free-Radical Scavenging, and Stomatal Behaviour in Two Malus Species under Drought Stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Su, X.; Chen, Y.; Fan, X.; He, L.; Guo, J.; Wang, Y.; Yang, Q. Melatonin Improves Drought Resistance in Maize Seedlings by Enhancing the Antioxidant System and Regulating Abscisic Acid Metabolism to Maintain Stomatal Opening Under PEG-Induced Drought. J. Plant Biol. 2021, 64, 299–312. [Google Scholar] [CrossRef]
- Waseem, M.; Hasan, M.M.; Hazzazi, Y.; Alharbi, B.M.; Ghani, M.U.; Ahmad, P.; Carriquí, M. Potential Mechanisms for the Rapid Post-Drought Reversal of ABA-Induced Stomatal Closure by Melatonin, 5-Aminolevulinic Acid, and Brassinosteroids. Photosynthetica 2025, 63, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Shreya, S.; Supriya, L.; Padmaja, G. Melatonin Induces Drought Tolerance by Modulating Lipoxygenase Expression, Redox Homeostasis and Photosynthetic Efficiency in Arachis hypogaea L. Front. Plant Sci. 2022, 13, 1069143. [Google Scholar] [CrossRef]
- Liu, X.; Chen, A.; Wei, Q.; Wang, C.; Zhao, Q.; Wang, Q.; Zheng, X.; He, T.; Qi, J.; Yin, H.; et al. Exogenous Melatonin Inhibits the Expression of GmABI5 and Enhances Drought Resistance in Fodder Soybean Through an ABA-Independent Pathway. Plant Cell Environ. 2025; Early View. [Google Scholar] [CrossRef]
- Back, K. Melatonin Metabolism, Signaling and Possible Roles in Plants. Plant J. 2021, 105, 376–391. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Orimoloye, I.R. Agricultural Drought and Its Potential Impacts: Enabling Decision-Support for Food Security in Vulnerable Regions. Front. Sustain. Food Syst. 2022, 6, 838824. [Google Scholar] [CrossRef]
- Satoh, Y.; Yoshimura, K.; Pokhrel, Y.; Kim, H.; Shiogama, H.; Yokohata, T.; Hanasaki, N.; Wada, Y.; Burek, P.; Byers, E.; et al. The Timing of Unprecedented Hydrological Drought under Climate Change. Nat. Commun. 2022, 13, 3287. [Google Scholar] [CrossRef]
- Daryanto, S.; Wang, L.; Jacinthe, P.-A. Global Synthesis of Drought Effects on Food Legume Production. PLoS ONE 2015, 10, e0127401. [Google Scholar] [CrossRef]
- Nunes, C.; de Sousa Araújo, S.; da Silva, J.M.; Fevereiro, M.P.S.; da Silva, A.B. Physiological Responses of the Legume Model Medicago truncatula cv. Jemalong to Water Deficit. Environ. Exp. Bot. 2008, 63, 289–296. [Google Scholar] [CrossRef]
- Amede, T.; Schubert, S.; Stahr, K. Mechanisms of Drought Resistance in Grain Legumes I: Osmotic Adjustment. SEJS 2004, 26, 37–46. [Google Scholar] [CrossRef]
- Gajardo, H.A.; Morales, M.; Larama, G.; Luengo-Escobar, A.; López, D.; Machado, M.; Nunes-Nesi, A.; Reyes-Díaz, M.; Planchais, S.; Savouré, A.; et al. Physiological, Transcriptomic and Metabolomic Insights of Three Extremophyte Woody Species Living in the Multi-Stress Environment of the Atacama Desert. Planta 2024, 260, 55. [Google Scholar] [CrossRef]
- Kumar, J.; Sen Gupta, D.; Djalovic, I.; Kumar, S.; Siddique, K.H.M. Root-Omics for Drought Tolerance in Cool-Season Grain Legumes. Physiol. Plant. 2021, 172, 629–644. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Horbowicz, M. Changes in Metabolic Profiles of Pea (Pisum sativum L.) as a Result of Repeated Short-Term Soil Drought and Subsequent Re-Watering. Int. J. Mol. Sci. 2022, 23, 1704. [Google Scholar] [CrossRef]
- Morin, A.; Maurousset, L.; Vriet, C.; Lemoine, R.; Doidy, J.; Pourtau, N. Carbon Fluxes and Environmental Interactions during Legume Development, with a Specific Focus on Pisum sativum. Physiol. Plant 2022, 174, e13729. [Google Scholar] [CrossRef] [PubMed]
- Rosales, M.A.; Ocampo, E.; Rodríguez-Valentín, R.; Olvera-Carrillo, Y.; Acosta-Gallegos, J.; Covarrubias, A.A. Physiological Analysis of Common Bean (Phaseolus vulgaris L.) Cultivars Uncovers Characteristics Related to Terminal Drought Resistance. Plant. Physiol. Biochem. 2012, 56, 24–34. [Google Scholar] [CrossRef]
- García-García, A.L.; García-Machado, F.J.; Borges, A.A.; Morales-Sierra, S.; Boto, A.; Jiménez-Arias, D. Pure Organic Active Compounds Against Abiotic Stress: A Biostimulant Overview. Front. Plant Sci. 2020, 11, 575829. [Google Scholar] [CrossRef] [PubMed]
- Mora, R.; Soto-Cerda, B.; Tighe-Neira, R.; Reyes-Díaz, M.; Alvarez, J.; Nunes-Nesi, A.; Ibáñez, C.; Inostroza-Blancheteau, C. Plant Resilience to Abiotic Stresses: Reveling the Role of Silicon in Drought and Metal(loid) Tolerance. J. Exp. Bot. 2025. [Google Scholar] [CrossRef]
- Sandoval, Y.; Tighe-Neira, R.; Inostroza-Blancheteau, C.; Soto-Cerda, B.; González-Villagra, J. Melatonin Improves Plant Water Status, Photosynthetic Performance, and Antioxidant Defense System in Highbush Blueberry (Vaccinium corymbosum L.) Plants Subjected to Drought Stress. Sci. Hortic. 2024, 323, 112528. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S.; et al. Melatonin Enhances Plant Growth and Abiotic Stress Tolerance in Soybean Plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef]
- Zhang, M.; He, S.; Zhan, Y.; Qin, B.; Jin, X.; Wang, M.; Zhang, Y.; Hu, G.; Teng, Z.; Wu, Y. Exogenous Melatonin Reduces the Inhibitory Effect of Osmotic Stress on Photosynthesis in Soybean. PLoS ONE 2019, 14, e0226542. [Google Scholar] [CrossRef]
- Zou, J.; Yu, H.; Yu, Q.; Jin, X.; Cao, L.; Wang, M.; Wang, M.; Ren, C.; Zhang, Y. Physiological and UPLC-MS/MS Widely Targeted Metabolites Mechanisms of Alleviation of Drought Stress-Induced Soybean Growth Inhibition by Melatonin. Ind. Crops Prod. 2021, 163, 113323. [Google Scholar] [CrossRef]
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.-M.; Lee, I.-J. Exogenous Melatonin Induces Drought Stress Tolerance by Promoting Plant Growth and Antioxidant Defence System of Soybean Plants. AoB PLANTS 2021, 13, plab026. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Qin, B.; Gong, Z.; Zhang, Y. Melatonin Improves Nitrogen Metabolism during Grain Filling under Drought Stress. Physiol. Mol. Biol. Plants 2022, 28, 1477–1488. [Google Scholar] [CrossRef]
- Oliveira-Spolaor, B.; Chiari-Bertoli, S.; Silva-Sukert, D.; Sala, H.R.; Picoli de Oliveira, B.F.; de Freitas, Í.R.; Lima-Moro, A. Exogenous Melatonin Induces Tolerance to Drought Stress Damage in Seedlings and Soybean Plants. Chil. J. Agric. Res. 2022, 82, 515–526. [Google Scholar] [CrossRef]
- Zhao, Q.; Zheng, X.; Wang, C.; Wang, Q.; Wei, Q.; Liu, X.; Liu, Y.; Chen, A.; Jiang, J.; Zhao, X.; et al. Exogenous Melatonin Improves Drought Tolerance by Regulating the Antioxidant Defense System and Photosynthetic Efficiency in Fodder Soybean Seedings. Plants 2025, 14, 460. [Google Scholar] [CrossRef]
- Kuppusamy, A.; Alagarswamy, S.; Karuppusami, K.M.; Maduraimuthu, D.; Natesan, S.; Ramalingam, K.; Muniyappan, U.; Subramanian, M.; Kanagarajan, S. Melatonin Enhances the Photosynthesis and Antioxidant Enzyme Activities of Mung Bean under Drought and High-Temperature Stress Conditions. Plants 2023, 12, 2535. [Google Scholar] [CrossRef]
- Yasmeen, S.; Wahab, A.; Saleem, M.H.; Ali, B.; Qureshi, K.A.; Jaremko, M. Melatonin as a Foliar Application and Adaptation in Lentil (Lens culinaris Medik.) Crops Under Drought Stress. Sustainability 2022, 14, 16345. [Google Scholar] [CrossRef]
- Abdoli, M.; Amerian, M.R.; Heidari, M.; Ebrahimi, A. Synergistic Effects of Melatonin and 24-Epibrassinolide on Chickpea Water Deficit Tolerance. BMC Plant Biol. 2024, 24, 671. [Google Scholar] [CrossRef]
- Kasapoğlu, A.G.; Muslu, S.; Aygören, A.S.; Öner, B.M.; Güneş, E.; İlhan, E.; Yiğider, E.; Aydin, M. Genome-Wide Characterization of the GPAT Gene Family in Bean (Phaseolus vulgaris L.) and Expression Analysis under Abiotic Stress and Melatonin. Genet. Resour. Crop Evol. 2024, 71, 4549–4569. [Google Scholar] [CrossRef]
- Aydınyurt, R.; Yağcı, S.; Yaprak, E.; Kasapoğlu, A.G.; Muslu, S.; Uçar, S.; Aygören, A.S.; Öner, B.M.; Yiğider, E.; İlhan, E.; et al. Epigenetic Evaluation of Melatonin Application in Bean (Phaseolus vulgaris L.) Genotypes Under Drought and Salt Stress Conditions. Plant Mol. Biol. Rep. 2025, 43, 1144–1162. [Google Scholar] [CrossRef]
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil Salinization Research in China: Advances and Prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Casanova, M.; Salazar, O.; Oyarzún, I.; Tapia, Y.; Fajardo, M. Field Monitoring of 2010-Tsunami Impact on Agricultural Soils and Irrigation Waters: Central Chile. Water Air Soil Pollut 2016, 227, 411. [Google Scholar] [CrossRef]
- Chen, J.; Mueller, V. Coastal Climate Change, Soil Salinity and Human Migration in Bangladesh. Nat. Clim. Change 2018, 8, 981–985. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Wang, M.; Ali, A.; Cheng, A.; Wang, X.; Ma, C. Grain Legumes and Fear of Salt Stress: Focus on Mechanisms and Management Strategies. Int. J. Mol. Sci. 2019, 20, 799. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Gogoi, N.; Hussain, M.; Barthakur, S.; Paul, S.; Bharadwaj, N.; Migdadi, H.M.; Alghamdi, S.S.; Siddique, K.H.M. Effects, Tolerance Mechanisms and Management of Salt Stress in Grain Legumes. Plant Physiol. Biochem. 2017, 118, 199–217. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Zhao, D.; Gao, S.; Zhang, X.; Zhang, Z.; Zheng, H.; Rong, K.; Zhao, W.; Khan, S.A. Impact of Saline Stress on the Uptake of Various Macro and Micronutrients and Their Associations with Plant Biomass and Root Traits in Wheat. Plant Soil Environ. 2021, 67, 61–70. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity Induced Physiological and Biochemical Changes in Plants: An Omic Approach towards Salt Stress Tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Manchanda, G.; Garg, N. Salinity and its effects on the functional biology of legumes. Acta Physiol. Plant. 2008, 30, 595–618. [Google Scholar] [CrossRef]
- Bouzroud, S.; Henkrar, F.; Fahr, M.; Smouni, A. Salt Stress Responses and Alleviation Strategies in Legumes: A Review of the Current Knowledge. 3 Biotech 2023, 13, 287. [Google Scholar] [CrossRef]
- Naz, S.; Bilal, A.; Saddiq, B.; Ejaz, S.; Ali, S.; Ain Haider, S.T.; Sardar, H.; Nasir, B.; Ahmad, I.; Tiwari, R.K.; et al. Foliar Application of Salicylic Acid Improved Growth, Yield, Quality and Photosynthesis of Pea (Pisum sativum L.) by Improving Antioxidant Defense Mechanism under Saline Conditions. Sustainability 2022, 14, 14180. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Ahanger, M.A.; Egamberdieva, D.; Wijaya, L.; Alam, P. Salicylic Acid (SA) Induced Alterations in Growth, Biochemical Attributes and Antioxidant Enzyme Activity in Faba Bean (Vicia faba L.) Seedlings under NaCl Toxicity. Russ. J. Plant Physiol. 2018, 65, 104–114. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, A.; Xu, B.; Wang, H.; Yu, J.; Liu, J.; Jian, L.; Quan, C.; Du, J. Exogenous Melatonin Enhances Salt Tolerance by Regulating the Phenylpropanoid Biosynthesis Pathway in Common Bean at Sprout Stage. Plant Stress. 2024, 14, 100589. [Google Scholar] [CrossRef]
- Adhikari, A.; Sapkota, M.; Savidya, R.N.; Tosin, A.T.; Adam, M.; Alam, M.N.; Kwon, E.-H.; Kang, S.-M.; Shaffique, S.; Lee, I.-J. Calcium Enhances the Effectiveness of Melatonin in Improving Nutritional Properties of Soybean Sprouts and Germination Under Salt and Cadmium Stress. Int. J. Mol. Sci. 2025, 26, 878. [Google Scholar] [CrossRef] [PubMed]
- Alinia, M.; Kazemeini, S.A.; Sepehri, M.; Dadkhodaie, A. Simultaneous Application of Rhizobium Strain and Melatonin Improves the Photosynthetic Capacity and Induces Antioxidant Defense System in Common Bean (Phaseolus vulgaris L.) Under Salinity Stress. J. Plant Growth Regul. 2022, 41, 1367–1381. [Google Scholar] [CrossRef]
- ElSayed, A.I.; Rafudeen, M.S.; Gomaa, A.M.; Hasanuzzaman, M. Exogenous Melatonin Enhances the Reactive Oxygen Species Metabolism, Antioxidant Defense-Related Gene Expression, and Photosynthetic Capacity of Phaseolus vulgaris L. to Confer Salt Stress Tolerance. Physiol. Plant. 2021, 173, 1369–1381. [Google Scholar] [CrossRef]
- Yang, X.; Liu, D.; Liu, C.; Li, M.; Yan, Z.; Zhang, Y.; Feng, G. Possible Melatonin-Induced Salt Stress Tolerance Pathway in Phaseolus vulgaris L. Using Transcriptomic and Metabolomic Analyses. BMC Plant Biol. 2024, 24, 72. [Google Scholar] [CrossRef]
- Zhang, Q.; Qin, B.; Wang, G.; Zhang, W.; Li, M.; Yin, Z.; Yuan, X.; Sun, H.; Du, J.; Du, Y.; et al. Exogenous Melatonin Enhances Cell Wall Response to Salt Stress in Common Bean (Phaseolus vulgaris) and the Development of the Associated Predictive Molecular Markers. Front. Plant Sci. 2022, 13, 1012186. [Google Scholar] [CrossRef]
- Askari, M.; Hamid, N.; Abideen, Z.; Zulfiqar, F.; Moosa, A.; Nafees, M.; El-Keblawy, A. Exogenous Melatonin Application Stimulates Growth, Photosynthetic Pigments and Antioxidant Potential of White Beans under Salinity Stress. S. Afr. J. Bot. 2023, 160, 219–228. [Google Scholar] [CrossRef]
- Dadasoglu, E.; Turan, M.; Ekinci, M.; Argin, S.; Yildirim, E. Alleviation Mechanism of Melatonin in Chickpea (Cicer arietinum L.) Under the Salt Stress Conditions. Horticulturae 2022, 8, 1066. [Google Scholar] [CrossRef]
- Song, Z.; Yang, Q.; Dong, B.; Li, N.; Wang, M.; Du, T.; Liu, N.; Niu, L.; Jin, H.; Meng, D.; et al. Melatonin Enhances Stress Tolerance in Pigeon Pea by Promoting Flavonoid Enrichment, Particularly Luteolin in Response to Salt Stress. J. Exp. Bot. 2022, 73, 5992–6008. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, H.; Abu-shahba, M.; Ali, G.; Mousa, M.; Sofy, M. Treatment with Melatonin and Titanium Oxide Nanoparticles Improves Limiting Sodium Uptake in Broad Beans Under Salt Stress. J. Soil Sci. Plant Nutr. 2025, 25, 4159–4182. [Google Scholar] [CrossRef]
- Dawood, M.G.; El-Awadi, M.E. Alleviation of salinity stress on Vicia faba L. plants via seed priming with melatonin. Acta Biológica Colomb. 2015, 20, 223–235. [Google Scholar] [CrossRef]
- Li, C.; Zhou, K.; Qin, W.; Tian, C.; Qi, M.; Yan, X.; Han, W. A Review on Heavy Metals Contamination in Soil: Effects, Sources, and Remediation Techniques. Soil. Sediment. Contam. An. Int. J. 2019, 28, 380–394. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Furini, A. An Overview of Heavy Metal Challenge in Plants: From Roots to Shoots. Metallomics 2013, 5, 1117. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Zhang, D.; Wang, Y. Probabilistic Human Health Risk Assessment of Heavy Metal Intake via Vegetable Consumption around Pb/Zn Smelters in Southwest China. Int. J. Environ. Res. Public Health 2019, 16, 3267. [Google Scholar] [CrossRef]
- Chukwu, E.C.; Gulser, C. Morphological, Physiological, and Anatomical Effects of Heavy Metals on Soil and Plant Health and Possible Remediation Technologies. Soil. Secur. 2025, 18, 100178. [Google Scholar] [CrossRef]
- Clemens, S. Toxic Metal Accumulation, Responses to Exposure and Mechanisms of Tolerance in Plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Song, J.; Sun, Z.; Saud, S.; Fahad, S.; Nawaz, T. Exploring the Deleterious Effects of Heavy Metal Cadmium on Antioxidant Defense and Photosynthetic Pathways in Higher Plants. Plant Stress 2025, 15, 100716. [Google Scholar] [CrossRef]
- Benhabiles, K.; Bellout, Y.; Amghar, F. Effect of Cadmium Stress on the Polyphenol Content, Morphological, Physiological, and Anatomical Parameters of Common Bean (Phaseolus vulgaris L.). Appl. Ecol. Environ. Res. 2020, 18, 3757–3774. [Google Scholar] [CrossRef]
- Zornoza, P.; Vázquez, S.; Esteban, E.; Fernández-Pascual, M.; Carpena, R. Cadmium-Stress in Nodulated White Lupin: Strategies to Avoid Toxicity. Plant Physiol. Biochem. 2002, 40, 1003–1009. [Google Scholar] [CrossRef]
- Shi, G.; Sun, L.; Wang, X.; Liu, C. Leaf Responses to Iron Nutrition and Low Cadmium in Peanut: Anatomical Properties in Relation to Gas Exchange. Plant Soil. 2014, 375, 99–111. [Google Scholar] [CrossRef]
- Armendariz, A.L.; Talano, M.A.; Travaglia, C.; Reinoso, H.; Wevar Oller, A.L.; Agostini, E. Arsenic Toxicity in Soybean Seedlings and Their Attenuation Mechanisms. Plant Physiol. Biochem. 2016, 98, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Pita-Barbosa, A.; Gonçalves, E.C.; Azevedo, A.A. Morpho-Anatomical and Growth Alterations Induced by Arsenic in Cajanus cajan (L.) DC (Fabaceae). Environ. Sci. Pollut Res. 2015, 22, 11265–11274. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular Mechanisms of Metal Hyperaccumulation in Plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Luo, J.-S.; Huang, J.; Zeng, D.-L.; Peng, J.-S.; Zhang, G.-B.; Ma, H.-L.; Guan, Y.; Yi, H.-Y.; Fu, Y.-L.; Han, B.; et al. A Defensin-like Protein Drives Cadmium Efflux and Allocation in Rice. Nat. Commun. 2018, 9, 645. [Google Scholar] [CrossRef]
- Boutahiri, S.; Benrkia, R.; Tembeni, B.; Idowu, O.E.; Olatunji, O.J. Effect of Biostimulants on the Chemical Profile of Food Crops under Normal and Abiotic Stress Conditions. Curr. Plant Biol. 2024, 40, 100410. [Google Scholar] [CrossRef]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin Inhibits Cadmium Translocation and Enhances Plant Tolerance by Regulating Sulfur Uptake and Assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-Mediated Nitric Oxide Improves Tolerance to Cadmium Toxicity by Reducing Oxidative Stress in Wheat Plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- Ren, R.; Cao, Z.; Ma, X.; Li, Z.; Zhao, K.; Cao, D.; Ma, Q.; Hou, M.; Zhao, K.; Zhang, L.; et al. Multi-Omics Analysis Reveals That AhNHL Contributes to Melatonin-Mediated Cadmium Tolerance in Peanut Plants. J. Pineal Res. 2025, 77, e70035. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.; Khan, R.; Liu, Z.; Ali, S.; Naseer, M.A.; Shah, M.A.; Ahmad, H.; Zhou, X.B. Melatonin Mitigates Nickel Oxide Nanoparticles Induced Phytotoxicity in Soybean by Reducing Metal Accumulation, Enhancing Antioxidant Defense and Promoting Nitrogen Assimilation. J. Hazard. Mater. 2025, 485, 136861. [Google Scholar] [CrossRef]
- Bhat, J.A.; Faizan, M.; Bhat, M.A.; Huang, F.; Yu, D.; Ahmad, A.; Bajguz, A.; Ahmad, P. Defense Interplay of the Zinc-Oxide Nanoparticles and Melatonin in Alleviating the Arsenic Stress in Soybean (Glycine max L.). Chemosphere 2022, 288, 132471. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Alamri, S.; Nasir Khan, M.; Corpas, F.J.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M.; Kalaji, H.M.; Ahmad, P. Melatonin and Calcium Function Synergistically to Promote the Resilience through ROS Metabolism under Arsenic-Induced Stress. J. Hazard. Mater. 2020, 398, 122882. [Google Scholar] [CrossRef]
- Khan, M.N.; Islam, S.; Siddiqui, M.H. Regulation of Anaplerotic Enzymes by Melatonin Enhances Resilience to Cadmium Toxicity in Vigna radiata (L.) R. Wilczek. Plant Physiol. Biochem. 2025, 220, 109522. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, Biochemical, and Molecular Mechanisms of Heat Stress Tolerance in Plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef] [PubMed]
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate Change Impact and Adaptation for Wheat Protein. Glob. Chang. Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gan, Y.T.; Clarke, F.; McDonald, C.L. Response of Chickpea Yield to High Temperature Stress during Reproductive Development. Crop Sci. 2006, 46, 2171–2178. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, D.; Nayyar, H. Comparative Response of Maize and Rice Genotypes to Heat Stress: Status of Oxidative Stress and Antioxidants. Acta Physiol. Plant 2012, 34, 75–86. [Google Scholar] [CrossRef]
- Siebert, S.; Ewert, F.; Eyshi Rezaei, E.; Kage, H.; Graß, R. Impact of Heat Stress on Crop Yield—On the Importance of Considering Canopy Temperature. Environ. Res. Lett. 2014, 9, 044012. [Google Scholar] [CrossRef]
- González-Villagra, J.; Ávila, K.; Gajardo, H.A.; Bravo, L.A.; Ribera-Fonseca, A.; Jorquera-Fontena, E.; Curaqueo, G.; Roldán, C.; Falquetto-Gomes, P.; Nunes-Nesi, A.; et al. Diurnal High Temperatures Affect the Physiological Performance and Fruit Quality of Highbush Blueberry (Vaccinium corymbosum L.) cv. Legacy. Plants 2024, 13, 1846. [Google Scholar] [CrossRef]
- Jiang, Y.; Lahlali, R.; Karunakaran, C.; Kumar, S.; Davis, A.R.; Bueckert, R.A. Seed Set, Pollen Morphology and Pollen Surface Composition Response to Heat Stress in Field Pea. Plant Cell Environ. 2015, 38, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Aslam, M.; Ahmed, S.R.; Tan, D.K.Y.; De Mastro, F.; Tariq, M.S.; Sakhawat, A.; Asad, M.A.; Liu, Y. An Overview of Heat Stress in Chickpea (Cicer arietinum L.): Effects, Mechanisms and Diverse Molecular Breeding Approaches for Enhancing Resilience and Productivity. Mol. Breed. 2025, 45, 18. [Google Scholar] [CrossRef]
- Sita, K.; Sehgal, A.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.M.; Nayyar, H. Identification of High-Temperature Tolerant Lentil (Lens culinaris Medik.) Genotypes Through Leaf Pollen Traits. Front. Plant Sci. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.R.A.; dos Santos, T.C.; Silva, E.G.F.; da Silva, W.O.; Guimarães, M.J.M.; Angelotti, F. Pollen Viability, and the Photosynthetic and Enzymatic Responses of Cowpea (Vigna unguiculata (L.) Walp., Fabaceae) in the Face of Rising Air Temperature: A Problem for Food Safety. Agronomy 2024, 14, 463. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J.; Zhu, Y.; Jones, A.; Rose, R.J.; Song, Y. Heat Stress in Legume Seed Setting: Effects, Causes, and Future Prospects. Front. Plant Sci. 2019, 10, 938. [Google Scholar] [CrossRef]
- Sher, A.; Noor, M.A.; Li, H.X.; Nasir, B.; Manzoor, M.A.; Hussain, S.; Zhang, J.; Riaz, M.W.; Hussain, S. Heat Stress Effects on Legumes: Challenges, Management Strategies and Future Insights. Plant Stress 2024, 13, 100537. [Google Scholar] [CrossRef]
- Priya, M.; Farooq, M.; Siddique, K.H.M. Enhancing Tolerance to Combined Heat and Drought Stress in Cool-Season Grain Legumes: Mechanisms, Genetic Insights, and Future Directions. Plant Cell Environ. 2025. Early View. [Google Scholar] [CrossRef]
- Kaushal, N.; Gupta, K.; Bhandhari, K.; Kumar, S.; Thakur, P.; Nayyar, H. Proline Induces Heat Tolerance in Chickpea (Cicer arietinum L.) Plants by Protecting Vital Enzymes of Carbon and Antioxidative Metabolism. Physiol. Mol. Biol. Plants 2011, 17, 203–213. [Google Scholar] [CrossRef]
- Pollastri, S.; Tsonev, T.; Loreto, F. Isoprene Improves Photochemical Efficiency and Enhances Heat Dissipation in Plants at Physiological Temperatures. J. Exp. Bot. 2014, 65, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Yadav, S.; Singh, M.P. Bioregulators Application Improved Heat Tolerance and Yield in Chickpea (Cicer arietinum L.) by Modulating Zeaxanthin Cycle. Plant Physiol. Rep. 2020, 25, 677–688. [Google Scholar] [CrossRef]
- Jianing, G.; Yuhong, G.; Yijun, G.; Rasheed, A.; Qian, Z.; Zhiming, X.; Mahmood, A.; Shuheng, Z.; Zhuo, Z.; Zhuo, Z.; et al. Improvement of Heat Stress Tolerance in Soybean (Glycine max L.), by Using Conventional and Molecular Tools. Front. Plant Sci. 2022, 13, 993189. [Google Scholar] [CrossRef] [PubMed]
- Brengi, S.H.; Abouelsaad, I.A.; Khadr, A.A.; Abdelghany, M. Enhancing the Growth and Yield of the Common Bean Cultivar ’Nebraska’ under High Temperature Conditions by Combining Different Magnesium Levels with Arginine, Glycine, and Melatonin. BMC Plant Biol. 2025, 25, 1156. [Google Scholar] [CrossRef]
- Imran, M.; Aaqil Khan, M.; Shahzad, R.; Bilal, S.; Khan, M.; Yun, B.-W.; Khan, A.L.; Lee, I.-J. Melatonin Ameliorates Thermotolerance in Soybean Seedling through Balancing Redox Homeostasis and Modulating Antioxidant Defense, Phytohormones and Polyamines Biosynthesis. Molecules 2021, 26, 5116. [Google Scholar] [CrossRef]
- Kumar, G.; Saad, K.R.; Arya, M.; Puthusseri, B.; Mahadevappa, P.; Shetty, N.P.; Giridhar, P. The Synergistic Role of Serotonin and Melatonin during Temperature Stress in Promoting Cell Division, Ethylene and Isoflavones Biosynthesis in Glycine max. Curr. Plant Biol. 2021, 26, 100206. [Google Scholar] [CrossRef]
- Anitha, K.; Senthil, A.; Kalarani, M.K.; Senthil, N.; Marimuthu, S.; Umapathi, M. Melatonin Mediated High-Temperature Tolerance at Seedling Stage in Green Gram (Vigna radiata L.). J. Appl. Nat. Sci. 2023, 15, 85–93. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Du, M.; Zang, D.; Men, Q.; Su, P.; Guo, S. Exogenous Melatonin Improves Drought Stress Tolerance via Regulating Tryptophan Metabolism and Flavonoid Biosynthesis Pathways in Wheat. Physiol. Plant. 2024, 176, e70006. [Google Scholar] [CrossRef]
- Muhammad, I.; Khan, A.; Mustafa, A.E.-Z.M.A.; Elshikh, M.S.; Shen, W. Elucidating the Modulatory Effect of Melatonin on Enzyme Activity and Oxidative Stress in Wheat: A Global Meta-Analysis. Physiol. Plant. 2024, 176, e14294. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an Antioxidant: Under Promises but over Delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Costa, E.J.; Lopes, R.H.; Lamy-Freund, M.T. Permeability of Pure Lipid Bilayers to Melatonin. J. Pineal Res. 1995, 19, 123–126. [Google Scholar] [CrossRef]
- Demidchik, V. Mechanisms of Oxidative Stress in Plants: From Classical Chemistry to Cell Biology. Environ. Exp. Bot. 2015, 109, 212–228. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, Oxidative Stress, and Signal Transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Foyer, C.H.; Noctor, G. Redox Regulation in Photosynthetic Organisms: Signaling, Acclimation, and Practical Implications. Antioxid. Redox Signal. 2009, 11, 861–905. [Google Scholar] [CrossRef]
- Ampofo, J.O.; Ngadi, M. Stimulation of the Phenylpropanoid Pathway and Antioxidant Capacities by Biotic and Abiotic Elicitation Strategies in Common Bean (Phaseolus vulgaris) Sprouts. Process Biochem. 2021, 100, 98–106. [Google Scholar] [CrossRef]
- Liu, G.; Hu, Q.; Zhang, X.; Jiang, J.; Zhang, Y.; Zhang, Z. Melatonin Biosynthesis and Signal Transduction in Plants in Response to Environmental Conditions. J. Exp. Bot. 2022, 73, 5818–5827. [Google Scholar] [CrossRef]
- Yang, X.; Ren, J.; Li, J.; Lin, X.; Xia, X.; Yan, W.; Zhang, Y.; Deng, X.; Ke, Q. Meta-Analysis of the Effect of Melatonin Application on Abiotic Stress Tolerance in Plants. Plant Biotechnol. Rep. 2023, 17, 39–52. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Gao, X.; Lan, J.; Fu, B. Comparative Physiological and Transcriptome Analysis Reveal the Molecular Mechanism of Melatonin in Regulating Salt Tolerance in Alfalfa (Medicago sativa L.). Front. Plant Sci. 2022, 13, 919177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gao, Y.; Du, Y.; Du, J.; Han, Y. Genome-Wide Analysis of the CML Gene Family and Its Response to Melatonin in Common Bean (Phaseolus vulgaris L.). Sci. Rep. 2023, 13, 1196. [Google Scholar] [CrossRef] [PubMed]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanati, F.; Allakhverdiev, S. Melatonin Improves the Photosynthesis in Dracocephalum kotschyi under Salinity Stress in a Ca2+/CaM-Dependent Manner. Funct. Plant Biol. 2021, 49, 89–101. [Google Scholar] [CrossRef]
- Chang, J.; Guo, Y.; Li, J.; Liu, L.; Liu, J.; Yuan, L.; Wei, C.; Ma, J.; Zhang, Y.; Ahammed, G.J.; et al. Cyclic Nucleotide-Gated Ion Channel 20 Regulates Melatonin-Induced Calcium Signaling and Cold Tolerance in Watermelon. Plant Physiol. 2025, 197, kiae630. [Google Scholar] [CrossRef]
- Arora, D.; Singh, N.; Bhatla, S.C. Calmodulin and Calcium-Mediated Melatonin Signaling Mechanisms in Plants. Theor. Exp. Plant Physiol. 2024, 36, 635–645. [Google Scholar] [CrossRef]
- Ikram, M.; Mehran, M.; Rehman, H.U.; Ullah, S.; Bakhsh, M.Z.M.; Tahira, M.; Maqsood, M.F.K.; Rauf, A.; Ghafar, S.; Haider, K.; et al. Mechanistic Review of Melatonin Metabolism and Signaling Pathways in Plants: Biosynthesis, Regulation, and Roles under Abiotic Stress. Plant Stress 2024, 14, 100685. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shu, H.; Hao, Y.; Mumtaz, M.A.; Lu, X.; Wang, Z. Melatonin Affects the Photosynthetic Performance of Pepper (Capsicum annuum L.) Seedl. Under Cold Stress. Antioxidants 2022, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Bai, Y.; Cheng, X.; Reiter, R.J.; Yin, X.; Shi, H. Lighting the Way: Advances in Transcriptional Regulation and Integrative Crosstalk of Melatonin Biosynthetic Enzymes in Cassava. J. Exp. Bot. 2021, 72, 161–166. [Google Scholar] [CrossRef]
- Back, K.; Tan, D.-X.; Reiter, R.J. Melatonin Biosynthesis in Plants: Multiple Pathways Catalyze Tryptophan to Melatonin in the Cytoplasm or Chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef]
- Wang, L.; Feng, C.; Zheng, X.; Guo, Y.; Zhou, F.; Shan, D.; Liu, X.; Kong, J. Plant Mitochondria Synthesize Melatonin and Enhance the Tolerance of Plants to Drought Stress. J. Pineal Res. 2017, 63, e12429. [Google Scholar] [CrossRef]
- Gajardo, H.A.; Gómez-Espinoza, O.; Boscariol Ferreira, P.; Carrer, H.; Bravo, L.A. The Potential of CRISPR/Cas Technology to Enhance Crop Performance on Adverse Soil Conditions. Plants 2023, 12, 1892. [Google Scholar] [CrossRef]
- Nivya, V.M.; Shah, J.M. Recalcitrance to Transformation, a Hindrance for Genome Editing of Legumes. Front. Genome Ed. 2023, 5, 1247815. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Lv, W.; Zhang, Y.; Bhat, J.A.; Kong, J.; Xing, H.; Zhao, J.; Zhao, T. GmNAC8 Acts as a Positive Regulator in Soybean Drought Stress. Plant Sci. 2020, 293, 110442. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Lv, P.; Antwi-Boasiako, A.; Begum, N.; Zhao, T.; Zhao, J. NAC Transcription Factor GmNAC12 Improved Drought Stress Tolerance in Soybean. Int. J. Mol. Sci. 2022, 23, 12029. [Google Scholar] [CrossRef]
- Zhong, X.; Hong, W.; Shu, Y.; Li, J.; Liu, L.; Chen, X.; Islam, F.; Zhou, W.; Tang, G. CRISPR/Cas9 Mediated Gene-Editing of GmHdz4 Transcription Factor Enhances Drought Tolerance in Soybean (Glycine max [L.] Merr.). Front. Plant Sci. 2022, 13, 988505. [Google Scholar] [CrossRef]
- Wang, T.; Xun, H.; Wang, W.; Ding, X.; Tian, H.; Hussain, S.; Dong, Q.; Li, Y.; Cheng, Y.; Wang, C.; et al. Mutation of GmAITR Genes by CRISPR/Cas9 Genome Editing Results in Enhanced Salinity Stress Tolerance in Soybean. Front. Plant Sci. 2021, 12, 779598. [Google Scholar] [CrossRef]
- Dong, L.; Hou, Z.; Li, H.; Li, Z.; Fang, C.; Kong, L.; Li, Y.; Du, H.; Li, T.; Wang, L.; et al. Agronomical Selection on Loss-of-Function of GIGANTEA Simultaneously Facilitates Soybean Salt Tolerance and Early Maturity. J. Integr. Plant Biol. 2022, 64, 1866–1882. [Google Scholar] [CrossRef] [PubMed]
- Badhan, S.; Ball, A.S.; Mantri, N. First Report of CRISPR/Cas9 Mediated DNA-Free Editing of 4CL and RVE7 Genes in Chickpea Protoplasts. Int. J. Mol. Sci. 2021, 22, 396. [Google Scholar] [CrossRef] [PubMed]
- de Koning, R.; Daryanavard, H.; Garmyn, J.; Kiekens, R.; Toili, M.E.M.; Angenon, G. Fine-Tuning CRISPR/Cas9 Gene Editing in Common Bean (Phaseolus vulgaris L.) Using a Hairy Root Transformation System and in Silico Prediction Models. Front. Plant Sci. 2023, 14, 1233418. [Google Scholar] [CrossRef] [PubMed]
- Augustine, S.M.; Cherian, A.V.; Paridhi, P.; Ugwuanyi, S.; Knoblauch, B.; Tzigos, S.; Pullamsetti, S.S.; Snowdon, R. Electrical Current-Mediated Transformation for Efficient Plant Genome Editing: A Case Study in Faba Bean. Legume Sci. 2025, 7, e70031. [Google Scholar] [CrossRef]
- Bhowmik, P.; Konkin, D.; Polowick, P.; Hodgins, C.L.; Subedi, M.; Xiang, D.; Yu, B.; Patterson, N.; Rajagopalan, N.; Babic, V.; et al. CRISPR/Cas9 Gene Editing in Legume Crops: Opportunities and Challenges. Legume Sci. 2021, 3, e96. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Chen, Y.; Jiang, W.; Zhang, J.; Wang, J.; Wu, Y.; Wang, S.; Yang, X.; Liu, M.; et al. Understanding the Mechanism of Red Light-Induced Melatonin Biosynthesis Facilitates the Engineering of Melatonin-Enriched Tomatoes. Nat. Commun. 2023, 14, 5525. [Google Scholar] [CrossRef]
- Park, H.; Kim, J. Activation of Melatonin Receptor 1 by CRISPR-Cas9 Activator Ameliorates Cognitive Deficits in an Alzheimer’s Disease Mouse Model. J. Pineal Res. 2022, 72, e12787. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Han, Z.; Zhang, W.; He, L.; Shi, Z.; Ma, X.; Zhou, J.; Si, Z.; Hu, Y.; Zhang, T. Enhancing Melatonin Biosynthesis in Crops through Synthetic Genetic Circuits: A Strategy for Nutritional Fortification in Soybean and Stress Resistance in Cotton. Plant Biotechnol. J. 2025, 23, 4428–4439. [Google Scholar] [CrossRef]
- Priti; Kapoor, P.; Mali, S.; Verma, V.; Katoch, M.; Zinta, G.; Bhargava, B. Genome-Wide Characterization of Melatonin Biosynthetic Pathway Genes in Carnation (Dianthus caryophyllus L.) and Their Expression Analysis in Response to Exogenous Melatonin. Sci. Hortic. 2024, 338, 113776. [Google Scholar] [CrossRef]
- Fang, S.; Li, W.; Wang, B.; Zhu, X.; Tian, H.; Zhu, T.; Sun, D.; Yang, A.; Duan, Y.; Yan, Y.; et al. Natural Variation and Association Analysis of Melatonin Synthesis Genes with Root-Related Traits in the Maize Seedling Stage. Agronomy 2024, 14, 2031. [Google Scholar] [CrossRef]
- Wang, X.; You, J.; Liu, A.; Qi, X.; Li, D.; Zhao, Y.; Zhang, Y.; Zhang, L.; Zhang, X.; Li, P. Variation in Melatonin Contents and Genetic Dissection of Melatonin Biosynthesis in Sesame. Plants 2022, 11, 2005. [Google Scholar] [CrossRef]
- Bai, Y.; Wei, Y.; Yin, H.; Hu, W.; Cheng, X.; Guo, J.; Dong, Y.; Zheng, L.; Xie, H.; Zeng, H.; et al. PP2C1 Fine-Tunes Melatonin Biosynthesis and Phytomelatonin Receptor PMTR1 Binding to Melatonin in Cassava. J. Pineal Res. 2022, 73, e12804. [Google Scholar] [CrossRef]
- Pranil, T.; Moongngarm, A.; Loypimai, P. Influence of pH, Temperature, and Light on the Stability of Melatonin in Aqueous Solutions and Fruit Juices. Heliyon 2020, 6, e03648. [Google Scholar] [CrossRef] [PubMed]
- Mirza-Aghazadeh-Attari, M.; Mihanfar, A.; Yousefi, B.; Majidinia, M. Nanotechnology-Based Advances in the Efficient Delivery of Melatonin. Cancer Cell Int. 2022, 22, 43. [Google Scholar] [CrossRef]
- Chen, J.; Qin, H.; Zhang, B.; Mao, W.; Lou, L.; Shen, C.; Mao, J.; Lin, Q. Development of Melatonin Nano-Delivery Systems to Reduce Cadmium Accumulation in Rice (Oryza sativa L.) Seedlings: Insights from Photosynthetic Efficiency, Antioxidative Response and Gene Expression. Environ. Exp. Bot. 2022, 196, 104822. [Google Scholar] [CrossRef]
- Mukherjee, S.; Roy, S.; Arnao, M.B. Nanovehicles for Melatonin: A New Journey for Agriculture. Trends Plant Sci. 2024, 29, 232–248. [Google Scholar] [CrossRef]
- Jiao, J.; Ma, Y.; Chen, S.; Liu, C.; Song, Y.; Qin, Y.; Yuan, C.; Liu, Y. Melatonin-Producing Endophytic Bacteria from Grapevine Roots Promote the Abiotic Stress-Induced Production of Endogenous Melatonin in Their Hosts. Front. Plant Sci. 2016, 7, 1387. [Google Scholar] [CrossRef]
- Jofre, M.F.; Mammana, S.B.; Appiolaza, M.L.; Silva, M.F.; Gomez, F.J.V.; Cohen, A.C. Melatonin Production by Rhizobacteria Native Strains: Towards Sustainable Plant Growth Promotion Strategies. Physiol. Plant. 2023, 175, e13852. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).