Characterization of Caleosin Genes in Carica papaya and Insights into Lineage-Specific Family Evolution in Brassicales
Abstract
1. Introduction
2. Results
2.1. Identification, Chromosomal Localization, and Duplication Event Analysis of Five Caleosin Genes in Papaya
2.2. Comparison of Caleosin Genes in Papaya and A. thaliana Revealed Species-Specific Evolution
2.3. Characterization of Caleosin Genes from Representative Plant Species and Insights into Lineage-Specific Family Evolution in Brassicales
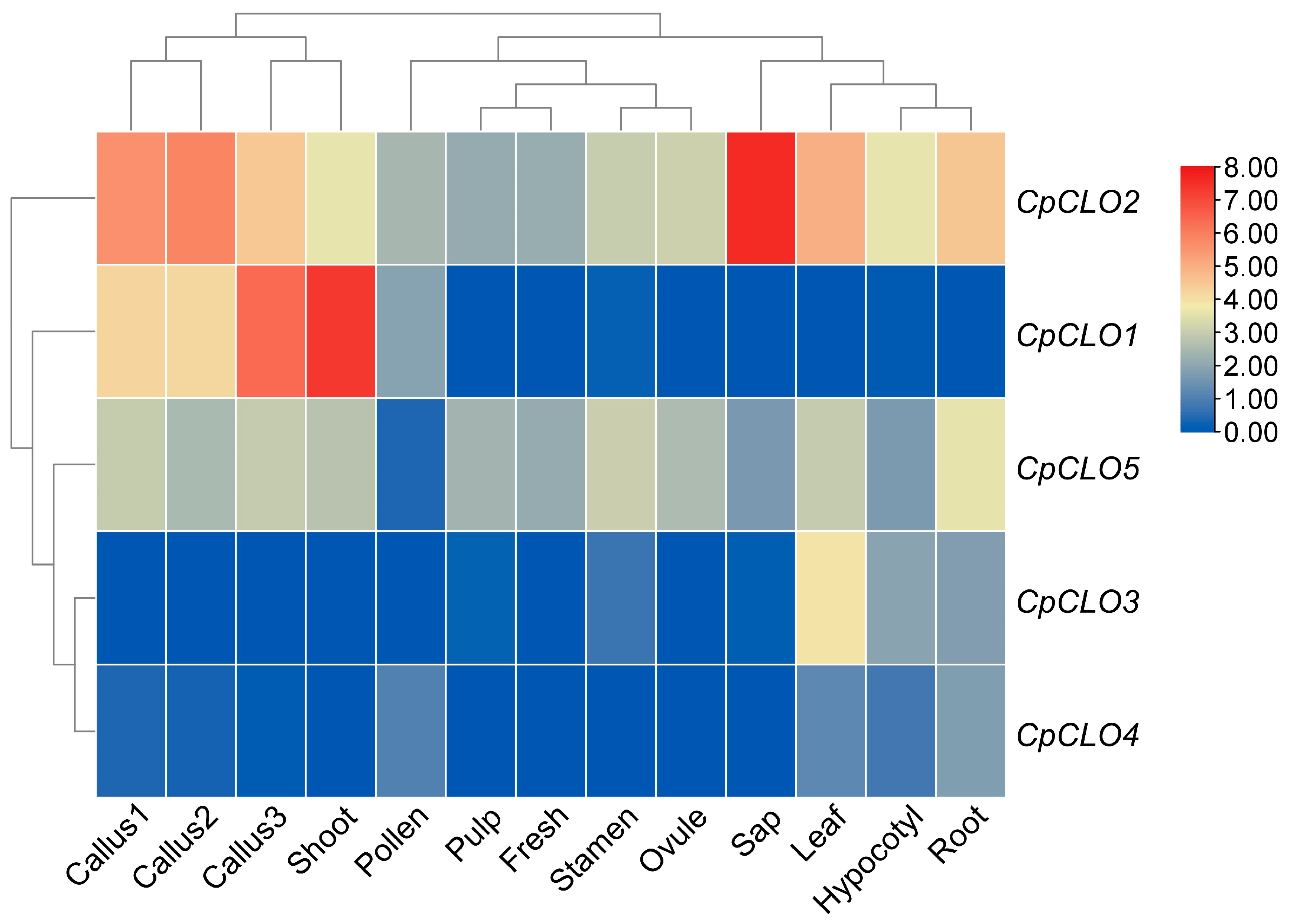
2.4. Caleosin Genes in Castor Bean and Papaya Underwent Apparent Expression Divergence
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Databases and Identification of Caleosin Family Genes
5.2. Multiple Sequence Alignment, Phylogenetic, and Conserved Motif Analyses
5.3. Definition of Orthogroups, Chromosomal Localization, Synteny Analysis, and Calculation of Evolutionary Rate
5.4. Gene Expression Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frandsen, G.; Müller-Uri, F.; Nielsen, M.; Mundy, J.; Skriver, K. Novel plant Ca2+-binding protein expressed in response to abscisic acid and osmotic stress. J. Biol. Chem. 1996, 271, 343–348. [Google Scholar] [CrossRef]
- Takahashi, S.; Katagiri, T.; Yamaguchi-Shinozaki, K.; Shinozaki, K. An Arabidopsis gene encoding a Ca2+-binding protein is induced by abscisic acid during dehydration. Plant Cell Physiol. 2000, 41, 898–903. [Google Scholar] [CrossRef]
- Poxleitner, M.; Rogers, S.W.; Lacey Samuels, A.; Browse, J.; Rogers, J.C. A role for caleosin in degradation of oil-body storage lipid during seed germination. Plant J. 2006, 47, 917–933. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.L.; Chen, J.C.; Chiu, S.T.; Tzen, J.T. Stable oil bodies sheltered by a unique caleosin in cycad megagametophytes. Plant Physiol. Biochem. 2009, 47, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.C.; Tai, S.S.; Peng, C.C.; Tzen, J.T. Identification of three novel unique proteins in seed oil bodies of sesame. Plant Cell Physiol. 1998, 39, 935–941. [Google Scholar] [CrossRef]
- Hernandez-Pinzon, I.; Patel, K.; Murphy, D.J. The Brassica napus calcium-binding protein, caleosin, has distinct endoplasmic reticulum- and lipid body-associated isoforms. Plant Physiol. Biochem. 2001, 39, 615–622. [Google Scholar] [CrossRef]
- Partridge, M.; Murphy, D.J. Roles of a membrane-bound caleosin and putative peroxygenase in biotic and abiotic stress responses in Arabidopsis. Plant Physiol. Biochem. 2009, 47, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Miklaszewska, M.; Zienkiewicz, K.; Klugier-Borowska, E.; Rygielski, M.; Feussner, I.; Zienkiewicz, A. CALEOSIN 1 interaction with AUTOPHAGY-RELATED PROTEIN 8 facilitates lipid droplet microautophagy in seedlings. Plant Physiol. 2023, 193, 2361–2380. [Google Scholar] [CrossRef]
- Naested, H.; Frandsen, G.I.; Jauh, G.Y.; Hernandez-Pinzon, I.; Nielsen, H.B.; Murphy, D.J.; Rogers, J.C.; Mundy, J. Caleosins: Ca2+-binding proteins associated with lipid bodies. Plant Mol. Biol. 2000, 44, 463–476. [Google Scholar] [CrossRef]
- Jiang, P.L.; Jauh, G.Y.; Wang, C.S.; Tzen, J.T. A unique caleosin in oil bodies of lily pollen. Plant Cell Physiol. 2008, 49, 1390–1395. [Google Scholar] [CrossRef]
- Zienkiewicz, K.; Castro, A.J.; Alché Jde, D.; Zienkiewicz, A.; Suárez, C.; Rodríguez-García, M.I. Identification and localization of a caleosin in olive (Olea europaea L.) pollen during in vitro germination. J. Exp. Bot. 2010, 61, 1537–1546. [Google Scholar] [CrossRef] [PubMed]
- Pasaribu, B.; Chung, T.Y.; Chen, C.S.; Wang, S.L.; Jiang, P.L.; Tzen, J.T. Identification of caleosin and two oleosin isoforms in oil bodies of pine megagametophytes. Plant Physiol. Biochem. 2014, 82, 142–150. [Google Scholar] [CrossRef]
- Pasaribu, B.; Chen, C.S.; Liao, Y.K.; Jiang, P.L.; Tzen, J.T. Identification of caleosin and oleosin in oil bodies of pine pollen. Plant Physiol. Biochem. 2017, 111, 20–29. [Google Scholar] [CrossRef]
- Pasaribu, B.; Fu, J.H.; Jiang, P.L. Identification and characterization of caleosin in Cycas revoluta pollen. Plant Signal Behav. 2020, 15, 1779486. [Google Scholar] [CrossRef]
- Lu, J.Y.; Xiong, S.X.; Yin, W.; Teng, X.D.; Lou, Y.; Zhu, J.; Zhang, C.; Gu, J.N.; Wilson, Z.A.; Yang, Z.N. MS1, a direct target of MS188, regulates the expression of key sporophytic pollen coat protein genes in Arabidopsis. J. Exp. Bot. 2020, 71, 4877–4889. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Tsai, C.C.; Tzen, J.T. Cloning and secondary structure analysis of caleosin, a unique calcium-binding protein in oil bodies of plant seeds. Plant Cell Physiol. 1999, 40, 1079–1086. [Google Scholar] [CrossRef]
- Huang, A.H. Plant lipid droplets and their associated proteins: Potential for rapid advances. Plant Physiol. 2018, 176, 1894–1918. [Google Scholar] [CrossRef]
- Hyun, T.K.; Kumar, D.; Cho, Y.Y.; Hyun, H.N.; Kim, J.S. Computational identification and phylogenetic analysis of the oil-body structural proteins, oleosin and caleosin, in castor bean and flax. Gene 2013, 515, 454–460. [Google Scholar] [CrossRef]
- Shen, Y.; Xie, J.; Liu, R.D.; Ni, X.F.; Wang, X.H.; Li, Z.X.; Zhang, M. Genomic analysis and expression investigation of caleosin gene family in Arabidopsis. Biochem. Biophys. Res. Commun. 2014, 448, 365–371. [Google Scholar] [CrossRef]
- Zou, Z.; Zhao, Y.; Zhang, L. Genomic insights into lineage-specific evolution of the oleosin family in Euphorbiaceae. BMC Genom. 2022, 23, 178. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zheng, Y.J.; Zhang, Z.T.; Xiao, Y.H.; Xie, Z.N.; Chang, L.L.; Zhang, L.; Zhao, Y.G. Molecular characterization oleosin genes in Cyperus esculentus, a Cyperaceae plant producing oil in underground tubers. Plant Cell Rep. 2023, 42, 1791–1808. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Fu, X.W.; Huang, J.Q.; Zhao, Y.G. Molecular characterization of CeOLE6, a diverged SH oleosin gene, preferentially expressed in Cyperus esculentus tubers. Planta 2024, 260, 122. [Google Scholar] [CrossRef]
- Zou, Z.; Zhang, L.; Zhao, Y. Integrative analysis of oleosin genes provides insights into lineage-specific family evolution in Brassicales. Plants 2024, 13, 280. [Google Scholar] [CrossRef]
- Hanano, A.; Burcklen, M.; Flenet, M.; Ivancich, A.; Louwagie, M.; Garin, J.; Blee, E. Plant seed peroxygenase is an original heme-oxygenase with an EF-hand calcium binding motif. J. Biol. Chem. 2006, 281, 33140–33151. [Google Scholar] [CrossRef]
- Chen, J.C.; Tzen, J.T. An in vitro system to examine the effective phospholipids and structural domain for protein targeting to seed oil bodies. Plant Cell Physiol. 2001, 42, 1245–1252. [Google Scholar] [CrossRef]
- Blée, E.; Flenet, M.; Boachon, B.; Fauconnier, M.L. A non-canonical caleosin from Arabidopsis efficiently epoxidizes physiological unsaturated fatty acids with complete stereoselectivity. FEBS J. 2012, 279, 3981–3995. [Google Scholar] [CrossRef]
- Song, W.; Qin, Y.; Zhu, Y.; Yin, G.; Wu, N.; Li, Y.; Hu, Y. Delineation of plant caleosin residues critical for functional divergence, positive selection and coevolution. BMC Evol. Biol. 2014, 14, 124. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Hassan, M.; Hanano, A.; Fitzpatrick, D.A.; McCarthy, C.P.; Murphy, D.J. Evolutionary, structural and functional analysis of the caleosin/peroxygenase gene family in the Fungi. BMC Genom. 2018, 19, 976. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.; Hassan, M.; Rosli, R.; Almousally, I.; Hanano, A.; Murphy, D.J. Evolutionary and genomic analysis of the caleosin/peroxygenase (CLO/PXG) gene/protein families in the Viridiplantae. PLoS ONE 2018, 13, e0196669. [Google Scholar] [CrossRef]
- Hanano, A.; Blée, E.; Murphy, D.J. Caleosin/peroxygenases: Multifunctional proteins in plants. Ann. Bot. 2023, 131, 387–409. [Google Scholar] [CrossRef]
- Khalil, H.B.; Brunetti, S.C.; Pham, U.M.; Maret, D.; Laroche, A.; Gulick, P.J. Characterization of the caleosin gene family in the Triticeae. BMC Genom. 2014, 15, 239. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, M.; Wang, L.; Li, Z.; Taylor, D.C.; Li, Z.; Zhang, M. Identification, duplication, evolution and expression analyses of caleosins in Brassica plants and Arabidopsis subspecies. Mol. Genet. Genom. 2016, 291, 971–988. [Google Scholar] [CrossRef]
- Bowers, J.E.; Chapman, B.A.; Rong, J.; Paterson, A.H. Unravelling angiosperm genome evolution by phylogenetic analysis of chromosomal duplication events. Nature 2003, 422, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Leebens-Mack, J.; Ayyampalayam, S.; Bowers, J.E.; McKain, M.R.; McNeal, J.; Rolf, M.; Ruzicka, D.R.; Wafula, E.; Wickett, N.J.; et al. A genome triplication associated with early diversification of the core eudicots. Genome Biol. 2012, 13, R3. [Google Scholar] [CrossRef] [PubMed]
- Cardinal-McTeague, W.M.; Sytsma, K.J.; Hall, J.C. Biogeography and diversification of Brassicales: A 103 million year tale. Mol. Phylogenet. Evol. 2016, 99, 204–224. [Google Scholar] [CrossRef]
- Mabry, M.E.; Brose, J.M.; Blischak, P.D.; Sutherland, B.; Dismukes, W.T.; Bottoms, C.A.; Edger, P.P.; Washburn, J.D.; An, H.; Hall, J.C.; et al. Phylogeny and multiple independent whole-genome duplication events in the Brassicales. Am. J. Bot. 2020, 107, 1148–1164. [Google Scholar] [CrossRef]
- Yue, J.; VanBuren, R.; Liu, J.; Fang, J.; Zhang, X.; Liao, Z.; Wai, C.M.; Xu, X.; Chen, S.; Zhang, S.; et al. SunUp and Sunset genomes revealed impact of particle bombardment mediated transformation and domestication history in papaya. Nat. Genet. 2022, 54, 715–724. [Google Scholar] [CrossRef]
- Zou, Z.; Li, M.Y.; Jia, R.Z.; Zhao, H.; He, P.P.; Zhang, Y.L.; Guo, A.P. Genes encoding light-harvesting chlorophyll a/b-binding proteins in papaya (Carica papaya L.) and insight into lineage-specific evolution in Brassicaceae. Gene 2020, 748, 144685. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Zheng, Y.J.; Xie, Z.N. Analysis of Carica papaya informs lineage-specific evolution of the aquaporin (AQP) family in Brassicales. Plants 2023, 12, 3847. [Google Scholar] [CrossRef]
- Cheng, S.; van den Bergh, E.; Zeng, P.; Zhong, X.; Xu, J.; Liu, X.; Hofberger, J.; de Bruijn, S.; Bhide, A.S.; Kuelahoglu, C.; et al. The Tarenaya hassleriana genome provides insight into reproductive trait and genome evolution of crucifers. Plant Cell 2013, 25, 2813–2830. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, Y.; Zhang, J.; Yang, C.; Yan, L.; Wang, X.; Shi, C.; Xie, J.; Dai, T.; Peng, L.; et al. High quality reference genome of drumstick tree (Moringa oleifera Lam.), a potential perennial crop. Sci. China Life Sci. 2015, 58, 627–638. [Google Scholar] [CrossRef]
- Filiault, D.L.; Ballerini, E.S.; Mandáková, T.; Aköz, G.; Derieg, N.J.; Schmutz, J.; Jenkins, J.; Grimwood, J.; Shu, S.; Hayes, R.D.; et al. The Aquilegia genome provides insight into adaptive radiation and reveals an extraordinarily polymorphic chromosome with a unique history. eLife 2018, 7, e36426. [Google Scholar] [CrossRef]
- Käfer, J.; Bewick, A.; Andres-Robin, A.; Lapetoule, G.; Harkess, A.; Caïus, J.; Fogliani, B.; Gâteblé, G.; Ralph, P.; dePamphilis, C.W.; et al. A derived ZW chromosome system in Amborella trichopoda, representing the sister lineage to all other extant flowering plants. New Phytol. 2022, 233, 1636–1642. [Google Scholar] [CrossRef]
- Lu, J.; Pan, C.; Fan, W.; Liu, W.; Zhao, H.; Li, D.; Wang, S.; Hu, L.; He, B.; Qian, K.; et al. A Chromosome-level genome assembly of wild castor provides new insights into its adaptive evolution in tropical desert. Genom. Proteom. Bioinf. 2022, 20, 42–59. [Google Scholar] [CrossRef]
- Wang, L.; Fan, L.; Zhao, Z.; Zhang, Z.; Jiang, L.; Chai, M.; Tian, C. The Capparis spinosa var. herbacea genome provides the first genomic instrument for a diversity and evolution study of the Capparaceae family. Gigascience 2022, 11, giac106. [Google Scholar] [CrossRef]
- Zhang, H.; Du, X.; Dong, C.; Zheng, Z.; Mu, W.; Zhu, M.; Yang, Y.; Li, X.; Hu, H.; Shrestha, N.; et al. Genomes and demographic histories of the endangered Bretschneidera sinensis (Akaniaceae). Gigascience 2022, 11, giac050. [Google Scholar] [CrossRef]
- Zhao, W.; Li, J.; Sun, X.; Zheng, Q.; Liu, J.; Hua, W.; Liu, J. Integrated global analysis in spider flowers illuminates features underlying the evolution and maintenance of C4 photosynthesis. Hortic. Res. 2023, 10, uhad129. [Google Scholar] [CrossRef]
- Huang, M.D.; Huang, A.H. Bioinformatics reveal five lineages of oleosins and the mechanism of lineage evolution related to structure/function from green algae to seed plants. Plant Physiol. 2015, 169, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Siloto, R.M.P.; Findlay, K.; Lopez, V.A.; Yeung, E.C.; Nykifork, C.L.; Moloney, M.M. The accumulation of oleosins determines the size of seed oil bodies in Arabidopsis. Plant Cell 2006, 18, 1961–1974. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Huang, A.C. Unique motifs and length of hairpin in oleosin target the cytosolic side of endoplasmic reticulum and budding lipid droplet. Plant Physiol. 2017, 174, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Wang, Y.; Shen, Y.; Jia, Q.; Zhao, C.; Zhang, M. Multiple caleosins have overlapping functions in oil accumulation and embryo development. J. Exp. Bot. 2022, 73, 3946–3962. [Google Scholar] [CrossRef]
- Toorop, P.E.; Barroco, R.M.; Engler, G.; Groot, S.P.; Hilhorst, H.W. Differentially expressed genes associated with dormancy or germination of Arabidopsis thaliana seeds. Planta 2005, 221, 637–647. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Jung, K.W.; Yoo, K.S.; Jeung, J.U.; Shin, J.S. A stress-responsive caleosin-like protein, AtCLO4, acts as a negative regulator of ABA responses in Arabidopsis. Plant Cell Physiol. 2011, 52, 874–884. [Google Scholar] [CrossRef]
- Zou, Z.; Yang, J.H. Genomic analysis of Dof transcription factors in Hevea brasiliensis, a rubber-producing tree. Ind. Crops Prod. 2019, 134, 271–283. [Google Scholar] [CrossRef]
- Zou, Z.; Gong, J.; An, F.; Xie, G.S.; Wang, J.K.; Mo, Y.Y.; Yang, L.F. Genome-wide identification of rubber tree (Hevea brasiliensis Muell. Arg.) aquaporin genes and their response to ethephon stimulation in the laticifer, a rubber-producing tissue. BMC Genom. 2015, 16, 1001. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Fu, X.; Li, C.; Yi, X.; Huang, J.; Zhao, Y. Integrative analysis provides insights into genes encoding LEA_5 domain-containing proteins in tigernut (Cyperus esculentus L.). Plants 2025, 14, 762. [Google Scholar] [CrossRef] [PubMed]
- Emms, D.M.; Kelly, S. OrthoFinder: Phylogenetic orthology inference for comparative genomics. Genome Biol. 2019, 20, 238. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Qiao, X.; Li, Q.; Yin, H.; Qi, K.; Li, L.; Wang, R.; Zhang, S.; Paterson, A.H. Gene duplication and evolution in recurring polyploidization-diploidization cycles in plants. Genome Biol. 2019, 20, 38. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z. PAML 4: Phylogenetic analysis by maximum likelihood. Mol. Biol. Evol. 2007, 24, 1586–1591. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
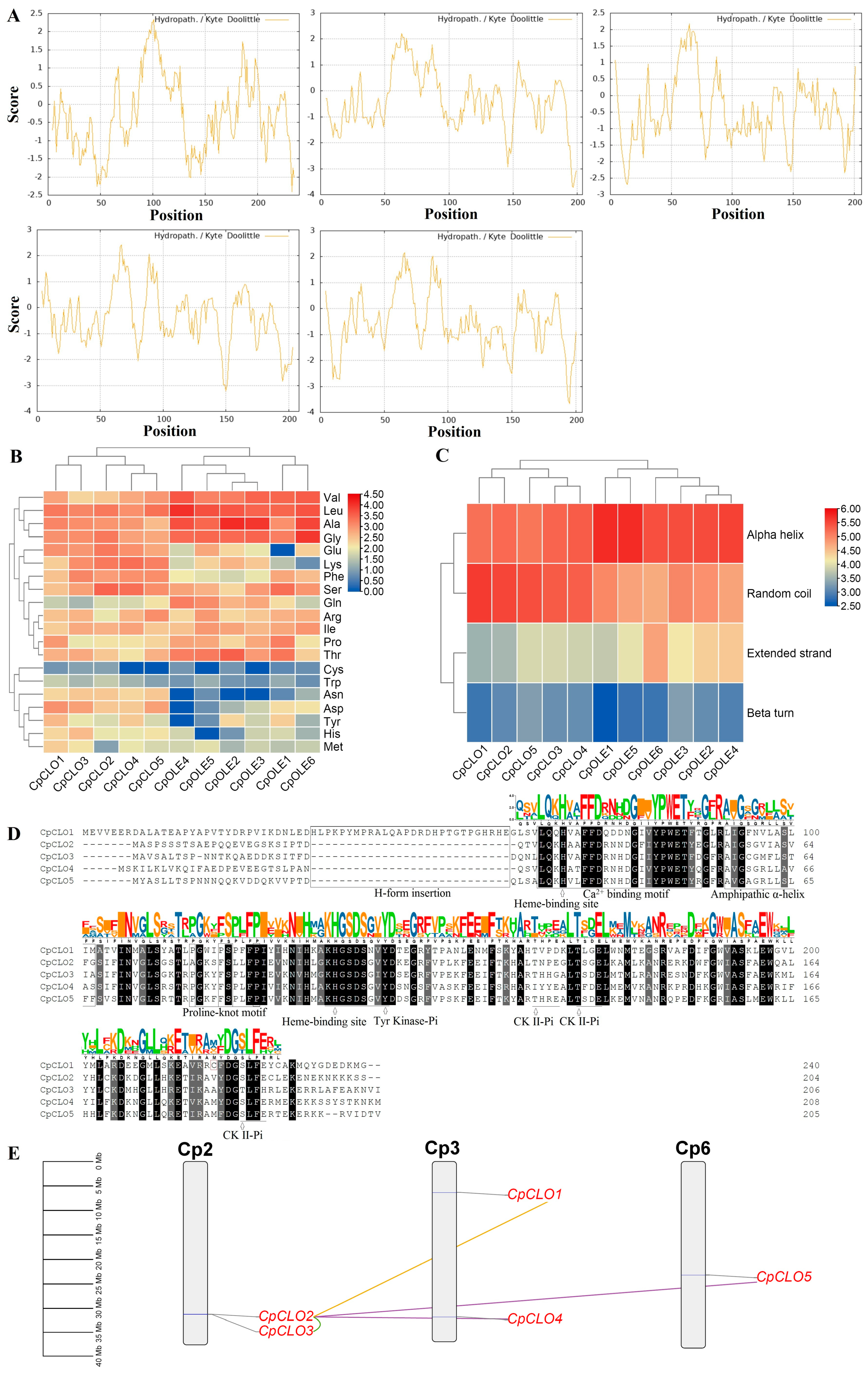
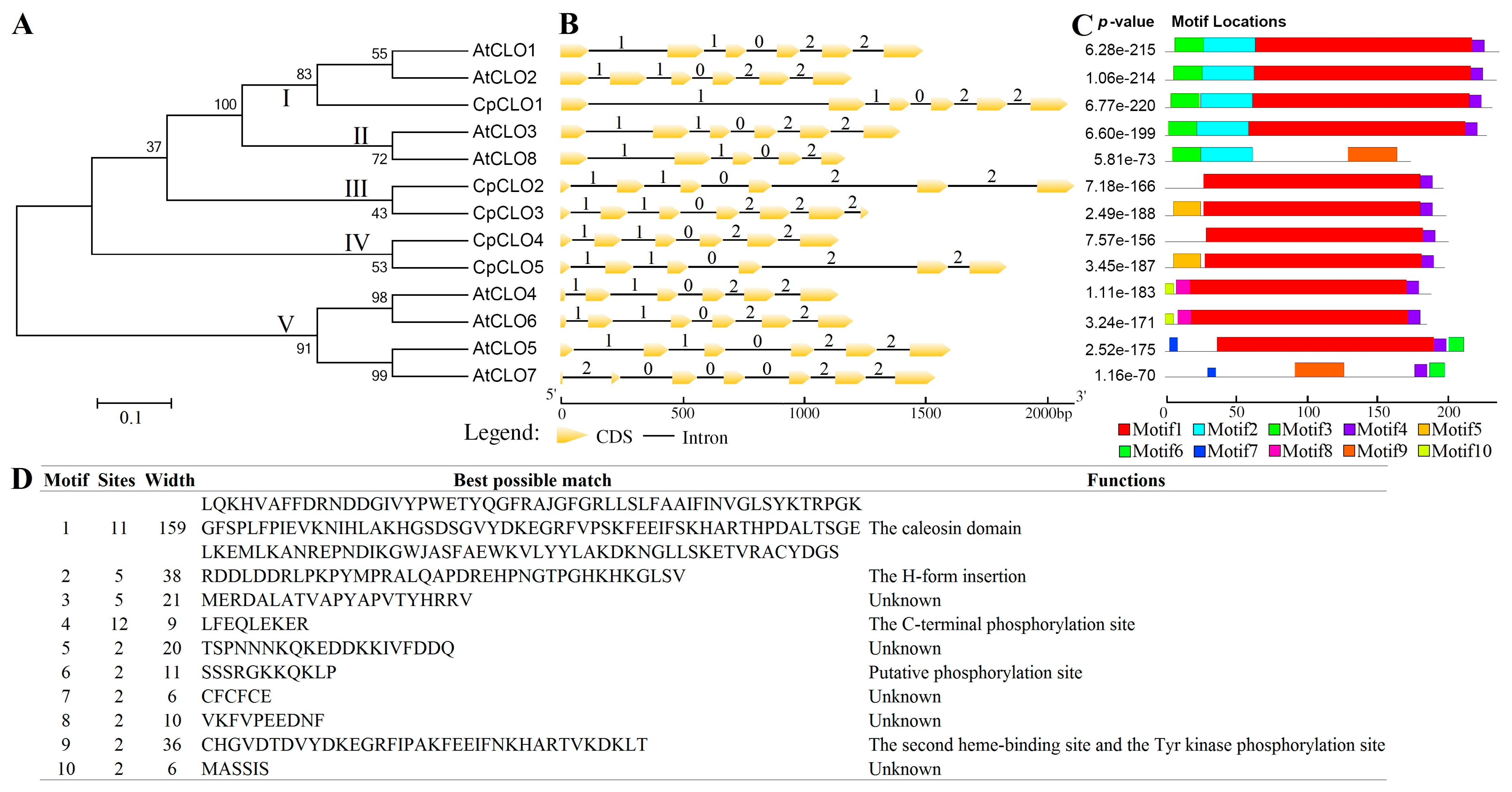
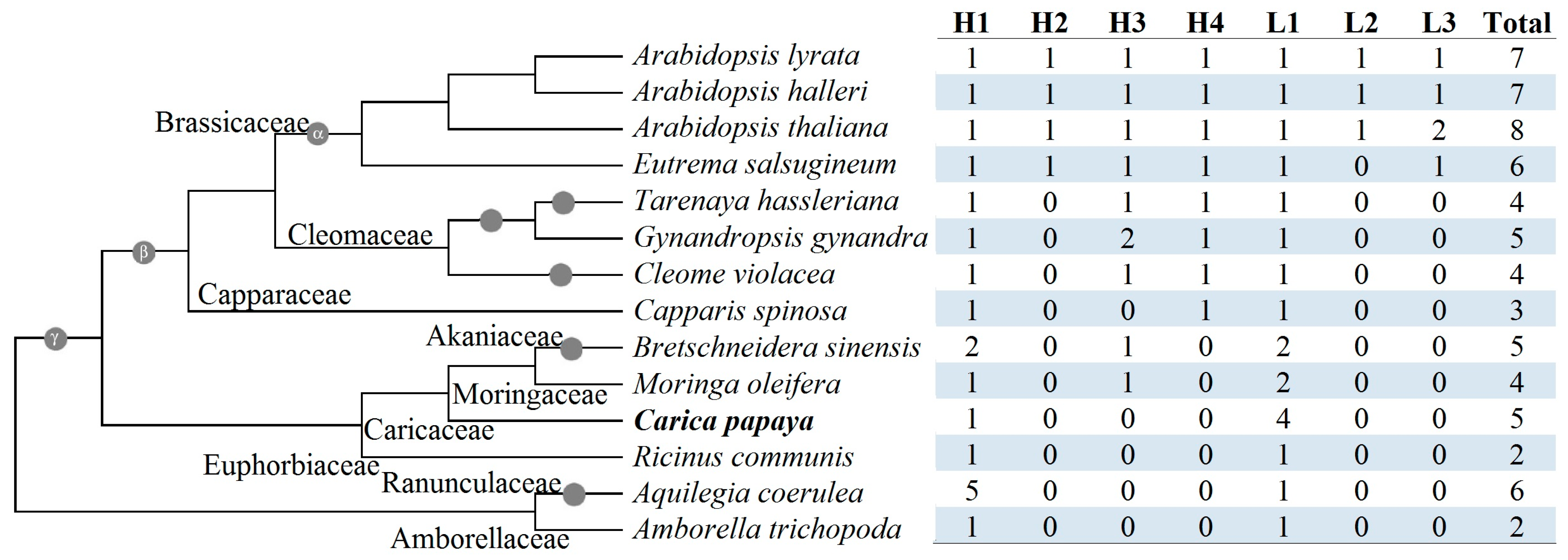
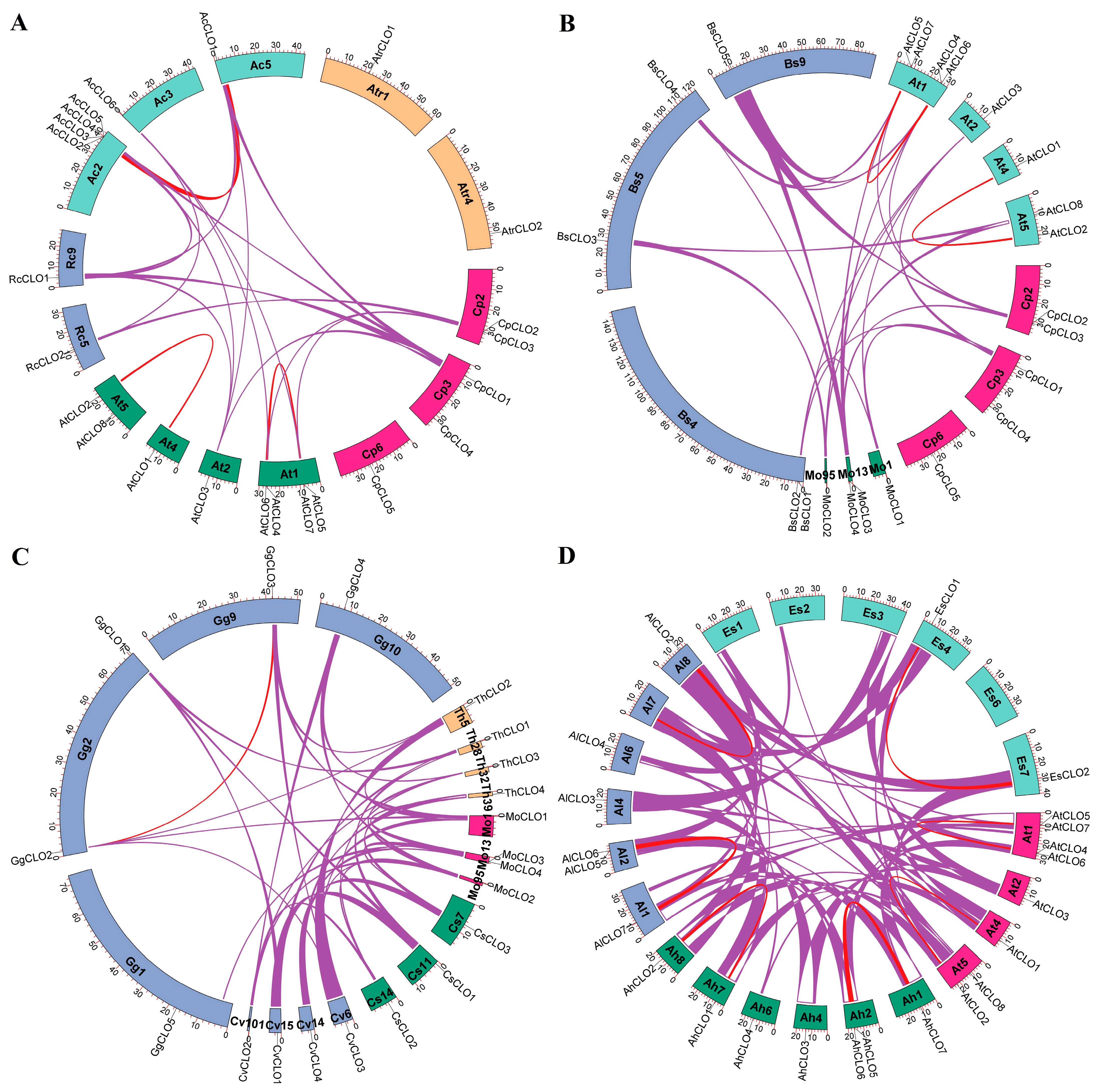
| Gene Name | Locus | Position | AA | MW (kDa) | pI | GRAVY | Caleosin Location | Clade |
|---|---|---|---|---|---|---|---|---|
| CpCLO1 | sunset03G0007900 | Chr3:6306411..6308488(+) | 240 | 27.24 | 5.05 | −0.329 | 62..228 | H |
| CpCLO2 | sunset02G0020020 | Chr2:31227224..31229504(+) | 204 | 22.67 | 6.09 | −0.419 | 25..192 | L |
| CpCLO3 | sunset02G0020030 | Chr2:31241163..31242710(+) | 206 | 23.57 | 6.80 | −0.402 | 25..192 | L |
| CpCLO4 | sunset03G0022160 | Chr3:31772679..31773742(+) | 208 | 23.89 | 9.41 | −0.401 | 27..194 | L |
| CpCLO5 | sunset06G0015560 | Chr6:23171674..23173752(+) | 205 | 23.67 | 9.57 | −0.509 | 26..192 | L |
| Gene1 | Gene2 | Identity (%) | Ka | Ks | Ka/Ks |
|---|---|---|---|---|---|
| CpCLO1 | CpCLO2 | 46.8 | 0.5152 | - | - |
| CpCLO2 | CpCLO3 | 71.2 | 0.2378 | 1.2020 | 0.1978 |
| CpCLO2 | CpCLO4 | 67.5 | 0.2646 | 1.2871 | 0.2056 |
| CpCLO2 | CpCLO5 | 69.6 | 0.2757 | 0.8983 | 0.3069 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, Z.; Fu, X.; Yi, X.; Li, C.; Zhao, Y. Characterization of Caleosin Genes in Carica papaya and Insights into Lineage-Specific Family Evolution in Brassicales. Plants 2025, 14, 3296. https://doi.org/10.3390/plants14213296
Zou Z, Fu X, Yi X, Li C, Zhao Y. Characterization of Caleosin Genes in Carica papaya and Insights into Lineage-Specific Family Evolution in Brassicales. Plants. 2025; 14(21):3296. https://doi.org/10.3390/plants14213296
Chicago/Turabian StyleZou, Zhi, Xiaowen Fu, Xiaoping Yi, Chunqiang Li, and Yongguo Zhao. 2025. "Characterization of Caleosin Genes in Carica papaya and Insights into Lineage-Specific Family Evolution in Brassicales" Plants 14, no. 21: 3296. https://doi.org/10.3390/plants14213296
APA StyleZou, Z., Fu, X., Yi, X., Li, C., & Zhao, Y. (2025). Characterization of Caleosin Genes in Carica papaya and Insights into Lineage-Specific Family Evolution in Brassicales. Plants, 14(21), 3296. https://doi.org/10.3390/plants14213296






