Abstract
Ahuehuete (Taxodium mucronatum Ten.) is a riparian tree species of significant ecological, cultural, and economic importance, demonstrating remarkable tolerance to prolonged flooding. However, the underlying mechanism of waterlogging adaptation remains unknown. In this study, we determined the physiological traits of the Ahuehuete leaves at 0, 15, 30, and 60 d under waterlogging conditions. The results showed that no significant difference in MDA content occurred between the Ahuehuete leaves subjected to waterlogging and those under well-watered (CK) conditions. In contrast, the contents of osmoprotectants (soluble sugar, soluble protein, and proline) and the activities of antioxidant enzymes (SOD, POD, and CAT) exhibited similar change trends under both waterlogging and CK conditions, despite minor quantitative differences between the two groups. Subsequent comparative transcriptome analysis was performed to investigate the transcriptional characteristics. A total of 3687 DEGs were expressed in all comparisons throughout the waterlogging process, while 2873, 4617, and 2710 DEGs were comparison group specific. KEGG enrichment analysis revealed that DEGs were enriched in various metabolic pathways, such as Plant hormone signal transduction (ko04075), MAPK signaling pathway-plant (ko04016), ABC transporter (ko02010), and Nitrogen metabolism (ko00910). WGCNA also identified key modules associated with physiological traits, simultaneously emphasizing the importance of plant hormone signal transduction and MAPK signal cascade. Overall, our findings revealed physiological and transcriptomic characteristics of the Ahuehuete under waterlogging conditions, and provided new insights to waterlogging adaptation in woody gymnosperm species.
1. Introduction
Taxodium mucronatum Ten., also called Ahuehuete, sabino, or Montezuma bald cypress, is a riparian tree species of significant ecological, cultural, and economic importance [1,2]. The term “Ahuehuete” originates from the Nahuatl word āhuēhuētl, and its etymology can be interpreted as “upright drum in water” or “old man of the water”, alluding to the typical habitat in moist environments [1]. Like other Taxodium species, such as baldcypress (T. distichum var. distichum (L.) Rich.) and T. ascendens Brongn., the Ahuehuete demonstrates remarkable tolerance to prolonged flooding [3,4]. It was introduced to southeastern China in the last century for ecological enhancement and ornamental purposes. Subsequent evaluations have also confirmed their adaptability to this region, leading to their widespread application in coastal shelterbelt establishment and wetland ecological restoration [5,6]. Previous studies on Taxodium species, such as T. distichum and Taxodium hybrid ‘Zhongshanshan’, have identified several important physiological and transcriptional adaptation mechanisms, including aerenchyma formation, photosynthesis adjustments, organic acid metabolism, inhibition of abscisic acid (ABA) and jasmonic acid (JA) biosynthesis, and activation of specific pathways like glycolysis and fermentation [7,8,9,10,11,12,13,14]. However, the physiological and molecular adaptation mechanisms of the Ahuehuete to waterlogged environments remain poorly understood. In particular, it remains unclear whether this species shares these mechanisms entirely or has evolved distinct regulatory strategies to cope with its specific riparian habitats.
Water is fundamental to the survival of plants, but excessive water, such as waterlogging or flooding, impedes gaseous exchange between the soil and the atmosphere, and reduces the oxygen supply to plant roots, resulting in hypoxic or anoxic situations [15,16]. Many plants possess the ability to cope with short-term flooding stress under natural conditions, and likely harbor evolutionarily conserved acute flooding stress-related regulatory mechanisms [3]. In particular, plants synthesize a diverse array of osmoprotectants, including proline, soluble sugars, and soluble proteins, in response to osmotic stress induced by waterlogging [16,17,18]. The activation of the antioxidant enzyme system is another important approach to cope with waterlogging-induced ROS generation [19,20]. In Styrax japonica Sieb. et Zucc., seedlings exhibited compensatory upregulation of catalase (CAT) and peroxidase (POD) activities under waterlogging and partial submergence conditions [21]. Similarly, the activity of superoxide dismutase (SOD) and CAT increased in Yulania stellata (Siebold & Zucc.) N.H. Xia at the early stages of flooding stress [22].
Notably, only highly tolerant plant species can endure prolonged inundation, such species have developed specialized regulatory mechanisms in response to persistent waterlogging stress. For example, Giant reed (Arundo donax L.) has developed adaptive mechanisms to waterlogging stress, which include enhancing the activity of antioxidant enzymes to reduce oxidative damage, alongside employing osmoprotectants and aquaporins to regulate water homeostasis [23]. In baldcypress, the activation of the mitogen-activated protein kinase (MAPK) signaling pathway, antioxidant systems, immune systems, and glycolytic pathways are conserved mechanisms that respond to flooding stress [3].
MAPKs represent a class of highly conserved signaling transduction modules that play critical roles in numerous signal transduction processes through MAPK cascade pathways [24]. A typical MAPK cascade consists of three sequentially acting kinases: MAPK kinase kinase (MAPKKK, also known as MAP3K or MEKK), MAPK kinase (MAPKK, MAP2K, MKK, or MEK), and MAPK (also referred to as MPK) [25,26]. They are involved in the biosynthesis and/or signal transduction pathways of several stress-related phytohormones (e.g., ethylene, ABA, JA), consequently mediating essential functions within plant abiotic stress response [27,28,29].
In this study, we examined the dynamics of physiological characteristics (osmoprotectants, antioxidant enzyme system, malondialdehyde (MDA)) of the Ahuehuete leaves under waterlogging condition. Subsequently, temporal transcriptomes were performed to identify key regulatory pathways and hub genes involved in waterlogging adaptation through comparative analysis and weighted correlation network analysis (WGCNA). On this basis, this study provides a new insight to adaptive responses to waterlogging stress at the physiological and molecular levels.
2. Results
2.1. Physiological Response of the Ahuehuete Leaves to Waterlogging
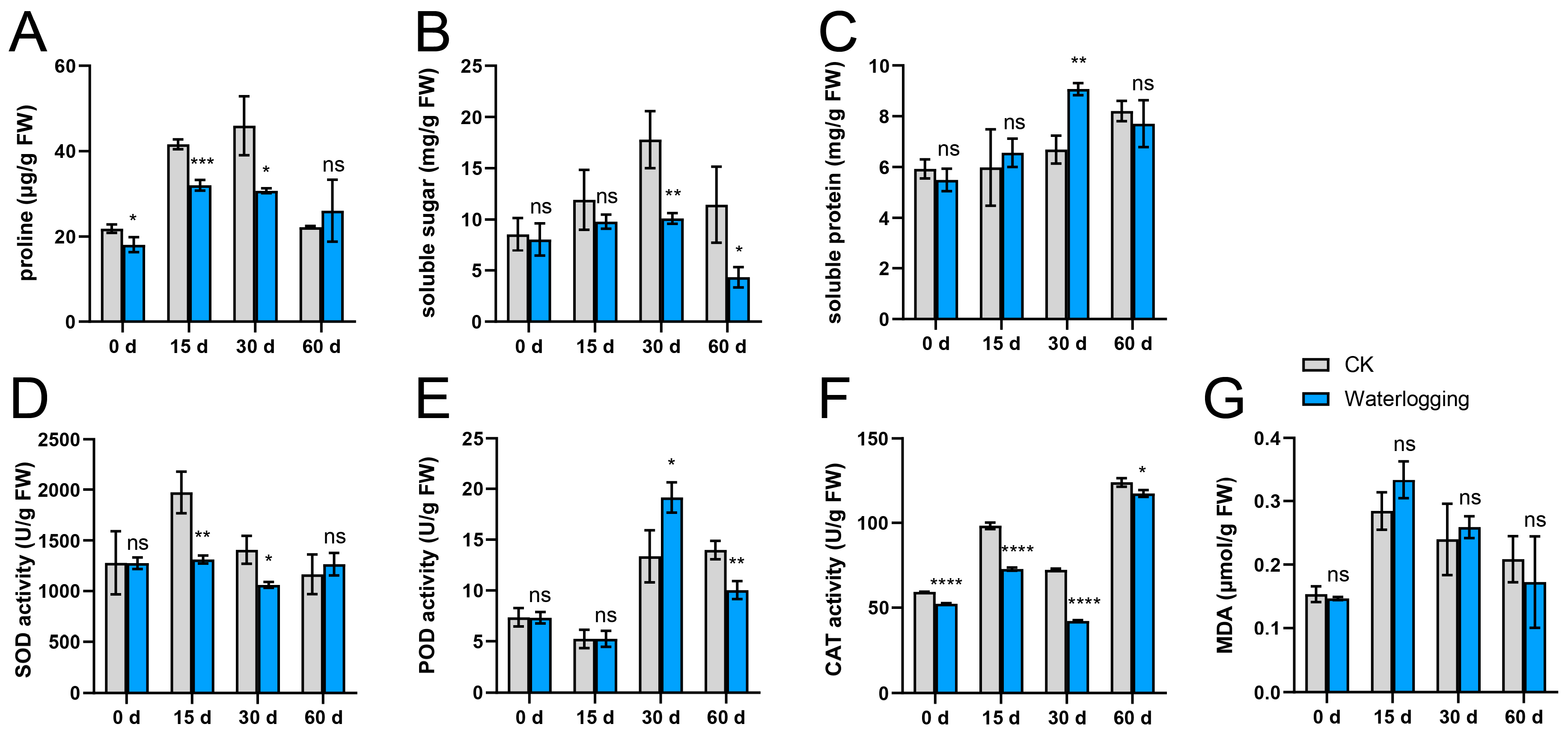
During the waterlogging treatment, the proline and soluble sugar contents exhibited an initial increase over the first 30 days, followed by a decline at 60 d. Throughout the experimental period, both parameters remained consistently lower than those observed in the control (CK) group (Figure 1A,B). In contrast to the CK group, the waterlogging treatment did not result in a significant difference in soluble protein content, with the exception of the measurement taken at 30 d (Figure 1C). Regarding antioxidant enzyme system, SOD activity showed relatively stable levels throughout the waterlogging treatment (Figure 1D). POD activity initially increased and subsequently decreased, reaching its peak at 30 d. Notably, the differences between the waterlogging and CK groups did not emerge until 30 d after the initiation of the experiment (Figure 1E). CAT activity exhibited a fluctuating upward trend throughout the experimental period, peaking at 60 d, with the waterlogging treatment consistently remaining lower than that of the CK group (Figure 1F). In addition, MDA content exhibited an initial increase followed by a subsequent decrease, with no significant difference observed between the waterlogging group and the CK group (Figure 1G).
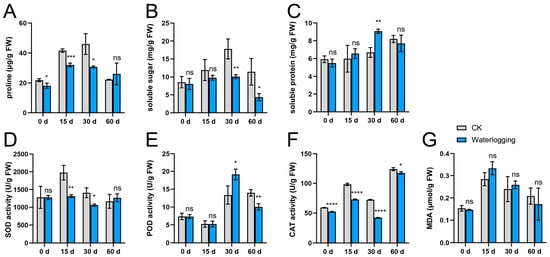
Figure 1.
Comparative analysis of physiological indexes of the ahuehuete leaves under well-watered (CK) and waterlogging conditions. (A) Proline content. (B) Soluble sugar content. (C) Soluble protein content. (D) Superoxide dismutase (SOD) activity. (E) Peroxidase (POD) activity. (F) Catalase (CAT) activity. (G) Malondialdehyde (MDA) content. Sample size: n = 3. Asterisks indicate statistical differences at the p < 0.05 (*), 0.01 (**), 0.001 (***), and 0.0001 (****) levels. ns: not significant.
2.2. Overview of Transcriptome Analysis
Transcriptomic samples were sequenced with three biological replicates per stage, yielding total raw reads ranging from 38,478,898 to 49,461,356 per sample (Table S1). After data filtering, between 38,342,710 and 49,071,202 clean reads were retained, with Q20 and Q30 values exceeding 98.25% and 94.90%, respectively, indicating high sequencing quality (Table S1). Subsequently, these clean reads were subjected to de novo assembly, generating a total of 101,585 unigenes (Table S2). Moreover, the length of the unigenes ranged from 201 bp to 23,773 bp, with an N50 length of 1703 bp (Table S2). Principal component analysis (PCA) demonstrated a clear separation between four different waterlogging stages, along with a strong correlation among three replicates at each stage, reflecting the high repeatability (Figure S1A). Clustering analysis also revealed that transcriptomic samples at D0 were separated from those at waterlogging conditions (D15, D30, and D60) (Figure S1B).
2.3. Analysis of Differentially Expressed Genes (DEGs) in the Ahuehuete Leaves Under Waterlogging Conditions
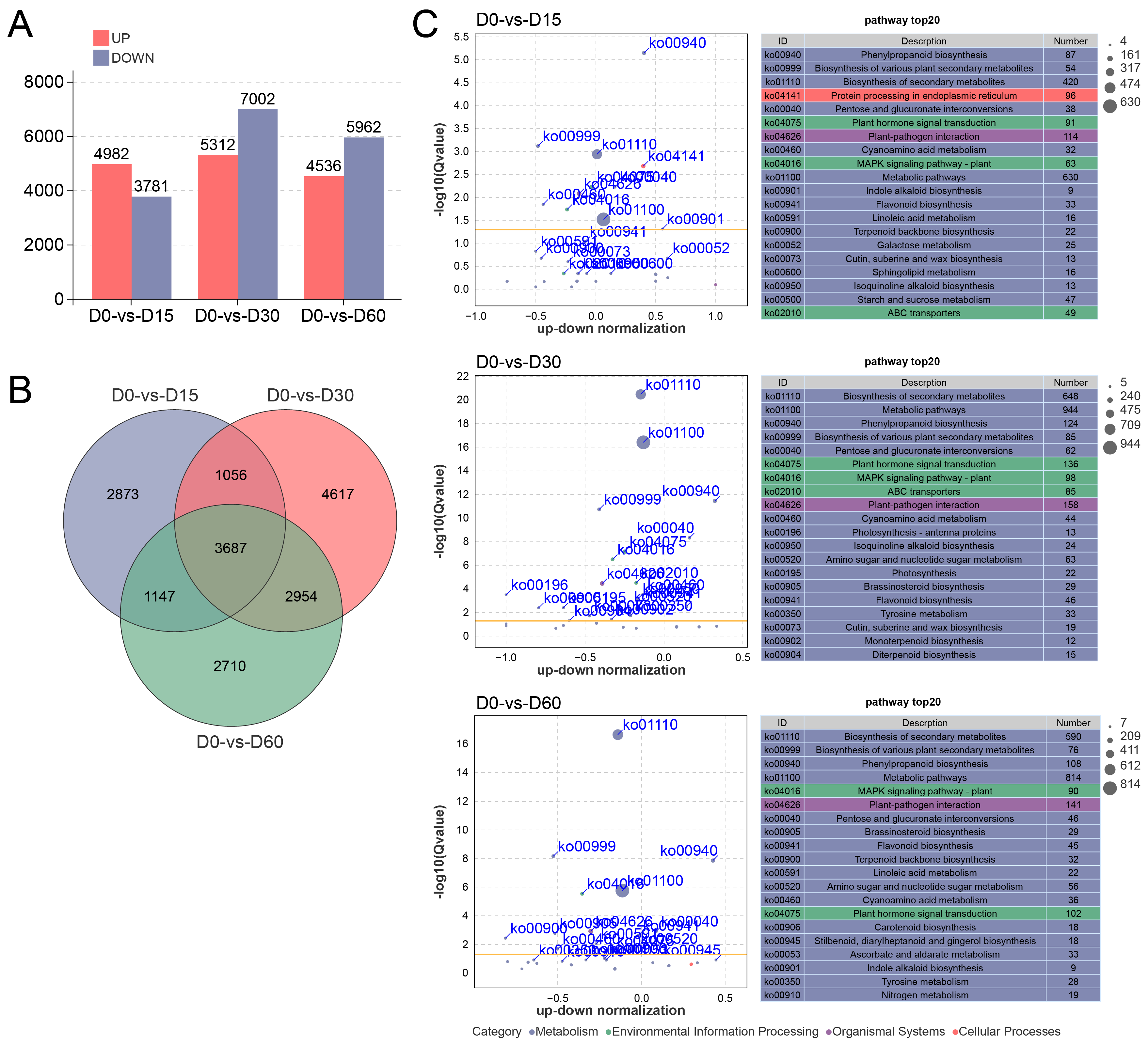
A total of 4982 up- and 3781 down-regulated DEGs were identified in the D0 versus D15 comparison. In the comparison of D0 versus D30, 5312 DEGs were preferentially expressed at D0, whereas 7002 DEGs were preferentially expressed at D30. The D0 versus D60 comparison yielded 4536 up- and 5962 down-regulated DEGs (Figure 2A). The Venn diagram revealed that a total of 3687 DEGs were expressed in all comparisons throughout the waterlogging process, while 2873, 4617, and 2710 DEGs were exclusively identified in the comparisons of D0 versus D15, D0 versus D30, and D0 versus D60, respectively (Figure 2B).
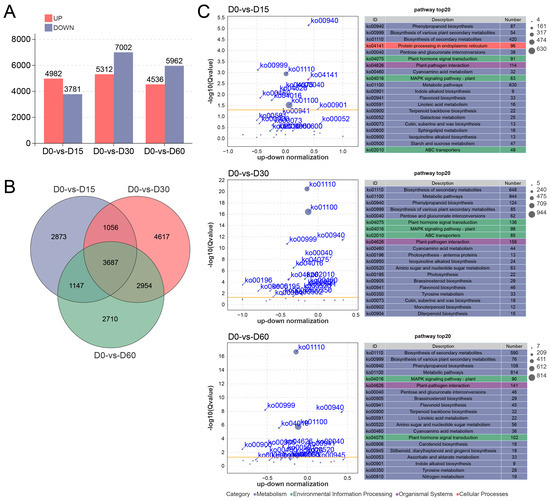
Figure 2.
Transcriptome sequencing analysis of the Ahuehuete leaves in response to waterlogging condition. (A) Statistic analysis of up-/down-regulated DEGs. (B) Venn diagram of DEGs. (C) KEGG enrichment analysis of DEGs reveals the top 20 significantly enriched pathways.
Subsequently, a GO enrichment analysis was conducted to explore the biological functions of DEGs involved in waterlogging response. In the D0 versus D15 comparison, DEGs were significantly enriched in terms related to oxidoreductase activity (GO:0016491), antioxidant activity (GO:0016209), peroxidase activity (GO:0004601), etc. DEGs in the D0 versus D30 comparison were mainly enriched oxidoreductase activity (GO:0016491) and DNA-binding transcription factor activity (GO:0003700). For the D0 versus D60 comparison, DEGs were involved in oxidoreductase activity (GO:0016491), antioxidant activity (GO:0016209), ATP hydrolysis activity (GO:0016887), etc. (Figure S2).
KEGG enrichment analysis revealed that pathways pertaining to the Metabolism category constituted the highest proportion, followed by those associated with Environmental Information Processing category (Figure 2C). Enriched pathways consistently identified across all comparative groups included Phenylpropanoid biosynthesis (ko00940), Flavonoid biosynthesis (ko00941), Plant hormone signal transduction (ko04075), and MAPK signaling pathway-plant (ko04016) (Figure 2C), indicating that these pathways may play a crucial role in the adaptation of the Ahuehuete to waterlogging. In addition, after 15 and 30 days of waterlogging stress, the ABC transporter (ko02010) was significantly enriched, and the Nitrogen metabolism (ko00910) was significantly enriched after 60 days (Figure 2C). These results implied that long-term waterlogging stress might induce nitrogen deficiency and alter gene expression of nitrogen metabolism-related pathways.
2.4. WGCNA Identified Key Module and Hub Genes Associated with Waterlogging Adaptation
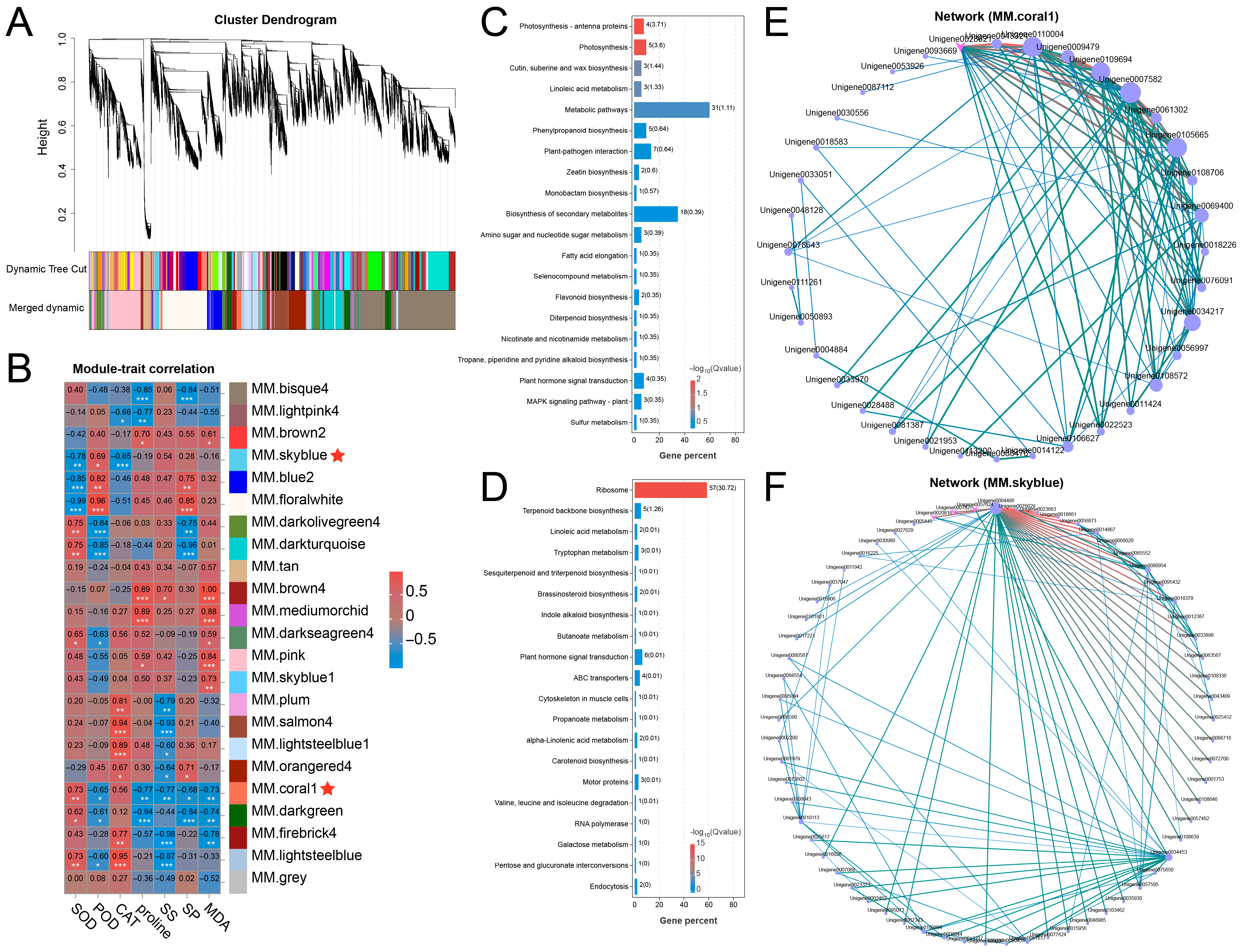
WGCNA was performed to identify gene clusters co-expressed in waterlogging conditions, setting the power value at 19 and the mean connectivity of 418.64688 (Figure S3A,B). A total of 19,044 DEGs were grouped into 23 modules, each comprising between 1 and 4909 genes (Figure 3A and Figure S3C). The module–trait correlation analysis demonstrated that MM.coral1 was significantly correlated with SOD, POD, proline, SS, SP, and MDA, and MM.skyblue was significantly correlated with SOD, POD, and CAT (Figure 3B). In addition, KEGG analysis showed that the MM.coral module harbored genes linked to various pathways, including Phenylpropanoid biosynthesis, Flavonoid biosynthesis, Plant hormone signal transduction, and MAPK signaling pathway-plant (Figure 3C). Genes from the MM.skyblue module were enriched in Plant hormone signal transduction, etc. (Figure 3D). Furthermore, co-expression network analysis identified several hub genes within distinct key modules. Specifically, the MM.coral1 module contained ERF017 (Unigene0108706), BGAL8 (Unigene0069400), and Histone H1.2 (Unigene0007582) (Figure 3E), whereas the MM.skyblue module included MYB4 (Unigene0007825) and MYB5 (Unigene0020816) (Figure 3F). These results imply that genes in MM.coral1 and MM.skyblue might play important roles in adaptation of the Ahuehuete to waterlogging.
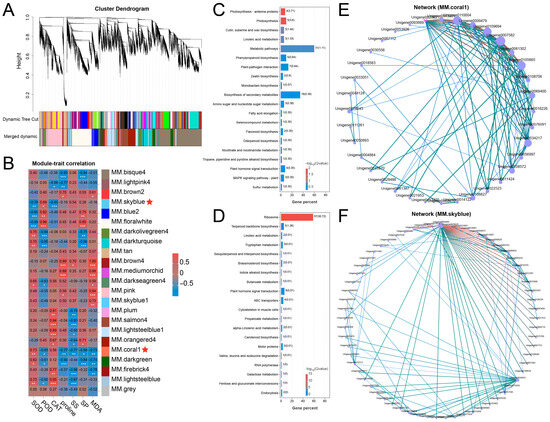
Figure 3.
WGCNA identifies key modules and candidate genes responsible for waterlogging adaptation. (A) Identification of modules. (B) Correlation analysis reveals key modules related to waterlogging adaptation. Asterisks indicate significance at p < 0.05 (*), p < 0.01 (**), and p < 0.001 (***). (C,D) KEGG analysis of genes from the coral1 (C) and skyblue (D) modules. (E,F) Co-expression network derived from the coral1 (E) and skyblue (F) module identifies hub genes associated with waterlogging adaptation.
2.5. Regulatory Road Maps for Plant Hormone Signal Transduction and MAPK Signaling Pathway
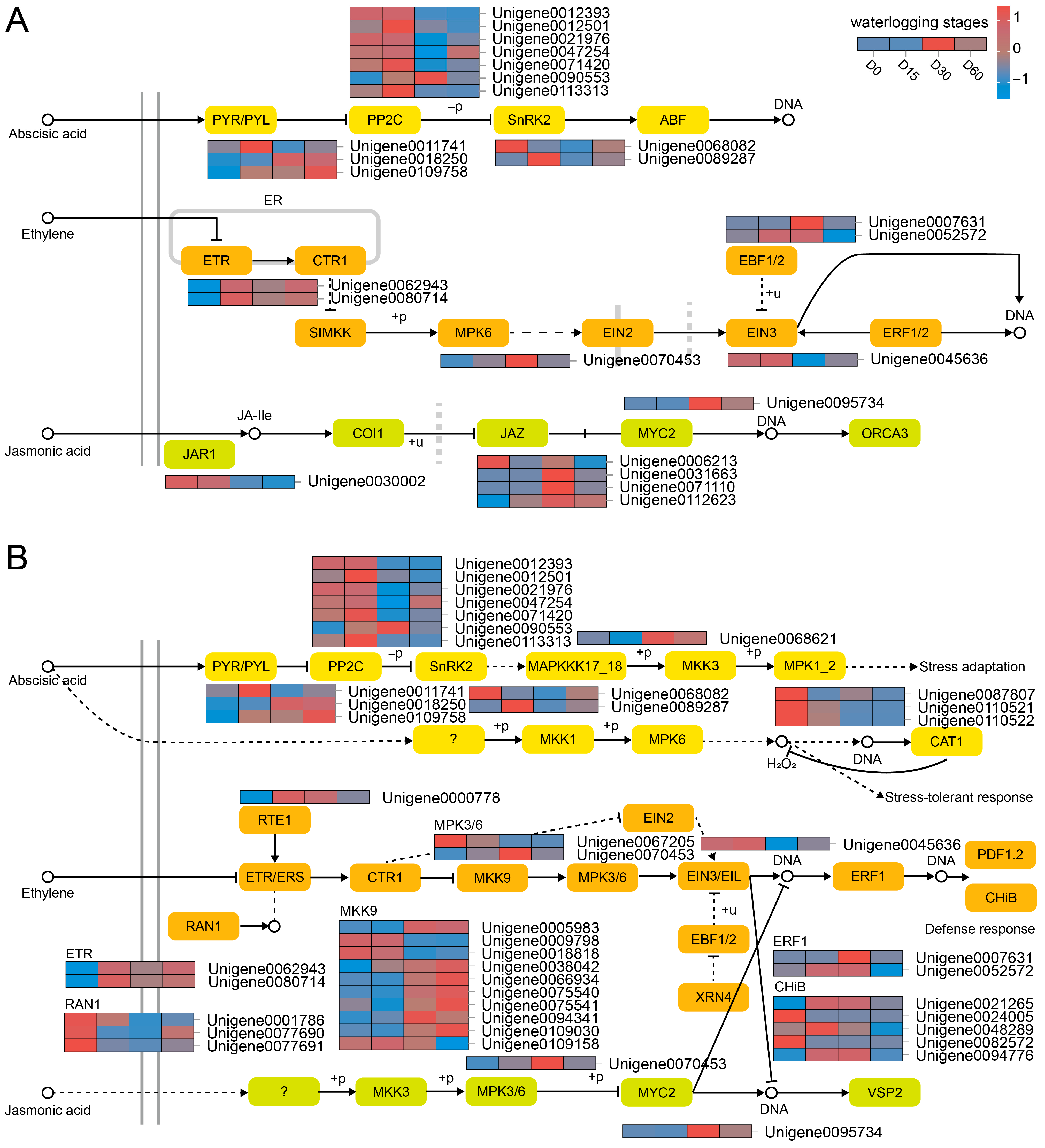
Comparative transcriptomic analysis and WGCNA have revealed that Plant hormone signal transduction (ko04075) and MAPK signaling pathway-plant (ko04016) might play important roles in waterlogging adaptation. Therefore, we further focused on the road maps of these pathways and identified key DEGs.
Consequently, our analysis identified a subset of DEGs implicated in the signal transduction pathways of ABA, ethylene (ET), and JA (Figure 4A). ABA signal transduction-related DEGs included PYL (Unigene0011741, Unigene0018250, and Unigene0109758), and PP2C (e.g., Unigene0012393), SnRK2 (Unigene0068082, Unigene0089287). ETR (Unigene0062943, Unigene0080714), MPK6 (Unigene0070453), EIN3 (Unigene0045636), and EBF1/2 (Unigene0007631, Unigene0052572) were involved in ET signal transduction. In addition, JAR1 (Unigene0030002), JAZ (e.g., Unigene0006213), and MYC2 (Unigene0095734) were associated with JA signal transduction.
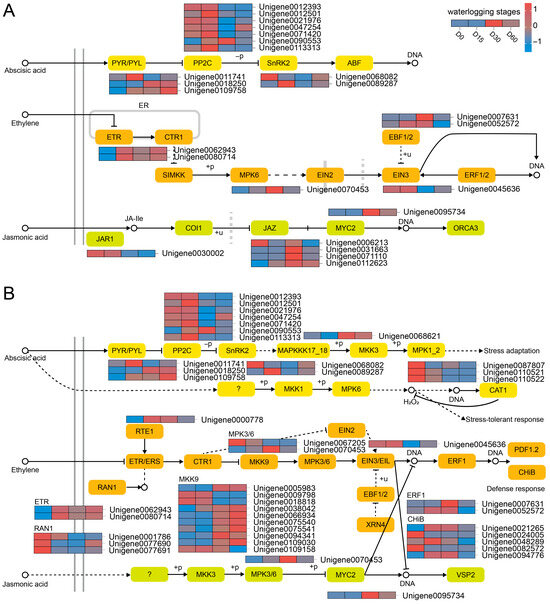
Figure 4.
Regulatory road maps for plant hormone signal transduction (A) and MAPK signaling pathway (B).
Furthermore, MAPK signal cascade related to ABA involved MAPKKK17_18 (Unigene0068621), and MPK1_2 (Unigene0087807, etc.) (Figure 4B). As for ET-associated MAPK signal pathway, the related DEGs were MPK3/6 (Unigene0067205, Unigene0070453), MKK9 (e.g., Unigene0005983), RAN1 (Unigene0001786, etc.), ERF1 (Unigene0007631, Unigene0052572), and CHiB (e.g., Unigene0021265). Additionally, MPK6 (Unigene0070453) participated in JA-related MAPK signal pathway.
2.6. RT-qPCR Verification of Key Genes Associated with Waterlogging Adaptation
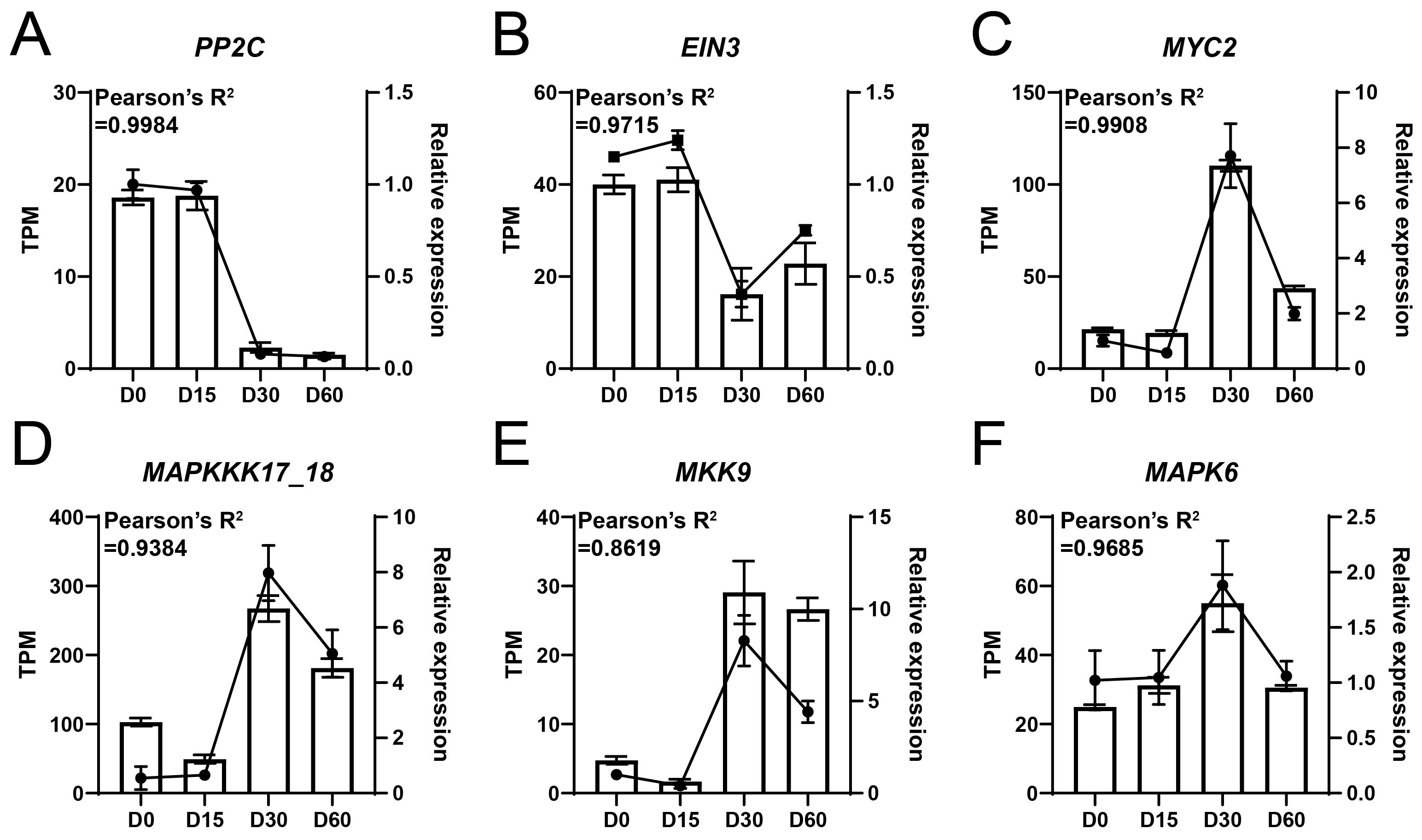
RT-qPCR assay was performed to assess the reliability of the high-throughput RNA-sequencing. The results showed that PP2C and EIN3 were highly expressed at D0 and D15, while their expression levels decreased at D30 and D60 (Figure 5A,B). In addition, the expression levels of the MYC2, MAPKKK17_18, MKK9, and MAPK6 genes were relatively low at D0 and D15, followed by an increase at D30 and a decrease at D60 (Figure 5C–F). Importantly, the correlation value between the results of RT-qPCR and RNA-sequencing exceeded 0.8619, suggesting the high reliability of RNA-seq data (Figure 5).
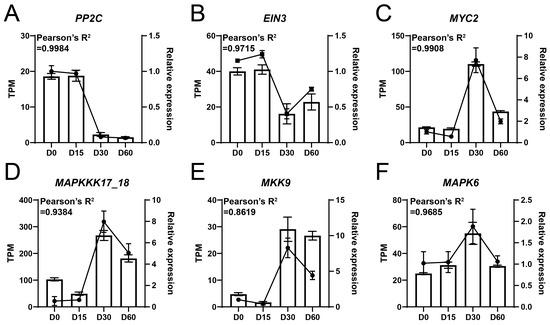
Figure 5.
RT-qPCR assay of key genes associated with waterlogging adaptation in the Ahuehuete. (A) PP2C. (B) EIN3. (C) MYC2. (D) MAPKKK17_18. (E) MKK9. (F) MAPK6. Bars represent TPM values (from RNA-seq), while dotted lines indicate relative expression levels (from RT-qPCR).
3. Discussion
Waterlogging stress represents one of the major abiotic constraints limiting global agricultural productivity and destabilizing natural ecosystems [30]. It induces soil hypoxia even anoxia, which in turn triggers a series of complex physiological, biochemical, and molecular cascades that severely impair plant survival, growth, and reproduction [31]. Waterlogging stress disrupts the electron transport chains in both mitochondria and chloroplasts, leading to the excessive accumulation of reactive oxygen species (ROS) such as superoxide anion () and hydrogen peroxide (H2O2), thereby inducing oxidative damage [32,33]. Compared to the control group, the leaves of tomato plants subjected to waterlogging stress showed a significant increase in the content of oxidative parameters (H2O2 and MDA) [34]. Conversely, our present study demonstrated no significant difference in MDA content between the Ahuehuete leaves subjected to waterlogging and those under well-watered conditions (Figure 1G). This indicates that the waterlogging did not cause damage to the Ahuehuete, or alternatively, that this species has developed an adaptation to waterlogging conditions.
To mitigate oxidative damage, plants have developed intricate antioxidant enzyme system in response to waterlogging stress, involving SOD, POD, CAT, etc. [35]. Under waterlogging conditions, the activities of CAT and SOD were enhanced in pigeonpea (Cajanus cajan (L.) Millsp.) roots. Notably, waterlogging-resistant lines exhibited significantly higher enzymatic activities compared to waterlogging-sensitive lines [36]. In Yulania stellata (Siebold & Zucc.) N.H. Xia, the activity of SOD and CAT was also increased by flooding stress [22]. Similarly, seedlings of Styrax japonica Sieb. et Zucc. exhibited compensatory increase in CAT and POD activities under waterlogging and partial submergence conditions [21]. As a waterlogging-tolerant species, the activities of antioxidant enzymes in the Ahuehuete under waterlogging condition were similar to those of well-watered condition. Moreover, at most time points, the enzymatic activity under waterlogging conditions was lower than that of the control (Figure 1D–F). These findings suggest that the Ahuehuete is well acclimated to waterlogging, necessitating only basal levels of antioxidant enzyme activity to cope with environmental stress. This contrasts with observations in waterlogging-sensitive species. For instance, in waterlogging-sensitive maize genotype K12, the POD activity is significantly inhibited by long-term waterlogging [37]. Similarly, in pigeon pea (Cajanus cajan L.), SOD and CAT activities continuously increase in the tolerant genotype ICPL 84023 as waterlogging persists, whereas they decline progressively in susceptible genotype ICP 7035 [38]. In the present study, antioxidant enzyme system of the Ahuehuete was still active after exposure to long-term waterlogging (Figure 1D–F). It may thus be inferred that the enhancement of antioxidant enzyme activities contributes to improved plant tolerance under long-term waterlogging conditions.
Waterlogging exerts a significant influence on the osmotic regulation processes in plants. A key physiological adaptation mechanism to short-term waterlogging stress involves the accumulation of osmoregulatory substances—including soluble proteins, sugars, and free proline [39,40]. After being subjected to waterlogging stress for 10 d, cucumber plants accumulated significantly higher levels of proline and glucose compared to those under non-waterlogged conditions [41]. In contrast, the levels of total and non-reducing sugars declined in mung bean (Vigna radiata) plants when subjected to an eight-day waterlogging treatment [42]. We observed an initial increase followed by the decrease in osmoprotectants during waterlogging conditions (Figure 1A–C). These results suggest that the Ahuehuete underwent dynamic adjustments in osmoprotectant levels in response to both short- and long-term waterlogging stress. The decrease in proline and soluble sugar content relative to the control may reflect a lower demand for osmotic adjustment under waterlogging conditions in this waterlogging-tolerant species, whereas the transient increase in soluble protein at D30 could represent a specific short-term adaptive mechanism.
High-resolution transcriptomic technology has been widely used to provide molecular insights into the genetic basis of plant responses to waterlogging or flooding stress [43,44,45,46]. To explore the molecular mechanism of the Ahuehuete in adaptation to waterlogging stress, we constructed time-order transcriptomic libraries for high-throughput sequencing. Comparative analysis and WGCNA identified key metabolic pathway, including Plant hormone signal transduction (ko04075), and MAPK signaling pathway-plant (ko04016) (Figure 2C and Figure 4). This phenomenon was consistent with the results observed in Actinidia valvata Dunn [46], barley [47], Sesamum indicum L. [48], and Secale cereale L. [49]. Phytohormones perform critical regulatory functions across a spectrum of molecular, morphological, biochemical, anatomical, and signaling processes that are essential for plant survival under oxygen-deprived stress conditions [50,51]. Under waterlogging conditions, 1-aminocyclopropane-1-carboxylic acid (ACC) is produced under the catalysis of ACC synthase (ACS), and then transferred from plant root system to the aerobic region, resulting in the production of ET by the catalysis of ACC oxidase (ACO) [52]. In the study on sweet potato, it was observed that the waterlogging-tolerant variety exhibited higher expression levels of genes associated with ET synthesis and signal transduction (ETR1, ERS1, and EIN2) under low-oxygen conditions [53]. Similarly, ETR, EIN3, and EBF1/2 were differentially expressed in the Ahuehuete leaves during waterlogging condition (Figure 4). In particular, MKK9-MPK3/MPK6 modules act downstream of the ET receptors, to control the key transcription factor EIN3 and downstream transcription cascades [54]. In addition to ET, other plant hormones such as ABA and JA also play critical roles in tolerance to waterlogging [55]. For example, Exogenous ABA application positively regulated the JA content in adventitious roots of Cleistocalyx operculatus and Syzygium jambos under long-term waterlogging, enhancing tolerance to stress [56]. In Actinidia deliciosa, a novel ethylene-responsive transcription factor named AdRAP2.3 could be induced by MeJA, ACC, ABA, waterlogging, etc. Overexpression of AdRAP2.3 in transgenic tobacco improved resistance to waterlogging through regulation of genes related to pyruvate decarboxylase (PDC) and alcohol dehydrogenase (ADH) [57]. We also identified several DEGs implicated in the signal transduction pathways of ABA and JA, and MAPK signal cascades, including PYL, PP2C, SnRK2, JAR1, JAZ, MYC2, MAPKKK17_18, MPK1_2 (Figure 4). These results imply that MAPK-dependent hormonal signaling pathway play an important role in adaptation of the Ahuehuete to waterlogging. Given the complexity of the hormonal regulatory network under flooding stress, the significance of hormones such as auxin and GA should not be overlooked and warrants further investigation, even though their signal transduction is not regulated by the MAPK cascade. Furthermore, the physiological and molecular changes induced by waterlogging are considerably more complex than previously discussed.
4. Materials and Methods
4.1. Plant Materials and Cultivation Conditions
The experimental materials were two-year-old container-grown seedlings of T. mucronatum, with an average plant height of approximately 100 cm. The seedlings were transplanted in the spring of 2024 into plastic nursery pots measuring 15 cm in height and 20 cm in diameter, with one seedling per pot. Garden soil (texture: clay; pH 5.13; N: 0.801 g/kg; P: 0.38 g/kg; K: 86.42 g/kg) was used as the growing medium.
The experimental site was located at the Baima Teaching and Research Base of Nanjing Forestry University in Lishui District, Nanjing City, Jiangsu Province (119°168′ E, 31°609′ N). This region experiences a subtropical monsoon climate, characterized by hot and rainy summers along with dry and cold winters. The mean annual temperature is approximately 15.4 °C, and the mean annual precipitation reaches 1204.3 mm. The experiment was initiated in July, during which the average temperature at the site was about 32 °C over the two-month experimental period. The seedling planting area was subjected to shading treatment using 16 needle shade net. Light intensity monitoring using a Temperature, humidity and light recorder DJL-18-G (Tuopuyunnong, Hangzhou, China) showed a shading rate of approximately 80% (15,930–17,458 Lux).
4.2. Waterlogging Treatment and Sample Collection
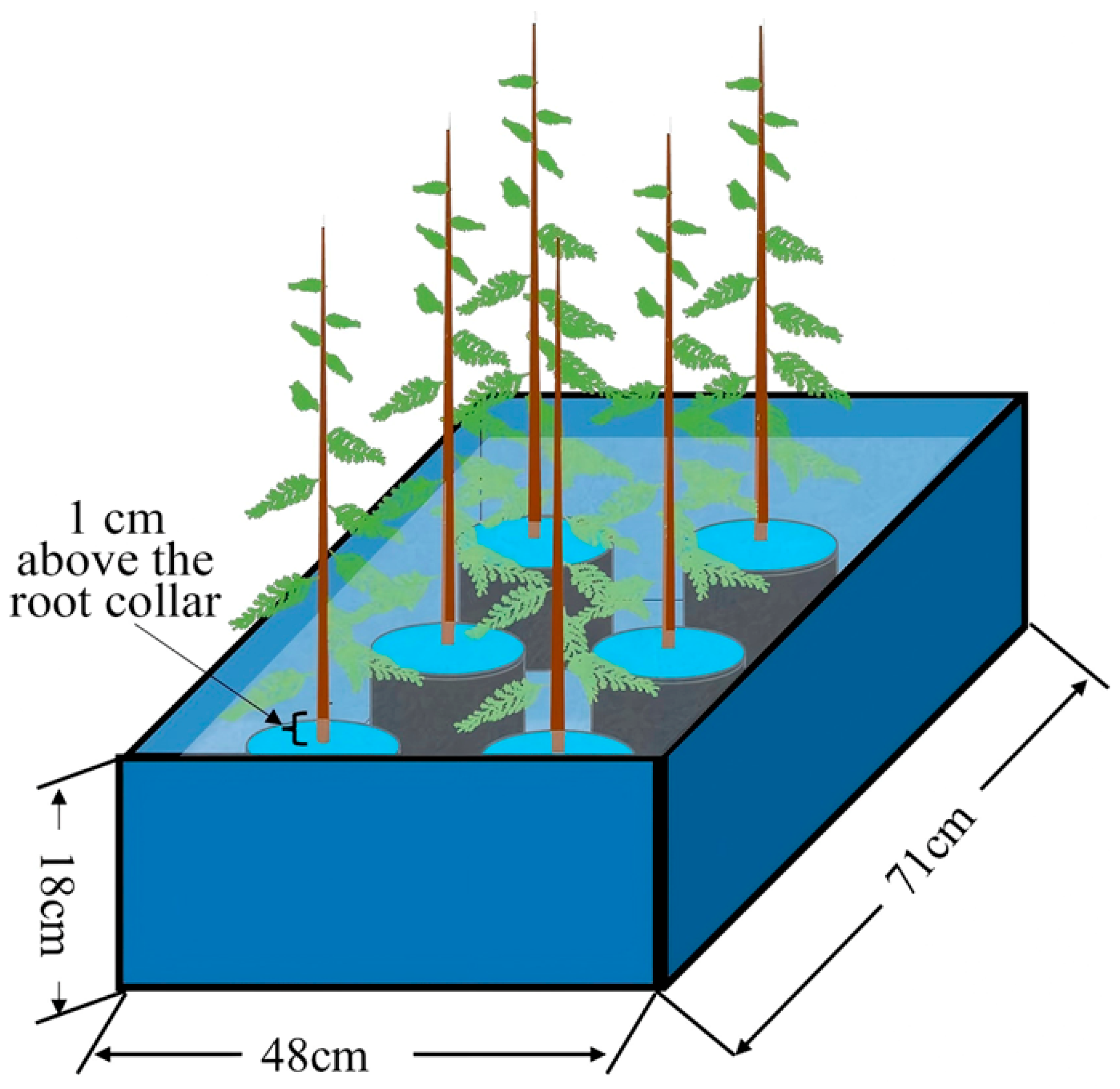
In late July 2024, robust seedlings with largely uniform growth were selected for waterlogging stress treatment. Two experimental conditions were established: waterlogged and control, with three replicates per condition. Each replicate consisted of 20 seedlings. Control (CK) treatment: Seedlings received conventional irrigation management, with the substrate moisture maintained at approximately 75% of field capacity. Waterlogging treatment: The waterlogging process was conducted using a double-pot method. Seedlings were placed in large plastic containers measuring 71 cm (length) × 48 cm (width) × 18 cm (height), with tap water filled to a level 1 cm above the root collar of the seedlings (Figure 6). The water level within the containers was monitored daily, and supplementary water was added as necessary to maintain the predetermined flooding depth. Importantly, several small holes were drilled into the side walls of the outer pot slightly above the intended water level, allowing excess water to drain once it exceeded the preset height. At 0, 15, 30, 60 d, fresh leaves were collected from three randomly selected seedlings per replicate and rapidly frozen using liquid nitrogen, and subsequently stored at −80 °C for future use.
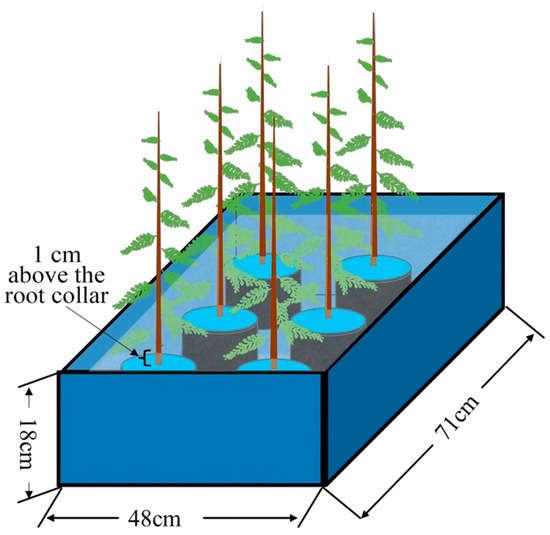
Figure 6.
Schematic diagram of waterlogging treatment.
4.3. Determination of MDA Content
The MDA content was quantified in accordance with an established protocol [58]. Briefly, leaf tissue samples (0.5 g fresh weight) were homogenized in 6 mL of 0.1 M phosphate buffer (pH 7.0). The resulting homogenate was transferred into a 10 mL centrifuge tube and centrifuged at 5500 rpm for 15 min at 4 °C. The supernatant was then collected and stored at 4 °C for subsequent analysis. For the assay, 1.0 mL of the supernatant was combined with 3 mL of 10% (w/v) trichloroacetic acid (TCA) and 1 mL of 0.6% (w/v) thiobarbituric acid (TBA), and the mixture was boiled for 15 min. After cooling to room temperature, the solution was centrifuged again under the same conditions. The absorbance of the final supernatant was measured spectrophotometrically at 450 nm, 532 nm, and 660 nm to quantify the MDA concentration using a PerkinElmer Lambda 950 spectrophotometer (PerkinElmer, Hopkinton, MA, USA).
4.4. Antioxidant Enzyme Activity Measurements
Superoxide dismutase (SOD) activity was quantified spectrophotometrically based on the inhibition of nitro blue tetrazolium (NBT) photochemical reduction, following the method described by Chen et al. [58]. The reaction mixture consisted of 0.1 M phosphate buffer (pH 7.8), 130 mM methionine, 750 µM NBT, 100 µM EDTA-Na2, and 20 µM riboflavin. Prior to absorbance measurement at 560 nm, 0.1 mL of enzyme supernatant was introduced into the assay system.
Peroxidase (POD) activity was determined using the guaiacol oxidation method. A mixture was prepared by adding 0.1 mL of supernatant to 3.8 mL of 0.3% (w/v) guaiacol and 0.1 mL of 3% (v/v) hydrogen peroxide, after which the absorbance was monitored at 470 nm.
Catalase (CAT) activity was assessed according to an established protocol wherein 0.2 mL of enzyme extract was reacted with the mixed solution (containing 1.5 mL of 0.1 M phosphate buffer (pH 7.8), 1 mL of ddH2O, and 0.3 mL of 0.1 M hydrogen peroxide solution). The decrease in absorbance at 240 nm was recorded over a 30 s period to evaluate catalytic activity.
4.5. Quantification of Soluble Sugar, Soluble Protein, and Proline
The soluble sugar content was determined using the anthrone–sulfuric acid method [59]. Leaf tissue samples (0.2 g FW) were ground and subjected to two consecutive heating cycles in a 100 °C water bath, followed by centrifugation at 3500× g for 10 min. The resulting supernatant was collected for subsequent analyses.
The soluble protein content was quantified using the Bradford method [58]. Specifically, 1.0 mL of the supernatant was combined with 5.0 mL of Coomassie Brilliant Blue solution (0.1 g/L), and the absorbance was measured at a wavelength of 595 nm.
Proline content was analyzed according to a modified spectrophotometric protocol [60]. Briefly, fresh leaf tissues (0.25 g) were ground and then extracted in 5 mL of 3% sulfosalicylic acid solution using a 100 °C water bath for 30 min. The extract was then mixed with an equal volume of glacial acetic acid and acidic ninhydrin reagent, and incubated in a boiling water bath for 30 min. After cooling to room temperature, 4 mL of toluene was added, and the mixture was vigorously vortexed for 60 s. Following a 10 min phase-separation period, the upper toluene layer containing the chromophore was collected, and its absorbance was measured at 520 nm for proline concentration calculation. All physiological index assays included three biological replicates.
4.6. RNA Extraction, Library Construction, and Transcriptome Sequencing
Total RNA extraction, library preparation, and RNA sequencing were performed in accordance with established protocols from our previous study [59]. Briefly, total RNA was isolated from the collected tissues using a Trizol Reagent Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA quality was then assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) and verified through RNase-free agarose gel electrophoresis. Following extraction, eukaryotic mRNA was enriched using Oligo (dT) magnetic beads and fragmented into short segments. cDNA synthesis was carried out using the NEBNext Ultra RNA Library Prep Kit for Illumina (New England Biolabs, Beverly, MA, USA). The resulting fragments underwent end repair, adenylation, and adapter ligation, followed by purification with 1.0× AMPure XP Beads. cDNA libraries were then constructed via polymerase chain reaction (PCR) amplification. Finally, transcriptome sequencing was conducted on the Illumina NovaSeq 6000 platform (Illumina, Inc., San Diego, CA, USA).
4.7. Transcriptomic Data Analysis
Raw sequencing reads were processed using Fastp (-q 20 -u 50 -N 15 -l 50, version 0.18.0) [61] to generate high-quality clean reads. These clean reads were then aligned to a ribosomal RNA (rRNA) reference database using Bowtie2 (--local, version 2.2.8) [62]. Reads mapped to rRNA were removed, and the remaining high-quality reads were de novo assembled into transcripts using StringTie (-f 0.3, v1.3.1) [63]. Gene abundance was quantified via the FPKM (fragments per kilobase of transcript per million mapped reads) method with RSEM software (default, version 1.3.3) [64].
Differentially expressed genes (DEGs) were identified based on a binomial distribution model implemented in DEGseq (default, v1.36.1), with thresholds set at |log2(Fold Change)| ≥ 1 and a Q value ≤ 0.05 [65]. Subsequently, Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses were conducted to elucidate the biological functions of the DEGs. Significantly enriched terms or pathways were defined using a Q value threshold of ≤0.05.
The gene set employed in the weighted correlation network analysis (WGCNA) was constructed from transcriptomic data, retaining all DEGs identified across any comparison group. WGCNA was implemented using the “WGCNA” package (version 1.73) in R software (version 4.3.1) [66]. The soft thresholding power was determined based on scale-free topology fit index analysis. A power value of 19 was selected, which satisfied the scale-free topology criterion with a fit index exceeding 0.800985 while preserving a high mean connectivity (418.64688) (Figure S3A,B). Module detection was conducted using default parameters, with the maximum module size and the minimum number of genes per module set to 20 and 50, respectively. In addition, the correlation between physiological traits and modules was generated based on Pearson correlation coefficients calculated between module membership values and trait data, thus identifying key modules. Subsequently, hub genes with high connectivity were identified in key modules.
4.8. RT-qPCR Validation
cDNA was synthesized from the total RNA using the Evo M-MLV RT Kit with gDNA Clean for qPCR (Accurate Biotechnology (Hunan) Co., Ltd., Changsha, China). Then, RT-qPCR was performed using the SYBR Green Premix Pro Taq HS qPCR Kit (Accurate Biotechnology (Hunan) Co., Ltd., Changsha, China) on the ABI StepOnePlus Real-Time PCR system (Applied Biosystems Inc., Foster City, CA, USA). All primers used in this study were listed in Table S3.
4.9. Statistical Analysis
Data visualization was conducted utilizing the online Omicsmart platform (http://www.omicsmart.com, accessed on 1 September 2025). Bar graphs were constructed using GraphPad Prism (Version 9.5.1; GraphPad Software, Boston, MA, USA). For statistical analysis, multiple unpaired t tests (p < 0.05) were performed with IBM SPSS Statistics 26 (International Business Machines Corporation, Armonk, NY, USA) to assess significant difference.
5. Conclusions
Based on the integrated physiological and transcriptomic analyses, this study demonstrates that Ahuehuete employs a sophisticated adaptive strategy to waterlogging stress. Physiologically, it exhibits a dynamic response with initial accumulation of osmoregulatory substances (soluble sugar, soluble protein, and proline) coupled with modulated activities of antioxidant enzymes (SOD, POD, and CAT) to mitigate oxidative damage. Transcriptome profiling revealed a significant reprogramming of gene expression, highlighting the crucial involvement of phenylpropanoid and flavonoid biosynthesis, plant hormone signal transduction, and the MAPK signaling pathway. Weighted Gene Co-expression Network Analysis (WGCNA) identified key modules (coral1, skyblue) highly correlated with physiological traits and pinpointed several hub genes, including ERF017 and MYB4. KEGG enrichment analysis of DEGs from key modules underlined the adaptive response by regulating ABA, ET, and JA signaling pathways, as well as MAPK cascades. Our findings provide a comprehensive molecular framework underlying Ahuehuete’s tolerance to waterlogging.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14213295/s1, Figure S1: (A) Principal component analysis of the Ahuehuete leaf samples using transcriptome data averaged from three replicates. The first two principal components are shown. (B) Clustering analysis. Figure S2: GO enrichment analysis of DEGs involved in waterlogging response; Figure S3: Soft threshold selection for WGCNA. (A) Scale independence shows the relationship between the soft threshold power and the scale-free topology fit index. (B) Mean connectivity in the right panel indicates the relationship between the soft threshold power and mean connectivity. (C) Gene number for each module; Table S1: Statistical information of transcriptome libraries of the Ahuehuete leaves under waterlogging conditions; Table S2: Statistical analysis of assembly quality; Table S3: Primers used for RT-qPCR assay.
Author Contributions
Conceptualization, H.W. and S.L.; methodology, C.L.; software, L.S.; validation, J.Z., S.W. and C.L.; formal analysis, L.S.; investigation, C.L.; resources, D.S.; data curation, L.S.; writing—original draft preparation, Y.G.; writing—review and editing, S.L.; visualization, L.S.; supervision, H.W.; project administration, D.S.; funding acquisition, S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Jiangsu Academy of Forestry (grant number ZZKY202102).
Data Availability Statement
The data are contained within this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Perez-Lopez, M.E.; Leal-Saenz, A.; Ortiz-Olivas, M.E.; Ramirez-Crescencio, F.; Arreola-Ortiz, A.E. The premature mortality of sabinos or Montezuma bald cypress (Taxodium mucronatum Ten.) in the state of Durango, Mexico. Sustainability 2024, 16, 10248. [Google Scholar] [CrossRef]
- Fierro-Cabo, A.; Plamann, A. Enhancing the seed germination process of Montezuma cypress (Taxodium mucronatum Ten.). J. For. Res. 2021, 26, 81–85. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, C.; Hua, J.; Wang, Z.; Chen, T.; Zhu, Q.; You, H.; Xuan, L.; Tang, C.; Yin, Y. The baldcypress genome provides insights into the adaptive evolution of flooding stress tolerance. New Phytol. 2025, 247, 979–997. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.Z.; Wu, L.; Tang, L.Z. Effects of flooding and endogenous hormone on the formation of knee roots in Taxodium ascendens. Front. Plant Sci. 2022, 13, 803619. [Google Scholar] [CrossRef]
- Guo, J.; Duan, H.; Xuan, L.; Wang, Z.; Hua, J.; Yu, C.; Yin, Y.; Li, M.; Yang, Y. Identification and functional analysis of LecRLK genes in Taxodium ‘Zhongshanshan’. PeerJ 2019, 7, e7498. [Google Scholar] [CrossRef]
- Zhang, F.; Xuan, L.; Chen, H.; Yu, C.; Chong, X.; Yin, Y.; Lu, X. Comparative transcriptome profiling of resistant and susceptible Taxodium trees in responding to the infection by Pestalotiopsis maculans. Forests 2021, 12, 1090. [Google Scholar] [CrossRef]
- He, X.R.; Wang, T.; Wu, K.J.; Wang, P.; Qi, Y.C.; Arif, M.; Wei, H. Responses of swamp cypress (Taxodium distichum) and Chinese willow (Salix matsudana) roots to periodic submergence in mega-reservoir: Changes in organic acid concentration. Forests 2021, 12, 203. [Google Scholar] [CrossRef]
- Guo, J.B.; Xue, J.H.; Yin, Y.L.; Pedersen, O.; Hua, J.F. Response of underwater photosynthesis to light, CO2, temperature, and submergence time of Taxodium distichum, a flood-tolerant tree. Front. Plant Sci. 2024, 15, 1355729. [Google Scholar] [CrossRef]
- Qi, B.Y.; Yang, Y.; Yin, Y.L.; Xu, M.; Li, H.G. De novo sequencing, assembly, and analysis of the Taxodium ‘Zhongshansa’ roots and shoots transcriptome in response to short-term waterlogging. BMC Plant Biol. 2014, 14, 201. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.B.; Cao, F.L.; Wang, Y.; Wang, J. Protein expression changes in leaves of bald cypress (Taxodium distichum (L.) Rich.) in response to waterlogging. Bangladesh J. Bot. 2015, 44, 869–880. [Google Scholar]
- Hua, J.F.; Han, L.W.; Wang, Z.Q.; Gu, C.S.; Yin, Y.L. Morpho-anatomical and photosynthetic responses of Taxodium hybrid ‘Zhongshanshan’ 406 to prolonged flooding. Flora 2017, 231, 29–37. [Google Scholar] [CrossRef]
- Lu, Y.; Xiang, P.; Zhang, S.Q.; Lu, Z.G.; Zhou, Z.D.; Yin, Y.L.; Hua, J.F.; Shi, Q.; Yu, W.W.; Yu, C.G. Physiological and transcriptional regulation in Taxodium hybrid ‘Zhongshanshan’ leaves in acclimation to prolonged partial submergence. Planta 2023, 258, 66. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, S.Q.; Xiang, P.; Yin, Y.L.; Yu, C.G.; Hua, J.F.; Shi, Q.; Chen, T.T.; Zhou, Z.D.; Yu, W.W.; et al. Integrated small RNA, transcriptome and physiological approaches provide insight into Taxodium hybrid ‘Zhongshanshan’ roots in acclimation to prolonged flooding. Tree Physiol. 2024, 44, tpae031. [Google Scholar] [CrossRef] [PubMed]
- Xiang, P.; Yu, W.W.; Li, L.B.; Zhang, S.Q.; Yu, C.G.; Yin, Y.L.; Lu, Z.G.; Shi, Q.; Wu, D.; Cai, M.Y.; et al. Dissecting lncRNA-mRNA networks underlying physiological alterations in Taxodium hybrid ‘Zhongshanshan’ leaves in acclimation to prolonged partial submergence. Ind. Crops Prod. 2024, 218, 119008. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, F.; Meng, Y.; Chandrasekaran, U.; Luo, X.; Yang, W.; Shu, K. Plant waterlogging/flooding stress responses: From seed germination to maturation. Plant Physiol. Biochem. 2020, 148, 228–236. [Google Scholar] [CrossRef]
- Hussain, M.A.; Jahan, M.S. Chapter 5—Mechanisms of waterlogging tolerance in plants. In Role of Antioxidants in Abiotic Stress Management; Ulhassan, Z., Hamid, Y., Zhou, W., Tripathi, D.K., Singh, V.P., Corpas, F.J., Eds.; Academic Press: Cambridge, MA, USA, 2025; pp. 69–77. [Google Scholar]
- Davosir, D.; Jahn, L.; ŠOla, I.; Ludwig-MÜLler, J. Variety-specific responses of young Brassica oleracea plants to waterlogging: Comparative biochemical, metabolic and gene expression analysis. Plant Physiol. Biochem. 2025, 227, 110098. [Google Scholar] [CrossRef] [PubMed]
- Argus, R.E.; Colmer, T.D.; Grierson, P.F. Early physiological flood tolerance is followed by slow post-flooding root recovery in the dryland riparian tree Eucalyptus camaldulensis subsp. refulgens. Plant Cell Environ. 2015, 38, 1189–1199. [Google Scholar] [CrossRef]
- Wang, H.M.; Huang, Y.L.; Hu, W.; Kou, Y.X.; Zhang, Z.Y. Plant tolerance to soil waterlogging was promoted by triggering osmotic adjustment and ROS scavenge system in peanut (Arachis hypogaea L.) seedlings. S. Afr. J. Bot. 2025, 184, 458–467. [Google Scholar] [CrossRef]
- Shao, D.Y.; Abubakar, A.S.; Chen, J.K.; Zhao, H.H.; Chen, P.; Chen, K.M.; Wang, X.F.; Shawai, R.S.; Chen, Y.; Zhu, A.G.; et al. Physiological, molecular, and morphological adjustment to waterlogging stress in ramie and selection of waterlogging-tolerant varieties. Plant Physiol. Biochem. 2024, 216, 109101. [Google Scholar] [CrossRef]
- Zhang, G.; Dong, J.; Han, C.; Liu, Z.; Liu, J.; Yu, F. Physiological and biochemical measurements reveal how Styrax japonica seedlings response to flooding stress. Forests 2025, 16, 634. [Google Scholar] [CrossRef]
- Xu, L.; Pan, L.L.; Ye, K.; Qin, J.; Hu, Y.H.; Guo, C.B.; Wu, D.R.; Wang, H.B. Physiological and transcriptome analyses in Yulania stellata under flooding stress. N. Z. J. Bot. 2025, 63, 1367–1390. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Huangfu, Y.; Chen, W.; Xie, Z.; Tian, B.; Wu, T.; Cao, G.; Guo, J.; Wei, F.; et al. The synergistic response of Arundo donax to multiple stressors: New insights from root genome-wide transcription analysis. Ind. Crops Prod. 2025, 228, 120893. [Google Scholar] [CrossRef]
- He, X.; Wang, C.; Wang, H.; Li, L.; Wang, C. The function of MAPK cascades in response to various stresses in horticultural plants. Front. Plant Sci. 2020, 11, 952. [Google Scholar] [CrossRef] [PubMed]
- Krysan, P.J.; Colcombet, J. Cellular complexity in MAPK signaling in plants: Questions and emerging tools to answer them. Front. Plant Sci. 2018, 9, 1674. [Google Scholar] [CrossRef]
- Manna, M.; Rengasamy, B.; Sinha, A.K. Revisiting the role of MAPK signalling pathway in plants and its manipulation for crop improvement. Plant Cell Environ. 2023, 46, 2277–2295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef]
- Smékalová, V.; Doskočilová, A.; Komis, G.; Šamaj, J. Crosstalk between secondary messengers, hormones and MAPK modules during abiotic stress signalling in plants. Biotechnol. Adv. 2014, 32, 2–11. [Google Scholar] [CrossRef]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Voesenek, L.A.C.J.; Bailey-Serres, J. Flood adaptive traits and processes: An overview. New Phytol. 2015, 206, 57–73. [Google Scholar] [CrossRef]
- Jia, W.; Ma, M.; Chen, J.; Wu, S. Plant Morphological, Physiological and anatomical adaption to flooding stress and the underlying molecular mechanisms. Int. J. Mol. Sci. 2021, 22, 1088. [Google Scholar] [CrossRef]
- Mudasir, M.; Shahzad, A. Decoding plant responses to waterlogging: From stress signals to molecular mechanisms and their future implications. Plant Mol. Biol. 2025, 115, 78. [Google Scholar] [CrossRef]
- Manghwar, H.; Hussain, A.; Alam, I.; Khoso, M.A.; Ali, Q.; Liu, F. Waterlogging stress in plants: Unraveling the mechanisms and impacts on growth, development, and productivity. Environ. Exp. Bot. 2024, 224, 105824. [Google Scholar] [CrossRef]
- Umicevic, S.; Kukavica, B.; Maksimovic, I.; Gasic, U.; Milutinovic, M.; Antic, M.; Misic, D. Stress response in tomato as influenced by repeated waterlogging. Front. Plant Sci. 2024, 15, 1331281. [Google Scholar] [CrossRef]
- Del Río, L.A. ROS and RNS in plant physiology: An overview. J. Exp. Bot. 2015, 66, 2827–2837. [Google Scholar] [CrossRef]
- Bansal, R.; Srivastava, J.P. Antioxidative defense system in pigeonpea roots under waterlogging stress. Acta Physiol. Plant. 2012, 34, 515–522. [Google Scholar] [CrossRef]
- Tang, B.; Xu, S.-Z.; Zou, X.-L.; Zheng, Y.-L.; Qiu, F.-Z. Changes of antioxidative enzymes and lipid peroxidation in leaves and roots of waterlogging-tolerant and waterlogging-sensitive maize genotypes at seedling stage. Agric. Sci. China 2010, 9, 651–661. [Google Scholar] [CrossRef]
- Sairam, R.K.; Kumutha, D.; Ezhilmathi, K.; Chinnusamy, V.; Meena, R.C. Waterlogging induced oxidative stress and antioxidant enzyme activities in pigeon pea. Biol. Plant. 2009, 53, 493–504. [Google Scholar] [CrossRef]
- Yang, L.; Li, N.; Liu, Y.; Miao, P.; Liu, J.; Wang, Z. Updates and prospects: Morphological, physiological, and molecular regulation in crop response to waterlogging stress. Agronomy 2023, 13, 2599. [Google Scholar] [CrossRef]
- Phukan, U.J.; Mishra, S.; Shukla, R.K. Waterlogging and submergence stress: Affects and acclimation. Crit. Rev. Biotechnol. 2016, 36, 956–966. [Google Scholar] [CrossRef]
- Barickman, T.C.; Simpson, C.R.; Sams, C.E. Waterlogging causes early modification in the physiological performance, carotenoids, chlorophylls, proline, and soluble sugars of cucumber plants. Plants 2019, 8, 160. [Google Scholar] [CrossRef]
- Kumar, P.; Pal, M.; Joshi, R.; Sairam, R.K. Yield, growth and physiological responses of mung bean [Vigna radiata (L.) Wilczek] genotypes to waterlogging at vegetative stage. Physiol. Mol. Biol. Plants 2013, 19, 209–220. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, M.; Zhang, L.; Chen, X.; Zhou, M.; Jiang, B.; Jia, Y.; Yong, X.; Tang, S.; Mou, L.; et al. Unraveling key factors for hypoxia tolerance in contrasting varieties of cotton rose by comparative morpho-physiological and transcriptome analysis. Physiol. Plant. 2024, 176, e14317. [Google Scholar] [CrossRef]
- Jiao, Y.; Sha, C.L.; Xie, R.J.; Shu, Q.Y. Comparative analysis of the potential physiological and molecular mechanisms involved in the response to root zone hypoxia in two rootstock seedlings of the Chinese bayberry via transcriptomic analysis. Funct. Integr. Genom. 2023, 23, 11. [Google Scholar] [CrossRef]
- Park, S.U.; Kim, Y.H.; Lee, C.J.; Kim, S.E.; Lim, Y.H.; Yoon, U.H.; Kim, H.S.; Kwak, S.S. Comparative transcriptome profiling of two sweetpotato cultivars with contrasting flooding stress tolerance levels. Plant Biotechnol. Rep. 2020, 14, 743–756. [Google Scholar] [CrossRef]
- Gao, Y.B.; Jiang, Z.Y.; Shi, M.Q.; Zhou, Y.F.; Huo, L.Q.; Li, X.L.; Xu, K. Comparative transcriptome provides insight into responding mechanism of waterlogging stress in Actinidia valvata Dunn. Gene 2022, 845, 146843. [Google Scholar] [CrossRef]
- Zhu, J.; Yin, H.; Cao, C.; Sun, C.; Zhang, M.; Hong, Y.; Zhang, Y.; Lv, C.; Guo, B.; Wang, F.; et al. Comparative transcriptome analyses reveal the mechanisms underlying waterlogging tolerance in barley. Plants 2025, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.D.; Cui, C.Q.; Liu, Y.Y.; Wu, K.; Du, Z.W.; Jiang, X.L.; Zhao, F.L.; Zhang, R.P.; Wang, J.J.; Mei, H.X.; et al. Physiological and transcriptional responses of sesame (Sesamum indicum L.) to waterlogging stress. Int. J. Mol. Sci. 2025, 26, 2603. [Google Scholar] [CrossRef] [PubMed]
- Bimpong, D.; Zhao, L.L.; Ran, M.Y.; Zhao, X.Z.; Wu, C.C.; Li, Z.Q.; Wang, X.; Cheng, L.; Fang, Z.W.; Hu, Z.M.; et al. Transcriptomic analysis reveals the regulatory mechanisms of messenger RNA (mRNA) and long non-coding RNA (lncRNA) in response to waterlogging stress in rye (Secale cereale L.). BMC Plant Biol. 2024, 24, 534. [Google Scholar] [CrossRef]
- Cheng, X.; Ruyter-Spira, C.; Bouwmeester, H. The interaction between strigolactones and other plant hormones in the regulation of plant development. Front. Plant Sci. 2013, 4, 199. [Google Scholar] [CrossRef]
- Bashar, K.K.; Tareq, M.Z.; Amin, M.R.; Honi, U.; Tahjib-Ul-Arif, M.; Sadat, M.A.; Hossen, Q.M.M. Phytohormone-mediated stomatal response, escape and quiescence strategies in plants under flooding stress. Agronomy 2019, 9, 43. [Google Scholar] [CrossRef]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef]
- Pan, R.; Han, H.; Medison, M.B.; Abou-Elwafa, S.F.; Liu, Y.; Yang, X.; Zhang, W. Aerenchyma formation in the root of leaf-vegetable sweet potato: Programmed cell death initiated by ethylene-mediated H2O2 accumulation. Physiol. Plant. 2021, 173, 2361–2375. [Google Scholar] [CrossRef]
- Yoo, S.-D.; Sheen, J. MAPK signaling in plant hormone ethylene signal transduction. Plant Signal. Behav. 2008, 3, 848–849. [Google Scholar] [CrossRef]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef]
- Cisse, E.M.; Zhang, J.; Li, D.D.; Miao, L.F.; Yin, L.Y.; Yang, F. Exogenous ABA and IAA modulate physiological and hormonal adaptation strategies in Cleistocalyx operculatus and Syzygium jambos under long-term waterlogging conditions. BMC Plant Biol. 2022, 22, 523. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.L.; Wang, G.; Wang, T.; Jia, Z.H.; Guo, Z.R.; Zhang, J.Y. AdRAP2.3, a novel Ethylene Response Factor VII from Actinidia deliciosa, enhances waterlogging resistance in transgenic tobacco through improving expression levels of PDC and ADH genes. Int. J. Mol. Sci. 2019, 20, 1189. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Du, H.; Sun, L.; Yang, A.; Nie, T.; Li, J.; Yin, Z. Selected crucial morpho-physiological indices of family may predict the rootstock quality in Magnolia ×soulangeana. Hortic. Environ. Biotechnol. 2025, 66, 205–217. [Google Scholar] [CrossRef]
- Sun, L.; Zu, X.; Cui, S.; Zhu, M.; Huang, T.; Duan, Y.; Ma, Q.; Yin, T.; Li, S. Postharvest water loss-induced transcriptomic reprogramming modulates non-structural carbohydrate remobilization in Quercus texana kernels. Ind. Crops Prod. 2025, 227, 120768. [Google Scholar] [CrossRef]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Zhen, J. Photosynthetic, antioxidant activities, and osmoregulatory responses in winter wheat differ during the stress and recovery periods under heat, drought, and combined stress. Plant Sci. 2023, 327, 111557. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, Z.; Wang, X.; Wang, X.; Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 2010, 26, 136–138. [Google Scholar] [CrossRef]
- Langfelder, P.; Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform. 2008, 9, 559. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).