Mechanisms Underlying the Cognitive Benefits of Solanum macrocarpon Leaf n-Butanol Extract: Acetylcholinesterase Inhibition and Oxidative Stress Modulation
Abstract
1. Introduction
2. Results
2.1. HPLC Quantification of Phenolic and Flavonoid Derivatives
2.2. Evaluation of the Chronic Exposure Safety of Solanum macrocarpon L. leaf n-butanol extract (SMB)
2.3. In Silico Evaluation of the Pharmacokinetic Properties of the Main Constituents
2.3.1. Physicochemical Properties of the Bioactive Compounds
2.3.2. Absorption of Bioactive Compounds
2.3.3. Distribution of Bioactive Compounds
2.3.4. Metabolism of Bioactive Compounds
2.3.5. Excretion of Bioactive Compounds
2.3.6. Toxicity of Bioactive Compounds
2.3.7. PASS Predictions of Neuropharmacological Activities and Safety Profile of Phenolic Compounds
2.4. Effects on Anxiety-like Behavior in the NTT
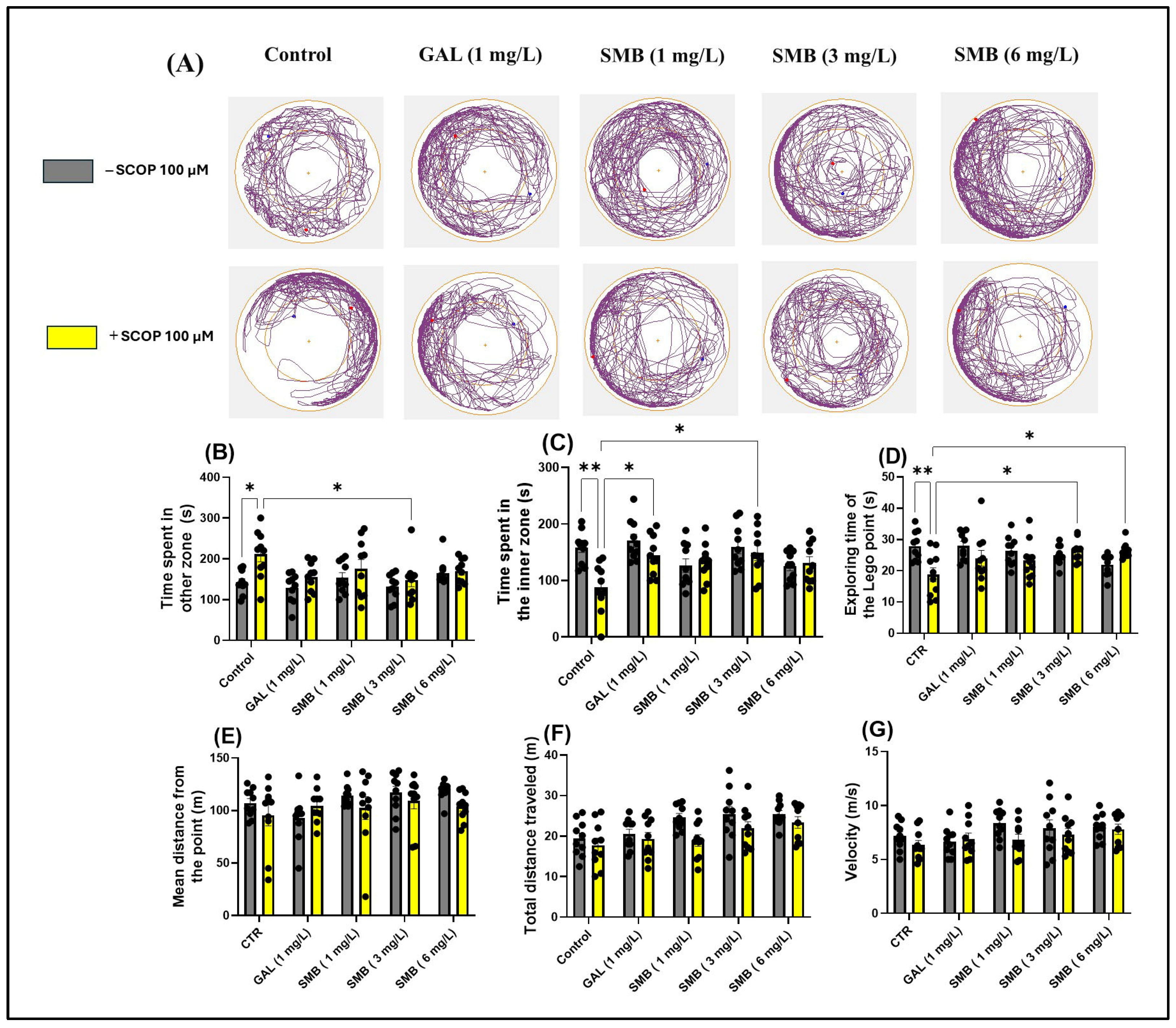
2.5. Effects on Anxiety-like Behavior in the NAT
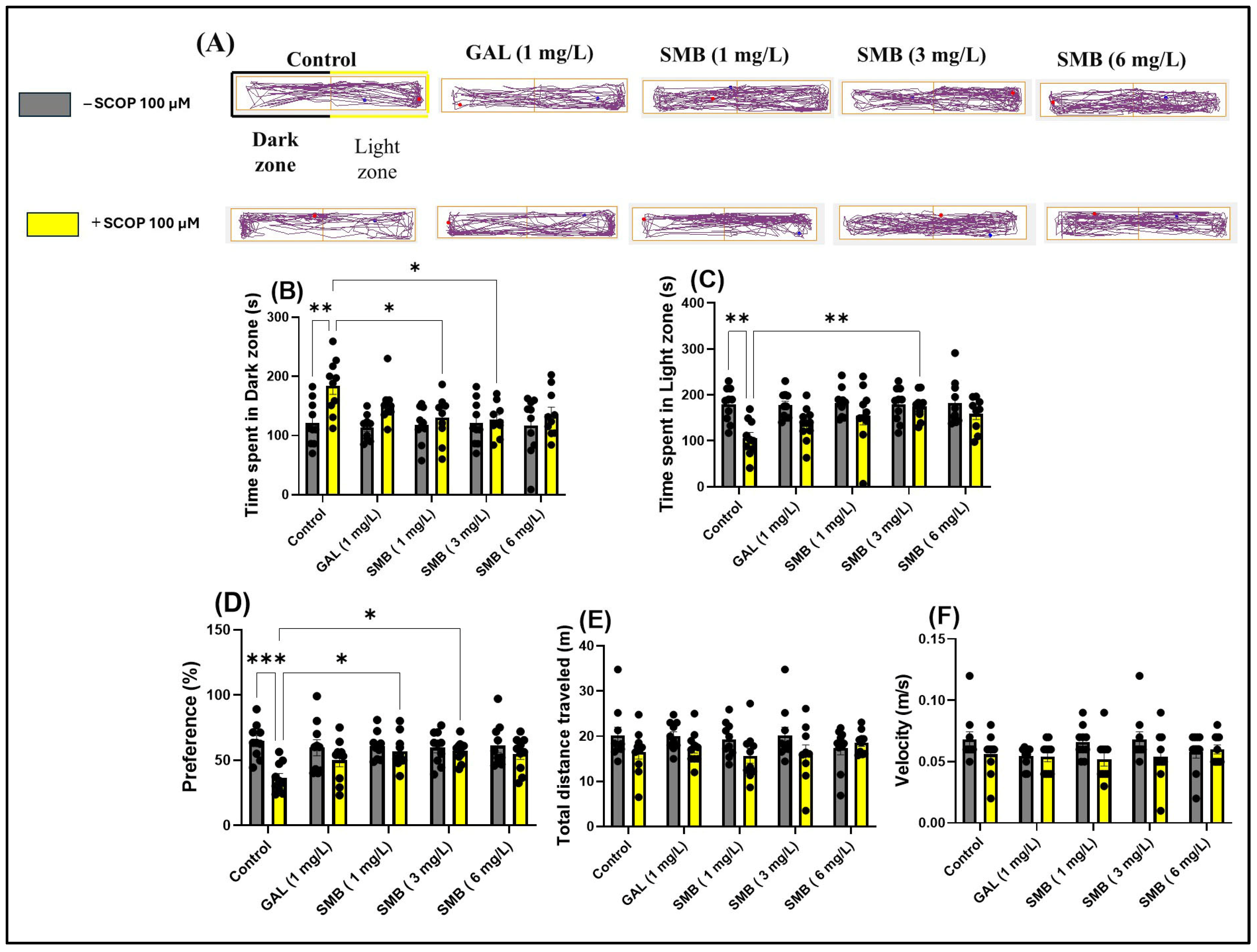
2.6. Effects on Anxiety-like Behavior in LDT
2.7. Effects on Spatial Memory in Y-Maze
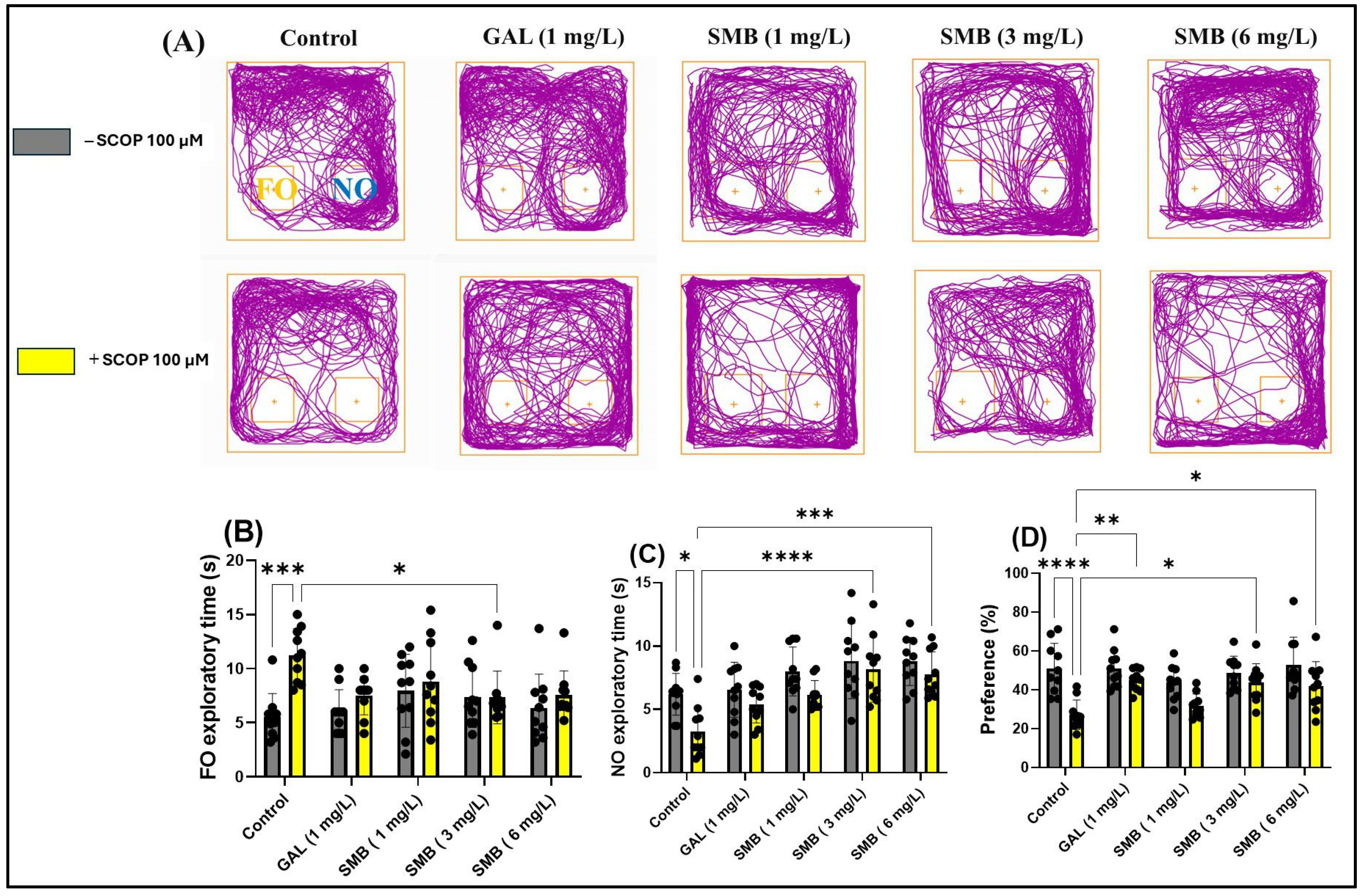
2.8. Effects on Recognition Memory in NOR
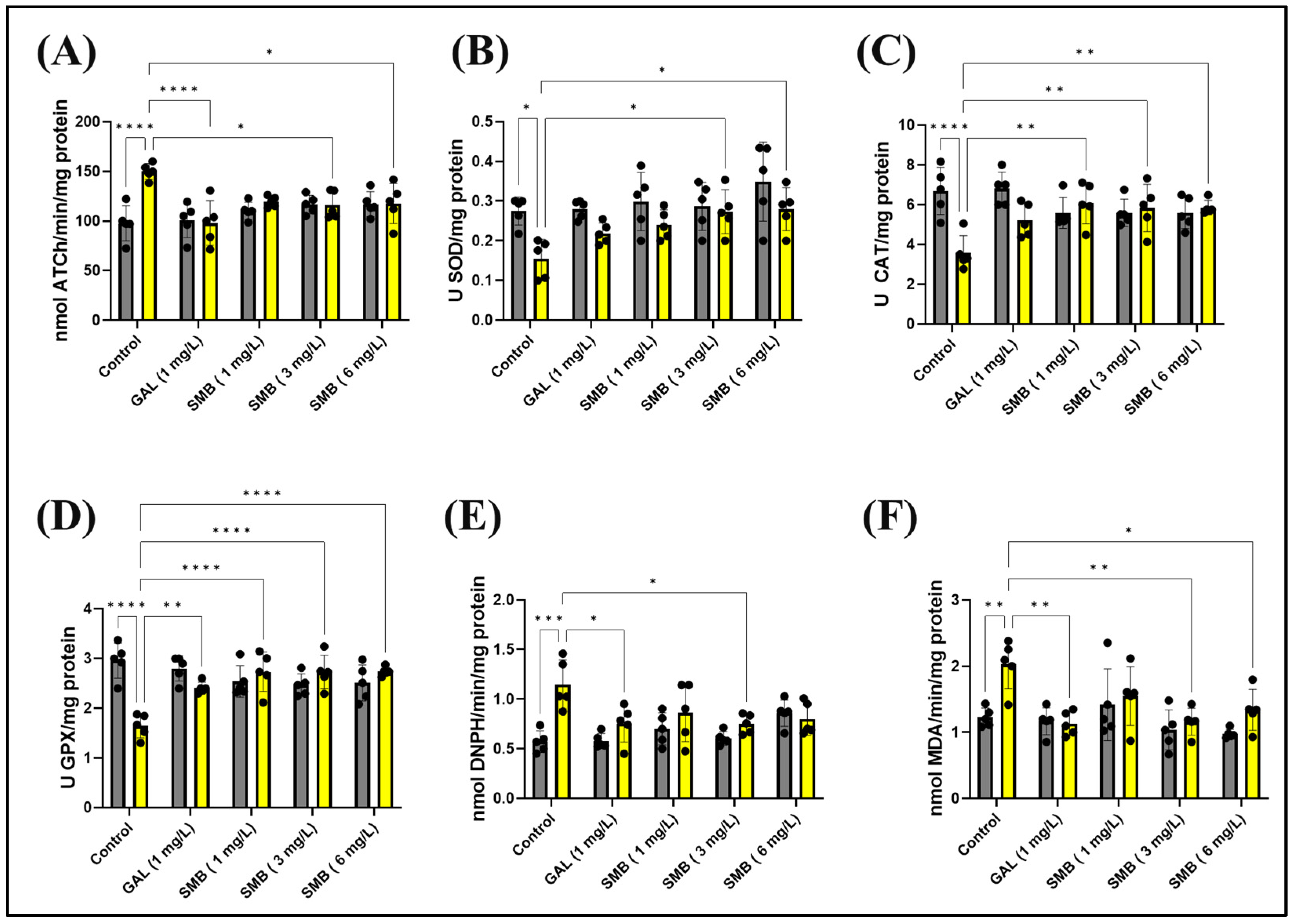
2.9. Effects on Brain AChE Activity
2.10. Effects on Brain Oxidative Status
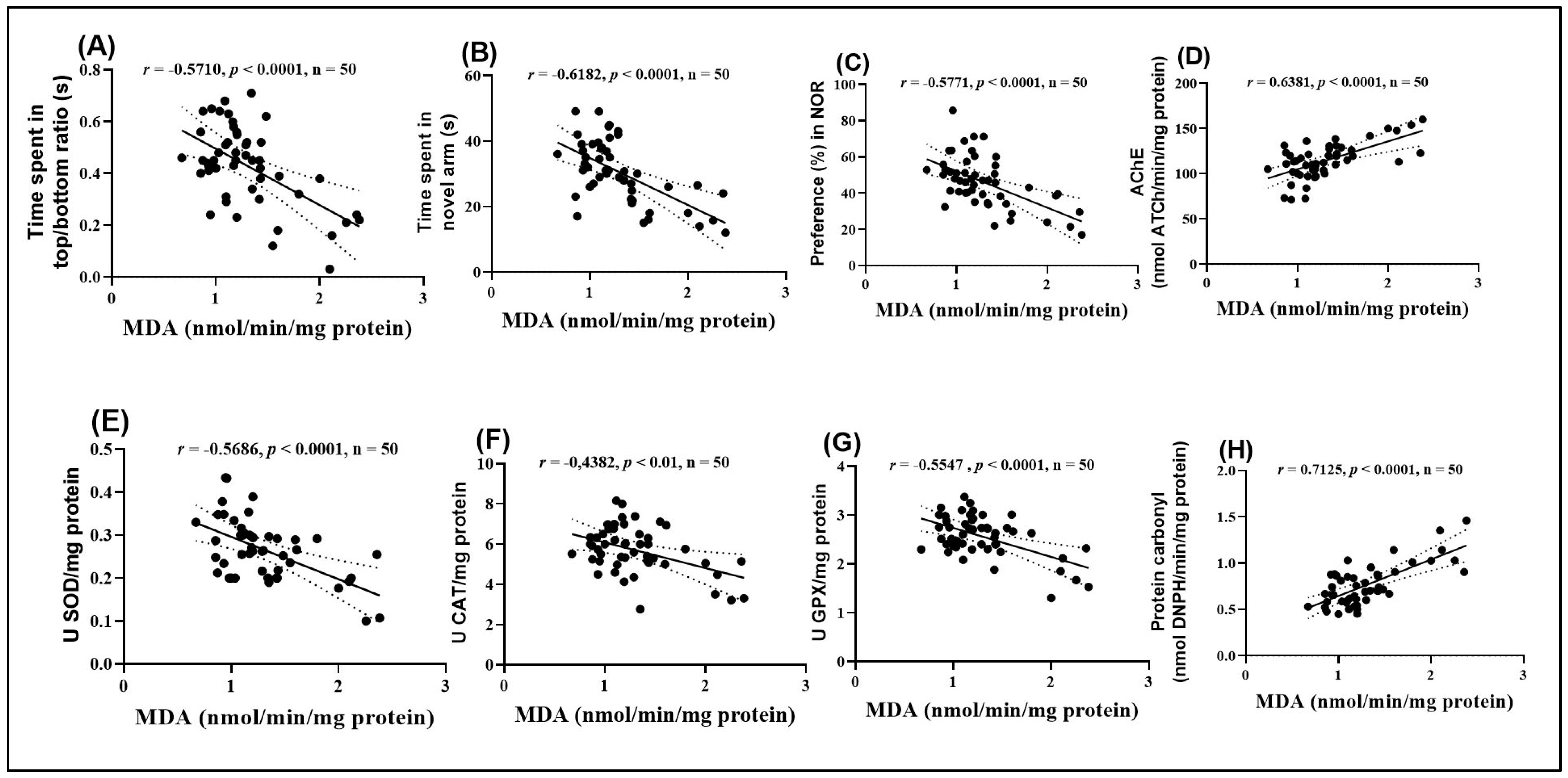
2.11. Pearson Correlations Between Behavioral and Biochemical Variables
3. Discussion
4. Materials and Methods
4.1. Retrieval of Molecular Formulas and Chemical Structures
4.2. In Silico Evaluation of Pharmacokinetic Properties of Major Constituents
4.3. In Silico Approach for the Evaluation of Neuropharmacological and Toxicological Properties Using the PASS Online Platform
4.4. Plant Material and Extraction
4.5. High-Performance Liquid Chromatography
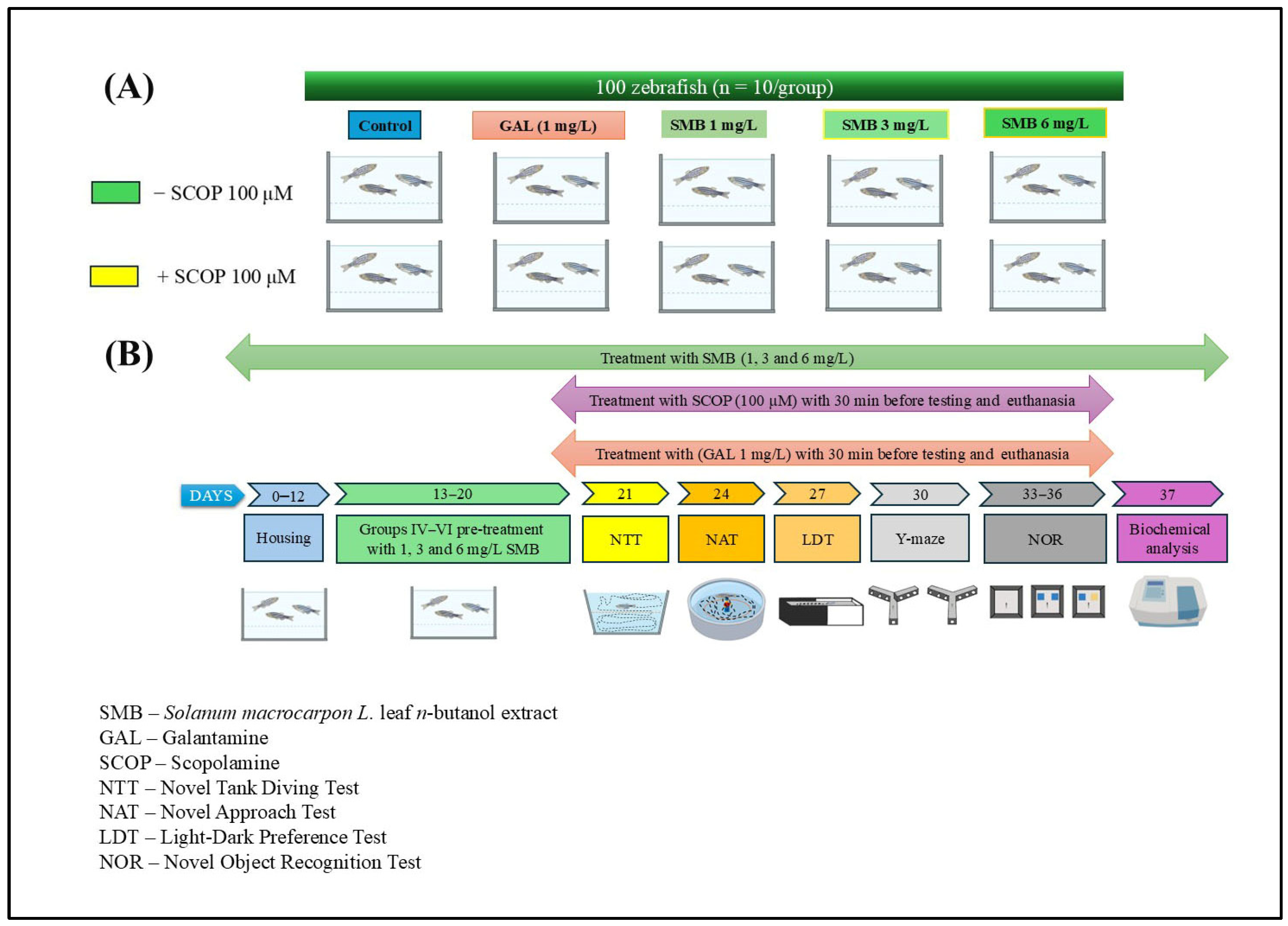
4.6. Study Design and Animal Care
4.7. Behavioral Assessments
4.7.1. Novel Tank Diving Test (NTT)
4.7.2. Novel Approach Test (NAT)
4.7.3. Light–Dark Transition Test (LDT)
4.7.4. The Novel Object Recognition (NOR)
4.8. Analysis of Biochemical Parameters
4.8.1. Acetylcholinesterase (AChE) Activity Assay
4.8.2. Superoxide Dismutase (SOD) Activity Assay
4.8.3. Catalase (CAT) Activity Assay
4.8.4. Glutathione Peroxidase (GPX) Activity Assay
4.8.5. Carbonylated Protein Content Assay
4.8.6. Malondialdehyde (MDA) Level Assay
4.9. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in Alzheimer’s Disease: Current Therapeutic Significance and Future Prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef]
- Bai, R.; Guo, J.; Ye, X.Y.; Xie, Y.; Xie, T. Oxidative Stress: The Core Pathogenesis and Mechanism of Alzheimer’s Disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef]
- Fronza, M.G.; Alves, D.; Praticò, D.; Savegnago, L. The Neurobiology and Therapeutic Potential of Multi-Targeting β-Secretase, Glycogen Synthase Kinase 3β and Acetylcholinesterase in Alzheimer’s Disease. Ageing Res. Rev. 2023, 90, 102033. [Google Scholar] [CrossRef] [PubMed]
- Gajendra, K.; Pratap, G.K.; Poornima, D.V.; Shantaram, M.; Ranjita, G. Natural Acetylcholinesterase Inhibitors: A Multi-Targeted Therapeutic Potential in Alzheimer’s Disease. Eur. J. Med. Chem. Rep. 2024, 11, 100154. [Google Scholar] [CrossRef]
- Majdi, A.; Sadigh-Eteghad, S.; Aghsan, S.R.; Farajdokht, F.; Vatandoust, S.M.; Namvaran, A.; Mahmoudi, J. Amyloid-β, Tau, and the Cholinergic System in Alzheimer’s Disease: Seeking Direction in a Tangle of Clues. Rev. Neurosci. 2020, 31, 391–413. [Google Scholar] [CrossRef]
- Kepp, K.P.; Robakis, N.K.; Høilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The Amyloid Cascade Hypothesis: An Updated Critical Review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef] [PubMed]
- Rawat, K.; Tewari, D.; Bisht, A.; Chandra, S.; Tiruneh, Y.K.; Hassan, H.M.; Al-Emam, A.; Sindi, E.R.; Al-Dies, A.A.M. Identification of AChE Targeted Therapeutic Compounds for Alzheimer’s Disease: An in-Silico Study with DFT Integration. Sci. Rep. 2024, 14, 30356. [Google Scholar] [CrossRef] [PubMed]
- Buccellato, F.R.; D’Anca, M.; Galimberti, D.; Fenoglio, C.; Scarpini, E. Role of Oxidative Damage in Alzheimer’s Disease and Neurodegeneration: From Pathogenic Mechanisms to Biomarker Discovery. Antioxidants 2021, 10, 1353. [Google Scholar] [CrossRef]
- Roy, R.G.; Mandal, P.K.; Maroon, J.C. Oxidative Stress Occurs Prior to Amyloid Aβ Plaque Formation and Tau Phosphorylation in Alzheimer’s Disease: Role of Glutathione and Metal Ions. ACS Chem. Neurosci. 2023, 14, 2944. [Google Scholar] [CrossRef]
- Wang, R.P.H.; Huang, J.; Chan, K.W.Y.; Leung, W.K.; Goto, T.; Ho, Y.S.; Chang, R.C.C. IL-1β and TNF-α Play an Important Role in Modulating the Risk of Periodontitis and Alzheimer’s Disease. J. Neuroinflamm. 2023, 20, 71. [Google Scholar] [CrossRef]
- Plantone, D.; Pardini, M.; Righi, D.; Manco, C.; Colombo, B.M.; De Stefano, N. The Role of TNF-α in Alzheimer’s Disease: A Narrative Review. Cells 2023, 13, 54. [Google Scholar] [CrossRef]
- Nafea, M.; Elharoun, M.; Abd-Alhaseeb, M.M.; Helmy, M.W. Leflunomide Abrogates Neuroinflammatory Changes in a Rat Model of Alzheimer’s Disease: The Role of TNF-α/NF-ΚB/IL-1β Axis Inhibition. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 485–498. [Google Scholar] [CrossRef]
- Walczak-Nowicka, Ł.J.; Herbet, M. Acetylcholinesterase Inhibitors in the Treatment of Neurodegenerative Diseases and the Role of Acetylcholinesterase in Their Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9290. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer Disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Sharma, C.; Kim, S.R.; Barone, E.; Trostchansky, A. Linking Oxidative Stress and Proteinopathy in Alzheimer’s Disease. Antioxidants 2021, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.H.; Biswas, P.; Hossain, M.S.; Islam, R.; Hannan, M.A.; Uddin, M.J.; Rhim, H. Potential Therapeutic Role of Phytochemicals to Mitigate Mitochondrial Dysfunctions in Alzheimer’s Disease. Antioxidants 2020, 10, 23. [Google Scholar] [CrossRef]
- Bhattacharya, R.S.; Singh, R.; Panghal, A.; Thakur, A.; Singh, L.; Verma, R.K.; Singh, C.; Goyal, M.; Kumar, J. Multi-Targeting Phytochemicals for Alzheimer’s Disease. Phytother. Res. 2025, 39, 1453–1483. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Aboulaghras, S.; El Menyiy, N.; El Omari, N.; Assaggaf, H.; Lee, L.H.; Montesano, D.; Gallo, M.; Zengin, G.; AlDhaheri, Y.; et al. Phytochemical Compounds and Nanoparticles as Phytochemical Delivery Systems for Alzheimer’s Disease Management. Molecules 2022, 27, 9043. [Google Scholar] [CrossRef] [PubMed]
- Calfio, C.; Gonzalez, A.; Singh, S.K.; Rojo, L.E.; MacCioni, R.B. The Emerging Role of Nutraceuticals and Phytochemicals in the Prevention and Treatment of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 77, 33–51. [Google Scholar] [CrossRef]
- Maqbool, Z.; Arshad, M.S.; Ali, A.; Aziz, A.; Khalid, W.; Afzal, M.F.; Bangar, S.P.; Addi, M.; Hano, C.; Lorenzo, J.M. Potential Role of Phytochemical Extract from Saffron in Development of Functional Foods and Protection of Brain-Related Disorders. Oxid. Med. Cell Longev. 2022, 2022, 6480590. [Google Scholar] [CrossRef]
- Nematzadeh, G.; Abdulhamid Abdulkareem, D.; Abdulhamid Abdulkareem, K.; Bello, A.; Abdul, N.; Olatunji Sidiq, K.; Umar Olayinka, B.; Kareem, I.; Muazu Danzaki, M.; Toyin Mustapha, O. Phylogenetic Analysis of Solanum macrocarpon: The Evolutionary Relationships and Species Diversification. J. Plant Mol. Breed. 2024, 12, 29–37. [Google Scholar] [CrossRef]
- Kayode, J.; Janet Ayeni, M. Growth, Yield, Nutritional and Mineral Composition of Solanum macrocarpon L. as Affected by Fertilizer Application. J. Biotechnol. Res. 2020, 6, 69–78. [Google Scholar] [CrossRef]
- Ogidi, O.I.; Ukamaka, E.; Ogidi, O.I.; Chukwudi, P.; Ibe, A.I.; Eze, P.U.; Canus, T.N.; History, A. Preliminary phytochemical profile and antimicrobial potentials of white-green african garden egg (Solanum macrocarpon) fruits obtained from Yenagoa. ASIO J. Pharm. Herb. Med. Res. 2021, 7, 1–5. [Google Scholar]
- Famuwagun, A.A.; Taiwo, K.A.; Gbadamosi, S.O.; Oyedele, D.J.; Aluko, R.E.; Adebooye, O.C. Extraction Optimization and Antioxidant Properties of African Eggplant (Solanum macrocarpon) Leaf Polyphenols. J. Food Qual. 2017, 2017, 2159183. [Google Scholar] [CrossRef]
- Anjorin, O.J.; Karigidi, K.O.; Agunloye, M.O.; Olaiya, C.O. Antidiabetic Effects of Fractions of Methanol Extract of Solanum macrocarpon Linn. On Streptozotocin—Induced Diabetic Male Wistar Rats. Vegetos 2023, 37, 2122–2130. [Google Scholar] [CrossRef]
- Adewale, O.; Onasanya, A.; Fadaka, A.; Iwere, H.; Anadozie, S.; Osukoya, O.; Olayide, I. In Vitro Antioxidant Effect of Aqueous Extract of Solanum macrocarpon Leaves in Rat Liver and Brain. Oxid. Antioxid. Med. Sci. 2014, 3, 225. [Google Scholar] [CrossRef]
- Ajiboye, B.O.; Akalabu, M.C.; Ojo, O.A.; Afolabi, O.B.; Okesola, M.A.; Olayide, I.; Oyinloye, B.E. Inhibitory Effect of Ethyl Acetate Fraction of Solanum macrocarpon L. Leaves on Cholinergic, Monoaminergic, and Purinergic Enzyme Activities. J. Food Biochem. 2018, 42, e12643. [Google Scholar] [CrossRef]
- Ogunsuyi, O.B.; Ademiluyi, A.O.; Oboh, G. Solanum Leaves Extracts Exhibit Antioxidant Properties and Inhibit Monoamine Oxidase and Acetylcholinesterase Activities (In Vitro) in Drosophila Melanogaster. J. Basic Clin. Physiol. Pharmacol. 2020, 31. [Google Scholar] [CrossRef]
- Idowu, G.P.; Obuotor, E.M.; Onajobi, F.D. In Vitro and in Silico Investigation of Cholinesterase Inhibition and Anti-Radical Properties of Solanum macrocarpon Leaf Extracts: A Preliminary Anti-Alzheimer’s Study. Alzheimer’s Dement. 2021, 17, e049605. [Google Scholar] [CrossRef]
- Ogunsuyi, O.B.; Omage, F.B.; Olagoke, O.C.; Oboh, G.; Rocha, J.B.T. Phytochemicals from African Eggplants (Solanum macrocarpon L.) and Black Nightshade (Solanum nigrum L.) Leaves as Acetylcholinesterase Inhibitors: An in-Silico Study. J. Biomol. Struct. Dyn. 2023, 41, 7725–7734. [Google Scholar] [CrossRef]
- Ogunsuyi, O.B.; Olagoke, O.C.; Afolabi, B.A.; Loreto, J.S.; Ademiluyi, A.O.; Aschner, M.; Oboh, G.; Barbosa, N.V.; da Rocha, J.B.T. Effect of Solanum Vegetables on Memory Index, Redox Status, and Expressions of Critical Neural Genes in Drosophila Melanogaster Model of Memory Impairment. Metab. Brain Dis. 2022, 37, 729–741. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Ogunsuyi, O.B.; Oluokun, O.O.; Oboh, G. Amnesiac (AMN) Gene and Cnc/Nrf2-Redox Responses in Fruit Fly Model of Memory Impairment Co-Administered Solanum Leaves and Donepezil. Pharmacol. Res.-Mod. Chin. Med. 2024, 10, 100361. [Google Scholar] [CrossRef]
- Okesola, M.A.; Ajiboye, B.O.; Oyinloye, B.E.; Osukoya, O.A.; Owero-ozeze, O.S.; Ekakitie, L.I.; Kappo, A.P. Effect of Solanum macrocarpon Linn Leaf Aqueous Extract on the Brain of an Alloxan-Induced Rat Model of Diabetes. J. Int. Med. Res. 2020, 48, 0300060520922649. [Google Scholar] [CrossRef]
- Shenoy, A.; Banerjee, M.; Upadhya, A.; Bagwe-Parab, S.; Kaur, G. The Brilliance of the Zebrafish Model: Perception on Behavior and Alzheimer’s Disease. Front. Behav. Neurosci. 2022, 16, 861155. [Google Scholar] [CrossRef]
- Boiangiu, R.S.; Mihasan, M.; Gorgan, D.L.; Stache, B.A.; Hritcu, L. Anxiolytic, Promnesic, Anti-Acetylcholinesterase and Antioxidant Effects of Cotinine and 6-Hydroxy-L-Nicotine in Scopolamine-Induced Zebrafish (Danio Rerio) Model of Alzheimer’s Disease. Antioxidants 2021, 10, 212. [Google Scholar] [CrossRef]
- Rathi, K.M.; Undale, V.R.; Wavhale, R.D.; Mohammed, F.S.; Karwa, P.N.; Patil, H. From Computational Screening to Zebrafish Testing: Repurposing of Doxazosin, Donepezil, and Dolutegravir for Neuroprotective Potential in Alzheimer’s Disease. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 10581–10595. [Google Scholar] [CrossRef]
- Sande, R.; Godad, A.; Doshi, G. Zebrafish Experimental Animal Models for AD: A Comprehensive Review. Curr. Rev. Clin. Exp. Pharmacol. 2024, 19, 295–311. [Google Scholar] [CrossRef]
- Salawu, S.; Akindahunsi, A.; Ibukun, E.; Duodu, K. Antioxidant Activities and Inhibitory Action of Solanum macrocarpon and Hibiscus esculentus Phenolic Containing Leaf Extracts against Lipid Oxidation. Int. J. Med. Plants Res. 2013, 2, 190–197. [Google Scholar]
- Brinza, I.; Guliev, C.; Oresanya, I.O.; Gok, H.N.; Orhan, I.E.; Hritcu, L. Solanum macrocarpon L. Ethanolic Leaf Extract Exhibits Neuroprotective and Anxiolytic Effects in Scopolamine-Induced Amnesic Zebrafish Model. Pharmaceuticals 2025, 18, 706. [Google Scholar] [CrossRef]
- Popovici, L.-F.; Brinza, I.; Oancea, S.; Hritcu, L. Chronic Administration of Calendula Officinalis Ethanolic Extract Mitigates Anxiety-like Behavior and Cognitive Impairment Induced by Acute Scopolamine Exposure in Zebrafish. Pharmaceuticals 2025, 18, 1483. [Google Scholar] [CrossRef]
- Coradini, K.; de Andrade, D.F.; Altenhofen, S.; Reolon, G.K.; Nery, L.R.; Silva, N.E.; Vianna, M.R.M.R.; Bonan, C.D.; Beck, R.C.R. Free and Nanoencapsulated Curcumin Prevents Scopolamine-Induced Cognitive Impairment in Adult Zebrafish. J. Drug Deliv. Sci. Technol. 2021, 66, 102781. [Google Scholar] [CrossRef]
- Fu, C.W.; Tong, S.K.; Liu, M.X.; Liao, B.K.; Chou, M.Y. Scopolamine Affects Fear Learning and Social Recognition in Adult Zebrafish. Neuroscience 2025, 568, 219–230. [Google Scholar] [CrossRef]
- Karunakaran, K.B.; Thiyagaraj, A.; Santhakumar, K. Novel Insights on Acetylcholinesterase Inhibition by Convolvulus Pluricaulis, Scopolamine and Their Combination in Zebrafish. Nat. Prod. Bioprospect. 2022, 12, 6. [Google Scholar] [CrossRef]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Nunes, C.; Santos, D. Polyphenols Beyond Barriers: A Glimpse into the Brain. Curr. Neuropharmacol. 2017, 15, 562–594. [Google Scholar] [CrossRef]
- Scuto, M.; Rampulla, F.; Reali, G.M.; Spanò, S.M.; Trovato Salinaro, A.; Calabrese, V. Hormetic Nutrition and Redox Regulation in Gut–Brain Axis Disorders. Antioxidants 2024, 13, 484. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.; Haleagrahara, N. Protective Effects of Quercetin Glycosides, Rutin, and Isoquercetrin against 6-Hydroxydopamine (6-OHDA)-Induced Neurotoxicity in Rat Pheochromocytoma (PC-12) Cells. Int. J. Immunopathol. Pharmacol. 2016, 29, 30–39. [Google Scholar] [CrossRef]
- Dogra, A. Phytotherapeutic Potential of Rutin Against Xenobiotic-Induced Toxicities in Preclinical Models. Food Rev. Int. 2024, 40, 2893–2916. [Google Scholar] [CrossRef]
- Suraweera, T.L.; Vasantha Rupasinghe, H.P.; Dellaire, G.; Xu, Z. Regulation of Nrf2/ARE Pathway by Dietary Flavonoids: A Friend or Foe for Cancer Management? Antioxidants 2020, 9, 973. [Google Scholar] [CrossRef]
- Popovici, L.F.; Brinza, I.; Gatea, F.; Badea, G.I.; Vamanu, E.; Oancea, S.; Hritcu, L. Enhancement of Cognitive Benefits and Anti-Anxiety Effects of Phytolacca Americana Fruits in a Zebrafish (Danio Rerio) Model of Scopolamine-Induced Memory Impairment. Antioxidants 2025, 14, 97. [Google Scholar] [CrossRef]
- Osakabe, N.; Anfuso, C.D.; Jacob, U.M.; Sidenkova, A.; Fritsch, T.; Abdelhameed, A.S.; Rashan, L.; Wenzel, U.; Calabrese, E.J.; Calabrese, V.; et al. Phytochemicals and Vitagenes for a Healthy Brain. In Brain and Mental Health in Ageing. Healthy Ageing and Longevity; Springer: Cham, Switzerland, 2024; pp. 215–253. [Google Scholar] [CrossRef]
- Sahebnasagh, A.; Eghbali, S.; Saghafi, F.; Sureda, A.; Avan, R. Neurohormetic Phytochemicals in the Pathogenesis of Neurodegenerative Diseases. Immun. Ageing 2022, 19, 36. [Google Scholar] [CrossRef]
- Mary, O.O.; Sebastine, O.U.; Ejuiwa, M.C.; Ikemefuna, O.I.; Dominic, E. Anxiolytic and Curative Effect of Solanum macrocarpon Leaves Extract on Acetaminophen Induced Brain Injury in Adult Wistar Rats. J. Pharmacogn. Phytochem. 2020, 9, 205–212. [Google Scholar]
- Ogunsuyi, O.B.; Ademiluyi, A.O.; Oboh, G.; Oyeleye, S.I.; Dada, A.F. Green Leafy Vegetables from Two Solanum Spp. (Solanum nigrum L. and Solanum macrocarpon L.) Ameliorate Scopolamine-Induced Cognitive and Neurochemical Impairments in Rats. Food Sci. Nutr. 2018, 6, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Usunobun, U.; Samuel, E.I. Phytochemical Analysis, Mineral Composition and in Vitro Antioxidant Activities of Celosia Argentea Leaves. Int. J. Sci. World 2016, 4, 19–22. [Google Scholar] [CrossRef]
- Kaur, R.; Sood, A.; Lang, D.K.; Bhatia, S.; Al-Harrasi, A.; Aleya, L.; Behl, T. Potential of Flavonoids as Anti-Alzheimer’s Agents: Bench to Bedside. Environ. Sci. Pollut. Res. 2022, 29, 26063–26077. [Google Scholar] [CrossRef] [PubMed]
- Dougnon, V.; Bankolé, H.; Edorh, P.; Klotoé, J.R.; Dougnon, J.; Fah, L.; Loko, F.; Boko, M. Acute Toxicity of Solanum macrocarpon Linn (Solanaceae) on Wistar Rats: Study about Leaves and Fruits. Am. J. Biochem. 2013, 2013, 84–88. [Google Scholar]
- Dougnon, T.V.; Bankolé, H.S.; Johnson, R.C.; Klotoé, J.R.; Dougnon, G.; Gbaguidi, F.; Assogba, F.; Gbénou, J.; Sahidou, S.; Atègbo, J.-M.; et al. Phytochemical Screening, Nutritional and Toxicological Analyses of Leaves and Fruits of Solanum macrocarpon Linn (Solanaceae) in Cotonou (Benin). Food Nutr. Sci. 2012, 3, 1595–1603. [Google Scholar] [CrossRef]
- Aryal, S.; Skinner, T.; Bridges, B.; Weber, J.T. The Pathology of Parkinson’s Disease and Potential Benefit of Dietary Polyphenols. Molecules 2020, 25, 4382. [Google Scholar] [CrossRef]
- Plazas, M.; Prohens, J.; Cuñat, A.N.; Vilanova, S.; Gramazio, P.; Herraiz, F.J.; Andújar, I. Reducing Capacity, Chlorogenic Acid Content and Biological Activity in a Collection of Scarlet (Solanum aethiopicum) and Gboma (S. macrocarpon) Eggplants. Int. J. Mol. Sci. 2014, 15, 17221–17241. [Google Scholar] [CrossRef]
- Wang, D.; Huang, J.; Wang, H.; Oresanya, I.O.; Orhan, I.E.; Heil, J.; Morlock, G.E. African Under-Utilized Medicinal Leafy Vegetables Studied by Microtiter Plate Assays and High-Performance Thin-Layer Chromatography–Planar Assays. Molecules 2024, 29, 733. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.; Walther, P.; Leitz, J.; Mukherjee, S.; Wu, J.C.; Shivnaraine, R.V.; Zou, J. ADMET-AI: A Machine Learning ADMET Platform for Evaluation of Large-Scale Chemical Libraries. Bioinformatics 2024, 40, btae416. [Google Scholar] [CrossRef]
- Way2Drug—Main. Available online: https://www.way2drug.com/PASSOnline/index.php (accessed on 6 November 2023).
- Oresanya, I.O.; Gök, H.N.; Akkol, E.K.; Sonibare, M.A.; Orhan, I.E. Phenolic Acids in Some Under-Utilized Medicinal and Leafy Vegetables, Their Anti-Inflammatory and Wound Healing Activities. S. Afr. J. Bot. 2025, 184, 827–837. [Google Scholar] [CrossRef]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Bate, S.T.; Clark, R.A. The Design and Statistical Analysis of Animal Experiments; Cambridge University Press: Cambridge, UK, 2014; pp. 1–310. [Google Scholar] [CrossRef]
- Cachat, J.M.; Canavello, P.R.; Elkhayat, S.I.; Bartels, B.K.; Hart, P.C.; Elegante, M.F.; Beeson, E.C.; Laffoon, A.L.; Haymore, W.A.M.; Tien, D.H.; et al. Video-Aided Analysis of Zebrafish Locomotion and Anxiety-Related Behavioral Responses. In Neuromethods; Humana Press: Totowa, NJ, USA, 2011; Volume 51, pp. 1–14. [Google Scholar]
- Hamilton, T.J.; Morrill, A.; Lucas, K.; Gallup, J.; Harris, M.; Healey, M.; Pitman, T.; Schalomon, M.; Digweed, S.; Tresguerres, M. Establishing Zebrafish as a Model to Study the Anxiolytic Effects of Scopolamine. Sci. Rep. 2017, 7, 15081. [Google Scholar] [CrossRef]
- Facciol, A.; Iqbal, M.; Eada, A.; Tran, S.; Gerlai, R. The Light-Dark Task in Zebrafish Confuses Two Distinct Factors: Interaction between Background Shade and Illumination Level Preference. Pharmacol. Biochem. Behav. 2019, 179, 9–21. [Google Scholar] [CrossRef]
- Faillace, M.; Pisera-Fuster, A.; Medrano, M.; Bejarano, A.; Bernabeu, R. Short- and Long-Term Effects of Nicotine and the Histone Deacetylase Inhibitor Phenylbutyrate on Novel Object Recognition in Zebrafish. Psychopharmacology 2017, 234, 943–955. [Google Scholar] [CrossRef]
- Gupta, T.; Mullins, M.C. Dissection of Organs from the Adult Zebrafish. JoVE (J. Vis. Exp.) 2010, e1717. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Artenie, V.; Ungureanu, E.; Negură, A.M. Metode de Investigare a Metabolismului Glucidic Și Lipidic; Editura Pim: Iași, Romania, 2008. [Google Scholar]
- Sinha, A.K. Colorimetric Assay of Catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Fukuzawa, K.; Tokumura, A. Glutathione Peroxidase Activity in Tissues of Vitamin E-Deficient Mice. J. Nutr. Sci. Vitaminol. 1976, 22, 405–407. [Google Scholar] [CrossRef]
- Luo, S.; Wehr, N.B. Protein Carbonylation: Avoiding Pitfalls in the 2,4-Dinitrophenylhydrazine Assay. Redox Rep. 2009, 14, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for Lipid Peroxides in Animal Tissues by Thiobarbituric Acid Reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
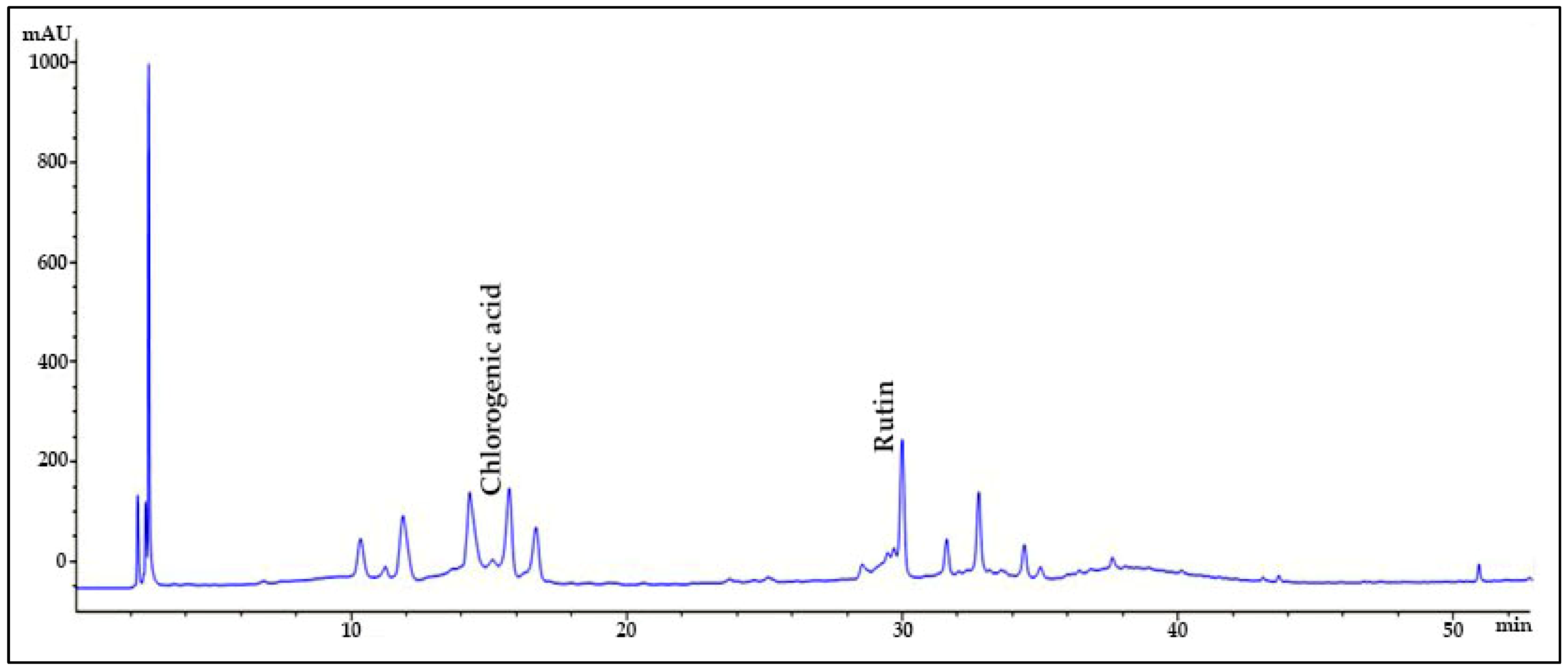



| Sample | Compounds | Retention Time (min) | Maximum Absorbance (nm) | Amount (mg/g, Mean ± SD) | Standard Curve | R2 |
|---|---|---|---|---|---|---|
| SMB | Chlorogenic acid | 15.595 | 320 | 162.39 ± 0.27 | y = 28.919x + 54.92 | 0.9974 |
| Rutin | 30.000 | 260 | 24.61 ± 0.56 | y = 36.127x + 150.42 | 0.9993 |
| Property | Chlorogenic Acid | Rutin |
|---|---|---|
| Molecular weight (Da) | 354.1 | 610.52 |
| Log P | –0.65 | –1.69 |
| TPSA (Å2) | 164.75 | 268.43 |
| HBA (H-bond Acceptors) | 8 | 16 |
| HBD (H-bond Donors) | 6 | 10 |
| Lipinski Rule Compliance | 3/4 | 1/4 |
| QED (Drug-likeness Score) | 0.23 | 0.14 |
| Stereocenters | 4 | 10 |
| Parameters | Chlorogenic Acid | Rutin |
|---|---|---|
| Human intestinal absorption | 0.88 | 0.09 |
| Oral bioavailability | 0.29 | 0.18 |
| Aqueous solubility (log mol/L) | –1.21 | –3.86 |
| Lipophilicity (logP) | –1.83 | 0.77 |
| Hydration free energy (kcal/mol) | –16.41 | –15.67 |
| Cell effective permeability (log cm/s) | –6.60 | –6.82 |
| PAMPA permeability | 0.07 | 0.09 |
| P-glycoprotein inhibition | 0.01 | 0.14 |
| Parameters | Chlorogenic Acid | Rutin |
|---|---|---|
| BBB permeability | 0.40 (24.35%) | 0.06 (3.02%) |
| PPB (%) | 59.12 (30.90%) | 84.88 (63.32%) |
| Vdss (L/kg) | 2.03 (54.36%) | 6.39 (81.74%) |
| Parameters | Chlorogenic Acid | Rutin |
|---|---|---|
| CYP1A2 Inhibition | 0.04 (49.71%) | 0.01 (34.74%) |
| CYP2C19 Inhibition | 0.04 (34.20%) | 0.03 (29.78%) |
| CYP2C9 Inhibition | 0.02 (43.23%) | 0.02 (36.91%) |
| CYP2D6 Inhibition | 0.03 (42.26%) | 0.03 (42.73%) |
| CYP3A4 Inhibition | 0.003 (24.47%) | 0.01 (33.11%) |
| CYP2C9 Substrate | 0.18 (56.69%) | 0.03 (9.42%) |
| CYP2D6 Substrate | 0.02 (17.29%) | 0.02 (12.95%) |
| CYP3A4 Substrate | 0.22 (25.28%) | 0.41 (42.50%) |
| Parameters | Chlorogenic Acid | Rutin |
|---|---|---|
| Half Life | 0.00 (16.98%) h | 49.51 (87.05%) h |
| Drug Clearance (Hepatocyte) | 22.94 (35.67%) µL/min/106 cells | 25.57 (38.66%) µL/min/106 cells |
| Drug Clearance (Microsome) | 0.00 (22.53%) µL/min/mg | 40.10 (70.84%) µL/min/mg |
| Parameters | Chlorogenic Acid | Rutin |
|---|---|---|
| hERG blocking | 0.06 (23.26%) | 0.65 (69.99%) |
| Clinical toxicity | 0.17 (63.28%) | 0.20 (65.99%) |
| Mutagenicity | 0.14 (42.88%) | 0.60 (88.02%) |
| Drug-induced liver injury | 0.48 (52.62%) | 0.75 (67.74%) |
| Carcinogenicity | 0.02 (7.91%) | 0.02 (10.62%) |
| Acute toxicity LD50 | 1.99 (19.39%) | 2.90 (73.40%) |
| Skin reaction | 0.28 (32.11%) | 0.21 (22.64%) |
| Androgen receptor (full length) | 0.12 (88.02%) | 0.08 (83.68%) |
| Androgen receptor (ligand binding domain) | 0.08 (88.68%) | 0.12 (91.47%) |
| Aryl hydrocarbon receptor | 0.03 (53.16%) | 0.11 (73.09%) |
| aromatase | 0.02 (49.09%) | 0.14 (79.80%) |
| Estrogen receptor (full length) | 0.11 (58.94%) | 0.29 (88.06%) |
| Estrogen receptor (ligand binding domain) | 0.07 (84.30%) | 0.21 (93.87%) |
| Peroxisome proliferator-activated receptor gamma | 0.02 (72.12%) | 0.01 (58.08%) |
| Nuclear factor (erythroid-derived 2)-like 2/antioxidant responsive element | 0.17 (57.31%) | 0.18 (58.67%) |
| ATPase family AAA domain-containing protein 5 (ATAD5) | 0.03 (72.51%) | 0.07 (85.03%) |
| Heat shock factor response element | 0.02 (54.28%) | 0.02 (55.29%) |
| Mitochondrial membrane potential | 0.03 (46.53%) | 0.12 (66.23%) |
| Tumor protein p53 | 0.06 (67.47%) | 0.22 (87.09%) |
| Parameters | Chlorogenic Acid | Rutin | ||
|---|---|---|---|---|
| Pa | Pi | Pa | Pi | |
| Antioxidant | 0.785 | 0.004 | 0.923 | 0.003 |
| Oxidoreductase inhibitor | 0.846 | 0.004 | 0.694 | 0.016 |
| Lipid peroxidase inhibitor | 0.855 | 0.003 | 0.987 | 0.001 |
| G-protein-coupled receptor kinase inhibitor | 0.716 | 0.021 | 0.257 | 0.184 |
| Dementia treatment | 0.258 | 0.209 | 0.541 | 0.008 |
| Age-related macular degeneration treatment | 0.210 | 0.161 | - | - |
| Glutamate (mGluR5) agonist | 0.133 | 0.106 | - | - |
| Dopamine precursors | 0.041 | 0.015 | 0.033 | 0.026 |
| NADH-kinase inhibitor | 0.199 | 0.108 | - | - |
| Neurotoxic | 0.874 | 0.008 | 0.882 | 0.007 |
| Dependence | 0.257 | 0.183 | - | - |
| Compound and Molecular Formula | 2D Structure | SMILES |
|---|---|---|
| Chlorogenic acid C16H18O9 | 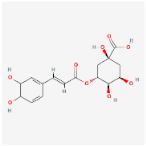 | C1[C@H]([C@H]([C@@H](C[C@@]1(C(=O)O)O) OC(=O)/C=C/C2=CC(=C(C=C2)O)O)O)O |
| Rutin C25H26O15 | 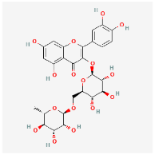 | C[C@H]1[C@@H]([C@H]([C@H]([C@@H](O1) OC[C@@H]2[C@H]([C@@H]([C@H]([C@@H] (O2)OC3=C(OC4=CC(=CC(=C4C3=O)O)O)C5=CC (=C(C=C5)O)O)O)O)O)O)O)O |
| p-Coumaric acid C9H8O3 | 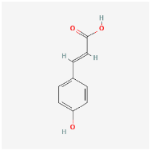 | C1=CC(=CC=C1/C=C/C(=O)O)O |
| Caffeic acid C9H8O4 | 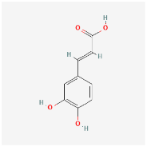 | C1=CC(=C(C=C1/C=C/C(=O)O)O)O |
| Resveratrol C14H12O3 | 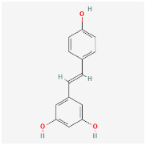 | C1=CC(=CC=C1/C=C/C2=CC(=CC(=C2)O)O)O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brinza, I.; Oresanya, I.O.; Orhan, I.E.; Gök, H.N.; Hritcu, L.; Boiangiu, R.S. Mechanisms Underlying the Cognitive Benefits of Solanum macrocarpon Leaf n-Butanol Extract: Acetylcholinesterase Inhibition and Oxidative Stress Modulation. Plants 2025, 14, 3283. https://doi.org/10.3390/plants14213283
Brinza I, Oresanya IO, Orhan IE, Gök HN, Hritcu L, Boiangiu RS. Mechanisms Underlying the Cognitive Benefits of Solanum macrocarpon Leaf n-Butanol Extract: Acetylcholinesterase Inhibition and Oxidative Stress Modulation. Plants. 2025; 14(21):3283. https://doi.org/10.3390/plants14213283
Chicago/Turabian StyleBrinza, Ion, Ibukun Oluwabukola Oresanya, Ilkay Erdogan Orhan, Hasya Nazlı Gök, Lucian Hritcu, and Razvan Stefan Boiangiu. 2025. "Mechanisms Underlying the Cognitive Benefits of Solanum macrocarpon Leaf n-Butanol Extract: Acetylcholinesterase Inhibition and Oxidative Stress Modulation" Plants 14, no. 21: 3283. https://doi.org/10.3390/plants14213283
APA StyleBrinza, I., Oresanya, I. O., Orhan, I. E., Gök, H. N., Hritcu, L., & Boiangiu, R. S. (2025). Mechanisms Underlying the Cognitive Benefits of Solanum macrocarpon Leaf n-Butanol Extract: Acetylcholinesterase Inhibition and Oxidative Stress Modulation. Plants, 14(21), 3283. https://doi.org/10.3390/plants14213283










