From Feedstock to Function: How Pyrolysis and Oxidation Shape Biochar Performance in Soil–Plant Interactions
Abstract
1. Introduction
2. Results
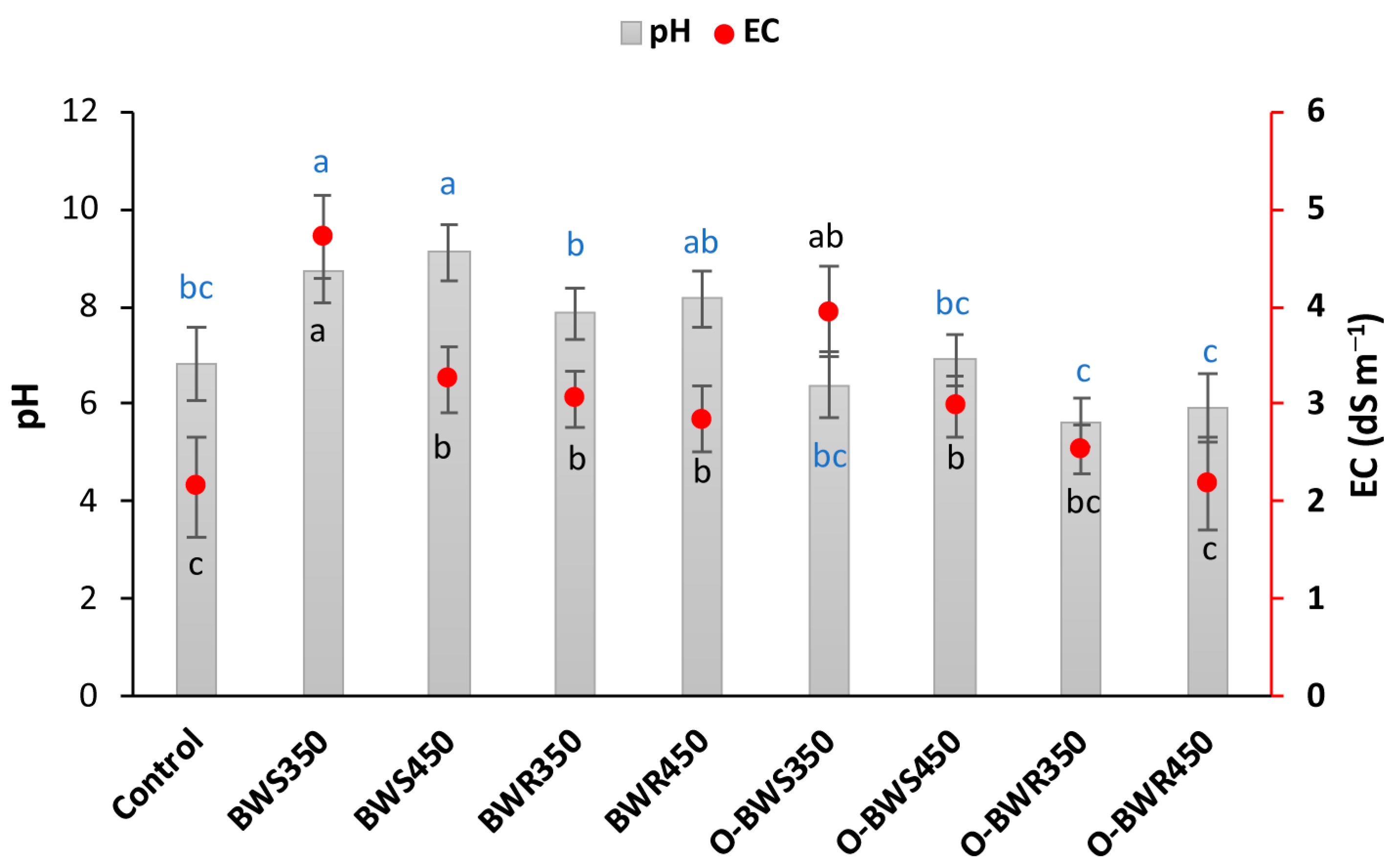
2.1. Changes in pH and EC
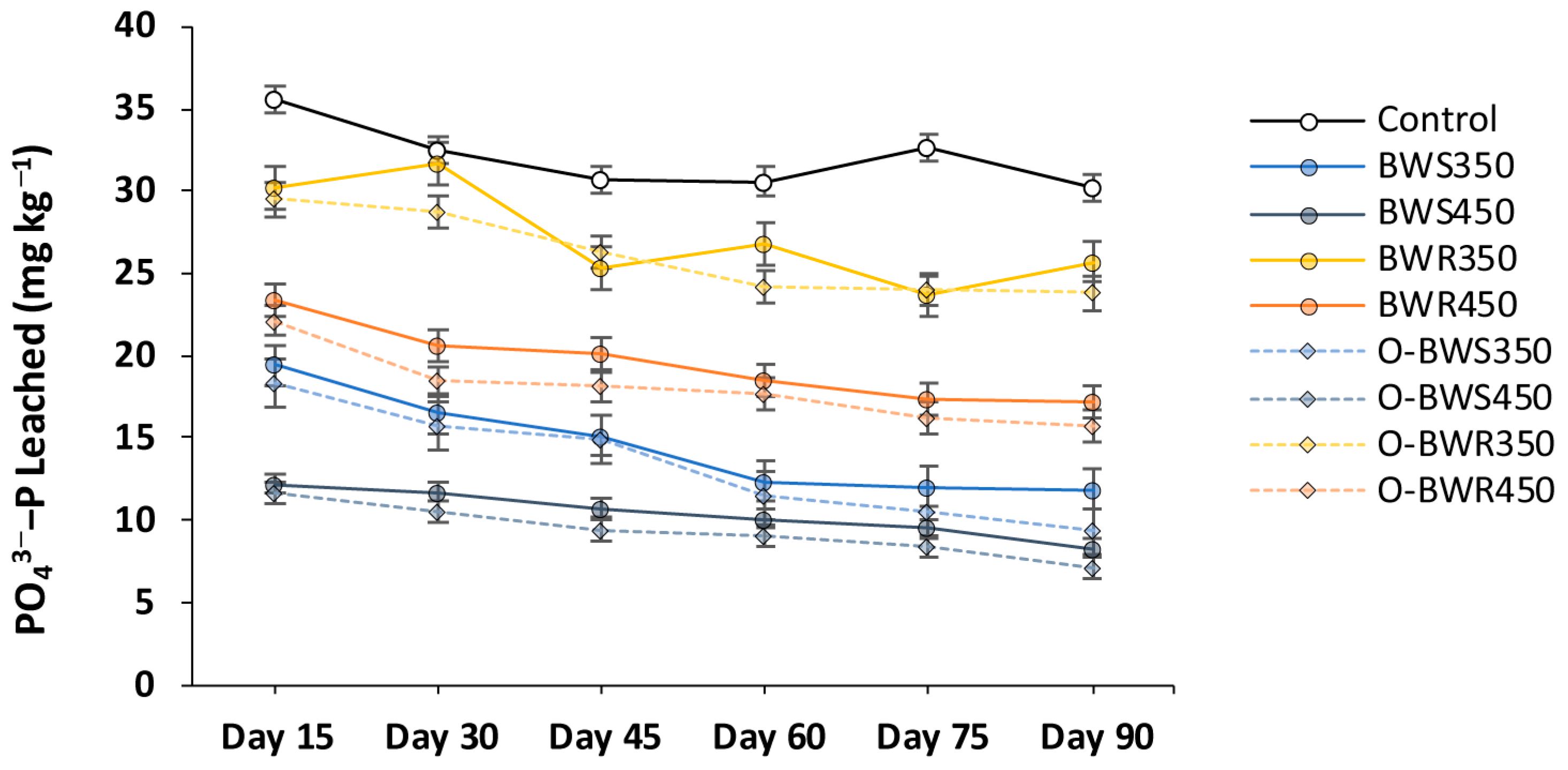
2.2. Changes in PO43− Leaching
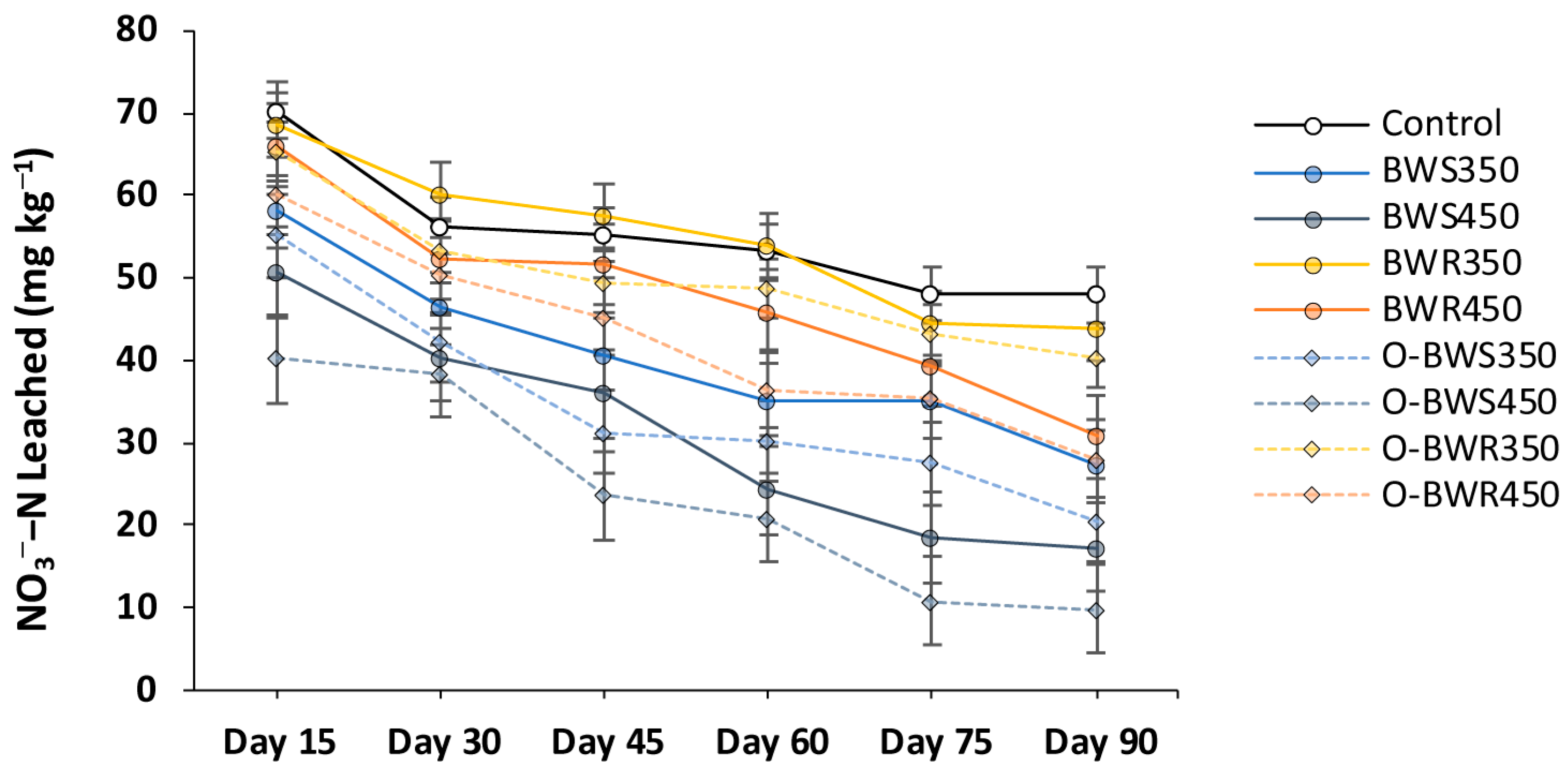
2.3. Changes in NO3− and NH4+ Leaching
2.4. Changes in N and P Uptakes in the Plant
3. Discussion
3.1. Changes in pH and EC
3.2. Changes in PO43− Leaching
3.3. Changes in NO3− and NH4+ Leaching
3.4. Changes in N and P Uptakes in the Plant
4. Materials and Methods
4.1. Experimental Setup
4.2. Biochar Production and Modification
4.3. Biochar Characterization
| pH | EC (dS m−1) | CEC (cmol+ kg−1) | C (%) | H (%) | N (%) | O (%) | VM (%) | AC (%) | SSA (m2 g−1) | Vt (cm3 g−1) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BWS350 | 7.04 | 1.97 | 62.7 | 55.4 | 4.32 | 2.31 | 21.2 | 46.7 | 16.42 | 103.9 | 0.031 |
| BWS450 | 7.75 | 2.12 | 52.8 | 65.8 | 3.72 | 1.24 | 15.1 | 34.6 | 13.83 | 132.4 | 0.037 |
| BWR350 | 8.41 | 1.06 | 45.7 | 68.5 | 4.23 | 1.36 | 18.3 | 37.1 | 7.14 | 89.62 | 0.019 |
| BWR450 | 8.89 | 1.11 | 34.8 | 72.8 | 3.84 | 1.15 | 13.3 | 32.9 | 8.49 | 96.81 | 0.023 |
| O-BWS350 | 4.22 | 1.91 | 74.2 | 52.2 | 4.24 | 1.38 | 26.3 | 42.1 | 15.6 | 136.1 | 0.095 |
| O-BWS450 | 4.68 | 2.16 | 68.4 | 62.6 | 2.13 | 1.29 | 17.2 | 29.8 | 16.59 | 159.8 | 0.125 |
| O-BWR350 | 5.34 | 1.09 | 43.5 | 66.8 | 3.94 | 0.95 | 18.2 | 28.4 | 9.74 | 113.7 | 0.094 |
| O-BWR450 | 6.63 | 1.22 | 34.6 | 75.5 | 3.18 | 0.66 | 12.3 | 25.1 | 8.07 | 158.4 | 0.116 |
4.4. Soil and Plant Analyses
4.5. Data Analyses
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Karlen, D.; Rice, C. Soil Degradation: Will Humankind Ever Learn? Sustainability 2015, 7, 12490–12501. [Google Scholar] [CrossRef]
- Pandian, K.; Mustaffa, M.R.A.F.; Mahalingam, G.; Paramasivam, A.; John Prince, A.; Gajendiren, M.; Rafiqi Mohammad, A.R.; Varanasi, S.T. Synergistic Conservation Approaches for Nurturing Soil, Food Security and Human Health towards Sustainable Development Goals. J. Hazard. Mater. Adv. 2024, 16, 100479. [Google Scholar] [CrossRef]
- Oni, B.A.; Oziegbe, O.; Olawole, O.O. Significance of Biochar Application to the Environment and Economy. Ann. Agric. Sci. 2019, 64, 222–236. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Ohoro, C.R.; Ojukwu, V.E.; Oniye, M.; Shaikh, W.A.; Biswas, J.K.; Seth, C.S.; Mohan, G.B.M.; Chandran, S.A.; Rangabhashiyam, S. Biochar for Ameliorating Soil Fertility and Microbial Diversity: From Production to Action of the Black Gold. iScience 2025, 28, 111524. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Sherpa, K.; Bui, X.-T.; Nguyen, V.-T.; Vo, T.-D.-H.; Ho, H.-T.-T.; Chen, C.-W.; Dong, C.-D. Biochar for Soil Remediation: A Comprehensive Review of Current Research on Pollutant Removal. Environ. Pollut. 2023, 337, 122571. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E. Biochar and Soil Contributions to Crop Lodging and Yield Performance—A Meta-Analysis. Plant Physiol. Biochem. 2024, 215, 109053. [Google Scholar] [CrossRef]
- Shyam, S.; Ahmed, S.; Joshi, S.J.; Sarma, H. Biochar as a Soil Amendment: Implications for Soil Health, Carbon Sequestration, and Climate Resilience. Discov. Soil 2025, 2, 18. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional Heterogeneity of Different Biochar: Effect of Pyrolysis Temperature and Feedstocks. J. Environ. Manag. 2021, 278, 111501. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Cornelis, W.; Zoroufchi Benis, K. Understanding the Physicochemical Structure of Biochar Affected by Feedstock, Pyrolysis Conditions, and Post-Pyrolysis Modification Methods—A Meta-Analysis. J. Environ. Chem. Eng. 2024, 12, 114885. [Google Scholar] [CrossRef]
- Adekiya, A.O.; Ogunbode, T.O.; Esan, V.I.; Adedokun, O.; Olatubi, I.V.; Ayegboyin, M.H. Short Term Effects of Biochar on Soil Chemical Properties, Growth, Yield, Quality, and Shelf Life of Tomato. Sci. Rep. 2025, 15, 24965. [Google Scholar] [CrossRef] [PubMed]
- Demo, A.H.; Gemeda, M.K.; Abdo, D.R.; Guluma, T.N.; Adugna, D.B. Impact of Soil Salinity, Sodicity, and Irrigation Water Salinity on Crop Production and Coping Mechanism in Areas of Dryland Farming. Agrosyst. Geosci. Environ. 2025, 8, e70072. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.-K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef]
- Afaf, A.; ALosaimi, J.S.R.; Alharby, H.F.; Alayafi, A.A.M. The Importance of Initial Application of Biochar On Soil Fertility to Improve Growth and Productivity of Tomato Plants (Solanum lycopersicum L.) Under Drought Stress. Gesunde Pflanz. 2023, 75, 2515–2524. [Google Scholar] [CrossRef]
- Ud Din, M.M.; Khan, M.I.; Azam, M.; Ali, M.H.; Qadri, R.; Naveed, M.; Nasir, A. Effect of Biochar and Compost Addition on Mitigating Salinity Stress and Improving Fruit Quality of Tomato. Agronomy 2023, 13, 2197. [Google Scholar] [CrossRef]
- Li, L.; Long, A.; Fossum, B.; Kaiser, M. Effects of Pyrolysis Temperature and Feedstock Type on Biochar Characteristics Pertinent to Soil Carbon and Soil Health: A Meta-analysis. Soil Use Manag. 2023, 39, 43–52. [Google Scholar] [CrossRef]
- Mengesha, T.T.; Ancha, V.R.; Sundar, L.S.; Pollex, A. Review on the Influence of Pyrolysis Process Parameters for Biochar Production with Minimized Polycyclic Aromatic Hydrocarbon Content. J. Anal. Appl. Pyrolysis 2024, 182, 106699. [Google Scholar] [CrossRef]
- Amirahmadi, E.; Ghorbani, M.; Adani, F. Biochar Contribution in Greenhouse Gas Mitigation and Crop Yield Considering Pyrolysis Conditions, Utilization Strategies and Plant Type—A Meta-Analysis. Field Crops Res. 2025, 333, 110040. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Insura, N.; Williams, P.T. Composition of Products from the Pyrolysis of Polyethylene and Polystyrene in a Closed Batch Reactor: Effects of Temperature and Residence Time. J. Anal. Appl. Pyrolysis 2009, 86, 293–303. [Google Scholar] [CrossRef]
- Sekimoto, K.; Koss, A.R.; Gilman, J.B.; Selimovic, V.; Coggon, M.M.; Zarzana, K.J.; Yuan, B.; Lerner, B.M.; Brown, S.S.; Warneke, C.; et al. High- and Low-Temperature Pyrolysis Profiles Describe Volatile Organic Compound Emissions from Western US Wildfire Fuels. Atmos. Chem. Phys. 2018, 18, 9263–9281. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Neugschwandtner, R.W.; Konvalina, P.; Kopecký, M.; Moudrý, J.; Perná, K.; Murindangabo, Y.T. The Impact of Pyrolysis Temperature on Biochar Properties and Its Effects on Soil Hydrological Properties. Sustainability 2022, 14, 14722. [Google Scholar] [CrossRef]
- Murtaza, G.; Ahmed, Z.; Valipour, M.; Ali, I.; Usman, M.; Iqbal, R.; Zulfiqar, U.; Rizwan, M.; Mahmood, S.; Ullah, A.; et al. Recent Trends and Economic Significance of Modified/Functionalized Biochars for Remediation of Environmental Pollutants. Sci. Rep. 2024, 14, 217. [Google Scholar] [CrossRef]
- Fakhar, A.; Canatoy, R.C.; Galgo, S.J.C.; Rafique, M.; Sarfraz, R. Advancements in Modified Biochar Production Techniques and Soil Application: A Critical Review. Fuel 2025, 400, 135745. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Soja, G.; Bárta, J.; Chen, W.-H.; Amirahmadi, E. How Do Different Feedstocks and Pyrolysis Conditions Effectively Change Biochar Modification Scenarios? A Critical Analysis of Engineered Biochars under H2O2 Oxidation. Energy Convers. Manag. 2024, 300, 117924. [Google Scholar] [CrossRef]
- Nepal, J.; Ahmad, W.; Munsif, F.; Khan, A.; Zou, Z. Advances and Prospects of Biochar in Improving Soil Fertility, Biochemical Quality, and Environmental Applications. Front. Environ. Sci. 2023, 11, 1114752. [Google Scholar] [CrossRef]
- Dong, X.; Chu, Y.; Tong, Z.; Sun, M.; Meng, D.; Yi, X.; Gao, T.; Wang, M.; Duan, J. Mechanisms of Adsorption and Functionalization of Biochar for Pesticides: A Review. Ecotoxicol. Environ. Saf. 2024, 272, 116019. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Bernas, J.; Konvalina, P. Testing Biochar’s Ability to Moderate Extremely Acidic Soils in Tea-Growing Areas. Agronomy 2024, 14, 533. [Google Scholar] [CrossRef]
- Liu, S.; Cen, B.; Yu, Z.; Qiu, R.; Gao, T.; Long, X. The Key Role of Biochar in Amending Acidic Soil: Reducing Soil Acidity and Improving Soil Acid Buffering Capacity. Biochar 2025, 7, 52. [Google Scholar] [CrossRef]
- Hansen, V.; Müller-Stöver, D.; Munkholm, L.J.; Peltre, C.; Hauggaard-Nielsen, H.; Jensen, L.S. The Effect of Straw and Wood Gasification Biochar on Carbon Sequestration, Selected Soil Fertility Indicators and Functional Groups in Soil: An Incubation Study. Geoderma 2016, 269, 99–107. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Kang, Y.; Ran, J.; Song, J.; Jiang, M.; Li, W.; Zhang, M. Effects of Wheat Straw-Derived Biochar on Soil Microbial Communities Under Phenanthrene Stress. Agriculture 2025, 15, 77. [Google Scholar] [CrossRef]
- Tag, A.T.; Duman, G.; Ucar, S.; Yanik, J. Effects of Feedstock Type and Pyrolysis Temperature on Potential Applications of Biochar. J. Anal. Appl. Pyrolysis 2016, 120, 200–206. [Google Scholar] [CrossRef]
- García-Jaramillo, M.; Cox, L.; Knicker, H.E.; Cornejo, J.; Spokas, K.A.; Hermosín, M.C. Characterization and Selection of Biochar for an Efficient Retention of Tricyclazole in a Flooded Alluvial Paddy Soil. J. Hazard. Mater. 2015, 286, 581–588. [Google Scholar] [CrossRef]
- Huff, M.D.; Lee, J.W. Biochar-Surface Oxygenation with Hydrogen Peroxide. J. Environ. Manag. 2016, 165, 17–21. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Yang, W.; Liu, L.; Pan, J. Adsorption of Elemental Mercury in Flue Gas Using Biomass Porous Carbons Modified by Microwave/Hydrogen Peroxide. Fuel 2021, 291, 120152. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.-M.; Dallmeyer, I.; Garcia-Perez, M. Modification of Biochar Surface by Air Oxidation: Role of Pyrolysis Temperature. Biomass Bioenergy 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Zhu, Z.; Duan, W.; Chang, Z.; Du, W.; Chen, F.; Li, F.; Oleszczuk, P. Stability of Functionally Modified Biochar: The Role of Surface Charges and Surface Homogeneity. Sustainability 2023, 15, 7745. [Google Scholar] [CrossRef]
- Zhang, N.; Xing, J.; Wei, L.; Liu, C.; Zhao, W.; Liu, Z.; Wang, Y.; Liu, E.; Ren, X.; Jia, Z.; et al. The Potential of Biochar to Mitigate Soil Acidification: A Global Meta-Analysis. Biochar 2025, 7, 49. [Google Scholar] [CrossRef]
- Cruz-Paredes, C.; Bang-Andreasen, T.; Christensen, S.; Ekelund, F.; Frøslev, T.G.; Jacobsen, C.S.; Johansen, J.L.; Mortensen, L.H.; Rønn, R.; Vestergård, M.; et al. Bacteria Respond Stronger Than Fungi Across a Steep Wood Ash-Driven pH Gradient. Front. For. Glob. Change 2021, 4, 781844. [Google Scholar] [CrossRef]
- Matsumoto, S.; Ogata, S.; Shimada, H.; Sasaoka, T.; Hamanaka, A.; Kusuma, G. Effects of pH-Induced Changes in Soil Physical Characteristics on the Development of Soil Water Erosion. Geosciences 2018, 8, 134. [Google Scholar] [CrossRef]
- Wang, X.; Ding, J.; Han, L.; Tan, J.; Ge, X.; Nan, Q. Biochar Addition Reduces Salinity in Salt-Affected Soils with No Impact on Soil pH: A Meta-Analysis. Geoderma 2024, 443, 116845. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, Y.; Tao, W.; Zhang, X.; Xu, Z.; Xu, C. The Biological Effects of Biochar on Soil’s Physical and Chemical Characteristics: A Review. Sustainability 2025, 17, 2214. [Google Scholar] [CrossRef]
- Gabhi, R.; Basile, L.; Kirk, D.W.; Giorcelli, M.; Tagliaferro, A.; Jia, C.Q. Electrical Conductivity of Wood Biochar Monoliths and Its Dependence on Pyrolysis Temperature. Biochar 2020, 2, 369–378. [Google Scholar] [CrossRef]
- Huang, L.; Liu, X.; Wang, Z.; Liang, Z.; Wang, M.; Liu, M.; Suarez, D.L. Interactive Effects of pH, EC and Nitrogen on Yields and Nutrient Absorption of Rice (Oryza sativa L.). Agric. Water Manag. 2017, 194, 48–57. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Q.; Zheng, H.; Li, M.; Liu, Y.; Wang, X.; Peng, Y.; Luo, X.; Li, F.; Li, X.; et al. Biochar as a Sustainable Tool for Improving the Health of Salt-Affected Soils. Soil Environ. Health 2023, 1, 100033. [Google Scholar] [CrossRef]
- Sharma, S.; Sekhon, B.S.; Singh, P.; Siddiqui, M.H.; Kesawat, M.S. Response of Biochar Derives from Farm Waste on Phosphorus Sorption and Desorption in Texturally Different Soils. Heliyon 2023, 9, e19356. [Google Scholar] [CrossRef] [PubMed]
- Truong, H.B.; Tran, T.C.P.; Nguyen, T.P.; Nguyen, T.T.N.; Oanh, D.T.; Thuy, D.T.; Nguyen, X.C. Biochar-Based Phosphorus Recovery from Different Waste Streams: Sources, Mechanisms, and Performance. Sustainability 2023, 15, 15376. [Google Scholar] [CrossRef]
- Kumari, S.; Dong, Y.; Safferman, S.I. Phosphorus Adsorption and Recovery from Waste Streams Using Biochar: Review of Mechanisms, Modifications, and Agricultural Applications. Appl. Water Sci. 2025, 15, 162. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, X.; Fang, J.; Zhang, X.; Zhang, H.; Zhang, Z.; Wu, X.; Zhu, X. Pyrolysis Temperature Dependent Effects of Biochar on Shifting Fluorescence Spectrum Characteristics of Soil Dissolved Organic Matter under Warming. Sci. Total Environ. 2023, 892, 164656. [Google Scholar] [CrossRef]
- Sarkar, D.; Panicker, T.F.; Kumar Mishra, R.; Srinivas Kini, M. A Comprehensive Review of Production and Characterization of Biochar for Removal of Organic Pollutants from Water and Wastewater. Water-Energy Nexus 2024, 7, 243–265. [Google Scholar] [CrossRef]
- Phuong Tran, T.C.; Nguyen, T.P.; Nguyen Nguyen, T.T.; Thao Tran, T.N.; Hang Nguyen, T.A.; Tran, Q.B.; Nguyen, X.C. Enhancement of Phosphate Adsorption by Chemically Modified Biochars Derived from Mimosa Pigra Invasive Plant. Case Stud. Chem. Environ. Eng. 2021, 4, 100117. [Google Scholar] [CrossRef]
- Strawn, D.G.; Crump, A.R.; Peak, D.; Garcia-Perez, M.; Möller, G. Reactivity of Fe-Amended Biochar for Phosphorus Removal and Recycling from Wastewater. PLoS Water 2023, 2, e0000092. [Google Scholar] [CrossRef]
- Ahmed, N.; Deng, L.; Wang, C.; Shah, Z.-H.; Deng, L.; Li, Y.; Li, J.; Chachar, S.; Chachar, Z.; Hayat, F.; et al. Advancements in Biochar Modification for Enhanced Phosphorus Utilization in Agriculture. Land 2024, 13, 644. [Google Scholar] [CrossRef]
- Shaaban, M.; Hu, R.; Wu, Y.; Younas, A.; Xu, X.; Sun, Z.; Jiang, Y.; Lin, S. Mitigation of N2O Emissions from Urine Treated Acidic Soils by Liming. Environ. Pollut. 2019, 255, 113237. [Google Scholar] [CrossRef] [PubMed]
- Aon, M.; Aslam, Z.; Hussain, S.; Bashir, M.A.; Shaaban, M.; Masood, S.; Iqbal, S.; Khalid, M.; Rehim, A.; Mosa, W.F.A.; et al. Wheat Straw Biochar Produced at a Low Temperature Enhanced Maize Growth and Yield by Influencing Soil Properties of Typic Calciargid. Sustainability 2023, 15, 9488. [Google Scholar] [CrossRef]
- Chandra, S.; Medha, I.; Bhattacharya, J. Potassium-Iron Rice Straw Biochar Composite for Sorption of Nitrate, Phosphate, and Ammonium Ions in Soil for Timely and Controlled Release. Sci. Total Environ. 2020, 712, 136337. [Google Scholar] [CrossRef]
- Tang, Z.; He, N.; Zhang, L.; Wang, L.; Gong, D.; Wang, C.; Wang, H.; Sui, G.; Zheng, W. Straw and Biochar Application Alters the Structure of Rhizosphere Microbial Communities in Direct-Seeded Rice (Oryza sativa L.) Paddies. Agronomy 2024, 14, 316. [Google Scholar] [CrossRef]
- Zhao, W.; Zhou, Q.; Tian, Z.; Cui, Y.; Liang, Y.; Wang, H. Apply Biochar to Ameliorate Soda Saline-Alkali Land, Improve Soil Function and Increase Corn Nutrient Availability in the Songnen Plain. Sci. Total Environ. 2020, 722, 137428. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, Q.; Guan, C.-Y.; Yang, X.; Jiang, X.; Wei, M.; Tan, J.; Li, X. Metal Oxide Modified Biochars for Fertile Soil Management: Effects on Soil Phosphorus Transformation, Enzyme Activity, Microbe Community, and Plant Growth. Environ. Res. 2023, 231, 116258. [Google Scholar] [CrossRef]
- Xue, Y.; Gao, B.; Yao, Y.; Inyang, M.; Zhang, M.; Zimmerman, A.R.; Ro, K.S. Hydrogen Peroxide Modification Enhances the Ability of Biochar (Hydrochar) Produced from Hydrothermal Carbonization of Peanut Hull to Remove Aqueous Heavy Metals: Batch and Column Tests. Chem. Eng. J. 2012, 200–202, 673–680. [Google Scholar] [CrossRef]
- Mia, S.; Dijkstra, F.A.; Singh, B. Aging Induced Changes in Biochar’s Functionality and Adsorption Behavior for Phosphate and Ammonium. Environ. Sci. Technol. 2017, 51, 8359–8367. [Google Scholar] [CrossRef] [PubMed]
- Sanford, J.R.; Larson, R.A.; Runge, T. Nitrate Sorption to Biochar Following Chemical Oxidation. Sci. Total Environ. 2019, 669, 938–947. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Reichel, R.; Wissel, H.; Brüggemann, N. Improving Nitrogen Retention of Cattle Slurry with Oxidized Biochar: An Incubation Study with Three Different Soils. J. Environ. Qual. 2023, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]

| N-Uptake (%) | P-Uptake (%) | |
|---|---|---|
| Control | 3.65 ± 0.13 c | 2.35 ± 0.11 c |
| BWS350 | 4.77 ± 0.21 bc | 2.41 ± 0.08 bc |
| BWS450 | 5.13 ± 0.75 ab | 2.67 ± 0.18 b |
| BWR350 | 4.64 ± 0.24 bc | 2.38 ± 0.12 bc |
| BWR450 | 4.94 ± 0.36 b | 2.54 ± 0.17 bc |
| O-BWS350 | 5.42 ± 0.48 ab | 3.52 ± 0.33 a |
| O-BWS450 | 6.71 ± 0.53 a | 3.89 ± 0.47 a |
| O-BWR350 | 5.25 ± 0.39 ab | 2.75 ± 0.16 b |
| Feedstock | Pyrolysis Temp (°C) | Oxidation Treatment | Description | |
|---|---|---|---|---|
| BWS350 | Wheat straw | 350 | None | Pristine wheat straw biochar |
| BWS450 | Wheat straw | 450 | None | Pristine wheat straw biochar |
| BWR350 | Wood residue | 350 | None | Pristine wood biochar |
| BWR450 | Wood residue | 450 | None | Pristine wood biochar |
| O-BWS350 | Wheat straw | 350 | H2O2 | Oxidized wheat straw biochar |
| O-BWS450 | Wheat straw | 450 | H2O2 | Oxidized wheat straw biochar |
| O-BWR350 | Wood residue | 350 | H2O2 | Oxidized wood biochar |
| O-BWR450 | Wood residue | 450 | H2O2 | Oxidized wood biochar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghorbani, M.; Amirahmadi, E.; Bernas, J.; Bárta, J. From Feedstock to Function: How Pyrolysis and Oxidation Shape Biochar Performance in Soil–Plant Interactions. Plants 2025, 14, 3278. https://doi.org/10.3390/plants14213278
Ghorbani M, Amirahmadi E, Bernas J, Bárta J. From Feedstock to Function: How Pyrolysis and Oxidation Shape Biochar Performance in Soil–Plant Interactions. Plants. 2025; 14(21):3278. https://doi.org/10.3390/plants14213278
Chicago/Turabian StyleGhorbani, Mohammad, Elnaz Amirahmadi, Jaroslav Bernas, and Jan Bárta. 2025. "From Feedstock to Function: How Pyrolysis and Oxidation Shape Biochar Performance in Soil–Plant Interactions" Plants 14, no. 21: 3278. https://doi.org/10.3390/plants14213278
APA StyleGhorbani, M., Amirahmadi, E., Bernas, J., & Bárta, J. (2025). From Feedstock to Function: How Pyrolysis and Oxidation Shape Biochar Performance in Soil–Plant Interactions. Plants, 14(21), 3278. https://doi.org/10.3390/plants14213278








