Modeling and Visualization of Nitrogen and Chlorophyll in Greenhouse Solanum lycopersicum L. Leaves with Hyperspectral Imaging for Nitrogen Stress Diagnosis
Abstract
1. Introduction
2. Results
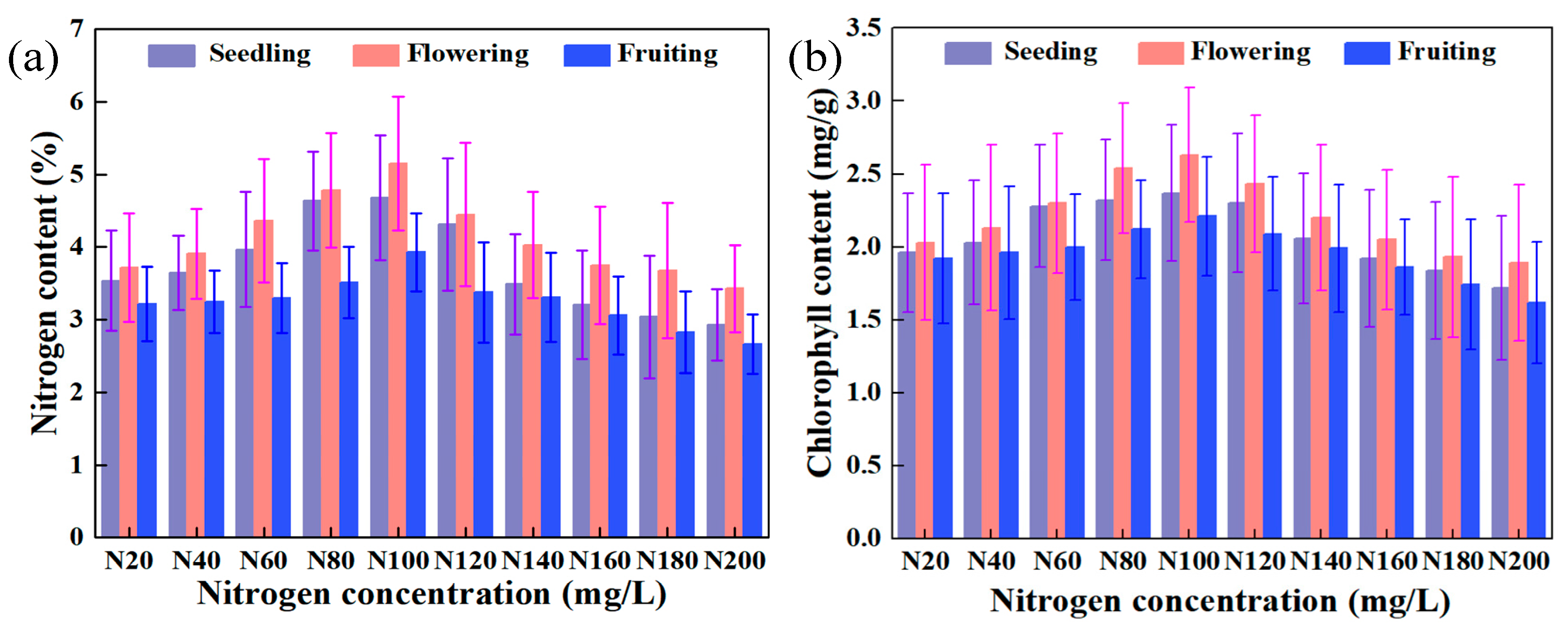
2.1. Results of Spatiotemporal Responses of Leaf Nitrogen and Chlorophyll Contents
- (1)
- Temporal responses of physiological parameters during growth stages
- (2)
- Responses of physiological parameters to spatial levels
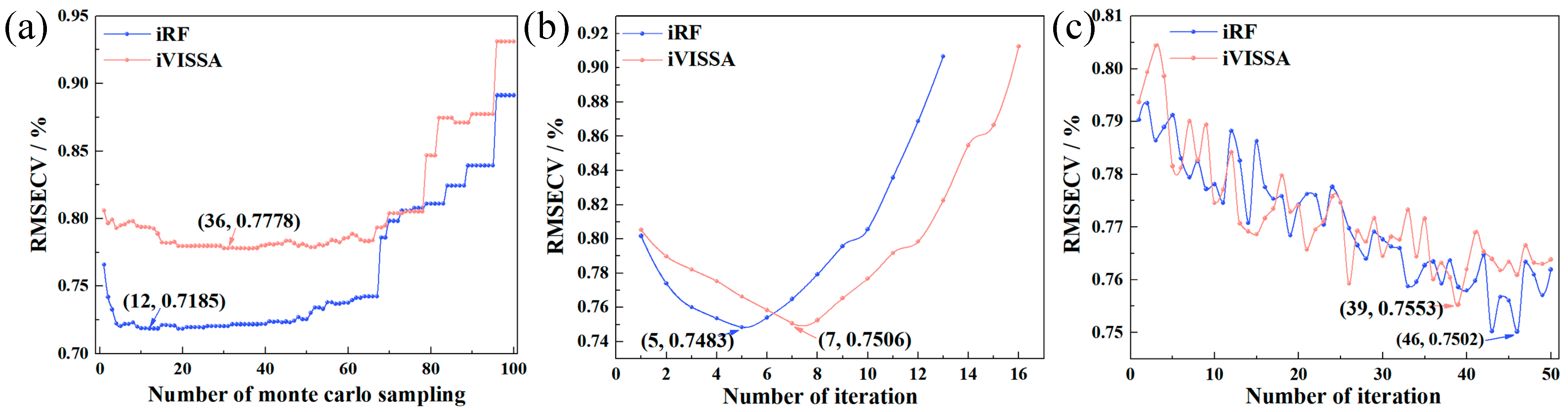
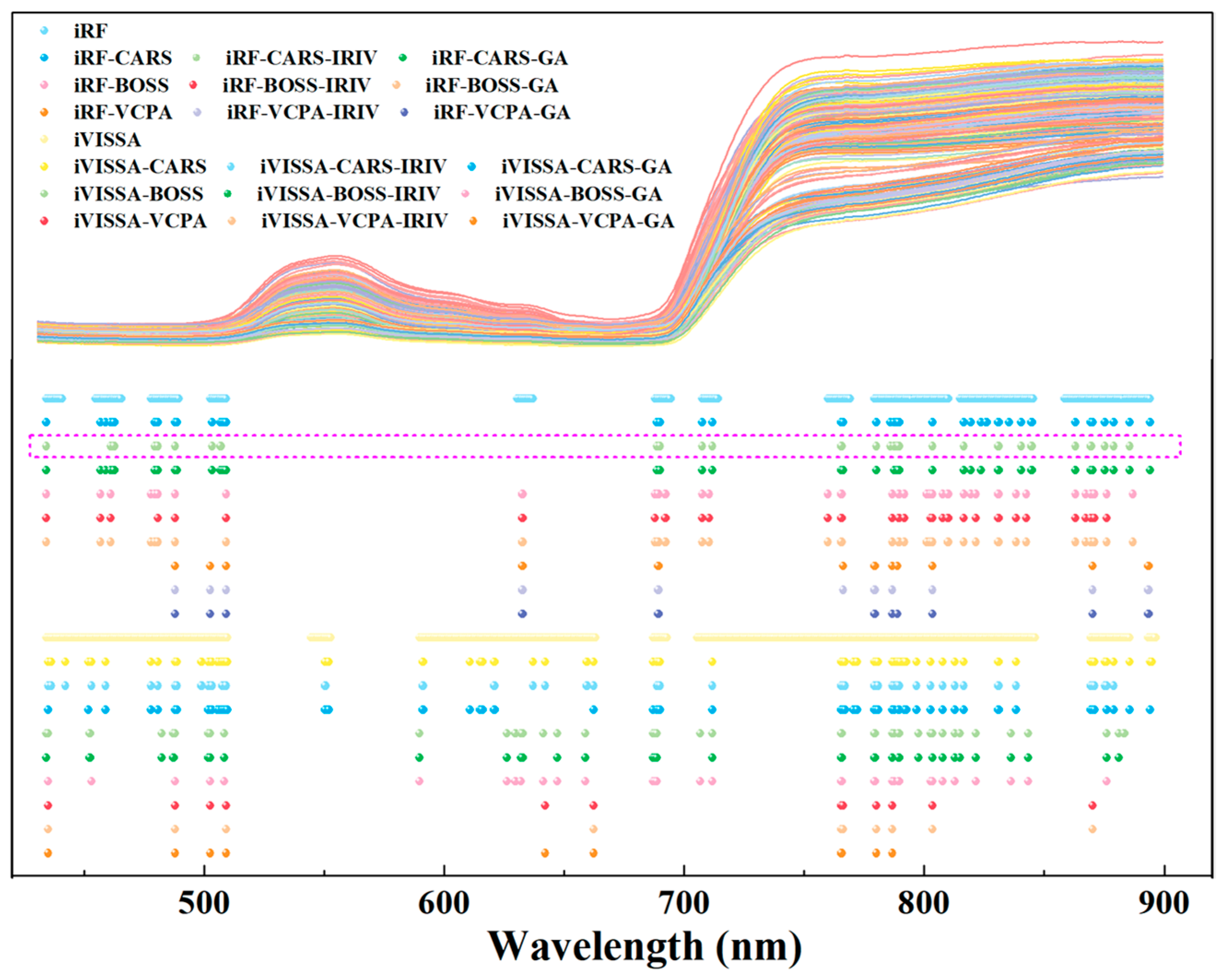
2.2. Results of Key Wavelengths and Detection Models of Nitrogen
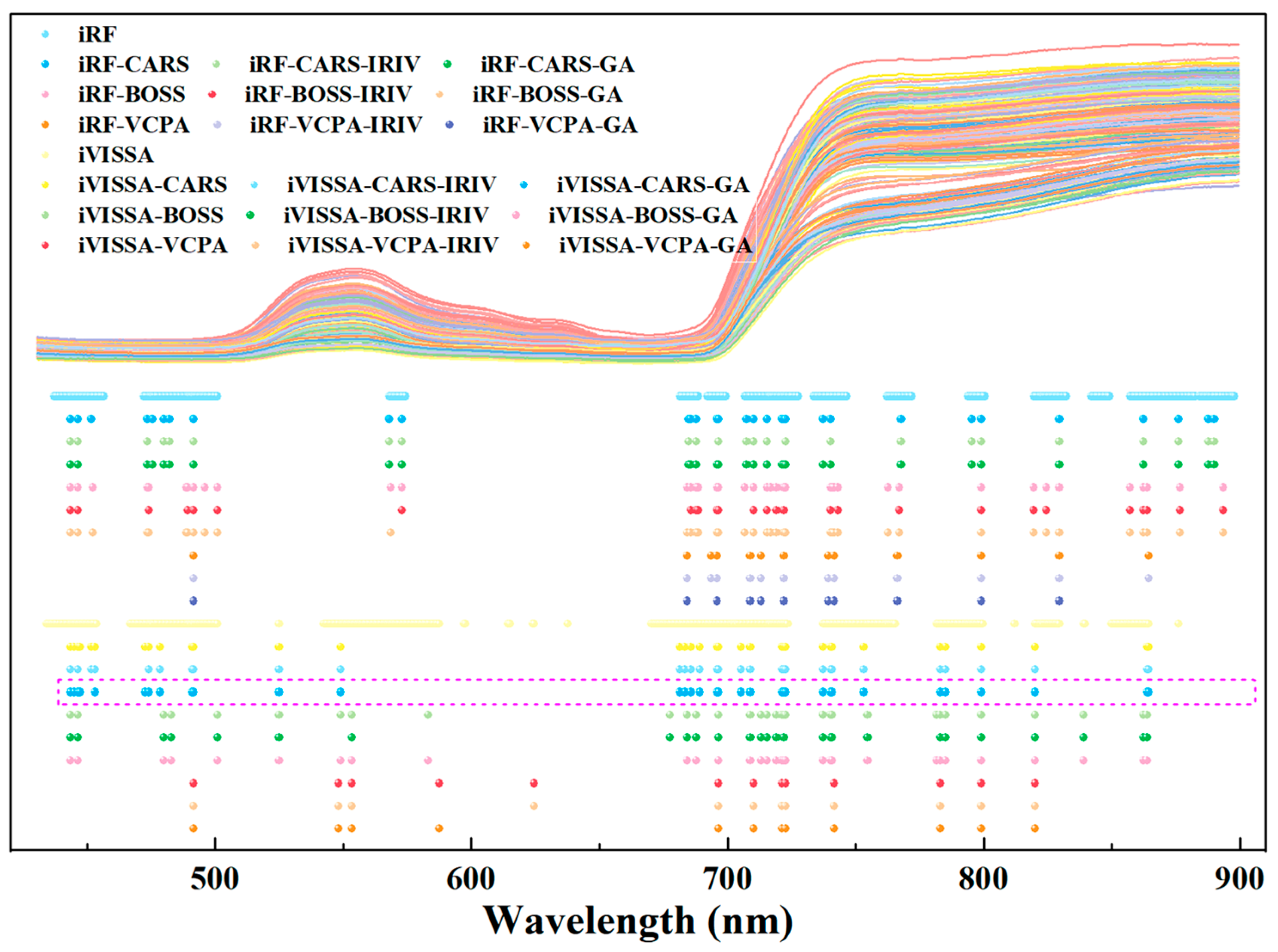
2.3. Results of Key Wavelengths and Detection Models of Chlorophyll
2.4. Results of Visualization of Content and Structural Distribution
3. Discussion
3.1. Screening of Key Wavelengths and Development of Non-Destructive Detection Models for Leaf Nitrogen and Chlorophyll
3.2. Interpretation of Key Wavelengths
3.3. Temporal Dynamics and Allocation Mechanisms of Nitrogen and Chlorophyll in Tomato Leaves
3.4. Spatial Distribution Patterns and Allocation Mechanisms of Nitrogen and Chlorophyll in Tomato Leaves
3.5. Distribution Mechanisms of Nitrogen and Chlorophyll in Leaf Tissues
3.6. Response Mechanisms of Nitrogen and Chlorophyll Under Nitrogen Stress
3.7. Advantages and Limitations of the Study
4. Materials and Methods
4.1. Experimental Scheme and Sampling Rules
4.2. Hyperspectral Imaging Acquisition Device
4.3. Determination of Nitrogen and Chlorophyll
4.4. Spectral Data Preprocessing
4.5. Key Wavelength Extraction and Model Construction
4.6. Hyperspectral Image Visualization
5. Conclusions and Future Work
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| S-G | Savitzky–Golay |
| FIR | Finite Impulse Response |
| SNV | Standard Normalized Variate |
| DT | Detrending |
| SPXY | Sample Set Partitioning Using The joint x–y Distance |
| iRF | Interval Random Forest |
| iVISSA | Interval Variable Iterative Space Shrinkage Approach |
| WBMS | Weighted Bootstrap Monte Carlo Sampling |
| CARS | Competitive Adaptive Reweighted Sampling |
| BOSS | Bootstrapping Soft Shrinkage |
| VCPA | Variable Combination Population Analysis |
| WBS | Weighted Bootstrap Sampling |
| BMS | Bootstrap Model Sampling |
| IRIV | Iteratively Retaining Informative Variables |
| GA | Genetic Algorithm |
| PLSR | Partial Least Squares Regression |
| R | Correlation Coefficient |
| RMSE | Root Mean Squared Error |
| RPD | Residual Prediction Deviation |
| RMSECV | Root Mean Square Error of Cross-Validation |
References
- Yao, J.; Gong, Y.; Xia, Z.; Nie, P.; Xu, H.; Zhang, H.; Chen, Y.; Li, X.; Li, Z.; Li, Y. Facility of tomato plant organ segmentation and phenotypic trait extraction via deep learning. Comput. Electron. Agric. 2025, 231, 109957. [Google Scholar] [CrossRef]
- Zhang, H.; Miao, C.; Zhu, C.; Ding, X.; Yang, S.; Chang, L.; Jiang, Y. Effects of Different Cultivation Facilities and Planting Densities on Tomato Seedling Production. Hortscience 2025, 60, 1065–1074. [Google Scholar] [CrossRef]
- Wang, C.; Wu, G.; Wang, H.; Wang, J.; Yuan, M.; Guo, X.; Liu, C.; Xing, S.; Sun, Y.; Talpur, M.M.A. Optimizing Tomato Cultivation: Impact of Ammonium–Nitrate Ratios on Growth, Nutrient Uptake, and Fertilizer Utilization. Sustainability 2024, 16, 5373. [Google Scholar] [CrossRef]
- He, X.; Wu, P.; Wang, Z.; Zhang, L.; Wu, S.; Liu, X.; Yang, X.; Hang, Y. Subsurface irrigation system with ceramic emitters stabilizes soil nitrogen availability to enhance tomato yield and nitrogen use efficiency in greenhouse systems. Plant Soil 2025, 1–17. [Google Scholar] [CrossRef]
- Farneselli, M.; Reale, L.; Falcinelli, B.; Akram, M.Z.; Cimarelli, S.; Cinti, E.; Paglialunga, M.; Carbone, F.; Pannacci, E.; Tei, F. A Multifaceted Approach to Optimizing Processed Tomato Production: Investigating the Combined Effects of Biostimulants and Reduced Nitrogen Fertilization. Horticulturae 2025, 11, 931. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Zhang, C.; Luo, J.; Jiang, N.; Zhang, F.; Zhu, W. Heat Stress Recovery of Chlorophyll Fluorescence in Tomato (Lycopersicon esculentum Mill.) Leaves through Nitrogen Levels. Agronomy 2023, 13, 2858. [Google Scholar] [CrossRef]
- Luo, J.; Yang, Z.; Zhang, F.; Li, C. Effect of nitrogen application on enhancing high-temperature stress tolerance of tomato plants during the flowering and fruiting stage. Front. Plant Sci. 2023, 14, 1172078. [Google Scholar] [CrossRef]
- Chormova, D.; Kavvadias, V.; Okello, E.; Shiel, R.; Brandt, K. Nitrogen Application Can Be Reduced without Affecting Carotenoid Content, Maturation, Shelf Life and Yield of Greenhouse Tomatoes. Plants 2023, 12, 1553. [Google Scholar] [CrossRef]
- Li, C.; Yang, Z.; Zhang, C.; Luo, J.; Zhang, F.; Qiu, R. Effects of Nitrogen Application in Recovery Period after Different High Temperature Stress on Plant Growth of Greenhouse Tomato at Flowering and Fruiting Stages. Agronomy 2023, 13, 1439. [Google Scholar] [CrossRef]
- Nie, J.; Li, Y.H.; Yang, X.; Zheng, J.R.; Xie, Y.M.; Shi, L.L. Effect of Fertilization Treatment on Growth, Yield, Fruit Quality, And Nutrition Accumulation of Cherry Tomato. Appl. Ecol. Environ. Res. 2023, 21, 3849. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Makraki, T.; Papadimitriou, D.; Nikoloudakis, N.; Taheri-Garavand, A.; Fanourakis, D. Non-Destructive Estimation of Area and Greenness in Leaf and Seedling Scales: A Case Study in Cucumber. Agronomy 2025, 15, 2294. [Google Scholar] [CrossRef]
- Atanassova, S.; Petrova, A.; Yorgov, D.; Mineva, R.; Veleva, P. Visible and Near-Infrared Spectroscopy for Investigation of Water and Nitrogen Stress in Tomato Plants. AgriEngineering 2025, 7, 155. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, S.; Li, Z.; Chang, T.; Xu, Q.; Xu, H.; Zhang, J. Effects of excessive nitrogen fertilizer and soil moisture deficiency on antioxidant enzyme system and osmotic adjustment in tomato seedlings. Int. J. Agric. Biol. Eng. 2022, 15, 127–134. [Google Scholar] [CrossRef]
- Fanourakis, D.; Papadakis, V.M.; Machado, M.; Psyllakis, E.; Nektarios, P.A. Non-invasive leaf hydration status determination through convolutional neural networks based on multispectral images in chrysanthemum. Plant Growth Regul. 2024, 102, 485–496. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A.; Zhang, H.; Zhu, Q.; Zhang, H.; Sun, W.; Niu, Y. Estimating Leaf Chlorophyll Content of Winter Wheat from UAV Multispectral Images Using Machine Learning Algorithms under Different Species, Growth Stages, and Nitrogen Stress Conditions. Agriculture 2024, 14, 1064. [Google Scholar] [CrossRef]
- Tan, C.; Du, Y.; Zhou, J.; Wang, D.; Luo, M.; Zhang, Y.; Guo, W. Analysis of different hyperspectral variables for diagnosing leaf nitrogen accumulation in wheat. Front. Plant Sci. 2018, 9, 674. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakaiya, E.; Zhu, Y.; Takahashi, W. Diagnostic mapping of canopy nitrogen content in rice based on hyperspectral measurements. Remote Sens. Environ. 2012, 126, 210–221. [Google Scholar] [CrossRef]
- Rasooli Sharabiani, V.; Soltani Nazarloo, A.; Taghinezhad, E.; Veza, I.; Szumny, A.; Figiel, A. Prediction of winter wheat leaf chlorophyll content based on VIS/NIR spectroscopy using ANN and PLSR. Food Sci. Nutr. 2023, 11, 2166–2175. [Google Scholar] [CrossRef]
- Zhai, W.; Li, C.; Cheng, Q.; Ding, F.; Chen, Z. Exploring Multisource Feature Fusion and Stacking Ensemble Learning for Accurate Estimation of Maize Chlorophyll Content Using Unmanned Aerial Vehicle Remote Sensing. Remote Sens. 2023, 15, 3454. [Google Scholar] [CrossRef]
- Liu, H.; Lei, X.; Liang, H.; Wang, X. Multi-Model Rice Canopy Chlorophyll Content Inversion Based on UAV Hyperspectral Images. Sustainability 2023, 15, 7038. [Google Scholar] [CrossRef]
- Picado, E.F.; Romero, K.F.; Heenkenda, M.K. Mapping Spatial Variability of Sugarcane Foliar Nitrogen, Phosphorus, Potassium and Chlorophyll Concentrations Using Remote Sensing. Geomatics 2025, 5, 3. [Google Scholar] [CrossRef]
- Ali, A.M.; Salem, H.M.; Bijay-Singh. Site-Specific Nitrogen Fertilizer Management Using Canopy Reflectance Sensors, Chlorophyll Meters and Leaf Color Charts: A Review. Nitrogen 2024, 5, 828–856. [Google Scholar] [CrossRef]
- Fiorio, P.R.; Silva, C.A.A.C.; Rizzo, R.; Demattê, J.A.M.; dos Santos Luciano, A.C.; da Silva, M.A. Prediction of leaf nitrogen in sugarcane (Saccharum spp.) by Vis-NIR-SWIR spectroradiometry. Heliyon 2024, 10, e26819. [Google Scholar] [CrossRef] [PubMed]
- Flores, W.O.; Hornung, F.; Zuffellato-Ribas, K.C.; Muller, M.; Fabris, J.L.; Lazzaretti, A.E. Machine Learning Models for Chlorophyll Content Estimation in Wheat Leaves from Multi-Angular Reflection Spectra. IEEE Sens. J. 2025, 25, 29252–29261. [Google Scholar] [CrossRef]
- Pan, W.; Cheng, X.; Du, R.; Zhu, X.; Guo, W. Detection of chlorophyll content based on optical properties of maize leaves. Spectrochim. Acta Part A 2024, 309, 123843. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, F.; Wang, X.; Yin, H.; Zhang, W.; Du, J.; Pu, H. Hyperspectral and Fluorescence Imaging Approaches for Nondestructive Detection of Rice Chlorophyll. Plants 2024, 13, 1270. [Google Scholar] [CrossRef]
- Kautsar, V.; Faizah, K.; Uktoro, A.I.; Khasanah, L.; Filiphus, F. Estimating SPAD, Nitrogen Concentration, and Chlorophyll Content in Rice Leaves using Calibrated Smartphone Digital Image. Planta Trop. 2024, 12, 127–138. [Google Scholar] [CrossRef]
- Chakraborty, M.; Pourreza, A.; Peanusaha, S.; Farajpoor, P.; Khalsa, S.D.S.; Brown, P.H. Integrating hyperspectral radiative transfer modeling and Machine learning for enhanced nitrogen sensing in almond leaves. Comput. Electron. Agric. 2025, 234, 110195. [Google Scholar] [CrossRef]
- Jia, M.; Colombo, R.; Rossini, M.; Celesti, M.; Zhu, J.; Cogliati, S.; Cheng, T.; Tian, Y.; Zhu, Y.; Cao, W.; et al. Estimation of leaf nitrogen content and photosynthetic nitrogen use efficiency in wheat using sun-induced chlorophyll fluorescence at the leaf and canopy scales. Eur. J. Agron. 2021, 122, 126192. [Google Scholar] [CrossRef]
- Liu, W.; Li, Y.; Tomasetto, F.; Yan, W.; Tan, Z.; Liu, J.; Jiang, J. Non-destructive measurements of Toona sinensis chlorophyll and nitrogen content under drought stress using near infrared spectroscopy. Front. Plant Sci. 2022, 12, 809828. [Google Scholar] [CrossRef]
- Jang, S.; Han, J.; Cho, J.; Jung, J.; Lee, S.; Lee, D.; Kim, J. Estimation of Apple Leaf Nitrogen Concentration Using Hyperspectral Imaging-Based Wavelength Selection and Machine Learning. Horticulturae 2024, 10, 35. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Tian, T.; Cui, J.; Shi, X.; Song, J.; Li, T.; Li, W.; Zhong, M.; Zhang, W. Construction of spectral index based on multi-angle spectral data for estimating cotton leaf nitrogen concentration. Comput. Electron. Agric. 2022, 201, 107328. [Google Scholar] [CrossRef]
- Raya-Sereno, M.D.; Alonso-Ayuso, M.; Pancorbo, J.L.; Gabriel, J.L.; Camino, C.; Zarco-Tejada, P.J.; Quemada, M. Residual effect and N fertilizer rate detection by high-resolution VNIR-SWIR hyperspectral imagery and solar-induced chlorophyll fluorescence in wheat. IEEE T Geosci. Remote 2021, 60, 4404017. [Google Scholar] [CrossRef]
- Gent, M.P.N.; Short, M.R. Effect on yield and quality of a simple system to recycle nutrient solution to greenhouse tomato. Hortscience 2012, 47, 1641–1645. [Google Scholar] [CrossRef]
- Kang, M.; Zhang, L.; Qin, T.; An, J.; Wang, C.; Wang, S.; Iftikhar, A.; Liu, B.; Liu, L.; Tang, L.; et al. Bridging chlorophyll content and vertical nitrogen distribution for accurate canopy photosynthesis simulation. Comput. Electron. Agric. 2025, 239, 110885. [Google Scholar] [CrossRef]
- Zhu, X.; Guo, H.; Huang, J.J.; Tian, S.; Zhang, Z. A hybrid decomposition and Machine learning model for forecasting Chlorophyll-a and total nitrogen concentration in coastal waters. J. Hydrol. 2023, 619, 129207. [Google Scholar] [CrossRef]
- Zhou, Z.; Struik, P.C.; Gu, J.; van der Putten, P.E.; Wang, Z.; Yin, X.; Yang, J. Enhancing leaf photosynthesis from altered chlorophyll content requires optimal partitioning of nitrogen. Crop Environ. 2023, 2, 24–36. [Google Scholar] [CrossRef]
- Xin, X.; Sun, J.; Shi, L.; Yao, K.; Zhang, B. Application of hyperspectral imaging technology combined with ECA-MobileNetV3 in identifying different processing methods of Yunnan coffee beans. J. Food Compos. Anal. 2025, 143, 107625. [Google Scholar] [CrossRef]
- Yang, J.; Dong, H.; Zhang, H.; Wang, J.; Dai, H.; Zhou, L.; Liang, T.; Zhang, Y. Enhancing the estimation of low-spectral-sensitivity soil properties: A case study of active organic carbon using vis-NIR hyperspectral data. Int. J. Remote Sens. 2025, 46, 4941–4958. [Google Scholar] [CrossRef]
- Cai, Y.; Ma, X.; Huang, B.; Zhang, R.; Wang, X. LIBS combined with SG-SPXY spectral data pre-processing for cement raw meal composition analysis. Appl. Optics 2024, 63, A24–A31. [Google Scholar] [CrossRef]
- Fan, N.; Ma, X.; Liu, G.; Ban, J.; Yuan, R.; Sun, Y. Rapid determination of TBARS content by hyperspectral imaging for evaluating lipid oxidation in mutton. J. Food Compos. Anal. 2021, 103, 104110. [Google Scholar] [CrossRef]
- Yang, X.; Liu, G.; He, J.; Kang, N.; Yuan, R.; Fan, N. Determination of sugar content in Lingwu jujube by NIR–hyperspectral imaging. J. Food Sci. 2021, 86, 1201–1214. [Google Scholar] [CrossRef]
- Li, P.; Jin, Q.; Liu, H.; Han, L.; Li, C.; Luo, Y. Determination of soluble solids content in loquat using near-infrared spectroscopy coupled with broad learning system and hybrid wavelength selection strategy. LWT 2024, 206, 116570. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, N.; Zhu, T.; Zhao, X.; Yuan, M.; Wang, Z.; Wang, G.; Li, Z.; Du, H. Simultaneous quantification and visualization of photosynthetic pigments in Lycopersicon esculentum mill. Under different levels of nitrogen application with visible-near infrared hyperspectral imaging technology. Plants 2023, 12, 2956. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, X.; Zeng, R.; Jiang, H. Rapid determination of free amino acids in Huangshan Maofeng tea using NIR spectra: Analysis and comparison of variable selection methods. Infrared Phys. Technol. 2025, 148, 105848. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, Z.; Ma, H.; Cheng, W.; Li, X.; Tang, L.; Zhao, G.; Wu, Y.; Liu, Z.; Wang, Q. Novel comprehensive variable selection algorithm based on multi-weight vector optimal selection and bootstrapping soft shrinkage. Infrared Phys. Technol. 2023, 133, 104800. [Google Scholar] [CrossRef]
- Chen, S.; Du, K.; Shan, B.; Xu, Q.; Zhang, F. A hybrid variable selection method combining Fisher’s linear discriminant combined population analysis and an improved binary cuckoo search algorithm. Anal. Methods 2024, 16, 1021–1033. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Sun, F.; Sun, Y.; Wang, S.; Meng, J. Comparison of benchtop near infrared and micro near infrared spectrometer for quality control of dried ginger and its different degrees of processed products. Spectrosc. Lett. 2022, 55, 514–526. [Google Scholar] [CrossRef]
- Yu, Y.; Yao, M.; Huang, J. A hybrid wavelength selection strategy-based quantitative analysis model for LIBS data from standard ground samples of the Curiosity rover on Mars. J. Anal. Atom Spectrom. 2022, 37, 2362–2376. [Google Scholar] [CrossRef]



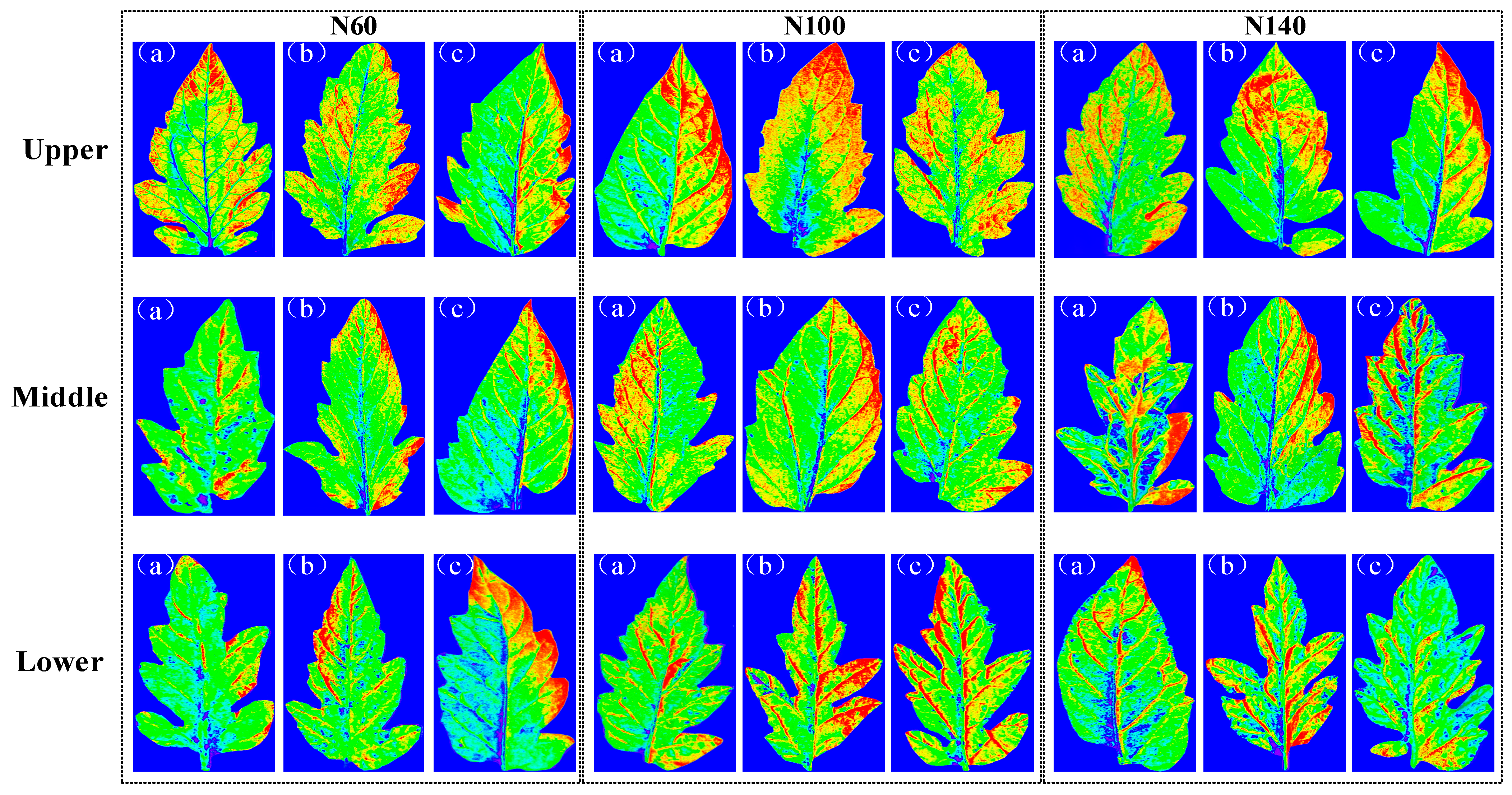
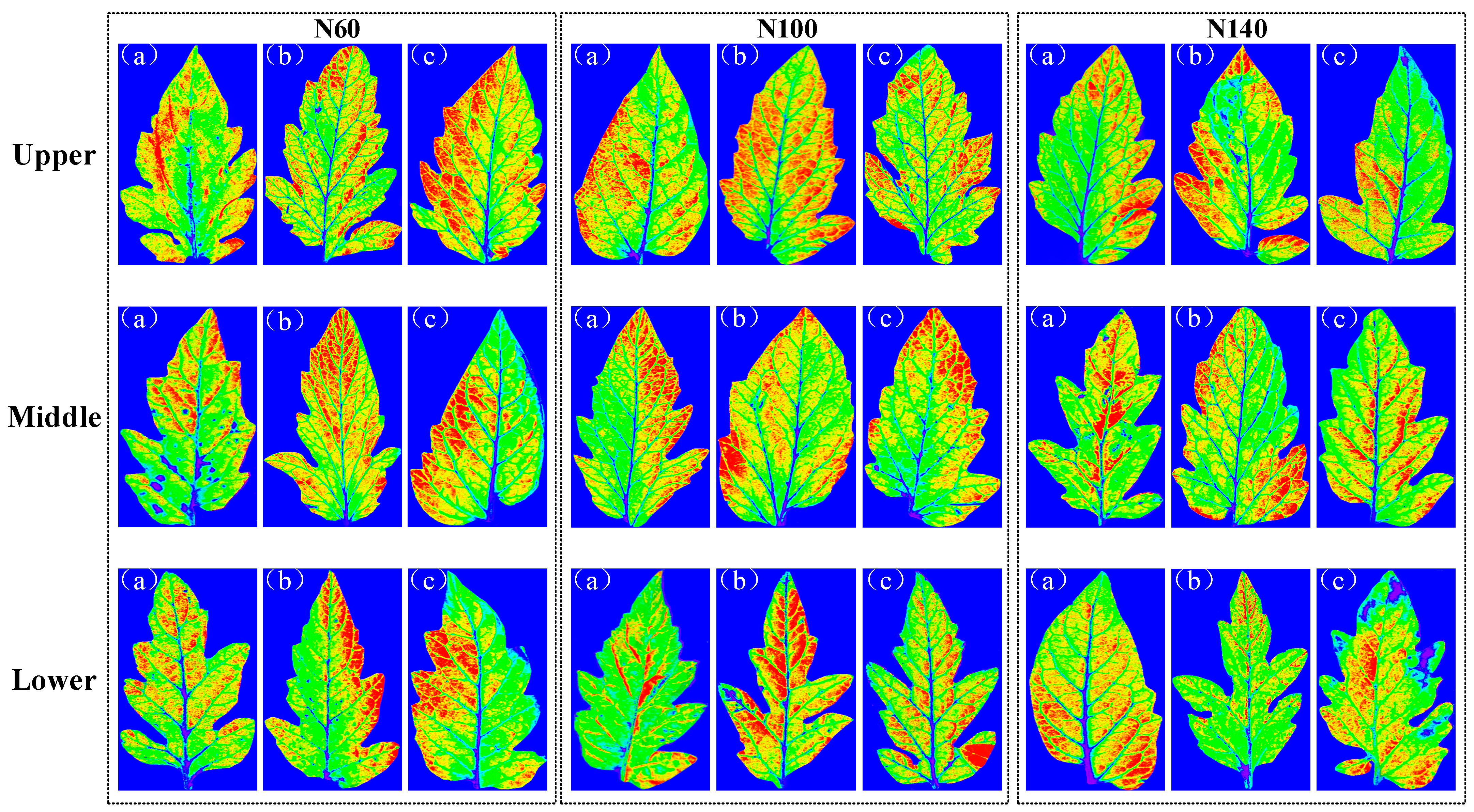

| Coarse | Fine | Optimization | NV 1 | Calibration | Prediction | ||||
|---|---|---|---|---|---|---|---|---|---|
| RC | RMSEC | RPDC | RP | RMSEP | RPDP | ||||
| iRF | - | - | 231 | 0.7434 | 0.7383 | 1.4951 | 0.8153 | 0.6481 | 1.7274 |
| CARS | - | 42 | 0.7637 | 0.7041 | 1.5490 | 0.8409 | 0.6367 | 1.8478 | |
| IRIV | 28 | 0.7983 | 0.6794 | 1.6604 | 0.8676 | 0.6287 | 2.0110 | ||
| GA | 41 | 0.7767 | 0.6953 | 1.5876 | 0.8433 | 0.6323 | 1.8607 | ||
| BOSS | - | 36 | 0.7541 | 0.7245 | 1.5226 | 0.8385 | 0.6358 | 1.8352 | |
| IRIV | 30 | 0.7763 | 0.7197 | 1.5864 | 0.8467 | 0.6326 | 1.8794 | ||
| GA | 34 | 0.7688 | 0.7114 | 1.5637 | 0.8304 | 0.6289 | 1.7948 | ||
| VCPA | - | 12 | 0.8351 | 0.6430 | 1.8179 | 0.8177 | 0.6718 | 1.7372 | |
| IRIV | 11 | 0.8639 | 0.6237 | 1.9855 | 0.8558 | 0.6281 | 1.9331 | ||
| GA | 11 | 0.8498 | 0.6302 | 1.8972 | 0.8419 | 0.6349 | 1.8531 | ||
| iVISSA | - | - | 451 | 0.7042 | 0.7879 | 1.4084 | 0.7464 | 0.7304 | 1.5026 |
| CARS | - | 66 | 0.7117 | 0.7770 | 1.4235 | 0.7697 | 0.7109 | 1.5664 | |
| IRIV | 47 | 0.7485 | 0.7346 | 1.5080 | 0.7882 | 0.6738 | 1.6249 | ||
| GA | 55 | 0.7103 | 0.8085 | 1.4207 | 0.7727 | 0.6572 | 1.5754 | ||
| BOSS | - | 38 | 0.7441 | 0.7374 | 1.4969 | 0.7534 | 0.7258 | 1.5208 | |
| IRIV | 31 | 0.7546 | 0.7224 | 1.5240 | 0.7967 | 0.6647 | 1.6546 | ||
| GA | 30 | 0.7451 | 0.7302 | 1.4994 | 0.7669 | 0.7198 | 1.5582 | ||
| VCPA | - | 12 | 0.8277 | 0.6614 | 1.7820 | 0.7651 | 0.7215 | 1.5530 | |
| IRIV | 10 | 0.8363 | 0.6573 | 1.8239 | 0.7798 | 0.7018 | 1.5974 | ||
| GA | 9 | 0.8316 | 0.6501 | 1.8006 | 0.7704 | 0.7164 | 1.5685 | ||
| Coarse | Fine | Optimization | NV | Calibration | Prediction | ||||
|---|---|---|---|---|---|---|---|---|---|
| RC | RMSEC | RPDC | RP | RMSEP | RPDP | ||||
| iRF | - | - | 280 | 0.8028 | 0.1117 | 1.6772 | 0.8430 | 0.0820 | 1.8590 |
| CARS | - | 34 | 0.8188 | 0.1102 | 1.7419 | 0.8515 | 0.0794 | 1.9071 | |
| IRIVs | 27 | 0.8391 | 0.1016 | 1.8383 | 0.8665 | 0.0705 | 2.0033 | ||
| GA | 29 | 0.8302 | 0.1053 | 1.7938 | 0.8598 | 0.0765 | 1.9584 | ||
| BOSS | - | 27 | 0.8381 | 0.1029 | 1.8331 | 0.8456 | 0.0808 | 1.8733 | |
| IRIVs | 21 | 0.8577 | 0.0927 | 1.9450 | 0.8723 | 0.0645 | 2.0451 | ||
| GA | 24 | 0.8452 | 0.0993 | 1.8711 | 0.8597 | 0.0765 | 1.9577 | ||
| VCPA | - | 14 | 0.8036 | 0.1112 | 1.6802 | 0.8452 | 0.0812 | 1.8711 | |
| IRIVs | 12 | 0.8104 | 0.1106 | 1.7068 | 0.8483 | 0.0798 | 1.8885 | ||
| GA | 14 | 0.8036 | 0.1112 | 1.6802 | 0.8452 | 0.0812 | 1.8711 | ||
| iVISSA | - | - | 475 | 0.7949 | 0.1132 | 1.6482 | 0.8419 | 0.0846 | 1.8531 |
| CARS | - | 49 | 0.8114 | 0.1109 | 1.7109 | 0.8429 | 0.0843 | 1.8585 | |
| IRIVs | 36 | 0.8244 | 0.1062 | 1.7668 | 0.8500 | 0.0805 | 1.8983 | ||
| GA | 46 | 0.8186 | 0.1102 | 1.7411 | 0.8505 | 0.0803 | 1.9012 | ||
| BOSS | - | 36 | 0.8325 | 0.1047 | 1.8050 | 0.8458 | 0.0800 | 1.8744 | |
| IRIVs | 32 | 0.8412 | 0.1004 | 1.8494 | 0.8543 | 0.0767 | 1.9239 | ||
| GA | 34 | 0.8411 | 0.1004 | 1.8488 | 0.8484 | 0.0798 | 1.8891 | ||
| VCPA | - | 12 | 0.8054 | 0.1110 | 1.6871 | 0.8472 | 0.0806 | 1.8937 | |
| IRIVs | 10 | 0.8183 | 0.1105 | 1.7398 | 0.8504 | 0.0802 | 1.9006 | ||
| GA | 11 | 0.8137 | 0.1075 | 1.7203 | 0.8499 | 0.8005 | 1.8977 | ||
| Fertilizer | N20 | N40 | N60 | N80 | N100 | N120 | N140 | N160 | N180 | N200 |
|---|---|---|---|---|---|---|---|---|---|---|
| Calcium nitrate | 0 | 307.48 | 605.68 | 913.15 | 1216 | 1216 | 1216 | 1216 | 1216 | 1216 |
| Chelated calcium fertilizer | 491.57 | 367.27 | 246.72 | 122.43 | 0 | 0 | 0 | 0 | 0 | 0 |
| Urea | 0 | 0 | 0 | 0 | 0 | 131.67 | 262.34 | 395.01 | 526.68 | 658.35 |
| Calcium ammonium nitrate | 42.1 | 42.1 | 42.1 | 42.1 | 42.1 | 42.1 | 42.1 | 42.1 | 42.1 | 42.1 |
| Potassium nitrate | 395 | 395 | 395 | 395 | 395 | 395 | 395 | 395 | 395 | 395 |
| Potassium dihydrogen phosphate | 208 | 208 | 208 | 208 | 208 | 208 | 208 | 208 | 208 | 208 |
| Potassium sulfate | 393 | 393 | 393 | 393 | 393 | 393 | 393 | 393 | 393 | 393 |
| Magnesium sulfate | 466 | 466 | 466 | 466 | 466 | 466 | 466 | 466 | 466 | 466 |
| Total nitrogen concentration | 59.64 | 121.14 | 181.7 | 242.27 | 302.84 | 363.41 | 423.98 | 484.54 | 545.54 | 605.68 |
| EC | 2.49 | 2.42 | 2.40 | 2.30 | 2.29 | 2.33 | 2.43 | 2.50 | 2.53 | 2.56 |
| pH | 6.88 | 6.84 | 6.80 | 6.95 | 6.99 | 7.05 | 7.00 | 6.95 | 6.96 | 6.98 |
| Target | Dataset | Number of Sample | Maximum | Minimum | Mean | Standard Deviation |
|---|---|---|---|---|---|---|
| Nitrogen | Calibration set | 551 | 7.6357 | 0.7374 | 4.1669 | 1.0721 |
| Prediction set | 184 | 6.4984 | 2.0761 | 4.2257 | 0.9627 | |
| Chlorophyll | Calibration set | 551 | 3.7026 | 0.2614 | 1.6102 | 0.6099 |
| Prediction set | 184 | 3.5208 | 0.6898 | 1.6795 | 0.5228 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Gao, A.; Wang, B.; Wen, J.; Duan, Y.; Wang, G.; Li, Z. Modeling and Visualization of Nitrogen and Chlorophyll in Greenhouse Solanum lycopersicum L. Leaves with Hyperspectral Imaging for Nitrogen Stress Diagnosis. Plants 2025, 14, 3276. https://doi.org/10.3390/plants14213276
Zhao J, Gao A, Wang B, Wen J, Duan Y, Wang G, Li Z. Modeling and Visualization of Nitrogen and Chlorophyll in Greenhouse Solanum lycopersicum L. Leaves with Hyperspectral Imaging for Nitrogen Stress Diagnosis. Plants. 2025; 14(21):3276. https://doi.org/10.3390/plants14213276
Chicago/Turabian StyleZhao, Jiangui, Anqi Gao, Boya Wang, Jiayi Wen, Yu Duan, Guoliang Wang, and Zhiwei Li. 2025. "Modeling and Visualization of Nitrogen and Chlorophyll in Greenhouse Solanum lycopersicum L. Leaves with Hyperspectral Imaging for Nitrogen Stress Diagnosis" Plants 14, no. 21: 3276. https://doi.org/10.3390/plants14213276
APA StyleZhao, J., Gao, A., Wang, B., Wen, J., Duan, Y., Wang, G., & Li, Z. (2025). Modeling and Visualization of Nitrogen and Chlorophyll in Greenhouse Solanum lycopersicum L. Leaves with Hyperspectral Imaging for Nitrogen Stress Diagnosis. Plants, 14(21), 3276. https://doi.org/10.3390/plants14213276






