Phytohormone Response to Exogenous Nitric Oxide in Cucumber Under Low-Temperature Stress
Abstract
1. Introduction
2. Results
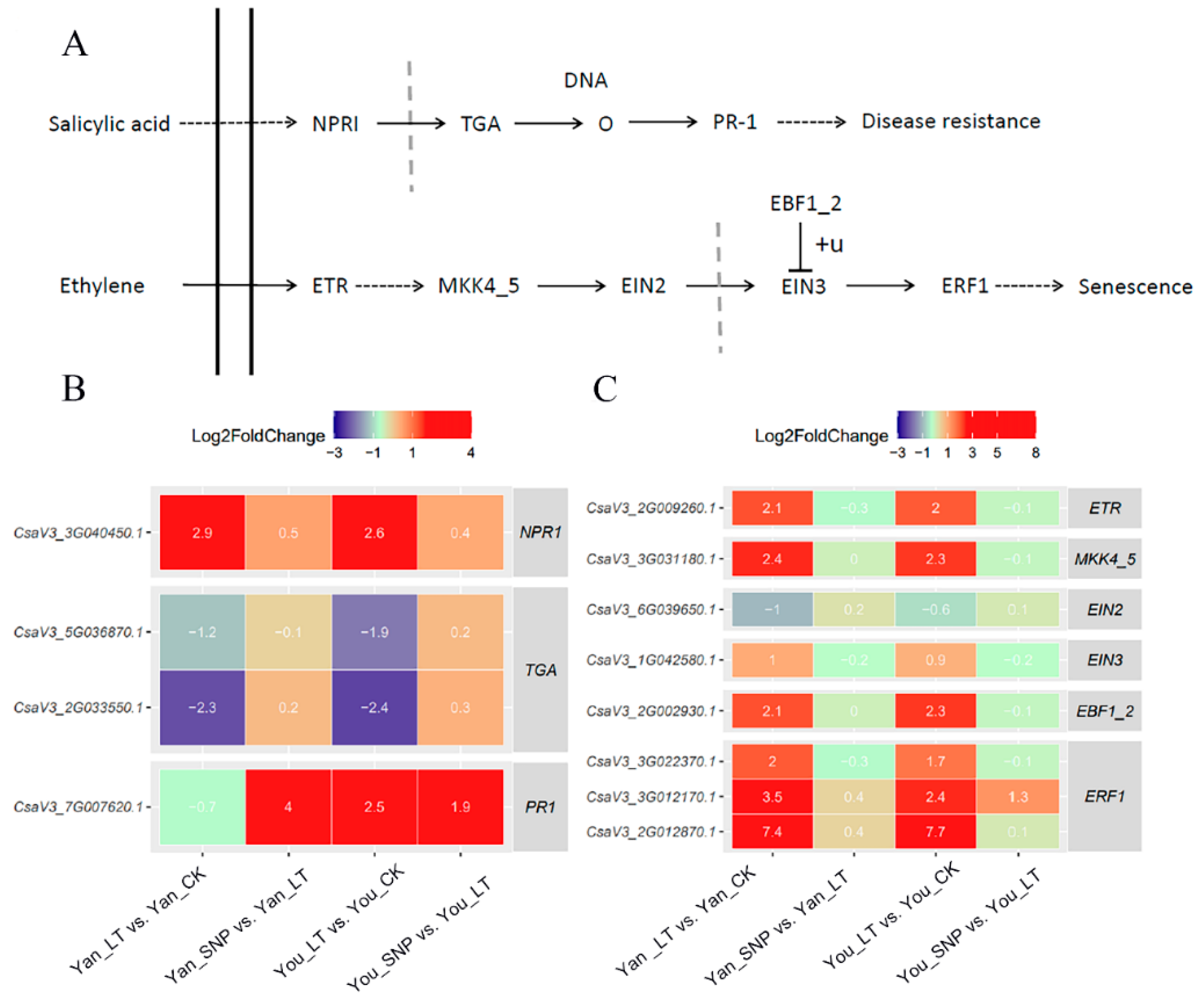
2.1. Expression Patterns of DEGs Enriched in SA Signal Transduction
2.2. Expression Patterns of DEGs Enriched in ETH Signal Transduction
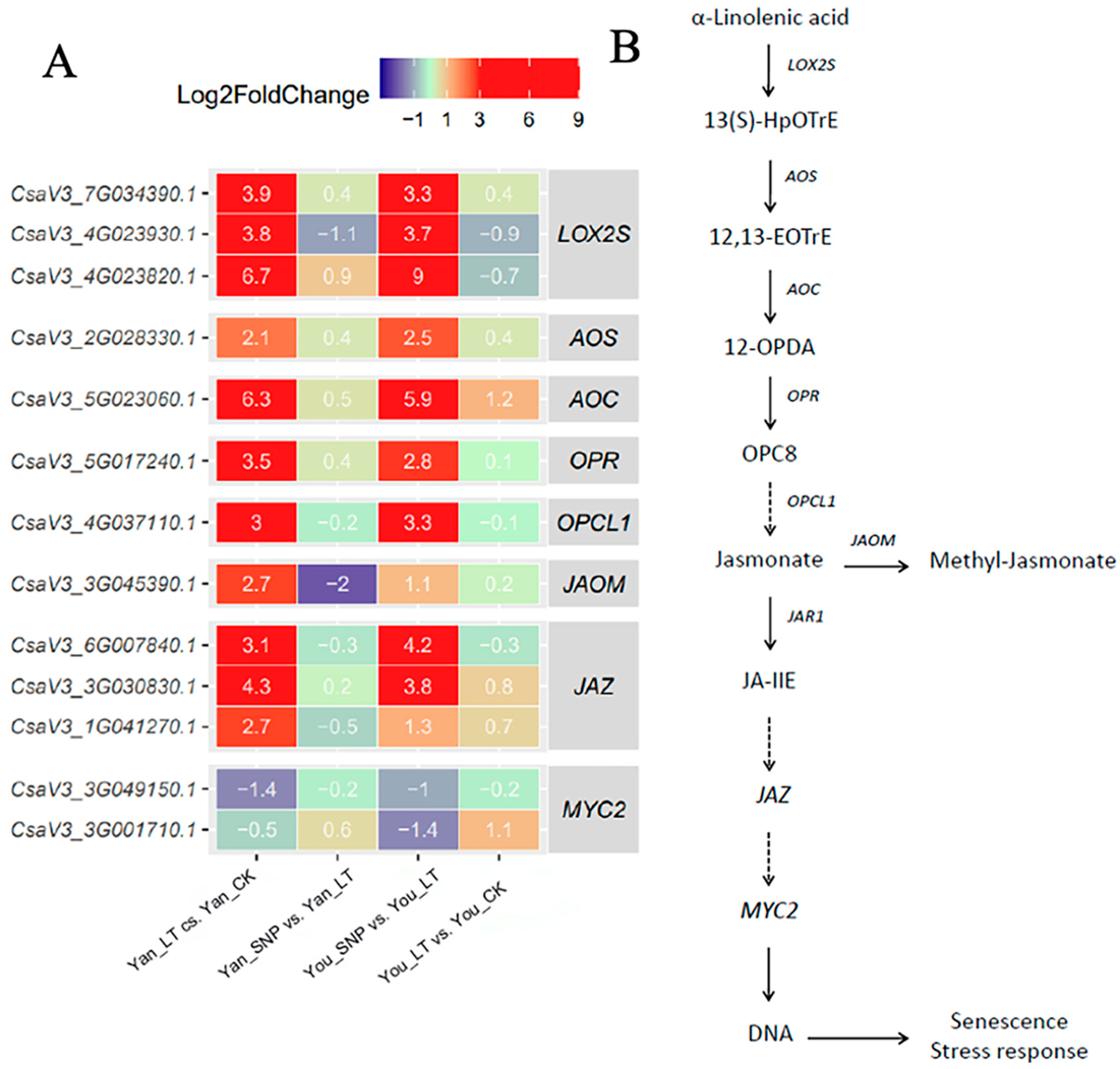
2.3. Expression Patterns of DEGs Enriched in JA and Methyl Jasmonate (MeJA) Synthesis, and the Signal Transduction Pathways of JA
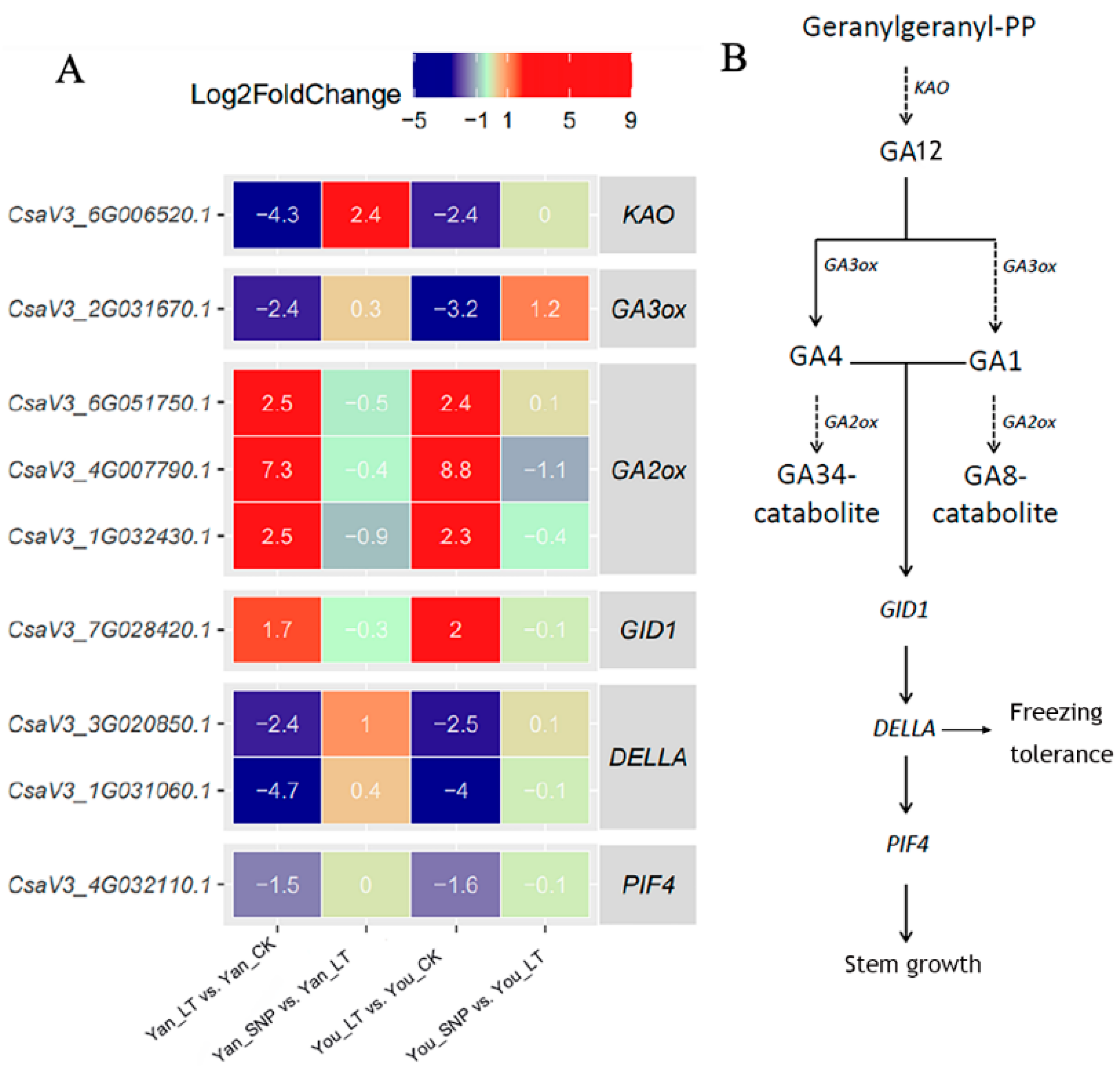
2.4. Expression Patterns of DEGs Enriched in Gibberellin (GA) Biosynthesis and Signal Transduction
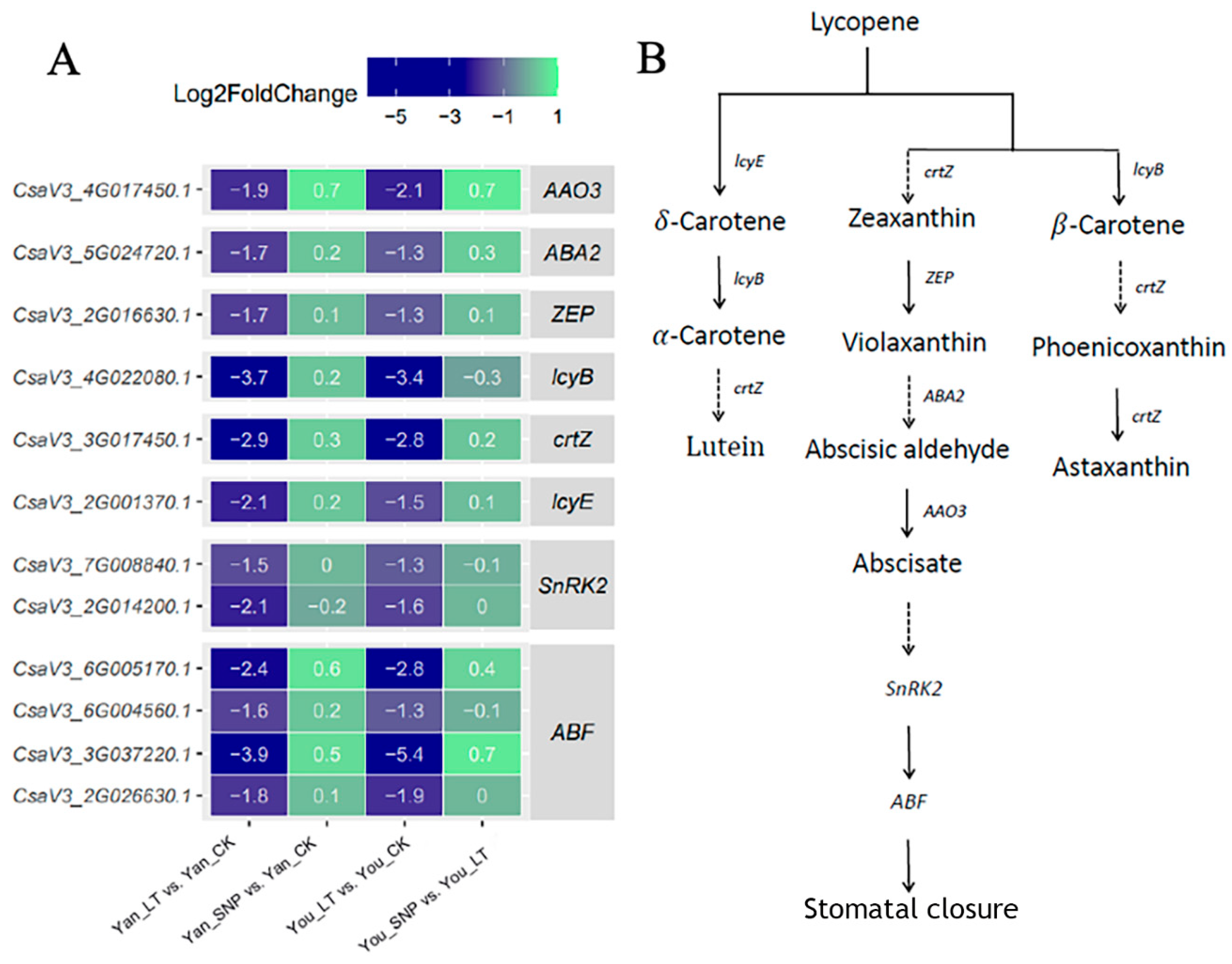
2.5. Expression Patterns of DEGs Enriched in ABA Biosynthesis and Signal Transduction Pathway
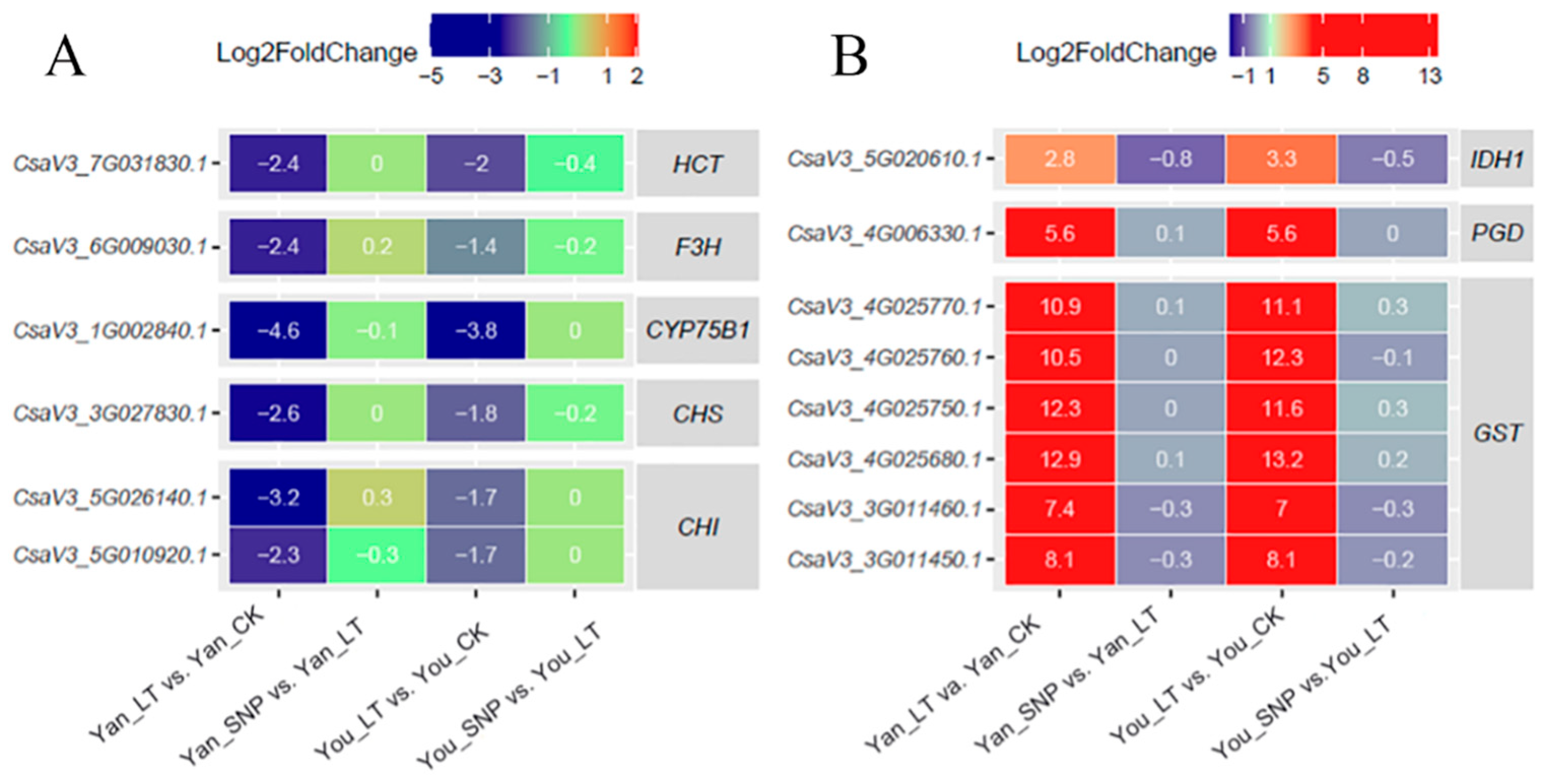
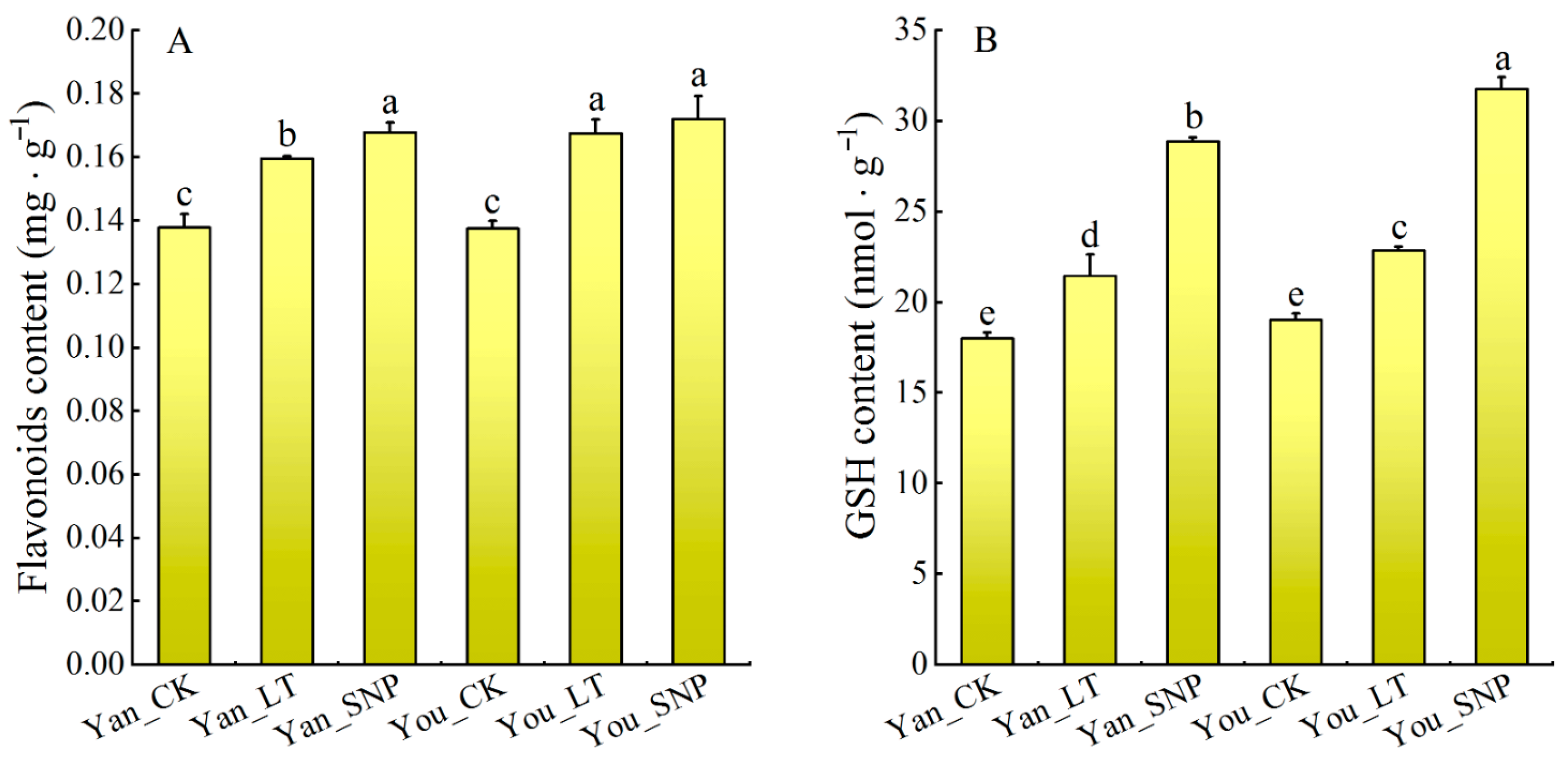
2.6. Expression Patterns of DEGs Were Involved in Flavonoid, and Glutathione (GSH) Metabolism Pathways
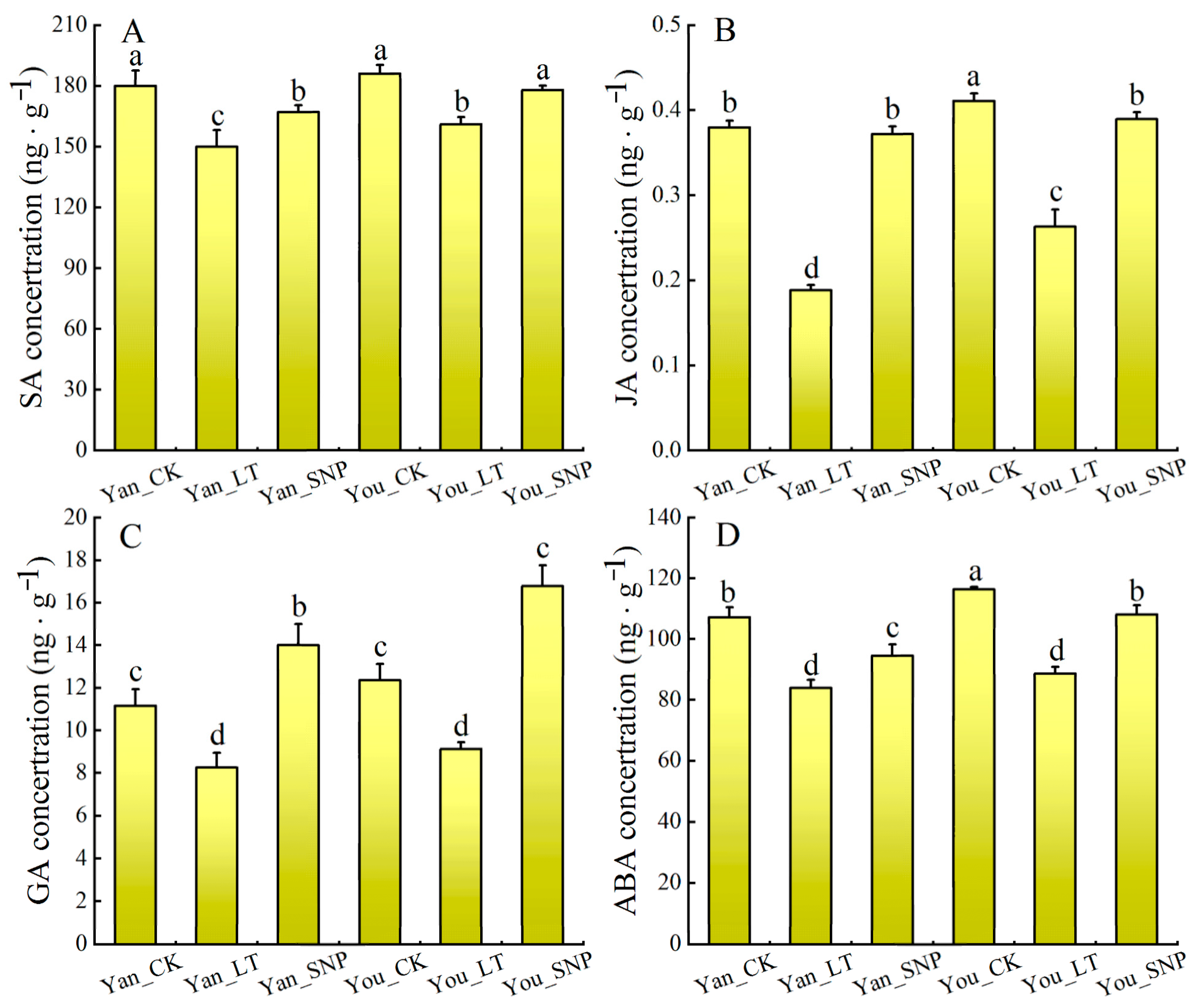
2.7. The Effect of Exogenous SNP on Phytohormone Content in Cucumber Leaves Under Low Temperature Stress
2.8. The Effect of Exogenous NO on Antioxidant Substances and Linoleic Acid Content in Cucumber Leaves Under Low Temperature Stress
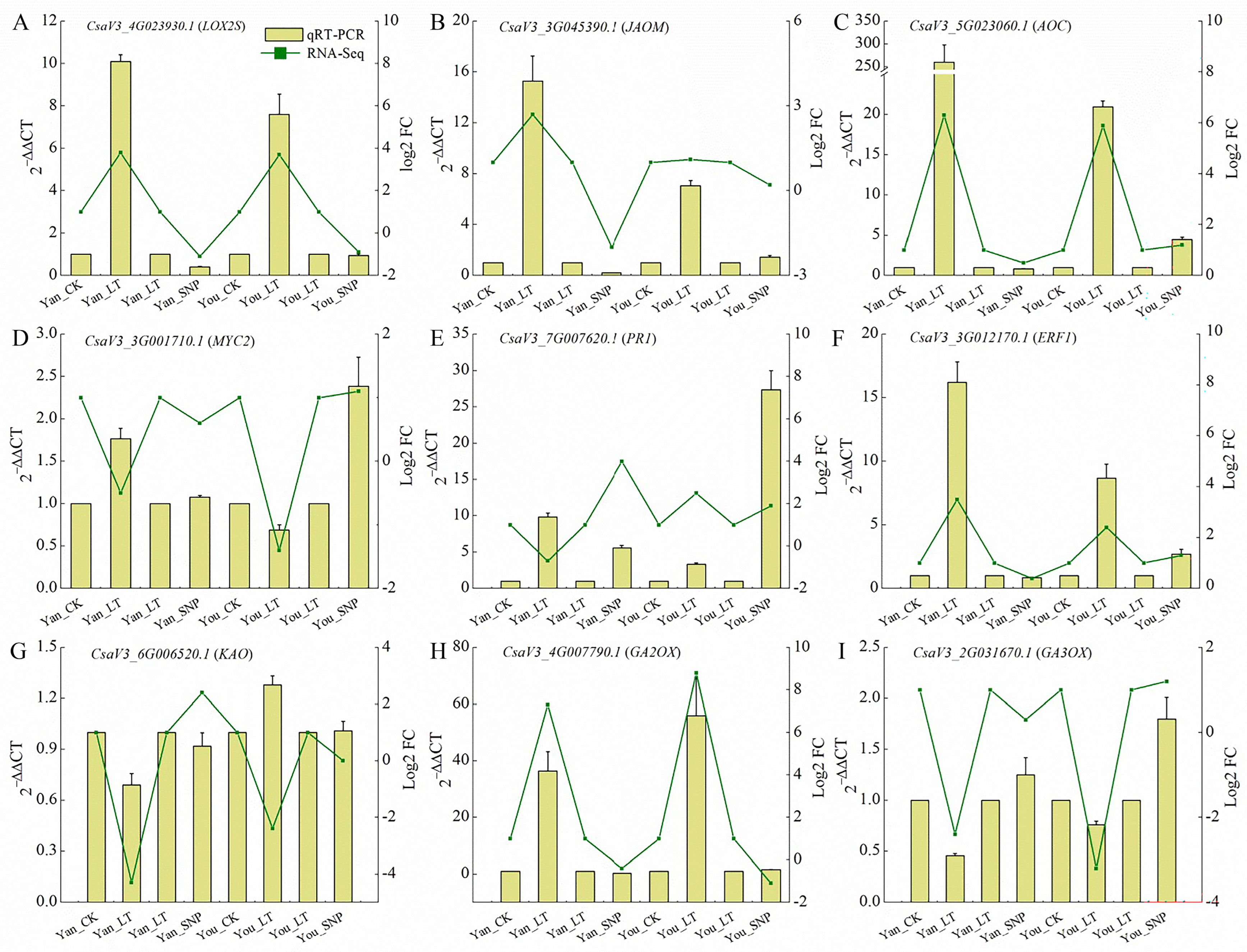
2.9. Validation of Transcriptomic Data Accuracy by qRT-PCR
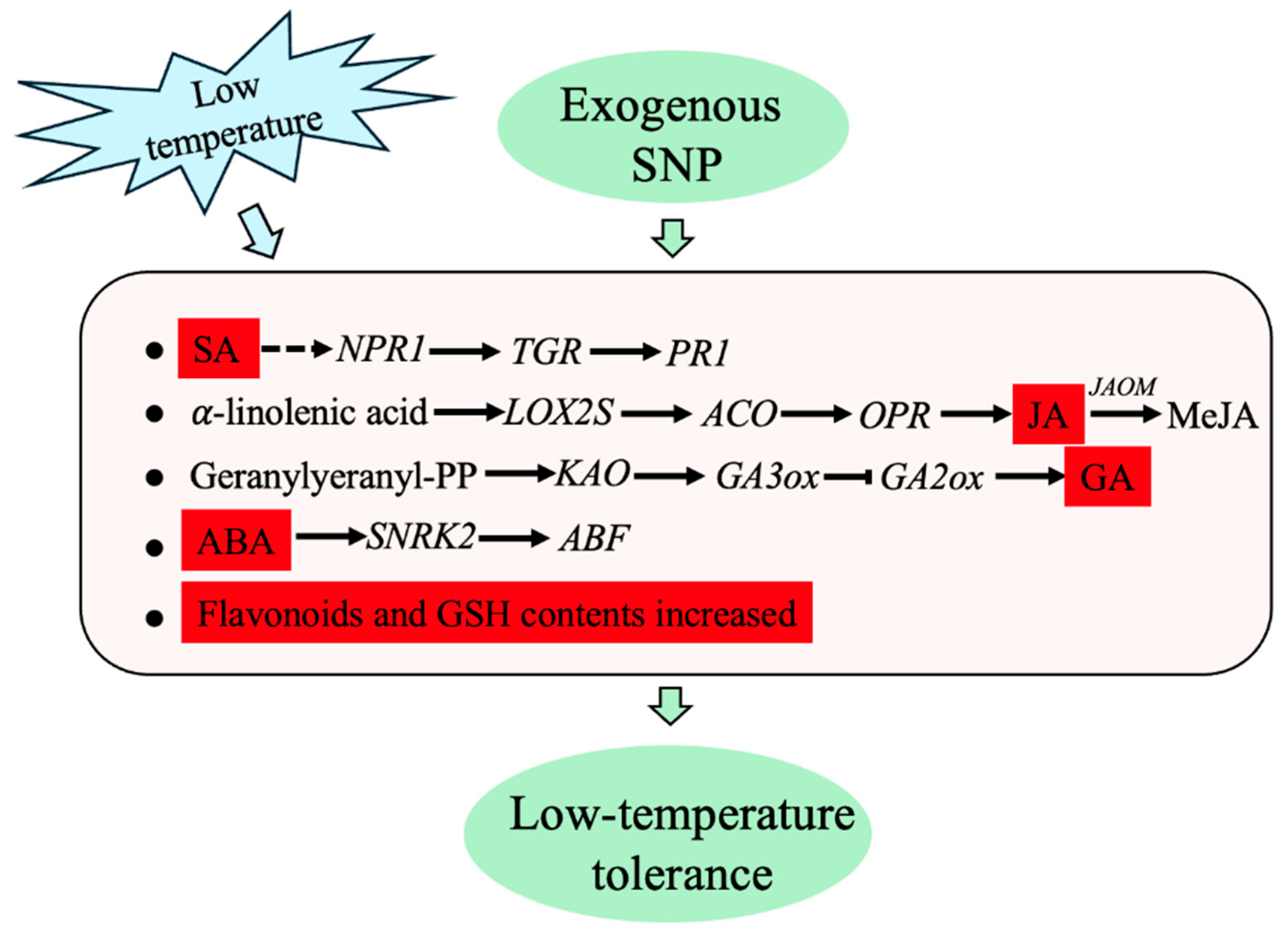
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Low Temperature Treatment
4.2. RNA Extraction and Strand-Specific Library Construction
4.3. Transcriptome Sequencing and Quality Control
4.4. DEG Identification and Enrichment Analysis
4.5. The Contents of Endogenous Hormones, Glutathione, Flavonoids, and Linoleic Acid Assays
4.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vojnovic, D.; Maksimovic, I.; Koprivica, G.; Horecki, A.T.; Milic, A.; Adamovic, B.; Sumic, Z.; Ilin, Z. Optimizing Greenhouse Cucumber Fertigation Through Grafting: Improving Yield, Bioactive Compounds, and Antioxidant Activity. Horticulturae 2024, 10, 1135. [Google Scholar] [CrossRef]
- Lay-Walters, A.M.; Dickson, R.W.; McWhirt, A.L. Short Report: Temperature Effects on Plant Development Rate and Quality of Compact Container-grown Cucumber and Hot Pepper. Hotttechnology 2025, 35, 671–673. [Google Scholar] [CrossRef]
- Kordi, S.; Ghanbari, F. Hardening Pretreatment by Drought and Low Temperature Enhanced Chilling Stress Tolerance of Cucumber Seedlings. Acta Sci. Pol. Hortorum Cultus 2019, 18, 29–37. [Google Scholar] [CrossRef]
- Miao, M.; Zhang, Z.; Xu, X.; Wang, K.; Cheng, H.; Cao, B. Different Mechanisms to Obtain Higher Fruit Growth Rate in Two Cold-Tolerant Cucumber (Cucumis sativus L.) Lines under Low Night Temperature. Sci. Hortic. 2009, 119, 357–361. [Google Scholar] [CrossRef]
- Xia, X.J.; Wang, Y.J.; Zhou, Y.H.; Tao, Y.; Mao, W.H.; Shi, K.; Asami, T.; Chen, Z.; Yu, J.Q. Reactive Oxygen Species Are Involved in Brassinosteroid-Induced Stress Tolerance in Cucumber. Plant Physiol. 2009, 150, 801–814. [Google Scholar] [CrossRef]
- Wu, P.; Xiao, C.; Cui, J.; Hao, B.; Zhang, W.; Yang, Z.; Ahammed, G.J.; Liu, H.; Cui, H. Nitric Oxide and Its Interaction with Hydrogen Peroxide Enhance Plant Tolerance to Low Temperatures by Improving the Efficiency of the Calvin Cycle and the Ascorbate–Glutathione Cycle in Cucumber Seedlings. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, P.; Zhang, W.; Yang, Z.; Liu, H.; Ahammed, G.J.; Cui, J. Calcium is Involved in Exogenous NO-Induced Enhancement of Photosynthesis in Cucumber (Cucumis sativus L.) Seedlings under Low Temperature. Sci. Hortic. 2020, 261, 108953. [Google Scholar] [CrossRef]
- Ma, C.; Pei, Z.; Zhu, Q.; Chai, C.; Xu, T.; Dong, C.; Wang, J.; Zheng, S.; Zhang, T. Melatonin-Mediated Low-Temperature Tolerance of Cucumber Seedlings Requires Ca2+/CPKs2+/CPKs signaling pathway. Plant Physiol. Biochem. 2024, 214, 108962. [Google Scholar] [CrossRef] [PubMed]
- Talanova, V.V.; Titov, A.F.; Topchieva, L.V.; Malysheva, I.E. Effect of Stress Factors on Expression of the Gene Encoding a CBF Transcription Factor in Cucumber Plants. Dokl. Biol. Sci. 2008, 423, 419–421. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, X.; Han, J.; Lu, W.; Ren, Z. Genome-Wide Analysis of the WRKY Gene Family in the Cucumber Genome and Transcriptome-Wide Identification of WRKY Transcription Factors that Respond to Biotic and Abiotic Stresses. BMC Plant Biol. 2020, 20, 443. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Yang, X.; Li, Q.; Ling, J.; Wang, H.; Gu, X.; Huang, S.; Jiang, W. CsWRKY46, a WRKY Transcription Factor from Cucumber, Confers Cold Resistance in Transgenic-Plant by Regulating a set of Cold-Stress Responsive Genes in an ABA-Dependent Manner. Plant Physiol. Biochem. PPB 2016, 108, 478–487. [Google Scholar] [CrossRef]
- Fan, J.; Chen, K.; Amombo, E.; Hu, Z.; Chen, L.; Fu, J. Physiological and Molecular Mechanism of Nitric Oxide (NO) Involved in Bermudagrass Response to Cold Stress. PLoS ONE 2015, 10, e0132991. [Google Scholar] [CrossRef]
- Anwar, A.; Bai, L.; Miao, L.; Liu, Y.; Li, S.; Yu, X.; Li, Y. 24-Epibrassinolide Ameliorates Endogenous Hormone Levels to Enhance Low-Temperature Stress Tolerance in Cucumber Seedlings. Int. J. Mol. Sci. 2018, 19, 2497. [Google Scholar] [CrossRef]
- Editorial: Nitric oxide in plants. Nitric Oxide 2017, 68, 1–4. [CrossRef]
- Fancy, N.N.; Bahlmann, A.K.; Loake, G.J. Nitric Oxide Function in Plant Abiotic Stress. Plant Cell Environ. 2016, 40, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Kong, Q.; Bian, J.; Ahammed, G.J.; Cui, H.; Xu, W.; Yang, Z.; Cui, J.; Liu, H. Unveiling molecular Mechanisms of Nitric Oxide-Induced Low-Temperature Tolerance in Cucumber by Transcriptome Profiling. Int. J. Mol. Sci. 2022, 23, 5615. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jing, H.K.; Feng, Z.H.; Huang, J.; Shen, R.F.; Zhu, X.F. Salicylic Acid Acts Upstream of Auxin and Nitric Oxide (NO) in Cell Wall Phosphorus Remobilization in Phosphorus Deficient Rice. Rice 2022, 15, 42. [Google Scholar] [CrossRef]
- Esim, N.; Atici, Ö. Effects of Exogenous Nitric Oxide and Salicylic Acid on Chilling-Induced Oxidative Stress in Wheat (Triticum aestivum). Front. Life Sci. 2015, 8, 124–130. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, Y.; Liu, J.; Zhang, H.; Chen, H. Heme Oxygenase-1 Delays Gibberellin-Induced Programmed Cell Death of Rice Aleurone Layers Subjected to Drought Stress by Interacting with Nitric Oxide. Front. Plant Sci. 2016, 6, 1267. [Google Scholar] [CrossRef]
- Zhao, H.; Yin, C.C.; Ma, B.; Chen, S.Y.; Zhang, J.S. Ethylene Signaling in Rice and Arabidopsis: New Regulators and Mechanisms. J. Integr. Plant Biol. 2021, 63, 102–125. [Google Scholar] [CrossRef]
- Olszewski, N.; Sun, T.; Gublerc, F. Gibberellin Signaling: Biosynthesis, Catabolism, and Response Pathways. Plant Cell 2002, 14, S61–S80. [Google Scholar] [CrossRef]
- Khan, T.A.; Fariduddin, Q.; Yusuf, M. Low-Temperature Stress: Is Pphytohormones Application a Remedy? Environ. Sci. Pollut. Res. 2017, 24, 21574–21590. [Google Scholar] [CrossRef] [PubMed]
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and Molecular Changes in Plants Grown at Low Temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef] [PubMed]
- Simontacchi, M.; Garcia-Mata, C.; Bartoli, C.G.; Santa-Maria, G.E.; Lamattina, L. Nitric Oxide as a Key Component in Hormone-Regulated Processes. Plant Cell Rep. 2013, 32, 853–866. [Google Scholar] [CrossRef]
- Neill, S.; Barros, R.; Bright, J.; Desikan, R.; Hancock, J.; Harrison, J.; Morris, P.; Ribeiro, D.; Wilson, I. Nitric Oxide, Stomatal Closure, and Abiotic Stress. J. Exp. Bot. 2008, 59, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, X.; Gao, C.; Wu, P.; Ahammed, G.J.; Liu, H.; Chen, S.; Cui, J. Glutathione is Required for Nitric Oxide-Induced Chilling Tolerance by Synergistically Regulating Antioxidant System, Polyamine Synthesis, and Mitochondrial Function in Cucumber (Cucumis sativus L.). Plant Physiol. Biochem. PPB 2024, 214, 108878. [Google Scholar] [CrossRef]
- Sánchez, A.G.; Andreu, L.S.; Manzano, S.G.; Buelga, C.S.; Lorenzo, O. Association between Flavonoids and Nitric Oxide in Salt Stress Responses of Plants. New Biotechnol. 2016, 33, 422. [Google Scholar] [CrossRef]
- Lu, X.; Yin, F.; Liu, C.; Liang, Y.; Song, M.; Shang, F.; Liu, Y.; Shuai, L. Nitric Oxide Alleviates Chilling Injury in Cucumber (Cucumis sativus L.) Fruit by Regulating Membrane Lipid and Energy Metabolism. Int. J. Food Prop. 2023, 26, 1047–1061. [Google Scholar] [CrossRef]
- Li, N.; Euring, D.; Cha, J.Y.; Lin, Z.; Lu, M.; Huang, L.j.; Kim, W.Y. Plant Hormone-Mediated Regulation of Heat Tolerance in Response to Global Climate Change. Front. Plant Sci. 2021, 11, 627969. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, J.; Zhang, J.; Ye, N.; Zhang, H.; Tan, M.; Jiang, M. Nitric Oxide Mediates Brassinosteroid-Induced ABA Biosynthesis Involved in Oxidative Stress Tolerance in Maize Leaves. Plant Cell Physiol. 2011, 52, 181–192. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Alsahli, A.A.; Alyemeni, M.N.; Ahmad, P. Influence of Exogenous Salicylic Acid and Nitric Oxide on Growth, Photosynthesis, and Ascorbate-Glutathione Cycle in Salt Stressed Vigna angularis. Biomolecules 2019, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Anwar, A.; Yan, Y.; Liu, Y.; Li, Y.; Yu, X. 5-Aminolevulinic Acid Improves Nutrient Uptake and Endogenous Hormone Accumulation, Enhancing Low-Temperature Stress Tolerance in Cucumbers. Int. J. Mol. Sci. 2018, 19, 3379. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.D.; Volko, S.M.; Ledford, H.; Ausubel, F.M.; Dong, X. Roles of Salicylic Acid, Jasmonic Acid, and Ethylene in cpr-Induced Resistance in Arabidopsis. Plant Cell 2000, 12, 2175–2190. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.R.; Chen, K.S.; Allan, A.C.; Wu, R.M.; Zhang, B.; Lallu, N.; Ferguson, I.B. Ethylene-Induced Modulation of Genes Associated with the ethylene signalling pathway in ripening kiwifruit. J. Exp. Bot. 2008, 59, 2097–2108. [Google Scholar] [CrossRef]
- Yin, X.R.; Allan, A.C.; Chen, K.S.; Ferguson, I.B. Kiwifruit EIL and ERF Genes Involved in Regulating Fruit Ripening. Plant Physiol. 2010, 153, 1280–1292. [Google Scholar] [CrossRef]
- Sin’kevich, M.S.; Naraikina, N.V.; Alieva, G.P.; Astakhova, N.V.; Trunova, T.I.; Moshkov, I.E. The Difference in Low-Temperature Tolerance of Arabidopsis thaliana Plants and Its Ethylene-Insensitive Mutants Is Related to Activities of Antioxidant Enzymes. Russ. J. Plant Physiol. 2020, 67, 1083–1093. [Google Scholar] [CrossRef]
- Sougrakpam, Y.; Babuta, P.; Deswal, R. Nitric oxide (NO) Modulates Low Temperature-Stress Signaling via S-nitrosation, a NO PTM, Inducing Ethylene Biosynthesis Inhibition Leading to Enhanced Post-Harvest Shelf-Life of Agricultural Produce. Physiol. Mol. Biol. Plants 2023, 29, 2051–2065. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The Role of Endogenous Nitric Oxide in Salicylic Acid-Induced up-Regulation of Ascorbate-Glutathione Cycle Involved in Salinity Tolerance of Pepper (Capsicum annuum L.) Plants. Plant Physiol. Biochem. PPB 2020, 147, 10–20. [Google Scholar] [CrossRef]
- Wasternack, C.; Parthier, B. Jasmonate-Signalled Plant Gene Expression. Trends Plant Sci. 1997, 2, 302–307. [Google Scholar] [CrossRef]
- Wang, J.W.; Wu, J.Y. Nitric Oxide is Involved in Methyl Jasmonate-induced Defense Responses and Secondary Metabolism Activities of Taxus Cells. Plant Cell Physiol. 2005, 46, 923–930. [Google Scholar] [CrossRef]
- Huang, X.; Stettmaier, K.; Michel, C.; Hutzler, P.; Mueller, M.J.; Durner, J. Nitric Oxide is Induced by Wounding And Influences Jasmonic Acid Signaling in Arabidopsis thaliana. Planta 2004, 218, 938–946. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Xiang, H.; Liu, Y.; Li, H.; Qi, M.; Li, T. Low Overnight Temperature-Induced Gibberellin Accumulation Increases Locule Number in Tomato. Int. J. Mol. Sci. 2019, 20, 3042. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, Y.; Hu, G.; Wang, X.; Chen, H.; Shi, Q.; Xiang, J.; Zhang, Y.; Zhu, D.; Zhang, Y. Reduced Bioactive Gibberellin Content in Rice Seeds Under Low Temperature Leads to Decreased Sugar Consumption and Low Seed Germination Rates. Plant Physiol. Biochem. 2018, 133, 1–10. [Google Scholar] [CrossRef]
- Nambara, E.; Marion-Poll, A. Abscisic Acid Biosynthesis and Catabolism. Annu. Rev. Plant Biol. 2005, 56, 165–185. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in Integrating Plant Responses to Drought and Salt Stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Li, P.; Dai, S.J.; Zhao, B.; Zhang, Y.S.; Liao, K.; Xu, F.; Du, C.L.; Leng, P. Effect of Low Temperatures on Pulp Browning and Endogenous Abscisic Acid and Ethylene Concentrations in Peach (Prunus persica L.) Fruit During Post-Harvest Storage. J. Hortic. Sci. Biotechnol. 2015, 89, 686–692. [Google Scholar] [CrossRef]
- Gao, T.M.; Katayama-Ikegami, A.; Matsuda, K.; Shindo, H.; Uemae, S.; Oyaizu, M. A Low Temperature Promotes Anthocyanin Biosynthesis But Does Not Accelerate Endogenous Abscisic Acid Accumulation in Red-Skinned Grapes. Plant Sci. 2019, 283, 165–176. [Google Scholar] [CrossRef]
- Mahajan, M.; Sahoo, R.N.; Khan, M.I.R. Lanthanum Supplementation with Abscisic Acid Modulates Rhizosphere Dynamics Through Changes in Nitric Oxide Synthesis in Wheat. Physiol. Plant 2025, 177, e70126. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An Ultra-Fast all-in-one FASTQ Preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Patro, R.; Duggal, G.; Love, M.I.; Irizarry, R.A.; Kingsford, C. Salmon Provides Fast and Bias-Aware Quantification of Transcript Expression. Nat. Methods 2017, 14, 417–419. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics-J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Nagalakshmi, N.; Prasad, M.N.V. Responses of glutathione cycle enzymes and glutathione metabolism to copper stress in Scenedesmus bijugatus. Plant Sci. 2001, 160, 291–299. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Yang, Z.; Kong, Q.; Cui, H.; Liu, Y.; Dong, R.; Zheng, C.; Liu, H.; Cui, J. Phytohormone Response to Exogenous Nitric Oxide in Cucumber Under Low-Temperature Stress. Plants 2025, 14, 3275. https://doi.org/10.3390/plants14213275
Wu P, Yang Z, Kong Q, Cui H, Liu Y, Dong R, Zheng C, Liu H, Cui J. Phytohormone Response to Exogenous Nitric Oxide in Cucumber Under Low-Temperature Stress. Plants. 2025; 14(21):3275. https://doi.org/10.3390/plants14213275
Chicago/Turabian StyleWu, Pei, Zhifeng Yang, Qiusheng Kong, Huimei Cui, Yumei Liu, Rongrong Dong, Caixia Zheng, Huiying Liu, and Jinxia Cui. 2025. "Phytohormone Response to Exogenous Nitric Oxide in Cucumber Under Low-Temperature Stress" Plants 14, no. 21: 3275. https://doi.org/10.3390/plants14213275
APA StyleWu, P., Yang, Z., Kong, Q., Cui, H., Liu, Y., Dong, R., Zheng, C., Liu, H., & Cui, J. (2025). Phytohormone Response to Exogenous Nitric Oxide in Cucumber Under Low-Temperature Stress. Plants, 14(21), 3275. https://doi.org/10.3390/plants14213275





