Optimization of Micropropagation and Metabolomic Analysis Under Different Light Qualities in Mussaenda pubescens Ait.f
Abstract
1. Introduction
2. Results
2.1. Establishment of Aseptic System and Optimization of Proliferation Medium
2.1.1. Establishment of an Aseptic Line
2.1.2. Optimization of Proliferation Medium: Impact of Hormonal Concentration Gradients on Mussaenda pubescens Shoot Multiplication
2.2. Effect of Hormone Concentration on In Vitro Rooting of Shoots and Subsequent Plantlet Growth in Mussaenda pubescens
2.3. Effect of Different Substrates on the Acclimatization of Mussaenda pubescens Plantlets
2.4. Differential Regulation of Morphogenesis in Mussaenda pubescens Micropropagated Shoots by Light Quality
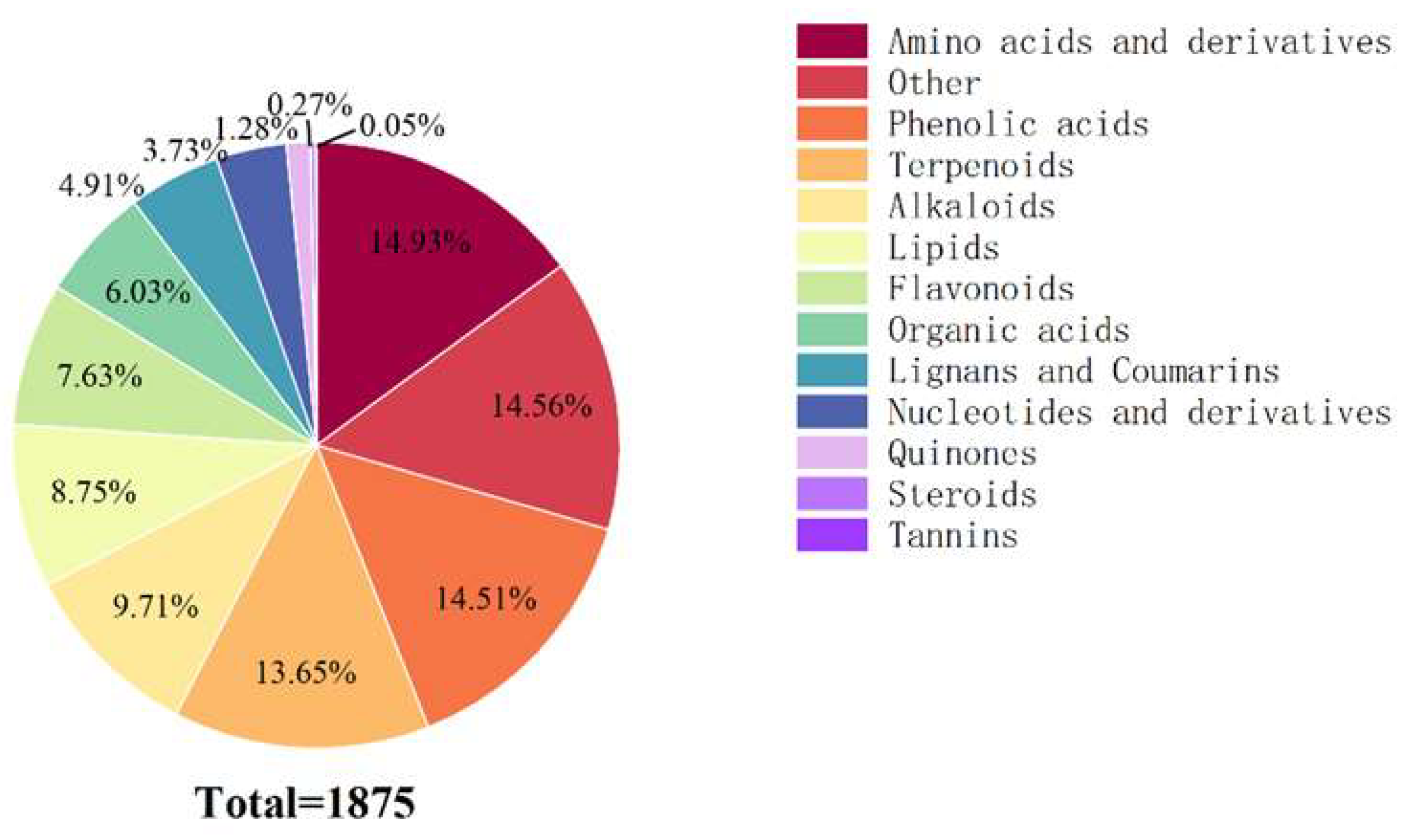
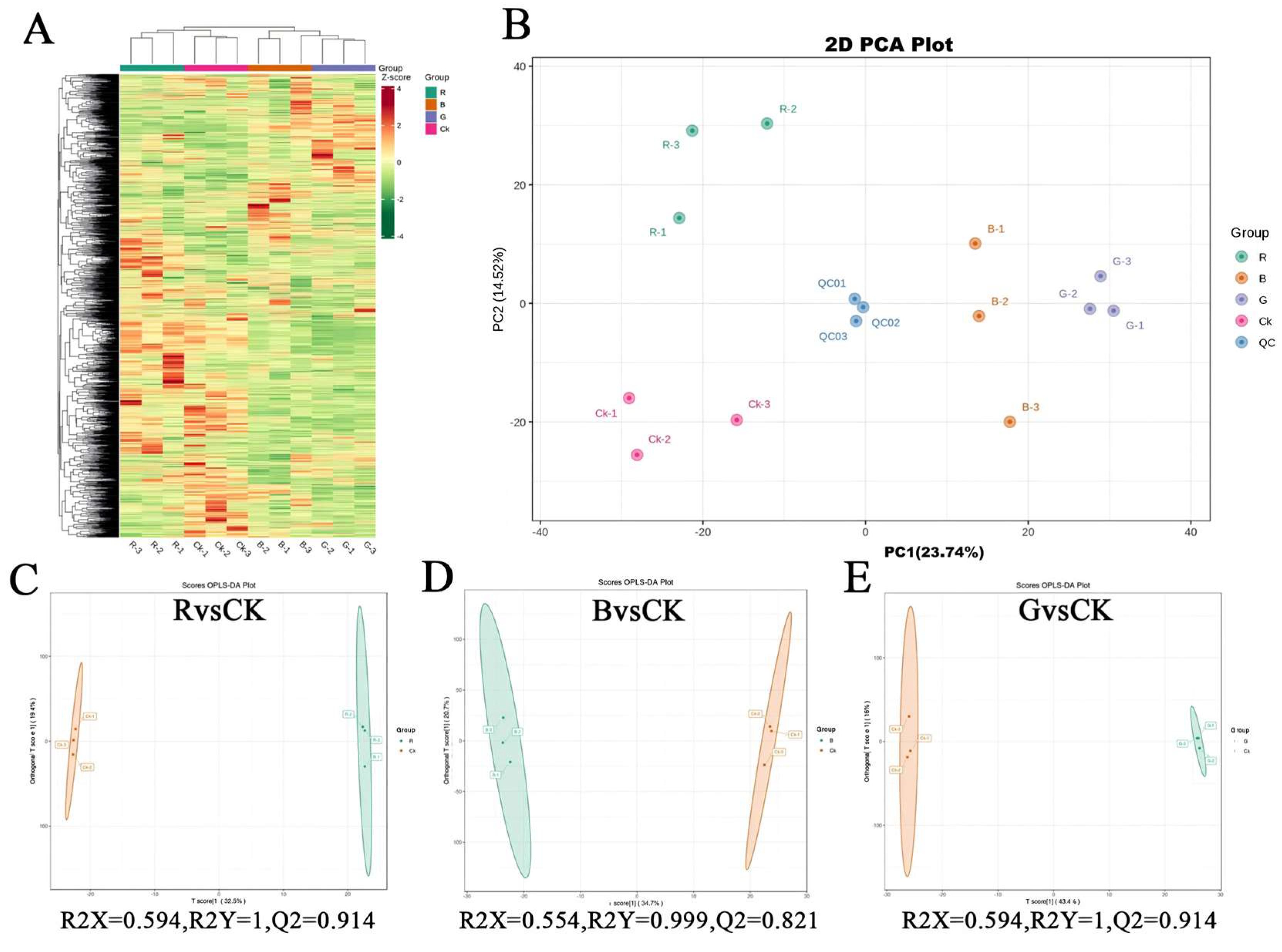
2.5. Foliar Metabolic Profiling of Mussaenda pubescens Micropropagated Shoots Under Different Light Qualities
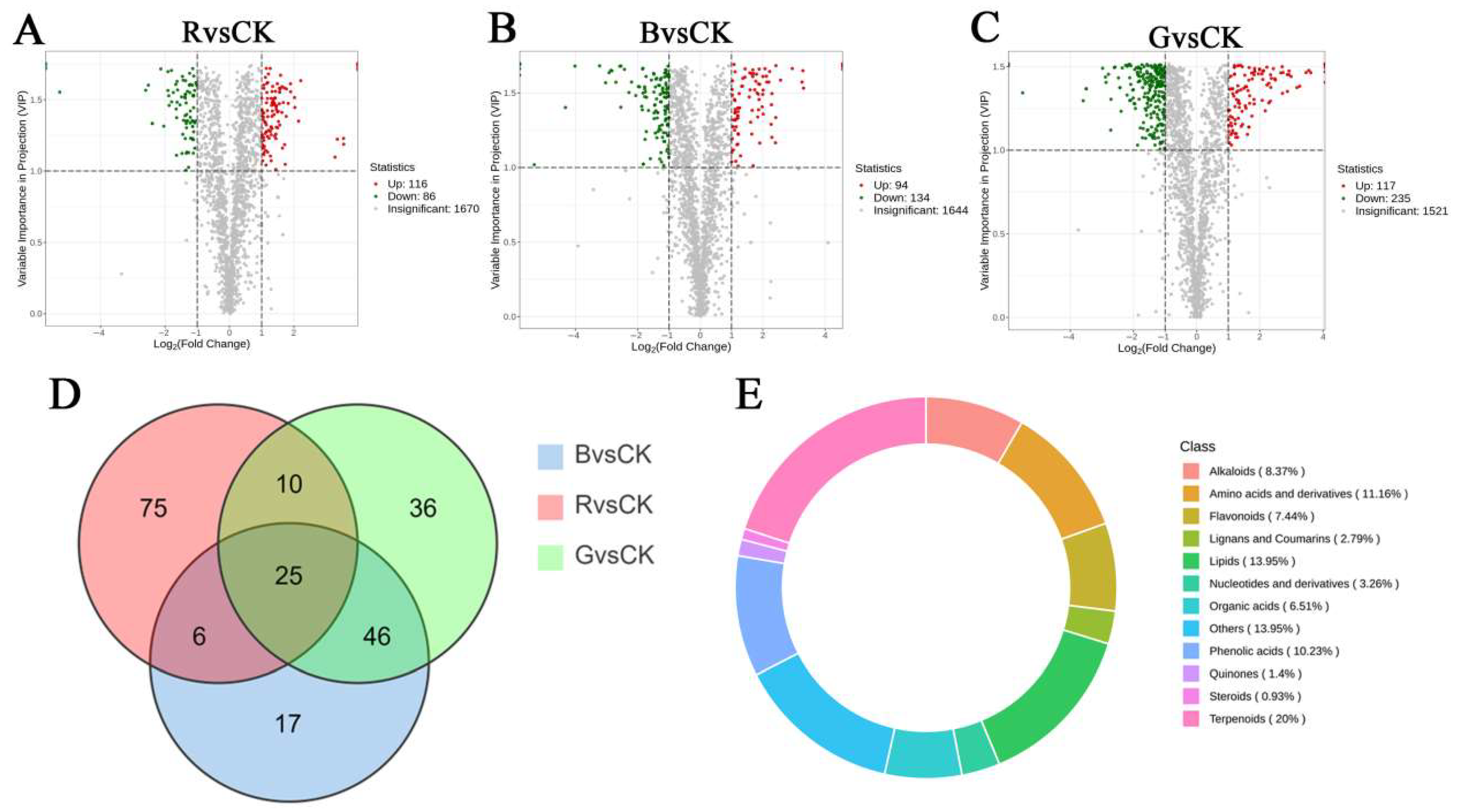
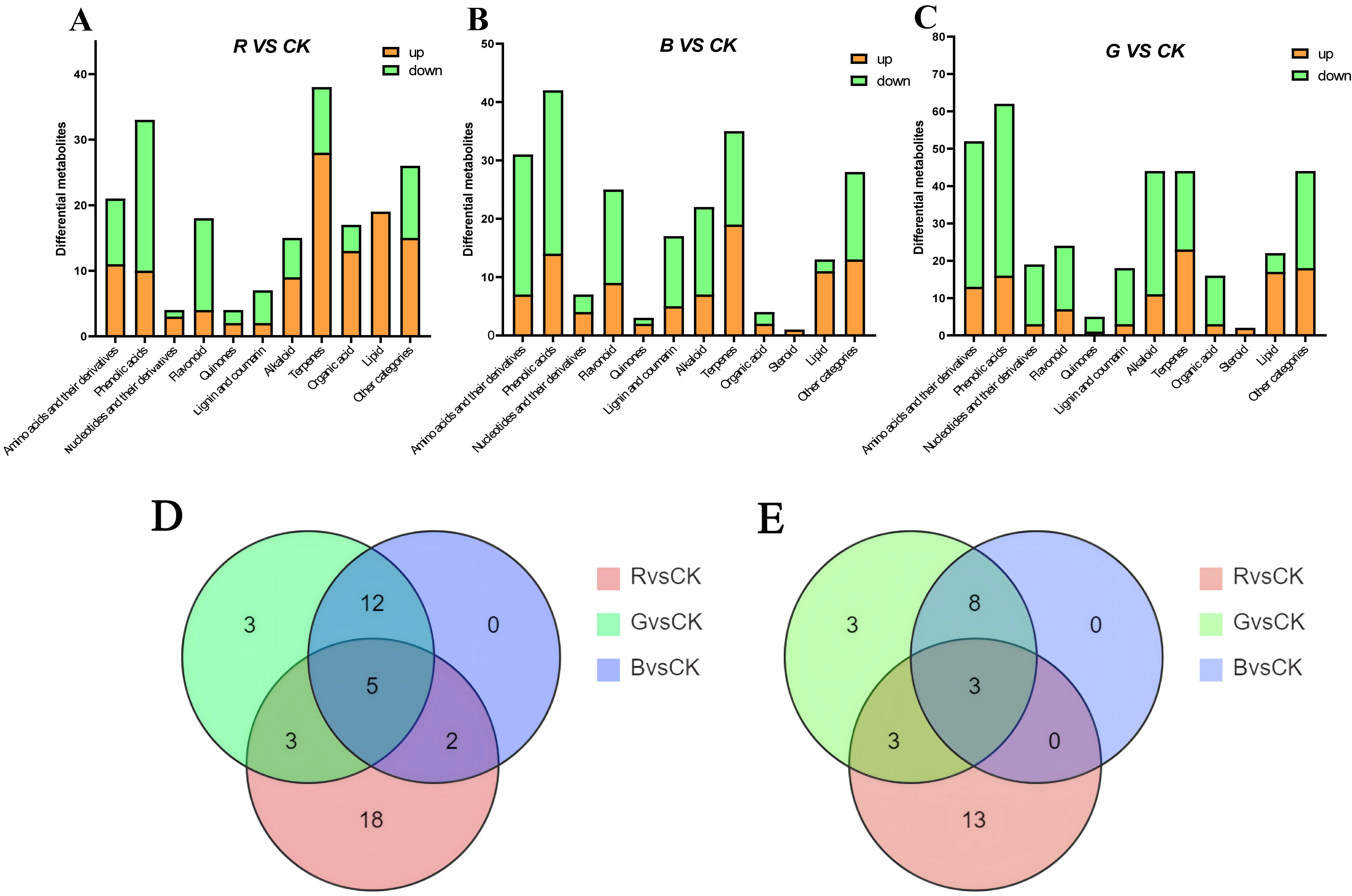
2.6. Identification of Differential Metabolites in Leaves of Mussaenda pubescens Micropropagated Shoots Under Different Light Qualities
2.7. Analysis of Key Differential Metabolites in Leaves of Mussaenda pubescens Micropropagated Shoots Under Different Light Qualities
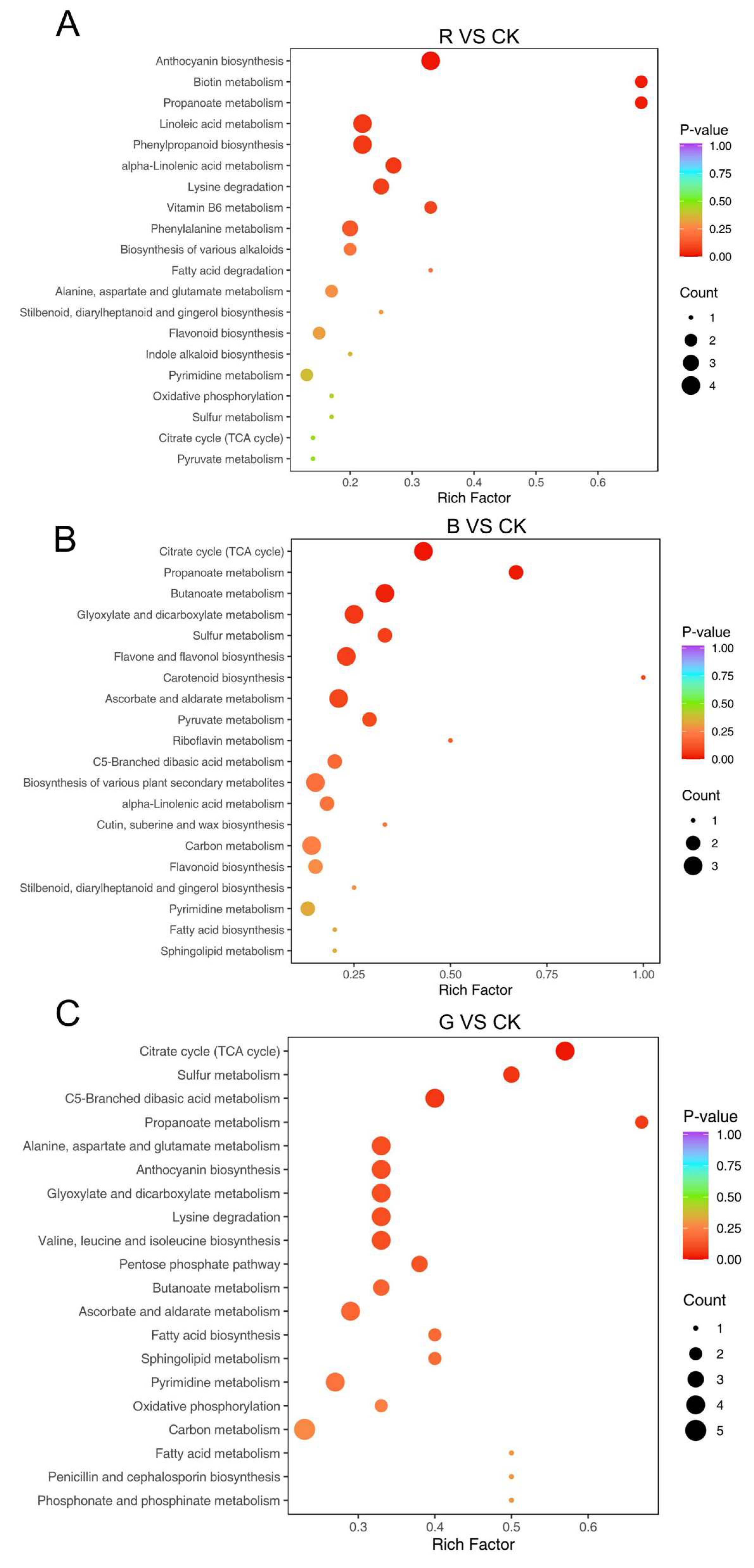
2.8. KEGG Pathway Enrichment Analysis of Differential Metabolites in Mussaenda pubescens Micropropagated Leaves Under Different Light Qualities
3. Discussion
3.1. Development and Hormonal Regulation of an Efficient Micropropagation System for Mussaenda pubescens
3.2. Light Quality Regulation of Morphogenesis in Mussaenda pubescens Micropropagated Shoots
3.3. Red Light-Induced Metabolic Pathways for Bioactive Compound Synthesis in Mussaenda pubescens
3.4. Photoresponsive Signatures and Regulatory Potential of the Anthocyanin-Phenylpropanoid Biosynthetic Pathway in Mussaenda pubescens
4. Materials and Methods
4.1. Materials
4.2. Sterilization of Explants
4.3. Screening of Optimal Medium for Axillary Shoot Proliferation
4.4. Effects of Different Media on Rooting of Shoots and Plantlet Growth
4.5. Screening for Optimal Substrate for Transplanting
4.6. Different Light Quality Treatments
4.7. Analysis by Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)
4.8. Data Analysis
4.8.1. Data Processing for In Vitro Fast Propagation Systems
- Proliferation coefficient = total number of shoots after proliferation culture/total number of inoculated explants
- Average plant height = total shoot height/total number of shoots
- Rooting rate = number of rooted explants/number of inoculated explants × 100%
- Average number of roots = total number of roots of all plantlets/total number of plantlets
- Mean stem thickness = total stem thickness of shoots/total number of shoots
4.8.2. Widely Targeted Metabolomics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| R | red light |
| B | blue light |
| G | green light |
| OPLS-DA | orthogonal partial least squares discriminant analysis |
| VIP | variable importance in projection |
| ESI | electrospray ionization |
| PPFD | photosynthetic photon flux density |
| d | days |
| 6-BA | 6-Benzylaminopurine |
| NAA | 1-naphthaleneacetic acid |
| MS | Murashige and Skoog basal medium |
| HSD | honestly significant difference |
| ANOVA | one-way analysis of variance |
| M. pubescens | Mussaenda pubescens |
| KT | Kinetin |
| FWHM | full width at half maximum |
| DEM | differentially expressed metabolites |
| MRM | Multiple Reaction Monitoring |
References
- Tang, D.Z. Research progress of Mussaenda pubescens. Pharm. J. Chin. PLA 2016, 32, 170–173. [Google Scholar]
- Zhang, Q.H.; Tang, M.; Hu, Z.Y.; Zhao, L.C. Overview of research on the chemical composition and pharmacological effects of Mussaenda pubescens. J. Guangxi Univ. Chin. Med. 2017, 20, 67–72. [Google Scholar]
- Zhao, W.M.; Yang, G.J.; Xu, R.S.; Qin, G.W. Three monoterpenes from Mussaenda pubescens. Phytochemistry 1996, 41, 1553–1555. [Google Scholar] [CrossRef]
- Gal, M.; Kim, O.; Tran, P.T.; Huong, L.T.; Nhiem, N.X.; Van Kiem, P.; Dang, N.H.; Lee, J. Mussaendoside O, a N-triterpene cycloartane saponin, attenuates RANKL-induced osteoclastogenesis and inhibits lipopolysaccharide-induced bone loss. Phytomedicine 2022, 105, 154378. [Google Scholar] [CrossRef]
- Huong, L.T.; Thao, T.P.; Trang, D.T.; Lee, J.H.; Tai, B.H.; Kiem, P.V.; Nhiem, N.X.; Dang, N.H. Anti-osteoclastogenic cycloartane saponins from Mussaenda pubescens. Nat. Prod. Res. 2022, 36, 4597–4604. [Google Scholar] [CrossRef]
- Wu, X.; Chen, S.; Zhang, D. Study on the breeding system of Mussaenda. In Proceedings of the Ninth National Symposium on Plant Structure and Reproductive Biology, Xian, China, 28–30 May 2010. [Google Scholar]
- Geng, S.; Zhao, X.X.; Tan, Y.; Wu, G.; Tao, G.L.; Peng, Z.C.; Yuan, D.; Li, Q.Y.; Wang, S.; Liu, Y.L. Effects of Basic Medium and Carbon and Nitrogen Source on Tissue Culture of Mussaenda anomala. Seed 2025, 44, 236–245. [Google Scholar]
- Zhang, W.-Q.; Wang, D.-J. A preliminary study on tissue culture of the rare and endangered plant Mussaenda anomala. J. Cent. South Univ. For. Technol. 2016, 36, 12–15, 47. [Google Scholar]
- Peng, G.T.; Huang, S.Z.; Li, H.G.; Fu, J.R. Tissue Culture and Plantlet Regeneration of Mussaenda pubescens. Plant Physiol. Commun. 2001, 37, 230–231. [Google Scholar]
- Hashim, M.; Ahmad, B.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. Comparative Effects of Different Light Sources on the Production of Key Secondary Metabolites in Plants In Vitro Cultures. Plants 2021, 10, 1521. [Google Scholar] [CrossRef]
- Weremczuk-Jeżyna, I.; Hnatuszko-Konka, K.; Lebelt, L.; Grzegorczyk-Karolak, I. The Protective Function and Modification of Secondary Metabolite Accumulation in Response to Light Stress in Dracocephalum forrestii Shoots. Int. J. Mol. Sci. 2021, 22, 7965. [Google Scholar] [CrossRef]
- Younas, M.; Drouet, S.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Differential accumulation of silymarin induced by exposure of Silybum marianum L. callus cultures to several spectres of monochromatic lights. J. Photochem. Photobiol. B 2018, 184, 61–70. [Google Scholar] [CrossRef]
- Tariq, U.; Ali, M.; Abbasi, B.H. Morphogenic and biochemical variations under different spectral lights in callus cultures of Artemisia absinthium L. J. Photochem. Photobiol. B 2014, 130, 264–271. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, T.; Zhang, K.; Zhang, M.; Ma, L.; Ren, W.; Bao, Y.; Ma, W. Effect of Light Treatment on Chemical Composition of Andrographis paniculata Seedlings. Front. Chem. 2022, 10, 889365. [Google Scholar] [CrossRef]
- Xiao, Q.; Mu, X.; Liu, J.; Li, T.; Li, Z.-L.; Zhou, L.; Zhang, L.; Xu, M.; Yang, W.-Z.; Guo, D.-A. A new strategy and tool for quality evaluation of Chinese medicinal materials. Plant Metab. 2022, 17, 45. [Google Scholar]
- Zhang, S.; Zhang, L.; Zou, H.; Qiu, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of Light on Secondary Metabolite Biosynthesis in Medicinal Plants. Front. Plant Sci. 2021, 12, 781236. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, I.; Barbaresi, A.; Barbanti, L.; Mandrone, M.; Poli, F.; Torreggiani, D.; Trenta, M.; Tassinari, P. Effects of LED supplemental lighting on the growth and metabolomic profile of Taxus baccata cultivated in a smart greenhouse. PLoS ONE 2022, 17, e266777. [Google Scholar] [CrossRef]
- Li, J.; Lu, Q.P.; Zhang, Y. Research progress on chemical constituents and pharmacological effects of Mussaenda plants. Chin. J. Ethnomed. Ethnopharm. 2016, 15, 39–48. [Google Scholar]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Lu, Y.; Gao, D.; Gao, X.; Huo, H.; Yang, Z.; Wang, J.; Hou, M.; Wu, Y.; Zhang, H.; Xie, H.; et al. Light quality modulates yields and secondary metabolite accumulation in Fritillaria cirrhosa: Insights from rhizosphere metabolomics and microbiomics. Ind. Crops Prod. 2025, 229, 120967. [Google Scholar] [CrossRef]
- Han, S.; Liu, M.; Wang, Y.; Chen, J. Tissue culture and rapid propagation technology for Gentiana rhodantha. Open Life Sci. 2023, 18, 20220567. [Google Scholar] [CrossRef]
- Li, M. Callus induction and multi-bud body formation from stem segment of Dioscorea opposita. Acta Agric. Boreali-Sin. 2000, 15, 85–88. [Google Scholar]
- Faisal, M.; Alatar, A.A.; Ahmad, N.; Anis, M.; Hegazy, A.K. Clonal Propagation of Dwarf Raspberry (Rubus pubescens Raf.) through in vitro Axillary Shoot Proliferation. Appl. Biochem. Biotechnol. 2012, 168, 1739–1752. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Gao, Z.; Chen, T.; Cao, L.; Wang, P. Callus Induction of Hybrid Broussonetia papyrifera Leaves. Henan Sci. 2020, 38, 1776–1780. [Google Scholar]
- Huang, Y.; Zhang, H.; Tao, X.; Sheng, M.; Zhang, J.; Zhanng, T.; Liu, H. Establishment of Tissue Culture and Rapid Propagation System of Hybrid Broussonetia papyrifera Leaves. Guizhou Agric. Sci. 2018, 46, 46–48. [Google Scholar]
- Shen, S.X.; Li, X.H.; He, A.L.; Fan, Y.M.; Zhou, Y.F. Establishment and optimization of tissue culture and rapid propagation system of Salvia miltiorrhiza Bunge stem segments. J. Chin. Med. Mater. 2017, 40, 2511–2515. [Google Scholar]
- Chen, J.; Chen, G.H. Study of the Concentration of 6-BA and NAA in the Rapid Propagation of Polygonum multiflorum Thunb. Chin. Agric. Sci. Bull. 2006, 22, 173–175. [Google Scholar]
- Li, N.; Han, H.Y.; Zhang, B. Cluster bud induction of base stem and establishment of high efficiency regeneration system of Lycium ruthenicum. Chin. Tradit. Herb. Drugs. 2020, 51, 3545–3553. [Google Scholar]
- Zhi, S.; Shi, L. Effects of Different Medium on Rooting of Broussonetia papyrifera (L.) Vent. Tissue Culture Seedlings. J. Shanxi Agric. Sci. 2020, 48, 1207–1210. [Google Scholar]
- Zhou, J.; Liu, Y.; Wu, L.; Zhao, Y.; Zhang, W.; Yang, G.; Xu, Z. Effects of Plant Growth Regulators on the Rapid Propagation System of Broussonetia papyrifera L. Vent Explants. Forests 2021, 12, 874. [Google Scholar] [CrossRef]
- Tang, W.X.; Yu, Y.Q.; Xu, T.D. The interplay between extracellular and intracellular auxin signaling in plants. J. Genet. Genom. 2025, 52, 14–23. [Google Scholar] [CrossRef]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Zhang, C.M.; Wang, Y.J.; Zhai, Y.N.; Lyu, S.; Wu, X.L.; Gao, H.B.; Gao, Y.H.; Gong, B.B. Mixed Substrate of Seedling Based on Mature Rice Husk and its Application Effect. China Veg. 2024, 3, 111–117. [Google Scholar]
- Shohael, A.M.; Ali, M.B.; Yu, K.W.; Hahn, E.J.; Islam, R.; Paek, K.Y. Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process Biochem. 2006, 41, 1179–1185. [Google Scholar] [CrossRef]
- Shimizu, H. Effect of light quality on secondary metabolite production in leafy greens and seedlings. In LED Lighting for Urban Agriculture; Springer: Singapore, 2016; pp. 239–260. [Google Scholar]
- Xie, D.; Chen, L.; Zhou, C.; Tarin, M.; Yang, D.; Ren, K.; He, T.; Rong, J.; Zheng, Y. Transcriptomic and metabolomic profiling reveals the effect of LED light quality on morphological traits, and phenylpropanoid-derived compounds accumulation in Sarcandra glabra seedlings. BMC Plant Biol. 2020, 20, 476. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Huang, Q.; Wei, K.; Yang, X.; Wei, F.; Miao, J. Identification of Differentially Expressed Genes and Pathways Involved in Growth and Development of Mesona chinensis Benth Under Red- and Blue-Light Conditions. Front. Plant Sci. 2021, 12, 809000. [Google Scholar] [CrossRef]
- Jung, H.W.; Tschaplinski, T.J.; Wang, L.; Glazebrook, J.; Greenberg, J.T. Priming in Systemic Plant Immunity. Science 2009, 324, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Ando, E.; Kinoshita, T. Red light-induced phosphorylation of plasma membrane H-ATPase in stomatal guard cells+. Plant Physiol. 2018, 178, 838–849. [Google Scholar] [CrossRef]
- Pashkovskiy, P.; Kreslavski, V.D.; Ivanov, Y.; Ivanova, A.; Kartashov, A.; Shmarev, A.; Strokina, V.; Kuznetsov, V.V.; Allakhverdiev, S.I. Influence of Light of Different Spectral Compositions on the Growth, Photosynthesis, and Expression of Light-Dependent Genes of Scots Pine Seedlings. Cells 2021, 10, 3284. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M. Long-Term Effects of Red- and Blue-Light Emitting Diodes on Leaf Anatomy and Photosynthetic Efficiency of Three Ornamental Pot Plants. Front. Plant Sci. 2017, 8, 1884. [Google Scholar] [CrossRef]
- Li, H.; Yu, C. Chloroplast Galactolipids: The Link Between Photosynthesis, Chloroplast Shape, Jasmonates, Phosphate Starvation and Freezing Tolerance. Plant Cell Physiol. 2018, 59, 1128–1134. [Google Scholar] [CrossRef]
- Inoue, S.I.; Kinoshita, T. Blue light regulation of stomatal opening and the plasma membrane H-ATPase+. Plant Physiol. 2017, 174, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Cowan, I.R.; Troughton, J.H. The relative role of stomata in transpiration and assimilation. Planta 1971, 97, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Sellaro, R.; Crepy, M.; Trupkin, S.A.; Karayekov, E.; Buchovsky, A.S.; Rossi, C.; Casal, J.J. Cryptochrome as a Sensor of the Blue/Green Ratio of Natural Radiation in Arabidopsis. Plant Physiol. 2010, 154, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Chung, I.; Hwang, M.H.; Kim, S.; Yu, C.Y.; Ghimire, B.K. Application of Light-Emitting Diodes for Improving the Nutritional Quality and Bioactive Compound Levels of Some Crops and Medicinal Plants. Molecules 2021, 26, 1477. [Google Scholar] [CrossRef]
- Appolloni, E.; Pennisi, G.; Zauli, I.; Carotti, L.; Paucek, I.; Quaini, S.; Orsini, F.; Gianquinto, G. Beyond vegetables: Effects of indoorLED light on specialized metabolite biosynthesis in medicinal and aromatic plants, edible flowers, and microgreens. J. Sci. Food Agric. 2022, 102, 472–487. [Google Scholar] [CrossRef]
- Kocsy, G.; Müller, M. Light-Dependent Control of Metabolism in Plants. Int. J. Mol. Sci. 2023, 24, 13861. [Google Scholar] [CrossRef]
- Yu, S.-Z.; Wang, J.-J.; Lu, W.-L.; Yang, Z.-D.; Chen, J. The Physiology of Mussaenda pubescens Ait.f. with Witches’-broom Disease. Hubei Agric. Sci. 2012, 51, 1808–1810. [Google Scholar]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Acta Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Zhou, C.; Li, P.; Fu, S.; You, Y.; Guo, S.; Piyaporn, C.; Mei, X.; Zhou, X.; Girdthai, T. Metabolomics reveals the importance of metabolites in Mussaenda pubescens for antioxidant properties and quality traits. Physiol. Plant. 2024, 176, e14299. [Google Scholar] [CrossRef]
- Zhang, D.; Sun, W.; Shi, Y.; Wu, L.; Zhang, T.; Xiang, L. Red and Blue Light Promote the Accumulation of Artemisinin in Artemisia Annua L. Molecules 2018, 23, 1329. Molecules 2018, 23, 1329. [Google Scholar] [CrossRef]
- Ren, Y.Y.; Niu, C.; Wang, J.J.; Yang, H.; Xu, Y.H.; Liu, Z. Effects of Different Light Qualities on Growth and Ginsenoside Contents in Callus of Panax ginseng. Spect. Anal. 2022, 42, 1318–1322. [Google Scholar]
- Shao, M.; Kuang, Z.; Wang, W.; Li, S.; Li, G.; Song, Y.; Li, H.; Cui, G.; Zhou, H. Aucubin Exerts Anticancer Activity in Breast Cancer and Regulates Intestinal Microbiota. Altern. Med. 2022, 2022, 3408208. [Google Scholar] [CrossRef]
- Shi, Y.; Pu, D.; Zhou, X.; Zhang, Y. Recent Progress in the Study of Taste Characteristics and the Nutrition and Health Properties of Organic Acids in Foods. Foods 2022, 11, 3408. [Google Scholar] [CrossRef]
- Tadda, S.A.; Li, C.; Ding, J.; Li, J.A.; Wang, J.; Huang, H.; Fan, Q.; Chen, L.; He, P.; Ahiakpa, J.K.; et al. Integrated metabolome and transcriptome analyses provide insight into the effect of red and blue LEDs on the quality of sweet potato leaves. Front. Plant Sci. 2023, 14, 1181680. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, Z.; Kong, H.; Li, Y.; Lu, X.; Xu, G. Exploring the mechanism of rhizoma coptidis in treating type II diabetes mellitus based on metabolomics by gas chromatography-mass spectrometry. Chin. J. Chromatogr. 2012, 30, 8–13. [Google Scholar] [CrossRef]
- Miyazaki, H.; Midorikawa, N.; Fujimoto, S.; Miyoshi, N.; Yoshida, H.; Matsumoto, T. Antimicrobial effects of lysophosphatidylcholine on methicillin-resistant Staphylococcus aureus. Ther. Adv. Infect. Dis. 2017, 4, 89–94. [Google Scholar] [CrossRef]
- Suh, D.H.; Kim, Y.X.; Jung, E.S.; Lee, S.; Park, J.; Lee, C.H.; Sung, J. Characterization of Metabolic Changes under Low Mineral Supply (N, K, or Mg) and Supplemental LED Lighting (Red, Blue, or Red–Blue Combination) in Perilla frutescens Using a Metabolomics Approach. Molecules 2020, 25, 4714. [Google Scholar] [CrossRef]
- Saddhe, A.A.; Potocký, M. Comparative phylogenomic and structural analysis of canonical secretory PLA2 and novel PLA2-like family in plants. Front. Plant Sci. 2023, 14, 1118670. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Tang, D.; Hu, B.; Zhang, Z.; Wu, H.; Fan, L.; Cai, K.; Tang, C.; Zhang, Y.; et al. Anthocyanin improves kidney function in diabetic kidney disease by regulating amino acid metabolism. J. Transl. Med. 2022, 20, 123. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Zhu, Q.; Liang, Q.; Wang, X.; Li, Y.; Sun, Y.; Zhang, X.; Liu, F.; Xue, F.; Sun, J. Transcriptome Analysis Reveals Differences in Anthocyanin Accumulation in Cotton (Gossypium hirsutum L.) Induced by Red and Blue Light. Plant Sci. 2022, 13, 788828. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.S.; Goud, K.V.; Sharma, R.; Reddy, A.R. Ultraviolet-B-Responsive Anthocyanin Production in a Rice Cultivar 1s Associated with a Specific Phase of Phenylalanine Ammonia Lyase Biosynthesis. Plant Physiol. 1994, 105, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, L.; Wang, Y.; Zhang, L.; Xu, S.; Wang, X.; He, W.; Zhang, Y.; Lin, Y.; Wang, Y.; et al. The blue light signal transduction module FaCRY1-FaCOP1-FaHY5 regulates anthocyanin accumulation in cultivated strawberry. Front. Plant Sci. 2023, 14, 1144273. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J. Effects of Tissue Culture and Light Quality on Metabolites in Mussaenda pubescens. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2024. [Google Scholar]




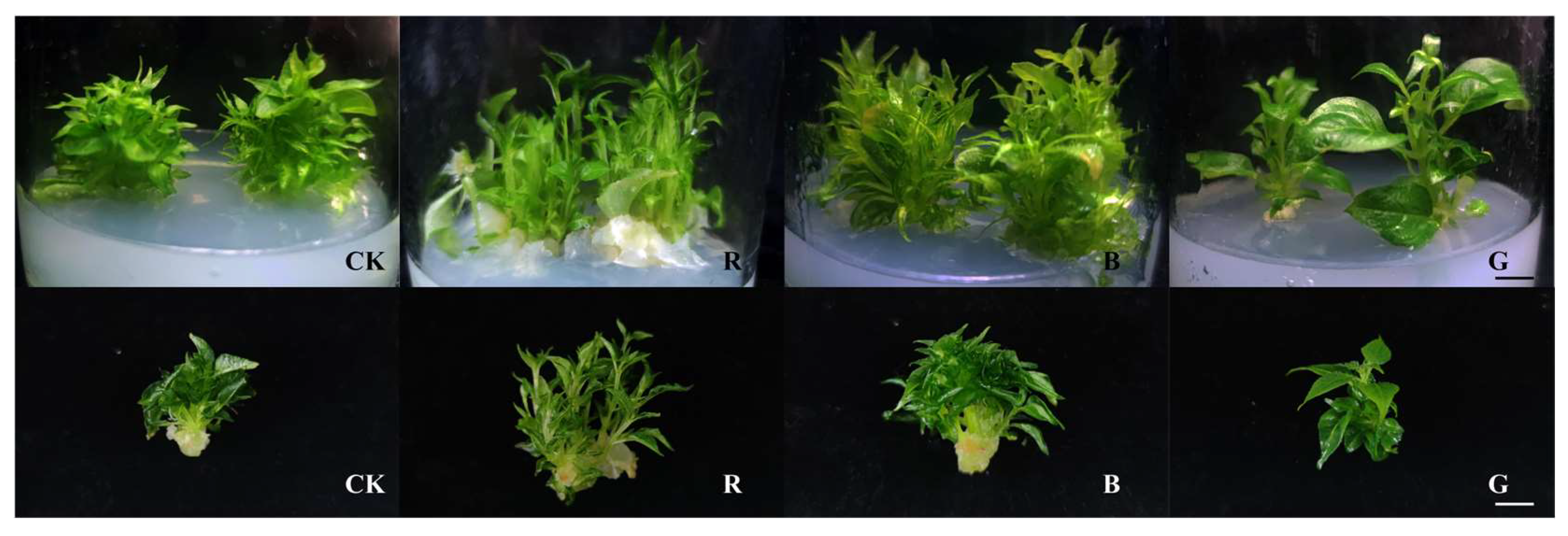

| Treatment Serial No. | Hormone Concentration (mg/L) | Value-Added Factor | Average Plant Height (cm) | Growth | ||
|---|---|---|---|---|---|---|
| 6-BA | NAA | Growth Momentum | Callus | |||
| 1 | 1 | 0.1 | 10.30 ± 2.56 a | 1.86 ± 0.08 bc | ** | + |
| 2 | 1 | 0.2 | 11.20 ± 3.14 ab | 1.89 ± 0.03 c | ** | + |
| 3 | 1 | 0.3 | 11.30 ± 2.68 ab | 1.85 ± 0.04 bc | ** | + |
| 4 | 2 | 0.1 | 11.80 ± 2.89 ab | 1.99 ± 0.03 d | *** | +++ |
| 5 | 2 | 0.2 | 12.20 ± 1.82 b | 2.07 ± 0.04 f | *** | +++ |
| 6 | 2 | 0.3 | 10.20 ± 2.78 a | 1.95 ± 0.07 d | *** | + |
| 7 | 3 | 0.1 | 11.50 ± 2.52 ab | 1.75 ± 0.03 a | ** | ++ |
| 8 | 3 | 0.2 | 11.40 ± 2.06 ab | 1.82 ± 0.02 bc | ** | ++ |
| 9 | 3 | 0.3 | 10.70 ± 2.71 ab | 1.80 ± 0.05 ab | * | |
| Treatment Serial No. | Hormone Concentration (mg/L) NAA | Average Plant Height (cm) | Rooting Rate (%) | Average Number of Roots |
|---|---|---|---|---|
| 1 | 0.05 | 3.11 ± 0.45 ab | 100 | 7.55 ± 0.70 ab |
| 2 | 0.10 | 3.46 ± 0.43 b | 100 | 9.33 ± 1.85 b |
| 3 | 0.15 | 3.26 ± 0.20 b | 100 | 8.44 ± 0.51 ab |
| 4 | 0.20 | 2.85 ± 0.11 a | 100 | 6.67 ± 0.67 a |
| Treatment Serial No. | Substrate for Transplanting | Average Plant Height (cm) | Average Stem Thickness (cm) | Survival Rate (%) |
|---|---|---|---|---|
| 1 | peat soil | 8.55 ± 0.47 a | 0.164 ± 0.009 b | 96.7 |
| 2 | perlite: vermiculite = 1:1 | 9.25 ± 0.88 ab | 0.18 ± 0.021 b | 93.3 |
| 3 | perlite: vermiculite: peat soil = 1:1:1 | 12.37 ± 1.76 b | 0.12 ± 0.013 a | 100 |
| KEGG Level 1 | KEGG Pathway | KoID | Metabolome Frequency |
|---|---|---|---|
| Metabolism | Metabolic pathways | ko01100 | 72.84% |
| Metabolism | Biosynthesis of secondary metabolites | ko01110 | 40.49% |
| Metabolism | Biosynthesis of cofactors | ko01240 | 13.83% |
| Metabolism | Biosynthesis of amino acids | ko01230 | 12.59% |
| Environmental Information Processing | ABC transporters | ko02010 | 11.11% |
| Metabolism | 2-Oxocarboxylic acid metabolism | ko01210 | 7.65% |
| Metabolism | Nucleotide metabolism | ko01232 | 5.93% |
| Metabolism | Carbon metabolism | ko01200 | 5.43% |
| Metabolism | Biosynthesis of various plant secondary metabolites | ko00999 | 4.94% |
| Metabolism | Linoleic acid metabolism | ko00591 | 4.44% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Wu, J.; Yang, Z.; Cai, R.; Xu, X.; Li, J.; Tong, N.; Awais, M.; Lin, Y.; Lai, Z. Optimization of Micropropagation and Metabolomic Analysis Under Different Light Qualities in Mussaenda pubescens Ait.f. Plants 2025, 14, 3268. https://doi.org/10.3390/plants14213268
Sun L, Wu J, Yang Z, Cai R, Xu X, Li J, Tong N, Awais M, Lin Y, Lai Z. Optimization of Micropropagation and Metabolomic Analysis Under Different Light Qualities in Mussaenda pubescens Ait.f. Plants. 2025; 14(21):3268. https://doi.org/10.3390/plants14213268
Chicago/Turabian StyleSun, Li, Jiajia Wu, Zilu Yang, Roudi Cai, Xiaoping Xu, Jiahui Li, Ning Tong, Muhammad Awais, Yuling Lin, and Zhongxiong Lai. 2025. "Optimization of Micropropagation and Metabolomic Analysis Under Different Light Qualities in Mussaenda pubescens Ait.f" Plants 14, no. 21: 3268. https://doi.org/10.3390/plants14213268
APA StyleSun, L., Wu, J., Yang, Z., Cai, R., Xu, X., Li, J., Tong, N., Awais, M., Lin, Y., & Lai, Z. (2025). Optimization of Micropropagation and Metabolomic Analysis Under Different Light Qualities in Mussaenda pubescens Ait.f. Plants, 14(21), 3268. https://doi.org/10.3390/plants14213268









