1. Introduction
Africa has the highest number of undernourished people in the world, with sub-Saharan Africa accounting for 92% of this population [
1,
2]. To address this issue, there is a need to grow new, highly nutritious crops. One such crop is vegetable soybean (
Glycine max L. Merr.), also called edamame, a type of soybean known for its large seeds. Vegetable soybean is an excellent source of protein, containing all essential amino acids, as well as being rich in oil, minerals, and dietary fibre [
3,
4]. Globally, its popularity as a nutritious snack and versatile ingredient is increasing, especially in Asia and Western countries, where there is a growing demand for plant-based protein options [
5]. In Africa, vegetable soybean has been introduced as a promising crop to combat malnutrition in South Africa (SA) and 25 other African countries [
2]. With its high nutritional value and adaptability to different growing conditions, vegetable soybean could play a big role in improving food security across the continent.
However, the successful cultivation of vegetable soybeans, particularly in regions prone to food insecurity, faces significant challenges due to environmental factors such as drought. While its nutritional benefits make it an excellent crop for combating malnutrition, vegetable soybean presents a challenge due to its substantial water needs throughout its developmental stages [
4]. This is particularly problematic in SA, a semi-arid country with unpredictable weather patterns, especially in soybean-producing regions [
6]. In 2015, SA declared a national state of disaster due to severe drought, resulting in economic losses exceeding 200 million US dollars and affecting over 2.7 million people [
7]. Therefore, it is important to highlight how drought, a major environmental stress associated with climate change, affects the physiological and biochemical responses of crops like vegetable soybean, as these changes can significantly affect yield and, in turn, the farming industry [
8].
Drought stress disrupts key physiological processes like photosynthesis, which in turn hinders crop growth, development, and yield [
9,
10,
11,
12,
13]. Furthermore, drought stress triggers the production of reactive oxygen species (ROS), which, in excess, can be harmful to plants. These ROS damage lipids, proteins, and nucleic acids, leading to cell damage and plant death [
14]. To combat such losses, efforts to breed drought-tolerant genotypes are ongoing, though this process can take time. Meanwhile, selenium has been found to help protect plants from various environmental stresses, offering an alternative approach to traditional breeding methods for improving plant resilience. This raises the question: Can selenium improve drought stress resilience in vegetable soybean to improve yield? While selenium is essential for both animals and humans, it is considered a beneficial element to plants because it helps enhance their tolerance to stress [
15,
16]. Recently, selenium has been suggested as a potential solution for improving drought tolerance in crops, including vegetable soybean [
17,
18]. According to Seleiman et al. [
17], selenium can promote plant growth, reduce oxidative stress damage, enhance antioxidant responses, and regulate water balance during drought stress.
A range of selenium (Se) compounds, including traditional forms (microparticles, such as selenite and selenate) and selenium nanoparticles (SeNPs), have been studied for their ability to mitigate drought stress in plants [
19,
20,
21]. Application of nano-selenium during the reproductive stage of soybean enhances drought tolerance by promoting biomass accumulation, maintaining water content, and increasing photosynthetic pigment levels, while simultaneously improving oxidative stress management and ultrastructural integrity [
22]. Both selenium microparticles and nanoparticles enhance drought tolerance by enhancing antioxidant enzyme activities (e.g., SOD, CAT, and POD); increasing osmoprotectant levels (e.g., proline, glycine betaine); improving photosynthetic pigment content and water status, and reducing oxidative damage and membrane lipid peroxidation [
22,
23,
24,
25].
Selenium, whether applied through foliar sprays, seed soaking, or soil incorporation, has consistently demonstrated its ability to increase relative water content (RWC), stimulate antioxidative defence, and improve crop resilience under stress. Foliar spraying with selenium nanoparticles has been shown to enhance drought tolerance in tomato by altering gene expression, upregulating antioxidant systems, and stimulating secondary metabolism [
26]. Similarly, both organic selenium and nanoselenium enhance drought resistance in pak choi by increasing photosynthetic capacity, maintaining water homeostasis, and promoting biomass accumulation through the up-regulation of metabolic pathways [
27]. In wheat, selenium foliar application has been reported to increase RWC and enhance photosynthesis [
19] while seed soaking reduced hydrogen peroxide (H
2O
2) accumulation [
20]. Soil application has also been associated with improved growth and yield in wheat [
21]. Additionally, foliar selenium application has been linked to enhanced growth parameters and yield in soybean [
22]. However, in vegetable soybean, foliar application at the vegetative stage increased antioxidative enzyme activity but did not improve yield [
18]. Moreover, de los Ángeles Sariñana-Navarrete et al. [
23] demonstrated that seed priming in Jalapeño with low concentrations of sodium selenite or selenium nanoparticles stimulates early growth (germination, radicle and plumule development, and seedling vigor) and enhances antioxidant activity, resulting in seedlings with greater stress tolerance. However, higher concentrations may be phytotoxic, emphasising the need to optimise selenium form and dose for improved crop resilience [
23].
Given these varying outcomes, evaluating the effectiveness of different selenium application methods is of practical importance. Such comparisons can help farmers to identify the most convenient and efficient method for their specific conditions. This study investigates how different selenium applications (through seed priming, soil drenching, and foliar spraying) affect the physio-biochemical responses and yield of two vegetable soybean cultivars, UVE17 (which is more sensitive to drought) and UVE14 (which is more drought-tolerant). Using two different cultivars allows for a comparison of their responses to selenium treatments under drought stress.
3. Discussion
Drought stress, exacerbated by climate change, is one of the biggest challenges to crop production. In legumes like vegetable soybean, stable yields under drought conditions depend on the ability of plants to maintain optimal physiological and biochemical function [
13,
28,
29,
30]. Many strategies have been explored to improve drought survival in legumes [
29]. However, little research has focused on the role of selenium, despite its great potential to improve plant tolerance to drought stress [
31]. Moloi and Khoza [
18] demonstrated that foliar spraying of selenium on vegetable soybean leaves during the vegetative stage enhanced drought tolerance by increasing APX activity. However, the efficacy of this application method appeared to decline over time, as only APX activity was elevated at the pod-filling stage without any corresponding improvement in yield. This highlights the need to investigate alternative selenium application methods, such as seed priming and soil treatments, which have shown potential to improve drought tolerance in plants [
21,
32]. This study investigates the effects of different selenium application methods (seed priming, soil drenching, and foliar spraying) on the physio-biochemical responses and yield traits of two vegetable soybean cultivars with varying drought tolerance levels. The effectiveness of these selenium application methods is measured over time (at flowering and pod-filling stages) because different developmental stages in plants experience different physiological and biochemical responses to drought stress [
33]. Since drought stress, especially during critical growth periods, can cause irreversible yield losses [
34], optimising selenium application methods is essential for finding alternative solutions to genetic engineering or conventional breeding for enhancing drought resilience and maximising yield in vegetable soybean.
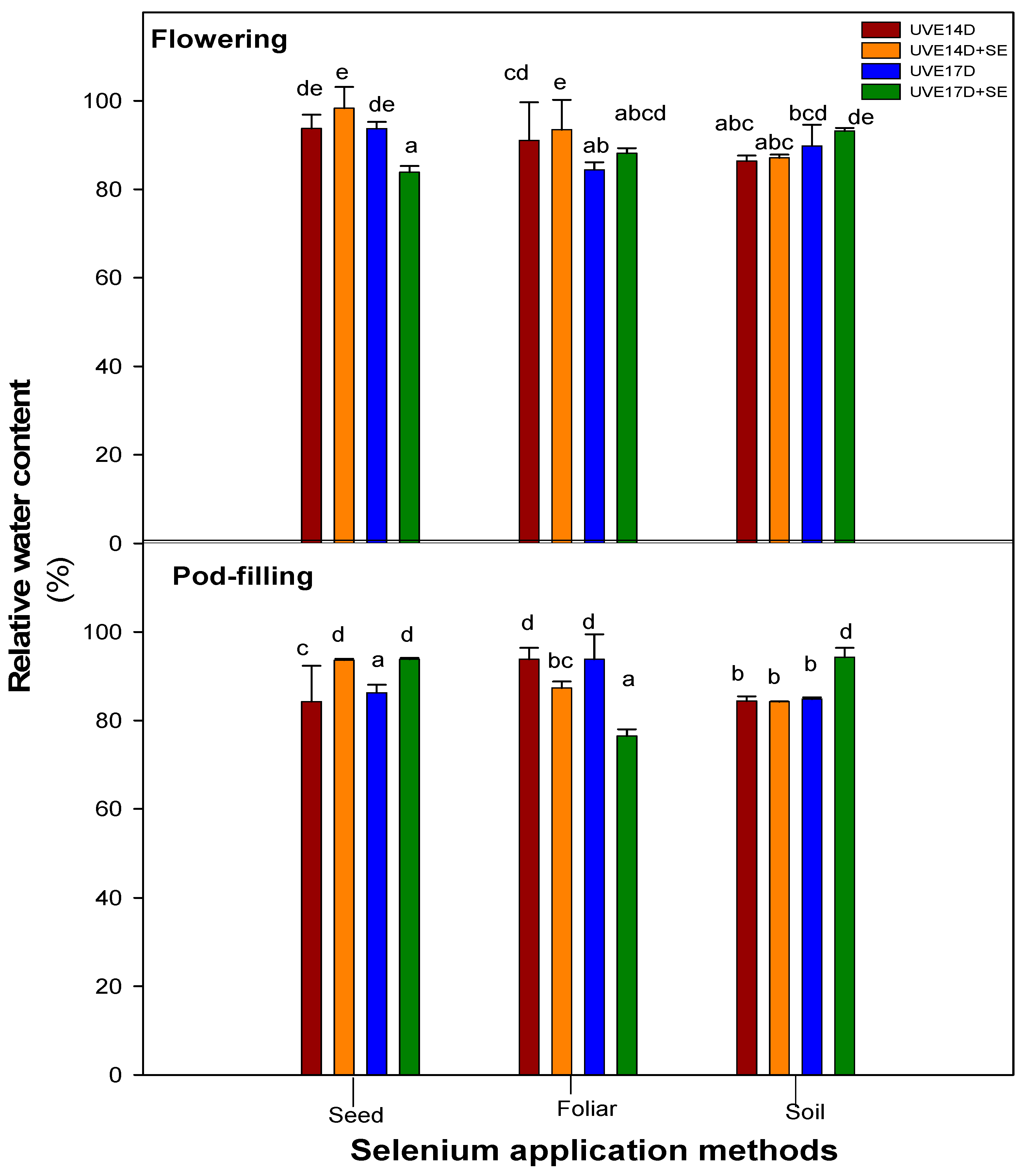
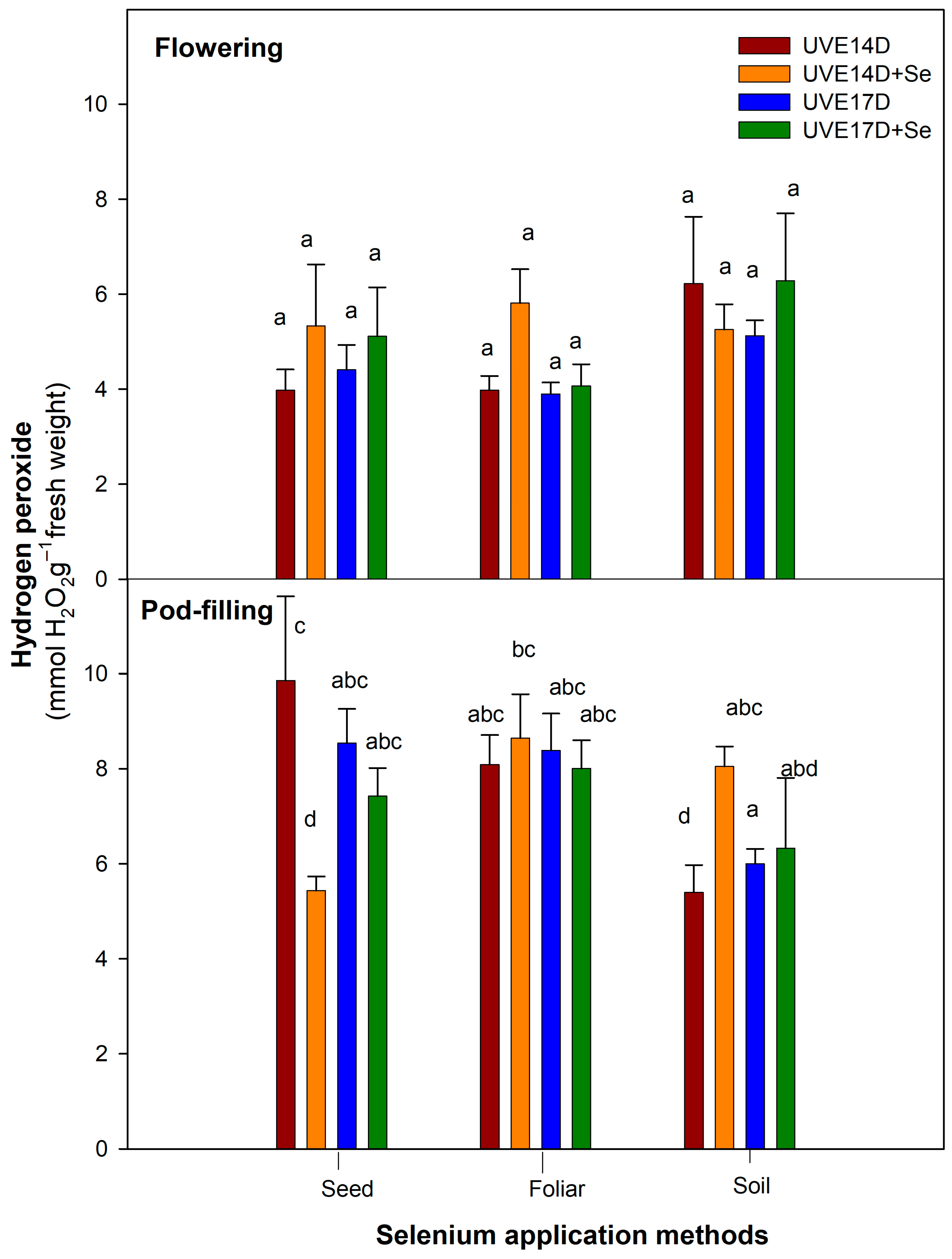
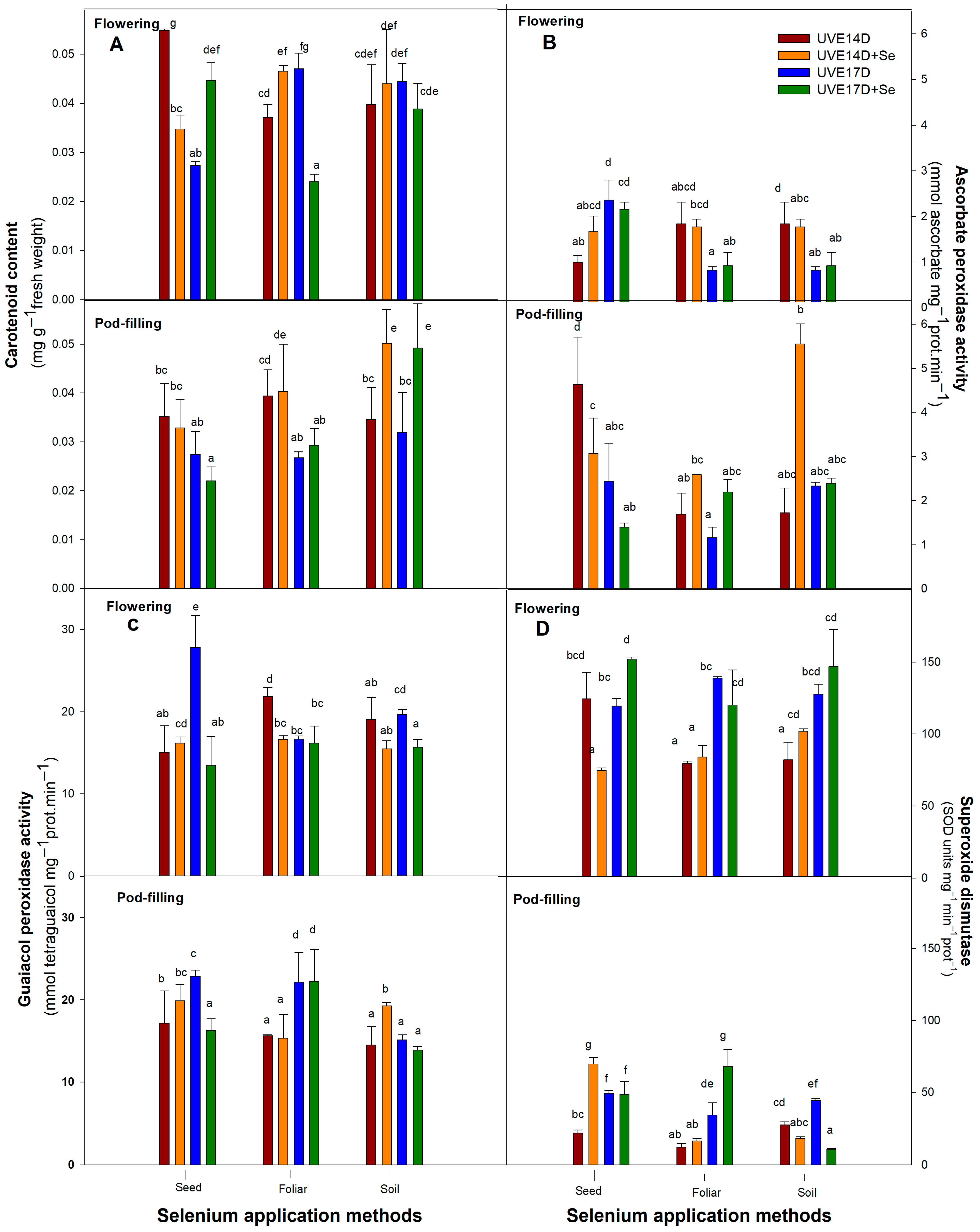
Selenium soil drench application improved photosynthetic efficiency under drought conditions, as evidenced by increased total chlorophyll content and photosystem performance (PItotal) in both vegetable soybean cultivars (UVE14 and UVE17) across growth stages. This suggests that selenium enhances the photosynthetic apparatus of and supports physiological stability under drought stress. However, the response was growth stage-dependent, with a notable increase in total chlorophyll accumulation observed only at the pod-filling stage, indicating that selenium effectiveness is influenced by developmental stage. Similarly, the application of selenium as a soil supplement increased the chlorophyll content of drought-stressed lentils [
35], indicating that selenium can regulate physiological processes [
36]. Under drought stress, plants respond by inducing stomatal closure to reduce transpiration. While this is a survival mechanism, stomatal closure reduces CO
2 fixation, leading to reduced plant growth [
37,
38]. Selenium soil application method effectively increased stomatal conductance in drought-stressed UVE17 at both growth stages. These imply that selenium soil application enhanced the light harvesting and light-dependent electron transport irrespective of the cultivar under drought conditions. Under drought stress, plants typically close their stomata to reduce water loss. However, this treatment increased stomatal conductance in UVE17 at pod-filling, suggesting higher CO
2 uptake and enhanced metabolite production that support crop growth and yield [
39]. Interestingly, soil-applied selenium decreased stomatal conductance in UVE14, indicating that the treatment’s ability to improve stomatal behaviour and drought tolerance is more pronounced in cultivars that are inherently more susceptible to water stress. The increased relative water content (RWC) observed in drought-stressed UVE17, at the pod-filling stage, suggests improved water retention, which supports survival and maintains optimal physiological processes [
40]. The observed increase in RWC in UVE17 under soil-applied selenium may be related to the reduced plant height, suggesting that shorter stature can help conserve water and support physiological stability under drought stress. Additionally, the ability of UVE17 to sustain high RWC despite elevated stomatal conductance with selenium soil drench treatment may be attributed to osmotic adjustment, which facilitates water retention under stress conditions [
41]. Nano-selenium application had the same effect on other crops’ RWC as in this study. In strawberry and pak choi, nano-selenium (and Se/SiO
2 nanocomposites) under drought stress significantly increased RWC, improved membrane stability, and enhanced antioxidant activity, supporting better water retention and physiological function during critical growth stages [
23,
25]. Studies also show that application of selenium on drought-stressed plants increases tolerance to drought by preventing oxidative damage through the activation of their antioxidative system [
15,
35]. Selenium application in the soil further increased hydrogen peroxide (H
2O
2) accumulation in the drought-stressed UVE14 cultivar. This increase may be linked to the observed reduction in stomatal conductance under selenium treatment, as stomatal closure can lead to the accumulation of reactive oxygen species (ROS), including H
2O
2 [
42]. Although H
2O
2 levels increased with selenium soil drench application in UVE14, the mean was lower compared to that of seed and foliar applications, indicating that soil drenching with selenium only induced moderate H
2O
2 levels, suggesting limited ROS damage from drought stress. Also, a lower H
2O
2 level is crucial for drought tolerance, as it serves as a signal molecule for activating drought tolerance in vegetable soybean [
30]. To keep ROS levels low under drought stress, plants need functional antioxidative responses to scavenge ROS, reducing the risk of oxidative stress that can damage vital biological molecules like lipids and proteins essential for cellular functions. In UVE14, selenium soil drench application under drought conditions enhanced most antioxidative responses, including carotenoids, ascorbate peroxidase, guaiacol peroxidase, and superoxide dismutase. Previous studies on vegetable soybean have shown that this cultivar enhances drought tolerance by activating ROS scavenging mechanisms [
13,
30]. Compared to the control, the observed increases in various antioxidative systems indicate that while the antioxidative response is inherently activated, selenium soil application further enhances ROS scavenging activity, strengthening UVE14’s ability to mitigate oxidative stress. In contrast, selenium soil application only increased carotenoid content in UVE17 under drought conditions, showing that enzymatic antioxidative mechanisms are not part of the drought tolerance responses in this susceptible cultivar. In addition, Zeeshan et al. [
22] also had similar findings using nano-selenium, they found that nano-selenium application improved oxidative stress management in drought-stressed soybean during the reproductive stage. Since yield is a critical factor in agriculture and food security, it is essential to determine whether the physiological and biochemical tolerance improvements observed under selenium soil application translate into enhanced growth and yield. Selenium supplementation through a soil treatment was beneficial for drought-stressed lentils, resulting in significant improvements in the pod number and seed yield per plant [
43]. For the yield parameters, selenium soil application could significantly increase the total seed number per plant (SPP) and total pods per plant (PPP) only in UVE17 under drought stress. Notably, in drought-stressed UVE17 at pod-filling, the increase in PPP under this selenium application method correlates positively with total chlorophyll content (r = 0.81) and RWC (0.85) (
Table S1), confirming that improved physiological traits could contribute to the observed yield benefits under drought conditions [
8]. In contrast, for drought-stressed UVE14, selenium soil application could only enhance one yield parameter, SPP. Furthermore, correlations show that an increased SPP in drought-stressed UVE14 under selenium soil treatment could be explained by a corresponding increase in the total chlorophyll (r = 0.89), stomatal conductance (r = 0.97) and SOD (r = 0.86) (
Table S1), suggesting that soil treatment was very efficient in improving tolerance for both cultivars.
Like selenium soil-drench, foliar application also increased total chlorophyll content in both UVE14 and UVE17 at the pod-filling stage. However, its effects on other traits depended on the cultivar and growth stage. In UVE17, selenium foliar spraying improved photosynthesis efficiency by increasing the maximum quantum efficiency of photosystem (PS) II (Fv/Fm) and lowering non-photochemical quenching, implying more efficient photochemical reactions. The reduced RWC in this cultivar, despite selenium foliar treatment under drought conditions, is linked to partially opened stomata, which suggests increased water loss with no osmotic adjustment. The selenium application consistently lowered H
2O
2 accumulation, with the strongest reductions observed for soil drenching, followed by seed priming and foliar spraying. The drought-susceptible cultivar (UVE17) exhibited slightly greater percentage decreases than the drought-tolerant cultivar (UVE14), indicating a potentially higher oxidative stress mitigation benefit from Se supplementation in this genotype (
Figure 3). These results correspond with the observation of Zeeshan et al. [
22]. They stated that foliar application of SeNPs during the reproductive stage in soybean significantly increases above-ground biomass, relative water content, and photosynthetic pigments under drought. SeNPs also boost antioxidant enzyme activity and osmolyte accumulation, reducing oxidative damage and improving cellular integrity. SeNPs improve stomatal function, mesophyll cell structure, and reduce reactive oxygen species (ROS) and membrane lipid peroxidation, leading to better drought resilience. Similarly, Sattar et al. [
44] reported an increased stomatal conductance in drought-stressed tomatoes under selenium foliar application. Of the antioxidative mechanisms studied, selenium foliar application increased UVE17’s potential of converting the superoxide anions to H
2O
2 through increased superoxide dismutase (SOD) activity, which, however, did not translate into improved yield parameters (no significant positive correlations with the yield parameters,
Table S2). The increased number of branches per plant (BPP) and pods per plant (PPP) in drought-stressed UVE17 under this selenium application method cannot be explained by the observed physio-biochemical responses (no correlations), suggesting the involvement of alternative mechanisms. In contrast to the previous study by Moloi and Khoza [
18], which showed no increase in the yield parameters when selenium was applied at V1 stage, foliar spraying of selenium slightly later (V2 stage) improves yield performance. This suggests that the efficacy of selenium when applied foliarly reduces with time. In support, Xia et al. [
43] suggested that the differences in yield parameters between soil and foliar in wheat under drought stress were influenced by the time of selenium application and the organ of the plant where selenium was applied (soil or foliar).
Selenium is known to enhance antioxidant defence, reduce oxidative stress, and help maintain chlorophyll and PSII efficiency under drought in various crops, but its impact is often context- and stage-dependent [
45,
46,
47,
48]. In drought-stressed UVE14, this application method also enhanced the light-harvesting capacity (total chlorophyll) and PSII (PI
abs) efficiency, along with increased stomatal conductance and better water retention. Improved leaf water status was also reported under combined heat and drought stress in maize when selenium was applied foliarly [
49]. Similarly, Rady et al. [
20] showed that foliar application of selenium increased RWC in drought-stressed wheat. This evidence suggests that while selenium application can mitigate drought stress by enhancing water retention in plant tissues [
50], its effectiveness varies by cultivar. Unlike soil application, foliar application in drought-stressed UVE14 increased H
2O
2 levels and carotenoid content. However, selenium foliar spraying decreased plant height (PH) but did not enhance any yield traits in this cultivar under drought stress. Furthermore, the findings correspond to the results of a study performed on quinoa under drought stress [
51], where it was found that while selenium foliar application improved physiological traits such as chlorophyll content and antioxidant activity, it did not increase the yield components. Xia et al. [
43] also illustrated that selenium application through foliar did not yield more grain in two purple wheat cultivars under drought stress.
Pre-treatment of vegetable soybean seeds with selenium had different effects on the physio-biochemical responses of drought-stressed vegetable soybean cultivars. In drought-stressed UVE14, selenium treatment enhanced the overall efficiency of PSII and PSI and increased the likelihood of CO
2 fixation, as indicated by higher stomatal conductance at the pod-filling stage. Notably, RWC at this stage improved despite increased stomatal opening, suggesting that this selenium application method may have activated additional drought tolerance mechanisms that prevent desiccation, thereby enhancing RWC, consistent with the findings of Sita et al. [
35]. Similarly, the selenium-mediated increase in maize yield was related to the maintenance of turgor and increased water retention, suggesting less cell membrane damage, which helps plants produce more biomass under water stress conditions [
36]. However, further investigations are needed to clarify the mechanisms used by drought-stressed UVE14 to maintain high RWC in drought-stressed UVE14 under the selenium seed priming method. Although carotenoid content declined in this cultivar under drought stress and selenium treatment at flowering, other ROS scavenging mechanisms, particularly increased GPX activity at flowering and SOD activity at pod-filling, effectively neutralised H
2O
2, as evidenced by its reduced levels. Similarly, Ibrahim [
52] reported that selenium pre-treatment through seed soaking reduced H
2O
2 accumulation in wheat, suggesting a crucial role for selenium in ROS inhibition during drought stress [
21]. While selenium seed-soaking effectively enhanced the physio-biochemical responses in drought-stressed UVE14, it did not significantly improve the yield traits, as no strong positive correlations were observed with these parameters (
Table S3). Pre-treatment of UVE17 seeds with selenium enhanced the light-harvesting capacity by increasing chlorophyll content at flowering and strengthened antioxidative responses, as indicated by higher carotenoid levels and SOD activity under drought stress. Selenium also mediated the increased carotenoid content of cold-stressed wheat seedlings [
53]. This selenium application method was effective in improving drought tolerance because not only did it increase the above physio-biochemical responses, but it also increased the SPP in a drought-susceptible UVE17. The strong positive correlations between these parameters at flowering and the SPP in drought-stressed UVE17 suggest that seed soaking with selenium induces distinct drought tolerance mechanisms across cultivars (
Table S3).
4. Materials and Methods
This section outlines the physiological and biochemical measurements conducted to achieve the study’s objectives. This included chlorophyll
a fluorescence parameters (to assess the efficiency of the light-dependent electron transport in photosynthesis), photosynthetic pigments (important for light harvesting and photoprotection of the photosynthesis apparatus [
54]), and stomatal conductance (regulates CO
2 entry for the light-independent reactions).
Additional analyses included relative water content (describes the amount of water in a leaf at sampling time, which is an important indicator of stress in plants [
55]) hydrogen peroxide content (a ROS produced under drought stress), electrolyte leakage (indicative of damage induced by overproduction of ROS), and antioxidative enzyme activities (involved in ROS scavenging to maintain cellular balance).
4.1. Plant Material, Growth Conditions, Selenium Application, and Drought Conditions
To ensure that the observed responses are not specific to a particular cultivar, two vegetable soybean varieties, namely the drought-susceptible (UVE17) and drought-tolerant (UVE14), as classified by van der Merwe et al. [
6], were used. Vegetable soybean seeds were provided by the South African Edamame Development Program as the breeder of the cultivars. This approach aimed to confirm if the measured responses are more likely to be general phenomena or specific to a particular cultivar. The seeds were germinated under controlled conditions (25 °C day and 18 °C night) in polystyrene seedling trays filled with Hygromix seedling mix (Hygrotech (Pty) Ltd., Pretoria, South Africa), with the chemical composition of peat moss > 40%, vermiculite > 20%, and polystyrene. At the unifoliate leaf (UV) stage, the seedlings were transplanted into potting bags with the following dimensions: 350 mm length, 150 mm width, filled with dried loamy sandy soil (10 kg), with one seedling per pot. The soil was watered to 100% water holding capacity (WHC) with 1.6 L H
2O, bringing the soil weight to 11.6 kg (Optika balance, Optika S.r.l., Ponteranica, province of Bergamo, Italy). The trial design was a completely randomised block design with three replications.
A Hydrosense II meter, attached to a 12 cm sensor probe (CS659; Campbell Scientific, Stellenbosch, South Africa) was used to determine the amount of water needed to maintain the soil at 100% WHC. The volumetric water content (VWC) of the soil at this level was used to calculate the amount of water required daily to avoid water stress. To avoid nutrient deficiencies, plants were fertilised with full-strength (200 mL) Hygrotech nutrient solution, consisting of essential macro- and micronutrients, every two weeks until the end of the pod-filling stage.
For seed treatment, six seeds per cultivar were submerged in 200 mL sodium selenate (Na
2SeO
4, 50 mg/L) solution, followed by sowing in the seedling trays as mentioned above (the control was submerged in double-distilled water). For the soil drench method, 200 mL of selenium (50 mg/L) was added to the soil (the control was drenched with 200 mL of double distilled water) at the V2 stage. Similarly, at V2 stage, foliar selenium (50 mg/L, prepared in 200 mL) was sprayed until leaf droplets formed without dripping; controls received double-distilled water. The selection of a 50 mg/L concentration was guided by a prior study [
18], which identified the dose as within the optimal range for inducing the specific physio-chemical parameters in vegetable soybean under drought stress. The soil had a pH of 8.64, a soil redox of 217 M/V, and an electrical conductivity (EC) of 41 μS. The water holding capacity (WHC) was 1.6 L per 10 kg of soil (100% WHC). The total selenium content in the untreated soil was measured at 487 mg/kg, and macronutrient and micronutrient levels included nitrogen (0.048%), phosphorus (10 mg/kg), potassium (261.9 mg/kg), iron (9.8 mg/kg), calcium (408.83 mg/kg), and copper (0.043 mg/kg).
The method of measuring soil pH was according to Black [
56]. A soil sample of 5 g was added to a 100 mL conical flask filled with 50 mL of distilled water. The pH was measured using a Hanna HI98121 pH/ORP/Temperature Tester (three calibrated at 20 °C).
The redox potential of the soil was determined according to Barnard [
57]. A Hanna HI98121 pH/ORP/Temperature Tester (three-point-calibrated at 20 °C) was inserted into distilled water (25 mL) with 12.5 g of soil in a conical flask. The solution was shaken for 30 min, and the reading was taken.
The soil electrical conductivity of a saturated paste was determined according to Barnard [
57]. Deionised water was added to a beaker containing a 250 g sample of soil until it was completely saturated. The soil was evaluated for the characteristics of a saturated paste, i.e., no surplus water on the surface of the soil. The saturated paste was let to stand for at least an hour before being filtered using Whatman No. 40 filter paper and a Buchman filter. A Metrohm Touch Control Swiss mode was used to test the temperature and conductivity of the filtrate.
Plants were continuously watered to 100% WHC until the initiation of drought stress. Drought stress was induced by withholding irrigation until 30% WHC at the third trifoliate leaf stage (V4). Irrigation was then maintained at either 30% or 100% WHC until the completion of the plant life cycle. Physiological and biochemical measurements were performed at the flowering and pod-filling stages, as these are the most sensitive to drought [
30]. Young but fully expanded trifoliate leaves were used for both non-destructive (chlorophyll fluorescence and stomatal conductance) and destructive (chloroplast pigments, hydrogen peroxide, and antioxidative enzymes) measurements, as they are actively growing. Leaf sampling and non-destructive measurements were conducted between 09:00 a.m. and noon.
4.2. The Physiological Measurements
A pocket photosynthesis efficiency analyser (PEA, Hansatech Instrument Ltd., Pentney, Norfolk, UK) was used to measure the effects of different selenium application methods on the photosynthetic efficiency of drought-stressed vegetable soybean at the flowering and pod-filling stages. To ensure repeatability, chlorophyll a fluorescence data were collected three times for each young, fully expanded trifoliate leaf. Sample leaves were dark-adapted for 30 min using lightweight leaf clips with closed shutter plates. The clips were placed to the middle part of leaves, avoiding the midrib and leaf edges. Measurements included the maximum quantum efficiency of photosystem II (Fv/Fm), total performance index (PItotal), performance index for absorbance (PIabs), and energy dissipation as heat (DI0/RC).
The method described by Su et al. [
58] was used to determine the concentrations of chlorophyll a (chl-
a), chlorophyll b (chl-
b), and carotenoid. Frozen leaf samples (0.1 g) were extracted with ice-cold 80% (
v/
v) acetone (5 mL) using a pestle and mortar on ice. The extract was centrifuged at 11,000×
g for 10 min, and the absorbance of the supernatant was measured at 663, 645, and 480 nm. The total chlorophyll, chl-
a, chl-
b, and carotenoid contents were calculated according to Pareek et al. [
59].
A leaf porometer SC-1 (Li-Cor. ADC BioScientific Ltd., Hoddesdon, UK), which quantifies the humidity gradient between the chamber and the leaf surface surroundings, was used to measure stomatal conductance on young, fully expanded trifoliate leaf.
Relative water content (RWC) was determined according to the method described by González and González-Vilar [
60]. Leaves were placed in airtight 50 mL Falcon tubes to prevent moisture loss. Fresh weight (FW) was recorded immediately in the laboratory. After adding 10 mL of double-distilled water, the tubes were incubated at 4 °C for 24 h and the turgid weight (TW) was recorded. After oven-drying at 72 °C for 72 h, dry weight (DW) was recorded. The RWC (%) was calculated using the formula; RWC (%) = (FW − DW)/(TW − DW) × 100.
4.3. The Biochemical Measurements
A modified method described by Velikova et al. [
61] was used for the H
2O
2 assay. Trichloroacetic acid (TCA, 0.1%
w/
v, 1 mL) was added to 0.1 g of frozen leaf tissue, homogenised on ice, and centrifuged at 12,000×
g for 15 min at 4 °C. To the supernatant (0.25 mL), 0.25 mL of 10 mM potassium phosphate buffer (pH 7.0) and 0.5 mL 1 M potassium iodide were added. For the blank, 0.25 mL of 0.1% (
w/
v) TCA was used in place of the supernatant. Absorbance was measured at 390 nm (Cary 100 Bio, Varian, Australia) after the reaction developed for 1 h in the dark at room temperature.
4.3.1. Enzyme Extract Preparation
Enzyme extracts were prepared according to Pukacka and Ratajczak [
62]. Leaves were ground in liquid nitrogen to fine powder using a mortar and pestle. Leaf powder (0.1 g) for each treatment was homogenised on ice in 1 mL of 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA, 2% (
w/
v) PVPP, 0.1% (
v/
v) Triton X-100. and 1 mM ascorbate (to oxidise the intracellular H
2O
2). The homogenate was centrifuged at 15,000×
g for 20 min at 4 °C. The supernatant was used as the enzyme extract and assayed in triplicate for all enzyme analyses.
4.3.2. Antioxidative Enzyme Assays and Protein Content
The ascorbate peroxidase (APX) assay was performed according to the modified method of Mishra et al. [
63]. The reaction mixture (1 mL) consisted of 500 μL of 50 mM potassium phosphate buffer (pH 7.0), 200 μL of 0.1 mM H
2O
2, 150 μL of 0.5 mM sodium ascorbate, and 150 μL of enzyme extract. Ascorbate oxidation was measured at 290 nm (Cary 100 Bio, Varian, Australia) for 3 min at 30 °C against a blank in which the enzyme extract was replaced with phosphate buffer.
Guaiacol peroxidase (GPX) activity was determined following the method of Zieslin and Ben-Zaken [
64]. The reaction mixture (1 mL) consisted of 500 µL of 80 mM potassium phosphate buffer (pH 5.5), 50 µL of 0.2 M H
2O
2, 100 µL of 50 mM guaiacol, 340 µL distilled water, and 10 µL of enzyme extract. The absorbance was monitored at 470 nm (Cary 100 Bio, Varian, Australia) over 3 min at 30 °C.
Superoxide dismutase (SOD) activity was determined using a modified method of Hwang et al. [
65]. Enzyme extract (15 μL) was mixed with 1.28 mL of 100 mM potassium phosphate buffer (pH 7.8), 40 μL of 55 mM methionine, 150 μL of 0.75 mM nitroblue tetrazolium (NBT), and 30 μL of 0.1 mM riboflavin. The cuvettes containing reaction mixtures were irradiated under two light bulbs (1000 kW) placed side by side (3 cm apart) and a high of 28 cm for 30 min. The control, prepared in the same way, contained all reactants except the enzyme. A non-irradiated reference was kept in the dark for the duration of the experiment. One unit of SOD activity was defined as the amount of enzyme required to inhibit 50% of NBT photoreduction.
Protein content was quantified using the Bradford method [
66], with gamma globulin serving as the standard (0.5 mg/ mL), in a 96-well microplate (Greiner Bio-One, Kremsmunster, Austria). The absorbance was measured at 595 nm using a microplate reader (Anthos Labtech Inc., GmbH, Salzburg, Austria).
Three replicates per treatment and three technical replicates per measured parameter.
4.4. Yield Traits
The following growth and yield traits were measured at R8 stage: plant height (PH), number of branches per plant (BPP), number of pods per plant (PPP) and number of seeds per plant (SPP).
4.5. Statistical Analysis
For the statistical analysis, data were subjected to a three-way analysis of variance (ANOVA) using Statistica version 7 (TIBCO Software Inc., Palo Alto, CA, USA) to test the effects of selenium treatment (Se), application method (seed, foliar, and soil), and cultivar (UVE14 and UVE17), as well as their interactions, on physiological and biochemical parameters. When significant main or interaction effects were detected, Fisher’s LSD was used for post hoc comparisons to determine differences between means at p ≤ 0.05.
For parameters showing significant three-way interactions (Se × Method × Cultivar), simple two-way interactions were further analysed within each cultivar to identify specific treatment differences. Means followed by different lowercase letters indicate significant differences (p ≤ 0.05) among treatment combinations within each cultivar and growth stage, whereas shared letters indicate no significant difference.
Statistical analyses were performed separately for each growth stage (flowering and pod-filling) to assess stage-specific responses. The relationship between yield and physio-biochemical parameters was analysed using correlation matrices, providing regression coefficients and p-values at a 95% confidence level.