Light Scattering of Leaf Surface and Spongy Mesophyll and Concentration of Anthocyanin Influence Typical and Modified Photochemical Reflectance Indices
Abstract
1. Introduction
2. Analytical Model of Light Reflectance and Transmittance in Leaves of Dicot Plants
2.1. Brief Description of the Analytical Model of Light Reflectance and Transmittance in Leaves
- -
- -
- Light transmittance and scattering of the palisade mesophyll layer: It was assumed that light scattering in this layer was low (in accordance with [70]). As a result, this scattering was not considered in the main description of light flows in the palisade mesophyll; the Beer–Bouguer–Lambert law was used for this description [71]. However, light scattering of the palisade mesophyll was described as the additional component of reflectance in the adaxial leaf surface (see Equation (S38) in File S1).
- -
- Light transmittance and scattering of the spongy mesophyll layer: It is known that this layer has a high coefficient of light scattering [70]. As a result, optical properties of the spongy mesophyll layer were described on the basis of the Kubelka–Munk model, which considered four light flows including forward- and backward-collimated light and forward- and backward-scattered light [72,73].
2.2. Parameters of the Analytical Model of Light Reflectance and Transmittance in Leaves
3. Results
3.1. Typical and Modified Photochemical Reflectance Indices Described by the Model at Basic Values of Parameters
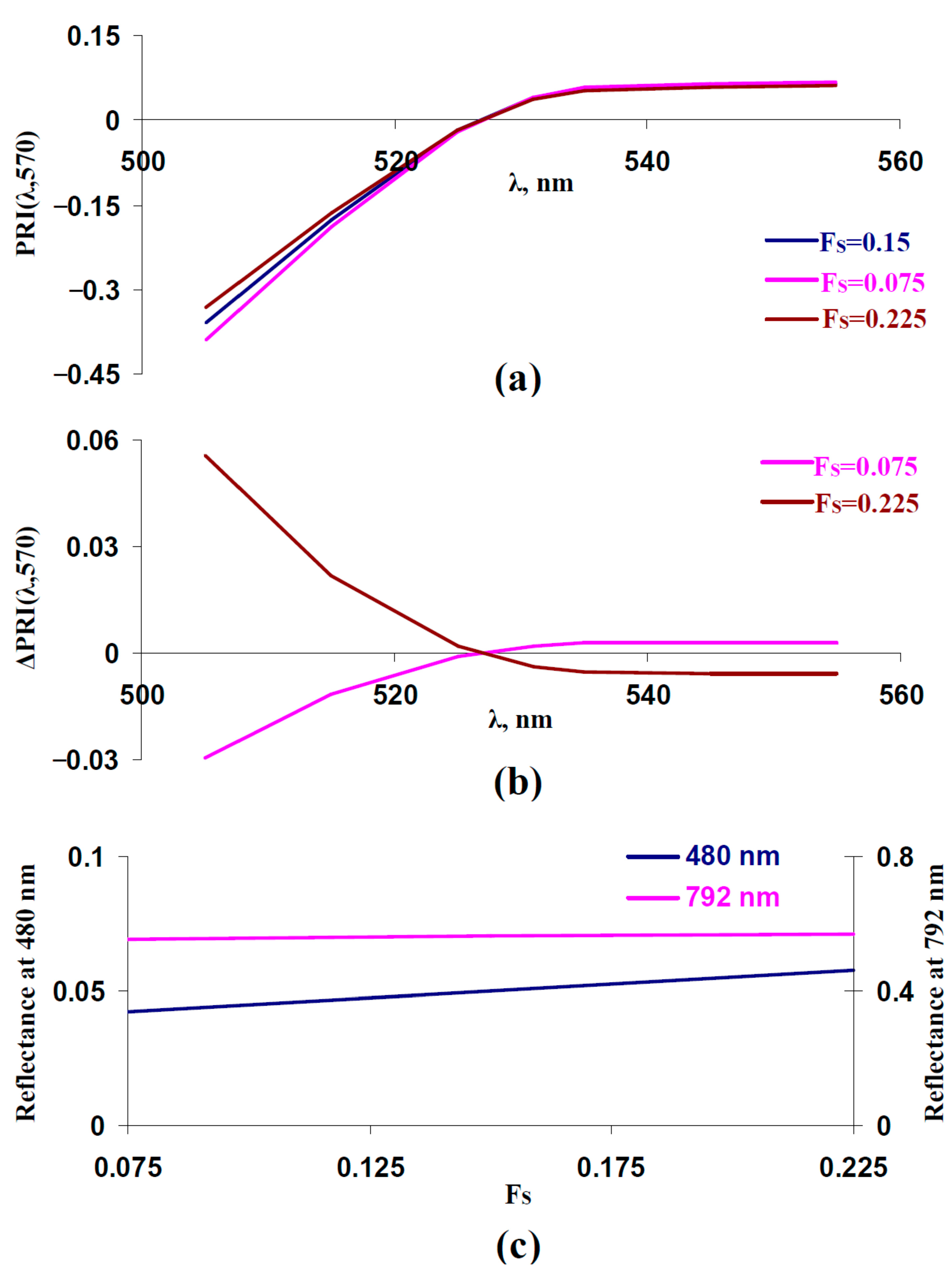
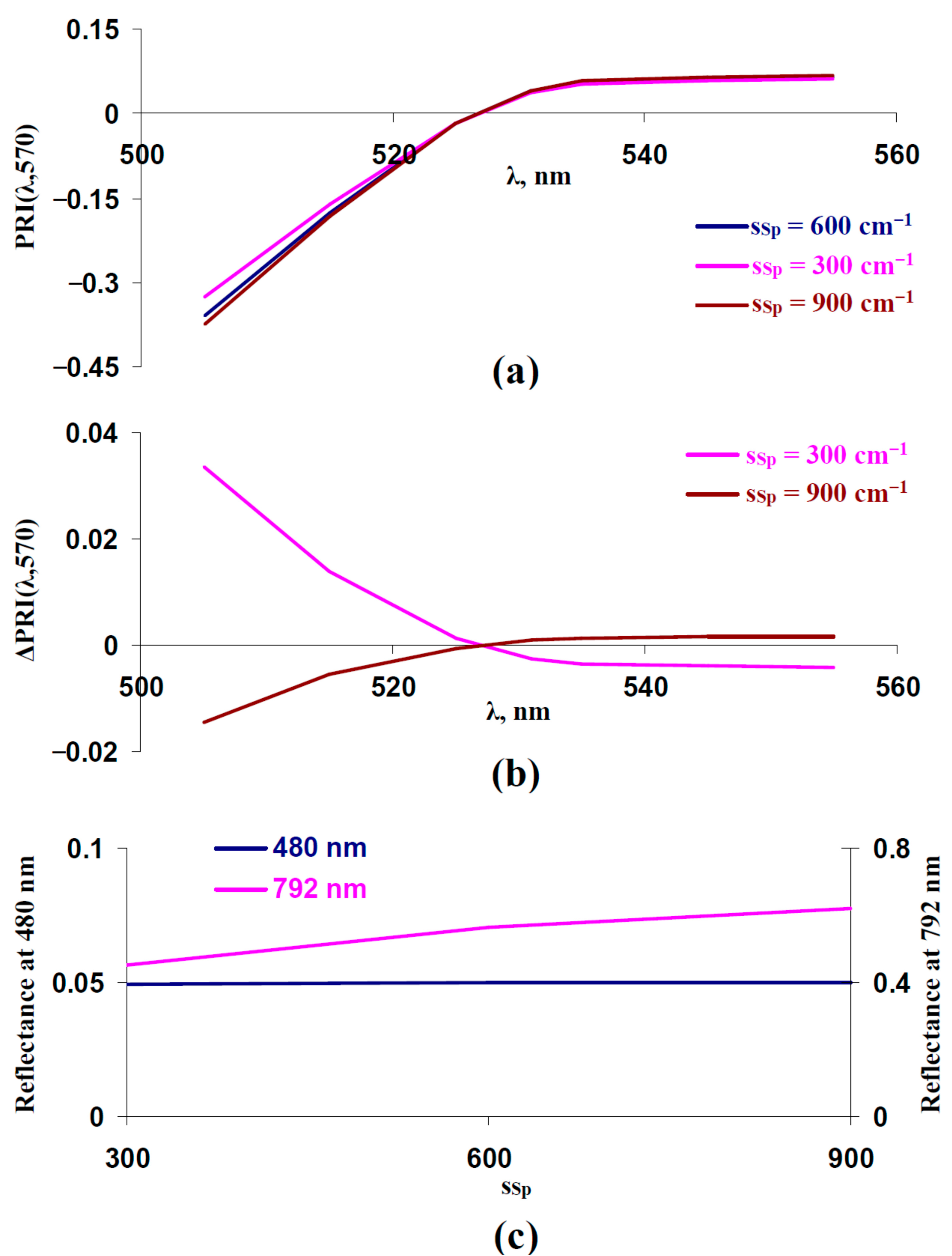
3.2. Influence of Light Scattering of Leaf Surface and Mesophyll Layers on Typical and Modified Photochemical Reflectance Indices
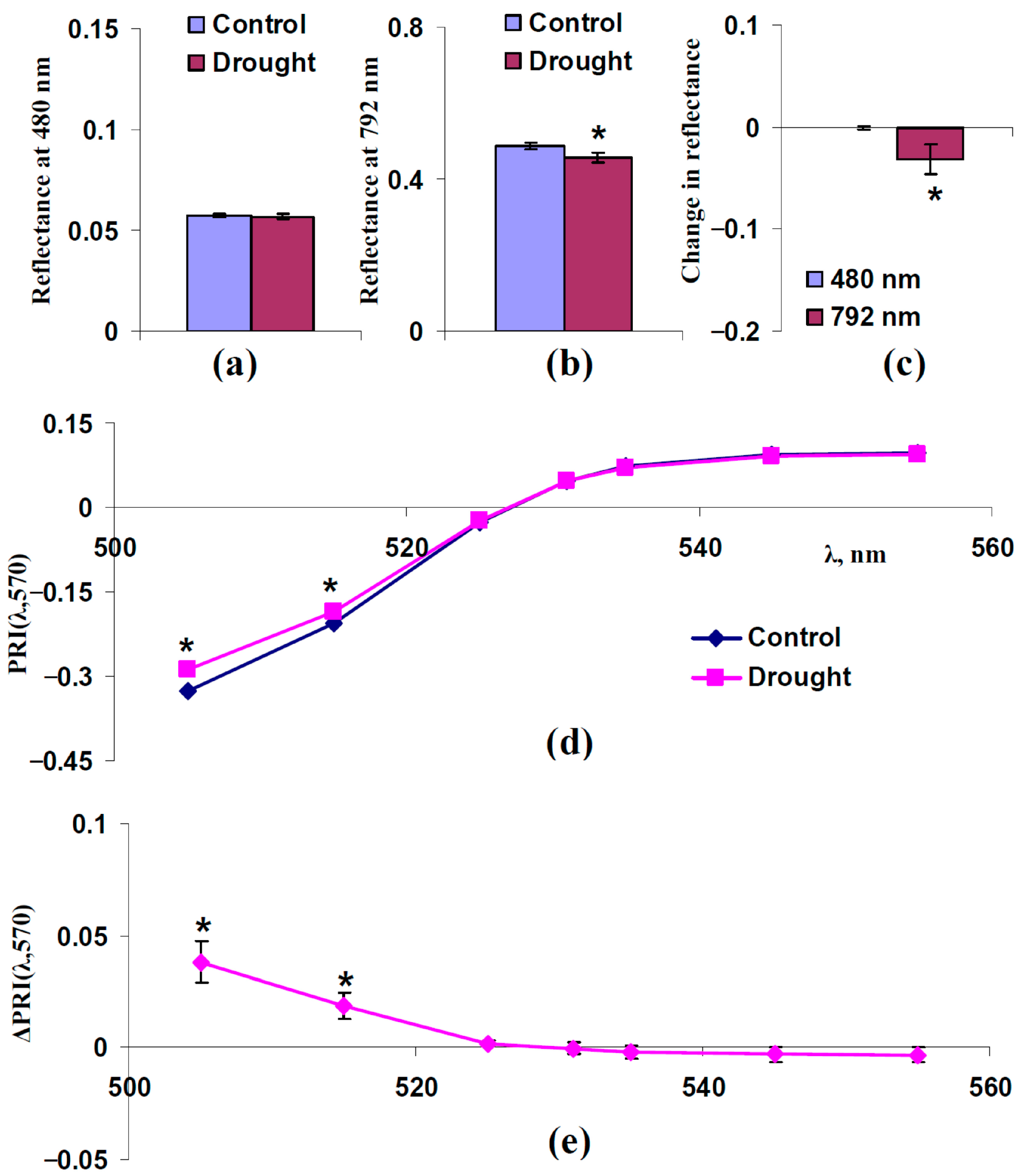
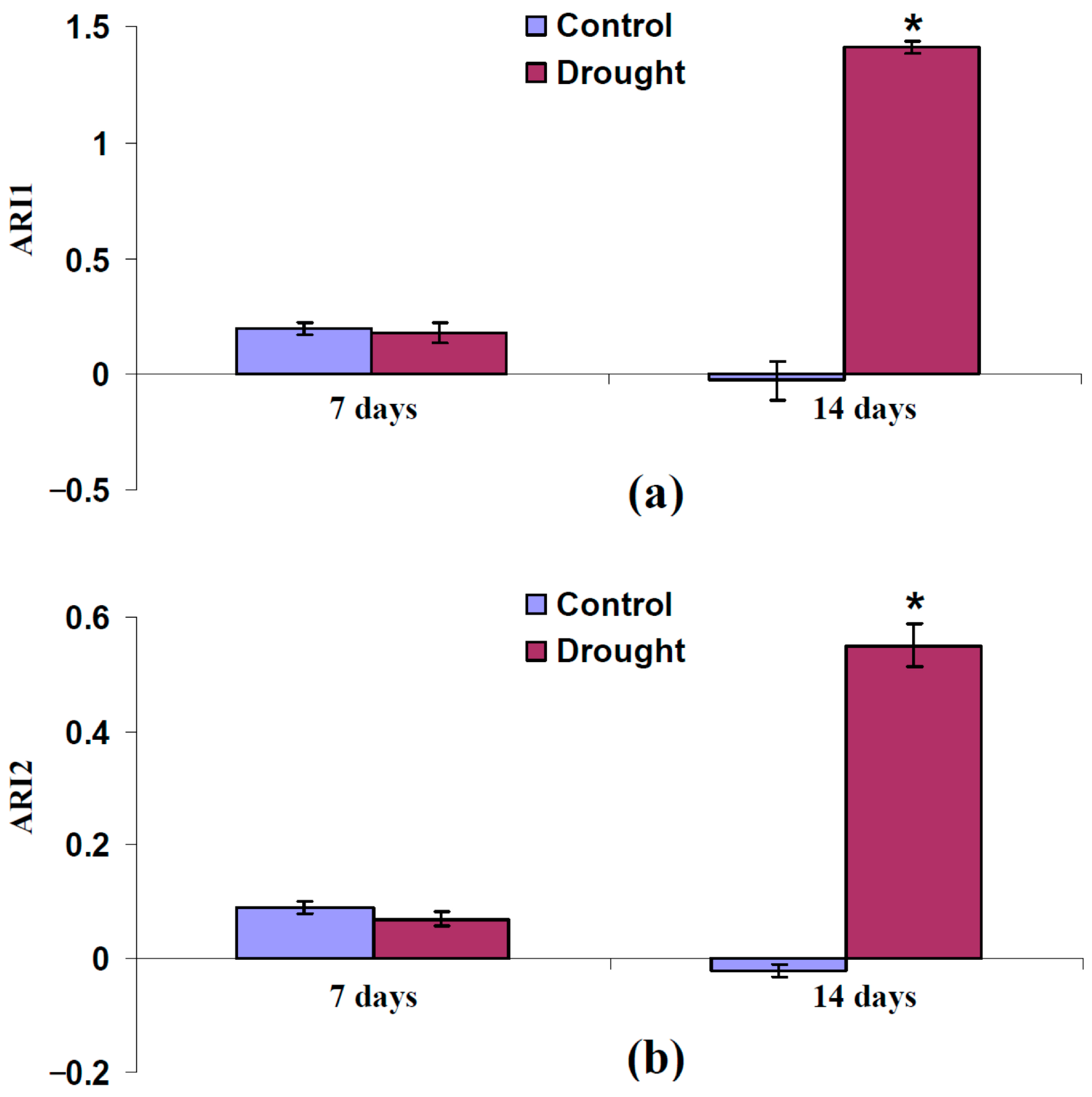
3.3. Analysis of Participation of Changes in Light Scattering of Leaf Surface and Spongy Mesophyll Layer in Changes in Typical and Modified Photochemical Reflectance Indices Under Drought
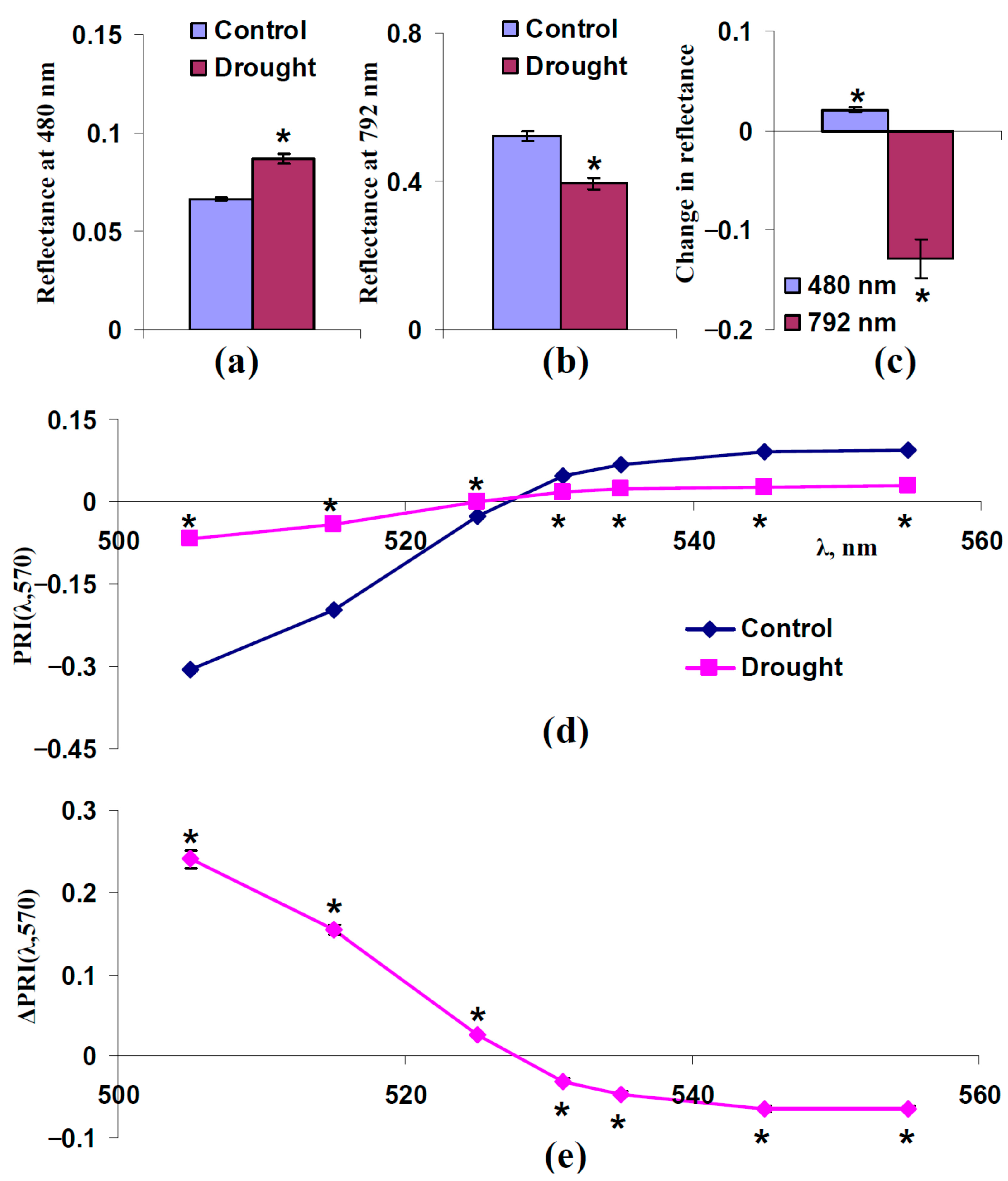
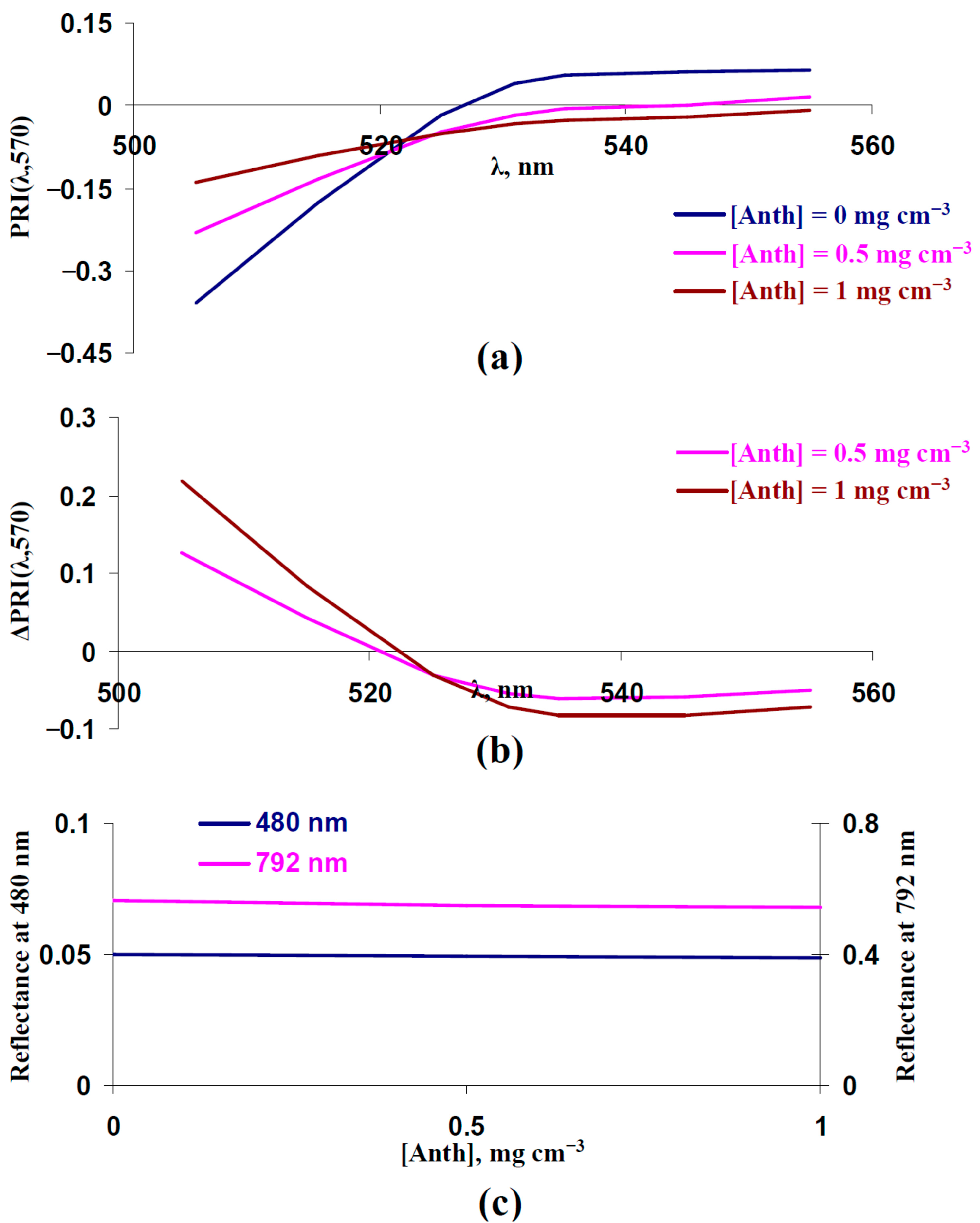
3.4. Analysis of Participation of Changes in Concentrations of Photosynthetic Pigments in Changes in Typical and Modified Photochemical Reflectance Indices Under Drought
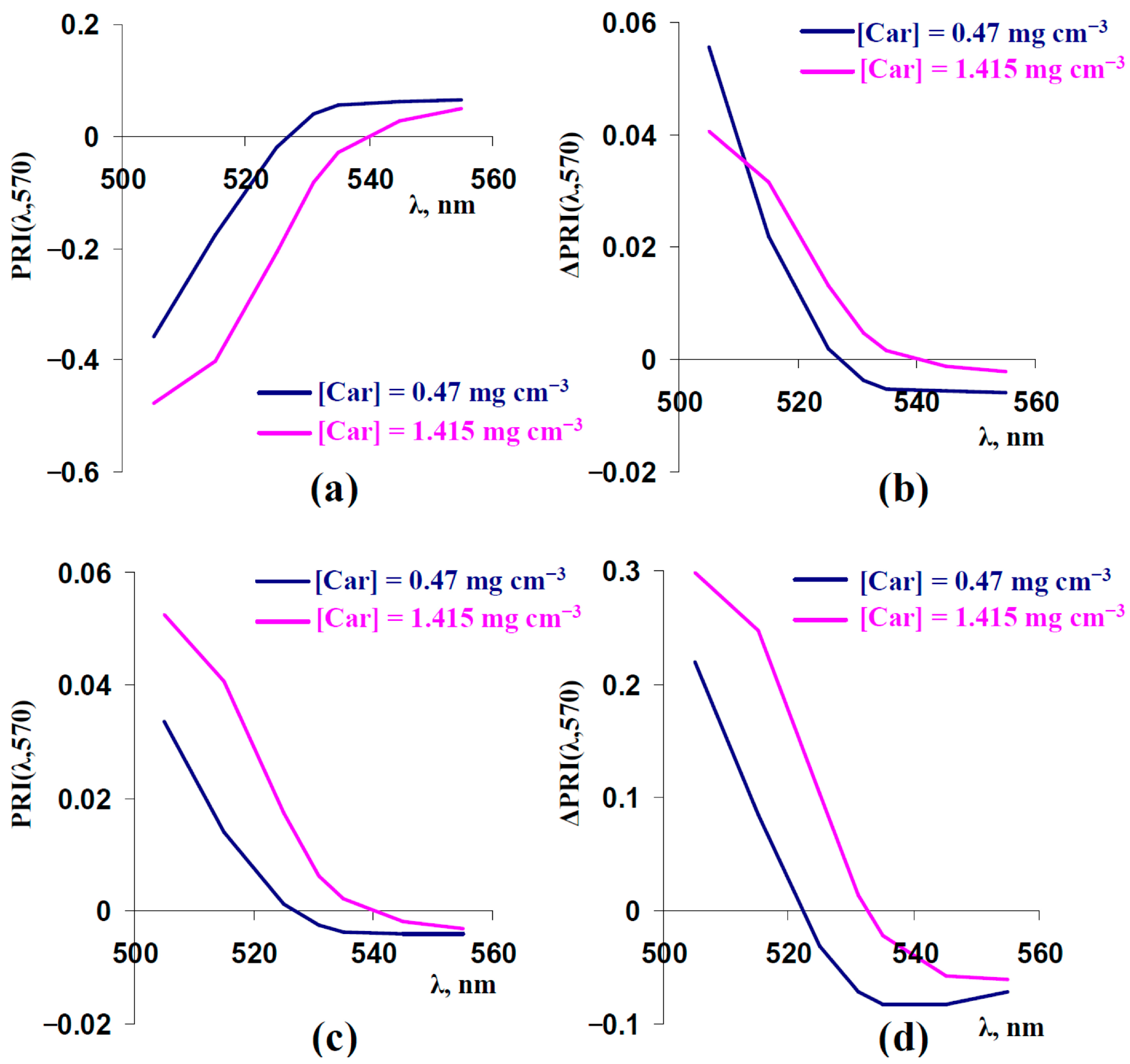
3.5. Analysis Changes in Typical and Modified Photochemical Reflectance Indices Under Increased Concentrations of Carotenoids
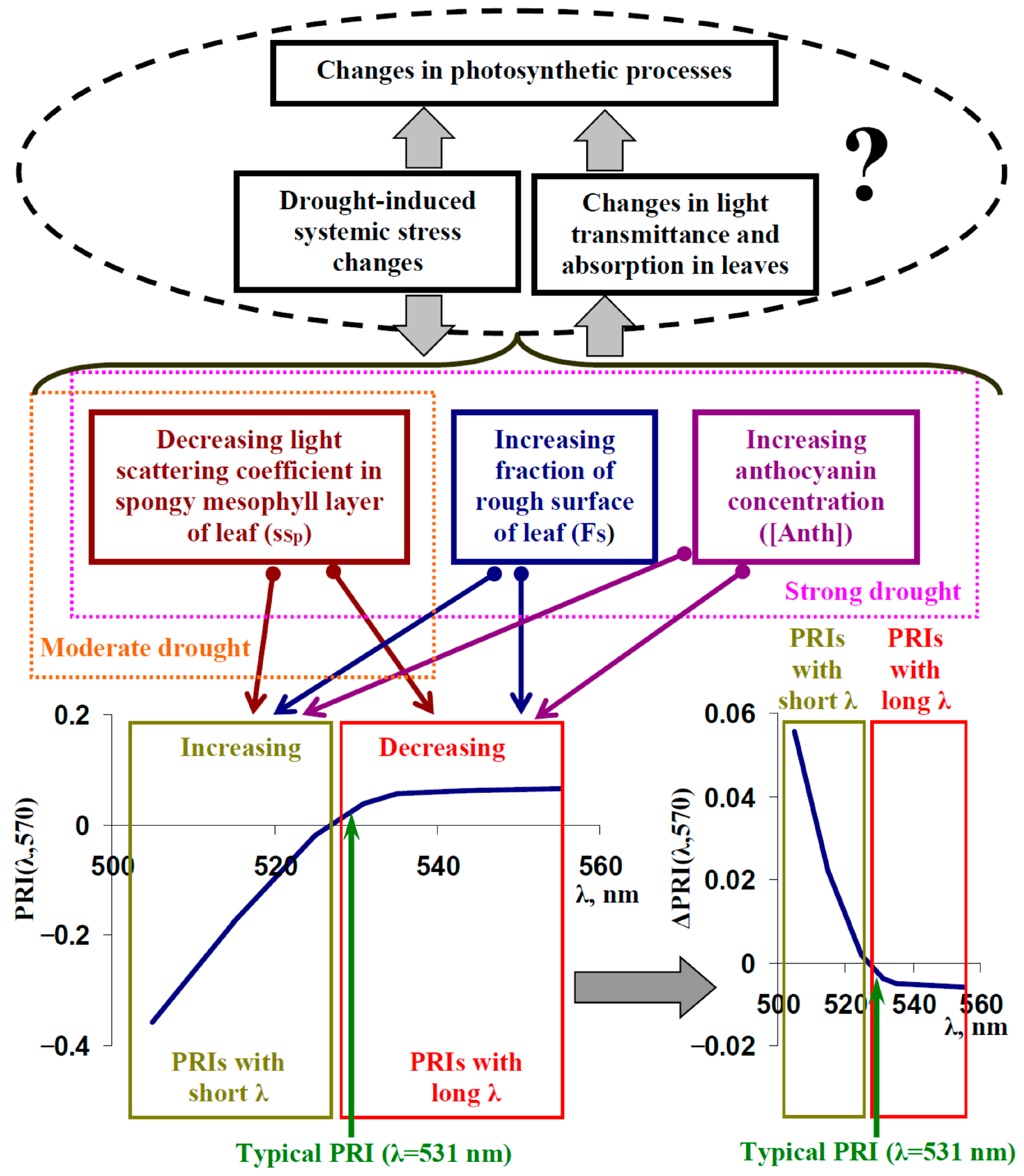
4. Discussion
5. Materials and Methods
5.1. Plant Cultivation and Drought
5.2. Measurements of Reflectance of Leaves and Calculation of Reflectance Indices
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PRI | Photochemical reflectance index |
| R531 | Light reflectance at 531 nm |
| R570 | Light reflectance at 570 nm |
| PRI(λ,570) | Photochemical reflectance index with measuring wavelength equaling to λ |
| ΔPRI(λ,570) | Changes in PRI(λ,570) |
| ARI1 | Anthocyanin reflectance index 1 |
| ARI2 | Anthocyanin reflectance index 2 |
| FS | Fraction of the rough surface |
| sSp | Light scattering coefficient in the spongy mesophyll layer |
| [Anth] | Average concentration of anthocyanin |
| [Chl a] | Average concentration of chlorophyll a |
| [Chl b] | Average concentration of chlorophyll b |
| [Car] | Average concentration of carotenoids |
References
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Bäurle, I. Plant heat adaptation: Priming in response to heat stress. F1000Research 2016, 5, 694. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Salaria, N.; Thakur, K.; Kukreja, S.; Gautam, S.; Goutam, U. Functional genomic approaches to improve crop plant heat stress tolerance. F1000Research 2019, 8, 1721. [Google Scholar] [CrossRef] [PubMed]
- Jha, U.C.; Bohra, A.; Jha, R. Breeding approaches and genomics technologies to increase crop yield under low-temperature stress. Plant Cell Rep. 2017, 36, 1–35. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, E.; Morales, A.; Harbinson, J. Fluctuating light takes crop photosynthesis on a rollercoaster ride. Plant Physiol. 2018, 176, 977–989. [Google Scholar] [CrossRef]
- Zeppel, M.J.B.; Wilks, J.V.; Lewis, J.D. Impacts of extreme precipitation and seasonal changes in precipitation on plants. Biogeosciences 2014, 11, 3083–3093. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef]
- Zivcak, M.; Brestic, M.; Balatova, Z.; Drevenakova, P.; Olsovska, K.; Kalaji, H.M.; Yang, X.; Allakhverdiev, S.I. Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 2013, 117, 529–546. [Google Scholar] [CrossRef]
- Allakhverdiev, S.I.; Kreslavski, V.D.; Klimov, V.V.; Los, D.A.; Carpentier, R.; Mohanty, P. Heat stress: An overview of molecular responses in photosynthesis. Photosynth. Res. 2008, 98, 541–550. [Google Scholar] [CrossRef]
- Zhang, R.; Sharkey, T.D. Photosynthetic electron transport and proton flux under moderate heat stress. Photosynth. Res. 2009, 100, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Evolution under the sun: Optimizing light harvesting in photosynthesis. J. Exp. Bot. 2015, 66, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from Photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [PubMed]
- Kior, A.; Sukhov, V.; Sukhova, E. Application of reflectance indices for remote sensing of plants and revealing actions of stressors. Photonics 2021, 8, 582. [Google Scholar] [CrossRef]
- Kior, A.; Yudina, L.; Zolin, Y.; Sukhov, V.; Sukhova, E. RGB imaging as a tool for remote sensing of characteristics of terrestrial plants: A review. Plants 2024, 13, 1262. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 2–17. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Signature analysis of leaf reflectance spectra: Algorithm development for remote sensing of chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive optical detection of pigment changes during leaf senescence and fruit ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Penuelas, J.; Pinol, J.; Ogaya, R.; Filella, I. Estimation of plant water concentration by the reflectance Water Index WI (R900/R970). Int. J. Remote Sens. 1997, 18, 2869–2875. [Google Scholar] [CrossRef]
- Wu, C.; Niu, Z.; Tang, Q.; Huang, W. Predicting vegetation water content in wheat using normalized difference water indices derived from ground measurements. J. Plant Res. 2009, 122, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Eitel, J.U.H.; Long, D.S.; Gessler, P.E.; Hunt, E.R. Combined spectral index to improve ground-based estimates of nitrogen status in dryland wheat. Agr. J. 2008, 100, 1694–1702. [Google Scholar] [CrossRef]
- Schlemmer, M.; Gitelson, A.; Schepers, J.; Ferguson, R.; Peng, Y.; Shanahana, J.; Rundquist, D. Remote estimation of nitrogen and chlorophyll contents in maize at leaf and canopy levels. Int. J. Appl. Earth Obser. Geoinform. 2013, 25, 47–54. [Google Scholar] [CrossRef]
- Jiang, J.; Chen, S.; Cao, S.; Wu, H.; Zhang, L.; Zhang, H. Leaf area index retrieval based on canopy reflectance and vegetation index in eastern China. J. Geogr. Sci. 2005, 15, 247–254. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, D. Improving forest aboveground biomass estimation using seasonal Landsat NDVI time-series. ISPRS J. Photogram. Remote Sens. 2015, 102, 222–231. [Google Scholar] [CrossRef]
- Xing, N.; Huang, W.; Xie, Q.; Shi, Y.; Ye, H.; Dong, Y.; Wu, M.; Sun, G.; Jiao, Q.A. Transformed triangular vegetation index for estimating winter wheat leaf area index. Remote Sens. 2020, 12, 16. [Google Scholar] [CrossRef]
- Gamon, J.A.; Field, C.B.; Bilger, W.; Björkman, O.; Fredeen, A.L.; Peñuelas, J. Remote sensing of the xanthophyll cycle and chlorophyll fluorescence in sunflower leaves and canopies. Oecologia 1990, 85, 1–7. [Google Scholar] [CrossRef]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A narrow-waveband spectral index that tracks diurnal changes in photosynthetic efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Gamon, J.A. Assessment of photosynthetic radiation-use efficiency with spectral reflectance. New Phytol. 1995, 131, 291–296. [Google Scholar] [CrossRef]
- Gamon, J.A.; Serrano, L.; Surfus, J.S. The photochemical reflectance index: An optical indicator of photosynthetic radiation use efficiency across species, functional types, and nutrient levels. Oecologia 1997, 112, 492–501. [Google Scholar] [CrossRef]
- Garbulsky, M.F.; Peñuelas, J.; Gamon, J.; Inoue, Y.; Filella, I. The photochemical reflectance index (PRI) and the remote sensing of leaf, canopy and ecosystem radiation use efficiencies. A review and meta-analysis. Remote Sens. Environ. 2011, 115, 281–297. [Google Scholar] [CrossRef]
- Zhang, C.; Filella, I.; Garbulsky, M.F.; Peñuelas, J. Affecting factors and recent improvements of the photochemical reflectance index (PRI) for remotely sensing foliar, canopy and ecosystemic radiation-use efficiencies. Remote Sens. 2016, 8, 677. [Google Scholar] [CrossRef]
- Sukhova, E.; Sukhov, V. Connection of the Photochemical Reflectance Index (PRI) with the photosystem ii quantum yield and nonphotochemical quenching can be dependent on variations of photosynthetic parameters among investigated plants: A meta-analysis. Remote Sens. 2018, 10, 771. [Google Scholar] [CrossRef]
- Evain, S.; Flexas, J.; Moya, I. A new instrument for passive remote sensing: 2. Measurement of leaf and canopy reflectance changes at 531 nm and their relationship with photosynthesis and chlorophyll fluorescence. Remote Sens. Environ. 2004, 91, 175–185. [Google Scholar] [CrossRef]
- Sarlikioti, V.; Driever, S.M.; Marcelis, L.F.M. Photochemical reflectance index as a mean of monitoring early water stress. Ann. Appl. Biol. 2010, 157, 81–89. [Google Scholar] [CrossRef]
- Osório, J.L.; Osório, M.L.; Romano, A. Reflectance indices as nondestructive indicators of the physiological status of Ceratonia siliqua seedlings under varying moisture and temperature regimes. Funct. Plant Biol. 2012, 39, 588–597. [Google Scholar] [CrossRef]
- Hmimina, G.; Dufrêne, E.; Soudani, K. Relationship between photochemical reflectance index and leaf ecophysiological and biochemical parameters under two different water statuses: Towards a rapid and efficient correction method using real-time measurements. Plant Cell Environ. 2014, 37, 473–487. [Google Scholar] [CrossRef]
- Sukhova, E.; Yudina, L.; Kior, A.; Kior, D.; Popova, A.; Zolin, Y.; Gromova, E.; Sukhov, V. Modified photochemical reflectance indices as new tool for revealing influence of drought and heat on pea and wheat plants. Plants 2022, 11, 1308. [Google Scholar] [CrossRef]
- Zinnert, J.C.; Nelson, J.D.; Hoffman, A.M. Effects of salinity on physiological responses and the photochemical reflectance index in two co-occurring coastal shrubs. Plant Soil. 2012, 354, 45–55. [Google Scholar] [CrossRef]
- Sukhova, E.; Zolin, Y.; Popova, A.; Yudina, L.; Sukhov, V. The influence of soil salt stress on modified photochemical reflectance indices in pea plants. Remote Sens. 2023, 15, 3772. [Google Scholar] [CrossRef]
- Shrestha, S.; Brueck, H.; Asch, F. Chlorophyll index, photochemical reflectance index and chlorophyll fluorescence measurements of rice leaves supplied with different N levels. J. Photochem. Photobiol. B Biol. 2012, 113, 7–13. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams III, W.W. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef]
- Kohzuma, K.; Hikosaka, K. Physiological validation of photochemical reflectance index (PRI) as a photosynthetic parameter using Arabidopsis thaliana mutants. Biochem. Biophys. Res. Commun. 2018, 498, 52–57. [Google Scholar] [CrossRef]
- Tikhonov, A.N. pH-dependent regulation of electron transport and ATP synthesis in chloroplasts. Photosynth. Res. 2013, 116, 511–534. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, A.N. The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol. Biochem. 2014, 81, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh-Bajgiran, P.; Munehiro, M.; Omasa, K. Relationships between the photochemical reflectance index (PRI) and chlorophyll fluorescence parameters and plant pigment indices at different leaf growth stages. Photosynth. Res. 2012, 113, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K.; Tsujimoto, K. Linking remote sensing parameters to CO2 assimilation rates at a leaf scale. J. Plant Res. 2021, 134, 695–711. [Google Scholar] [CrossRef]
- Murakami, K.; Ibaraki, Y. Time course of the photochemical reflectance index during photosynthetic induction: Its relationship with the photochemical yield of photosystem II. Physiol. Plant 2019, 165, 524–536. [Google Scholar] [CrossRef] [PubMed]
- Hikosaka, K. Photosynthesis, chlorophyll fluorescence and photochemical reflectance index in photoinhibited leaves. Funct. Plant Biol. 2021, 48, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.; Tomimatsu, H.; Hikosaka, K. The relationships between photochemical reflectance index (PRI) and photosynthetic status in radish species differing in salinity tolerance. J. Plant Res. 2025, 138, 231–241. [Google Scholar] [CrossRef]
- Sukhov, V.; Sukhova, E.; Khlopkov, A.; Yudina, L.; Ryabkova, A.; Telnykh, A.; Sergeeva, E.; Vodeneev, V.; Turchin, I. Proximal imaging of changes in photochemical reflectance index in leaves based on using pulses of green-yellow light. Remote Sens. 2021, 13, 1762. [Google Scholar] [CrossRef]
- Filella, I.; Porcar-Castell, A.; Munné-Bosch, S.; Bäck, J.; Garbulsky, M.F.; Peñuelas, J. PRI assessment of long-term changes in carotenoids/chlorophyll ratio and short-term changes in de-epoxidation state of the xanthophyll cycle. Int. J. Remote Sens. 2009, 30, 4443–4455. [Google Scholar] [CrossRef]
- Porcar-Castell, A.; Garcia-Plazaola, J.I.; Nichol, C.J.; Kolari, P.; Olascoaga, B.; Kuusinen, N.; Fernández-Marín, B.; Pulkkinen, M.; Juurola, E.; Nikinmaa, E. Physiology of the seasonal relationship between the photochemical reflectance index and photosynthetic light use efficiency. Oecologia 2012, 170, 313–323. [Google Scholar] [CrossRef]
- Wong, C.Y.; Gamon, J.A. Three causes of variation in the photochemical reflectance index (PRI) in evergreen conifers. New Phytol. 2015, 206, 187–195. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gamon, J.A.; Solovchenko, A. Multiple drivers of seasonal change in PRI: Implications for photosynthesis 1. Leaf level. Remote Sens. Environ. 2017, 191, 110–116. [Google Scholar] [CrossRef]
- Kováč, D.; Veselovská, P.; Klem, K.; Večeřová, K.; Ač, A.; Peñuelas, J.; Urban, O. Potential of photochemical reflectance index for indicating photochemistry and light use efficiency in leaves of European beech and Norway spruce trees. Remote Sens. 2018, 10, 1202. [Google Scholar] [CrossRef]
- Kováč, D.; Veselá, B.; Klem, K.; Večeřová, K.; Kmecová, Z.M.; Peñuelas, J.; Urban, O. Correction of PRI for carotenoid pigment pools improves photosynthesis estimation across different irradiance and temperature conditions. Remote Sens. Environ. 2020, 244, 111834. [Google Scholar] [CrossRef]
- Kohzuma, K.; Tamaki, M.; Hikosaka, K. Corrected photochemical reflectance index (PRI) is an effective tool for detecting environmental stresses in agricultural crops under light conditions. J. Plant Res. 2021, 134, 683–694. [Google Scholar] [CrossRef]
- Kior, A.; Yudina, L.; Zolin, Y.; Popova, A.; Sukhova, E.; Sukhov, V. A small-scale spatial heterogeneity in photochemical reflectance index and intensity of reflected light at 530 nm in pea (Pisum sativum) leaves is sensitive to action of salinization. Funct. Plant Biol. 2024, 51, FP24254. [Google Scholar] [CrossRef]
- Sukhova, E.; Sukhov, V. Relation of photochemical reflectance indices based on different wavelengths to the parameters of light reactions in photosystems I and II in pea plants. Remote Sens. 2020, 12, 1312. [Google Scholar] [CrossRef]
- Yu, Y.; Piao, J.; Fan, W.; Yang, X. Modified photochemical reflectance index to estimate leaf maximum rate of carboxylation based on spectral analysis. Environ. Monit. Assess. 2020, 192, 788. [Google Scholar] [CrossRef]
- Peters, R.D.; Noble, S.D. Spectrographic measurement of plant pigments from 300 to 800 nm. Remote Sens. Environ. 2014, 148, 119–123. [Google Scholar] [CrossRef]
- Sukhova, E.; Zolin, Y.; Grebneva, K.; Berezina, E.; Bondarev, O.; Kior, A.; Popova, A.; Ratnitsyna, D.; Yudina, L.; Sukhov, V. Development of analytical model to describe reflectance spectra in leaves with palisade and spongy mesophyll. Plants 2024, 13, 3258. [Google Scholar] [CrossRef]
- Prahl, S.A.; Keijzer, M.; Jacques, S.L.; Welch, A.J. A Monte Carlo model of light propagation in tissue. SPIE Inst. Ser. 1989, 5, 102–111. [Google Scholar]
- Lim, H.S.; Kenar, N. Estimation of photon distribution within biological tissue using Monte Carlo simulation. Biomed. J. Sci. Tech. Res. 2017, 1, 1021–1023. [Google Scholar] [CrossRef][Green Version]
- Maier, S.W.; Lüdeker, W.; Günther, K.P. SLOP: A revised version of the stochastic model for leaf optical properties. Remote Sens. Environ. 1999, 68, 273–280. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Ustin, S. Leaf Optical Properties; Cambridge University Press: Cambridge, UK, 2019; pp. 229–264. [Google Scholar]
- Fukshansky, L.; Fukshansky-Kazarinova, N.; Remisowsky, A.M. Estimation of optical parameters in a living tissue by solving the inverse problem of the multiflux radiative transfer. Appl. Opt. 1991, 30, 3145–3153. [Google Scholar] [CrossRef]
- Richter, T.; Fukshansky, L. Optics of a Bifacial Leaf: 1. A novel combined procedure for deriving the optical parameters. Photochem. Photobiol. 1996, 63, 507–516. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The role of anthocyanins in plant tolerance to drought and salt stresses. Plants 2023, 12, 2558. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Ouzounidou, G.; Moustakas, M. Hormesis responses of photosystem II in Arabidopsis thaliana under water deficit stress. Int. J. Mol. Sci. 2023, 24, 9573. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Sohail; Zaman, S.; Li, G.; Fu, M. Adaptive responses of plants to light stress: Mechanisms of photoprotection and acclimation. A review. Front. Plant Sci. 2025, 16, 1550125. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 71, 38–45. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Chivkunova, O.B.; Merzlyak, M.N. Nondestructive estimation of anthocyanins and chlorophylls in anthocyanic leaves. Am. J. Bot. 2009, 96, 1861–1868. [Google Scholar] [CrossRef]
- Zolin, Y.; Popova, A.; Yudina, L.; Grebneva, K.; Abasheva, K.; Sukhov, V.; Sukhova, E. RGB indices can be used to estimate NDVI, PRI, and Fv/Fm in wheat and pea plants under soil drought and salinization. Plants 2025, 14, 1284. [Google Scholar] [CrossRef]
- Govindjee; Shevela, D.; Björn, L.O. Evolution of the Z-scheme of photosynthesis: A perspective. Photosynth. Res. 2017, 133, 5–15. [Google Scholar] [CrossRef]
- Landi, M.; Zivcak, M.; Sytar, O.; Brestic, M.; Allakhverdiev, S.I. Plasticity of photosynthetic processes and the accumulation of secondary metabolites in plants in response to monochromatic light environments: A review. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148131. [Google Scholar] [CrossRef]
- Ptushenko, O.S.; Ptushenko, V.V.; Solovchenko, A.E. Spectrum of light as a determinant of plant functioning: A historical perspective. Life 2020, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, K.; Hikosaka, K. Estimating leaf photosynthesis of C3 plants grown under different environments from pigment index, photochemical reflectance index, and chlorophyll fluorescence. Photosynth Res. 2021, 148, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Kováč, D.; Novotný, J.; Šigut, L.; Ač, A.; Peñuelas, J.; Grace, J.; Urban, O. Estimation of photosynthetic dynamics in forests from daily measured fluorescence and PRI data with adjustment for canopy shadow fraction. Sci. Total Environ. 2023, 898, 166386. [Google Scholar] [CrossRef] [PubMed]




| Parameter | Value | Unit |
|---|---|---|
| Intensity of the forward-collimated light directed to adaxial leaf surface (I0) | 1000 | µmol m−2s−1 |
| Angle of light incidence on adaxial leaf surface (βO1) | 35 | ° |
| Intensity of the backward-collimated light directed to abaxial leaf surface (J0) | 0 | µmol m−2s−1 |
| Angle of light incidence on abaxial leaf surface (βO2) | 35 | ° |
| Quantity of iterations which is necessary to approximately describe the reflectance and transmittance spectra of leaves (N) | 6 | - |
| Refractive index in air (nO) | 1 | - |
| Refractive index in leaf (nI) | 1.415 | - |
| Transmittance coefficient for the scattered light transfer from air to leaf (TsOI) | 0.866 | - |
| Transmittance coefficient for the scattered light transfer from leaf to air (TsIO) | 0.469 | - |
| Fraction of the rough surface (FS) | 0.15 | - |
| Thickness of the palisade mesophyll layer (h) | 35.5 | µm |
| Thickness of the spongy mesophyll layer (l) | 58.6 | µm |
| Light scattering coefficient in the palisade mesophyll layer () | 5 | cm−1 |
| Light scattering coefficient in the spongy mesophyll layer () | 600 | cm−1 |
| Asymmetry factor (f) | 0.5 | - |
| Average concentration of chlorophyll a ([Chl a]) | 1.6 | mg cm−3 |
| Average concentration of chlorophyll b ([Chl b]) | 1.05 | mg cm−3 |
| Average concentration of carotenoids ([Car]) | 0.47 | mg cm−3 |
| Average concentration of anthocyanin ([Anth]) | 0 | mg cm−3 |
| Ratio between concentrations of photosynthetic pigments in the spongy and palisade mesophyll (NSp/P) | 0.2 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukhova, E.; Yudina, L.; Zolin, Y.; Popova, A.; Grebneva, K.; Abasheva, K.; Kozlova, E.; Sukhov, V. Light Scattering of Leaf Surface and Spongy Mesophyll and Concentration of Anthocyanin Influence Typical and Modified Photochemical Reflectance Indices. Plants 2025, 14, 3255. https://doi.org/10.3390/plants14213255
Sukhova E, Yudina L, Zolin Y, Popova A, Grebneva K, Abasheva K, Kozlova E, Sukhov V. Light Scattering of Leaf Surface and Spongy Mesophyll and Concentration of Anthocyanin Influence Typical and Modified Photochemical Reflectance Indices. Plants. 2025; 14(21):3255. https://doi.org/10.3390/plants14213255
Chicago/Turabian StyleSukhova, Ekaterina, Lyubov Yudina, Yuriy Zolin, Alyona Popova, Kseniya Grebneva, Karina Abasheva, Elizaveta Kozlova, and Vladimir Sukhov. 2025. "Light Scattering of Leaf Surface and Spongy Mesophyll and Concentration of Anthocyanin Influence Typical and Modified Photochemical Reflectance Indices" Plants 14, no. 21: 3255. https://doi.org/10.3390/plants14213255
APA StyleSukhova, E., Yudina, L., Zolin, Y., Popova, A., Grebneva, K., Abasheva, K., Kozlova, E., & Sukhov, V. (2025). Light Scattering of Leaf Surface and Spongy Mesophyll and Concentration of Anthocyanin Influence Typical and Modified Photochemical Reflectance Indices. Plants, 14(21), 3255. https://doi.org/10.3390/plants14213255






