Analysis of the Toxicological Profile of Heracleum sosnowskyi Manden. Metabolites Using In Silico Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Preparation
2.2. Establishing Canonical SMILES
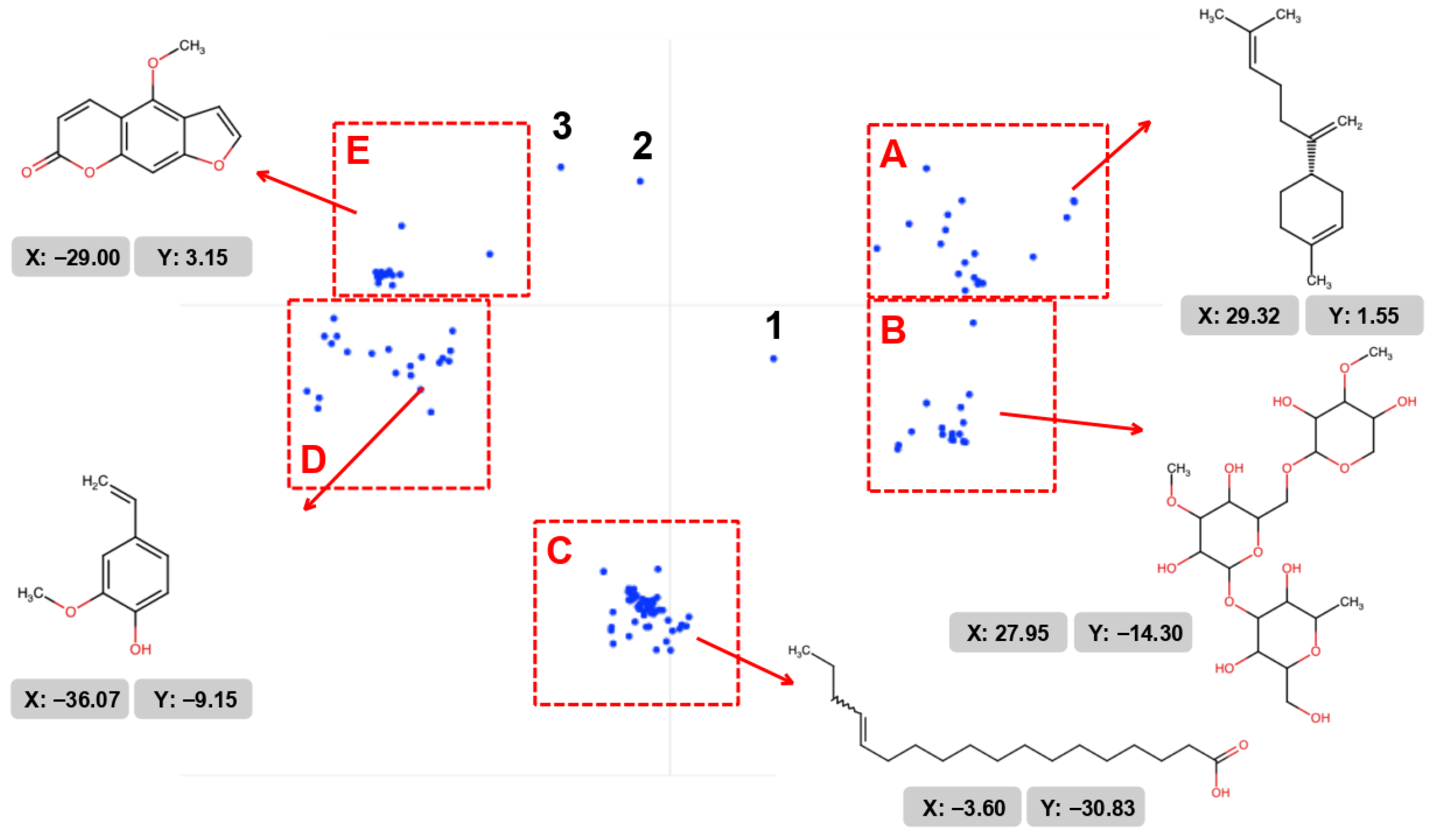
2.3. Clusterization of Data
2.4. In Silico Toxicity Analysis
2.5. Assessment of the Applicability Domain
2.6. Cost of Synthesis
2.7. Statistical Analysis
3. Results
3.1. Dataset on Heracleum sosnowskyi Metabolites
3.2. Clustering of Metabolites
3.3. In Silico Toxicity Prediction and Applicability Domain Assessment
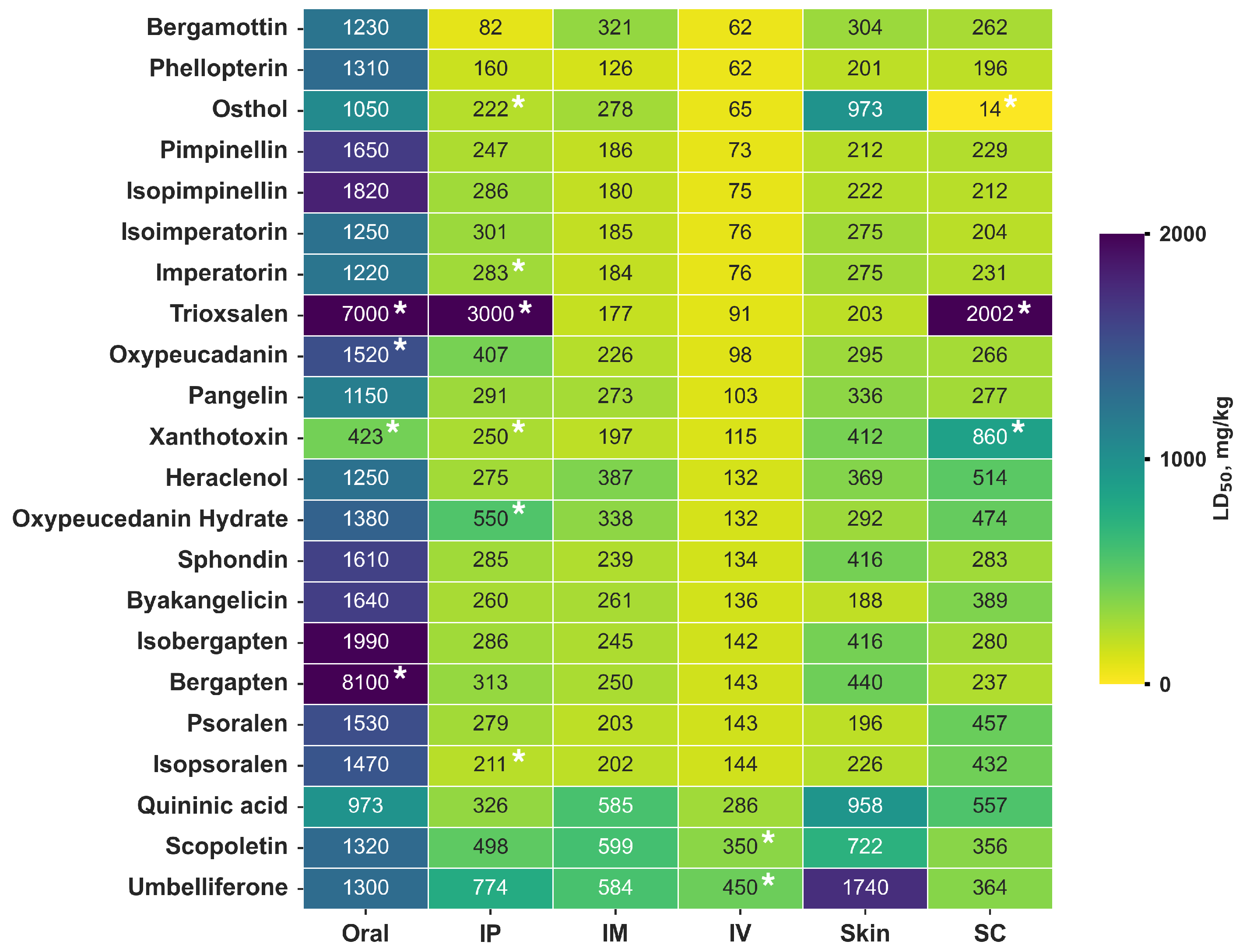
3.4. Analysis of H. sosnowskyi Metabolite Toxicity
3.5. Prediction of General Toxicity In Silico
3.6. Estimation of the Cost of Synthesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dalke, I.V.; Chadin, I.F.; Zakhozhiy, I.G. Control of Sosnowskyi’s hogweed (Heracleum sosnowskyi Manden.) invasion on the territory of the Russian Federation. Russ. J. Biol. Invasions 2018, 9, 331–344. [Google Scholar] [CrossRef]
- Chadin, I.; Dalke, I.; Zakhozhiy, I.; Malyshev, R.; Madi, E.; Kuzivanova, O.; Kirillov, D.; Elsakov, V. Distribution of the invasive plant species Heracleum sosnowskyi Manden. in the Komi Republic (Russia). PhytoKeys 2017, 77, 71. [Google Scholar] [CrossRef]
- Žalnierius, T.; Šveikauskas, V.; Aphalo, P.J.; Gavelienė, V.; Būda, V.; Jurkonienė, S. Gibberellic acid (GA3) applied to flowering Heracleum sosnowskyi decreases seed viability even if seed development is not inhibited. Plants 2022, 11, 314. [Google Scholar] [CrossRef]
- Paramonova, K.; Chaloupkova, V.; Ivanova, T.A. Invasive Heracleum sosnowskyi as a potential feedstock for biorefineries: A review. Ind. Crops Prod. 2024, 216, 118754. [Google Scholar] [CrossRef]
- Rysiak, A.; Dresler, S.; Hanaka, A.; Hawrylak-Nowak, B.; Strzemski, M.; Kováčik, J.; Sowa, I.; Latalski, M.; Wójciak, M. High temperature alters secondary metabolites and photosynthetic efficiency in Heracleum sosnowskyi. Int. J. Mol. Sci. 2021, 22, 4756. [Google Scholar] [CrossRef]
- Afonin, A.N.; Luneva, N.N.; Li, Y.S.; Kotsareva, N.V. Ecological-geographical analysis of distribution pattern and occurrence of cow-parsnip (Heracleum sosnowskyi Manden) with respect to area aridity and its mapping in European Russia. Russ. J. Ecol. 2017, 48, 86–89. [Google Scholar] [CrossRef]
- Jahodová, Š.; Trybush, S.; Pyšek, P.; Wade, M.; Karp, A. Invasive species of Heracleum in Europe: An insight into genetic relationships and invasion history. Divers. Distrib. 2007, 13, 99–114. [Google Scholar] [CrossRef]
- Madariaga-Mazón, A.; Hernández-Alvarado, R.B.; Noriega-Colima, K.O.; Osnaya-Hernández, A.; Martinez-Mayorga, K. Toxicity of secondary metabolites. Phys. Sci. Rev. 2019, 4, 20180116. [Google Scholar] [CrossRef]
- Hosseinzadeh, Z.; Ramazani, A.; Razzaghi-Asl, N. Plants of the genus Heracleum as a source of coumarin and furanocoumarin. Chem. Rev. 2019, 1, 78–98. [Google Scholar]
- Gordina, E.N.; Kuznetsov, S.P.; Golovchenko, V.V.; Zlobin, A.A. Preliminary structural characteristic of polysaccharides extracted from the callus tissue of Sosnowskyi’s hogweed (Heracleum sosnowskyi Manden) stem by aqueous ammonium oxalate. Russ. J. Bioorganic Chem. 2019, 45, 522–527. [Google Scholar] [CrossRef]
- Mironova, D.Y.; Varadarajan, V.; Timakhovich, I.V.; Barakova, N.V.; Tokbaeva, A.A.; Rumiantceva, O.N.; Pomazkova, E.E.; Baranov, I.V.; Tishchenko, L.I. Methods of commercialization and usage of Sosnovsky hogweed processing. Recycling 2022, 7, 77. [Google Scholar] [CrossRef]
- Punegov, V.V.; Gruzdev, I.V.; Triandafilov, A.F. Analysis of the composition of lipophilic substances in Heracleum sosnowskyi juice before and after electric discharge cavitation treatment. Khimiya Rastit. Syr’Ya 2019, 3, 61–68. [Google Scholar] [CrossRef]
- Andreeva, L.V. Content of coumarins in various organs of Sosnovsky’s hogweed (Heracleum sosnowskyi Manden). IOP Conf. Ser. Earth Environ. Sci. 2021, 852, 012006. [Google Scholar] [CrossRef]
- Borska, E.; Kviesis, J.; Ramata-Stunda, A.; Nikolajeva, V.; Ansone-Bertina, L.; Boroduskis, M.; Klavins, M. Bioactive lipids and allelopathic potential of the invasive plant Heracleum sosnowskyi: Insights into its fatty acid composition, antimicrobial and cytotoxic effects. Front. Pharmacol. 2025, 16, 1582694. [Google Scholar] [CrossRef] [PubMed]
- Shakhmatov, E.G.; Toukach, P.V.; Kuznetsov, S.P.; Makarova, E.N. Structural characteristics of water-soluble polysaccharides from Heracleum sosnowskyi Manden. Carbohydr. Polym. 2014, 102, 521–528. [Google Scholar] [CrossRef]
- Ivanova, T.A.; Matveeva, T.N.; Chanturia, V.A.; Ivanova, E.N. Composition of multicomponent heracleum extracts and its effect on flotation of gold-bearing sulfides. J. Min. Sci. 2015, 51, 819–824. [Google Scholar] [CrossRef]
- Grzędzicka, E. Invasion of the giant hogweed and the Sosnowsky’s hogweed as a multidisciplinary problem with unknown future—A review. Earth 2022, 3, 287–312. [Google Scholar] [CrossRef]
- Vickackaite, V.; Pilaityte, K.; Poskus, V. Extraction, isolation, and purification of furanocoumarins from invasive Heracleum sosnowskyi. Separations 2025, 12, 175. [Google Scholar] [CrossRef]
- Kulikov, O.A.; Shlyapkina, V.I.; Brodovskaya, E.P.; Al-khadj Aioub, A.M.; Ageev, V.P.; Zharkov, M.N.; Yakobson, D.E.; Sokushev, D.S.; Pyataev, N.A.; Sukhorukov, G.B. Phototoxicity in vitro and safety in vivo of the emulsion photosensitizer based on furanocoumarins of Heracleum sosnowskyi. Eur. J. Pharm. Biopharm. 2024, 198, 114257. [Google Scholar] [CrossRef] [PubMed]
- Lagey, K.; Duinslaeger, L.; Vanderkelen, A. Burns induced by plants. Burns 1995, 21, 542–543. [Google Scholar] [CrossRef]
- Maugeri, A.; Lombardo, G.E.; Musumeci, L.; Russo, C.; Gangemi, S.; Calapai, G.; Cirmi, S.; Navarra, M. Bergamottin and 5-geranyloxy-7-methoxycoumarin cooperate in the cytotoxic effect of Citrus bergamia (bergamot) essential oil in human neuroblastoma SH-SY5Y cell line. Toxins 2021, 13, 275. [Google Scholar] [CrossRef]
- Osipova, E.S.; Gladkov, E.A. Heracleum sosnowskyi Manden. as a source of valuable chemicals (Elimination with Utility). Chem. Methodol. 2024, 8, 944–956. [Google Scholar]
- Li, S.; Xu, H.; Liu, F.; Ni, R.; Shi, Y.; Li, X. In silico prediction of drug-induced cardiotoxicity with ensemble machine learning and structural pattern recognition. Mol. Divers. 2025, 1–12. [Google Scholar] [CrossRef]
- Melough, M.M.; Cho, E.; Chun, O.K. Furocoumarins: A review of biochemical activities, dietary sources and intake, and potential health risks. Food Chem. Toxicol. 2018, 113, 99–107. [Google Scholar] [CrossRef]
- Bruni, R.; Barreca, D.; Protti, M.; Brighenti, V.; Righetti, L.; Anceschi, L.; Mercolini, L.; Benvenuti, S.; Gattuso, G.; Pellati, F. Botanical sources, chemistry, analysis, and biological activity of furanocoumarins of pharmaceutical interest. Molecules 2019, 24, 2163. [Google Scholar] [CrossRef]
- Shtratnikova, V.Y. Furanocoumarins: History of research, diversity, synthesis, physiological role in the plant, and medical application. Russ. J. Plant Physiol. 2023, 70, 169. [Google Scholar] [CrossRef]
- Shao, X.; Zhang, Z.; Qian, X.; Wang, L.; Zhang, Y.; Luo, Y. Potential biochemical pesticide—Synthesis of neofuranocoumarin and inhibition the proliferation of Spodoptera frugiperda cells through activating the mitochondrial pathway. Toxins 2022, 14, 677. [Google Scholar] [CrossRef]
- Frumin, G.T. Toxicity of juice of Heracleum sosnowskyi. Russ. J. Gen. Chem. 2023, 93, 3483–3487. [Google Scholar] [CrossRef]
- Noonan, J. Tomatine and furocoumarins: Toxins in commonly consumed plants. Chem. Biochem. Stud. Proj. 2018, 15, 1–30. [Google Scholar]
- Khokhlov, I.; Krasnov, L.; Fedorov, M.V.; Sosnin, S. Image2SMILES: Transformer-based molecular optical recognition engine. Chem.-Methods 2022, 2, e202100069. [Google Scholar] [CrossRef]
- Cavasotto, C.N.; Scardino, V. Machine learning toxicity prediction: Latest advances by toxicity end point. ACS Omega 2022, 7, 47536–47546. [Google Scholar] [CrossRef] [PubMed]
- Sosnin, S.; Vashurina, M.; Withnall, M.; Karpov, P.; Fedorov, M.; Tetko, I.V. A survey of multi-task learning methods in chemoinformatics. Mol. Inf. 2019, 38, 1800108. [Google Scholar] [CrossRef]
- Rim, K.T. In silico prediction of toxicity and its applications for chemicals at work. Toxicol. Environ. Health Sci. 2020, 12, 191–202. [Google Scholar] [CrossRef]
- Syntelly: Artificial Intelligence for the Analysis and Processing of Scientific Data. Available online: https://syntelly.com/ (accessed on 1 July 2025).
- Karlov, D.S.; Sosnin, S.; Tetko, I.V.; Fedorov, M.V. Chemical space exploration guided by deep neural networks. RSC Adv. 2019, 9, 5151–5157. [Google Scholar] [CrossRef]
- Shkil, D.O.; Muhamedzhanova, A.A.; Petrov, P.I.; Skorb, E.V.; Aliev, T.A.; Steshin, I.S.; Tumanov, A.V.; Kislinskiy, A.S.; Fedorov, M.V. Expanding predictive capacities in toxicology: Insights from hackathon-enhanced data and model aggregation. Molecules 2024, 29, 1826. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, L.; Khokhlov, I.; Fedorov, M.V.; Sosnin, S. Transformer-based artificial neural networks for the conversion between chemical notations. Sci. Rep. 2021, 11, 14798. [Google Scholar] [CrossRef]
- Andronov, M.; Fedorov, M.V.; Sosnin, S. Exploring chemical reaction space with reaction difference fingerprints and parametric t-SNE. ACS Omega 2021, 6, 30743–30751. [Google Scholar] [CrossRef]
- Karlov, D.S.; Sosnin, S.; Fedorov, M.V.; Popov, P. graphDelta: MPNN scoring function for the affinity prediction of protein–ligand complexes. ACS Omega 2020, 5, 5150–5159. [Google Scholar] [CrossRef]
- Shtratnikova, V.Y.; Bogdanov, V.P.; Schelkunov, M.I.; Klepikova, A.V.; Kulbachnaya, M.A.; Obukhova, E.N.; Ptitsyna, E.V.; Ezhova, M.A.; Penin, A.A.; Logacheva, M.D. Furanocoumarins in two European species of Heracleum: Transcriptomic and metabolomic study. BMC Plant Biol. 2025, 25, 1091. [Google Scholar] [CrossRef]
- Mathea, M.; Klingspohn, W.; Baumann, K. Chemoinformatic classification methods and their applicability domain. Mol. Inf. 2016, 35, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Kornicka, A.; Balewski, Ł.; Lahutta, M.; Kokoszka, J. Umbelliferone and its synthetic derivatives as suitable molecules for the development of agents with biological activities: A review of their pharmacological and therapeutic potential. Pharmaceuticals 2023, 16, 1732. [Google Scholar] [CrossRef]
- Cruz, L.F.; Figueiredo, G.F.; Pedro, L.P.; Amorin, Y.M.; Andrade, J.T.; Passos, T.F.; Rodrigues, F.F.; Souza, I.L.A.; Gonçalves, T.P.R.; Lima, L.A.R.S.; et al. Umbelliferone (7-hydroxycoumarin): A non-toxic antidiarrheal and antiulcerogenic coumarin. Biomed. Pharmacother. 2020, 129, 110432. [Google Scholar] [CrossRef]
- Barratt, M.D.; Rodford, R.A. The computational prediction of toxicity. Curr. Opin. Chem. Biol. 2001, 5, 383–388. [Google Scholar] [CrossRef]
- Sahigara, F.; Ballabio, D.; Todeschini, R.; Consonni, V. Defining a novel k-nearest neighbours approach to assess the applicability domain of a QSAR model for reliable predictions. J. Chem. 2013, 5, 27. [Google Scholar] [CrossRef]
- Potemkin, O.I. Use of Artificial Intelligence in chemical technology based on the Syntelly platform. Acad. J. Sci. Electron. J. 2025, 4-2, 32–36. [Google Scholar]
- Budarin, S.N.; Kondratyev, M.N. The Use of Secondary Metabolites of Heracleum sosnowskyi Manden. in Agriculture. Int. J. Second. Metab. 2014, 1, 16. [Google Scholar]
- Meng, D.; Dong, Y.; Shang, Q.; Sun, Z. Anti-tumor effect and hepatotoxicity mechanisms of psoralen. Front. Pharmacol. 2024, 15, 1442700. [Google Scholar] [CrossRef]
- Liang, Y.; Xie, L.; Liu, K.; Cao, Y.; Dai, X.; Wang, X.; Lu, J.; Zhang, X.; Li, X. Bergapten: A review of its pharmacology, pharmacokinetics, and toxicity. Phytother. Res. 2021, 35, 6131–6147. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Walter, M.; Wright, P.; Bartosik, A.; Dolciami, D.; Elbasir, A.; Yang, H.; Bender, A. Prediction and mechanistic analysis of drug-induced liver injury (DILI) based on chemical structure. Biol. Direct 2021, 16, 6. [Google Scholar] [CrossRef] [PubMed]
- Synowiec, A.; Kalemba, D. Composition and herbicidal effect of Heracleum sosnowskyi essential oil. Open Life Sci. 2015, 10, 1. [Google Scholar] [CrossRef]

| Parts of the Plant | Substance | Content, mg/g * | References |
|---|---|---|---|
| Triterpenes | 7.4 | [14] | |
| Leaves | Terpenes | 0.6 | [14] |
| Furanocoumarins | >6.4 | [18] | |
| Seeds | Carboxylic acids | 21.0 | [14] |
| Coumarins | 22.8 | [14] | |
| Carboxylic acids | 1.1 | [14] | |
| Stems | Terpenes | <0.1 | [14] |
| Alcohols | <0.1 | [14] |
| Substance | Hepatotoxicity | DILI | Cardiotoxicity | Carcinogenicity | ||||
|---|---|---|---|---|---|---|---|---|
| Effect | AD,% | Effect | AD,% | Effect | AD,% | Effect | AD,% | |
| Bergamottin a | Toxic | 90 | Toxic | 41 | Nontoxic | 67 | Nontoxic | 48 |
| Phellopterin a | Toxic | 91 | Toxic | 41 | Nontoxic | PyTDC | Nontoxic | 58 |
| Osthol b | Toxic | TOXRIC * | Toxic | 48 | Nontoxic | PyTDC | Toxic | 79 |
| Pimpinellin a | Toxic | 88 | Toxic | 40 | Nontoxic | 51 | Nontoxic | 51 |
| Isopimpinellin a | Toxic | 79 | Toxic | 37 | Nontoxic | PyTDC | Nontoxic | 45 |
| Isoimperatorin a | Toxic | TOXRIC | Toxic | 46 | Nontoxic | PyTDC | Toxic | 76 |
| Imperatorin a | Toxic | TOXRIC | Toxic | 43 | Nontoxic | PyTDC | Nontoxic | 61 |
| Trioxsalen a | Toxic | TOXRIC | Toxic | 53 | Nontoxic | NCATS-Flux | Toxic | 88 |
| Oxypeucedanin a | Toxic | 38 | Toxic | 29 | Nontoxic | PyTDC | Toxic | 26 |
| Pangelin a | Toxic | 61 | Toxic | 41 | Nontoxic | PyTDC | Nontoxic | 77 |
| Xanthotoxin a | Toxic | 89 | Toxic | 41 | Nontoxic | PyTDC | Toxic | 45 |
| Heraclenol a | Nontoxic | 92 | Toxic | 41 | Nontoxic | PyTDC | Nontoxic | 58 |
| Oxypeucedanin Hydrate a | Nontoxic | 90 | Toxic | 41 | Nontoxic | PyTDC | Nontoxic | 57 |
| Sphondin a | Toxic | 89 | Toxic | 46 | Nontoxic | PyTDC | Toxic | 61 |
| Byakangelicin a | Nontoxic | 89 | Toxic | 40 | Nontoxic | PyTDC | Nontoxic | 47 |
| Isobergapten a | Toxic | 82 | Toxic | 42 | Nontoxic | 54 | Toxic | 47 |
| Bergapten a | Toxic | TOXRIC | Toxic | 41 | Nontoxic | NCATS-Flux | Toxic | 48 |
| Psoralen a | Toxic | TOXRIC | Toxic | 51 | Nontoxic | PyTDC | Toxic | 79 |
| Isopsoralen a | Toxic | 93 | Toxic | 42 | Nontoxic | 23 | Toxic | 54 |
| Quininic acid c | Nontoxic | 29 | Toxic | 63 | Nontoxic | 66 | Nontoxic | 49 |
| Scopoletin b | Nontoxic | TOXRIC | Toxic | 51 | Nontoxic | PyTDC | Nontoxic | PyTDC |
| Umbelliferone b | Toxic | TOXRIC | Toxic | 57 | Nontoxic | 67 | Nontoxic | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rassabina, A.E.; Fedorov, M.V. Analysis of the Toxicological Profile of Heracleum sosnowskyi Manden. Metabolites Using In Silico Methods. Plants 2025, 14, 3253. https://doi.org/10.3390/plants14213253
Rassabina AE, Fedorov MV. Analysis of the Toxicological Profile of Heracleum sosnowskyi Manden. Metabolites Using In Silico Methods. Plants. 2025; 14(21):3253. https://doi.org/10.3390/plants14213253
Chicago/Turabian StyleRassabina, Anna E., and Maxim V. Fedorov. 2025. "Analysis of the Toxicological Profile of Heracleum sosnowskyi Manden. Metabolites Using In Silico Methods" Plants 14, no. 21: 3253. https://doi.org/10.3390/plants14213253
APA StyleRassabina, A. E., & Fedorov, M. V. (2025). Analysis of the Toxicological Profile of Heracleum sosnowskyi Manden. Metabolites Using In Silico Methods. Plants, 14(21), 3253. https://doi.org/10.3390/plants14213253





