Physiological Response to Nitrogen Deficit in Potato Under Greenhouse Conditions
Abstract
1. Introduction
2. Results
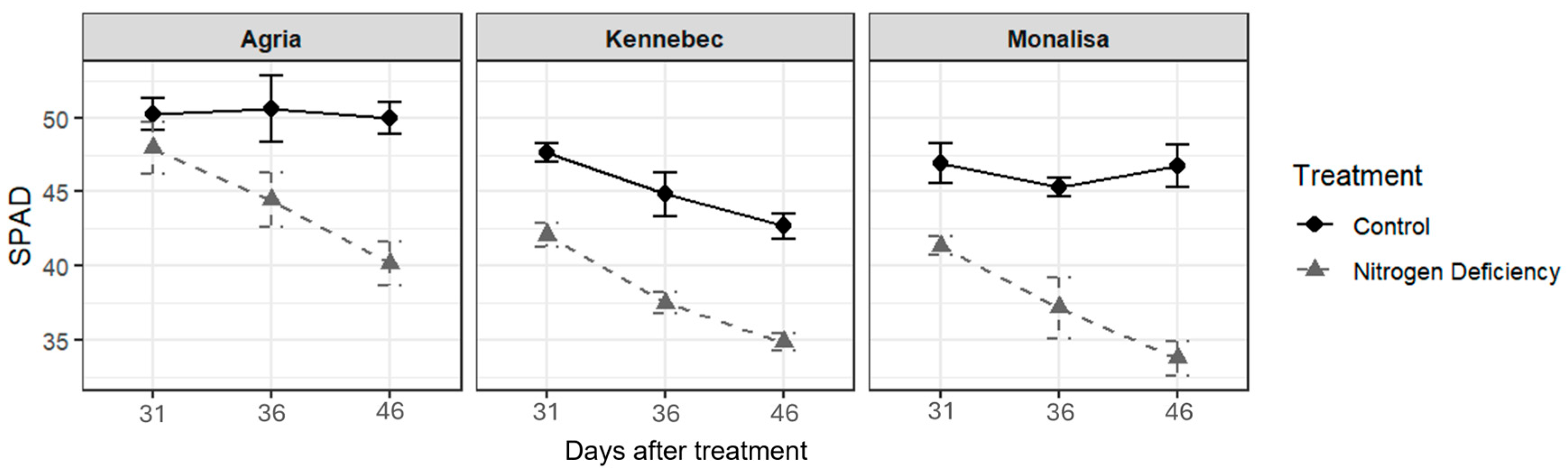
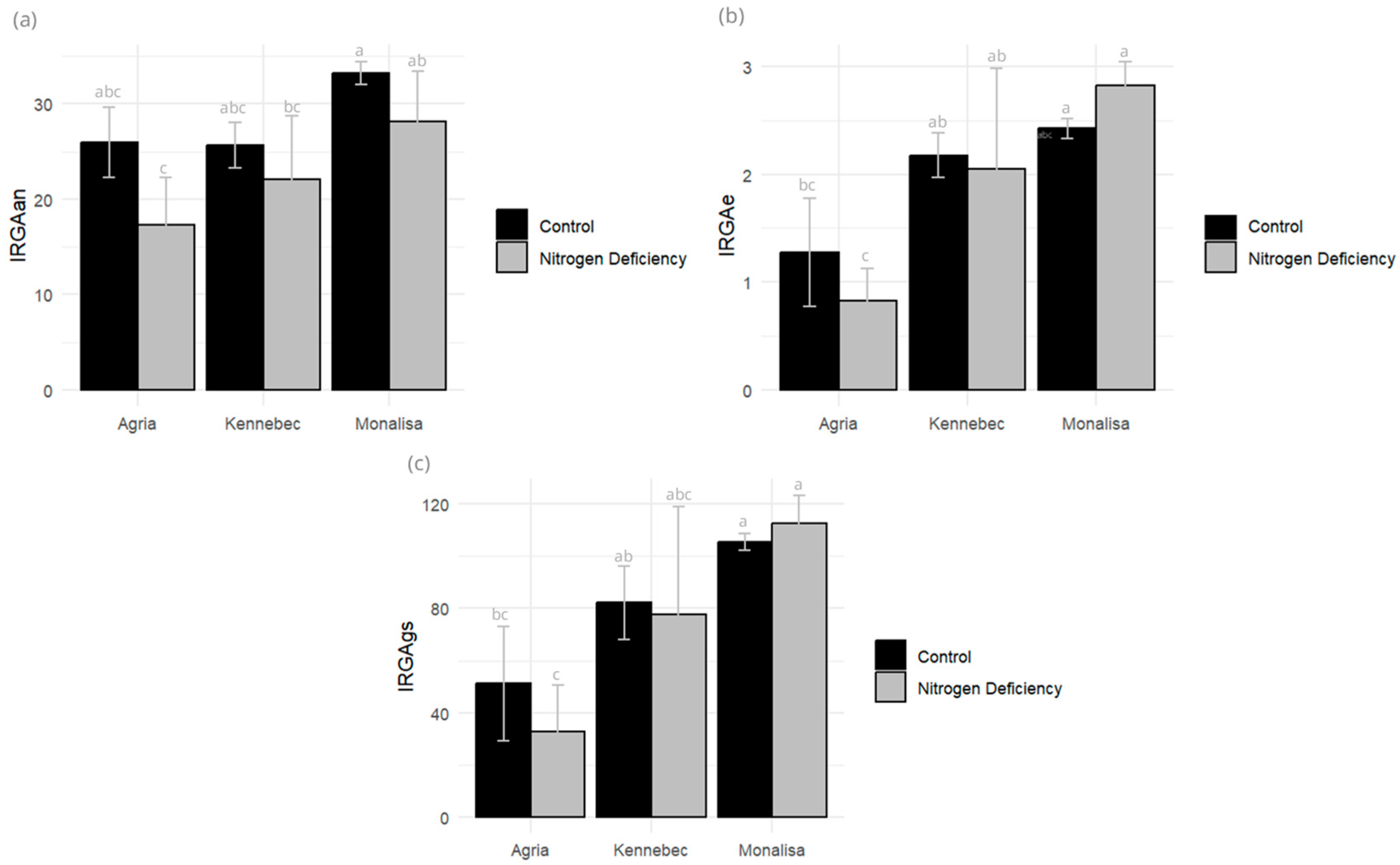
2.1. Physiological Responses to Nitrogen Deficit
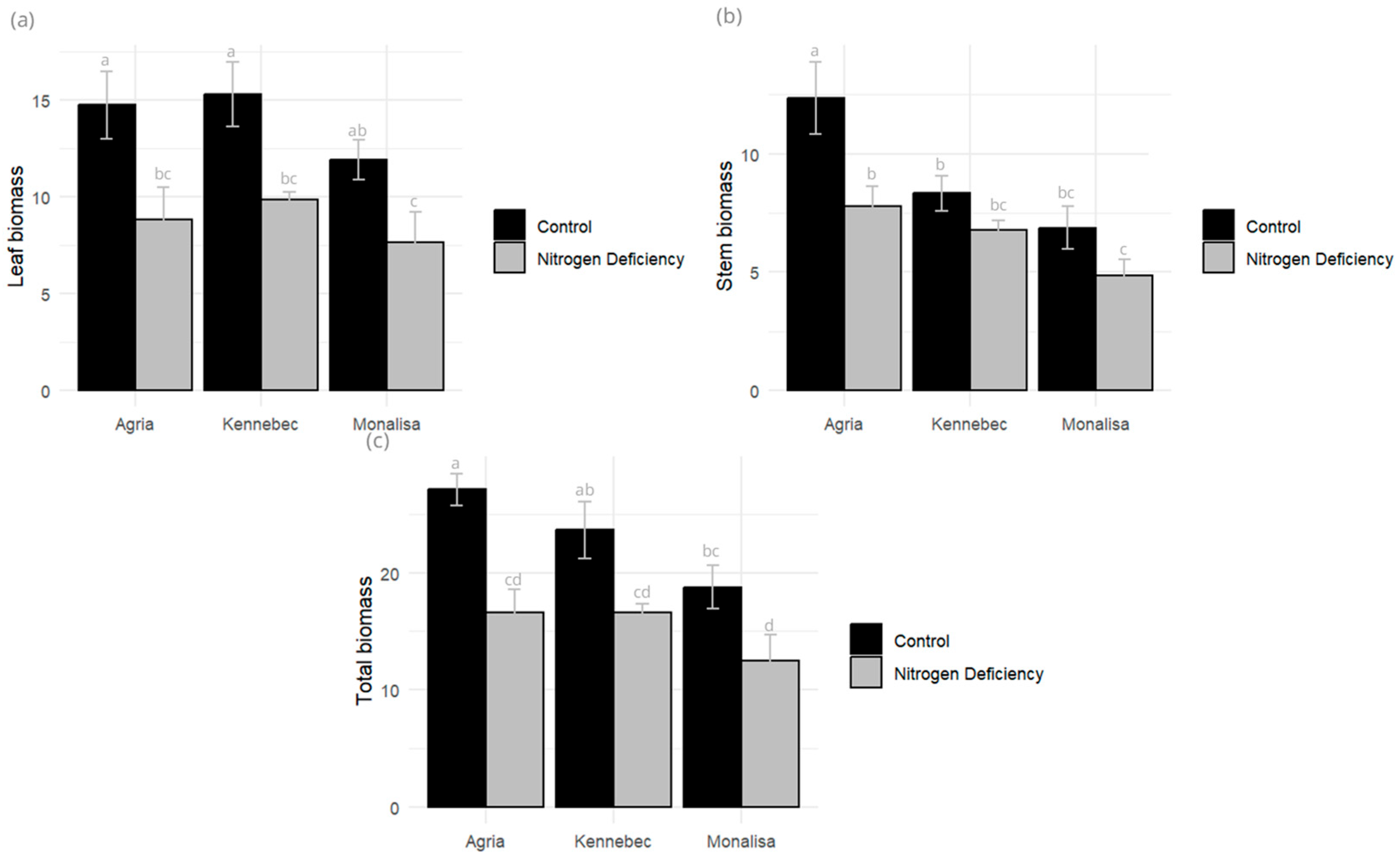
2.2. Biomass Changes
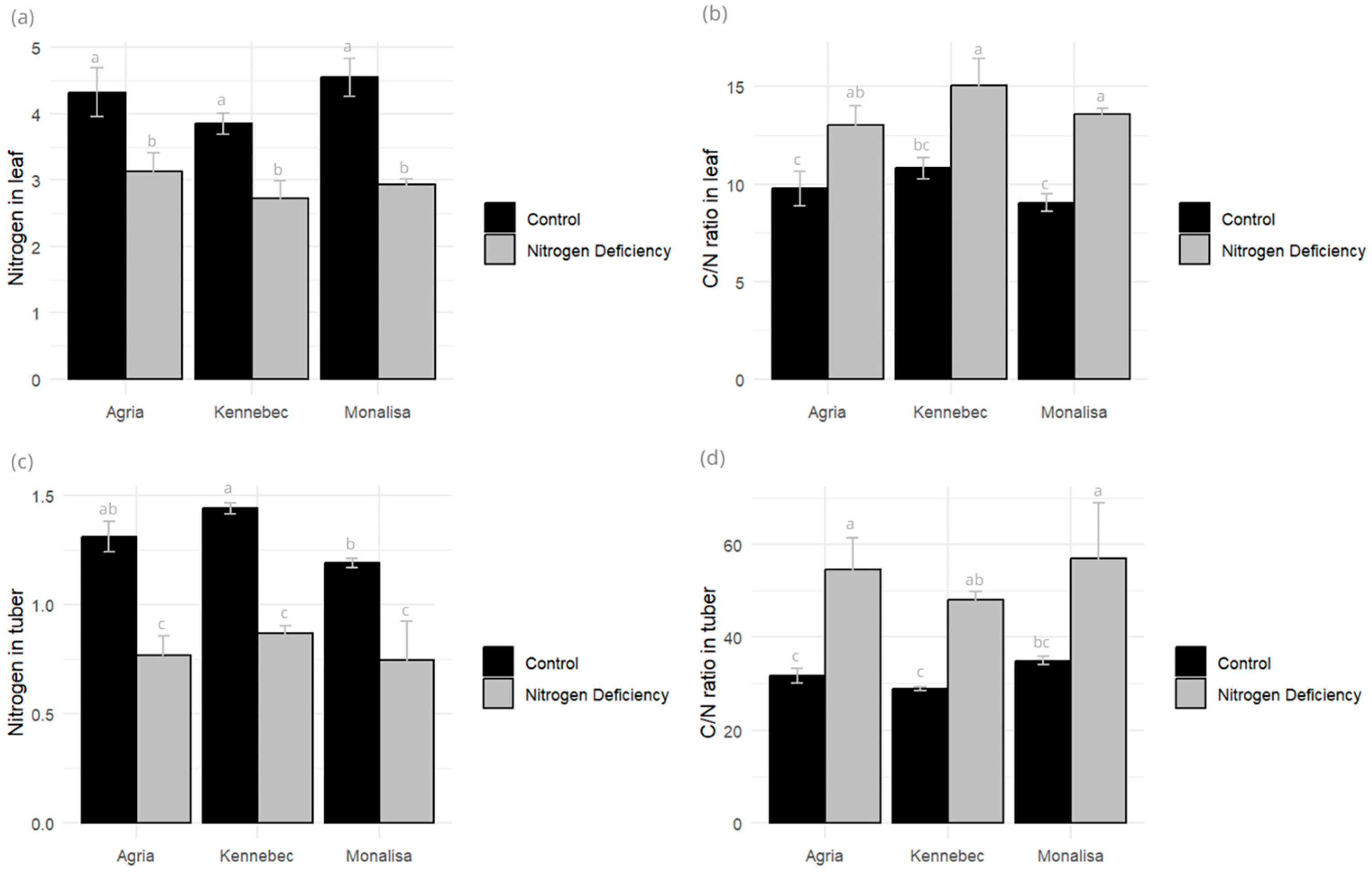
2.3. Nitrogen Content and C/N Ratio
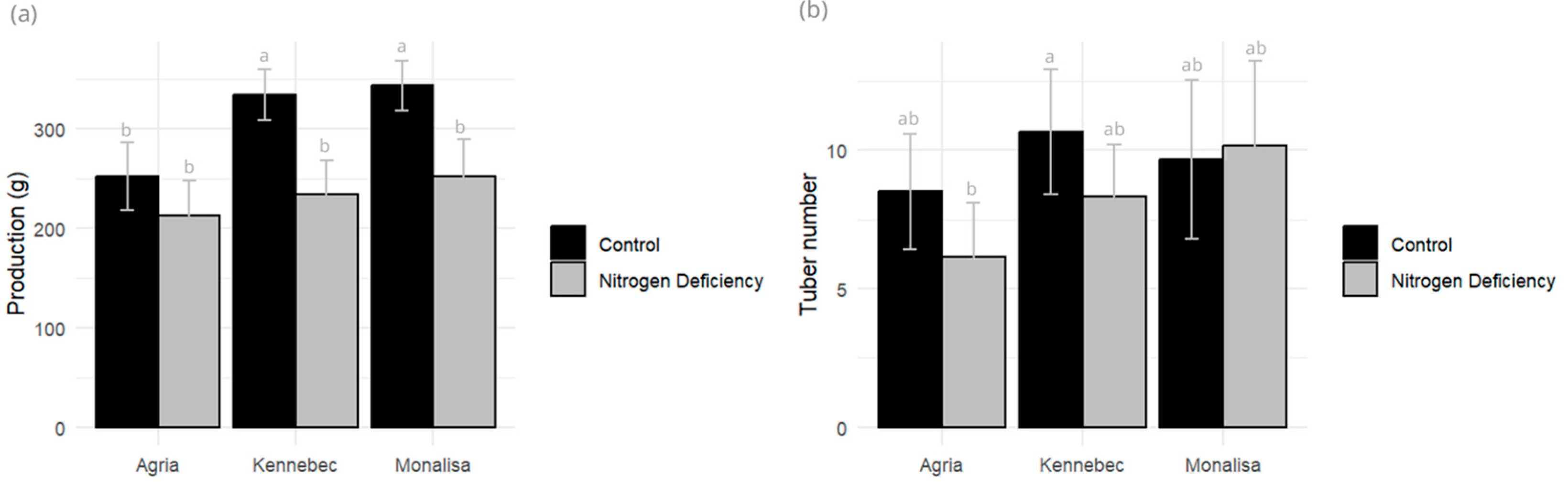
2.4. Yield Components
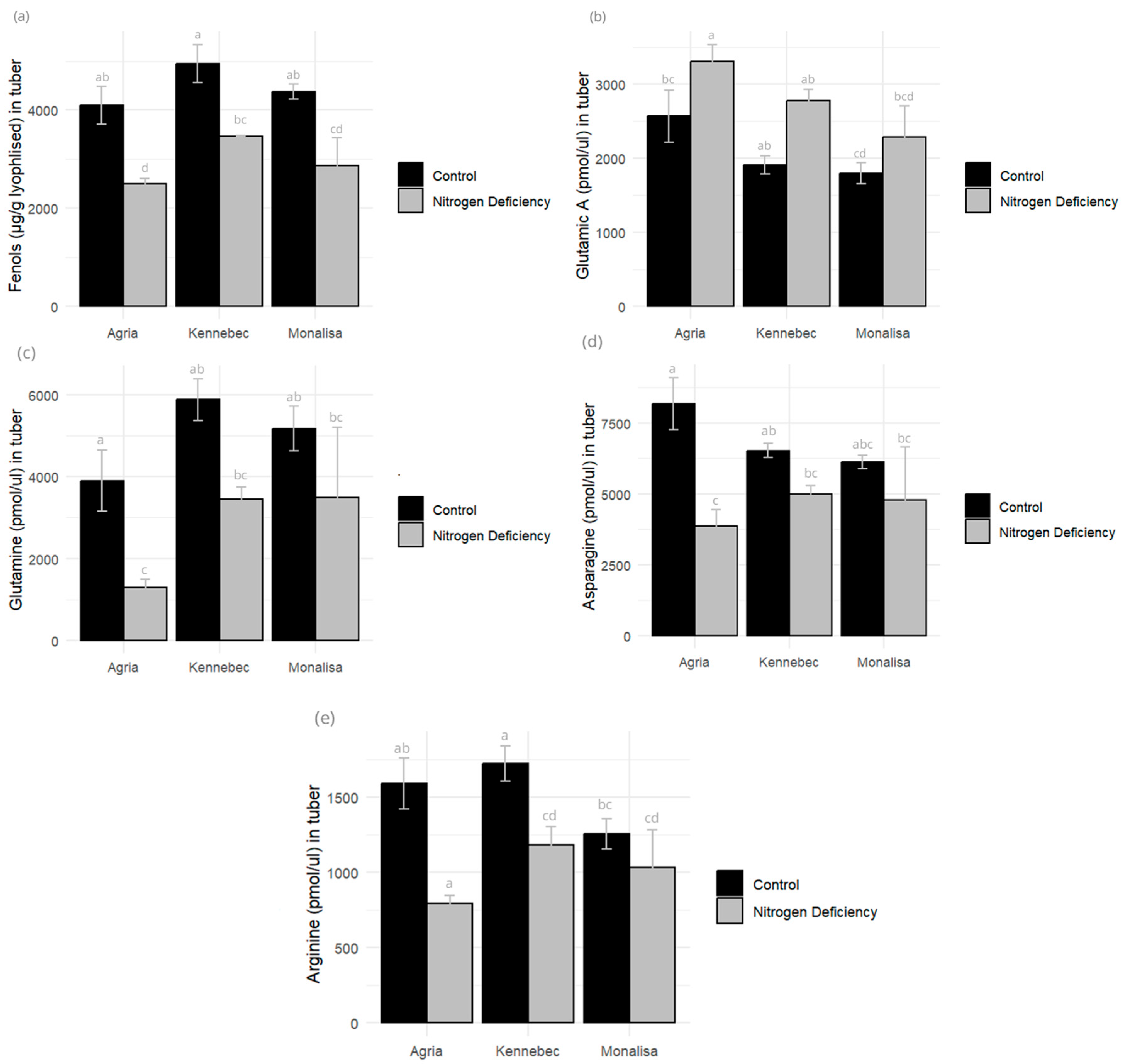
2.5. Amino Acid and Phenolic Compound Profiles
2.6. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Physiological Parameters
4.3. Agronomic Parameters
4.4. Mineral, Carbon, Amino Acid, and Phenol Contents
4.5. Antioxidant Capacity and Vitamin C
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meise, P.; Seddig, S.; Uptmoor, R.; Ordon, F.; Schum, A. Assessment of yield and yield components of starch potato cultivars (Solanum tuberosum L.) under nitrogen deficiency and drought stress conditions. Potato Res. 2019, 62, 193–220. [Google Scholar] [CrossRef]
- Ospina, C.A.; van Bueren, E.T.L.; Allefs, J.J.H.M.; Engel, B.; van der Putten, P.E.L.; van der Linden, C.G.; Struik, P.C. Diversity of crop development traits and nitrogen use efficiency among potato cultivars grown under contrasting nitrogen regimes. Euphytica 2014, 199, 13–29. [Google Scholar] [CrossRef]
- Mauromicale, G.; Ierna, A.; Marchese, M. Chlorophyll fluorescence and chlorophyll content in field-grown potato as affected by nitrogen supply, genotype, and plant age. Photosynthetica 2006, 44, 76–82. [Google Scholar] [CrossRef]
- Ahmed, A.; El-Baky, M.; El-Abd, S.; Riad, G.; Ghoname, A. Potato Tuber Quality as Affected by Nitrogen Form and Rate. Middle East. Russ. J. Plant Sci. Biotechnol. 2009, 3, 47–52. [Google Scholar]
- Zebarth, B.J.; Tai, G.; Tarn, R.; de Jong, H.; Milburn, P.H. Nitrogen use efficiency characteristics of commercial potato cultivars. Can. J. Plant Sci. 2004, 84, 589–598. [Google Scholar] [CrossRef]
- Fontes, P.C.R.; Braun, H.; Busato, C.; Cecon, P.R. Economic Optimum Nitrogen Fertilization Rates and Nitrogen Fertilization Rate Effects on Tuber Characteristics of Potato Cultivars. Potato Res. 2010, 53, 167–179. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Optimizing nitrogen fertilization to improve qualitative performances and physiological and yield responses of potato (Solanum tuberosum L.). Agronomy 2020, 10, 352. [Google Scholar] [CrossRef]
- Akkamis, M.; Caliskan, S. Responses of yield, quality and water use efficiency of potato grown under different drip irrigation and nitrogen levels. Sci. Rep. 2023, 13, 9911. [Google Scholar] [CrossRef]
- Zhang, C.; Gu, B.; Liang, X.; Lam, S.K.; Zhou, Y.; Chen, D. The role of nitrogen management in achieving global sustainable development goals. Resour. Conserv. Recycl. 2024, 201, 107304. [Google Scholar] [CrossRef]
- Ierna, A.; Mauromicale, G. Sustainable and Profitable Nitrogen Fertilization Management of Potato. Agronomy 2019, 9, 582. [Google Scholar] [CrossRef]
- Milroy, S.; Wang, P.; Sadras, V. Defining upper limits of nitrogen uptake and nitrogen use efficiency of potato in response to crop N supply. Field Crop. Res. 2019, 239, 38–46. [Google Scholar] [CrossRef]
- Opena, G.B.; Porter, G.A. Soil Management and Supplemental Irrigation Effects on Potato: II. Root Growth. Agron. J. 1999, 91, 426–431. [Google Scholar] [CrossRef]
- Roberts, T.L. Improving Nutrient Use Efficiency. Turk. J. Agric. For. 2008, 32, 177–182. [Google Scholar]
- Bundy, L.G.; Andraski, T.W. Recovery of Fertilizer Nitrogen in Crop Residues and Cover Crops on an Irrigated Sandy Soil. Soil. Sci. Soc. Am. J. 2005, 69, 640–648. [Google Scholar] [CrossRef]
- Moll, R.H.; Kamprath, E.J.; Jackson, W.A. Analysis and Interpretation of Factors Which Contribute to Efficiency of Nitrogen Utilization. Agron. J. 1982, 74, 562–564. [Google Scholar] [CrossRef]
- Mishra, S.; Levengood, H.; Fan, J.; Zhang, C. Plants Under Stress: Exploring Physiological and Molecular Responses to Nitrogen and Phosphorus Deficiency. Plants 2024, 13, 3144. [Google Scholar] [CrossRef]
- Iribar, C.; Alvarez-Morezuelas, A.; Barandalla, L.; de Galarreta, J.I.R. Genome-Wide Association Analysis of Traits Related to Nitrogen Deficiency Stress in Potato. Horticulturae 2025, 11, 889. [Google Scholar] [CrossRef]
- Wellpott, K.; Jozefowicz, A.M.; Meise, P.; Schum, A.; Seddig, S.; Mock, H.-P.; Winkelmann, T.; Bündig, C. Combined nitrogen and drought stress leads to overlapping and unique proteomic responses in potato. Planta 2023, 257, 58. [Google Scholar] [CrossRef]
- Ding, K.; Shan, Y.; Wang, L.; Zhang, Y.; Tian, G. Transcriptomics combined with physiological analysis and metabolomics revealed the response of potato tuber formation to nitrogen. BMC Plant Biol. 2024, 24, 1109. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Pu, X.; Jia, H.; Zhou, Y.; Ye, G.; Yang, Y.; Na, T.; Wang, J. Transcriptome analysis reveals multiple effects of nitrogen accumulation and metabolism in the roots, shoots, and leaves of potato (Solanum tuberosum L.). BMC Plant Biol. 2022, 22, 282. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Artins, A.; Martins, M.C.M.; Meyer, C.; Fernie, A.R.; Caldana, C. Sensing and regulation of C and N metabolism—Novel features and mechanisms of the TOR and SnRK1 signaling pathways. Plant J. 2024, 118, 1268–1280. [Google Scholar] [CrossRef]
- Margalha, L.; Confraria, A.; Baena-González, E. SnRK1 and TOR: Modulating growth–defense trade-offs in plant stress responses. J. Exp. Bot. 2019, 70, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-T.; Zheng, Z.-C.; Hua, D.; Chen, X.-F.; Zhang, J.; Chen, H.-H.; Ye, X.; Guo, J.-X.; Yang, L.-T.; Chen, L.-S. Adaptive responses of carbon and nitrogen metabolisms to nitrogen-deficiency in Citrus sinensis seedlings. BMC Plant Biol. 2022, 22, 370. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Fernández, I.; De Diego, N.; Brzobohatý, B.; Muñoz-Rueda, A.; Lacuesta, M. The imbalance between C and N metabolism during high nitrate supply inhibits photosynthesis and overall growth in maize (Zea mays L.). Plant Physiol. Biochem. 2017, 120, 213–222. [Google Scholar] [CrossRef]
- Chen, Z.; Dolfing, J.; Zhuang, S.; Wu, Y. Periphytic biofilms-mediated microbial interactions and their impact on the nitrogen cycle in rice paddies. Eco-Environ. Health 2022, 1, 172–180. [Google Scholar] [CrossRef]
- Anas, M.; Liao, F.; Verma, K.K.; Sarwar, M.A.; Mahmood, A.; Chen, Z.L.; Li, Q.; Zeng, X.P.; Liu, Y.; Li, Y.R. Fate of nitrogen in agriculture and environment: Agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol. Res. 2020, 53, 47. [Google Scholar] [CrossRef]
- Fu, Y.; Mason, A.S.; Song, M.; Ni, X.; Liu, L.; Shi, J.; Wang, T.; Xiao, M.; Zhang, Y.; Fu, D.; et al. Multi-omics strategies uncover the molecular mechanisms of nitrogen, phosphorus and potassium deficiency responses in Brassica napus. Cell. Mol. Biol. Lett. 2023, 28, 63. [Google Scholar] [CrossRef]
- Nguyen, T.N.P.; Serwaa, R.N.; Sung, J. Primary Metabolic Variations in Maize Plants Affected by Different Levels of Nitrogen Supply. Metabolites 2025, 15, 519. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, W.; Lyu, D.; Su, F.; Liu, S.; Li, H.; Wang, X.; Liu, Z.; Hu, L. Effects of Different Nitrogen Application Rates on Starch Accumulation, Starch Synthase Gene Expression and Enzyme Activity in Two Distinctive Potato Cultivars. Potato Res. 2018, 61, 309–326. [Google Scholar] [CrossRef]
- Tedone, L. Influence of storage temperature, tuber size and nitrogen nutrition on the content of vitamin C in potato. In Proceedings of the V International Postharvest Symposium, Verona, Italy, 6–11 June 2004. [Google Scholar]
- Hamouz, K.; Lachman, J.; Dvořák, P.; Orsák, M.; Hejtmánková, K.; Čížek, M. Effect of selected factors on the content of ascorbic acid in potatoes with different tuber flesh colour. Plant Soil. Environ. 2009, 55, 281–287. [Google Scholar] [CrossRef]
- Fang, X.; Xiang, Z.; Ma, H.; Wang, F.; Wang, Q.; Li, P.; Zheng, S. Effect of N fertilizer dosage and base/topdressing ratio on potato growth characteristics and yield. Agronomy 2023, 13, 909. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Lachman, J.; Hamouz, K.; Orsák, M.; Pivec, V.; Dvořák, P. The influence of flesh colour and growing locality on polyphenolic content and antioxidant activity in potatoes. Sci. Hortic. 2008, 117, 109–114. [Google Scholar] [CrossRef]
- Tierno, R.; Hornero-Méndez, D.; Gallardo-Guerrero, L.; López-Pardo, R.; de Galarreta, J.I.R. Effect of boiling on the total phenolic, anthocyanin and carotenoid concentrations of potato tubers from selected cultivars and introgressed breeding lines from native potato species. J. Food Compos. Anal. 2015, 41, 58–65. [Google Scholar] [CrossRef]
- Michalska, A.; Wojdyło, A.; Bogucka, B. The influence of nitrogen and potassium fertilisation on the content of polyphenolic compounds and antioxidant capacity of coloured potato. J. Food Compos. Anal. 2016, 47, 69–75. [Google Scholar] [CrossRef]
- Vásquez, M.R.S.; Trujillo, J.D.R. Capacidad antioxidante in vitro de cuatro variedades de tubérculos de Solanum tuberosum L. “Papa”(cruda y cocida, con y sin cáscara) frente al 2, 2-Difenil-1-picrilhidrazil. TZHOECOEN 2014, 6, 243–259. [Google Scholar]
- Liu, K.; Du, J.; Zhong, Y.; Shen, Z.; Yu, X. The response of potato tuber yield, nitrogen uptake, soil nitrate nitrogen to different nitrogen rates in red soil. Sci. Rep. 2021, 11, 22506. [Google Scholar] [CrossRef]
- Goffart, D.; Ben Abdallah, F.; Curnel, Y.; Planchon, V.; Defourny, P.; Goffart, J.-P. In-Season potato crop nitrogen status assessment from satellite and meteorological data. Potato Res. 2022, 65, 729–755. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, N.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil. Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar] [CrossRef]
- Goffart, J.P.; Olivier, M.; Frankinet, M. Potato crop nitrogen status assessment to improve N fertilization management and efficiency: Past-present-future. Potato Res. 2008, 51, 355–383. [Google Scholar] [CrossRef]
- Akkamis, M.; Caliskan, S. Effects of Different Irrigation Levels and Nitrogen Fertilization on Some Physiological Indicators of Potato. Potato Res. 2024, 67, 815–831. [Google Scholar] [CrossRef]
- Carruthers, L.C.; Congreves, K.A. High yield and efficiency: Cultivar selection to improve potato nitrogen use efficiency. Front. Agron. 2025, 7, 1617873. [Google Scholar] [CrossRef]
- Tsai, O.; Stammer, A.; Ruark, M.D.; Wang, Y. Potato (Solanum tuberosum L.) Responses to Nitrogen Fertilisation in Different Groundwater Nitrate Environments. Potato Res. 2025. [Google Scholar] [CrossRef]
- Müller, K.; Cervenkova, I. Die Ermittlung des Stärke- und Trockensubstanzgehaltes in Kartoffelknollen Nach Bestimmung Des Unterwassergewichtes an Hand modifizierter Tabellenwerte. Starch-Starke 1978, 30, 12–20. [Google Scholar] [CrossRef]
- Minocha, R.; Long, S. Simultaneous separation and quantitation of amino acids and polyamines of forest tree tissues and cell cultures within a single high-performance liquid chromatography run using dansyl derivatization. J. Chromatogr. A 2004, 1035, 63–73. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Tierno, R. Mejora Genética de Patata Para Compuestos Bioactivos y Capacidad Antioxidante. Master’s Thesis, University of the Basque Country, Vitoria-Gasteiz, Spain, 2017. [Google Scholar]
| Chlorophyll Content | Transpiration | Stomatal Conductance | Nitrogen in Leaf | Nitrogen in Tuber | Leaf Area | Yield | Tuber Number | Leaf Biomass | Tuber Biomass | |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (Tr) | 622.201 *** | 177.670 *** | 70.041 | 7.748 *** | 44,909.38 ** | 153.125 *** | 44,909.38 *** | 36.380 ** | 122.200 *** | 33.3472 *** |
| Variety (V) | 86.640 *** | 165.100 *** | 8214.041 *** | 0.398 * | 14,971.33 ** | 130.172 *** | 14,971.33 *** | 25.330 ** | 12.600 * | 26.8072 *** |
| VxTr | 13.481 | 9.0804 | 192.791 | 0.103 | 4035.78 * | 13.297 *** | 4035.78 * | 4.750 | 1.097 | 4.0372 * |
| Mn l | Zn l | C l | Ca l | K l | Mg l | C/N Ratio l | Ash l | |
|---|---|---|---|---|---|---|---|---|
| Treatment (Tr) | 552.330 *** | 57.501 * | 6.125 *** | 0.007 | 72.407 *** | 4.603 | 72.320 *** | 18.120 *** |
| Variety (V) | 281.032 *** | 75.660 * | 1.131 | 21.590 ** | 129.841 *** | 22.608 *** | 5.024 * | 3.343 |
| VxTr | 337.513 *** | 0.490 | 0.171 | 0.325 | 10.964 | 1.184 | 0.700 | 18.120 *** |
| Ile l | Glu t | Asp t | Gly t | Arg t | CAox | Fen l | Fen t | |
|---|---|---|---|---|---|---|---|---|
| Treatment (Tr) | 214.038 * | 2,651,551.3 *** | 27,157,123.4 *** | 78,100.7 *** | 1,314,052.1 *** | 0.006 * | 6,448,837.5 * | 10,609,506,5 *** |
| Variety (V) | 2.106 | 1,021,543.1 *** | 668,194.1 | 186,990.1 *** | 186,990.1 ** | 0.012 *** | 22,494,377.3 *** | 1,275,354.2 *** |
| VxTr | 55.934 | 11,665.1 | 392,550.7 * | 98,429.2 * | 10.96 | 0.001 | 4,396,253.7 * | 4704.2 |
| Chlorophyll Content | Transpiration | CO2 Assimilation | Stomatal Conductance | Leaf Biomass | Tuber Biomass | Leaf Micronutrients | Reducing Sugars | Dry Matter | Tuber Number | Average Tuber Weight | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Chlorophyll content | 1 | −0.394 | −0.641 * | −0.599 | −0.369 | 0.707 * | 0.640 * | −0.719 * | −0.309 | 0.081 | −0.308 |
| Transpiration | 1 | 0.873 ** | 0.921 ** | 0.602 | −0.518 | −0.647 * | 0.621 | −0.164 | −0.029 | 0.001 | |
| CO2 assimilation | 1 | 0.991 ** | 0.626 | −0.734 * | −0.742 * | 0.810 ** | −0.028 | 0.174 | −0.060 | ||
| Stomatal conductance | 1 | 0.647 * | −0.699 * | −0.742 * | 0.775 * | −0.060 | 0.137 | −0.044 | |||
| Leaf biomass | 1 | −0.725 * | −0.845 ** | 0.698 * | −0.340 | 0.455 | −0.377 | ||||
| Tuber biomass | 1 | 0.826 ** | −0.769 * | −0.077 | −0.459 | 0.208 | |||||
| Leaf micronutrients | 1 | −0.698 * | −0.034 | −0.298 | 0.107 | ||||||
| Reducing sugars | 1 | −0.223 | 0.248 | −0.237 | |||||||
| Dry matter | 1 | −0.360 | 0.676 * | ||||||||
| Tuber number | 1 | −0.849 ** | |||||||||
| Average tuber weight | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barandalla, L.; Alvarez-Morezuelas, A.; Iribar, C.; Ritter, E.; Riga, P.; Lacuesta, M.; de Galarreta, J.I.R. Physiological Response to Nitrogen Deficit in Potato Under Greenhouse Conditions. Plants 2025, 14, 3237. https://doi.org/10.3390/plants14213237
Barandalla L, Alvarez-Morezuelas A, Iribar C, Ritter E, Riga P, Lacuesta M, de Galarreta JIR. Physiological Response to Nitrogen Deficit in Potato Under Greenhouse Conditions. Plants. 2025; 14(21):3237. https://doi.org/10.3390/plants14213237
Chicago/Turabian StyleBarandalla, Leire, Alba Alvarez-Morezuelas, Carmen Iribar, Enrique Ritter, Patrick Riga, Maite Lacuesta, and Jose Ignacio Ruiz de Galarreta. 2025. "Physiological Response to Nitrogen Deficit in Potato Under Greenhouse Conditions" Plants 14, no. 21: 3237. https://doi.org/10.3390/plants14213237
APA StyleBarandalla, L., Alvarez-Morezuelas, A., Iribar, C., Ritter, E., Riga, P., Lacuesta, M., & de Galarreta, J. I. R. (2025). Physiological Response to Nitrogen Deficit in Potato Under Greenhouse Conditions. Plants, 14(21), 3237. https://doi.org/10.3390/plants14213237






