1. Introduction
Helleborus L. is a perennial evergreen herbaceous ornamental plant belonging to the
Ranunculaceae family, comprising approximately 22 species native to Western Asia and Southeastern Europe [
1]. Species in this genus exhibit strong adaptability to cool climates, characterized by notable shade tolerance and cold hardiness [
2]. Blooming primarily in winter and early spring, they are prized as flowering groundcovers for understory planting due to their elegant flowers and evergreen foliage [
3]. Additionally,
Helleborus L. is also an endangered medicinal resource. Its rhizomes contain diverse bioactive compounds, demonstrating significant potential for pharmaceutical research and development [
4]. Among these species,
H. orientalis L. is highly valued in horticulture and widely cultivated as a high-quality potted plant and cut flower [
5]. It is distinguished by its vibrant flower colors, ease of hybridization, vigorous growth, rapid development, drought tolerance at maturity, and low susceptibility to pests and diseases. Consequently, it has become a mainstream choice for commercial cultivation and low-maintenance garden landscaping across Europe, North America, and other regions worldwide.
Current research on
Helleborus L. has advanced in areas such as garden cultivation [
6], tissue culture [
7], introduction and domestication, heat tolerance [
8], and the pharmacology of rhizome extracts [
9]. However, challenges persist in its practical application and breeding. Although existing
H. orientalis L. hybrids demonstrate strong cold tolerance, most are sensitive to high temperature and humidity, which limits their large-scale cultivation in subtropical monsoon climate regions [
8]. Meanwhile, market demand for ornamental traits of
Helleborus L. continues to rise. There is an urgent need for novel cultivars featuring rare colors, unique sepal markings, double flower type, and more compact architectures suited to potted environments. Therefore, studying the ornamental traits and ecological adaptability of
Helleborus L. hybrid progenies is crucial for cultivating and promoting superior germplasm.
Plant phenotypic traits are determined by both genetic background and environmental factors [
10]. They are key indicators of ornamental value and are essential for parental selection and variety screening in hybrid breeding [
11]. This has been confirmed in species such as
Pennisetum alopecuroides,
Lagerstroemia indica, and
Manihot esculenta [
12,
13,
14]. Phenotypic traits are closely related to genetic diversity. Therefore, analyzing these traits can provide valuable insights into germplasm genetic diversity and help identify germplasm with superior traits. Currently, genetic diversity is typically studied through morphological, cytological, and molecular marker methods [
15]. Although SSR marker analysis has been conducted on young leaf tissues of
Helleborus L. [
2], the genetic basis of floral organs and plant phenotypes remains unclear. Moreover, the absence of unified ornamental evaluation standards and a dedicated Distinctness, Uniformity, and Stability (DUS) testing system for new
Helleborus L. varieties has severely limited cultivar protection and commercial application. Thus, systematically elucidating the correlations among phenotypic traits and establishing a comprehensive ornamental value evaluation system for
Helleborus L. are crucial for effective genetic resource utilization and breeding.
The Analytic Hierarchy Process (AHP) is a decision analysis method that combines qualitative judgment with quantitative calculation [
16]. It breaks down complex problems into a hierarchical structure, using pairwise comparisons and matrix calculations to quantify subjective experience [
17]. Currently, AHP has been successfully applied in evaluating plants such as
Rhododendron [
18],
Calcareimontana [
19], and
Cornus spp. [
20] and selecting superior varieties of
Camellia spp. [
21]. Since
Helleborus L. is an important ornamental flowering plant, qualitative traits such as flower color, type, sepal stripe pattern, and flower posture are more significant than quantitative traits such as flower diameter and bloom count in its evaluation. Thus, the AHP method, which combines qualitative and quantitative assessments, is well-suited for the comprehensive evaluation of
Helleborus L. germplasm. Its application will provide a scientific approach for establishing a robust evaluation framework for
Helleborus L. resources.
This study systematically evaluated phenotypic diversity and trait variation in 51 Helleborus L. hybrid individuals, aiming to address the research gap in phenotypic trait inheritance in this genus. Furthermore, this study established a comprehensive evaluation system for ornamental value to support the development of DUS testing guidelines and germplasm assessment.
2. Results
2.1. Analysis of Phenotypic Trait Diversity in Helleborus × hybridus L. Progeny
2.1.1. Quantitative Trait Diversity
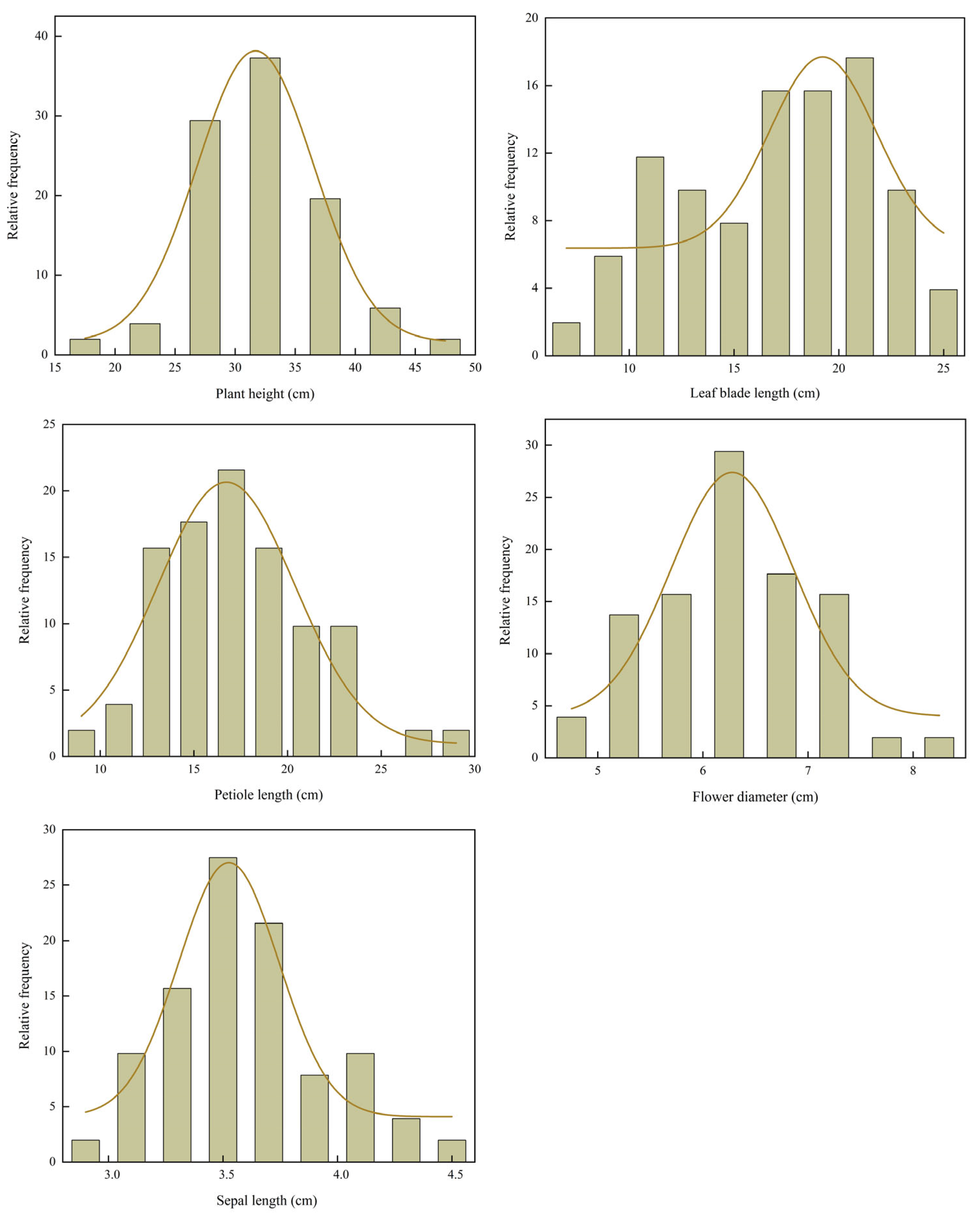
Frequency distribution histograms of seven quantitative traits (
Figure 1) revealed that plant height (PHt), petiole length (PL), flower diameter (FD), and sepal length (SL) followed unimodal and approximately symmetric distributions, which did not significantly deviate from normality (
p > 0.05, Shapiro–Wilk test). Their respective peaks were observed at approximately 33.00 cm, 17.00 cm, 6.50 cm, and 3.50 cm. In contrast, leaf blade length (LBL) significantly deviated from normality (
p < 0.05). LBL was right-skewed, with a concentration of values between 17.00–21.00 cm and a broad spread toward higher values. Collectively, these distribution patterns suggest that traits such as PHt, PL, FD, and SL show high genetic stability in the hybrid progeny. Conversely, the skewed distributions of LBL imply that they may be influenced by environmental factors or major-effect genes.
To further investigate the variation patterns and genetic characteristics of quantitative traits, we analyzed the variation and genetic diversity of seven quantitative traits. As shown in
Table 1, all traits displayed a wide range of variation, with coefficients of variation (CV) ranging from 9.48% to 37.99%. Among them, TFC displayed the highest CV (37.99%), followed by PSFC (35.04%), while SL showed the lowest (9.48%). The Shannon–Weaver diversity index (H′) revealed that LBL had the highest diversity (H′ = 1.51), followed by TFC (H′ = 1.35) and PSFC (H′ = 1.29), whereas SL had the lowest (H′ = 1.09). Notably, substantial phenotypic variation was observed in PHt and TFC. The difference between the tallest and shortest individuals in PHt was 28.60 cm, and the maximum TFC reached 46, which was 2.26 times the mean value. These findings demonstrate clear differences in both phenotypic variability (CV) and genetic diversity (H′) among the quantitative traits across the 51 hybrid progenies. Floral abundance-related traits such as TFC and PSFC exhibited high CV and H′ values, indicating strong potential for selecting high-yielding individuals. In contrast, SL and FD displayed low CV and H′ values, reflecting high genetic conservation and phenotypic stability. LBL, though moderately variable, showed the highest H′ value, indicating rich underlying genetic diversity. Moreover, the extreme phenotypes identified in this population—such as a PL of 45.60 cm and a TFC of 46—constitute valuable breeding resources for the genetic improvement of
Helleborus L.
2.1.2. Qualitative Trait Diversity
Frequency distribution analysis of ten qualitative traits (
Table 2) indicated that flower type (FT) and curvature of sepal edges (CSE) were highly skewed in their distribution: 84.31% of individuals displayed the double flower type, and 76.47% exhibited flat sepal edges. Moderate distributional skewness was observed in plant habit (PlH), flower bearing posture (FBP), and growth vigor (GV), whereas sprouting ability (SA), heat resistance (HR), and disease and pest resistance (DPR) showed relatively balanced distributions. The main color of the sepal upper surface (MC) was highly variable, with white (33.33%) and purple (31.37%) being the most frequent, followed by pink (17.65%). Rare colors such as green and orange each accounted for less than 2%. Regarding sepal stripe pattern type (SSPT), the absence of patterns was most common (43.14%), stellate patterns were not observed, and the remaining types were distributed relatively evenly.
The Shannon–Weaver diversity index (H′) was calculated for each qualitative trait based on frequency distribution data (
Table 2). The H′ values ranged from 0.52 to 1.55, reflecting considerable variation in genetic diversity across traits. Sepal-related traits generally exhibited relatively high diversity, with MC showing the highest value (H′ = 1.55), followed by SSPT (H′ = 1.31) and CSE (H′ = 1.08). SA, GV, and DPR displayed moderate diversity, whereas PlH and HR were comparatively low. The lowest diversity indices were observed for FBP (H′ = 0.66) and FT (H′ = 0.52).
In summary, qualitative traits in Helleborus × hybridus L. progeny displayed complex segregation and significant genetic differentiation. Sepal-related traits such as MC and SSPT showed high genetic diversity, while FT, FBP, and CSE (with flat edges accounting for 76.47%) exhibited high genetic conservation. Rare colors and semi-involute phenotypes occurred at very low frequencies, indicating considerable challenges for breeding improvement.
2.2. Correlation Analysis of Phenotypic Traits in Helleborus × hybridus L. Progeny
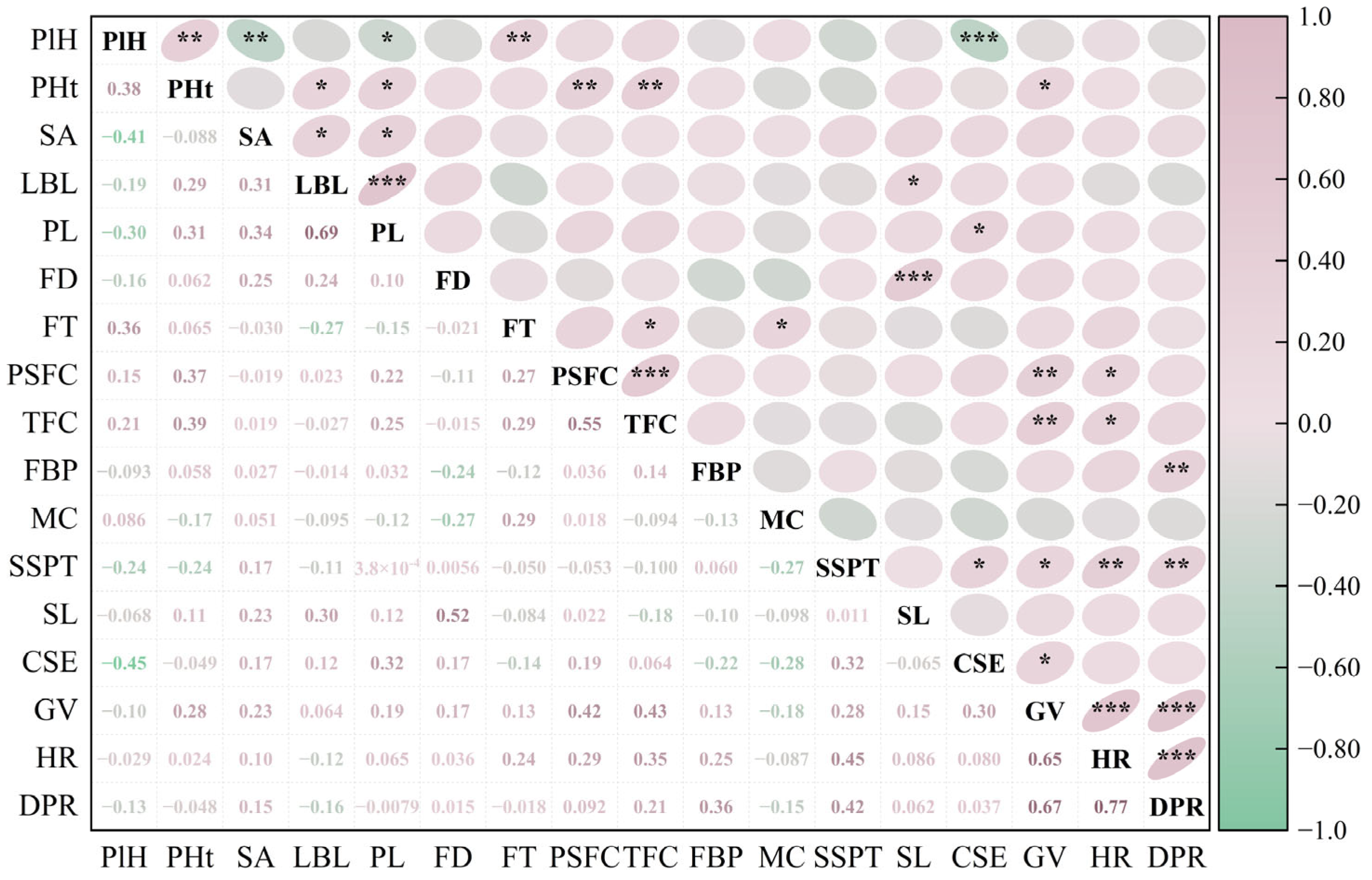
Spearman correlation analysis was performed on 17 phenotypic traits of 51 hybrid individuals. The results showed varying degrees of correlation among these traits, with some showing significant associations. Among all 136 trait pairs analyzed, 32 pairs (23.5%) showed significant correlations, with 26 of these pairs (19.1% of the total) having an absolute correlation coefficient |ρ| ≥ 0.3 (
Figure 2).
Specifically, HR and DPR showed the highest correlation (ρ = 0.77), followed by LBL and PL (ρ = 0.69), as well as GV and DPR (ρ = 0.67). PlH was highly significantly positively correlated with FT, but significantly negatively correlated with SA, PL, and CSE. SA was significantly positively correlated with LBL and PL but not significantly correlated with other traits. Both PSFC and TFC were highly significantly positively correlated with PHt and GV. In addition, TFC was also significantly positively correlated with HR and negatively correlated with SL. SSPT was significantly positively correlated with CSE, HR, and DPR. Meanwhile, CSE was also significantly positively correlated with GV. Overall, phenotypic traits in the Helleborus × hybridus L. progeny were predominantly positively correlated, with few negative correlations. However, the proportion of strong correlations (|ρ| ≥ 0.3) was relatively low (19.10%), indicating a considerable degree of genetic independence among traits.
2.3. Principal Component Analysis of Phenotypic Traits in Helleborus × hybridus L. Progeny
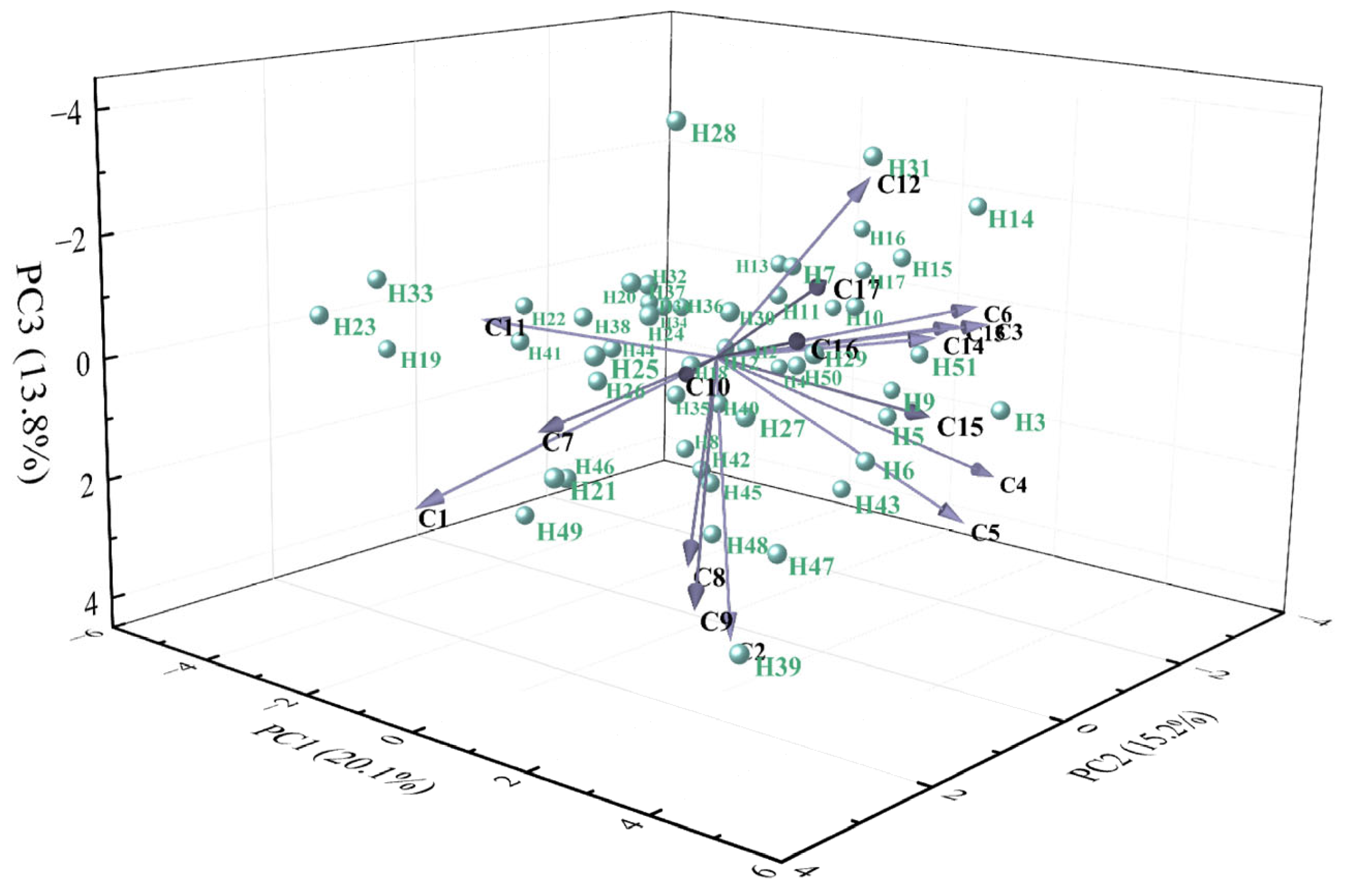
Principal component analysis (PCA) was conducted on 17 phenotypic traits of 51 hybrid individuals (
Table 3). Based on the criterion of eigenvalues greater than 1, six principal components (PCs) were extracted, with eigenvalues of 3.41, 2.58, 2.34, 1.68, 1.41, and 1.25, and variance contribution rates of 20.06%, 15.19%, 13.77%, 9.86%, 8.26%, and 7.36%, respectively. The cumulative contribution rate reached 74.50%, indicating that these six PCs retained most of the information from the original traits and effectively explained the main characteristics of the 17 phenotypic traits in the hybrid progeny.
PC1 (20.06% variance) was heavily loaded with GV, HR, and DPR, reflecting overall resistance performance. PC2 (15.19%) was associated with PHt, PSFC, and TFC, representing growth and floral productivity. PC3 (13.77%) was linked to SA, LBL, and PL, indicating vegetative growth capacity. PC4 (9.86%) was dominated by FD and SL, representing floral organ size. PC5 (8.26%) was primarily characterized by CSE, while PC6 (7.36%) showed high loadings on FT and MC, corresponding to floral morphology.
In the three-dimensional space defined by the first three PCs, the 51 hybrids showed a dispersed distribution (
Figure 3), indicating clear phenotypic segregation among progeny. Overall, these results demonstrate substantial phenotypic variation in the hybrid population. Notably, DPR, HR, GV, PHt, TFC, and PSFC were identified as key traits underlying phenotypic diversity, providing important criteria for germplasm evaluation and cultivar breeding.
2.4. Cluster Analysis of Helleborus × hybridus L. Progeny
R-type and Q-type cluster analyses were performed using average linkage hierarchical clustering based on Euclidean distance, for the 17 phenotypic traits and the 51 hybrid individuals, respectively (
Figure 4 and
Figure 5).
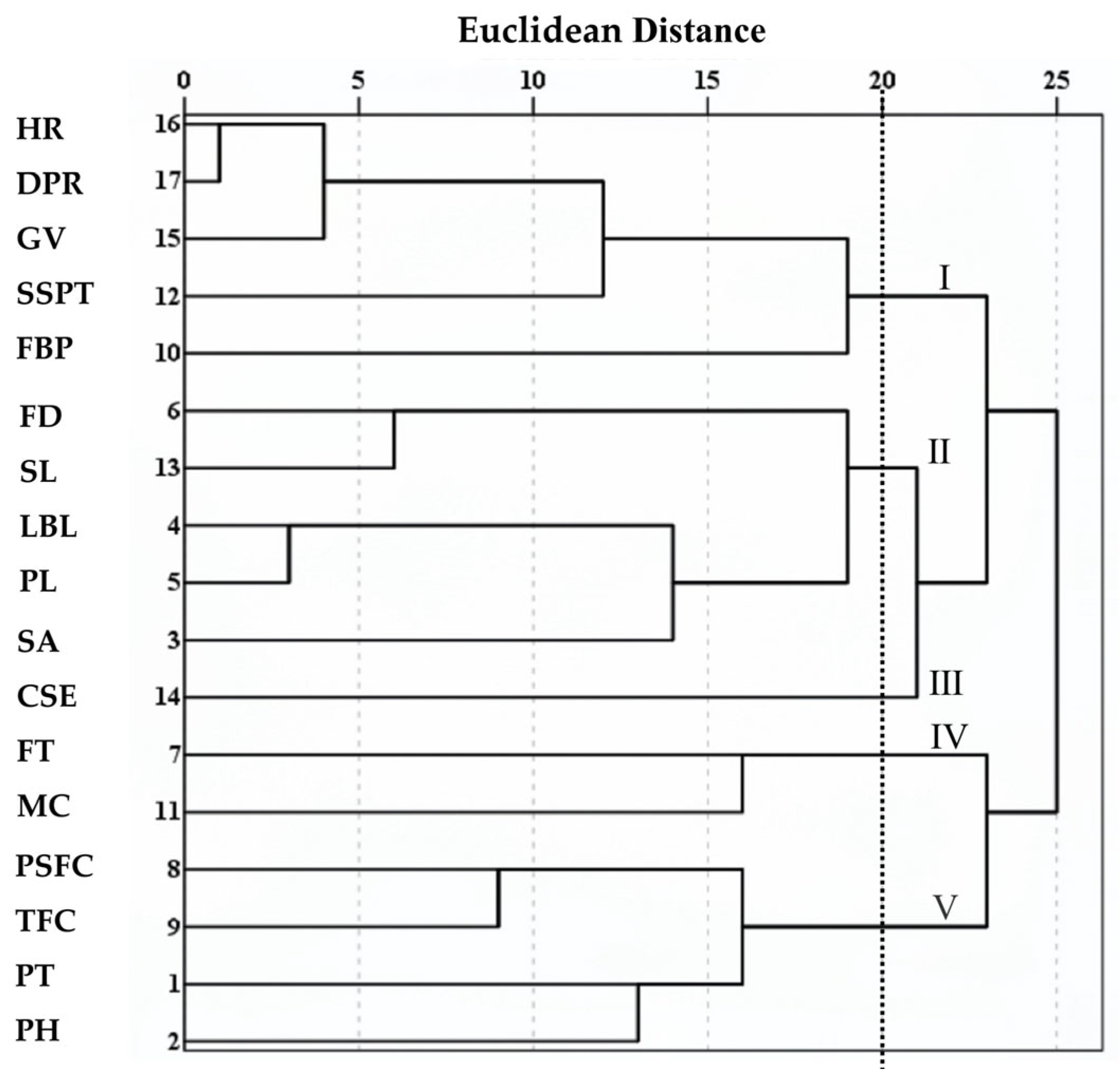
The R-type clustering results (
Figure 4) showed that at a Euclidean distance of 20, the 17 phenotypic traits were divided into five major clusters. Cluster I included HR, DPR, GV, SSPT, and FBP, which are associated with stress resistance and ecological adaptability. Cluster II consisted of FD, SL, LBL, PL, and SA, reflecting characteristics of nutritional and reproductive organs. Cluster III contained only CSE. Cluster IV comprised FT and MC, representing floral traits. Cluster V included PSFC, TFC, PlH, and PHt, related to flower production and plant conformation. Notably, within Cluster I, HR and DPR clustered together first, indicating the strongest correlation between the two traits, followed by the clustering of HR and GV. In Cluster II, LBL and PL were grouped initially, followed by FD and SL, which was highly consistent with the previous correlation analysis (
Figure 2). The R-type clustering results revealed groups of traits with coordinated variation among specific phenotypic trait combinations. However, most other traits showed larger Euclidean distances and dispersed clustering suggest relatively independent evolution. These traits can effectively distinguish germplasm and are suitable for the classification and evaluation studies of
Helleborus L. genetic resources.
The Q-type clustering results showed that at a Euclidean distance of 15, 51 hybrid individuals were divided into five major clusters (
Figure 5):
Cluster I contained 41 hybrids, characterized by superior plant height (PHt; mean: 32.90 cm), larger flower diameter (FD; mean: 6.22 cm), and higher total flower count per plant (TFC; mean: 21.50), as shown in
Table 4. The majority of individuals exhibited a dispersive plant habit (PlH; 58.54%), double flowers (FT; 85.37%), and flat sepal edges (CSE; 73.17%). However, this cluster generally displayed intermediate performance in ecological adaptability traits, as summarized in
Table 5. Representative individuals were H3 and H39.
Cluster II comprised two hybrids, distinguished by the flower diameter (FD; mean: 8.03 cm) and a high number of flowers per stem (PSFC; mean: 4.17), as confirmed in
Table 4. All individuals in this cluster showed double flowers (FT; 100%) and sepal edges with margined spotting patterns (SSPT; 100%). In addition, they were rated as strong in ecological adaptability (
Table 5).
Cluster III contained two hybrids, which exhibited high plant height (PHt; mean: 32.60 cm), along with longer leaf blade length (LBL; mean: 20.10 cm), petiole length (PL; mean: 16.55 cm), and sepal length (SL; mean: 4.50 cm), as supported by
Table 4. All individuals displayed single flowers (FT; 100%) and horizontal flower bearing posture (FBP; 100%). Traits related to ecological adaptability, including GV, HR, and DPR, were generally rated as medium, as detailed in
Table 5.
Cluster IV included five hybrids characterized by intermediate plant height (PHt; mean: 28.61 cm) and relatively short leaf blade length (LBL; mean: 10.97 cm) and petiole length (PL; mean: 12.03 cm), as indicated in
Table 4. All plants had double flowers (FT; 100%), with yellow as the predominant main color of sepal upper surface (MC; 60%). This cluster was consistently rated as poor in ecological adaptability (
Table 5).
Cluster V contained one hybrid, which showed greater plant height (PHt; mean: 32.00 cm) and a high total flower count (TFC; mean: 21.00), according to
Table 4. This individual displayed black as the main color of sepal upper surface (MC; 100%) and semi-involute sepal edges (CSE; 100%). It also showed strong performance in ecological adaptability, as summarized in
Table 5.
Overall, the
Helleborus × hybridus L. individuals exhibited a relatively dispersed structure with indistinct subpopulation differentiation, consistent with the R-type clustering results which showed that most traits did not form strong, cohesive clusters, as evidenced by the long branches and dispersed distribution in the dendrogram (
Figure 4).
2.5. Comprehensive Evaluation of Ornamental Value of Helleborus × hybridus L. Progeny
2.5.1. Construction of Judgment Matrix and Consistency Test
Experts were invited to evaluate the ornamental value indicators of
Helleborus × hybridus L. progeny. The Saaty 1–9 scale method (
Table 6) was used to conduct pairwise comparisons of the evaluation indicators within the same level, with independent scoring based on the relative importance of each indicator.
Based on the expert scoring results, four judgment matrices were constructed: Target Layer A-Criteria Layer (A-B
1-B
3), Criteria Layer B
1-Index Layer (B
1-C
1-C
5), Criteria Layer B
2-Index Layer (B
2-C
6-C
14), and Criteria Layer B
3-Indicator Layer (B
3-C
15-C
17) (
Table 7). The consistency ratio (CR) of each judgment matrix was calculated using yaahp 10.1 software. When CR < 0.1, the judgment matrix was considered to have acceptable consistency, and the results were valid. The results showed that all four judgment matrices had CR values below 0.1, passing the consistency test.
2.5.2. Ranking of Phenotypic Trait Weights
The judgment matrices that passed the consistency test were subjected to weighted calculation using Excel 2019 to determine the weight values (
Wi) of each element in the criteria layer (B
1–B
3) and the index layer (C
1–C
17) (
Table 8).
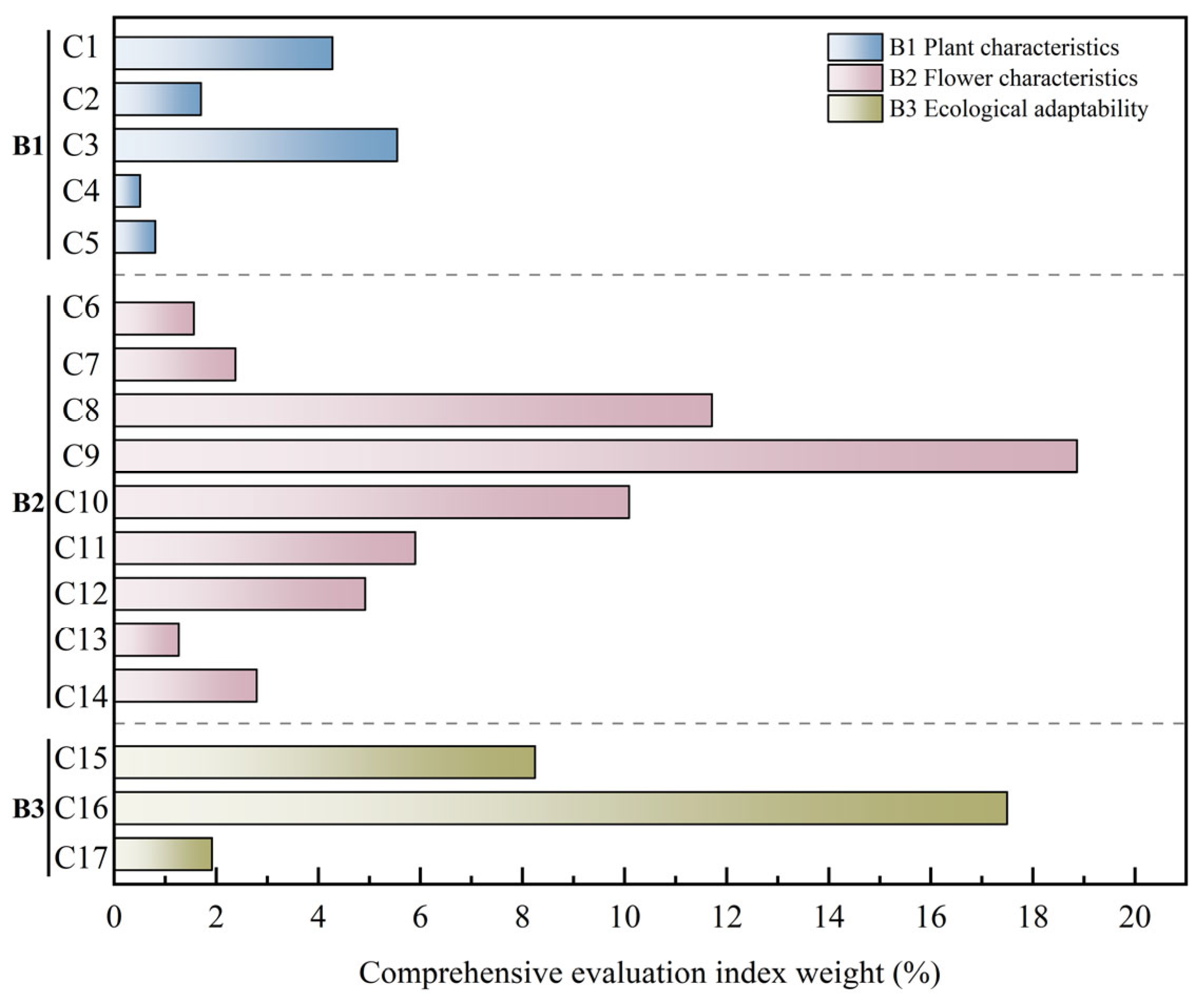
Results of weight analysis showed that among the criteria layers, flower traits (B
2) dominated with a weight of 0.60, indicating their greatest influence on the ornamental value of
Helleborus × hybridus L. progeny; ecological adaptability (B
3) was of secondary importance, while plant traits (B
1) had the lowest weight. Within the index layer, TFC (C
9) had the highest total weight (0.19) making it the primary selection indicator in
Helleborus L. hybrid breeding; HR (C
16) ranked second (0.17). In addition, PSFC (C
8) and FBP (C
10) also had relatively high comprehensive weights, at 0.12 and 0.10, respectively, whereas indicators such as LBL (C
4), PL (C
5), and FD (C
6) had relatively low comprehensive weights (
Figure 6).
Overall, in the comprehensive evaluation system for ornamental value of Helleborus × hybridus L. progeny, the weight of flower trait indicators was significantly higher than that of other trait indicators, indicating that flower-related traits are the decisive factors in ornamental value assessment. This weight distribution reflects the biological characteristics of Helleborus L. as an ornamental flowering plant and provides a reference for subsequent germplasm evaluation and selection of superior individuals.
2.5.3. Comprehensive Evaluation of Hybrid Progeny
According to the scoring criteria, the 51 hybrid individuals were quantitatively evaluated for each trait.
Combined with the total weight values (
Wi) of each evaluation indicator in
Table 8, the comprehensive score (S) of each individual was calculated using the weighted summation formula:
(S represents the comprehensive evaluation score of the individual, Wi is the comprehensive weight value of the i-th trait, and Xi is its quantitative value).
The results (
Table 9) showed that the comprehensive scores of the 51 individuals ranged from 3.08 to 4.36. Among them, H31 received the highest score, while H2 had the lowest. Based on the comprehensive scores, the hybrid progeny were classified into three grades: Grade I (S ≥ 4.0) included 13 individuals with outstanding ornamental value, representing core candidate materials for new cultivar development, such as H31, H3, and H39; Grade II (4.0 > S ≥ 3.5) consisted of 29 individuals with good ornamental value, suitable as foundational material for breeding improvement, including representatives H43, H20, and H51; Grade III (S < 3.5) contained 9 individuals with relatively poor comprehensive ornamental performance, which require careful selection in hybrid breeding, exemplified by H11, H16, and H34. The detailed scores for each trait per individual are provided in
Supplementary Table S1.
Based on the comprehensive evaluation of phenotypic traits, the top three superior germplasms were H31 (score: 4.36), H3 (4.26), and H39 (4.25), which exhibited outstanding performance in multiple traits including plant height (PHt), sprouting ability (SA), flower diameter (FD), total flower count (TFC), and ecological adaptability. In contrast, the three lowest-ranked germplasms—H2 (3.08), H19 (3.10), and H23 (3.26)—were characterized by relatively short plant height, small flower diameter, absence of sepal spots, and poor ecological adaptability. Detailed phenotypic values for all individuals are provided in
Supplementary Table S1.
Statistical analysis revealed that in 51 hybrid individuals, Grade I superior individuals accounted for 25.49%, Grade II for 56.86%, and Grade III for 17.65%. The combined proportion of superior and promising individuals (Grade I + Grade II) reached 82.35%, indicating significant improvement in ornamental traits within this hybrid population. These results demonstrate abundant favorable genetic resources, providing valuable germplasm for subsequent breeding of elite cultivars and selection of hybrid parents in Helleborus L.
2.6. Phenotypic Characterization of Elite Selections of Helleborus × hybridus L. Progeny
Based on comprehensive evaluation scores, 13 Grade I
Helleborus × hybridus L. individuals were selected for variety rights application, and their phenotypic traits were characterized in detail (
Figure 7). At the plant trait level, these superior accessions predominantly exhibited a dispersive plant habit, with H39 and H47 showing a bushy form. Plant height ranged from 30.00 to 45.00 cm, suitable for group planting in landscapes. They also displayed strong sprouting ability, promoting rapid clump formation and enhancing horticultural value. Additionally, these selections showed well-expanded leaves and long petioles, further contributing to their ornamental superiority.
Flower traits clearly highlighted the core ornamental advantages of these elite individuals. The average flower diameter reached 6.41 cm, with H14 attaining a maximum of 8.20 cm. All 13 accessions were double-flowered with rich petal layers and dense blooming, resulting in outstanding visual appeal. Flowers were primarily horizontally oriented, improving upon the drooping habit of parental plants. Sepal colors were mainly pink, white, or yellow, some with margined patterning, and relatively long sepals with curved edges enhanced esthetic quality.
In terms of ecological adaptability, the superior selections exhibited vigorous growth and demonstrated breakthroughs in heat tolerance through cultivation practice. Notably, H3 has become the first Helleborus L. cultivar capable of large-scale open-field summer cultivation in regions south of the Yangtze River.
In summary, these elite Helleborus × hybridus L. progeny display stable growth, outstanding floral traits, and high comprehensive ornamental value, making them promising candidates for winter and understory landscaping in the lower Yangtze River region of China.
5. Conclusions
In summary, the 51 Helleborus L. hybrid individuals displayed rich phenotypic diversity, including extreme phenotypes with high breeding potential. Analysis of trait variation revealed that total flower count (TFC), primary stem flower count (PSFC), main sepal color of sepal upper surface (MC), and sepal stripe pattern type (SSPT) showed particularly high diversity, offering a genetic basis for selecting high-yield individuals and novel floral traits. Correlation analysis indicated the strongest association between heat tolerance (HR) and disease and pest resistance (DPR). Although most traits were positively correlated, the low proportion of strong correlations suggested relative trait independence. PCA extracted six principal components explaining 74.50% of total variance, identifying disease and pest resistance (DPR), heat tolerance (HR), and growth vigor (GV) as key drivers of phenotypic variation. Q-type cluster analysis classified the hybrids into five distinct groups. The AHP-based evaluation confirmed the predominant role of floral traits—particularly total flower count, primary stem flower count, and flower bearing posture—in determining ornamental value. The superior individuals selected through comprehensive scoring provide valuable germplasm resources for future Helleborus L. breeding programs.