An Integrated Approach for the Comprehensive Characterization of Metabolites in Broccoli (Brassica oleracea var. Italica) by Liquid Chromatography High-Resolution Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results
2.1. Optimization of Sample Preparation Methods
2.2. The Widely Targeted Metabolomics Database from Public Data
2.2.1. A Database on Published Components in Broccoli
2.2.2. A Database on Published Glucosinolates and Related Compounds
2.2.3. A Database on Possible Metabolites in Plants
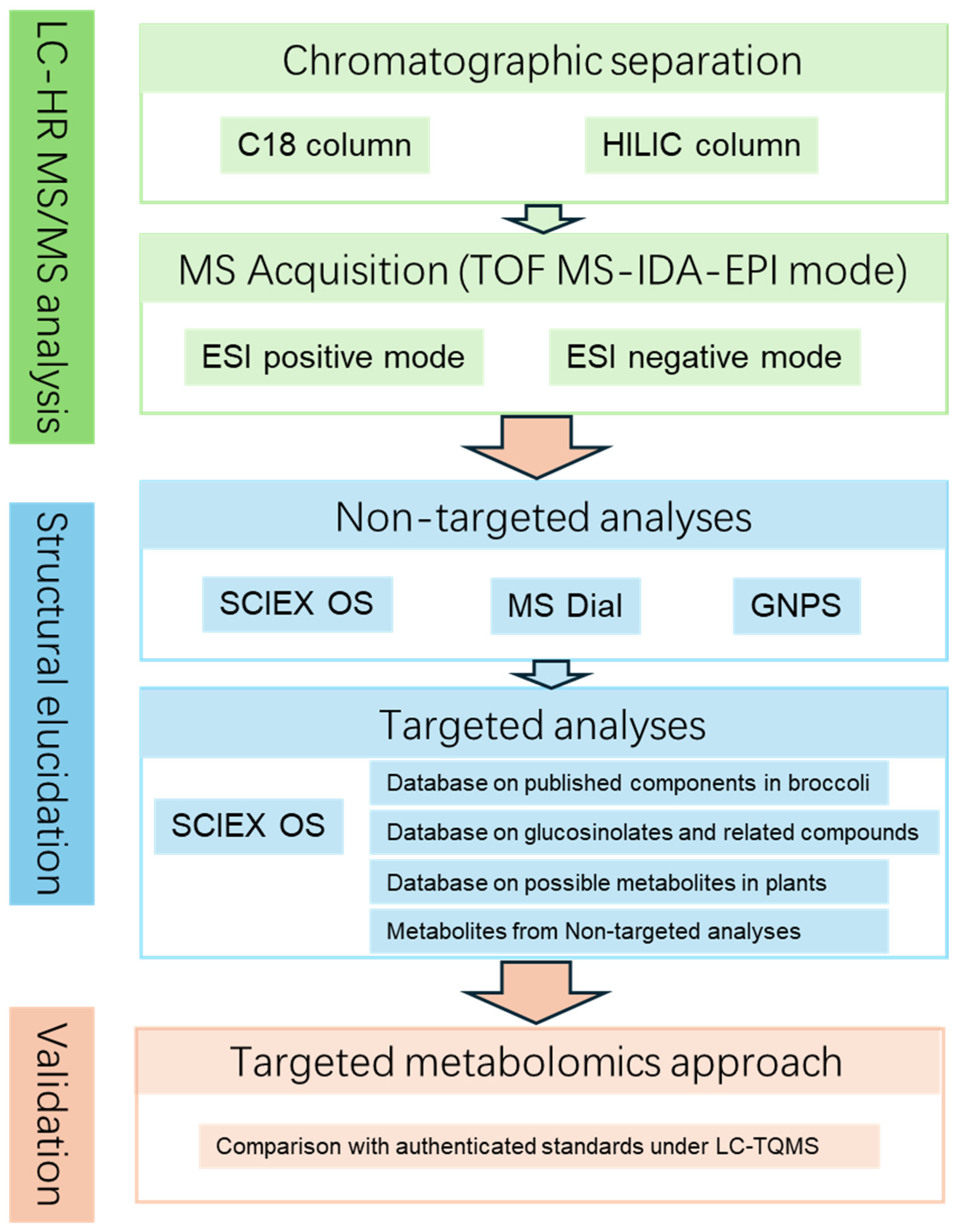
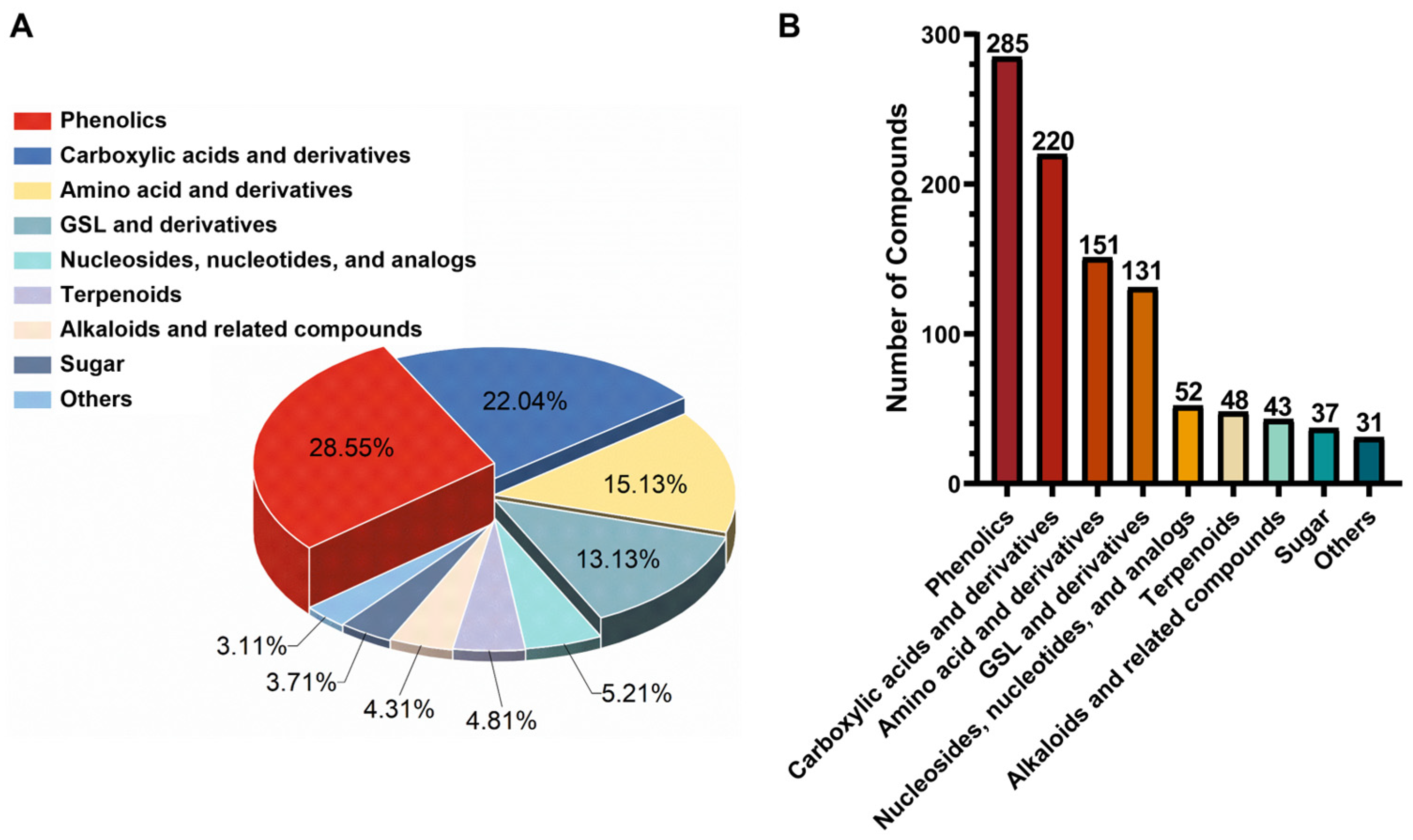
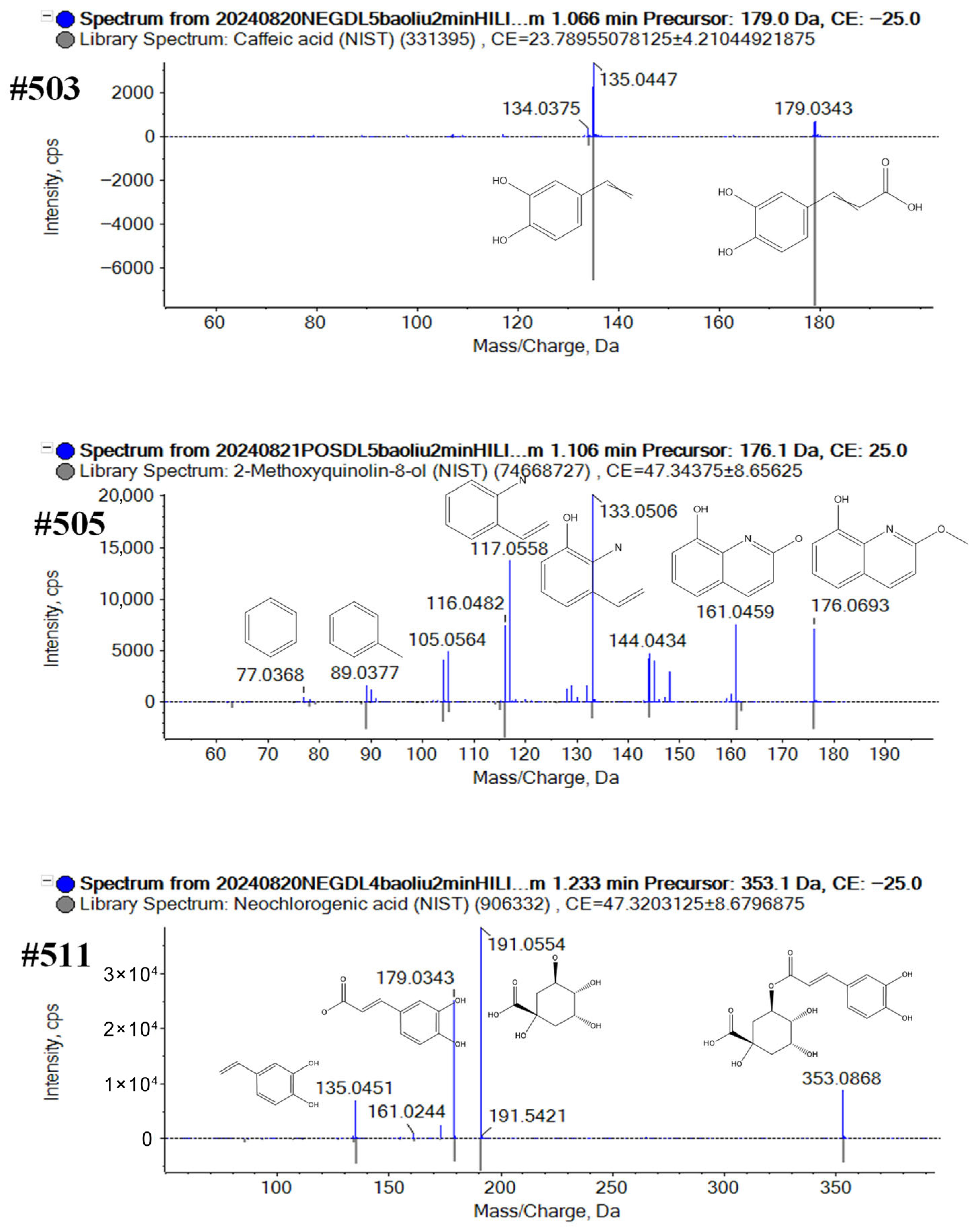
2.3. Comprehensive Characterization of the Metabolites from Broccoli
3. Discussion
3.1. Optimization of Sample Preparation Methods
3.2. Orthogonal Chromatographic Separation
3.3. Integration of Widely Targeted Metabolomics Databases
3.4. Linking Methods to Comprehensive Metabolite Characterization
3.5. Summary of Comprehensive Metabolite Characterization
3.6. Physiological, Nutritional, and Pharmacological Significance
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Materials and Sample Preparation
4.3. UHPLC-Q-TOF MS/MS Analysis Conditions
4.4. Establishment of Published Broccoli Component Database
4.5. Establishment of Published Glucosinolates and Related Compounds Database
4.6. The Integrated Identification
4.6.1. Non-Targeted Analysis
4.6.2. Targeted Metabolite Identification
4.6.3. Final Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABF | Analysis Base File |
| CDS | Calibration delivery system |
| EPI | Enhanced Product Ion |
| ESI | Electrospray ionization |
| FDR | False Discovery Control |
| GNPS | Global Natural Products Social Molecular Networking |
| GSLs | Glucosinolates |
| HILIC | Hydrophilic interaction |
| HR-MS/MS | High-resolution tandem mass spectrometry |
| IDA | Information Dependent Acquisition |
| LC-HRMS | Liquid Chromatography High Resolution Mass Spectrometry |
| LC-MS | Liquid chromatography Mass spectrometry |
| MRM | Multiple Reaction Monitoring |
| TOF MS | Time of Flight Mass Spectrometry |
| UHPLC | Ultra High Performance Liquid Chromatography |
| UHPLC-QTOF MS/MS | Ultra High Performance Liquid Chromatography Quadrupole Time of Flight Tandem Mass Spectrometry |
| XIC | Extracted Ion Chromatograms |
References
- Christ, B.; Pluskal, T.; Aubry, S.; Weng, J.-K. Contribution of untargeted metabolomics for future assessment of biotech crops. Trends Plant Sci. 2018, 23, 1047–1056. [Google Scholar] [CrossRef]
- Dabbousy, R.; Rima, M.; Roufayel, R.; Rahal, M.; Legros, C.; Sabatier, J.-M.; Fajloun, Z. Plant metabolomics: The future of anticancer drug discovery. Pharmaceuticals 2024, 17, 1307. [Google Scholar] [CrossRef]
- Li-Beisson, Y.; Hirai, M.Y.; Nakamura, Y. Plant metabolomics. J. Exp. Bot. 2024, 75, 1651–1653. [Google Scholar] [CrossRef]
- Prosapio, V.; Lopez-Quiroga, E. Freeze-drying technology in foods. Foods 2020, 9, 920. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Ma, L.J.; Ma, N.; Cao, J.L.; Wan, J.B. Characterizing the influence of different drying methods on chemical components of Panax notoginseng leaves by heart-cutting two-dimensional liquid chromatography coupled to Orbitrap high-resolution mass spectrometry. Food Chem. 2022, 369, 130965. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Daka, Z.; Yao, L.; Dong, J.; Zhang, Y.; Li, P.; Zhang, K.; Ji, S. Recent progress in the application of chromatography-coupled mass-spectrometry in the analysis of contaminants in food products. Food Chem. X 2025, 27, 102397. [Google Scholar] [CrossRef] [PubMed]
- Giera, M.; Yanes, O.; Siuzdak, G. Metabolite discovery: Biochemistry’s scientific driver. Cell Metab. 2022, 34, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Bi, J.H.; Xie, M.; Zhang, H.; Shi, Z.Q.; Guo, H.; Yin, H.B.; Zhang, J.N.; Xin, G.Z.; Song, H.P. Classification-based strategies to simplify complex traditional Chinese medicine researches through liquid chromatography–mass spectrometry in the last decade (2011–2020): Theory, technical route and difficulty. J. Chromatogr. A 2021, 1651, 462307. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, L.; Yang, F.; Wang, X.; Hu, Y.; Wang, T.; Lu, X.; Lu, J.; Hu, C.; Tu, H.; et al. High-sensitivity profiling of dipeptides in sauce-flavor Baijiu Daqu by chemical derivatization and ultrahigh-performance liquid chromatography coupled with high-resolution mass spectrometry. Food Chem. X 2023, 21, 101097. [Google Scholar] [CrossRef]
- Pan, H.; Zhou, H.; Miao, S.; Cao, J.; Liu, J.; Lan, L.; Hu, Q.; Mao, X.; Ji, S. An integrated approach for global profiling of multi-type constituents: Comprehensive chemical characterization of Lonicerae Japonicae Flos as a case study. J. Chromatogr. A 2020, 1613, 460674. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.F.; Zhou, H.; Wang, R.; Yu, D.P.; Guo, Z.M.; Liang, X.M. A guide of column selection for two-dimensional liquid chromatography method development of natural alkaloids. Talanta 2023, 251, 123738. [Google Scholar] [CrossRef] [PubMed]
- Iguiniz, M.; Corbel, E.; Roques, N.; Heinisch, S. Quantitative aspects in online comprehensive two-dimensional liquid chromatography for pharmaceutical applications. Talanta 2019, 195, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Wang, S.; Xu, H.S.; Su, Z.Y.; Tang, D.Q.; Qiao, X. The application of on-line two-dimensional liquid chromatography (2DLC) in the chemical analysis of herbal medicines. J. Pharm. Biomed. Anal. 2018, 160, 301–313. [Google Scholar] [CrossRef]
- Cao, J.L.; Wang, S.S.; Hu, H.; He, C.W.; Wan, J.B.; Su, H.X.; Wang, Y.T.; Li, P. Online comprehensive two-dimensional hydrophilic interaction chromatography × reversed-phase liquid chromatography coupled with hybrid linear ion trap Orbitrap mass spectrometry for the analysis of phenolic acids in Salvia miltiorrhiza. J. Chromatogr. A 2018, 1536, 216–227. [Google Scholar] [CrossRef]
- Goyon, A.; Scott, B.; Crittenden, C.M.; Zhang, K. Analysis of pharmaceutical drug oligomers by selective comprehensive two-dimensional liquid chromatography-high resolution mass spectrometry. J. Pharm. Biomed. Anal. 2022, 208, 114466. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, M.; Sun, H.; Xu, X.; Wang, S.; Wang, H.; Li, X.; Jiang, M.; Chen, B.; Zhao, Y.; et al. A multidimensional chromatography/high-resolution mass spectrometry approach for the in-depth metabolites characterization of two Astragalus species. J. Chromatogr. A 2023, 1688, 463718. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Yan, M.; Song, C.; Su, S.; Li, J.; Hu, Z.; Lin, S.; Zou, H.; Tang, Z.; Yan, X. Quantification and diversity analyses of glucosinolates in 191 broccoli genotypes highlight valuable genetic resources for molecular breeding. Agronomy 2023, 13, 2928. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Y.; Yang, H.; Xu, Z.; Li, Z.; Zhang, Z.; Zhang, W.; Deng, J. A comparative metabolomics analysis of phytochemicals and antioxidant activity between broccoli floret and by-products (leaves and stalks). Food Chem. 2024, 443, 138517. [Google Scholar] [CrossRef]
- Nagraj, G.S.; Chouksey, A.; Jaiswal, S.; Jaiswal, A.K. Broccoli. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2020; pp. 5–17. [Google Scholar]
- Gasmi, A.; Gasmi Benahmed, A.; Shanaida, M.; Chirumbolo, S.; Menzel, A.; Anzar, W.; Arshad, M.; Cruz-Martins, N.; Lysiuk, R.; Beley, N.; et al. Anticancer activity of broccoli, its organosulfur and polyphenolic compounds. Crit. Rev. Food Sci. Nutr. 2024, 64, 8054–8072. [Google Scholar] [CrossRef]
- Radünz, M.; Camargo, T.M.; Raphaelli, C.O.; Radünz, A.L.; Gandra, E.Á.; Zavareze, E.D.R. Chemical composition, antimicrobial and antioxidant activities of broccoli, kale, and cauliflower extracts. Plant Foods Hum. Nutr. 2024, 79, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of phenolic compounds and antioxidant activity of 12 cruciferous vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.M.; El-Shiekh, R.A.; Mohamed, O.G.; Al-Karmalawy, A.A.; Tripathi, A.; Abdel-Baki, P.M. LC/MS-based metabolomics reveals chemical variations of two broccoli varieties in relation to their anticholinesterase activity: In vitro and in silico studies. Plant Foods Hum. Nutr. 2024, 79, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Supikova, K.; Kosinova, A.; Vavrusa, M.; Koplikova, L.; François, A.; Pospisil, J.; Zatloukal, M.; Wever, R.; Hartog, A.; Gruz, J. Sulfated phenolic acids in plants. Planta 2022, 255, 124. [Google Scholar] [CrossRef]
- Lopez-Sanchez, P.; de Vos, R.C.; Jonker, H.H.; Mumm, R.; Hall, R.D.; Bialek, L.; Leenman, R.; Strassburg, K.; Vreeken, R.; Hankemeier, T.; et al. Comprehensive metabolomics to evaluate the impact of industrial processing on the phytochemical composition of vegetable purees. Food Chem. 2015, 168, 348–355. [Google Scholar] [CrossRef]
- Ferruzza, S.; Natella, F.; Ranaldi, G.; Murgia, C.; Rossi, C.; Trošt, K.; Mattivi, F.; Nardini, M.; Maldini, M.; Giusti, A.M.; et al. Nutraceutical improvement increases the protective activity of broccoli sprout juice in a human intestinal cell model of gut inflammation. Pharmaceuticals 2016, 9, 48. [Google Scholar] [CrossRef]
- Lin, H.; Sun, J.; Hu, Z.; Cheng, C.; Lin, S.; Zou, H.; Yan, X. Variation in glucosinolate accumulation among different sprout and seedling stages of broccoli (Brassica oleracea var. italica). Plants 2022, 11, 1563. [Google Scholar] [CrossRef]
- Aloo, S.O.; Ofosu, F.K.; Muchiri, M.N.; Vijayalakshmi, S.; Pyo, C.G.; Oh, D.H. In vitro bioactivities of commonly consumed cereal, vegetable, and legume seeds as related to their bioactive components: An untargeted metabolomics approach using UHPLC-QTOF-MS2. Antioxidants 2023, 12, 1501. [Google Scholar] [CrossRef]
- Dębski, H.; Wiczkowski, W.; Szablińska-Piernik, J.; Horbowicz, M. The application of Fe-EDTA and sodium silicate affects the polyphenols content in broccoli and radish sprouts. Biomolecules 2021, 11, 1190. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2010, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, J.; Wan, J.; Pham, Q.; Zhang, Z.; Sun, J.; Yu, L.; Luo, Y.; Wang, T.T.; Chen, P. Profiling of polyphenols and glucosinolates in kale and broccoli microgreens grown under chamber and windowsill conditions by ultrahigh-performance liquid chromatography high-resolution mass spectrometry. ACS Food Sci. Technol. 2021, 2, 101–113. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Identification of the phenolic components of collard greens, kale, and Chinese broccoli. J. Agric. Food Chem. 2009, 57, 7401–7408. [Google Scholar] [CrossRef] [PubMed]
- Choe, U.; Li, Y.; Gao, B.; Yu, L.U.; Wang, T.T.; Sun, J.; Chen, P.; Liu, J.; Yu, L. Chemical compositions of cold-pressed broccoli, carrot, and cucumber seed flours and their in vitro gut microbiota modulatory, anti-inflammatory, and free radical scavenging properties. J. Agric. Food Chem. 2018, 66, 9309–9317. [Google Scholar] [CrossRef]
- Lee, K.B.; Kim, Y.J.; Kim, H.J.; Choi, J.; Kim, J.K. Phytochemical profiles of Brassicaceae vegetables and their multivariate characterization using chemometrics. Appl. Biol. Chem. 2018, 61, 131–144. [Google Scholar] [CrossRef]
- Ren, H.; Bao, H.; Endo, H.; Hayashi, T. Antioxidative and antimicrobial activities and flavonoid contents of organically cultivated vegetables. Nippon. Shokuhin Kagaku Kogaku Kaishi 2001, 48, 246–252. [Google Scholar] [CrossRef][Green Version]
- Vale, A.P.; Santos, J.; Melia, N.; Peixoto, V.; Brito, N.V.; Beatriz, M.; Oliveira, P.P. Phytochemical composition and antimicrobial properties of four varieties of Brassica oleracea sprouts. Food Control 2015, 55, 248–256. [Google Scholar] [CrossRef]
- Zabaras, D.; Roohani, M.; Krishnamurthy, R.; Cochet, M.; Delahunty, C.M. Characterisation of taste-active extracts from raw Brassica oleracea vegetables. Food Funct. 2013, 4, 592–601. [Google Scholar] [CrossRef]
- Lai, J.; Li, C.; Zhang, Y.; Wu, Z.; Li, W.; Zhang, Z.; Ye, W.; Guo, H.; Wang, C.; Long, T.; et al. Integrated transcriptomic and metabolomic analyses reveal the molecular and metabolic basis of flavonoids in Areca catechu L. J. Agric. Food Chem. 2023, 71, 4851–4862. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, L.; Ong, C.N. Untargeted metabolomic analysis of nonvolatile and volatile glucosinolates in Brassicaceae. In Plant Secondary Metabolism Engineering; Fett-Neto, A.G., Ed.; Humana: Louisville, KY, USA, 2022; Volume 2469, pp. 219–229. [Google Scholar]
- Zhu, G.; Wang, S.; Huang, Z.; Zhang, S.; Liao, Q.; Zhang, C.; Lin, T.; Qin, M.; Peng, M.; Yang, C.; et al. Rewiring of the fruit metabolome in tomato breeding. Cell 2018, 172, 249–261. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, Y.; Wu, X.; Zhu, Y.; Mupunga, J.; Bao, W.; Huang, J.; Mao, J.; Liu, S.; You, Y. Hydrolysis before stir-frying increases the isothiocyanate content of broccoli. J. Agric. Food Chem. 2018, 66, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Blažević, S.; Montaut, F.; Burčul, I.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate structural diversity, identification, chemical synthesis and metabolism in plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, H.; Zhao, Z.; Sheng, X.; Shen, Y.; Gu, H. Natural variation of glucosinolates and their breakdown products in broccoli (Brassica oleracea var. italica) seeds. J. Agric. Food Chem. 2019, 67, 12528–12537. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Thi, K.O.; Nguyen, N.L.; Pham, H.N.; Sawada, Y.; Hirai, M.Y.; Dauwe, R.; Dijoux-Franca, M.G. Development of a Pteris vittata L. compound database by widely targeted metabolomics profiling. Biomed. Chromatogr. 2021, 35, e5110. [Google Scholar] [CrossRef]
- Sawada, Y.; Akiyama, K.; Sakata, A.; Kuwahara, A.; Otsuki, H.; Sakurai, T.; Saito, K.; Hirai, M.Y. Widely targeted metabolomics based on large-scale MS/MS data for elucidating metabolite accumulation patterns in plants. Plant Cell Physiol. 2009, 50, 37–47. [Google Scholar] [CrossRef]
- Xiao, Y.; Vecchi, M.M.; Wen, D. Distinguishing Between Leucine and Isoleucine by Integrated LC-MS Analysis Using an Orbitrap Fusion Mass Spectrometer. Anal. Chem. 2016, 88, 10757–10766. [Google Scholar] [CrossRef]
- Wang, J.; Gu, H.; Yu, H.; Zhao, Z.; Sheng, X.; Zhang, X. Genotypic variation of glucosinolates in broccoli (Brassica oleracea var. italica) florets from China. Food Chem. 2012, 133, 735–741. [Google Scholar] [CrossRef]
- Guo, R.; Yuan, G.; Wang, Q. Sucrose enhances the accumulation of anthocyanins and glucosinolates in broccoli sprouts. Food Chem. 2011, 129, 1080–1087. [Google Scholar] [CrossRef]
- Jo, J.S.; Bhandari, S.R.; Kang, G.H.; Shin, Y.K.; Lee, J.G. Selection of broccoli (Brassica oleracea var. italica) on composition and content of glucosinolates and hydrolysates. Sci. Hortic. 2022, 298, 110984. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]





| No | RT | Metabolites | Formula | Calculated [M+H]+/[M−H]− | Observed [M+H]+/[M−H]− | Mass Error (ppm) | Column and Mode * | Class ** |
|---|---|---|---|---|---|---|---|---|
| 2 | 1.55 | Pyroglutamic acid | C5H7NO3 | 128.0353 | 128.0354 | 0.7 | HILIC, NEG | A |
| 4 | 1.8 | 2-Aminoadipic acid | C6H11NO4 | 162.0761 | 162.0756 | −2.8 | HILIC, POS | A |
| 9 | 3.7 | S-Methyl-cysteine | C4H9NO2S | 136.0427 | 136.0428 | 1.2 | HILIC, POS | A |
| 13 | 4.18 | Leucine | C6H13NO2 | 132.1019 | 132.1013 | −4.3 | HILIC, POS | A |
| 14 | 4.29 | Tryptophane | C11H12N2O2 | 205.0972 | 205.0969 | −1.3 | HILIC, POS | A |
| 14 | 2.64 | Tryptophane | C11H12N2O2 | 205.0972 | 205.0973 | 0.9 | T3, POS | A |
| 15 | 4.43 | 5-Methylcytosine hydrochloride | C5H7N3O | 126.0662 | 126.0659 | −2 | HILIC, POS | A |
| 19 | 6.33 | Phenylalanine | C9H11NO2 | 166.0863 | 166.0857 | −3.6 | HILIC, POS | A |
| 19 | 6.35 | Phenylalanine | C9H11NO2 | 164.0723 | 164.0718 | 0.8 | HILIC, NEG | A |
| 20 | 6.51 | Isoleucine | C6H13NO2 | 130.0874 | 130.0876 | 1.9 | HILIC, NEG | A |
| 21 | 6.6 | Methionine | C5H11NO2S | 148.0438 | 148.0438 | 0.4 | HILIC, NEG | A |
| 22 | 6.61 | Tyrosine | C9H11NO3 | 180.0666 | 180.0667 | 0.6 | HILIC, NEG | A |
| 22 | 6.74 | Tyrosine | C9H11NO3 | 182.0812 | 182.0805 | −3.7 | HILIC, POS | A |
| 23 | 6.65 | β-Homovaline | C6H13NO2 | 132.1019 | 132.1013 | −4.7 | HILIC, POS | A |
| 25 | 6.68 | 5-Aminovaleric acid | C5H11NO2 | 116.0717 | 116.0717 | 0.2 | HILIC, NEG | A |
| 27 | 6.69 | N-Acetyl-glutamic acid | C7H11NO5 | 188.0564 | 188.0562 | −1.3 | HILIC, NEG | A |
| 30 | 6.86 | Norvaline | C5H11NO2 | 116.0717 | 116.0718 | 0.8 | HILIC, NEG | A |
| 31 | 6.87 | Threonine | C4H9NO3 | 118.0510 | 118.0511 | 0.9 | HILIC, NEG | A |
| 35 | 7.27 | 2-Aminobutyric acid | C4H9NO2 | 102.0561 | 102.0562 | 1.1 | HILIC, NEG | A |
| 36 | 7.28 | N-Acetyl-serine | C5H9NO4 | 146.0459 | 146.0462 | 2 | HILIC, NEG | A |
| 38 | 7.39 | Aspartic acid | C4H7NO4 | 132.0302 | 132.0304 | 1.6 | HILIC, NEG | A |
| 39 | 7.39 | Allo-threonine | C4H9NO3 | 118.0510 | 118.0510 | 0 | HILIC, NEG | A |
| 41 | 7.59 | Pyridoxal hydrochrolide | C8H9NO3 | 168.0655 | 168.0653 | −1.5 | HILIC, POS | A |
| 42 | 7.61 | Carnitine HCl | C7H15NO3 | 162.1125 | 162.1123 | −1 | HILIC, POS | A |
| 45 | 7.64 | Homocarnosine | C10H16N4O3 | 241.1295 | 241.1292 | −1.2 | HILIC, POS | A |
| 47 | 7.65 | Serine | C3H7NO3 | 104.0353 | 104.0354 | 0.8 | HILIC, NEG | A |
| 50 | 7.67 | Glutamate | C5H9NO4 | 148.0604 | 148.0599 | −3.7 | HILIC, POS | A |
| 58 | 7.97 | Asparagine | C4H8N2O3 | 131.0462 | 131.0463 | 0.5 | HILIC, NEG | A |
| 62 | 8.14 | Glutamine | C5H10N2O3 | 145.0619 | 145.0618 | −0.3 | HILIC, NEG | A |
| 62 | 8.32 | Glutamine | C5H10N2O3 | 147.0759 | 147.0759 | −3.5 | HILIC, POS | A |
| 65 | 8.32 | Ala-Ala | C6H12N2O3 | 159.0775 | 159.0774 | −0.9 | HILIC, NEG | A |
| 68 | 8.44 | Glutathione (reduced form) | C10H17N3O6S | 306.0765 | 306.0764 | −0.4 | HILIC, NEG | A |
| 70 | 8.47 | Citrulline | C6H13N3O3 | 174.0884 | 174.0888 | 2.4 | HILIC, NEG | A |
| 70 | 8.6 | Citrulline | C6H13N3O3 | 176.1030 | 176.103 | 0.4 | HILIC, POS | A |
| 71 | 8.48 | Anserine | C10H16N4O3 | 241.1295 | 241.1297 | 0.6 | HILIC, POS | A |
| 72 | 8.51 | Methionine sulfoxide | C5H11NO3S | 164.0387 | 164.0388 | 0.6 | HILIC, NEG | A |
| 74 | 8.52 | Cystathionine | C7H14N2O4S | 221.0602 | 221.0602 | 0.1 | HILIC, NEG | A |
| 77 | 8.59 | Gly-Gly | C4H8N2O3 | 131.0462 | 131.0462 | 0.1 | HILIC, NEG | A |
| 78 | 8.67 | Urocanic acid | C6H6N2O2 | 137.0357 | 137.0357 | 0.4 | HILIC, NEG | A |
| 79 | 8.69 | S-Adenosyl- homocysteine | C14H20N6O5S | 385.1289 | 385.1282 | −1.7 | HILIC, POS | A |
| 80 | 8.72 | Ornithine monohydrochloride | C5H12N2O2 | 131.0826 | 131.0827 | 0.8 | HILIC, NEG | A |
| 81 | 8.75 | Histidine | C6H9N3O2 | 156.0768 | 156.0762 | −3.5 | HILIC, POS | A |
| 81 | 8.82 | Histidine | C6H9N3O2 | 154.0624 | 154.0624 | 1.5 | HILIC, NEG | A |
| 90 | 9.44 | 3-Methyl- histidine | C7H11N3O2 | 170.0924 | 170.0919 | −2.9 | HILIC, POS | A |
| 93 | 9.54 | Pipecolinic acid | C6H11NO2 | 130.0863 | 130.0861 | −1 | HILIC, POS | A |
| 95 | 9.57 | Lysine | C6H14N2O2 | 147.1128 | 147.1123 | −3.7 | HILIC, POS | A |
| 96 | 9.96 | Homomethionine | C6H13NO2S | 164.0740 | 164.0732 | −4.8 | HILIC, POS | A |
| 97 | 10.65 | Saccharopine | C11H20N2O6 | 277.1393 | 277.1393 | −0.4 | HILIC, POS | A |
| 99 | 10.69 | 2,3-Diaminopropionic acid monohydrochloride | C3H9CLN2O2 | 141.0425 | 141.0426 | 0.3 | HILIC, POS | A |
| 100 | 10.93 | Glutathione (oxidized form) | C20H32N6O12S2 | 611.1447 | 611.1454 | −2 | HILIC, NEG | A |
| 100 | 10.93 | Glutathione (oxidized form) | C20H32N6O12S2 | 613.1592 | 613.158 | −2 | HILIC, POS | A |
| 107 | 2.14 | Proline | C5H9NO2 | 116.0706 | 116.0707 | 0.7 | T3, POS | A |
| 108 | 2.14 | 1-Amino-1-cyclopentanecarboxylic acid | C6H11NO2 | 130.0863 | 130.0867 | 3.5 | T3, POS | A |
| 151 | 38.51 | Argininosuccinic acid disodium salt | C10H18N4O6 | 291.1299 | 291.13 | 0.3 | T3, POS | A |
| 162 | 1.14 | Succinic acid | C4H6O4 | 117.0193 | 117.0194 | 0.8 | HILIC, NEG | B |
| 171 | 1.26 | Malic acid | C4H6O5 | 133.0143 | 133.0143 | 0.7 | HILIC, NEG | B |
| 171 | 2.25 | Malic acid | C4H6O5 | 133.0144 | 133.0144 | 1 | T3, NEG | B |
| 174 | 1.44 | Glyceric acid | C3H6O4 | 105.0193 | 105.0193 | −0.4 | HILIC, NEG | B |
| 186 | 2.08 | Citric acid, Anhydrous | C6H8O7 | 191.0199 | 191.0199 | 1.1 | HILIC, NEG | B |
| 186 | 2.3 | Citric acid, Anhydrous | C6H8O7 | 191.0201 | 191.0201 | 2.1 | T3, NEG | B |
| 199 | 5.14 | Quinic acid | C7H12O6 | 191.0561 | 191.056 | −0.3 | HILIC, NEG | B |
| 206 | 7.6 | 1-Aminocyclopropane-1-carboxylic acid | C4H7NO2 | 100.0404 | 100.0404 | 0.3 | HILIC, NEG | B |
| 209 | 7.93 | Trigonelline hydrochloride | C7H7NO2 | 138.0550 | 138.0543 | −4.7 | HILIC, POS | B |
| 226 | 2.28 | Citramalic acid | C5H8O5 | 147.0299 | 147.0298 | −0.8 | T3, NEG | B |
| 239 | 4.15 | Threonic acid hemicalcium salt | C4H8O5 | 135.0299 | 135.0299 | 0.3 | T3, NEG | B |
| 241 | 4.68 | Pimelic acid | C7H12O4 | 159.0663 | 159.0664 | 0.8 | T3, NEG | B |
| 270 | 16.69 | Vanillin | C8H8O3 | 151.0401 | 151.0402 | 0.8 | T3, NEG | B |
| 276 | 18.14 | Sebacic acid | C10H18O4 | 201.1132 | 201.1132 | 0.1 | T3, NEG | B |
| 368 | 36.67 | Adipic acid | C6H10O4 | 147.0653 | 147.0653 | 0.8 | T3, POS | B |
| 376 | 1.35 | Indol-3-ylmethyl-glucosinolate | C16H20N2O9S2 | 447.0537 | 447.0541 | 0.8 | HILIC, NEG | C |
| 376 | 19.77 | Indol-3-ylmethyl-glucosinolate | C16H20N2O9S2 | 447.0537 | 447.0553 | 3.5 | T3, NEG | C |
| 393 | 3.39 | But-3-enylglucosinolate | C11H19NO9S2 | 372.0428 | 372.043 | 0.4 | HILIC, NEG | C |
| 444 | 7.89 | Sulforaphane | C6H11NOS2 | 178.0355 | 178.0354 | −0.7 | T3, POS | C |
| 477 | 20.85 | Gluconasturtiin | C15H21NO9S2 | 422.0585 | 422.0587 | 0.5 | T3, NEG | C |
| 511 | 8.88 | Chlorogenic acid Hemihydrate | C16H18O9 | 353.0878 | 353.0881 | 1 | T3, NEG | D |
| 521 | 1.87 | 4-Pyridoxate | C8H9NO4 | 182.0459 | 182.046 | 0.9 | HILIC, NEG | D |
| 529 | 4.44 | Kynurenic acid | C10H7NO3 | 188.0353 | 188.0351 | −1.1 | HILIC, NEG | D |
| 529 | 4.48 | Kynurenic acid | C10H7NO3 | 190.0499 | 190.0495 | −2 | HILIC, POS | D |
| 529 | 15.82 | Kynurenic acid | C10H7NO3 | 188.0353 | 188.0355 | 0.7 | T3, NEG | D |
| 534 | 5.14 | Luteolin-3′,7-di-O-glucoside | C27H30O16 | 609.1461 | 609.146 | −0.2 | HILIC, NEG | D |
| 547 | 8.08 | 2′,6′-Dihydroxy-4-methoxychalcone-4′-O-neohesperidoside | C28H34O14 | 593.1876 | 593.1874 | −0.4 | HILIC, NEG | D |
| 549 | 8.27 | 3-Hydroxyanthranilic acid | C7H7NO3 | 154.0499 | 154.0498 | −0.5 | HILIC, POS | D |
| 549 | 4.04 | 3-Hydroxyanthranilic acid | C7H7NO3 | 154.0499 | 154.0499 | 0 | T3, POS | D |
| 555 | 8.94 | Shikimic acid | C7H10O5 | 175.0601 | 175.0602 | 0.7 | HILIC, POS | D |
| 576 | 3.22 | Esculin sesquihydrate | C15H16O9 | 341.0867 | 341.0869 | −0.1 | T3, POS | D |
| 600 | 6.99 | cis or trans-4-Hydroxy-3-methoxycinnamic acid_Ferulic acid | C10H10O4 | 195.0652 | 195.0658 | 3.3 | T3, POS | D |
| 606 | 7.36 | Aureusidin | C15H10O6 | 287.0550 | 287.055 | 0 | T3, POS | D |
| 611 | 8.29 | Glucopyranosyl sinapate | C17H22O10 | 387.1286 | 387.1287 | 0.2 | T3, POS | D |
| 611 | 8.32 | Glucopyranosyl sinapate | C17H22O10 | 385.1140 | 385.1142 | 0.5 | T3, NEG | D |
| 629 | 12.3 | Quercitrin | C21H20O11 | 449.1078 | 449.1082 | 0.8 | T3, POS | D |
| 665 | 15.63 | 5-Hydroxyindole-3-acetate | C10H9NO3 | 190.0510 | 190.051 | 0.3 | T3, NEG | D |
| 665 | 15.63 | 5-Hydroxyindole-3-acetate | C10H9NO3 | 192.0655 | 192.0656 | 0.2 | T3, POS | D |
| 696 | 17.18 | Kaempferol-3-rhamnoside-4″-rhamnoside,-7-rhamnoside | C33H40O18 | 723.2142 | 723.2168 | 3.6 | T3, NEG | D |
| 717 | 18.33 | Salicylic acid | C7H6O3 | 137.0244 | 137.0249 | 3.8 | T3, NEG | D |
| 737 | 20.62 | Sissotrin | C22H22O10 | 447.1286 | 447.1284 | −0.3 | T3, POS | D |
| 790 | 1.67 | Nicotinamide | C6H6N2O | 123.0547 | 123.0547 | −4.9 | HILIC, POS | E |
| 792 | 2.13 | Riboflavin | C17H20N4O6 | 377.1456 | 377.1458 | 0.6 | HILIC, POS | E |
| 792 | 10.74 | Riboflavin | C17H20N4O6 | 377.1456 | 377.1459 | 0.9 | T3, POS | E |
| 793 | 3.34 | Diethanolamine | C4H11NO2 | 106.0863 | 106.0859 | −3.6 | HILIC, POS | E |
| 800 | 2.13 | Pyridoxamine dihydrochloride | C8H12N2O2 | 167.0826 | 167.082 | −3.4 | T3, NEG | E |
| 802 | 2.13 | N-Acetyl putrescine hydrochloride | C6H14N2O | 131.1179 | 131.1185 | 4.9 | T3, POS | E |
| 810 | 2.4 | Pyridoxine | C8H11NO3 | 170.0812 | 170.081 | −0.8 | T3, POS | E |
| 824 | 21.29 | Indole-3-carboxyaldehyde | C9H7NO | 146.0600 | 146.0601 | 0.3 | T3, POS | E |
| 831 | 1.44 | Uridine | C9H12N2O6 | 243.0623 | 243.0623 | 0.2 | HILIC, NEG | F |
| 833 | 2.07 | 5′-Deoxy-5′-Methylthioadenosine | C11H15N5O3S | 298.0968 | 298.0966 | −0.9 | HILIC, POS | F |
| 833 | 2.2 | 5′-Deoxy-5′-Methylthioadenosine | C11H15N5O3S | 298.0968 | 298.098 | 4 | T3, POS | F |
| 835 | 2.21 | Guanosine | C10H13N5O5 | 284.0989 | 284.0987 | −0.7 | HILIC, POS | F |
| 836 | 2.36 | Adenosine-3′,5′-cyclicmonophosphate | C10H12N5O6P | 330.0598 | 330.0595 | −0.8 | HILIC, POS | F |
| 837 | 2.42 | 2′-Deoxyguanosine monohydrate | C10H13N5O4 | 268.1040 | 268.1038 | −0.7 | HILIC, POS | F |
| 838 | 3.71 | Cytidine | C9H13N3O5 | 244.0926 | 244.0926 | −0.8 | HILIC, POS | F |
| 842 | 6.61 | Inosine | C10H12N4O5 | 267.0735 | 267.0728 | −2.7 | HILIC, NEG | F |
| 845 | 7.12 | Uridine-5′-monophosphate | C9H13N2O9P | 323.0286 | 323.029 | 1.2 | HILIC, NEG | F |
| 845 | 2.48 | Uridine-5′-monophosphate | C9H13N2O9P | 323.0286 | 323.0284 | −0.5 | T3, NEG | F |
| 846 | 7.59 | β-Nicotinamide mononucleotide | C11H15N2O8P | 335.0639 | 335.0634 | −1.5 | HILIC, POS | F |
| 852 | 8.08 | 2′-Deoxyadenosine monohydrate | C10H13N5O3 | 250.0946 | 250.0943 | −1 | HILIC, NEG | F |
| 853 | 8.12 | Adenosine | C10H13N5O4 | 266.0895 | 266.0886 | HILIC, NEG | F | |
| 855 | 8.16 | Thymidine | C10H14N2O5 | 243.0975 | 243.0974 | −0.6 | HILIC, POS | F |
| 859 | 8.74 | 2′-Deoxycytidine | C9H13N3O4 | 226.0833 | 226.0834 | 0.2 | HILIC, NEG | F |
| 867 | 2.25 | Zeatin | C10H13N5O | 218.1047 | 218.1044 | −1.4 | T3, NEG | F |
| 870 | 5.35 | Zeatin-9-glucoside | C16H23N5O6 | 380.1576 | 380.1574 | −0.5 | T3, NEG | F |
| 871 | 5.89 | Adenine | C5H5N5 | 136.0618 | 136.0616 | −0.9 | T3, POS | F |
| 876 | 10.8 | Inosine-5′-monophosphate | C10H13N4O8P | 349.0544 | 349.0528 | −4.5 | T3, POS | F |
| 888 | 2.88 | Sucrose | C12H22O11 | 341.1089 | 341.1088 | −0.5 | HILIC, NEG | G |
| 890 | 4.37 | Cellobiose | C12H22O11 | 341.1089 | 341.1087 | −0.8 | HILIC, NEG | G |
| 891 | 5.05 | Glucoheptose | C7H14O7 | 209.0667 | 209.0669 | 1 | HILIC, NEG | G |
| 891 | 2.22 | Glucoheptose | C7H14O7 | 209.0667 | 209.0665 | −0.7 | T3, NEG | G |
| 896 | 5.19 | 1-Kestose | C18H32O16 | 503.1618 | 503.1618 | 0.1 | HILIC, NEG | G |
| 898 | 5.36 | Melibiose | C12H22O11 | 341.1089 | 341.1088 | −0.5 | HILIC, NEG | G |
| 900 | 6.76 | α-Lactose monohydrate | C12H22O11 | 341.1089 | 341.1087 | −0.8 | HILIC, NEG | G |
| 903 | 7.64 | N-Acetyl-glucosamine | C8H15NO6 | 222.0972 | 222.0972 | −0.1 | HILIC, POS | G |
| 906 | 8.02 | Raffinose pentahydrate | C18H32O16 | 503.1618 | 503.1618 | 0.1 | HILIC, NEG | G |
| No. | Cultivar | Provider |
|---|---|---|
| Q276 | D2206 | Wuhan jiutouniao seedling Co., Ltd., Wuhan, China |
| Q121 | Zheqing 227 | Zhejiang Academy of Agricultural Sciences, Hangzhou, China |
| Q287 | Zhongqing 15 | Institute of vegetables and flowers, Chinese academy of agricultural Sciences, Beijing, China |
| Q175 | W5 | Wenzhou Academy of Agricultural Sciences, Wenzhou, China |
| Q258 | B2043 | Henan Oulande Seed Industry Co., Ltd., Zhengzhou, China |
| Q134 | Xilanhua 75 | Fujian ZhuBo Agriculture Science & Technology Co., Ltd., Ningde, China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Yan, M.; Song, C.; Sato, M.; Su, S.; Lin, S.; Li, J.; Zou, H.; Tang, Z.; Hirai, M.Y.; et al. An Integrated Approach for the Comprehensive Characterization of Metabolites in Broccoli (Brassica oleracea var. Italica) by Liquid Chromatography High-Resolution Tandem Mass Spectrometry. Plants 2025, 14, 3223. https://doi.org/10.3390/plants14203223
Hu Z, Yan M, Song C, Sato M, Su S, Lin S, Li J, Zou H, Tang Z, Hirai MY, et al. An Integrated Approach for the Comprehensive Characterization of Metabolites in Broccoli (Brassica oleracea var. Italica) by Liquid Chromatography High-Resolution Tandem Mass Spectrometry. Plants. 2025; 14(20):3223. https://doi.org/10.3390/plants14203223
Chicago/Turabian StyleHu, Zhiwei, Meijia Yan, Chenxue Song, Muneo Sato, Shiwen Su, Sue Lin, Junliang Li, Huixi Zou, Zheng Tang, Masami Yokota Hirai, and et al. 2025. "An Integrated Approach for the Comprehensive Characterization of Metabolites in Broccoli (Brassica oleracea var. Italica) by Liquid Chromatography High-Resolution Tandem Mass Spectrometry" Plants 14, no. 20: 3223. https://doi.org/10.3390/plants14203223
APA StyleHu, Z., Yan, M., Song, C., Sato, M., Su, S., Lin, S., Li, J., Zou, H., Tang, Z., Hirai, M. Y., & Yan, X. (2025). An Integrated Approach for the Comprehensive Characterization of Metabolites in Broccoli (Brassica oleracea var. Italica) by Liquid Chromatography High-Resolution Tandem Mass Spectrometry. Plants, 14(20), 3223. https://doi.org/10.3390/plants14203223







