Effects of Top-Pruning Intensity Gradient on Root System Architecture and Allometric Patterns in Pinus yunnanensis Franch. Seedlings
Abstract
1. Introduction
2. Results
2.1. Effects of Different Top-Pruning Intensities on Root Biomass Accumulation, Allocation, and Relative Water Content
2.2. Effects of Different Top-Pruning Intensities on Root Diameter Distribution
2.3. Effects of Different Top-Pruning Intensities on Fine and Coarse Roots
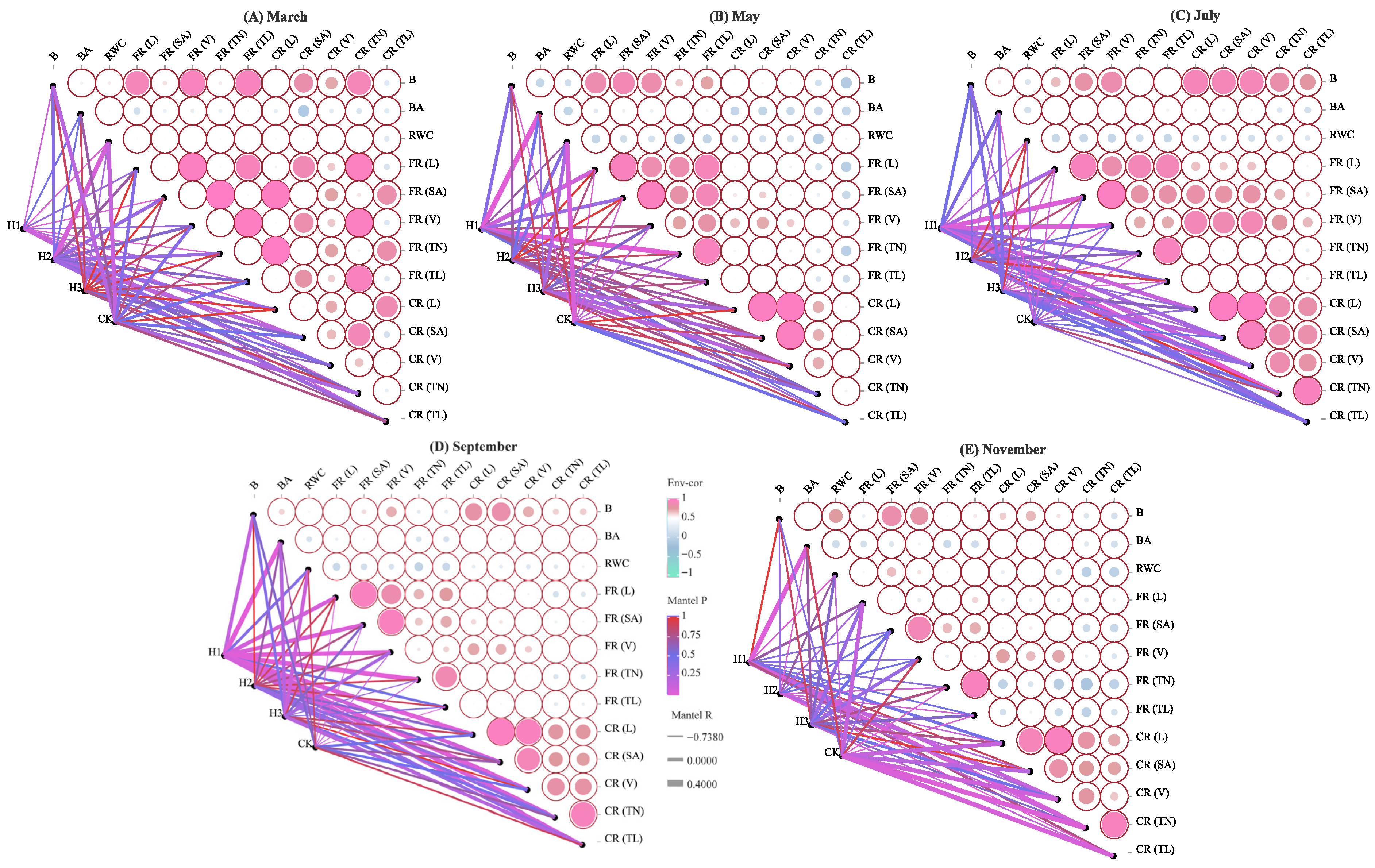
2.4. Correlation Analysis of Root Morphological Traits
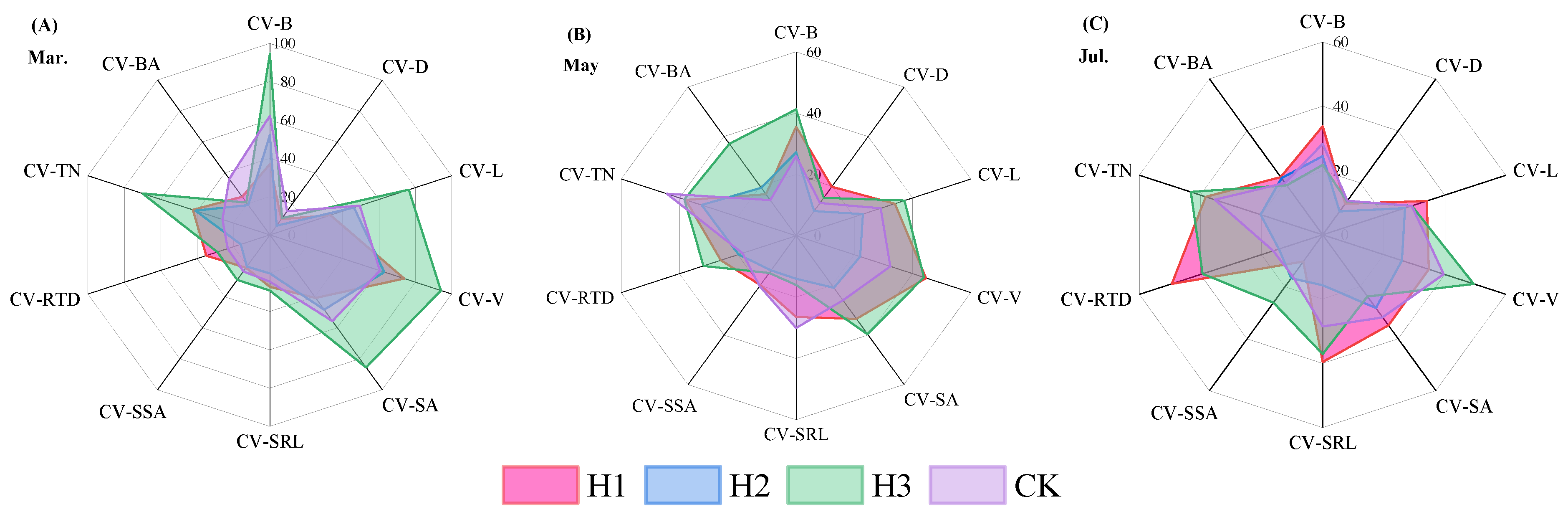
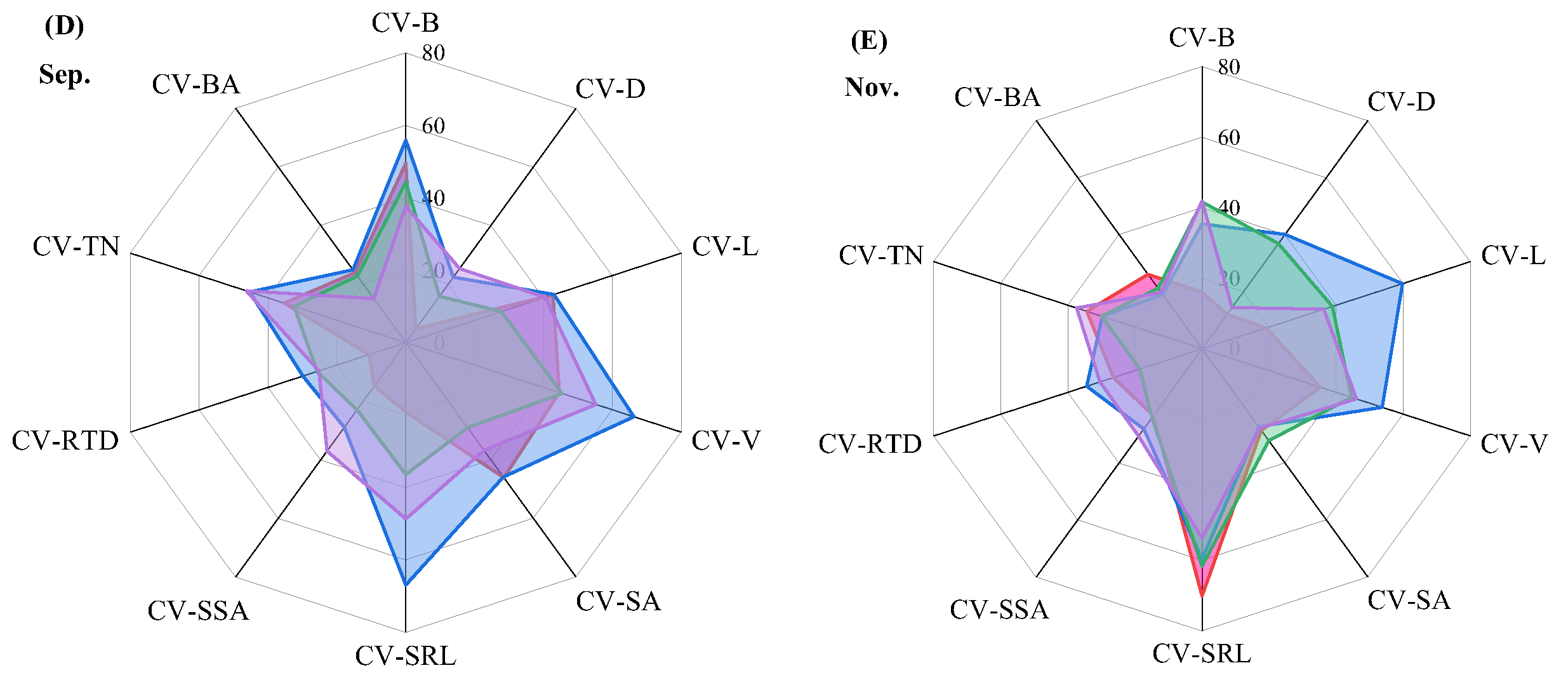
2.5. Variation Analysis of Root Morphological Traits Under Different Top-Pruning Intensities
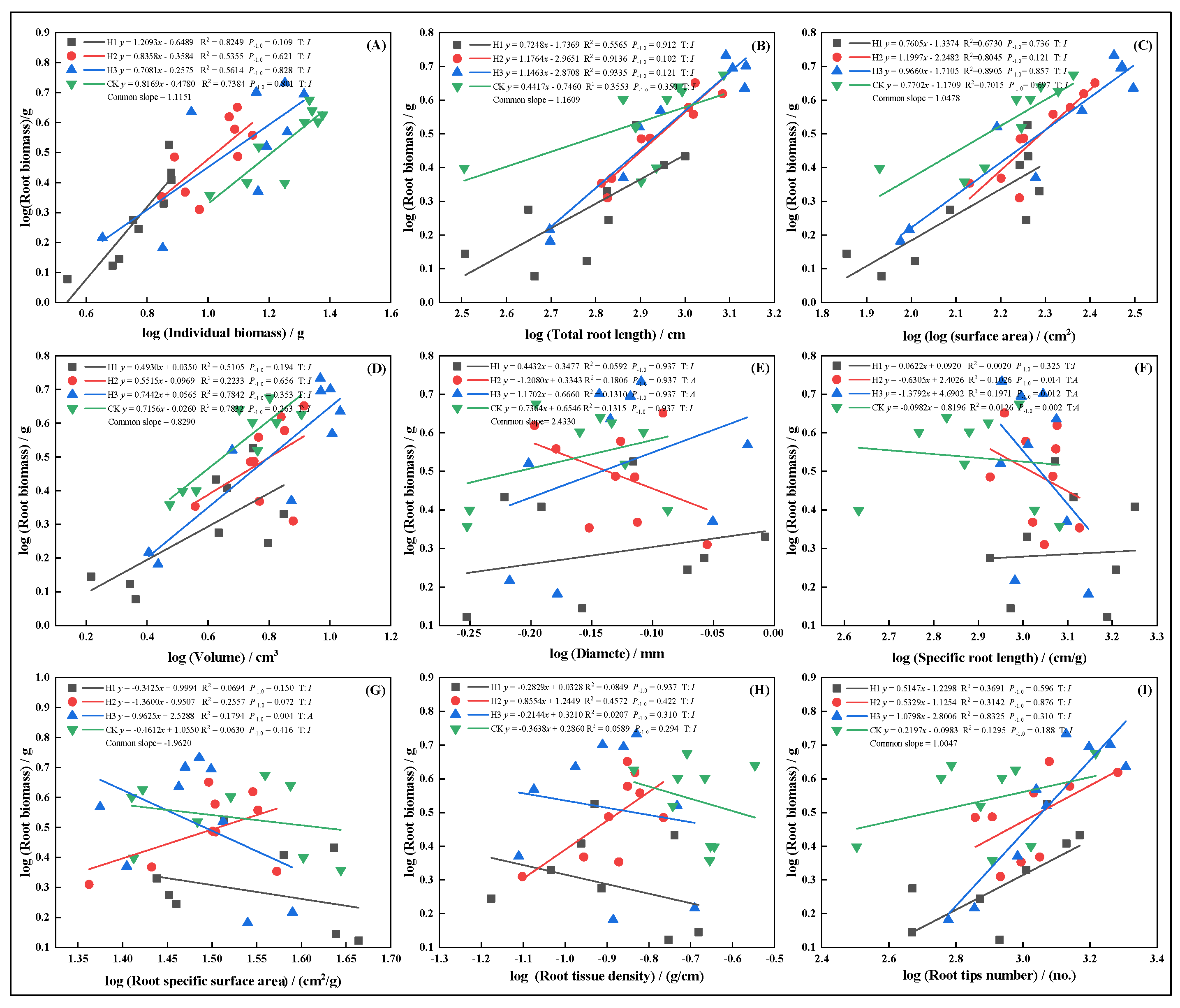
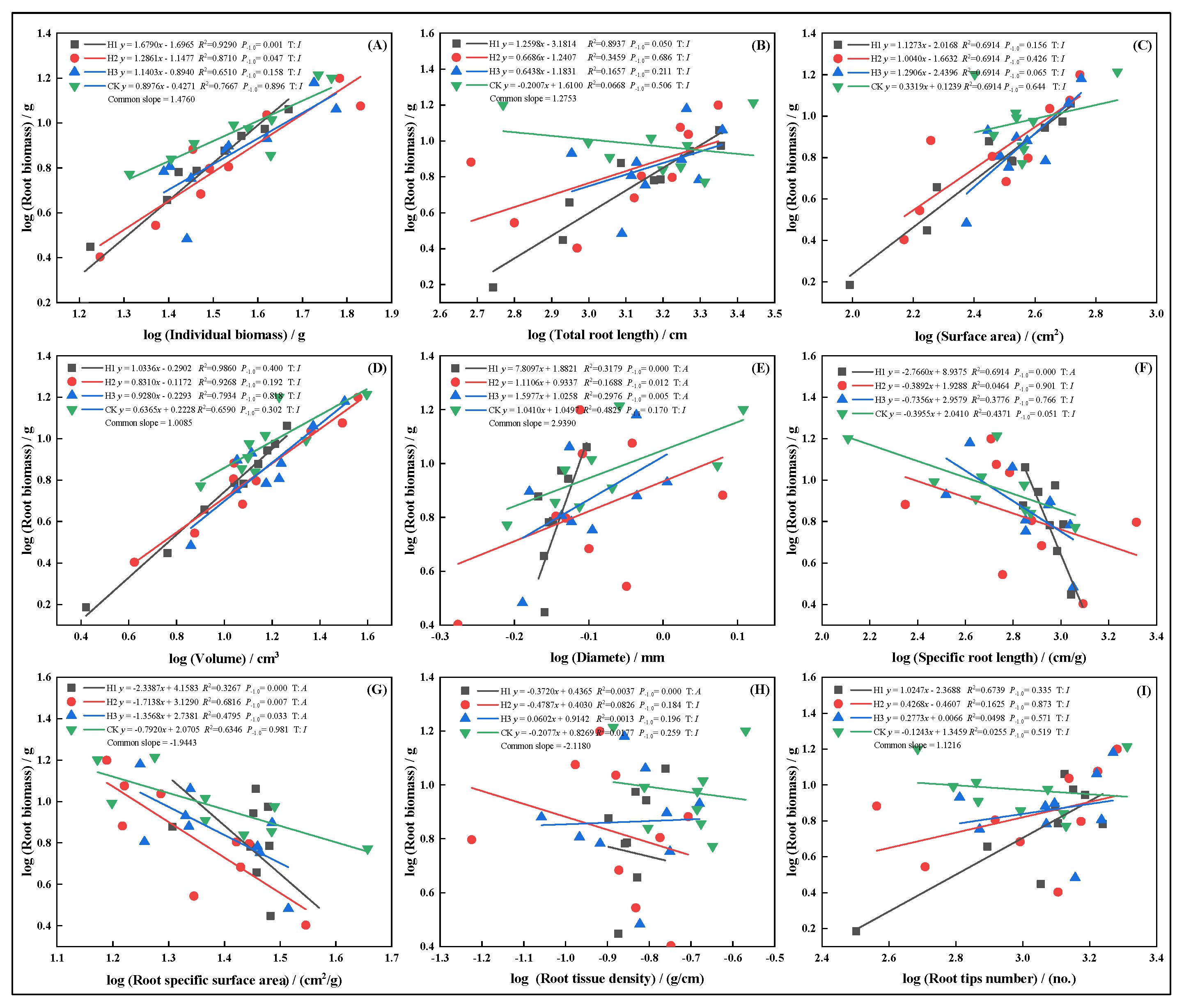
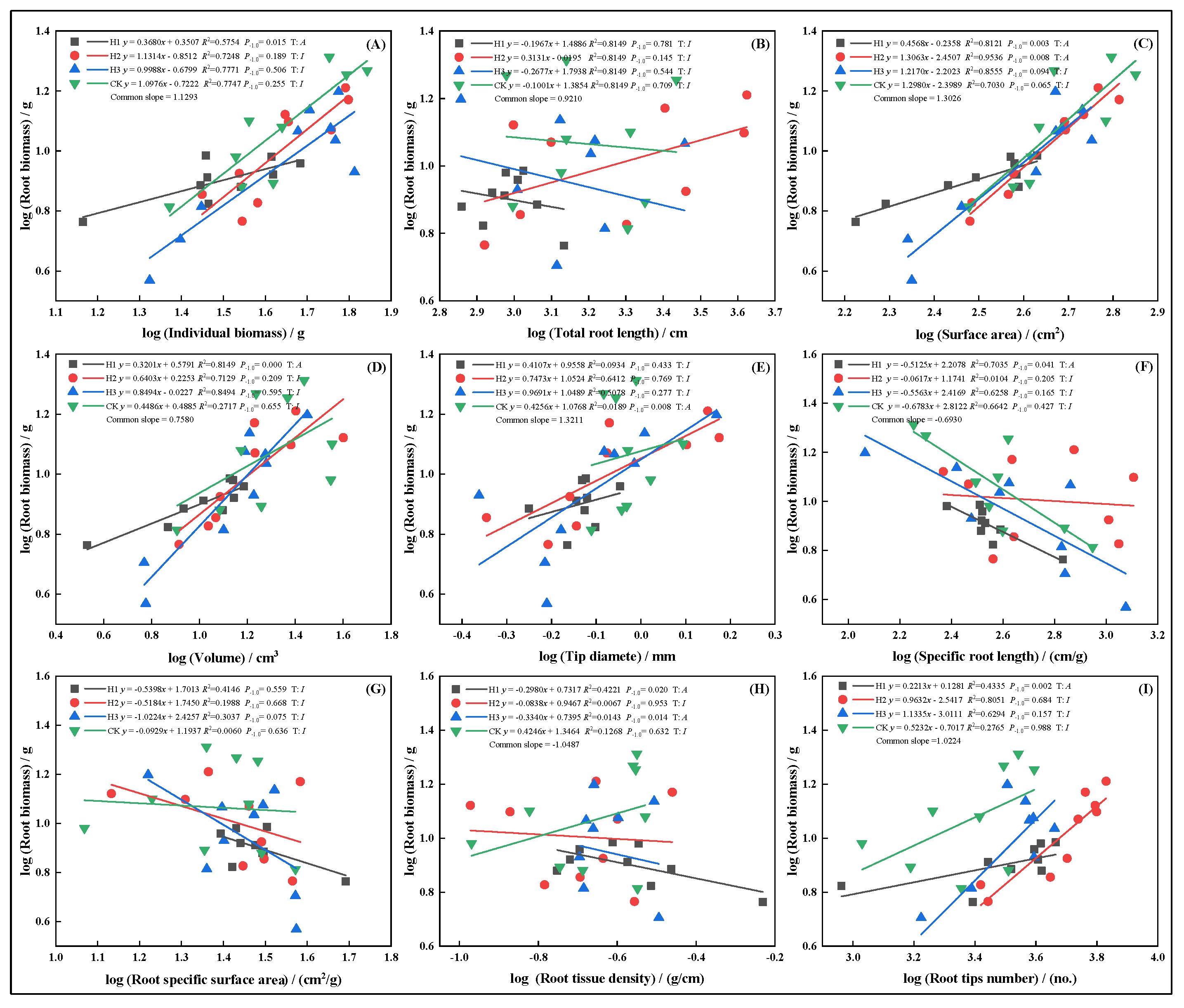
2.6. Allometric Growth Relationships of Root Traits Under Different Top-Pruning Intensities
3. Discussion
3.1. Response of Root Morphological Traits to Different Top-Pruning Intensities
3.2. Response of Seedling Allometric Growth to Different Top-Pruning Intensities
4. Materials and Methods
4.1. Study Area
4.2. Experimental Design
4.3. Root Measurement
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, J.; Li, Z.; Gao, C.; Li, S.; Huang, X.; Lang, X.; Su, J. Radial growth response of Pinus yunnanensis to rising temperature and drought stress on the Yunnan Plateau, southwestern China. For. Ecol. Manag. 2020, 474, 118357. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, M.; Li, P.; Sun, G.; Shi, S.; Xu, C. Root morphological plasticity determing the adaptive strategies of Cotinus coggygria seedlings in barren soil environment. J. Beijing Foesty Univ. 2017, 39, 60–69. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, H.; Wu, J. Non-structural carbohydrate (NSC) content and C: N: P stoichiometry of Pinus yunnanensis seedling needles in response to shade treatment. Ind. Crops Prod. 2024, 210, 118138. [Google Scholar] [CrossRef]
- Bangerth, F.; Li, C.-J.; Gruber, J. Mutual interaction of auxin and cytokinins in regulating correlative dominance. Plant Growth Regul. 2000, 32, 205–217. [Google Scholar] [CrossRef]
- Matsui, H.; Inui, T.; Oka, K.; Fukui, N. The influence of pruning and harvest timing on hop aroma, cone appearance, and yield. Food Chem. 2016, 202, 15–22. [Google Scholar] [CrossRef]
- Dong, Y.; Xiao, W.; Guo, W.; Liu, Y.; Nie, W.; Huang, R.; Tan, C.; Jia, Z.; Liu, J.; Jiang, Z. Effects of donor ages and propagation methods on seedling growth of Platycladus orientalis (L.) Franco in winter. Int. J. Mol. Sci. 2023, 24, 7170. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y. Plant grafting and its application in biological research. Chin. Sci. Bull. 2011, 56, 3511–3517. [Google Scholar] [CrossRef]
- Krasnoperova, V.; Bukharina, I. The study into the method of culture in vitro as a method of vegetative propagation of coniferous trees. Russ. Agric. Sci. 2020, 46, 19–22. [Google Scholar] [CrossRef]
- Zhang, A.; Ji, Y.; Sun, M.; Lin, C.; Zhou, P.; Ren, J.; Luo, D.; Wang, X.; Ma, C.; Zhang, X. Research on the drought tolerance mechanism of Pennisetum glaucum (L.) in the root during the seedling stage. BMC Genom. 2021, 22, 568. [Google Scholar] [CrossRef]
- Wei, R.; Ma, L.; Lu, X.; Xu, L.; Feng, X.; Ma, Y.; Li, S.; Ma, S.; Chai, Q.; Zhang, X. Research advances in plant root geotropism. Plant Growth Regul. 2024, 102, 237–250. [Google Scholar] [CrossRef]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Picard, N. The role of spatial competitive interactions between trees in shaping forest patterns. Theor. Popul. Biol. 2021, 142, 36–45. [Google Scholar] [CrossRef]
- Begon, M.; Harper, J.L.; Townsend, C.R. Ecology: Individuals, Populations and Communities; Blackwell Science Ltd.: Oxford, UK, 1996. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Xu, J.; Qi, J.; Liu, X.; Guo, L.; Zhang, H. Research on the Mechanisms of Phytohormone Signaling in Regulating Root Development. Plants 2024, 13, 3051. [Google Scholar] [CrossRef] [PubMed]
- Brunner, I.; Herzog, C.; Dawes, M.A.; Arend, M.; Sperisen, C. How tree roots respond to drought. Front. Plant Sci. 2015, 6, 547. [Google Scholar] [CrossRef]
- Wang, L.; Pang, X.; Li, N.; Qi, K.; Huang, J.; Yin, C. Effects of vegetation type, fine and coarse roots on soil microbial communities and enzyme activities in eastern Tibetan plateau. Catena 2020, 194, 104694. [Google Scholar] [CrossRef]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Prentice, C. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Steele, S.J.; Gower, S.T.; Vogel, J.G.; Norman, J.M. Root mass, net primary production and turnover in aspen, jack pine and black spruce forests in Saskatchewan and Manitoba, Canada. Tree Physiol. 1997, 17, 577–587. [Google Scholar] [CrossRef]
- Hendrick, R.L.; Pregitzer, K.S. The demography of fine roots in a northern hardwood forest. Ecology 1992, 73, 1094–1104. [Google Scholar] [CrossRef]
- Norby, R.J.; Jackson, R.B. Root dynamics and global change: Seeking an ecosystem perspective. New Phytol. 2000, 147, 3–12. [Google Scholar] [CrossRef]
- Resh, S.C.; Battaglia, M.; Worledge, D.; Ladiges, S. Coarse root biomass for eucalypt plantations in Tasmania, Australia: Sources of variation and methods for assessment. Trees 2003, 17, 389–399. [Google Scholar] [CrossRef]
- Normand, F.; Bissery, C.; Damour, G.; Lauri, P.É. Hydraulic and mechanical stem properties affect leaf–stem allometry in mango cultivars. New Phytol. 2008, 178, 590–602. [Google Scholar] [CrossRef]
- Niklas, K.J. Plant Allometry: The Scaling of Form and Process; University of Chicago Press: Chicago, IL, USA, 1994. [Google Scholar] [CrossRef]
- Cornelissen, J. A triangular relationship between leaf size and seed size among woody species: Allometry, ontogeny, ecology and taxonomy. Oecologia 1999, 118, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Abbo, S.; van-Oss, R.P.; Gopher, A.; Saranga, Y.; Ofner, I.; Peleg, Z. Plant domestication versus crop evolution: A conceptual framework for cereals and grain legumes. Trends Plant Sci. 2014, 19, 351–360. [Google Scholar] [CrossRef]
- Reich, P.B.; Tjoelker, M.G.; Machado, J.-L.; Oleksyn, J. Universal scaling of respiratory metabolism, size and nitrogen in plants. Nature 2006, 439, 457–461. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Uhl, E.; Hense, P. Coarse root–shoot allometry of Pinus radiata modified by site conditions in the Western Cape province of South Africa. South. For. J. For. Sci. 2012, 74, 237–246. [Google Scholar] [CrossRef]
- Enquist, B.J.; Niklas, K.J. Invariant scaling relations across tree-dominated communities. Nature 2001, 410, 655–660. [Google Scholar] [CrossRef]
- Müller, I.; Schmid, B.; Weiner, J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect. Plant Ecol. Evol. Syst. 2000, 3, 115–127. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Funct. Plant Biol. 2000, 27, 1191. [Google Scholar] [CrossRef]
- McConnaughay, K.; Coleman, J. Biomass allocation in plants: Ontogeny or optimality? A test along three resource gradients. Ecology 1999, 80, 2581–2593. [Google Scholar] [CrossRef]
- Yu, Q.; Elser, J.J.; He, N.; Wu, H.; Chen, Q.; Zhang, G.; Han, X. Stoichiometric homeostasis of vascular plants in the Inner Mongolia grassland. Oecologia 2011, 166, 1–10. [Google Scholar] [CrossRef]
- Rudgers, J.A.; Hallmark, A.; Baker, S.R.; Baur, L.; Hall, K.M.; Litvak, M.E.; Muldavin, E.H.; Pockman, W.T.; Whitney, K.D. Sensitivity of dryland plant allometry to climate. Funct. Ecol. 2019, 33, 2290–2303. [Google Scholar] [CrossRef]
- Espeleta, J.; Donovan, L. Fine root demography and morphology in response to soil resources availability among xeric and mesic sandhill tree species. Funct. Ecol. 2002, 16, 113–121. [Google Scholar] [CrossRef]
- Malhotra, A.; Brice, D.J.; Childs, J.; Graham, J.D.; Hobbie, E.A.; Vander Stel, H.; Feron, S.C.; Hanson, P.J.; Iversen, C.M. Peatland warming strongly increases fine-root growth. Proc. Natl. Acad. Sci. USA 2020, 117, 17627–17634. [Google Scholar] [CrossRef]
- Warton, D.I.; Duursma, R.A.; Falster, D.S.; Taskinen, S. Smatr 3—An R package for estimation and inference about allometric lines. Methods Ecol. Evol. 2012, 3, 257–259. [Google Scholar] [CrossRef]
- Hendrick, R.L.; Pregitzer, K.S. The dynamics of fine root length, biomass, and nitrogen content in two northern hardwood ecosystems. Can. J. For. Res. 1993, 23, 2507–2520. [Google Scholar] [CrossRef]
- Jama, B.; Ndufa, J.; Buresh, R.; Shepherd, K. Vertical distribution of roots and soil nitrate: Tree species and phosphorus effects. Soil Sci. Soc. Am. J. 1998, 62, 280–286. [Google Scholar] [CrossRef]
- Zhao, N.; Yu, G.; Wang, Q.; Wang, R.; Zhang, J.; Liu, C.; He, N. Conservative allocation strategy of multiple nutrients among major plant organs: From species to community. J. Ecol. 2020, 108, 267–278. [Google Scholar] [CrossRef]
- Yang, X.; Tang, Z.; Ji, C.; Liu, H.; Ma, W.; Mohhamot, A.; Shi, Z.; Sun, W.; Wang, T.; Wang, X. Scaling of nitrogen and phosphorus across plant organs in shrubland biomes across Northern China. Sci. Rep. 2014, 4, 5448. [Google Scholar] [CrossRef]
- Dinh, T.T.; Kajikawa, C.; Akaji, Y.; Yamada, K.; Matsumoto, T.K.; Makimoto, T.; Miki, N.H.; Hirobe, M.; Sakamoto, K. Stump sprout dynamics of Quercus serrata Thunb. and Q. acutissima Carruth. four years after cutting in an abandoned coppice forest in western Japan. For. Ecol. Manag. 2019, 435, 45–56. [Google Scholar] [CrossRef]
- Warton, D.I.; Wright, I.J.; Falster, D.S.; Westoby, M. Bivariate line-fitting methods for allometry. Biol. Rev. 2006, 81, 259–291. [Google Scholar] [CrossRef]
- Vasseur, F.; Exposito-Alonso, M.; Ayala-Garay, O.J.; Wang, G.; Enquist, B.J.; Vile, D.; Violle, C.; Weigel, D. Adaptive diversification of growth allometry in the plant Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2018, 115, 3416–3421. [Google Scholar] [CrossRef]
- Lindmark, M.; Ohlberger, J.; Huss, M.; Gårdmark, A. Size-based ecological interactions drive food web responses to climate warming. Ecol. Lett. 2019, 22, 778–786. [Google Scholar] [CrossRef]
- Malerba, M.E.; White, C.R.; Marshall, D.J. Eco-energetic consequences of evolutionary shifts in body size. Ecol. Lett. 2018, 21, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.E.; Marshall, D.J. Size-abundance rules? Evolution changes scaling relationships between size, metabolism and demography. Ecol. Lett. 2019, 22, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- Hersbach, H.; Bell, B.; Berrisford, P.; Biavati, G.; Horányi, A.; Muñoz Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Rozum, I.; et al. ERA5 Hourly Data on Single Levels from 1959 to Present. Copernicus Climate Change Service (C3S) Climate Data Store (CDS). 2018. Available online: https://www.xihe-energy.com (accessed on 25 January 2025).
- Boyer, J. Measurement of the water status of plants. Annu. Rev. Plant Physiol. 1969, 20, 351–364. [Google Scholar] [CrossRef]
- Liao, T.; Wang, Y.; Xu, C.; Li, Y.; Kang, X. Adaptive photosynthetic and physiological responses to drought and rewatering in triploid Populus populations. Photosynthetica 2018, 56, 578–590. [Google Scholar] [CrossRef]
- Enquist, B.J.; Niklas, K.J. Global allocation rules for patterns of biomass partitioning in seed plants. Science 2002, 295, 1517–1520. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, G.; Liao, J.; Xu, Y.; Cai, N. Effects of Top-Pruning Intensity Gradient on Root System Architecture and Allometric Patterns in Pinus yunnanensis Franch. Seedlings. Plants 2025, 14, 3210. https://doi.org/10.3390/plants14203210
Tang G, Liao J, Xu Y, Cai N. Effects of Top-Pruning Intensity Gradient on Root System Architecture and Allometric Patterns in Pinus yunnanensis Franch. Seedlings. Plants. 2025; 14(20):3210. https://doi.org/10.3390/plants14203210
Chicago/Turabian StyleTang, Guangpeng, Jianzhen Liao, Yulan Xu, and Nianhui Cai. 2025. "Effects of Top-Pruning Intensity Gradient on Root System Architecture and Allometric Patterns in Pinus yunnanensis Franch. Seedlings" Plants 14, no. 20: 3210. https://doi.org/10.3390/plants14203210
APA StyleTang, G., Liao, J., Xu, Y., & Cai, N. (2025). Effects of Top-Pruning Intensity Gradient on Root System Architecture and Allometric Patterns in Pinus yunnanensis Franch. Seedlings. Plants, 14(20), 3210. https://doi.org/10.3390/plants14203210





