The Impact of Reduced Nitrogen Fertilizer Application and Arbuscular mycorrhizal fungi Inoculation on Nitrogen Utilization in Intercropped Areca catechu L. and Vanilla planifolia Andrews
Abstract
1. Introduction
2. Results
2.1. Effects of Nitrogen Fertilizer Reduction and AMF Inoculation on the Photosynthetic Characteristics of Vanilla and Areca
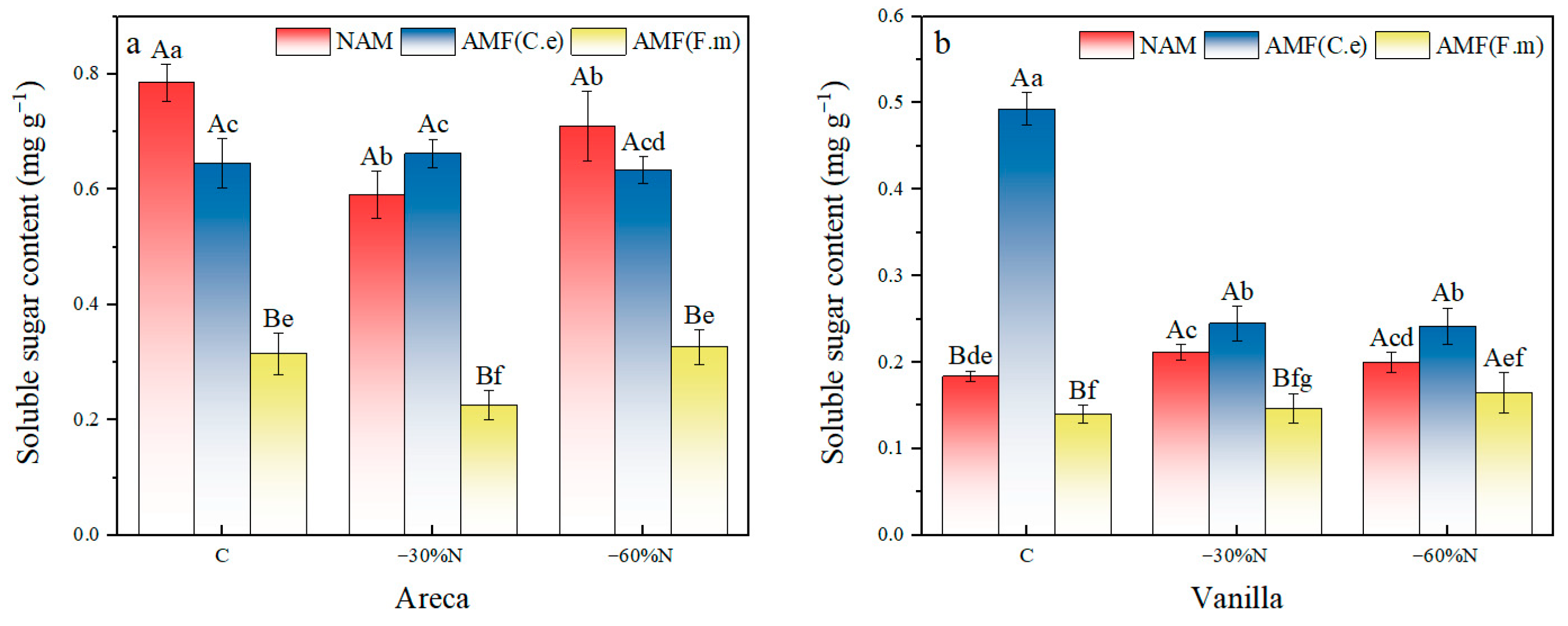
2.2. The Effects of Nitrogen Fertilizer Reduction and AMF Inoculation on the Soluble Sugar Content of Plants
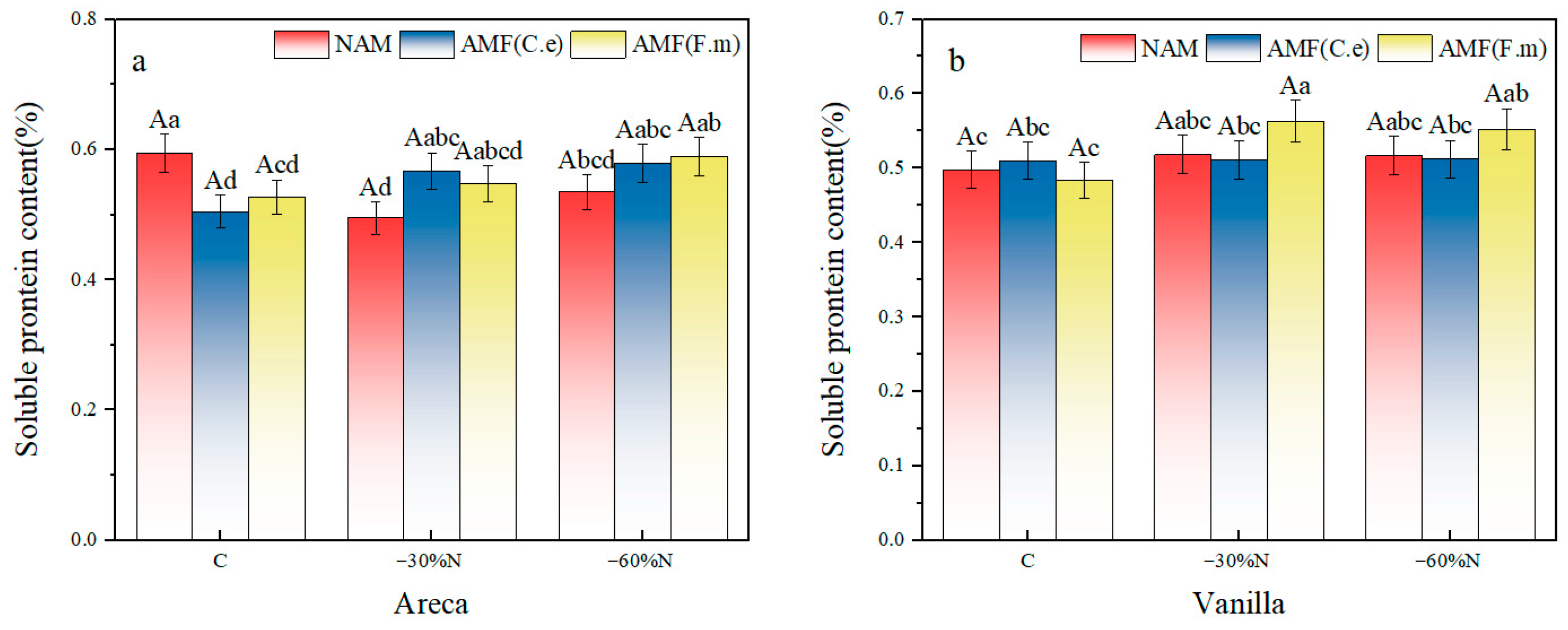
2.3. The Effect of Reduced Nitrogen Fertilizer and AMF Inoculation on the Content of Soluble Protein in Plants
2.4. The Effect of Reduced Nitrogen Fertilizer and AMF Inoculation on the Activity of Glutamine Synthetase in Plants
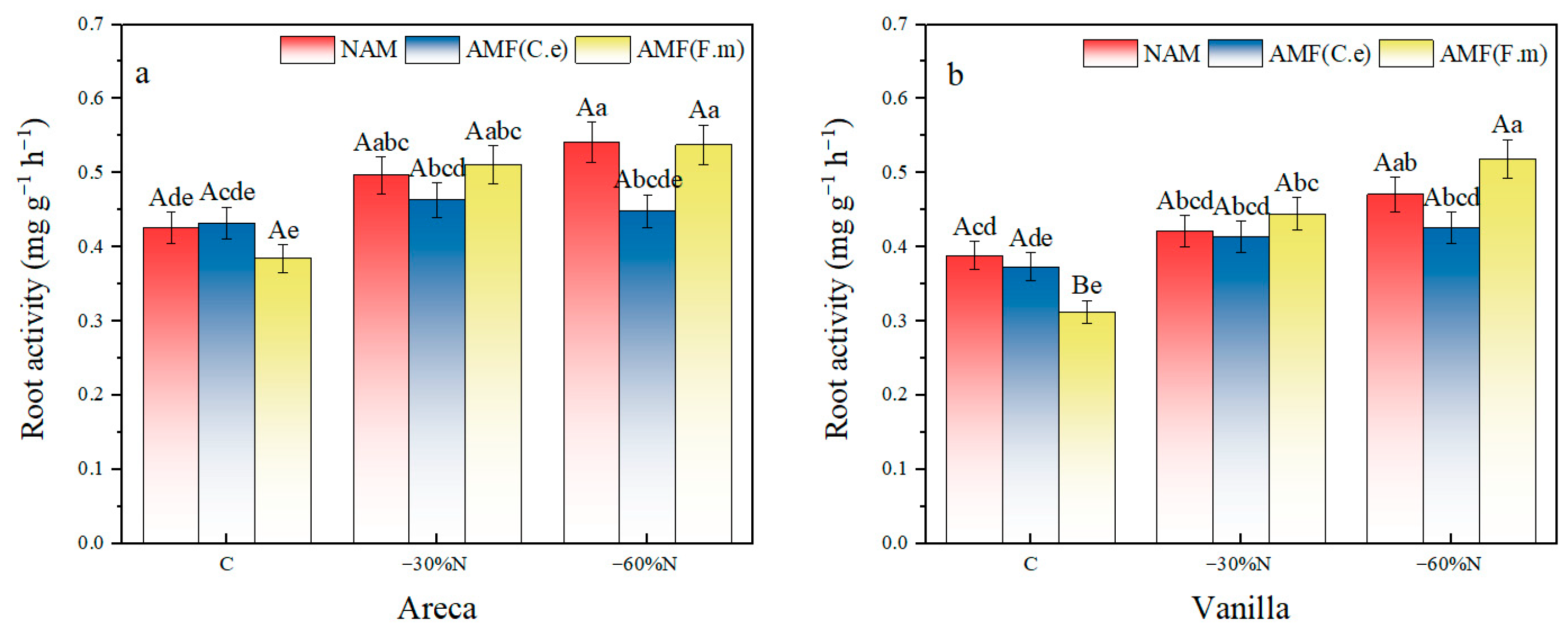
2.5. The Effect of Synergistic Regulation of Reduced Nitrogen Fertilizer and AMF Inoculation on the Vitality of Plant Root System
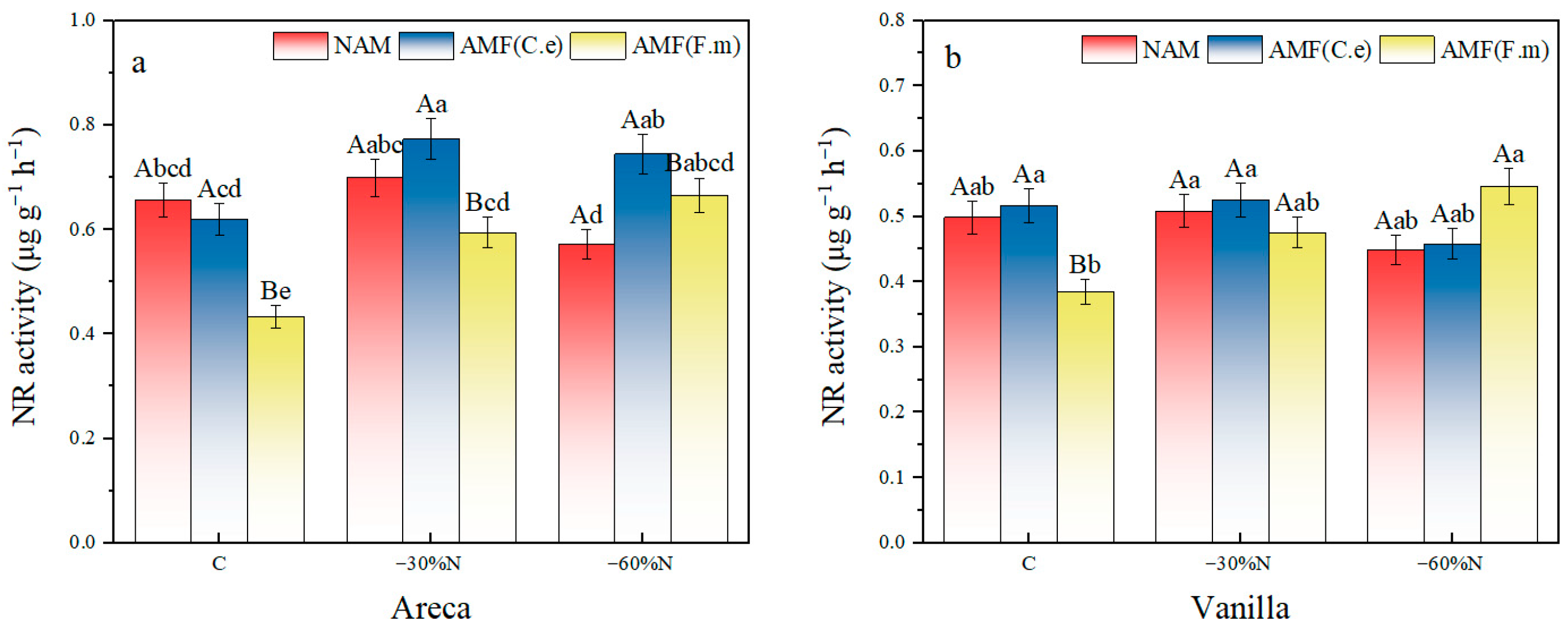
2.6. The Effect of Reduced Nitrogen Fertilizer and AMF Inoculation on the Activity of Nitrate Reductase in Plants
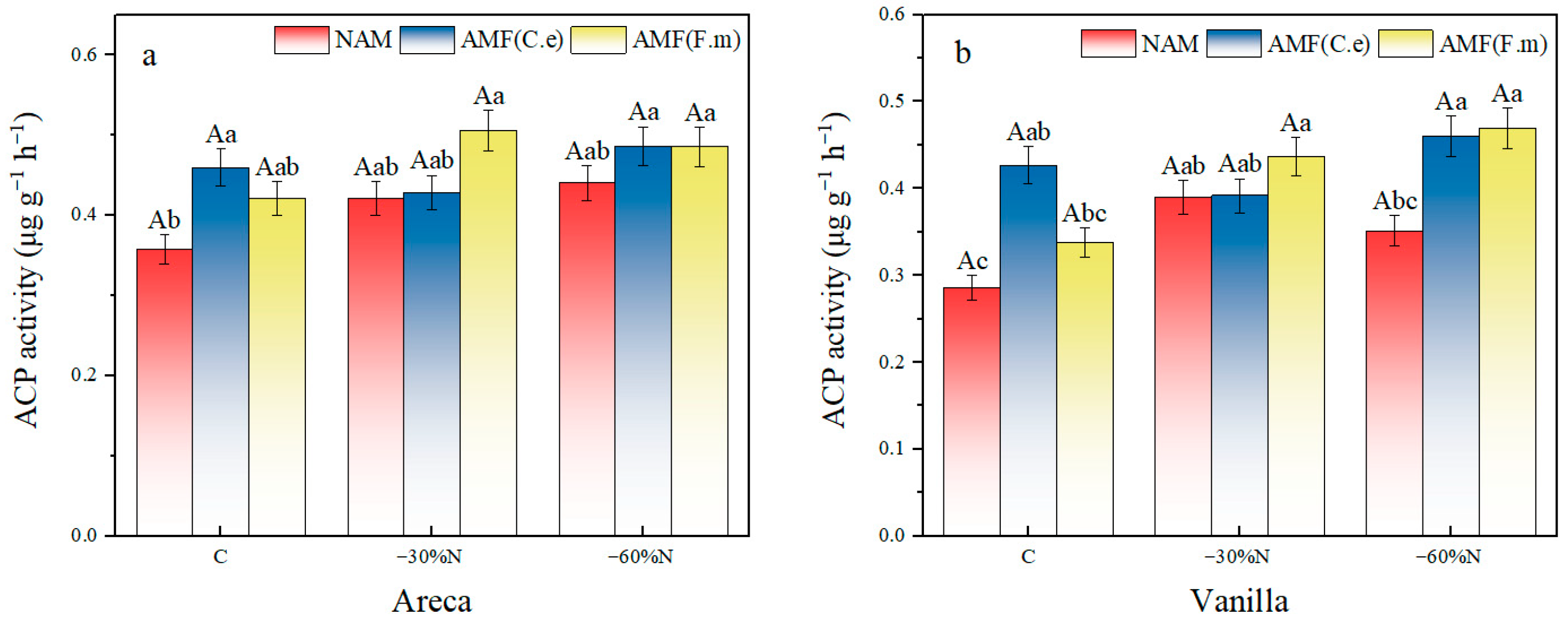
2.7. The Effects of Reduced Nitrogen Fertilizer and AMF Inoculation on the Activity of Acid Phosphatase in Plants
2.8. Under the Coordinated Regulation of Reduced Nitrogen Fertilization and AMF Inoculation, the Relationship Between Net Photosynthetic Rate and Soluble Protein Content and Root Activity Has Been Established
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Experimental Treatments
4.2.2. Measured Indicators and Methods
Chlorophyll Content Determination
Photosynthetic Characteristics Determination
Determination of Soluble Sugar Content
Determination of Soluble Protein Content
Determination of Glutamine Synthetase (GS) Activity
Determination of Root Activity
Determination of Plant Nitrate Reductase (NR) Activity
Determination of Acid Phosphatase Ctivity in Soil
4.2.3. Data Processing and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMF | Arbuscular mycorrhizal fung |
| NAM | non-inoculated control |
| AMF (C.e) | Claroideoglomus etunicatum |
| AMF (F.m) | Funneliformis mosseae |
| C | conventional fertilization |
| −30%N | 30% reduction |
| −60%N | 60% reduction |
| Pn | net photosynthesis |
| Tr | transpiration rate |
| Gs | stomatal conductance |
| Ci | intercellular CO2 concentration |
| GS | glutamine synthetase |
| NR | nitrate reductase |
| ACP | acid phosphatase |
| TTC | 2,3,5-triphenyltetrazolium chloride |
References
- Wang, Y.; Wang, L.; Liu, H.; Gou, B.; Hu, W.; Qin, L.; Shen, W.; Wang, A.; Cui, H.; Dai, Z. Direct Leaf-Peeling Method for Areca Protoplasts: A Simple and Efficient System for Protoplast Isolation and Transformation in Areca Palm (Areca catechu). BMC Plant Biol. 2023, 23, 56. [Google Scholar] [CrossRef]
- Sun, H.; Yu, W.; Li, H.; Hu, X.; Wang, X. Bioactive Components of Areca Nut: An Overview of Their Positive Impacts Targeting Different Organs. Nutrients 2024, 16, 695. [Google Scholar] [CrossRef]
- Rath, S.; Zamora-Re, M.; Graham, W.; Dukes, M.; Kaplan, D. Quantifying Nitrate Leaching to Groundwater from a Corn-Peanut Rotation under a Variety of Irrigation and Nutrient Management Practices in the Suwannee River Basin, Florida. Agric. Water Manag. 2021, 246, 106634. [Google Scholar] [CrossRef]
- Palama, T.L.; Grisoni, M.; Fock-Bastide, I.; Jade, K.; Bartet, L.; Choi, Y.H.; Verpoorte, R.; Kodja, H. Metabolome of Vanilla Planifolia (Orchidaceae) and Related Species under Cymbidium Mosaic Virus (CymMV) Infection. Plant Physiol. Biochem. 2012, 60, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Manyatsi, T.-S.; Lin, Y.-H.; Sung, P.-H.; Jou, Y.-T. Exploring the Volatile Profile of Vanilla Planifolia after Fermentation at Low Temperature with Bacillus Isolates. Foods 2024, 13, 2777. [Google Scholar] [CrossRef] [PubMed]
- Palama, T.L.; Khatib, A.; Choi, Y.H.; Payet, B.; Fock, I.; Verpoorte, R.; Kodja, H. Metabolic Changes in Different Developmental Stages of Vanilla Planifolia Pods. J. Agric. Food Chem. 2009, 57, 7651–7658. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, F.; Bassil, E.; Crane, J.H.; Shahid, M.A.; Vincent, C.I.; Schaffer, B. Spectral Light Distribution Affects Photosynthesis, Leaf Reflective Indices, Antioxidant Activity and Growth of Vanilla Planifolia. Plant Physiol. Biochem. 2022, 182, 145–153. [Google Scholar] [CrossRef]
- Zhu, B.; Gao, T.; Zhang, D.; Ding, K.; Li, C.; Ma, F. Functions of Arbuscular Mycorrhizal Fungi in Horticultural Crops. Sci. Hortic. 2022, 303, 111219. [Google Scholar] [CrossRef]
- Zhou, G.; Fan, K.; Li, G.; Gao, S.; Chang, D.; Liang, T.; Li, S.; Liang, H.; Zhang, J.; Che, Z.; et al. Synergistic Effects of Diazotrophs and Arbuscular Mycorrhizal Fungi on Soil Biological Nitrogen Fixation after Three Decades of Fertilization. iMeta 2023, 2, e81. [Google Scholar] [CrossRef]
- Cruz-Paredes, C.; Diera, T.; Davey, M.; Rieckmann, M.M.; Christensen, P.; Cruz, M.D.; Laursen, K.H.; Joner, E.J.; Christensen, J.H.; Nybroe, O.; et al. Disentangling the Abiotic and Biotic Components of AMF Suppressive Soils. Soil Biol. Biochem. 2021, 159, 108305. [Google Scholar] [CrossRef]
- Mu, X.; Chen, Y. The Physiological Response of Photosynthesis to Nitrogen Deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef]
- Qiang, B. Effect of Nitrogen Application Levels on Photosynthetic Nitrogen Distribution and Use Efficiency in Soybean Seedling Leaves. J. Plant Physiol. 2023, 287, 154051. [Google Scholar] [CrossRef] [PubMed]
- Diez, M.C.; Osorio, N.W.; Moreno, F. Effect of Dose and Type of Fertilizer on Flowering and Fruiting of Vanilla Plants. J. Plant Nutr. 2016, 39, 1297–1310. [Google Scholar] [CrossRef]
- Yang, H.; Fang, C.; Li, Y.; Wu, Y.; Fransson, P.; Rillig, M.C.; Zhai, S.; Xie, J.; Tong, Z.; Zhang, Q.; et al. Temporal Complementarity between Roots and Mycorrhizal Fungi Drives Wheat Nitrogen Use Efficiency. New Phytol. 2022, 236, 1168–1181. [Google Scholar] [CrossRef] [PubMed]
- Hamzehzadeh, H.; Abbaspour, H.; Afshar, A.; Hamdi, S. AMF-Mediated Salinity Adaptation in Pistachio Plants: Photosynthetic Efficiency and Ionic Balance. Biologia 2025, 80, 1–17. [Google Scholar] [CrossRef]
- Tang, H.; Hassan, M.U.; Feng, L.; Nawaz, M.; Shah, A.N.; Qari, S.H.; Liu, Y.; Miao, J. The Critical Role of Arbuscular Mycorrhizal Fungi to Improve Drought Tolerance and Nitrogen Use Efficiency in Crops. Front. Plant Sci. 2022, 13, 919166. [Google Scholar] [CrossRef]
- Jerbi, M.; Labidi, S.; Laruelle, F.; Tisserant, B.; Ben Jeddi, F.; Sahraoui, A.L.-H. Mycorrhizal Biofertilization Improves Grain Yield and Quality of Hulless Barley (Hordeum vulgare ssp. nudum L.) under Water Stress Conditions. J. Cereal Sci. 2022, 104, 103436. [Google Scholar] [CrossRef]
- An, J.; Huo, H.; Liu, Q.; Jiang, Y.; Luo, H.; Hao, Y. Physiological and Molecular Mechanisms of Nitrogen in Alleviating Drought Stress in Phoebe Bournei. Sci. Rep. 2025, 15, 14684. [Google Scholar] [CrossRef]
- Wang, X.; Feng, J.; Zhang, J.; Wang, H.; Guo, Y.; Ma, W.; Lyu, Y.; Wang, C.; Han, X.; Ru, J.; et al. Effects of Nitrogen and Phosphorus Inputs on Photosynthesis of Three Emergent Macrophytes in a Freshwater Wetland. Environ. Exp. Bot. 2025, 230, 106086. [Google Scholar] [CrossRef]
- Govindasamy, P.; Muthusamy, S.K.; Bagavathiannan, M.; Mowrer, J.; Jagannadham, P.T.K.; Maity, A.; Halli, H.M.; Sujayananad, G.K.; Vadivel, R.; Das, T.K.; et al. Nitrogen Use Efficiency—A Key to Enhance Crop Productivity under a Changing Climate. Front. Plant Sci. 2023, 14, 1121073. [Google Scholar] [CrossRef]
- Perchlik, M.; Tegeder, M. Leaf Amino Acid Supply Affects Photosynthetic and Plant Nitrogen Use Efficiency under Nitrogen Stress. Plant Physiol. 2018, 178, 174–188. [Google Scholar] [CrossRef]
- Xia, Z.; Gong, Y.; Lyu, X.; Lin, J.; Yang, Y.; Lu, H. Split Nitrogen Application Increases Maize Root Growth, Yield, and Nitrogen Use Efficiency under Soil Warming Conditions. Crop J. 2025, 13, 565–575. [Google Scholar] [CrossRef]
- Luo, Y.; Yin, H.; Ma, Y.; Wang, J.; Che, Q.; Zhang, M.; Chen, B.; Feng, G. Optimizing Nitrogen Fertilizer for Improved Root Growth, Nitrogen Utilization, and Yield of Cotton under Mulched Drip Irrigation in Southern Xinjiang, China. Sci. Rep. 2024, 14, 23223. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Wang, Z.; Wu, M.; Li, H.; Gu, J.; Yang, J.; Zhang, H.; Zhang, Z. Optimized Leaf Anatomy Improves Photosynthetic Producing Capacity of Mid-Season Indica Rice in the Yangtze River Basin during the Genetic Improvement. Eur. J. Agron. 2024, 158, 127196. [Google Scholar] [CrossRef]
- Sharma, S.; Bennett, M.J.; Mehra, P. Roles of Hormones in Regulating Root Growth–Water Interactions. J. Exp. Bot. 2025, 76, 1987–1995. [Google Scholar] [CrossRef]
- Gou, X.; Ren, Y.; Qin, X.; Wei, X.; Wang, J. Global Patterns of Soil Phosphatase Responses to Nitrogen and Phosphorus Fertilization. Pedosphere 2024, 34, 200–210. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, G.; Luo, X.; Hou, E.; Zheng, M.; Zhang, L.; He, X.; Shen, W.; Wen, D. Mycorrhizal Fungi and Phosphatase Involvement in Rhizosphere Phosphorus Transformations Improves Plant Nutrition during Subtropical Forest Succession. Soil Biol. Biochem. 2021, 153, 108099. [Google Scholar] [CrossRef]
- Burni, T.; Hussain, F.; Bibi, S.; Ullah, R.; Lalay, G. The Salutary Impacts of AMF Species, Rock Phosphates (RP), and Organic Matter (FYM) Fertilizers on the Development and Chemical Behavior of Mentha arvensis L. Acta Ecol. Sin. 2023, 43, 835–841. [Google Scholar] [CrossRef]
- Riches, M.; Lee, D.; Farmer, D.K. Simultaneous Leaf-Level Measurement of Trace Gas Emissions and Photosynthesis with a Portable Photosynthesis System. Atmos. Meas. Tech. 2020, 13, 4123–4139. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, S.; Zhao, L. Evaluation of the ENVI-Met Vegetation Model of Four Common Tree Species in a Subtropical Hot-Humid Area. Atmosphere 2018, 9, 198. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.; Hamilton, J.K.; Rebers, P.A.; Smith, F. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Zhang, Y. Determination of Glucose, Fructose, Sucrose, and Starch in Fruits and Vegetables by Anthrone Spectrophotometry. Chin. J. Anal. Chem. 1977, 3, 5–9. [Google Scholar] [CrossRef]
- Wan, D. Study on the Determination of Protein Content in Milk Powder Using the Coomassie Brilliant Blue Method. China Food Ind. 2024, 88–90. [Google Scholar] [CrossRef]
- Cheng, Y.; Liu, Y.; Wei, H.; Sun, R.; Tian, Z.; Zheng, X. Quantitation of Low Concentrations of Polysorbates 80 in Protein Formulations by Coomassie Brilliant Blue. Anal. Biochem. 2019, 573, 67–72. [Google Scholar] [CrossRef]
- Peng, I.-C.; Bott, A.; Zong, W.-X. Spectrophotometric Determination of Glutamine Synthetase Activity in Cultured Cells. Bio-Protocol 2016, 6, e1959. [Google Scholar] [CrossRef]
- Vorhaben, J.E.; Wong, L.; Campbell, J.W. Assay for Glutamine Synthetase Activity. Biochem. J. 1973, 135, 893–896. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, C.; Liu, R.; Ni, W. Effects of Three Phenolic Compounds on Mitochondrial Function and Root Vigor of Cotton (Gossypium hirsutum L.) Seedling Roots. Acta Physiol. Plant. 2019, 41, 60. [Google Scholar] [CrossRef]
- Lea, U.S.; Leydecker, M.-T.; Quilleré, I.; Meyer, C.; Lillo, C. Posttranslational Regulation of Nitrate Reductase Strongly Affects the Levels of Free Amino Acids and Nitrate, Whereas Transcriptional Regulation Has Only Minor Influence. Plant Physiol. 2006, 140, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic Stoichiometry of Microbial Organic Nutrient Acquisition in Soil and Sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Pan, X.; Li, H.; Wu, J.; Li, Q.; Zhou, Y.; Yang, P. Mycorrhiza Inoculation and Soil-Available Phosphorus Modulate Phosphorus Acquisition Strategies of Alfalfa. J. Agric. Food Chem. 2025, 73, 14265–14279. [Google Scholar] [CrossRef]


| Treatments | Fertilization Level | SPAD Value (mg g−1) | Pn (μmol m−2 s−1) | Tr (mmol m−2 s−1) | Ci (μmol mol−1) | Gs (mmol m−2 s−1) |
|---|---|---|---|---|---|---|
| NAM | C | 27.07 ± 3.04 Aa | 7.05 ± 0.85 ABb | 0.53 ± 0.07 Aa | 1878.02 ± 18.12 Bbc | 0.005 ± 0.001 Bc |
| −30%N | 15.27 ± 2.85 Ab | 2.52 ± 0.79 Bd | 0.31 ± 0.02 Bbc | 192.78 ± 8.78 Cc | 0.010 ± 0.003 Ab | |
| −60%N | 13.85 ± 2.94 Abc | 2.09 ± 0.46 Bd | 0.28 ± 0.02 Bc | 130.77 ± 5.97 Cc | 0.008 ± 0.002 Bbc | |
| AMF (C.e) | C | 13.81 ± 3.17 Bbc | 4.27 ± 0.26 Bc | 0.35 ± 0.06 BCb | 1571.65 ± 16.06 Cc | 0.009 ± 0.001 ABbc |
| −30%N | 12.27 ± 2.44 Ac | 7.54 ± 0.63 Ab | 0.45 ± 0.06 Aab | 2135.20 ± 15.17 Bbc | 0.012 ± 0.003 Ab | |
| −60%N | 11.05 ± 2.12 Ac | 7.51 ± 0.77 Ab | 0.37 ± 0.05 Ab | 2663.64 ± 18.69 Bb | 0.017 ± 0.003 Aa | |
| AMF (F.m) | C | 6.45 ± 1.55 Bd | 7.51 ± 0.86 Ab | 0.28 ± 0.01 Bc | 2633.05 ± 12.28 Ab | 0.007 ± 0.001 ABbc |
| −30%N | 6.20 ± 0.91 Bd | 8.43 ± 0.97 Aa | 0.49 ± 0.03 Aa | 2736.51 ± 13.91 Ab | 0.012 ± 0.002 Ab | |
| −60%N | 5.54 ± 0.90 Be | 7.74 ± 0.76 Ab | 0.36 ± 0.03 Ab | 3274.11 ± 14.93 Aa | 0.015 ± 0.002 Aa |
| Treatments | Fertilization Level | SPAD Value (mg g−1) | Pn (μmol m−2 s−1) | Tr (mmol m−2 s−1) | Ci (μmol mol−1) | Gs (mmol m−2 s−1) |
|---|---|---|---|---|---|---|
| NAM | C | 28.39 ± 2.06 Bb | 6.58 ± 1.16 Aa | 0.22 ± 0.06 Bb | 1581.63 ± 24.10 Acd | 0.007 ± 0.001 Bc |
| −30%N | 15.96 ± 2.56 Bcd | 1.25 ± 0.13 Cd | 0.30 ± 0.03 Aab | 193.91 ± 3.79 Cf | 0.006 ± 0.001 Bc | |
| −60%N | 14.86 ± 3.79 Bd | 1.29 ± 0.09 Cd | 0.16 ± 0.03 Ac | 160.61 ± 5.21 Cf | 0.005 ± 0.001 Bc | |
| AMF (C.e) | C | 36.17 ± 3.82 Aa | 2.96 ± 0.21 Bc | 0.19 ± 0.02 Bbc | 617.81 ± 12.69 Ce | 0.010 ± 0.002 Ab |
| −30%N | 29.11 ± 3.41 Ab | 5.61 ± 0.24 Ab | 0.31 ± 0.04 Aab | 1428.59 ± 21.15 Bd | 0.016 ± 0.002 Aab | |
| −60%N | 20.89 ± 2.21 Ac | 5.46 ± 0.37 Bb | 0.05 ± 0.01 Bd | 1884.95 ± 31.00 Bb | 0.019 ± 0.002 Aa | |
| AMF (F.m) | C | 19.18 ± 2.90 Cc | 6.36 ± 0.34 Aa | 0.38 ± 0.03 Aa | 1333.88 ± 14.85 Bde | 0.010 ± 0.001 Ab |
| −30%N | 16.78 ± 2.91 Bcd | 5.32 ± 0.43 Bb | 0.27 ± 0.05 Ab | 1682.97 ± 12.94 Ac | 0.018 ± 0.003 Aa | |
| −60%N | 14.55 ± 1.43 Bd | 6.16 ± 0.53 Aab | 0.16 ± 0.02 AAc | 3179.21 ± 22.99 a | 0.019 ± 0.002 Aa |
| Measurement Indicators | Treatments | Areca | Vanilla |
|---|---|---|---|
| soluble sugar content | N | 546.64 *** | 466.99 *** |
| A | 23.02 ** | 94.71 *** | |
| N × A | 12.18 *** | 152.22 *** |
| Measurement Indicators | Treatments | Areca | Vanilla |
|---|---|---|---|
| soluble protein content | N | 0.41 | 2.02 |
| A | 2.66 ^ | 4.05 * | |
| N × A | 6.70 ** | 1.97 |
| Measurement Indicators | Treatments | Areca | Vanilla |
|---|---|---|---|
| GS activity | N | 3.17 ^ | 4.65 * |
| A | 1.05 | 0.98 | |
| N × A | 0.59 | 1.32 |
| Measurement Indicators | Treatments | Areca | Vanilla |
|---|---|---|---|
| root activity | N | 2.73 ^ | 1.03 |
| A | 16.09 *** | 20.84 *** | |
| N × A | 2.91 * | 3.81 |
| Measurement Indicators | Treatments | Areca | Vanilla |
|---|---|---|---|
| NR activity | N | 0.72 | 0.55 |
| A | 1.28 | 0.75 | |
| N × A | 2.24 | 2.98 * |
| Measurement Indicators | Treatments | Areca | Vanilla |
|---|---|---|---|
| ACP activity | N | 3.67 * | 8.34 ** |
| A | 2.74 ^ | 6.31 ** | |
| N × A | 1.08 | 2.62 ^ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, H.; Tang, X.; Ning, Z.; Zhou, C.; Zhao, Q.; Wang, H.; Xing, Y.; Zhang, A. The Impact of Reduced Nitrogen Fertilizer Application and Arbuscular mycorrhizal fungi Inoculation on Nitrogen Utilization in Intercropped Areca catechu L. and Vanilla planifolia Andrews. Plants 2025, 14, 3207. https://doi.org/10.3390/plants14203207
Zhuang H, Tang X, Ning Z, Zhou C, Zhao Q, Wang H, Xing Y, Zhang A. The Impact of Reduced Nitrogen Fertilizer Application and Arbuscular mycorrhizal fungi Inoculation on Nitrogen Utilization in Intercropped Areca catechu L. and Vanilla planifolia Andrews. Plants. 2025; 14(20):3207. https://doi.org/10.3390/plants14203207
Chicago/Turabian StyleZhuang, Huifa, Xinyu Tang, Ziwei Ning, Chengjun Zhou, Qingyun Zhao, Hui Wang, Yizhang Xing, and Ang Zhang. 2025. "The Impact of Reduced Nitrogen Fertilizer Application and Arbuscular mycorrhizal fungi Inoculation on Nitrogen Utilization in Intercropped Areca catechu L. and Vanilla planifolia Andrews" Plants 14, no. 20: 3207. https://doi.org/10.3390/plants14203207
APA StyleZhuang, H., Tang, X., Ning, Z., Zhou, C., Zhao, Q., Wang, H., Xing, Y., & Zhang, A. (2025). The Impact of Reduced Nitrogen Fertilizer Application and Arbuscular mycorrhizal fungi Inoculation on Nitrogen Utilization in Intercropped Areca catechu L. and Vanilla planifolia Andrews. Plants, 14(20), 3207. https://doi.org/10.3390/plants14203207






