Abstract
This study reports the discovery of four novel Pinnularia species—P. latocentra sp. nov., P. rhombocentra sp. nov., P. seouloflexuosa sp. nov., and P. paristriata sp. nov.—from urban freshwater streams in South Korea. Species delimitation was achieved using a polyphasic approach that integrated light and scanning electron microscopy, ecological profiling, and molecular evidence from SSU rRNA and rbcL sequences. Each taxon was confirmed as morphologically and genetically distinct from its closest congeners. Our findings broaden the recognized diversity of Pinnularia in East Asia and demonstrate that urban streams, often regarded as degraded habitats, can harbor hidden diatom diversity and ecological complexity. By clarifying diagnostic traits, validating type material in a recognized repository, and aligning molecular and morphological evidence, this study contributes to a more robust taxonomy of Pinnularia. These results also highlight the importance of polyphasic taxonomy and the strategic inclusion of urban habitats in diatomological surveys and biodiversity assessments.
1. Introduction
Diatoms (Bacillariophyta) represent one of the most diverse and ecologically significant algal lineages, with over 100,000 estimated species worldwide. They are key contributors to global primary production, silica cycling, and climate regulation, while their siliceous frustules provide a robust foundation for taxonomy, bioassessment, and paleoenvironmental reconstruction [1,2,3,4,5]. Their short generation times and sensitivity to physicochemical gradients make them indispensable bioindicators in both lentic and lotic ecosystems [6,7,8,9].
Among diatoms, the genus Pinnularia Ehrenberg is especially noteworthy for its global distribution, ecological versatility, and long-standing taxonomic complexity. According to AlgaeBase [5], the genus currently comprises about 880 accepted species, though many additional names have been historically proposed [10]. Importantly, Pinnularia species are not confined to freshwater habitats; they also occur in marine and terrestrial environments [11]. They inhabit diverse climates and substrate types, ranging from glacial meltwaters to highly eutrophic or anthropogenically disturbed rivers [12,13,14]. Some taxa, such as P. borealis and P. viridiformis, are cosmopolitan, while others are restricted to acidic, high-altitude, or polar systems [15,16,17]. This combination of broad distribution and ecological specialization has complicated species delimitation, particularly in the absence of integrative approaches [18].
In South Korea, diatom research has historically emphasized alpine wetlands, reservoirs, and forested mountain streams, yielding records of approximately 40–60 Pinnularia taxa [19,20,21]. However, these estimates likely underrepresent actual diversity, because urban and peri-urban streams remain comparatively underexplored despite Korea’s dense urbanization and hydrological modification of major watersheds such as the Han, Nakdong, and Yeongsangang Rivers. Urban streams, subject to nutrient enrichment, conductivity shifts, and sedimentation from impervious runoff and weir regulation, may act as both ecological filters and evolutionary hotspots, fostering overlooked or specialized diatom lineages [22,23,24].
Recent methodological advances have facilitated more rigorous taxonomic resolution. Multi-gene phylogenies (e.g., SSU, rbcL) have revealed substantial cryptic diversity within Pinnularia [25,26,27], while scanning electron microscopy (SEM) of valve ultrastructure has refined species-level boundaries and enabled critical reassessment of type material [28,29,30]. Experimental studies on physiology, such as freezing tolerance, and comparative analyses across polar, alpine, and temperate habitats have further underscored the ecological breadth of the genus [3,31]. Together, these approaches highlight the necessity of a polyphasic framework that integrates morphology, molecular data, and ecology in Pinnularia taxonomy [4,32,33,34].
Against this background, the present study describes four novel Pinnularia species—P. latocentra sp. nov., P. rhombocentra sp. nov., P. seouloflexuosa sp. nov., and P. paristriata sp. nov.—based on monoclonal strains isolated from urban freshwater streams across South Korea. By combining LM, SEM, molecular markers (SSU, rbcL), and ecological profiling, we aim to contribute not only to the refinement of regional taxonomic inventories but also to a broader understanding of how anthropogenically influenced habitats sustain hidden diatom diversity and inform bioindicator development.
2. Results
This section presents the morphological, ecological, and phylogenetic characteristics of four novel species of the genus Pinnularia—P. latocentra sp. nov., P. rhombocentra sp. nov., P. seouloflexuosa sp. nov., and P. paristriata sp. nov.—isolated from urban and peri-urban freshwater streams across the Korean Peninsula (Figure 1). Detailed observations were conducted using light microscopy (LM) and scanning electron microscopy (SEM), and molecular analyses were based on the SSU and rbcL gene sequences. Diagnostic morphological traits, ecological preferences, and phylogenetic positions were provided for each taxon, along with comparisons with morphologically similar species.
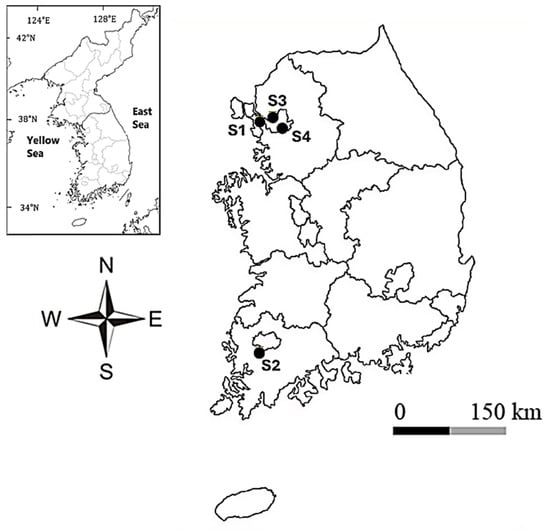
Figure 1.
Geographic locations of the four urban freshwater stream sampling sites in South Korea where the Pinnularia strains were isolated. S1 = Wonjokssan (Seoul), S2 = Yeongsangang River (Gwangju), S3 = Hongjecheon (Seoul), S4 = Yangjaecheon (Seoul).
For clarity, we note that GenBank accession numbers (rbcL, SSU rDNA) represent DNA sequence records registered in NCBI (National Center for Biotechnology Information, USA). Deposition Numbers (DN) indicate type materials—permanent slides and living cultures—preserved at KCTC (Korean Collection for Type Cultures, KRIBB, Jeongeup, Korea). Holotypes are designated as permanent slides and cultures at KCTC; isotypes are duplicate slides and cultures derived from the same original strains, also preserved at KCTC. Ex-type strains (living cultures corresponding to type material) are maintained at KCTC under the same accession numbers. Laboratory duplicates of all strains are preserved at Hanyang University (HYU, Seoul, Korea), but these are not name-bearing types.
A summary of the site-specific environmental conditions, GenBank accession numbers, and type material deposition is presented in Table 1.

Table 1.
Sampling information, environmental parameters, GenBank accession numbers, and type material deposition for four Pinnularia species isolated from urban freshwater streams in South Korea.
2.1. Pinnularia latocentra Y. Li & B.H. Kim, sp. nov.
Diagnosis: Valves linear to linear-elliptical with nearly parallel sides, slightly concave at the mid-region, and broadly rounded apices. Length 29.3–31.3 µm, width 5.55–6.76 µm (wild and cultured material). Axial area narrowly lanceolate, expanding asymmetrically into a broad fascia. Striae finely punctate, weakly radiate at the center and slightly convergent toward the apices, 14–16 in 10 µm.
Description (Table 2).

Table 2.
Comparative morphological characteristics of Pinnularia latocentra sp. nov. and morphologically similar species based on valve structure and striae patterns.
LM (Figure 2A–H): Valves linear to linear-elliptical, with a distinctly broad, asymmetrical central fascia. Mid-regions subtly concave; apices broadly rounded. Striae finely punctate, slightly radiate at the center, weakly convergent toward apices.
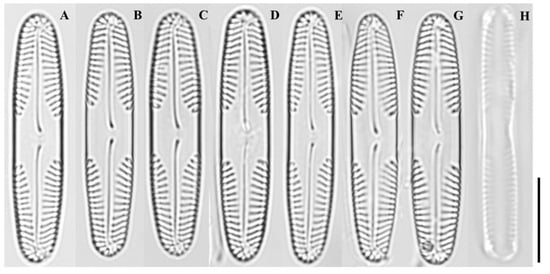
Figure 2.
Light micrographs of Pinnularia latocentra sp. nov. (A–E) Valves showing linear to linear-elliptical outlines with broad, asymmetrical central fascia and slightly concave sides; (F–H) Larger valves with nearly parallel margins, broadly rounded apices, and finely punctate striae that are weakly radiate at the center and slightly convergent near the ends. Scale bar = 10 µm.
SEM (Figure 3A–F): Raphe lateral with drop-shaped proximal ends, deflected unilaterally. Terminal fissures hook-shaped externally; raphe internally ending in small helictoglossae. Five alveoli per valve, ghost striae absent.
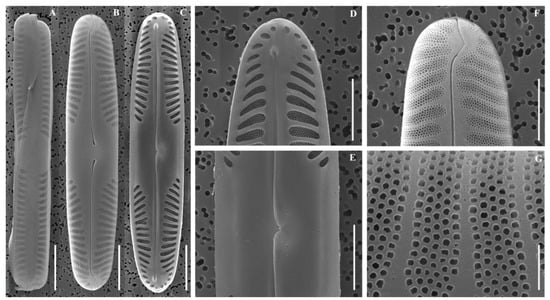
Figure 3.
Scanning electron micrographs (SEM) of Pinnularia latocentra sp. nov. (A) Girdle view; (B) External valve view; (C) Internal valve view; (D) Internal view of valve apices; (E) Central area; (F) External view of valve apices; (G) External detail of an alveolus. Scale bars: (A–F) = 10 µm; (G) = 5 µm.
Type material.
Holotype: KCTC (Korean Collection for Type Cultures, KRIBB, Jeongeup, Korea), permanent slide, accession no. AG61352.
Isotypes: KCTC, duplicate slides and cultures, accession nos. AG61363 (strain WJS230803B5A3) and AG61364 (strain WJS230803B5A4).
Type locality: Hongjecheon, Seoul, South Korea (37°34′03.29″ N, 126°54′57.87″ E).
Etymology: The epithet latocentra derives from the Latin latus (broad) and centrum (center). It refers to the broad, asymmetrical central fascia that is the most characteristic feature of this species, readily distinguishing it from morphologically allied taxa.
Ecology: Found in low-turbidity urban streams with moderate dissolved oxygen (~5.51 mg L−1) and neutral to slightly alkaline pH (~6.19).
2.2. Pinnularia rhombocentra Y. Li & B.H. Kim, sp. nov.
Diagnosis: Valves rhombic-lanceolate with broadly rounded ends. Length 31.4–48.2 µm, width 7.0–9.3 µm. Axial area distinctly rhombic, expanded at the center. Central area narrow and slightly asymmetric. Striae radiate throughout, 16–20 in 10 µm.
Description (Table 3).

Table 3.
Diagnostic morphological features of Pinnularia rhombocentra sp. nov. compared with closely related taxa from the P. parvulissima complex.
LM (Figure 4A–D): Valves rhombic-lanceolate with distinct rhombic central area. Apices broadly rounded. Striae radiate across the valve, denser at apices.
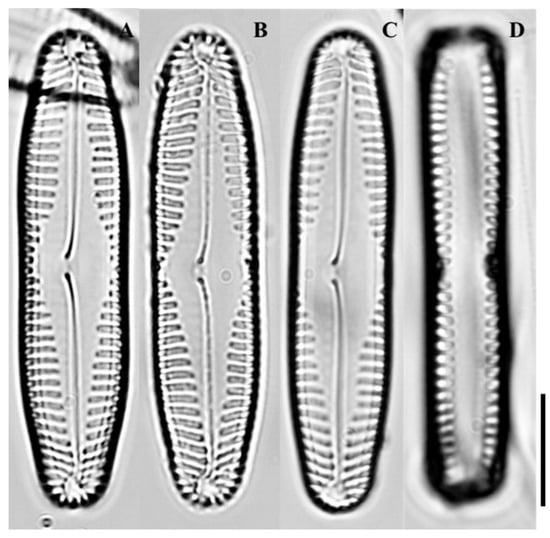
Figure 4.
Light micrographs of Pinnularia rhombocentra sp. nov. (A) Valve in girdle view showing overall outline; (B) Valve in external view highlighting the rhombic central area; (C) Valve showing radiate striae across the central region; (D) Valve apex showing rounded ends and striae arrangement. Scale bar = 10 µm.
SEM (Figure 5A–G): Raphe filiform, externally straight with expanded proximal ends. Terminal fissures slightly deflected. Internally, raphe endings with small helictoglossae. Well-defined alveoli visible.
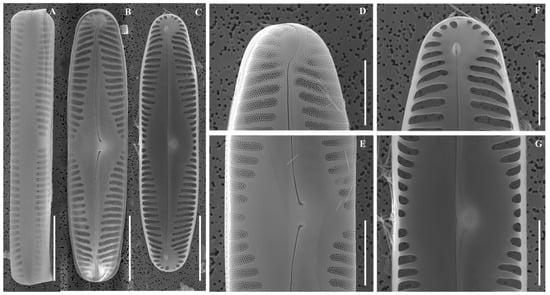
Figure 5.
Scanning electron micrographs (SEM) of Pinnularia rhombocentra sp. nov. (A) Girdle view; (B) External valve view; (C) Internal valve view; (D) External view of valve apices; (E) Central area; (F) Internal view of valve apices; (G) Central area. Scale bars: (A–C) = 10 µm; (D–G) = 5 µm.
Type material.
Holotype: KCTC, permanent slide, accession no. AG61353.
Isotypes: KCTC, duplicate slides and cultures, accession nos. AG61365 (strain YSG231115B7A1) and AG61366 (strain YSG231115B7A2).
Type locality: Yeongsan-gang, Gwangju, South Korea (35°00′20.71″ N, 126°44′28.47″ E).
Etymology: The epithet rhombocentra combines the Greek rhombos (diamond-shaped) and Latin centrum (center). It highlights the distinctive rhombic outline of the central area, which is a defining character that separates this taxon from other similar Pinnularia species.
Ecology: Recorded in moderately turbid rivers with higher conductivity (362 µS cm−1) and slightly alkaline pH (~7.8).
2.3. Pinnularia seouloflexuosa Y. Li & B.H. Kim, sp. nov.
Diagnosis: Valves distinctly flexuous, linear-lanceolate with capitate ends. Length 147–151 µm, width 19–21 µm. Axial area broad, slightly eccentric; central area fascia-like. Striae weakly radiate at center, parallel to slightly convergent near apices, 14–18 in 10 µm.
Description (Table 4).

Table 4.
Morphological delineation of Pinnularia seouloflexuosa sp. nov. in comparison with other large-celled linear Pinnularia species.
LM (Figure 6A–G): Valves linear-lanceolate, distinctly flexuous, apices capitate. Central area fascia-like. Striae weakly radiate at center, convergent near apices.
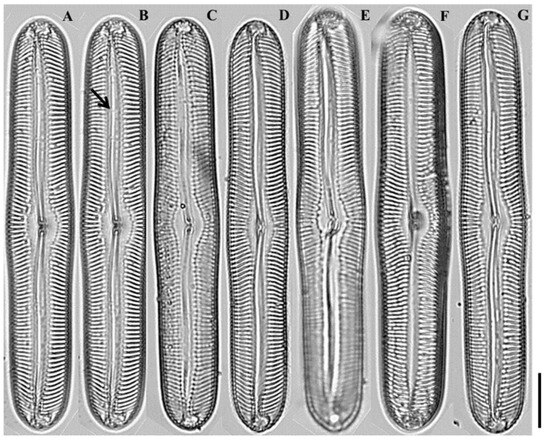
Figure 6.
Light micrographs of Pinnularia seouloflexuosa sp. nov. (A–C) Smaller valves showing slightly flexuous linear-lanceolate outlines with narrow central fascia; (D–E) Medium-sized valves exhibiting more distinct curvature and broader fascia; (F–G) Larger valves with capitate apices and weakly radiate striae near the center. Scale bar = 20 µm.
SEM (Figure 7A–I): Raphe filiform, proximal ends externally expanded, deflected laterally. Terminal fissures hook-shaped. Internally ending in small helictoglossae. Striae uniseriate, areolae elongated transapically.
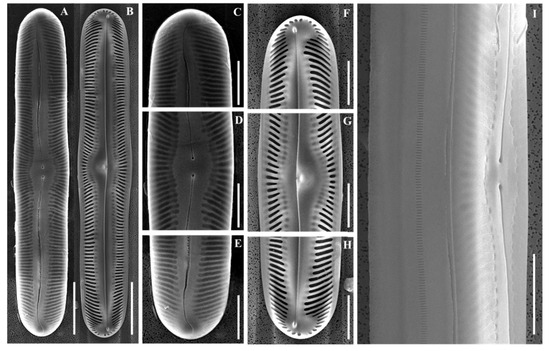
Figure 7.
Scanning electron micrographs (SEM) of Pinnularia seouloflexuosa sp. nov. (A) External and (B) internal views of whole valves; (C–E) External valve details; (F–H) Internal valve views; (D,G) Central area close-ups; (I) External central area shown in the girdle view. Scale bars: (A,B) = 20 µm; (C–I) = 10 µm.
Type material.
Holotype: KCTC, permanent slide, accession no. AG61340.
Isotypes: KCTC, duplicate slides and living cultures, accession nos. AG61343 (strain HJC230531B3A1) and AG61344 (strain HJC230531B3A2).
Type locality: Hongjecheon, Seoul, South Korea (37°34′03.29″ N, 126°54′57.88″ E).
Etymology: The epithet seouloflexuosa commemorates the type locality (Seoul) and Latin flexuosus (bent, sinuous). It emphasizes the rare flexuous valve outline, a prominent morphological trait within the genus, and reflects its origin in an urban environment.
Ecology: Occurs in moderately warm waters (~25 °C), with very low turbidity (1.2 NTU) and circumneutral pH (~7.6).
2.4. Pinnularia paristriata Y. Li & B.H. Kim, sp. nov.
Diagnosis: Valves linear-elliptical to narrowly lanceolate, apices subrostrate. Length 57.6–75.1 µm, width 13.8–16.5 µm. Axial area narrow to moderately broad, central area fascia-like. Striae parallel to weakly radiate at center, radiate toward apices, 20–24 in 10 µm.
Description (Table 5).

Table 5.
Comparative morphology of Pinnularia paristriata sp. nov. and related species exhibiting linear to linear-elliptic valve shapes.
LM (Figure 8A–F): Valves linear-elliptical to lanceolate, apices subrostrate. Central area broad fascia-like. Striae parallel to weakly radiate at center, denser toward apices.
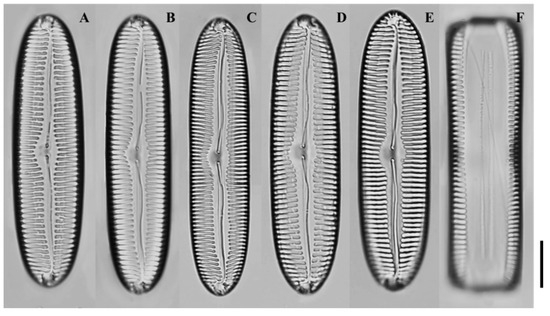
Figure 8.
Light micrographs of Pinnularia paristriata sp. nov. (A–D) Valves showing linear-elliptical to narrowly lanceolate outlines with parallel to slightly convex margins and broad central fascia; (E–F) Larger valves with subrostrate apices and striae that are parallel to weakly radiate at the center and denser toward the ends. Scale bar = 10 µm.
SEM (Figure 9A–I): Raphe lateral, proximal ends expanded, slightly deflected. Terminal fissures hook-shaped. Internally ending in small helictoglossae. Areolae arranged linearly, striae punctate.
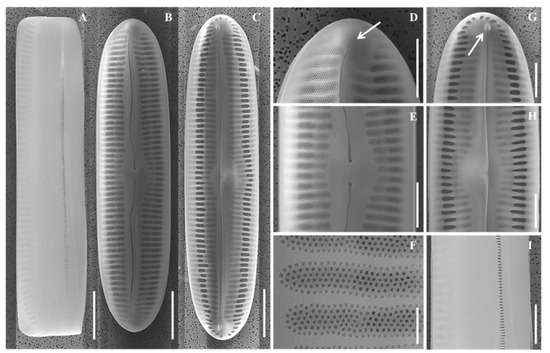
Figure 9.
Scanning electron micrographs (SEM) of Pinnularia paristriata sp. nov., showing external and internal valve structure, including apices and central area architecture. (A) External and (B) internal views of whole valves; (C–E) External valve details; (F–H) Internal valve views; (D,G) Central area close-ups; (I) External central area shown in the girdle view. Scale bars: (A–C) = 10 µm; (D,E, G–I) = 5 µm; (F) = 1 µm.
Type material.
Holotype: KCTC, permanent slide, accession no. AG61387.
Isotypes: KCTC, duplicate slides and living cultures, accession nos. AG61390 (strain YJC241112B2A1) and AG61391 (strain YJC241112B2A2).
Type locality: Yangjaecheon, Seoul, South Korea (37°29′23.38″ N, 127°03′52.23″ E).
Etymology: The epithet paristriata is derived from Latin para (beside, near) and striata (striated). It refers to the diagnostic pattern of closely spaced, nearly parallel striae that dominate the valve surface.
Ecology: Found in streams with moderate conductivity (~518 µS cm−1), slightly alkaline pH (~8.0), and dissolved oxygen around 11 mg L−1.
2.5. Phylogenetic Placement
Partial SSU rRNA and rbcL sequences were successfully obtained from all four novel Pinnularia species. Maximum likelihood (ML) analyses based on both markers consistently resolved each species as a distinct and well-supported lineage that was evidently separated from all previously described congeners. These results provide strong genetic evidence that supports the recognition of each taxon as a novel species.
In the SSU rRNA phylogeny (Figure 10), Pinnularia seouloflexuosa clustered with P. neglectiformis and P. viridis, forming a clade with moderate bootstrap support (62%). Despite this proximity, P. seouloflexuosa was separated as an independent lineage from P. neglectiformis and P. viridis with moderate bootstrap support (62%). P. rhombocentra was grouped with P. subanglica and P. nodosa, supported by bootstrap values of 73–79%. Each of these placements indicates that the novel taxa are genetically distinct from their morphologically similar relatives. P. latocentra formed a separate, well-supported branch (bootstrap 81%) adjacent to P. obscura and P. cf. marchica but was readily distinguished by its broad asymmetrical fascia and lack of ghost striae, which are features rarely observed in related taxa. P. paristriata was placed as an independent basal lineage of the P. microstauron complex and P. cf. borealis with high support (bootstrap, 88%), exhibiting evident differentiation.
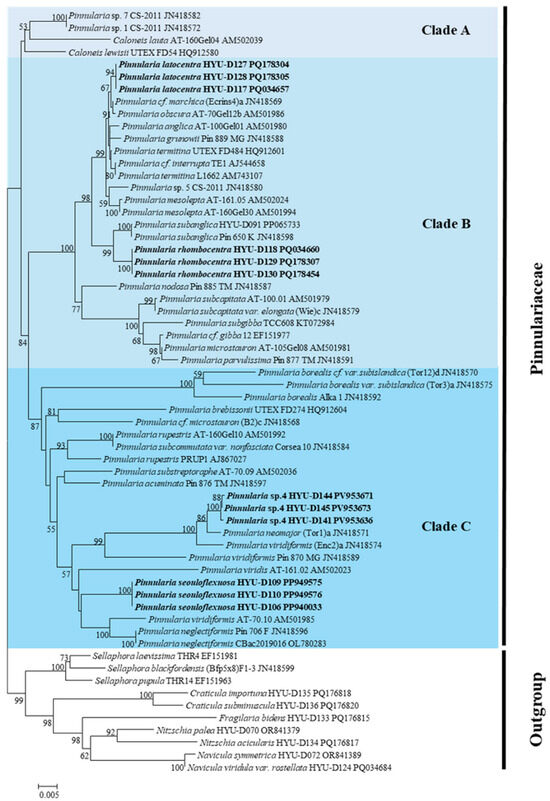
Figure 10.
Maximum likelihood (ML) phylogenetic tree based on nuclear 18S small subunit ribosomal RNA (SSU rRNA) sequences, showing the position of P. latocentra, P. rhombocentra, P. seouloflexuosa, and P. paristriata among related Pinnularia species.
To complement the SSU rRNA tree, pairwise evolutionary divergences among the 20 closest Pinnularia species were calculated using the Kimura 2-parameter model (Table 6). These values ranged from 0.006 to 0.042 among the new taxa and their nearest relatives, well within the expected interspecific range for diatoms and apparently above the commonly observed intraspecific threshold (<0.005), which is consistent with previous studies establishing molecular divergence thresholds in Pinnularia [15,16,40,41,42].

Table 6.
Estimates of evolutionary divergence among 20 closely related Pinnularia species based on SSU rRNA (1678 bp).
The rbcL phylogeny (Figure 11) was generally consistent with the SSU results, confirming that each new species occupied a distinct evolutionary position: P. seouloflexuosa (bootstrap, 78%), P. rhombocentra (68%), P. latocentra (64%), and P. paristriata (84%). Pairwise distances based on the rbcL sequences (Table 7) ranged from 0.005 to 0.078. Notably, the smallest observed distance (0.005) was between P. rhombocentra and P. subanglica, whereas other comparisons exceeded 0.02, supporting robust genetic divergence even within the same clade. Together with the SSU results, these data support species-level differentiation and highlight lineage independence.
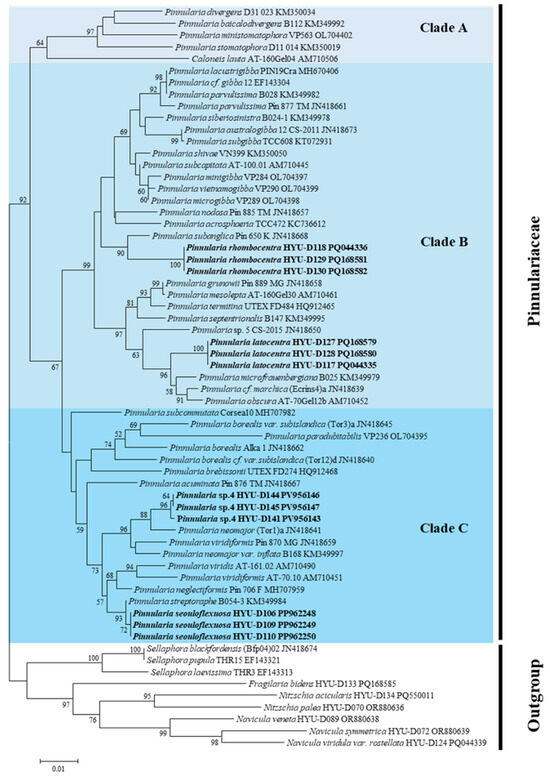
Figure 11.
Maximum likelihood (ML) phylogenetic tree based on chloroplast-encoded ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit (rbcL) gene sequences, illustrating the placement of P. latocentra, P. rhombocentra, P. seouloflexuosa, and P. paristriata within the genus Pinnularia.

Table 7.
Estimates of evolutionary divergence among 20 closely related Pinnularia species based on rbcL (752 bp).
Full strain names and GenBank accession numbers used in the phylogenetic analyses are provided in Supplementary Table S1, which includes both novel and comparative taxa used in the SSU and rbcL trees. Secondary structure comparisons corresponding to the SSU- and rbcL-based clades are shown in Supplementary Figures S1–S4. Table 1 summarizes the strain type information and site-specific environmental conditions of the four novel species described in this study. The consistent correspondence between molecular data, diagnostic morphology, and ecological preferences reinforces the polyphasic taxonomic framework and highlights the previously undescribed diversity within Pinnularia in urbanized freshwater habitats.
2.6. Distribution and Habitat Preferences
The four novel Pinnularia species described in this study exhibit distinct ecological preferences and habitat specializations across multiple freshwater systems in South Korea, comprising urban, peri-urban, and forested tributaries. Their distributions indicate ecological differentiation through niche partitioning and environmental filtering under varying degrees of anthropogenic and natural disturbance.
P. latocentra was consistently recorded in clear, low-turbidity streams (~3.3 NTU) with neutral to slightly alkaline pH (~7.2) and moderate dissolved oxygen (~9.4 mg/L). Its broad, asymmetrical central fascia and thickened valve walls suggest an adaptation to stable hydraulic regimes and low sediment abrasion.
P. rhombocentra occurred in urban streams subjected to episodic sediment influx with moderate turbidity and conductivity levels. Its rhombic axial area and intermediate striae density (14–15 per 10 µm), combined with distinct alveolation (4–6 alveoli per valve), indicate morphological adjustments to variable flow and sediment regimes.
P. seouloflexuosa dominated eutrophic urban streams characterized by high turbidity (>10 NTU), low dissolved oxygen, and elevated conductivity. Its large size (up to 151 µm), flexuous axial area, and high length-to-width ratio (7.01–7.66) demonstrate tolerance to physicochemical stressors and sediment resuspension.
P. paristriata was isolated from shaded, forested tributaries with slightly acidic pH (~6.8), low conductivity, and minimal anthropogenic influence. It is distinguished by a wide central fascia and short striae, which indicate an adaptation to low-light, low-flow environments.
Collectively, these four Pinnularia species exhibit non-overlapping ecological niches, consistent morphometric adaptation, and evident phylogenetic separation, reinforcing their taxonomic novelty. These findings are concordant with ecological niche partitioning and habitat specialization reported for Pinnularia taxa in contrasting acidic, polar, and eutrophic systems [24,26,30], further supporting the robustness of our conclusions.
3. Discussion
This study establishes an integrative framework for delimiting four novel Pinnularia species—P. latocentra, P. rhombocentra, P. seouloflexuosa, and P. paristriata—from Korean urban streams. By combining LM/SEM-based morphology, ecological profiling, and molecular markers (SSU, rbcL), we highlight the overlooked diversity of this genus in anthropogenically influenced habitats, consistent with findings from underexplored regions [10,12,30,32,36].
Morphological differentiation and taxonomic coherence. Each taxon is morphologically distinct from its closest congeners (Table 2, Table 3, Table 4 and Table 5). Pinnularia latocentra differs from P. microfrauenbergiana and P. siberiosinistra by its broader fascia and absence of ghost striae [12,36]. P. rhombocentra is distinguished from members of the P. parvulissima complex, such as P. subanglica and P. nodosa, by its rhombic central area and radiate striae [10,37,43]. P. seouloflexuosa exhibits a strongly flexuous outline, unlike the straight or weakly curved valves of P. streptoraphe and P. spinifera [10,44,45]. P. paristriata possesses parallel striae and a narrow axial area, separating it from P. viridis and P. viridiformis [34,46]. These differences were observed consistently in both field-collected and cultured material, reinforcing the validity of each species [47].
Molecular corroboration with cautious interpretation. Pairwise divergences in SSU and rbcL sequences (Table 6 and Table 7) exceeded typical intraspecific thresholds for diatoms [20,48]. For example, rbcL distances between Pinnularia rhombocentra and P. subanglica exceeded 0.02, consistent with species-level separation [12,36]. The congruence of LM/SEM traits with molecular discontinuities supports their recognition as distinct taxa, in agreement with recent integrative studies in Pinnularia [12,20,49]. In accordance with reviewer recommendations, the phylogenetic trees (Figure 10 and Figure 11) are presented solely as supporting evidence for species-level distinctiveness, without making inferences about deeper evolutionary relationships.
Ecological and biogeographic context. The four taxa exhibit non-overlapping ecological associations. Pinnularia paristriata was recorded in shaded, oligotrophic tributaries, while P. seouloflexuosa occurred in nutrient-rich, high-conductivity channels. P. latocentra was associated with low-turbidity, moderately conductive streams, and P. rhombocentra occurred in sandy-bottom channels under episodic disturbance. Such niche partitioning parallels patterns reported for specialized Pinnularia in polar, alpine, and tropical ecosystems [11,14,41,50]. Urban rivers may therefore function as reservoirs of cryptic diatom diversity, consistent with observations from East Asia [51], studies of pseudocryptic diversity in Europe [52], and recent surveys in the Damavand River basin, Iran [53].
Implications for taxonomy and biomonitoring. This study emphasizes the necessity of a polyphasic approach in Pinnularia taxonomy [23,49,54]. Morphological convergence and phenotypic plasticity alone have historically confounded species recognition in this genus [10,36,55]. Our findings further underscore the importance of reassessing historical type specimens for taxonomic clarity, as emphasized by Jahn [56] in the rediscovery of the Pinnularia gastrum type specimen. By integrating SEM ultrastructure and molecular markers, we improved diagnostic precision. Furthermore, the habitat fidelity of P. latocentra and P. paristriata suggests their potential as sensitive indicators of conductivity and sediment changes, complementing existing diatom-based biomonitoring frameworks [6,57].
Synthesis. Recognition of Pinnularia latocentra, P. rhombocentra, P. seouloflexuosa, and P. paristriata extends the known diversity of Pinnularia in East Asia and demonstrates that even heavily modified urban streams can harbor novel diatom lineages. By aligning morphology, molecular evidence, and ecology, this study contributes to a conservative yet robust taxonomy and underscores the ecological importance of urban freshwater habitats in sustaining hidden microbial diversity [30,32,58].
4. Materials and Methods
4.1. Study Area and Diatom Isolation
Between May 2023 and November 2024, epilithic diatom samples were collected from four urban freshwater streams in South Korea: Wonjokssan (Seoul), Hongjecheon (Seoul), Yangjaecheon (Seoul), and the Yeongsangang River (Gwangju). These sites represent a range of physicochemical conditions and anthropogenic disturbance, from nutrient-enriched urban channels to moderately impacted mid-gradient river segments (Figure 1; Table S1). At each location, submerged cobbles (ca. 5 × 5 cm) were sampled from shallow flowing areas (<0.3 m depth). Epilithic biofilms were gently brushed from cobble surfaces with sterilized soft-bristle brushes and transferred into sterile 200 mL polypropylene containers partially filled with ambient stream water to preserve cell integrity during transport.
On-site physicochemical parameters—including water temperature (WT), pH, turbidity (Turb), dissolved oxygen (DO), and electrical conductivity (EC)—were measured using a portable multiparameter probe (U-50 Series; HORIBA, Kyoto, Japan). These environmental data were used to characterize the ecological niches of each strain. Twelve clonal strains of the four novel Pinnularia taxa were successfully isolated by micropipette selection under an inverted microscope (IX73, Olympus, Tokyo, Japan) and maintained in WC medium at 20 °C under a 14:10 h light: dark cycle. These clonal isolates were used for morphological, ecological, and molecular analyses [52,59].
4.2. Isolation and Cultivation
Single diatom cells were isolated with fine-tipped glass microcapillaries using an inverted microscope (Eclipse Ts2; Nikon, Tokyo, Japan). Clonal strains were first established in 96-well plates containing Diatom Medium (DM; CCAP formulation) and incubated under controlled conditions (20 °C, 12:12 h light: dark cycle, light intensity 120 µmol m−2 s−1). After 2–4 weeks of initial growth, cultures were successively transferred to larger volumes (24-well plates, 50 mL flasks). Strains were subcultured every 30–45 days to preserve genetic and physiological stability [60,61].
4.3. Morphological and Ultrastructural Examination
For frustule cleaning, dense culture material was digested with a 1:3 (v/v) mixture of nitric and sulfuric acids at low heat (3–5 min), followed by repeated rinsing with distilled water until neutral pH, following established diatom protocols [62,63]. Cleaned material was mounted in Naphrax® for LM observations using a Nikon Eclipse E600 microscope with a DS-Fi3 digital camera (Nikon Corporation, Tokyo, Japan). For each taxon, at least 60 valves were measured to assess intrapopulation variability. Parameters recorded included valve length, width, central and axial area features, striae and areolar densities, and raphe morphology.
For SEM, frustules were filtered onto 0.2 µm polycarbonate membranes, mounted on aluminum stubs with conductive adhesive, and sputter-coated with platinum. Imaging was conducted with a field-emission SEM (Apreo S, Thermo Fisher Scientific, Waltham, MA, USA) to resolve fine ultrastructural features critical for taxonomic differentiation in Pinnularia (e.g., internal distal raphe ends, areolar occlusions, virgae and vimines) [22,59].
4.4. DNA Extraction and Phylogenetic Analysis
DNA was extracted from actively growing cultures using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) with minor modifications to improve lysis of silica-encased cells. Two loci were targeted: nuclear SSU rRNA and plastid-encoded rbcL. PCR was performed with standard primers and conditions (95 °C for 4 min; 35 cycles of 95 °C for 30 s, 56 °C for 30 s, 72 °C for 1 min; final extension 72 °C for 7 min). Products were checked on 1% agarose gels, purified using the QIAquick Gel Extraction Kit (Qiagen GmbH, Hilden, Germany), and sequenced (Bionics, Seoul, Korea). Sequences were assembled, aligned using ClustalW in MEGA7 [64], and analyzed under Maximum Likelihood with 1000 bootstrap replicates using the Kimura 2-parameter model [65], with bootstrap support values calculated following Felsenstein [66].
4.5. Taxonomic Validation
Species diagnoses were established under a polyphasic framework integrating LM and SEM morphology, ecological context, and molecular evidence (SSU, rbcL). Morphological comparisons were made against published monographs and regional floras [59,61], and nomenclatural validation was cross-checked with AlgaeBase [5] and Diatoms of North America [2]. Type material (holotypes and isotypes) has been deposited in the Korean Collection for Type Cultures (KCTC) with unique accession numbers (Table 1; Supplementary Table S1). This approach improves the resolution of morphologically cryptic diversity and ensures reproducibility in taxonomic practice.
5. Conclusions
This study provides a comprehensive taxonomic and ecological characterization of four novel Pinnularia species—P. latocentra, P. rhombocentra, P. seouloflexuosa, and P. paristriata—discovered in urban streams of South Korea. Species delimitation was achieved using a polyphasic framework that integrated high-resolution LM and SEM morphology, ecological data, and molecular evidence (SSU rRNA, rbcL). These combined datasets confirm the distinctiveness and novelty of the four taxa. Our results broaden the recognized diversity of Pinnularia in East Asia and demonstrate that urban streams, often regarded as degraded or marginal habitats, can harbor cryptic diatom lineages. This highlights not only the hidden taxonomic diversity but also the frequently overlooked ecological complexity of anthropogenically influenced aquatic systems. By clarifying diagnostic traits, validating type material in a recognized repository, and aligning molecular and morphological data, this work contributes to a more robust taxonomy of Pinnularia. Beyond taxonomy, the findings underscore the importance of strategically including urban habitats in diatomological surveys and biomonitoring programs. Such efforts are essential for detecting cryptic species, refining biodiversity assessments, and improving the ecological management of freshwater ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants14203206/s1. Figure S1: Secondary structure model of 18S rRNA (Clade B) in Pinnularia species, inferred from phylogenetic relationships shown in Figure 10 of the main text. Figure S2: Secondary structure model of 18S rRNA (Clade C) in Pinnularia species, corresponding to phylogenetic Clade C shown in Figure 10. Figure S3: Secondary structure model of rbcL (Clade B) in Pinnularia species, based on the phylogenetic analysis in Figure 11. Figure S4: Secondary structure model of rbcL (Clade C) in Pinnularia species, inferred from the rbcL phylogeny shown in Figure 11. Table S1: Strains and GenBank accession numbers used for phylogenetic analyses based on SSU rRNA and rbcL genes.
Author Contributions
Y.L. performed investigation, formal analysis, and original draft writing; W.W. contributed investigation, methodology, and formal analysis; B.-H.H. and S.-O.H. conducted investigation and methodology; B.-H.K. conceived the study, acquired funding, supervised the project, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institute of Biological Resources (NIBR), grant number NNIBR202201205. However, the authors did not receive any direct funding for manuscript preparation or publication.
Data Availability Statement
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors express their sincere gratitude to the three anonymous reviewers for their insightful and constructive comments, which greatly improved the clarity and scientific quality of this manuscript. We also extend our thanks to In-Hwan Cho, Ha-Kyung Kim, Eun-A Hwang, Jeong-Hwan Byun, and Cheon Lee for their valuable advice and technical assistance during this study.
Conflicts of Interest
Author Byeong-Hun Han was employed by the company Dongmoon ENT (Seoul, Republic of Korea). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| DN | Deposition Number (type material at KCTC) |
| DO | Dissolved oxygen (mg L−1) |
| EC | Electrical conductivity (µS cm−1) |
| GPS | Global Positioning System (latitude, longitude in decimal degrees) |
| HYU | Hanyang University (Seoul, Korea) |
| KCTC | Korean Collection for Type Cultures (KRIBB, Jeongeup, Korea) |
| LM | Light microscopy |
| ML | Maximum likelihood |
| NCBI | National Center for Biotechnology Information (USA) |
| NIBR | National Institute of Biological Resources (Incheon, Korea) |
| NTU | Nephelometric Turbidity Unit |
| rbcL | Ribulose-1,5-bisphosphate carboxylase/oxygenase large subunit gene |
| SEM | Scanning electron microscopy |
| SSU rDNA | Small subunit ribosomal DNA |
| Turb | Turbidity (NTU) |
| WT | Water temperature (°C) |
References
- Rimet, F.; Bouchez, A. Life-forms, cell-sizes and ecological guilds of diatoms in European rivers. Knowl. Manag. Aquat. Ecosyst. 2012, 406, 1. [Google Scholar] [CrossRef]
- Simaika, J.P.; Stribling, J.; Lento, J.; Bruder, A.; Poikane, S.; Moretti, M.S.; Rivers-Moore, N.; Meissner, K.; Macadam, C.R. Towards harmonized standards for freshwater biodiversity monitoring and biological assessment using benthic macroinvertebrates. Sci. Total Environ. 2024, 918, 170360. [Google Scholar] [CrossRef] [PubMed]
- Hejduková, E.; Kollár, J.; Nedbalová, L. Freezing stress tolerance of benthic freshwater diatoms from the genus Pinnularia: Comparison of strains from polar, alpine, and temperate habitats. J. Phycol. 2024, 60, 1105–1120. [Google Scholar] [CrossRef] [PubMed]
- Pinseel, E.; Vanormelingen, P.; Hamilton, P.B.; Vyverman, W.; Van de Vijver, B.; Kopalová, K. Molecular and morphological characterization of the Achnanthidium minutissimum complex (Bacillariophyta) in Petuniabukta (Spitsbergen, High Arctic) including the description of A. digitatum sp. nov. Eur. J. Phycol. 2017, 52, 264–280. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. National University of Ireland, Galway. 2023. Available online: https://www.algaebase.org (accessed on 3 September 2025).
- Krammer, K. The Genus Pinnularia. In Diatoms of Europe: Diatoms of the European Inland Waters and Comparable Habitats; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2000; Volume 1. [Google Scholar]
- Van de Vijver, B.; Zidarova, R. Five new taxa in the genus Pinnularia sectio Distantes (Bacillariophyta) from Livingston Island (South Shetland Islands). Phytotaxa 2011, 24, 39–50. [Google Scholar] [CrossRef]
- Kulikovskiy, M.; Glushchenko, A.; Kezlya, E.; Kuznetsova, I.; Kociolek, J.P.; Maltsev, Y. The genus Pinnularia Ehrenberg (Bacillariophyta) from the Transbaikal area (Russia, Siberia): Description of seven new species on the basis of morphology and molecular data with discussion of the phylogenetic position of Caloneis. Plants 2023, 12, 3552. [Google Scholar] [CrossRef] [PubMed]
- Veselá, J.; Johansen, J.R. The diatom flora of ephemeral headwater streams in the Elbsandsteingebirge region of the Czech Republic. Diatom Res. 2009, 24, 443–477. [Google Scholar] [CrossRef]
- Pinseel, E.; Vanormelingen, P.; Vyverman, W.; Kopalová, K.; Van de Vijver, B. Pinnularia catenaborealis sp. nov. from Antarctica. Phycologia 2016, 56, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Vyverman, W.; Verleyen, E.; Sabbe, K.; Vanhoutte, K.; Sterken, M. The evolution of highly diversified diatom communities in polar lakes. Biodivers. Conserv. 2007, 16, 327–344. [Google Scholar] [CrossRef]
- Kwon, D.; Park, M.; Lee, H.; Lee, J.-Y.; Lee, S.D. New recorded diatoms in Holocene sediment cores from the Gonggeom-ji Wetland in Korea. Appl. Microsc. 2023, 53, 3. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Yun, S.M.; Cho, P.Y.; Yang, H.-W.; Kim, O.J. Newly recorded species of diatoms in the source of Han and Nakdong Rivers, South Korea. Phytotaxa 2019, 403, 145–160. [Google Scholar] [CrossRef]
- Lee, J.H. Historical review and prospect on diatoms in Korea. Algae 1996, 11, 247–267. Available online: https://www.e-algae.org/m/journal/view.php?number=2090 (accessed on 12 August 2025).
- Souffreau, C.; Verbruggen, H.; Wolfe, A.P.; Vanormelingen, P.; Siver, P.A.; Cox, E.J.; Mann, D.G.; Van de Vijver, B.; Sabbe, K.; Vyverman, W. A time-calibrated multi-gene phylogeny of the diatom genus Pinnularia. Mol. Phylogenet. Evol. 2011, 61, 866–879. [Google Scholar] [CrossRef] [PubMed]
- Evans, K.M.; Wortley, A.H.; Mann, D.G. An assessment of potential diatom ‘barcode’ genes (cox1, rbcL, 18S and ITS rDNA) and their effectiveness in determining relationships in Sellaphora (Bacillariophyta). Protist 2007, 158, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Kezlya, E.; Tseplik, N.; Kulikovskiy, M. Genetic markers for metabarcoding of freshwater microalgae: Review. Biology 2023, 12, 1038. [Google Scholar] [CrossRef] [PubMed]
- Chepurnov, V.A.; Mann, D.G.; Sabbe, K.; Vyverman, W. Experimental studies on sexual reproduction in diatoms. Int. Rev. Cytol. 2004, 237, 91–154. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Wang, P.; Cho, I.-H.; Hwang, E.-A.; Lee, H.; Kim, B.-H. Morphology and phylogenetic position of three new raphid diatoms (Bacillariophyceae) from Hangang River, South Korea. Phytotaxa 2020, 442, 153–182. [Google Scholar] [CrossRef]
- Hwang, E.-A.; Kim, H.-K.; Cho, I.-H.; Yi, C.; Kim, B.-H. Morphological and molecular studies of three new diatom species from mountain streams in South Korea. Diversity 2022, 14, 790. [Google Scholar] [CrossRef]
- Lee, H.; Yun, S.M.; Lee, J.-Y.; Lee, S.D.; Lim, J.; Cho, P.Y. Late Holocene climate changes from diatom records in the historical Reservoir Gonggeomji, Korea. J. Appl. Phycol. 2018, 30, 3205–3219. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, P.; Kim, H.K.; Lee, H.; Han, M.S.; Kim, B.H. Lemnicola hungarica (Bacillariophyceae) and the new monoraphid diatom Lemnicola uniseriata sp. nov. (Bacillariophyceae) from South Korea. Diatom Res. 2018, 33, 69–87. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Han, B.-H.; Hwang, S.-O.; Kim, B.-H. Morphology and phylogenetic positions of two novel Gogorevia species (Bacillariophyta) from the Han River, South Korea. Plants 2025, 14, 1272. [Google Scholar] [CrossRef] [PubMed]
- Ciniglia, C.; Cennamo, P.; De Stefano, M.; Pinto, G.; Caputo, P.; Pollio, A. Pinnularia obscura Krasske (Bacillariophyceae, Bacillariophyta) from acidic environments: Characterization and comparison with other acid-tolerant Pinnularia species. Arch. Hydrobiol. 2007, 170, 29–47. [Google Scholar] [CrossRef]
- Xu, S.-M.; Liu, B.; Rioual, P.; Yi, M.-Q.; Ma, Y.-D. A new freshwater species of Pinnularia (Bacillariophyta) from Hunan Province, China. PhytoKeys 2024, 237, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Noga, T.; Stanek-Tarkowska, J.; Kochman-Kędziora, N.; Peszek, Ł.; Pajączek, A.; Woźniak, M. The Pinnularia genus in south-eastern Poland with consideration of rare and new taxa to Poland. Oceanol. Hydrobiol. Stud. 2014, 43, 77–99. [Google Scholar] [CrossRef]
- Levkov, Z.; Krstic, S.; Nakov, T.; Melovski, L. Diatom assemblages on Shara and Nidze Mountains, Macedonia. Nova Hedwigia 2005, 81, 501–538. [Google Scholar] [CrossRef]
- Sonneman, J.A. An Illustrated Guide to Common Stream Diatom Species from Temperate Australia; Cooperative Research Centre for Freshwater Ecology & Environment Protection Authority of Victoria: Melbourne, Australia, 2000. Available online: https://www.epa.vic.gov.au/ (accessed on 3 September 2025).
- Kezlya, E.; Maltsev, Y.; Genkal, S.; Krivova, Z.; Kulikovskiy, M. Phylogeny and fatty acid profiles of new Pinnularia (Bacillariophyta) species from soils of Vietnam. Cells 2022, 11, 2446. [Google Scholar] [CrossRef] [PubMed]
- Leira, M.; López-Rodríguez, M.C.; Carballeira, R. Epilithic diatoms (Bacillariophyceae) from running waters in NW Iberian Peninsula (Galicia, Spain). An. Jard. Bot. Madr. 2017, 74, e062. [Google Scholar] [CrossRef]
- Tremarin, P.I.; Moreira-Filho, H.; Ludwig, T.A.V. Pinnulariaceae (Bacillariophyceae) do Rio Guaraguaçu, bacia hidrográfica litorânea paranaense, Brasil. Acta Bot. Bras. 2010, 24, 335–353. [Google Scholar] [CrossRef][Green Version]
- Hirota, M.; Ohtsuka, T. Epilithic diatoms of Sendai River, Tottori Prefecture, Japan. Diatom 2009, 25, 52–72. [Google Scholar] [CrossRef]
- Metzeltin, D.; Lange-Bertalot, H. Tropical Diatoms of South America I: About 700 Predominantly Rarely Known or New Taxa Representative of the Neotropical Flora. In Iconographia Diatomologica, Annotated Diatom Micrographs; Lange-Bertalot, H., Ed.; Koeltz Scientific Books: Königstein, Germany, 1998; Volume 5. [Google Scholar]
- Pereira, A.C.; Torgan, L.C.; Melo, S. Four new Pinnularia Ehrenberg (Bacillariophyta, Pinnulariaceae) species from Amazonian black water (Tupé Lake, Amazonas State, Brazil). Phytotaxa 2014, 158, 109–123. [Google Scholar] [CrossRef][Green Version]
- Serieyssol, K.K. Diatoms of Europe: Diatoms of the European Inland Waters and Comparable Habitats. Diatom Res. 2012, 27, 103. [Google Scholar] [CrossRef]
- Patrick, R.; Reimer, C.W. The Diatoms of the United States. In Vol. 1, Monographs of the Academy of Natural Sciences of Philadelphia, No. 13; Academy of Natural Sciences of Philadelphia: Philadelphia, PA, USA, 1966; pp. 1–688. [Google Scholar]
- Krammer, K.; Lange-Bertalot, H. Bacillariophyceae. 1. Teil: Naviculaceae. In Süßwasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Gerloff, J., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer Verlag: Stuttgart, Germany, 1986; Volume 2, pp. 1–876. [Google Scholar]
- Potapova, M.G.; Kersey, M.H.; Aycock, L.L. Diversity and distribution of spine-bearing species of Pinnularia in eastern North America. Diatom Res. 2023, 38, 33–53. [Google Scholar] [CrossRef]
- Krammer, K. Pinnularia: Eine Monographie der europäischen Taxa; Volume 26 of Bibliotheca Diatomologica; Gebruder Borntraeger Verlagsbuchhandlung: Berlin–Stuttgart, Germany, 1992; 353p. [Google Scholar]
- Kollár, J.; Pinseel, E.; Vyverman, W.; Poulíčková, A. A polyphasic approach to the delimitation of diatom species: A case study for the genus Pinnularia (Bacillariophyta). J. Phycol. 2019, 55, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Jahn, R.; Gemeinholzer, B. Barcoding diatoms: Evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Org. Divers. Evol. 2011, 11, 173–192. [Google Scholar] [CrossRef]
- Kollár, J.; Pinseel, E.; Vyverman, W.; Poulíčková, A. A time-calibrated multi-gene phylogeny provides insights into the evolution, taxonomy and DNA barcoding of the Pinnularia gibba group (Bacillariophyta). Fottea 2021, 21, 62–72. [Google Scholar] [CrossRef]
- Pinseel, E.; Ruck, E.C.; Nakov, T.; Jonsson, P.R.; Kourtchenko, O.; Kremp, A.; Pinder, M.I.M.; Roberts, W.R.; Sjöqvist, C.; Töpel, M.; et al. Genome-wide adaptation to a complex environmental gradient in a keystone phytoplankton species. Mol. Ecol. 2025, 34, e17817. [Google Scholar] [CrossRef]
- Smol, J.P.; Wolfe, A.P.; Birks, H.J.B.; Douglas, M.S.V.; Jones, V.J.; Korhola, A.; Pienitz, R.; Rühland, K.; Sorvari, S.; Antoniades, D.; et al. Climate-driven regime shifts in the biological communities of arctic lakes. Proc. Natl. Acad. Sci. USA 2005, 102, 4397–4402. [Google Scholar] [CrossRef] [PubMed]
- Smol, J.P.; Rühland, K.M.; Michelutti, N.; Evans, M.S. From Arctic ponds to the ‘Northern Great Lakes’: Algae as first responders of climate-driven regime shifts. J. Phycol. 2024, 60, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Weiskopf, S.R.; Rubenstein, M.A.; Crozier, L.G.; Gaichas, S.; Griffis, R.; Halofsky, J.E.; Hyde, K.J.W.; Morelli, T.L.; Morisette, J.T.; Muñoz, R.C.; et al. Climate change effects on biodiversity, ecosystems, ecosystem services, and natural resource management in the United States. Sci. Total Environ. 2020, 733, 137782. [Google Scholar] [CrossRef] [PubMed]
- Anim, D.O.; Banahene, P. Urbanization and stream ecosystems: The role of flow hydraulics towards an improved understanding in addressing urban stream degradation. Environ. Rev. 2021, 29, 401–414. [Google Scholar] [CrossRef]
- An, S.M.; Choi, D.H.; Lee, J.H.; Lee, H.; Noh, J.H. Identification of benthic diatoms isolated from the eastern tidal flats of the Yellow Sea: Comparison between morphological and molecular approaches. PLoS ONE 2017, 12, e0179422. [Google Scholar] [CrossRef] [PubMed]
- Nistal-García, A.; García-García, P.; García-Girón, J.; Borrego-Ramos, M.; Blanco, S.; Bécares, E. DNA metabarcoding and morphological methods show complementary patterns in the metacommunity organization of lentic epiphytic diatoms. Sci. Total Environ. 2021, 786, 147410. [Google Scholar] [CrossRef] [PubMed]
- Turk Dermastia, T.; Cerino, F.; Stanković, D.; Francé, J.; Ramšak, A.; Žnidarič Tušek, M.; Beran, A.; Natali, V.; Cabrini, M.; Mozetič, P. Ecological time series and integrative taxonomy unveil seasonality and diversity of the toxic diatom Pseudo-nitzschia H.Peragallo in the northern Adriatic Sea. Harmful Algae 2020, 93, 101773. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Ohtsuka, T.; Tuji, A.; Houki, A. Picture Book and Ecology of the Freshwater Diatoms; Uchida Rokakuho Publishing: Tokyo, Japan, 2005; 666p. [Google Scholar]
- Poulíčková, A.; Veselá, J.; Neustupa, J.; Škaloud, P. Pseudocryptic diversity versus cosmopolitanism in diatoms: A case study on Navicula cryptocephala Kütz.(Bacillariophyceae) and morphologically similar taxa. Protist 2010, 161, 353–369. [Google Scholar] [CrossRef]
- Kheiri, S.; Siavash, B.; Safavi, S.R. Spatial and temporal patterns of diatom diversity in the Damavand River basin, Central Alborz, Iran. In Proceedings of the Abstracts of the Online International Diatom Symposium, Online, 23–25 August 2021; Jordan, R., Sato, S., Eds.; International Society for Diatom Research: Yamagata, Japan, 2021; p. 27. Available online: https://www.researchgate.net/publication/356602882 (accessed on 3 September 2025).
- Souffreau, C.; Vanormelingen, P.; Van de Vijver, B.; Isheva, T.; Verleyen, E.; Sabbe, K.; Vyverman, W. Molecular evidence for distinct Antarctic lineages in the cosmopolitan terrestrial diatoms Pinnularia borealis and Hantzschia amphioxys. Protist 2013, 164, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, H.; Schimani, K.; Al-Shaheen, M.; Abarca, N.; Jahn, R.; Al-Handal, A.; Kusber, W.-H.; Zimmermann, J. Comparison of the biodiversity of epiphytic diatoms in the Euphrates–Tigris Rivers using morphological and metabarcoding analyses. Metabarcoding Metagenomics 2024, 8, e135082. [Google Scholar] [CrossRef]
- Jahn, R. Discovery of Pinnularia gastrum type specimen. Diatom Res. 2004, 19, 229–234. [Google Scholar] [CrossRef]
- Lee, K.-L.; Choi, J.S.; Lee, J.-H.; Jung, K.-Y.; Kim, H.S. Response of epilithic diatom assemblages to weir construction on the Nakdong River, Republic of Korea. Ecol. Indic. 2021, 126, 107711. [Google Scholar] [CrossRef]
- Lewandowicz, W.; Grabowska, M.; Wojtal, A.Z.; Puczko, K.; Więcko, A. Taxonomic diversity: Importance, threats, and status of diatoms from lowland urban springs (Northeast Poland). Water 2025, 17, 2293. [Google Scholar] [CrossRef]
- Pennesi, C.; Caputo, A.; Lobban, C.S.; Poulin, M.; Totti, C. Morphological discoveries in the genus Diploneis (Bacillariophyceae) from the tropical west Pacific, including the description of new taxa. Diatom Res. 2017, 32, 195–228. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques. Elsevier Academic Press: New York, NY, USA, 2005; p. 578. [Google Scholar]
- Hasle, G.R.; Fryxell, G.A. Diatoms: Cleaning and mounting for light and electron microscopy. Trans. Am. Microsc. Soc. 1970, 89, 469–474. [Google Scholar] [CrossRef]
- Friedrichs, L. A simple cleaning and fluorescent staining protocol for recent and fossil diatom frustules. Diatom Res. 2013, 28, 317–327. [Google Scholar] [CrossRef]
- Saxena, A.; Mazumder, A.; Singh, D.S.; Bera, S.K. Relation between Present Freshwater Diatom Assemblage and Grain Size of the Lake: A Comparative Case Study from the Two Swamps of Rani-Garbhanga Reserve Forest, Assam. Appl. Ecol. Environ. Sci. 2022, 10, 665–678. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).