Thermal Vulnerability and Potential Cultivation Areas of Four Day-Neutral Strawberries in Chile: Implications for Climate Adaptation
Abstract
1. Introduction
2. Results
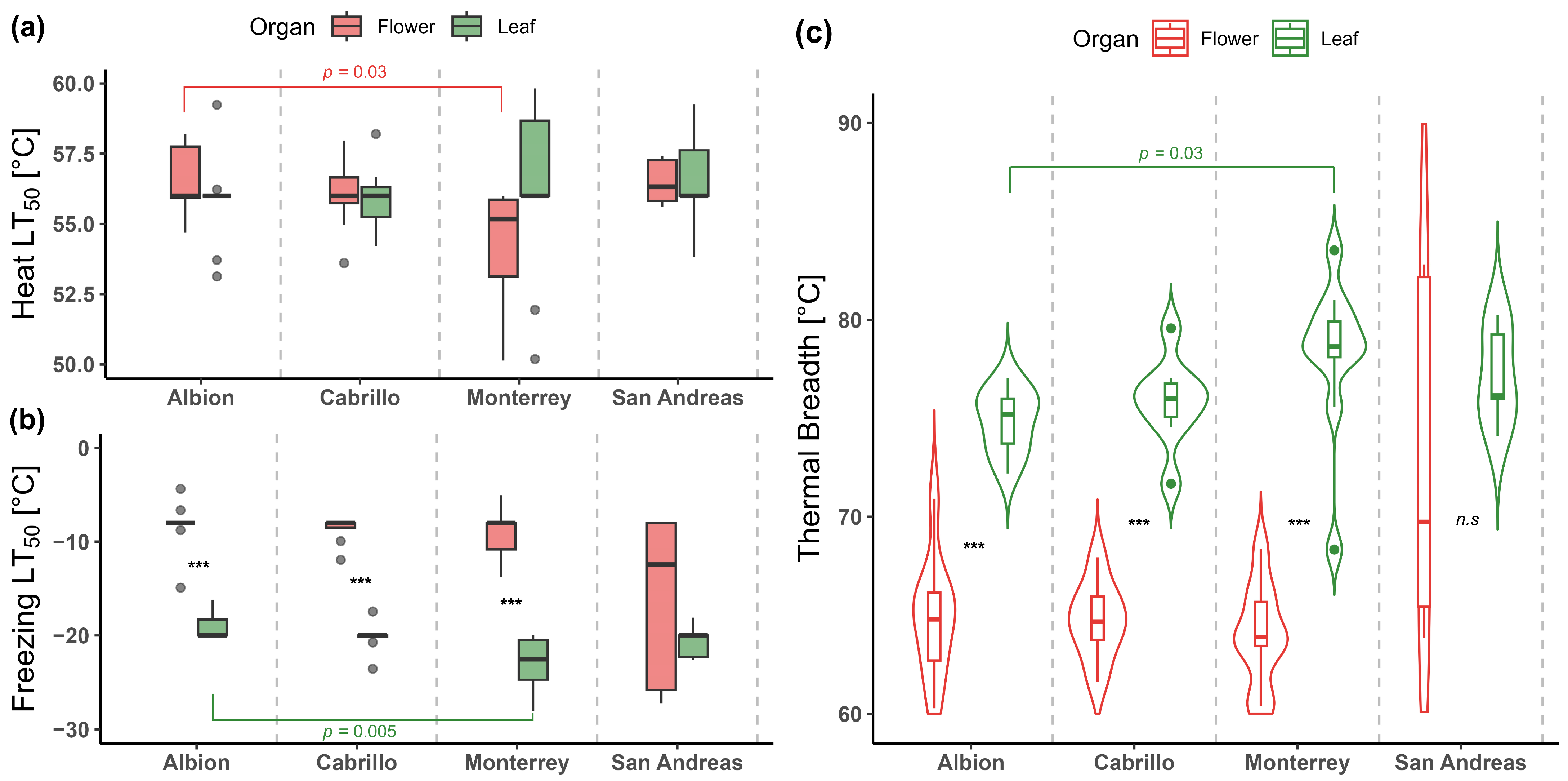
2.1. Differences in Heat and Freezing Tolerance, and Thermal Tolerance Breadth Between Leaves and Flowers of Strawberry Varieties
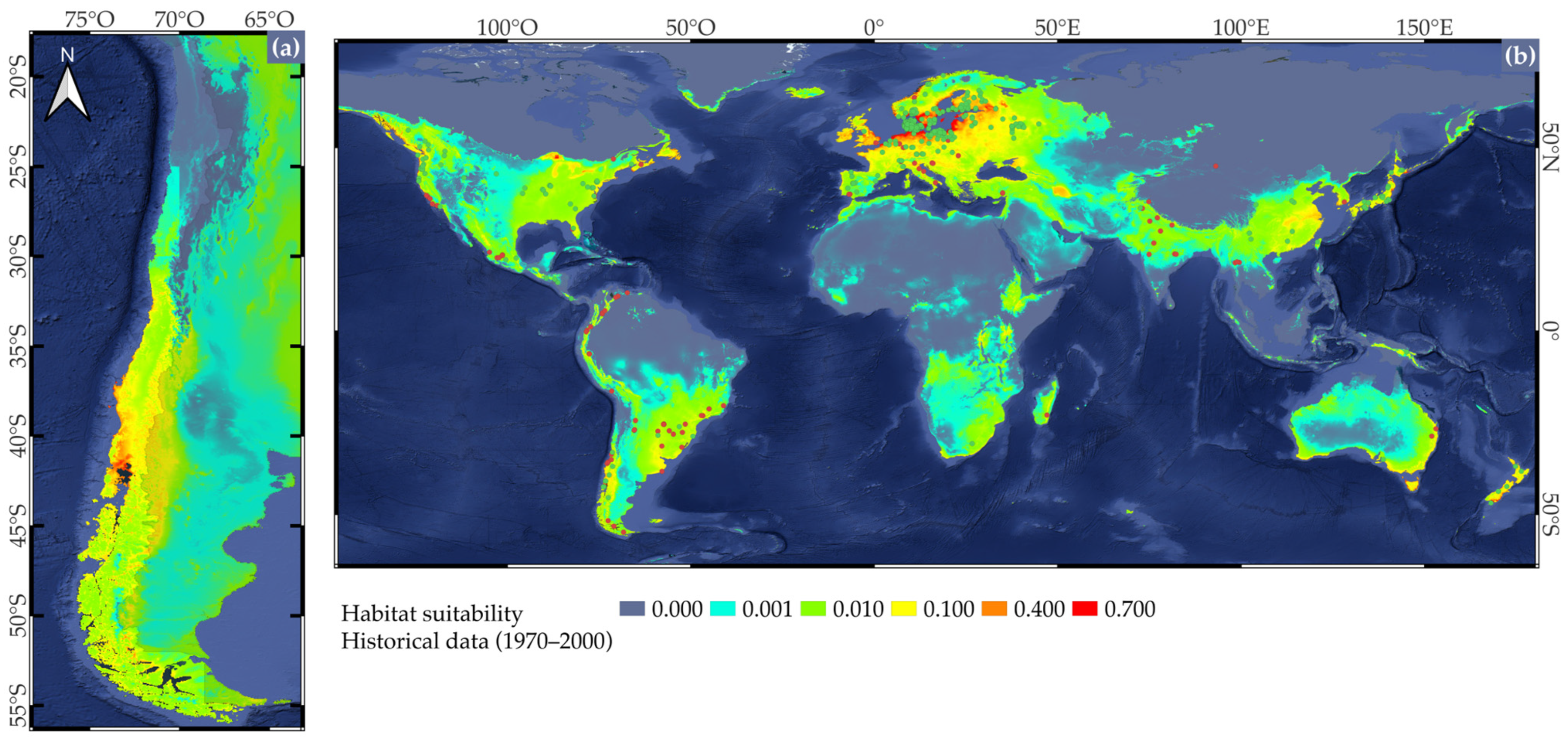
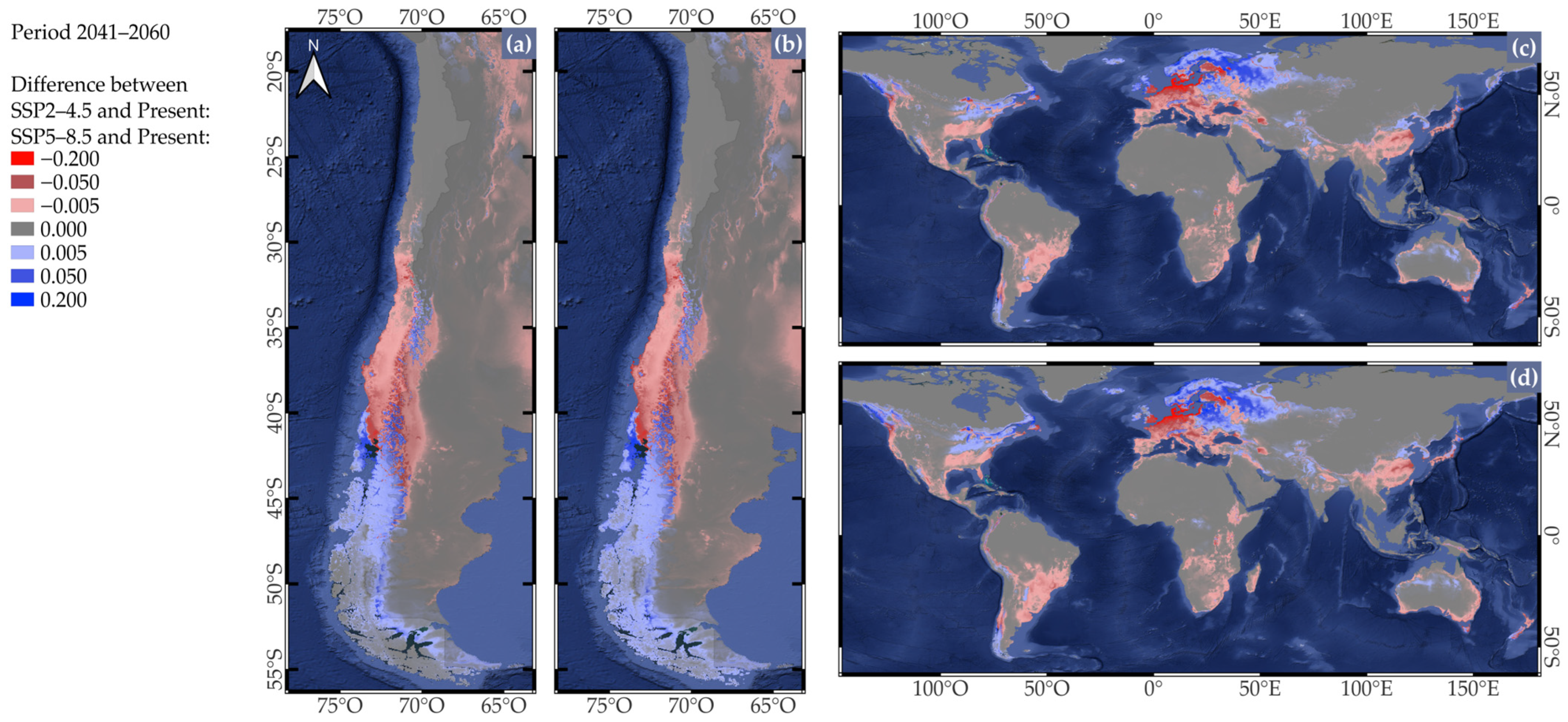
2.2. Potential Shifts in Suitable Cultivation Areas Based on Niche Models
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Protocol for Determining Thermal Tolerance in Strawberries
4.3. Assessing Plant Thermal Damage
4.4. Climatic Data and Modeling
4.4.1. Occurrence Data Processing and Definition of the Study Area
4.4.2. Climatic Variables and Filtering
4.4.3. Model Construction and Configuration
4.4.4. Climate Change Scenarios
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EC | Electrical Conductivity |
| REC | Relative Electrical Conductivity |
| Corrected REC | Corrected Relative Electrical Conductivity |
| LT50 freezing | Freezing Temperature that Produces 50% Tissue Damage (°C) |
| LT50 heat | High Temperature that Produces 50% Tissue Damage (°C) |
| TTB | Thermal Tolerance Breadth |
| GBIF | Global Biodiversity Information Facility |
| MaxEnt | Maximum Entropy |
| SSP2-4.5 | Shared Socioeconomic Pathway 2, Intermediate Development Scenario with ~4.5 W/m2 |
| SSP5-8.5 | Shared Socioeconomic Pathway 5, Fossil-Fueled Development Scenario with ~8.5 W/m2 Radiative Forcing by 2100 |
References
- Malhi, G.S.; Kaur, M.; Kaushik, P. Impact of climate change on agriculture and its mitigation strategies: A review. Sustainability 2021, 13, 1318. [Google Scholar] [CrossRef]
- Bacelar, E.; Pinto, T.; Anjos, R.; Morais, M.C.; Oliveira, I.; Vilela, A.; Cosme, F. Impacts of Climate Change and Mitigation Strategies for Some Abiotic and Biotic Constraints Influencing Fruit Growth and Quality. Plants 2024, 13, 1942. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.R.; Araújo, F.H.V.; Ferreira, S.R.; dos Santos, J.C.B.; de Abreu, C.M.; Siqueira da Silva, R.; Regina da Costa, M. Strawberries in a warming world: Examining the ecological niche of Fragaria × ananassa Duch-Across different climate scenarios. J. Berry Res. 2024, 14, 193–208. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. In Core Writing Team; Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Geange, S.R.; Arnold, P.A.; Catling, A.A.; Coast, O.; Cook, A.M.; Gowland, K.M.; Leigh, A.; Notarnicola, R.F.; Posch, B.C.; Venn, S.E.; et al. The thermal tolerance of photosynthetic tissues: A global systematic review and agenda for future research. New Phytol. 2021, 229, 2497–2513. [Google Scholar] [CrossRef]
- Hummer, K.E.; Williams, K.A.; Bushakra, J.M. North American Crop Wild Relatives of Temperate Berries (Fragaria L., Ribes L., Rubus L., and Vacciniun L.). In North American Crop Wild Relatives; Greene, S., Williams, K., Khoury, C., Kantar, M., Marek, L., Eds.; Springer: Cham, Switzerland, 2019; Volume 2, pp. 283–328. [Google Scholar] [CrossRef]
- Cossio, A.J.; Flores, A.A. Competitividad de la fresa mexicana en el mercado estadounidense de 1992 a 2017. Cienc. Tecnol. Agropecu. 2021, 22, e1414. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O.M. Long-day control of flowering in everbearing strawberries. J. Hortic. Sci. Biotechnol. 2007, 82, 875–884. [Google Scholar] [CrossRef]
- Yoneda, A.; Yasutake, D.; Hidaka, K.; Muztahidin, N.I.; Miyoshi, Y.; Kitano, M.; Okayasu, T. Effects of supplemental lighting during the period of rapid fruit development on the growth, yield, and energy use efficiency in strawberry plant production. Int. Agrophys. 2020, 34, 233–239. [Google Scholar] [CrossRef]
- Morales, A.; Vargas, S. Manual de Manejo Agronómico de la Frutilla; Boletín INIA-Instituto de Investigaciones Agropecuarias: Villa Alegre, Chile, 2017; pp. 1–98. Available online: https://hdl.handle.net/20.500.14001/6713 (accessed on 29 July 2021).
- Sønsteby, A.; Heide, O.M. Dormancy relations and flowering of the strawberry cultivars Korona and Elsanta as influenced by photoperiod and temperature. Sci. Hortic. 2006, 110, 57–67. [Google Scholar] [CrossRef]
- Heide, O.M.; Stavang, J.A.; Sønsteby, A. Physiology and genetics of flowering in cultivated and wild strawberries—A review. J. Hortic. Sci. Biotechnol. 2013, 88, 1–18. [Google Scholar] [CrossRef]
- Rowley, D.; Black, B.L.; Drost, D.; Feuz, D. Late-season Strawberry Production Using Day-neutral Cultivars in High-elevation High Tunnels. Hortscience 2011, 46, 1480–1485. [Google Scholar] [CrossRef]
- Gude, K.; Stanley, H.; Rivard, C.L.; Cunningham, B.; Kang, Q.; Pliakoni, E.D. Quality of day-neutral strawberries grown in a high tunnel system. Sci. Hortic. 2021, 275, 109726. [Google Scholar] [CrossRef]
- Menzel, C.M. A review of fruit development in strawberry: High temperatures accelerate flower development and decrease the size of the flowers and fruit. J. Hortic. Sci. Biotechnol. 2023, 98, 409–431. [Google Scholar] [CrossRef]
- Jiang, N.; Yang, Z.; Luo, J.; Wang, C. Quantifying Chilling Injury on the Photosynthesis System of Strawberries: Insights from Photosynthetic Fluorescence Characteristics and Hyperspectral Inversion. Plants 2023, 12, 3138. [Google Scholar] [CrossRef]
- Seymour, Z.J.; Mercedes, J.F. Effect of heat acclimation on thermotolerance of in vitro strawberry plantlet. Folia Hortic. 2024, 36, 135–147. [Google Scholar] [CrossRef]
- Maughan, T.L.; Black, B.L.; Drost, D. Critical temperature for sub-lethal cold injury of strawberry leaves. Sci. Hortic. 2015, 183, 8–12. [Google Scholar] [CrossRef]
- Cui, M.; Pham, M.D.; Hwang, H.; Chun, C. Flower development and fruit malformation in strawberries after short-term exposure to high or low temperature. Sci. Hortic. 2021, 288, 110308. [Google Scholar] [CrossRef]
- Bo, Y.; Zhang, H.; Tong, Y.; Jia, Y.; Liu, X.; Yang, L.; Zuo, Z.; Wang, Y. Growth, antioxidant enzyme activity and transcriptome response to low-temperature induction of flowering in cultivated strawberry. Plant Stress 2024, 12, 100453. [Google Scholar] [CrossRef]
- Xu, C.; Wang, M.T.; Yang, Z.Q.; Wang, M.T. Low temperature and low irradiation induced irreversible damage of strawberry seedlings. Photosynthetica 2020, 58, 156–164. [Google Scholar] [CrossRef]
- Roussos, P.A.; Ntanos, E.; Tsafouros, A.; Denaxa, N.K. Strawberry physiological and biochemical responses to chilling and freezing stress and application of alleviating factors as countermeasures. J. Berry Res. 2020, 10, 437–457. [Google Scholar] [CrossRef]
- Ariza, M.T.; Soria, C.; Martínez-Ferri, E. Developmental stages of cultivated strawberry flowers in relation to chilling sensitivity. AoB Plants 2015, 7, plv012. [Google Scholar] [CrossRef]
- Goswami, A.K.; Singh, S.K.; Pradhan, S. Physiological disorders. In Strawberries, Production, Postharvest Management and Protection, 1st ed.; Sharma, R.M., Yamdagni, R., Dubey, A.K., Pandey, V., Eds.; CRS Press: Boca Raton, FL, USA, 2019; pp. 445–452. [Google Scholar]
- Menzel, C.M. Temperatures above 30 °C decrease leaf growth in strawberry under global warming. J. Hortic. Sci. Biotechnol. 2024, 99, 507–530. [Google Scholar] [CrossRef]
- Ullah, I.; Toor, M.D.; Yerlikaya, B.A.; Mohamed, H.-I.; Yerlikaya, S.; Basit, A.; ur Rehman, A. High-temperature stress in strawberry: Understanding physiological, biochemical and molecular responses. Planta 2024, 260, 118. [Google Scholar] [CrossRef]
- Carlen, C.; Potel, A.M.; Ançay, A. Photosynthetic response of strawberry leaves to changing temperatures. Acta Hortic. 2009, 838, 73–76. [Google Scholar] [CrossRef]
- Nathewet, P. Effects of heat stress on physiological index in cultivated strawberries. Khon Kaen Agric. J. 2017, 45, 314–320. [Google Scholar]
- Ledesma, N.A.; Nakata, M.; Sugiyama, N. Effect of high temperature stress on the reproductive growth of strawberry cvs. ‘Nyoho’ and ‘Toyonoka’. Sci. Hortic. 2008, 116, 186–193. [Google Scholar] [CrossRef]
- Maughan, T.L. Optimizing Systems for Cold-Climate Strawberry Production. Master’s Thesis, Utah State University, Logan, UT, USA, 2013. [Google Scholar]
- Zini, L.M.; Galati, B.G.; Carrera, C.S. High temperatures during late floral bud stages decrease fertilization in strawberry (Fragaria × ananassa): Pollen-pistil interaction and anatomical evidences. Plant Biosyst. 2023, 157, 367–378. [Google Scholar] [CrossRef]
- Kadir, S.; Sidhu, G.; Al-Khatib, K. Strawberry (Fragaria ×·ananassa Duch.) Growth and Productivity as Affected by Temperature. HortScience 2006, 41, 1423–1430. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Ledesma, N.A.; Kawabata, S. Responses of two strawberry cultivars to severe high temperature stress at different flower development stages. Sci. Hortic. 2016, 211, 319–327. [Google Scholar] [CrossRef]
- Paquin, R.; Bolduc, R.; Zizka, J.; Pelletier, G.; Lechasseur, P. Tolerance au gel et teneur en sucres et en proline du collet du fraisier (Fragaria annassa Duch) durant L’hiver. Can. J. Plant Sci. 1989, 69, 945–954. [Google Scholar] [CrossRef]
- O’Neill, S.D.; Priestley, D.A.; Chabot, B.F. Temperature and Aging Effects on Leaf Membranes of a Cold Hardy Perennial, Fragaria virginiana. Plant Physiol. 1981, 68, 1409–1415. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.L.; Thomashow, M.F.; Hancock, J.F.; Iezzoni, A.F. CBF1 orthologs in sour cherry and the strawberry and heterologous expression of CBF1 in strawberry. J. Am. Soc. Hortic. Sci. 2002, 127, 489–494. [Google Scholar] [CrossRef]
- Turhan, E.; Aydoğan, Ç.; Akoglu, A.; Baykul, A.; Evrenosoğlu, Y. Relationship of seasonal changes in antioxidative enzymes and cold hardiness in strawberry plants. J. Food Agric. Environ. 2012, 10, 445–450. [Google Scholar]
- Rajashekar, C.B.; Panda, M. Water stress is a component of cold acclimation process essential for inducing full freezing tolerance in strawberry. Sci. Hortic. 2014, 174, 54–59. [Google Scholar] [CrossRef]
- Sarikhani, H.; Safariyan-Nejad, M.-S. Improving of Winter Cold Hardiness by Glycine Betaine in Strawberry. Int. J. Hortic. Sci. Technol. 2021, 8, 401–413. [Google Scholar] [CrossRef]
- Ourecky, D.K.; Reich, J.E. Frost Tolerance in Strawberry Cultivars. HortScience 1976, 1, 413–414. [Google Scholar] [CrossRef]
- Boyce, B.R.; Strater, J.B. Comparison of frost injury in strawberry buds, blossoms and immature fruit. Adv. Strawb. Prod. 1984, 3, 8–10. [Google Scholar]
- Ki, W.K.; Warmund, M.R. Low-temperature Injury to Strawberry Floral Organs at Several Stages of Development. HortScience 1992, 27, 1302–1304. [Google Scholar] [CrossRef]
- Hummel, R.L.; Moore, P.P. Freeze resistance of Pacific Northwest Strawberry flowers. J. Am. Soc. Hortic. Sci. 1997, 122, 179–182. [Google Scholar] [CrossRef]
- Gulen, H.; Eris, A. Some physiological changes in strawberry (Fragaria × ananassa ‘Camarosa’) plants under heat stress. J. Hortic. Sci. Biotechnol. 2003, 78, 894–898. [Google Scholar] [CrossRef]
- Kesici, M.; Gulen, H.; Ergin, S.; Turhan, E.; Ipek, A.; Koksal, N. Heat-stress Tolerance of Some Strawberry (Fragaria × ananassa) Cultivars. Not. Bot. Horti Agrobot. Cluj 2013, 41, 244–249. [Google Scholar] [CrossRef]
- Ergin, S.; Gülen, H.; Kesici, M.; Turhan, E.; Ipek, A.; Köksal, N. Effects of high temperature stress on enzymatic and nonenzymatic antioxidants and proteins in strawberry plants. Turk. J. Agric. For. 2016, 40, 11. [Google Scholar] [CrossRef]
- Proexant. El Cultivo de la Fresa; Corporación Proexant: Quito, Ecuador, 2002. [Google Scholar]
- ICAMEX. Guía Técnica Para el Cultivo de la Fresa; Esquivel, M.A., Ed.; Instituto de Investigación y Capacitación Agropecuaria, Acuícola y Forestal del Estado de México: México, Mexico, 2006; pp. 12–20. [Google Scholar]
- Vitasse, Y.; Rebetez, M. Unprecedented risk of spring frost damage in Switzerland and Germany in 2017. Clim. Change 2018, 149, 233–246. [Google Scholar] [CrossRef]
- Garreaud, R. Cambio Climático: Bases Físicas e Impactos en Chile. Rev. Tierra Adentro-INIA 2011, 93, 1–14. [Google Scholar]
- Pica-Téllez, A.; Garreaud, R.; Meza, F.; Bustos, S.; Falvey, M.; Ibarra, M.; Duarte, K.; Ormazábal, R.; Dittborn, R.; Silva, I. Informe Proyecto ARClim: Atlas de Riesgos Climáticos para Chile; Centro de Ciencia del Clima y la Resiliencia, Centro de Cambio Global UC y Meteodata para el Ministerio del Medio Ambiente a través de La Deutsche Gesellschaft für Internationale Zusammenarbeit (GIZ): Santiago, Chile, 2020; pp. 12–181. [Google Scholar]
- González-Reyes, Á.; Jacques-Coper, M.; Bravo, C.; Rojas, M.; Garreaud, R. Evolution of heatwaves in Chile since 1980. Weather Clim. Extrem. 2023, 41, 100588. [Google Scholar] [CrossRef]
- Fernandez, E.; Whitney, C.; Cuneo, I.F.; Luedeling, E. Prospects of decreasing winter chill for deciduous fruit production in Chile throughout the 21st century. Clim. Change 2020, 159, 423–439. [Google Scholar] [CrossRef]
- DGAC. Reporte anual de la evolución del clima en Chile. In Documento elaborado por la Oficina Cambio Climático de la Sección Climatología de la Dirección Meteorológica de Chile; Villarroel, C., Ed.; Dirección Meteorológica de Chile: Santiago, Chile, 2023; pp. 3–52. [Google Scholar]
- Asagrin. Estrategias Regionales de Competitividad por Rubro. “FRUTILLAS REGIÓN METROPOLITANA”; Instituto de Desarrollo Agropecuario (INDAP): Santiago, Chile, 2007; pp. 1–42. [Google Scholar]
- González, C. Frutillas y Moras Procesadas: La Irrupción de los Otros Berries; Oficina De Estudios Y Políticas Agrarias (ODEPA): Santiago, Chile, 2013; pp. 1–7. [Google Scholar]
- SAG. Lista de Variedades Oficialmente Descritas. Servicio Agrícola y Ganadero (SAG). Available online: https://www.sag.gob.cl/ambitos-de-accion/lista-de-variedades-oficialmente-descritas (accessed on 1 April 2025).
- ODEPA. Boletín de Hortalizas. Oficina de Estudios y políticas Agrarias (ODEPA). Available online: https://www.odepa.gob.cl/publicaciones/boletines/boletin-de-hortalizas-octubre-2024 (accessed on 1 April 2025).
- Red Agrícola. Manual del Cultivo de Frambuesas y Frutillas en Chile; Instituto de Desarrollo Agropecuario (INDAP): Santiago, Chile, 2015; pp. 1–8. [Google Scholar]
- Muñoz, M. Evolución de los Frutales; Oficina de Estudios y Políticas Agrarias (ODEPA): Santiago, Chile, 2016; pp. 1–7. [Google Scholar]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.h2ynb6 (accessed on 10 September 2025).
- Houde, M.; Dallaire, S.; N’Dong, D.; Sarhan, F. Overexpression of the acidic dehydrin WCOR410 improves freezing tolerance in transgenic strawberry leaves. Plant Biotechnol. J. 2004, 2, 381–387. [Google Scholar] [CrossRef]
- Zareei, E.; Karami, F.; Gholami, M.; Ershadi, A.; Avestan, S.; Aryal, R.; Gohari, G.; Farooq, M. Physiological and biochemical responses of strawberry crown and leaf tissues to freezing stress. BMC Plant Biol. 2021, 21, 532. [Google Scholar] [CrossRef]
- Balasooriya, B.L.H.N.; Dassanayake, K.B.; Ajlouni, S. High temperature effects on strawberry fruit quality and antioxidant contents. Acta Hortic. 2020, 1278, 225–234. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Mazzoni, A.; Lescano, M.N.; Fernandez, A.; Reiner, G.; Langenheim, M.E.; Mattera, G.; Robredo, N.; Garibaldi, L.; Quintero, C. Wild vs. cultivated strawberries: Differential fruit quality traits and antioxidant properties in Fragaria chiloensis and Fragaria × ananassa. Discov. Food 2025, 5, 71. [Google Scholar] [CrossRef]
- Ledesma, N.; Kawabata, S.; Sugiyama, N. Effect of High Temperature on Protein Expression in Strawberry Plants. Biol. Plant. 2004, 48, 73–79. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.-S. Effect of plant growth temperature on membrane lipids in strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2006, 108, 35–42. [Google Scholar] [CrossRef]
- Sakai, A.; Larcher, W. Frost Survival of Plants. Responses and Adaptation to Freezing Stress, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 1–321. [Google Scholar]
- Fields, P.A. Review: Protein function at thermal extremes: Balancing stability and flexibility. Comp. Biochem. Physiol. Part A 2001, 129, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, R.; Bhandari, K.; Nayyar, H. Temperature stress and redox homeostasis in agricultural crops. Front. Environ. Sci. 2015, 3, 11. [Google Scholar] [CrossRef]
- Arora, R.; Taulavuori, K. Increased Risk of Freeze Damage in Woody Perennials VIS-À-VIS Climate Change: Importance of Deacclimation and Dormancy Response. Front. Environ. Sci. 2016, 4, 44. [Google Scholar] [CrossRef]
- Jiang, N.; Yang, Z.; Zhang, H.; Xu, J.; Li, C. Effect of Low Temperature on Photosynthetic Physiological Activity of Different Photoperiod Types of Strawberry Seedlings and Stress Diagnosis. Agronomy 2023, 13, 1321. [Google Scholar] [CrossRef]
- Larcher, W. Physiological Plant Ecology. Ecophysiology and Stress Physiology of Functional Groups; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1–514. [Google Scholar]
- Yu, M.; Zhaxi, L.; Deqing, Z.; Wei, X.; Tang, Y. Advances in plant response to low-temperature stress. Plant Growth Regul. 2025, 105, 167–185. [Google Scholar] [CrossRef]
- Gervini Menezes, F.O., Jr.; Vieira Neto, J. Evaluation of neutral day strawberry cultivars “Albion” and “San Andreas” under semi-hydroponic cultivation in the Alto Vale do Itajaí–SC. Rev. Thema 2019, 16, 845–854. [Google Scholar] [CrossRef]
- Hernández-Martínez, N.; Salazar-Gutiérrez, M.; Chaves-Córdoba, B.; Wells, D.; Foshee, W.; McWhirt, A. Model Development of the Phenological Cycle from Flower to Fruit of Strawberries (Fragaria × ananassa). Agronomy 2023, 13, 2489. [Google Scholar] [CrossRef]
- O’Sullivan, O.S.; Heskel, M.A.; Reich, P.B.; Tjoelker, M.G.; Weerasinghe, L.K.; Penillard, A.; Zhu, L.; Egerton, J.J.G.; Bloomfield, K.J.; Creek, D.; et al. Thermal limits of leaf metabolism across biomes. Glob. Change Biol. 2017, 23, 209–223. [Google Scholar] [CrossRef]
- Sastry, A.; Barua, D. Leaf thermotolerance in tropical trees from a seasonally dry climate varies along the slow-fast resource acquisition spectrum. Sci. Rep. 2017, 7, 11246. [Google Scholar] [CrossRef]
- Kitudom, N.; Fauset, S.; Zhou, Y.; Fan, Z.; Li, M.; He, M.; Zhang, S.; Xu, K.; Lin, H. Thermal safety margins of plant leaves across biomes under a heatwave. Sci. Total Environ. 2022, 806, 150416. [Google Scholar] [CrossRef] [PubMed]
- Curtis, E.M.; Gollan, J.; Murray, B.R.; Leigh, A. Native microhabitats better predict tolerance to warming than latitudinal macro-climatic variables in arid-zone plants. J. Biogeogr. 2016, 43, 1156–1165. [Google Scholar] [CrossRef]
- Körner, C.; Hiltbrunner, E. The 90 Ways to Describe Plant Temperature. Perspect. Plant Ecol. Evol. Syst. 2018, 30, 16–21. [Google Scholar] [CrossRef]
- Menzel, C.M. A review of strawberry under protected cultivation: Yields are higher under tunnels than in the open field. J. Hortic. Sci. Biotechnol. 2025, 100, 286–313. [Google Scholar] [CrossRef]
- Rahimi, E.; Jung, C. A global estimation of potential climate change effects on pollinator-dependent crops. Agric. Res. 2024. [Google Scholar] [CrossRef]
- Perez, T.M.; Feeley, K.J. Photosynthetic heat tolerances and extreme leaf temperatures. Funct. Ecol. 2020, 34, 2236–2245. [Google Scholar] [CrossRef]
- Zhu, L.L.; Bloomfield, K.J.; Hocart, C.H.; Egerton, J.J.G.; O’Sullivan, O.S.; Penillard, A.; Weerasinghe, L.K.; Atkin, O. Plasticity of photosynthetic heat tolerance in plants adapted to thermally contrasting biomes. Plant Cell Environ. 2018, 41, 1251–1262. [Google Scholar] [CrossRef]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.; et al. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Rawat, N.; Singla-Pareek, S.L.; Pareek, A. Membrane dynamics during individual and combined abiotic stresses in plants and tools to study the same. Physiol. Plant. 2021, 171, 653–676. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, L.; Zhou, J.; Rogers, M.; Jin, Z. Predicting the growth trajectory and yield of greenhouse strawberries based on knowledge-guided computer vision. Comput. Electron. Agric. 2024, 220, 108911. [Google Scholar] [CrossRef]
- Casadebaig, P.; Mestries, E.; Debaeke, P. A model-based approach to assist variety assessment in sunflower crop. Eur. J. Agron. 2016, 81, 92–105. [Google Scholar] [CrossRef]
- Villagrán, V. Morfología y fisiología. In Frutilla, Consideraciones Productivas y Manejo; Reyes, M., Zschau, B., Eds.; Instituto de Investigaciones Agropecuarias INIA: Raihuen, Chile, 2012; Boletín INIA Nº 252; pp. 17–30. [Google Scholar]
- Zschau, B.; Legarraga, M. Variedades. In Frutilla, Consideraciones Productivas y Manejo; Reyes, M., Zschau, B., Eds.; Instituto de Investigaciones Agropecuarias INIA: Raihuen, Chile, 2012; Boletín INIA Nº 252; pp. 63–74. [Google Scholar]
- Wilner, J. Relative and absolute electrolytic conductance tests for frost hardiness of apple varieties. Can. J. Plant Sci. 1960, 40, 630–637. [Google Scholar] [CrossRef]
- Lipp, C.C.; Goldstein, G.; Meinzer, F.C.; Niemczura, W. Freezing tolerance and avoidance in high-elevation Hawaiian plants. Plant Cell Environ. 1994, 17, 1035–1044. [Google Scholar] [CrossRef]
- Arias, N.S.; Bucci, S.J.; Scholz, F.G.; Goldstein, G. Freezing avoidance by supercooling in Olea europaea cultivars: The role of apoplastic water, solute content and cell wall rigidity. Plant Cell Environ. 2015, 38, 2061–2070. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Bucci, S.J.; Arias, N.S.; Scholz, F.G.; Hao, G.Y.; Cao, K.F.; Goldstein, G. Freezing resistance in Patagonian woody shrubs: The role of cell wall elasticity and stem vessel size. Tree Physiol. 2016, 36, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.V.; Alvear, C.; Sanfuentes, C.; Saldaña, A.O.; Sierra-Almeida, A. Does the life-history strategy determine the freezing resistance of flowers and leaves of alpine herbaceous species? Alp. Bot. 2020, 130, 157–168. [Google Scholar] [CrossRef]
- Chamberlain, S.; Barve, V.; Mcglinn, D.; Oldoni, D.; Desmet, P.; Geffert, L.; Ram, K. rgbif: Interface to the Global Biodiversity Information Facility API.R Package Version 3.8.1. 2025. Available online: https://CRAN.R-project.org/package=rgbif (accessed on 10 September 2025).
- Zizka, A.; Silvestro, D.; Andermann, T.; Azevedo, J.; Ritter, C.D.; Edler, D.; Farooq, H.; Herdean, A.; Ariza, M.; Scharn, R.; et al. Coordinate Cleaner: Standardized Cleaning of Occurrence Records from Biological Collection Databases. Methods Ecol. Evol. 2019, 10, 744–751. [Google Scholar] [CrossRef]
- Sierra-Almeida, A.; Morales, L.V.; Guerrero, D.; Hasbún, R.J.N.; Retamal, L.; Tamburrino, I.; Garrido-Bigotes, A. Global Records of Cultivated Fragaria × ananassa for Climate Modeling and Adaptive Management in Chile. Mendeley Data V2 2025. [Google Scholar] [CrossRef]
- Boria, R.A.; Olson, L.E.; Goodman, S.M.; Anderson, R.P. Spatial Filtering to Reduce Sampling Bias Can Improve the Performance of Ecological Niche Models. Ecol. Model. 2014, 275, 73–77. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by randomForest. R News 2002, 2, 18–22. Available online: https://cran.r-project.org/doc/Rnews/ (accessed on 10 September 2025).
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Valavi, R.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Predictive performance of presence-only species distribution models: A benchmark study with reproducible code. Ecol. Monogr. 2021, 91, e01486. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; García Márquez, J.R.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, C.; Liu, Y.; Liu, G.; He, P.; Liao, G.; Shao, Z. Simulation of Citrus Production Space Based on MaxEnt. Front. Environ. Sci. 2022, 10, 993920. [Google Scholar] [CrossRef]
- Fitzgibbon, A.; Pisut, D.; Fleisher, D. Evaluation of Maximum Entropy (Maxent) Machine Learning Model to Assess Relationships between Climate and Corn Suitability. Land 2022, 11, 1382. [Google Scholar] [CrossRef]
- Ali, S.; Makanda, T.A.; Umair, M.; Ni, J. MaxEnt Model Strategies to Studying Current and Future Potential Land Suitability Dynamics of Wheat, Soybean and Rice Cultivation under Climatic Change Scenarios in East Asia. PLoS ONE 2023, 18, e0296182. [Google Scholar] [CrossRef]
- Ali, S.; Umair, M.; Makanda, T.A.; Shi, S.; Hussain, S.A.; Ni, J. Modeling Current and Future Potential Land Distribution Dynamics of Wheat, Rice, and Maize under Climate Change Scenarios Using MaxEnt. Land 2024, 13, 1156. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- QGIS Development Team. QGIS Geographic Information System. Version 3.40.5 “Bratislava”. Open Source Geospatial Foundation Project. 2024. Available online: http://qgis.org (accessed on 15 September 2025).
- Gateño, F.; Mendoza, P.A.; Vásquez, N.; Lagos-Zúñiga, M.; Jiménez, H.; Jerez, C.; Montserrat, S. Screening CMIP6 Models for Chile Based on Past Performance and Code Genealogy. Clim. Change 2024, 177, 87. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 13 March 2025).
- Dinno, A. dunn.test: Dunn’s Test of Multiple Comparisons Using Rank Sums. 2017. Available online: https://cran.r-project.org/web/packages/dunn.test/dunn.test.pdf (accessed on 13 March 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]


| Time | ||||||
|---|---|---|---|---|---|---|
| Variable | Oct. | Nov. | Dec. | Jan. | Feb. | Mar. |
| Mean air T (°C) | 14.1 ± 0.5 | 17.2 ± 0.5 | 20.1 ± 0.5 | 20.2 ± 0.4 | 20.2 ± 0.4 | 17.5 ± 0.5 |
| Minimum air T (°C) | 6.9 ± 0.5 (2.6) | 9.3 ± 0.4 (4.6) | 11.8 ± 0.4 (7.8) | 11.4 ± 0.4 (6) | 11.1 ± 0.3 (8.4) | 8.6 ± 0.4 (5.1) |
| 1 Freq. T <5 °C (%) | 32.3 | 10 | 0 | 0 | 0 | 0 |
| Maximum air T (°C) | 21.2 ± 0.7 (28.5) | 25.1 ± 0.7 (31.3) | 28.4 ± 0.8 (36.8) | 29 ± 0.6 (34.7) | 29.4 ± 0.6 (36.2) | 26.4 ± 0.7 (33.1) |
| 2 Freq. T >30 °C (%) | 0 | 3.3 | 51.6 | 38.7 | 42.9 | 22.6 |
| Mean RH (%) | 70.5 ± 1 | 66.5 ± 1.4 | 62.2 ± 1.5 | 65.9 ± 1.2 | 62.3 ± 1.7 | 63.3 ± 1.4 |
| Minimum RH (%) | 39.2 ± 1.9 | 33.3 ± 1.8 | 31.3 ± 2.3 | 31.1 ± 1.7 | 27.5 ± 1.6 | 28 ± 1.4 |
| Maximum RH (%) | 94.8 ± 0.7 | 93.3 ± 0.9 | 89.8 ± 1.2 | 93.7 ± 0.8 | 90.3 ± 1.6 | 92.2 ± 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Almeida, A.; Morales, L.V.; Guerrero, D.; Hasbún, R.J.N.; Retamal, L.; Garrido-Bigotes, A.; Tamburrino, Í.; Maruri, A. Thermal Vulnerability and Potential Cultivation Areas of Four Day-Neutral Strawberries in Chile: Implications for Climate Adaptation. Plants 2025, 14, 3205. https://doi.org/10.3390/plants14203205
Sierra-Almeida A, Morales LV, Guerrero D, Hasbún RJN, Retamal L, Garrido-Bigotes A, Tamburrino Í, Maruri A. Thermal Vulnerability and Potential Cultivation Areas of Four Day-Neutral Strawberries in Chile: Implications for Climate Adaptation. Plants. 2025; 14(20):3205. https://doi.org/10.3390/plants14203205
Chicago/Turabian StyleSierra-Almeida, Angela, Loreto V. Morales, Diego Guerrero, Rodrigo J. N. Hasbún, Luis Retamal, Adrián Garrido-Bigotes, Ítalo Tamburrino, and Andrea Maruri. 2025. "Thermal Vulnerability and Potential Cultivation Areas of Four Day-Neutral Strawberries in Chile: Implications for Climate Adaptation" Plants 14, no. 20: 3205. https://doi.org/10.3390/plants14203205
APA StyleSierra-Almeida, A., Morales, L. V., Guerrero, D., Hasbún, R. J. N., Retamal, L., Garrido-Bigotes, A., Tamburrino, Í., & Maruri, A. (2025). Thermal Vulnerability and Potential Cultivation Areas of Four Day-Neutral Strawberries in Chile: Implications for Climate Adaptation. Plants, 14(20), 3205. https://doi.org/10.3390/plants14203205







