Advances in Research on the Biological Characteristics of Weedy Rice
Abstract
1. Introduction
2. Biological Characteristics of Weedy Rice
2.1. Phenotypic Diversity of Weedy Rice
2.2. Seed Shattering
2.3. Seed Dormancy
2.4. Strong Competitiveness
2.5. Stress Resistance
2.6. Early Maturity
3. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roma-Burgos, N.; Butts, T.R.; Werle, I.S.; Bottoms, S.; Mauromoustakos, A. Weedy rice update in Arkansas, USA, and adjacent locales. Weed Sci. 2021, 69, 514–525. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, V.; Saharawat, Y.S.; Gathala, M.; Ladha, J.K.; Chauhan, B.S. Weedy rice: An emerging threat for direct-seeded rice production systems in India. Rice Res. 2013, 1, 106–112. [Google Scholar] [CrossRef]
- Ziska, L.H.; Gealy, D.R.; Burgos, N.; Caicedo, A.L.; Gressel, J.; Lawton-Rauh, A.L.; Avila, L.A.; Theisen, G.; Norsworthy, J.; Ferrero, A.; et al. Chapter three—Weedy (red) rice: An emerging constraint to global rice production. Adv. Agron. 2015, 129, 181–228. [Google Scholar] [CrossRef]
- Karim, R.S.; Man, A.B.; Sahid, I.B. Weed problems and their management in rice fields of Malaysia: An overview. Weed Biol. Manag. 2004, 4, 177–186. [Google Scholar] [CrossRef]
- Jamjod, S.; Maneechote, C.; Pusadee, T.; Rerkasem, B. Emergence of invasive weedy rice in Southeast Asia. A review. Agron. Sustain. Dev. 2025, 45, 23. [Google Scholar] [CrossRef]
- Nadir, S.; Xiong, H.-B.; Zhu, Q.; Zhang, X.-L.; Xu, H.-Y.; Li, J.; Dongchen, W.; Henry, D.; Guo, X.-Q.; Khan, S.; et al. Weedy rice in sustainable rice production. A review. Agron. Sustain. Dev. 2017, 37, 46. [Google Scholar] [CrossRef]
- Vaughan, L.K.; Ottis, B.V.; Prazak-Havey, A.M.; Bormans, C.A.; Sneller, C.; Chandler, J.M.; Park, W.D. Is all red rice found in commercial rice really Oryza sativa? Weed Sci. 2001, 49, 468–476. [Google Scholar] [CrossRef]
- Olsen, K.M.; Caicedo, A.L.; Jia, Y. Evolutionary genomics of weedy rice in the USA. J. Integr. Plant Biol. 2007, 49, 811–816. [Google Scholar] [CrossRef]
- Shivrain, V.K.; Burgos, N.R.; Scott, R.C.; Gbur Jr, E.E.; Estorninos, L.E., Jr.; McClelland, M.R. Diversity of weedy red rice (Oryza sativa L.) in Arkansas, USA in relation to weed management. Crop Prot. 2010, 29, 721–730. [Google Scholar] [CrossRef]
- Baki, B.B.; Chin, D.V.; Mortimer, M. Wild and weedy rice in rice ecosystems in Asia—A review. Proc. Int. Rice Res. Inst. 2000, 124, 124. [Google Scholar]
- Xu, C.; Wu, W. Ecological investigating and identifying of weedy rices in hainan island. Chin. J. Rice Sci. 1996, 10, 247–249. [Google Scholar]
- Rathore, M.; Singh, R.; Kumar, B.; Chauhan, B. Characterization of functional trait diversity among Indian cultivated and weedy rice populations. Sci. Rep. 2016, 6, 24176. [Google Scholar] [CrossRef]
- Abraham, C.T.; Jose, N. Weedy rice invasion in rice fields of India and management options. J. Crop Weed 2014, 10, 365–374. [Google Scholar]
- Grimm, A.; Sahi, V.P.; Amann, M.; Vidotto, F.; Fogliatto, S.; Devos, K.M.; Ferrero, A.; Nick, P. Italian weedy rice—A case of de-domestication? Ecol. Evol. 2020, 10, 8449–8464. [Google Scholar] [CrossRef]
- Ratnasekera, D.; Perera, U.I.; He, Z.; Senanayake, S.G.; Wijesekara, G.A.; Yang, X.; Lu, B. High level of variation among Sri Lankan weedy rice populations, as estimated by morphological characterization. Weed Biol. Manag. 2014, 14, 68–75. [Google Scholar] [CrossRef]
- Juliano, L.M.; Donayre, D.K.M.; Martin, E.C.; Beltran, J.C. Weedy rice: An expanding problem in direct-seeded rice in the philippines. Weed Biol. Manag. 2020, 20, 27–37. [Google Scholar] [CrossRef]
- Prathepha, P. Seed morphological traits and genotypic diversity of weedy rice (Oryza sativa f. spontanea) populations found in the Thai Hom Mali rice fields of north-eastern Thailand. Weed Biol. Manag. 2009, 9, 1–9. [Google Scholar] [CrossRef]
- Nagao, S.; Takahashi, M. Trial construction of twelve linkage groups in Japanese rice. J. Fac. Agric. Hokkaido Univ. 1963, 53, 72–130. [Google Scholar]
- Akasaka, M.; Konishi, S.; Izawa, T.; Ushiki, J. Histological and genetic characteristics associated with the seed-shattering habit of weedy rice (Oryza sativa L.) from Okayama, Japan. Breed. Sci. 2011, 61, 168–173. [Google Scholar] [CrossRef]
- Merotto Jr, A.; Goulart, I.C.; Nunes, A.L.; Kalsing, A.; Markus, C.; Menezes, V.G.; Wander, A.E. Evolutionary and social consequences of introgression of nontransgenic herbicide resistance from rice to weedy rice in Brazil. Evol. Appl. 2016, 9, 837–846. [Google Scholar] [CrossRef]
- Olajumoke, B.; Juraimi, A.S.; Uddin, M.K.; Husni, M.H.; Alam, M.A. Competitive ability of cultivated rice against weedy rice biotypes: A review. Chil. J. Agric. Res. 2016, 76, 243–252. [Google Scholar] [CrossRef]
- Delouche, J.C.; Burgos, N.R.; Gealy, D.R.; de San Martin, G.Z.; Labrada, R.; Larinde, M.; Rosell, C. Weedy rices: Origin, biology, ecology and control. In FAO Plant Production and Protection Paper 188; FAO: Rome, Italy, 2007; pp. 3–15. [Google Scholar]
- Andres, A.; Fogliatto, S.; Bastiaans, L.; Vidotto, F. Dynamics of weedy rice soil seedbank under different control strategies in Italian rice fields: Survey and model study. Weed Sci. 2021, 69, 575–584. [Google Scholar] [CrossRef]
- Fogliatto, S.; Vidotto, F.; Ferrero, A. Germination of weedy rice in response to field conditions during winter. Weed Technol. 2011, 25, 252–261. [Google Scholar] [CrossRef]
- Kehinde, B.O.; Xie, L.; Song, B.K.; Zheng, X.; Fan, L. African cultivated, wild and weedy rice (Oryza spp.): Anticipating further genomic studies. Biology 2024, 13, 697. [Google Scholar] [CrossRef] [PubMed]
- Noldin, J.A.; Chandler, J.M.; McCAULEY, G.N. Red rice (Oryza sativa) biology. I. Characterization of red rice ecotypes. Weed Technol. 1999, 13, 12–18. [Google Scholar] [CrossRef]
- Arrieta-Espinoza, G.; Sanchez, E.; Vargas, S. The weedy rice complex in costa rica. i. morphological study of relationships between commercial rice varieties, wild oryza relatives and weedy types. Genet. Resour. Crop Evol. 2005, 52, 575–587. [Google Scholar] [CrossRef]
- Suh, H.S.; Sato, Y.I.; Morishima, H. Genetic characterization of weedy rice (Oryza sativa L.) based on morpho-physiology, isozymes and RAPD markers. Theor. Appl. Genet. 1997, 94, 316–321. [Google Scholar] [CrossRef]
- Ferrero, A.; Vidotto, F. Shattering ability of red rice seeds [Oryza sativa L. var. sylvatica a weedy biotype of cultivated rice] in cultural conditions. In Proceedings of the International Symposium on Crop Protection, Ghent, Belgium, 5 May 1998; Mededelingen-Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen Universiteit Gent: Ghent, Belgium, 1998; Volume 63. [Google Scholar]
- Ajaykumar, R.; Sivasabari, K.; Vigneshwaran, R.; Kumaresan, P. Weedy rice (Oryza sativa f. spontanea) complexes on direct seeded rice (dsr) and its management strategy: A review. Agric. Rev. 2025, 46, 62–69. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, J.; Foley, M.E.; Gu, X.Y. Comparative mapping of seed dormancy loci between tropical and temperate ecotypes of weedy rice (Oryza sativa L.). G3 Genes Genet. 2017, 7, 2605–2614. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, V.; Plaza, G.; Caicedo, A.L. Characterization of the phenotypic variability in Colombian weedy rice (Oryza spp.). Weed Sci. 2019, 67, 441–452. [Google Scholar] [CrossRef]
- Karn, E.; De Leon, T.; Espino, L.; Al-Khatib, K.; Brim-DeForest, W. Phenotypic diversity of weedy rice (Oryza sativa f. spontanea) biotypes found in California and implications for management. Weed Sci. 2020, 68, 485–495. [Google Scholar] [CrossRef]
- Lejr, E.; Gealy, D.R.; Gbur, E.E.; Talbert, R.E.; McClelland, M.R. Rice and red rice interference. II. Rice response to population densities of three red rice (Oryza sativa) ecotypes. Weed Sci. 2005, 53, 683–689. [Google Scholar] [CrossRef]
- Fogliatto, S.; Ferrero, A.; Vidotto, F. How can weedy rice stand against abiotic stresses? A review. Agronomy 2020, 10, 1284. [Google Scholar] [CrossRef]
- Wang, H.Q.; Dai, W.M.; Zhang, Z.X.; Li, M.S.; Meng, L.C.; Zhang, Z.; Lu, H.; Song, X.L.; Qiang, S. Occurrence Pattern and Morphological Polymorphism of Chinese Weedy Rice. J. Integr. Agric. 2023, 22, 149–169. [Google Scholar] [CrossRef]
- Yang, H.M.; Feng, L.; Tian, X.S.; Lu, Y.L.; Yue, M.F.; Yang, C.H.; Yu, L.Q. Biological characteristics of the weedy rice in Leizhou of Guangdong province. Guangdong Agric. Sci. 2011, 38, 95–98. [Google Scholar] [CrossRef]
- Zhang, Z.; Dai, W.M.; Zhang, C.B.; Qiang, S. Investigation of the biological characteristics and harmfulness of weedy rice (Oryza sativa L. f. spontanea) occurred in the regions along the Yangtze river of Jiangsu province. Sci. Agric. Sin. 2012, 45, 2856–2866. [Google Scholar] [CrossRef]
- Gao, Q.; Ma, D.R.; Kong, D.X.; Wang, W.J.; Tong, H.; Zhao, M.H.; Xu, Z.J.; Chen, W.F. Photosynthetic and water physiological characteristics of weedy rice in northern China. Chin. J. Appl. Ecol. 2013, 24, 3131–3136. [Google Scholar] [CrossRef]
- Sun, J.C.; Wang, X.L.; Ma, J.; Wang, X.S.; Yang, S.L. Analysis of Agronomic Characteristics of Ningxia Weedy Rice. Crops 2014, 22, 129–132. [Google Scholar] [CrossRef]
- Diarra, A.; Rjjr, S.; Talbert, R.E. Growth and morphological characteristics of red rice (Oryza sativa) biotypes. Weed Sci. 1985, 33, 310–314. [Google Scholar] [CrossRef]
- Kwon, S.L.; Rjjr, S.; Talbert, R.E. Comparative growth and development of red rice (Oryza sativa) and rice (O. sativa). Weed Sci. 1992, 40, 57–62. [Google Scholar] [CrossRef]
- Suh, H.S.; Park, S.Z.; Heu, M.H. Collection and evaluation of Korean red rices I. Regional distribution and seed characteristics. Korean J. Crop Sci. 1992, 37, 425–430. [Google Scholar]
- Dai, L.; Dai, W.M.; Song, X.L.; Lu, B.R.; Qiang, S. A comparative study of competitiveness between different genotypes of weedy rice (Oryza sativa) and cultivated rice. Pest Manag. Sci. 2014, 70, 113–122. [Google Scholar] [CrossRef]
- Dai, L.; Song, X.L.; He, B.Y.; Valverde, B.E.; Qiang, S. Enhanced photosynthesis endows seedling growth vigor contributing to the competitive dominance of weedy rice over cultivated rice. Pest Manag. Sci. 2017, 73, 1410–1420. [Google Scholar] [CrossRef]
- Thurber, C.S.; Reagon, M.; Olsen, K.M.; Jia, Y.; Caicedo, A.L. The evolution of flowering strategies in US weedy rice. Am. J. Bot. 2014, 101, 1737–1747. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, H.; Yang, Z.; Zhang, Z.; Li, M.; Zhang, Z.; Dai, W.; Song, X.; Olsen, K.M.; Qiang, S. Characterization of lodging variation of weedy rice. J. Exp. Bot. 2023, 74, 1403–1419. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Sharma, G.; Stallworth, S.; Redona, E.D.; Tseng, T.M. Exploring the genetic diversity among weedy rice accessions differing in herbicide tolerance and allelopathic potential. Diversity 2022, 14, 44. [Google Scholar] [CrossRef]
- Turra, G.M.; Li, X.; Nunes, A.L.; Markus, C.; Caicedo, A.L.; Merotto, A., Jr. Experimental methods for phenotypic and molecular analyses of seed shattering in cultivated and weedy rice. Adv. Weed Sci. 2024, 41, e020230049. [Google Scholar] [CrossRef]
- Li, X.; Lowey, D.; Lessard, J.; Caicedo, A.L. Comparative histology of abscission zones reveals the extent of convergence and divergence in seed shattering in weedy and cultivated rice. J. Exp. Bot. 2024, 75, 4837–4850. [Google Scholar] [CrossRef]
- Harlan, J.R.; de Wet, J.M.J.; Price, E.C. Comparative evolution of cereals. Evolution 1973, 27, 311–325. [Google Scholar] [CrossRef]
- Gao, P.L.; Zhang, Z.; Sun, G.J.; Yu, H.Y.; Qiang, S. The within-field and between-field dispersal of weedy rice by combine harvesters. Agron. Sustain. Dev. 2018, 38, 55. [Google Scholar] [CrossRef]
- Vidotto, F.; Ferrero, A. Germination behaviour of red rice (Oryza sativa L.) seeds in field and laboratory conditions. Agronomie 2000, 20, 375–382. [Google Scholar] [CrossRef]
- Sastry, M.V.S.; Seetharaman, R. Inheritance of grain shattering and lazy habit and their interrelationship in rice. Indian J. Genet. Plant Breed. 1978, 38, 318–321. [Google Scholar]
- Thurber, C.S.; Hepler, P.K.; Caicedo, A.L. Timing is everything: Early degradation of abscission layer is associated with increased seed shattering in US weedy rice. BMC Plant Biol. 2011, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Htun, T.M.; Inoue, C.; Chhourn, O.; Ishii, T.; Ishikawa, R. Effect of quantitative trait loci for seed shattering on abscission layer formation in Asian wild rice Oryza rufipogon. Breed. Sci. 2014, 64, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Li, C.B.; Zhou, A.L.; Sang, T. Rice domestication by reducing shattering. Science 2006, 311, 1936–1939. [Google Scholar] [CrossRef]
- Konishi, S.; Izawa, T.; Lin, S.Y.; Ebana, K.; Fukuta, Y.; Sasaki, T.; Yano, M. An SNP caused loss of seed shattering during rice domestication. Science 2006, 312, 1392–1396. [Google Scholar] [CrossRef]
- Lin, Z.; Griffith, M.E.; Li, X.; Zhu, Z.F.; Tan, L.B.; Fu, Y.C.; Zhang, W.X.; Wang, X.K.; Xie, D.X.; Sun, C.Q. Origin of seed shattering in rice (Oryza sativa L.). Planta 2007, 226, 11–20. [Google Scholar] [CrossRef]
- Wang, J.; Xu, X.; Yang, J.; Wang, F.Q.; Fan, F.J.; Zhu, J.Y.; Li, W.Q.; Zhong, W.G. Sequence analysis of genes associated with seed shattering of weedy rice in region of Jiangsu and northeast of China. Mol. Plant Breed. 2014, 12, 1097–1102. [Google Scholar]
- Zhu, Y.; Ellstrand, N.C.; Lu, B.R. Sequence polymorphisms in wild, weedy, and cultivated rice suggest seed-shattering locus sh4 played a minor role in Asian rice domestication. Ecol. Evol. 2012, 2, 2106–2113. [Google Scholar] [CrossRef]
- Wedger, M.J.; Olsen, K.M. Evolving insights on weedy rice. Ecol. Genet. Genom. 2018, 7, 23–26. [Google Scholar] [CrossRef]
- Akasaka, M.; Ushiki, J.; Iwata, H.; Ishikawa, R.; Ishii, T. Genetic relationships and diversity of weedy rice (Oryza sativa L.) and cultivated rice varieties in Okayama Prefecture, Japan. Breed. Sci. 2009, 59, 401–409. [Google Scholar] [CrossRef]
- Nunes, A.L.; Markus, C.; Delatorre, C.A.; Merotto, A. Nucleotide variability and gene expression reveal new putative genes related to seed shattering in weedy rice. Ann. Appl. Biol. 2015, 166, 39–52. [Google Scholar] [CrossRef]
- Ji, H.; Kim, S.R.; Kim, Y.H.; Kim, H.B.; Eun, M.Y.; Cha, Y.S.; Yun, D.; Ahn, B.O.; Lee, M.C.; Lee, G.S.; et al. Inactivation of the CTD phosphatase-like gene OsCPL1 enhances the development of the abscission layer and seed shattering in rice. Plant J. 2010, 61, 96–106. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, D.; Li, C.; Luo, J.; Zhu, B.F.; Zhu, J.; Han, B. Genetic control of seed shattering in rice by the APETALA2 transcription factor SHATTERING ABORTION1. Plant Cell 2012, 24, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Lang, H.; He, Y.; Li, F.; Ma, D.; Sun, J. Integrative hormone and transcriptome analysis underline the role of abscisic acid in seed shattering of weedy rice. Plant Growth Regul. 2021, 94, 261–273. [Google Scholar] [CrossRef]
- Yuan, X.D.; Zhao, G.C.; Liu, C.K.; Wu, M.G. Evaluation of main characteristics of weedy rice in the northeast of China. J. Jilin Agric. Sci. 2006, 31, 6–9. [Google Scholar]
- Xu, J.Y.; Wang, X.S.; Lai, C.K.; Wu, B.; Ma, J.; Sun, J.C. Research of habits and characteristics about survival and spread of weedy rice in Ningxia ii—Identification on seedling emergence and wintering ability of weedy rice seed. Seed 2015, 34, 88–90. [Google Scholar]
- Ma, J.; Sun, J.C.; Wu, B.; Wang, X.S. Research of habits and characteristics about survival and spread of weedy rice in Ningxia iii—Dormancy and viability of weedy rice seed. Seed 2016, 35, 75–77. [Google Scholar]
- Gu, X.Y.; Foley, M.E. Multiple loci and epistases control genetic variation for seed dormancy in weedy rice (Oryza sativa). Genetics 2004, 166, 1503–1516. [Google Scholar] [CrossRef]
- Yu, L.Q.; Mortimer, A.M.; Xu, A.S.; Lu, Y.L.; Zhou, Y.J. Stress-resistance of weedy rice Luolijing (Oryza sativa). Chin. J. Appl. Ecol. 2005, 16, 717–720. [Google Scholar]
- Fogliatto, S.; Vidotto, F.; Ferrero, A. Morphological characterisation of Italian weedy rice (Oryza sativa) populations. Weed Res. 2012, 52, 60–69. [Google Scholar] [CrossRef]
- Gu, X.Y.; Chen, Z.X.; Foley, M.E. Inheritance of seed dormancy in weedy rice. Crop Sci. 2003, 43, 835–843. [Google Scholar] [CrossRef]
- Gu, X.Y.; Kianian, S.F.; Foley, M.E. Seed dormancy imposed by covering tissues interrelates to shattering and seed morphological characteristics in weedy rice. Crop Sci. 2005, 45, 948–955. [Google Scholar] [CrossRef]
- Xia, H.B.; Xia, H.; Ellstrand, N.C.; Yang, C.; Lu, B.R. Rapid evolutionary divergence and ecotypic diversification of germination behavior in weedy rice populations. New Phytol. 2011, 191, 1119–1127. [Google Scholar] [CrossRef]
- Sugimoto, K.; Takeuchi, Y.; Ebana, K.; Miyao, A.; Hirochika, H.; Hara, N.; Yano, M. Molecular cloning of Sdr4, a regulator involved in seed dormancy and domestication of rice. Proc. Natl. Acad. Sci. USA 2010, 107, 5792–5797. [Google Scholar] [CrossRef]
- Gu, X.Y.; Foley, M.E.; Horvath, D.P.; Anderson, J.V.; Feng, J.H.; Zhang, L.H.; Mowry, C.R.; Ye, H.; Suttle, J.C.; Kadowaki, K.; et al. Association between seed dormancy and pericarp color is controlled by a pleiotropic gene that regulates abscisic acid and flavonoid synthesis in weedy red rice. Genetics 2011, 189, 1515–1524. [Google Scholar] [CrossRef]
- Nguyen, T.; Fu, K.; Mou, C.; Yu, J.; Zhu, X.; Huang, Y.; Zhou, C.; Hao, Q.; Zhang, F.; Song, W.; et al. Fine mapping of qSdr9, a novel locus for seed dormancy (SD) in weedy rice, and development of NILs with a strong SD allele. Mol. Breed. 2020, 40, 81. [Google Scholar] [CrossRef]
- Zulrushdi, A.Q.; Rejab, N.A.; Mispan, M.S. Seed dormancy status of tropical weedy rice population in Malaysia. Weed Res. 2022, 62, 277–286. [Google Scholar] [CrossRef]
- Jiang, X.Q.; Zhu, X.Y.; Lu, B.R. Soil burial induced dormancy in weedy rice seeds through hormone level changes: Implications in adaptive evolution and weed control. J. Syst. Evol. 2022, 60, 1049–1061. [Google Scholar] [CrossRef]
- Bakar, S.N.S.; Saiman, M.Z.; Mohamed, Z.; Mispan, M.S. Impact of burial and duration on seed viability of weedy rice. Int. J. Pest Manag. 2025, 61, 1–7. [Google Scholar] [CrossRef]
- Fukuda, M.; Imaizumi, T.; Koarai, A. Seed germination responses to temperature and water availability in weedy rice. Pest Manag. Sci. 2023, 79, 870–880. [Google Scholar] [CrossRef]
- Ma, D.R.; Ding, G.H.; Liu, X.L.; Tang, L.; Yang, Q.; Xu, Z.J.; Chen, W.F. Effects of weedy rice interference on dry matter accumulation and grain filling of the cultivated rice. J. Shenyang Agric. Univ. 2012, 43, 736–740. [Google Scholar]
- Wang, N.; Ma, D.R.; Jia, D.T.; Wang, Y.; Chen, W.F. Germination characteristics of northern weedy Rice. J. Huazhong Agric. Univ. 2007, 26, 755–758. [Google Scholar]
- Dai, L.; Dai, W.M.; Song, X.L.; Qiang, S. Diversity of Botanical Characters of Weedy Rice (Oryza sativa L. f. spontanea) in Jiangsu Province. Weed Sci. 2014, 32, 10–18. [Google Scholar]
- Andres, A.; Fogliatto, S.; Ferrero, A.; Vidotto, F. Growth variability of Italian weedy rice populations grown with or without cultivated rice. Crop Sci. 2015, 55, 394–402. [Google Scholar] [CrossRef]
- Jindalouang, R.; Maneechote, C.; Prom-u-Thai, C.; Rerkasem, B.; Jamjod, S. Growth and nutrients competition between weedy rice and crop rice in a replacement series study. Int. J. Agric. Biol. 2018, 20, 784–790. [Google Scholar]
- Schaedler, C.; Lubian, W.; Lima, P.; Chiapinotto, D. Relative competitiveness between cultivated and weedy rice under full and low light. Planta Daninha 2020, 38, e020189605. [Google Scholar] [CrossRef]
- Balbinot, A.; Viana, V.E.; Feijó, A.R.; Fipke, M.V.; Heck, T.; Ziska, L.H.; Markus, C.; Merotto, A.; Avila, L.A. Elevated CO2 concentration enhances Oryza sativa seed shattering and affects seed shattering gene expression. Weed Res. 2023, 63, 207–217. [Google Scholar] [CrossRef]
- Miao, W.; Wang, G.J.; Ma, D.R.; Wang, J.Y.; Xu, Z.J.; Chen, W.F. Physiological responses of weedy rice to cold stress at seedling stage in Liaoning province, China. China J. Rice Sci. 2011, 25, 639–644. [Google Scholar]
- Gao, N.; Ma, D.R.; Chen, W.F. Preliminary study on the cold tolerance of seed germination in northern weedy rice. China Rice 2007, 3, 5–7. [Google Scholar]
- Zhang, Z.L.; Tan, X.L.; Deng, A.F. Comprehensive evaluation of weedy rice germplasm resources. J. Plant Genet. Resour. 2002, 3, 47–50. [Google Scholar]
- Shao, G.S.; Xie, Z.K.; Zhang, G.P. Different responses to cadmium stress in nitrogen metabolism between weedy rice and cultivated rice (Oryza sativa). Chin. J. Rice Sci. 2006, 20, 189–193. [Google Scholar]
- Yang, Y.; Jung, J.Y.; Song, W.Y.; Suh, H.S.; Lee, Y. Identification of rice varieties with high tolerance or sensitivity to lead and characterization of the mechanism of tolerance. Plant Physiol. 2000, 124, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Ku, Y.C.; Kim, K.H.; Hahn, S.J.; Chung, I.M. Allelopathic potential of rice germplasm against barnyardgrass. Allelopath. J. 2004, 13, 17–28. [Google Scholar]
- Chen, H.Z.; Xuan, S.N.; Wang, W.X.; Shao, G.S.; Sun, Z.X. Freezing tolerance and germination ability at low temperature of dandong weedy rice. Chin. J. Rice Sci. 2004, 18, 109–112. [Google Scholar]
- Han, Y.H.; Li, X.Y.; Xie, H.J.; Dai, W.M.; Qiang, S. Adaptability of rice (Oryza sativa) varieties to cold tolerance associated with methylation variation of ice1 gene. J. Agric. Biotechnol. 2017, 25, 1381–1390. [Google Scholar]
- Yang, J.L.; Qiang, S.; Zhang, B.H.; Song, X.L.; Shi, Z.H.; Jiang, Q.; Dai, W.M. Cold tolerance of seed germination for weedy rice populations in China. J. Plant Genet. Resour. 2017, 18, 1–9. [Google Scholar] [CrossRef]
- Xie, H.; Han, Y.; Li, X.; Dai, W.; Song, X.; Olsen, K.M.; Qiang, S. Climate-dependent variation in cold tolerance of weedy rice and rice mediated by OsICE1 promoter methylation. Mol. Ecol. 2019, 29, 121–137. [Google Scholar] [CrossRef]
- Baek, J.S.; Chung, N.J. Seed wintering and deterioration characteristics between weedy and cultivated rice. Rice 2012, 5, 21. [Google Scholar] [CrossRef]
- Zhao, N.; Ma, D.R.; Chen, W.F. Preliminary evaluation of salt tolerance at germination stage of northern weedy rice. China Rice 2007, 13, 20–24. [Google Scholar]
- Suh, J.P.; Sang, N.A.; Choi, I.S. Identification of QTLs for cold tolerance at seedling stage of Korean weedy rice. Korean J. Breed. 2003, 35, 96–101. [Google Scholar]
- Balbinot, A.; Feijó, A.d.R.; Fipke, M.V.; Gehrke, V.R.; Agostinetto, D.; Kruse, N.D.; Ziska, L.H.; Camargo, E.R.; de Avila, L.A. Rising atmospheric CO2 concentration affect weedy rice growth, seed shattering and seedbank longevity. Weed Res. 2022, 62, 277–286. [Google Scholar] [CrossRef]
- Wang, Y.; Mo, S.-D.; Kong, M.-Y.; Chao, J.; Chen, X.-F.; Yang, J.-L.; Yan, Y.-J.; Shi, Z.-H.; Qiang, S.; Song, X.-L.; et al. Better performance of germination in hyperosmotic solutions in conspecific weedy rice than cultivated rice. J. Syst. Evol. 2019, 57, 519–529. [Google Scholar] [CrossRef]
- Puteh, A.B.; Jali, N.; Ismail, M.R.; Juraimi, A.S.; Samsudin, N. Pollen and seed yield components of water-stressed cultivated and weedy rice. Pertanika J. Trop. Agric. Sci. 2009, 32, 293–303. [Google Scholar]
- Jeong, J.-M.; Cho, Y.-C.; Jeong, J.-U.; Mo, Y.-J.; Kim, C.-S.; Kim, W.-J.; Baek, M.-K.; Kim, S.-M. QTL mapping and effect confirmation for anaerobic germination tolerance derived from the japonica weedy rice landrace PBR. Plant Breed. 2020, 139, 83–92. [Google Scholar] [CrossRef]
- Zou, D.T. Characteristic of weedy rice and cold tolerance in Heilongjiang province. Res. Agric. Modern. 2008, 29, 235–238. [Google Scholar]
- Jia, Y.; Gealy, D. Weedy red rice has novel sources of resistance to biotic stress. Crop J. 2018, 6, 443–450. [Google Scholar] [CrossRef]
- Mohd Hanafiah, N.; Mispan, M.S.; Lim, P.E.; Baisakh, N.; Cheng, A. The 21st century agriculture: When rice research draws attention to climate variability and how weedy rice and underutilized grains come in handy. Plants 2020, 9, 365. [Google Scholar] [CrossRef]
- Ruzmi, R.; Ahmad-Hamdani, M.S.; Abidin, M.Z.Z.; Roma-Burgos, N. Evolution of imidazolinone-resistant weedy rice in Malaysia: The current status. Weed Sci. 2021, 69, 598–608. [Google Scholar] [CrossRef]
- Dilipkumar, M.; Burgos, N.R.; Chuah, T.S.; Ismail, S. Cross-resistance to imazapic and imazapyr in a weedy rice (Oryza sativa) biotype found in Malaysia. Planta Daninha 2018, 36, e018182239. [Google Scholar] [CrossRef]
- Li, J.; Yu, J.; Yin, S.; Gao, H.; Hou, X.; Dong, L. Target site–resistance mechanisms to imazamox in imidazolinone herbicide-resistant weedy rice (Oryza sativa f. spontanea) in China. Weed Sci. 2025, 73, e78. [Google Scholar] [CrossRef]
- Goulart, I.C.; Borba, T.C.; Menezes, V.G.; Merotto, A., Jr. Distribution of weedy red rice (Oryza sativa) resistant to imidazolinone herbicides and its relationship to rice cultivars and wild Oryza species. Weed Sci. 2014, 62, 280–293. [Google Scholar] [CrossRef]
- Wu, C.; Dai, W.M.; Song, X.L.; Qiang, S. Diversity of plant traits of weedy rice in Liaoning and Jiangsu provinces. Biodivers. Sci. 2010, 18, 29–36. [Google Scholar] [CrossRef]
- Sun, J.C.; Li, Y.H.; Wang, X.S.; Wu, B.; Ma, J. Comparison of biological characteristics between weedy rice and cultivated rice in Ningxia. J. Jiangsu Agric. Sci. 2015, 43, 158–161. [Google Scholar]
- Sun, J.D.; Xiao, Y.C.; Huang, X.F.; Jiao, W.C.; Chen, J.C.; Zhou, Y.Y. Preliminary study on the occurrence characteristics and control techniques of weedy rice in medium japonica rice fields. Weed Sci. 2005, 23, 21–23. [Google Scholar]
- Xu, S.L.; Chen, D.H.; Li, Q.; Zhang, P. Occurrence patterns and control techniques of weedy rice in paddy fields. Crop Res. 2007, 21, 35–37. [Google Scholar]
- Xu, W.R.; Zhao, C.; Qiang, S.; Dai, W.M.; Song, X.L. The chlorophyll fluorescence characteristics of flag leaf of weedy rice during grain filling. Plant Physiol. J. 2017, 53, 1742–1752. [Google Scholar] [CrossRef]
- Ouk, M.; Basnayake, J.; Tsubo, M.; Fukai, S.; Fischer, K.; Cooper, M.; Nesbitt, H. Competitive N uptake between rice and weedy rice. Field Crops Res. 2006, 99, 96–105. [Google Scholar] [CrossRef]
- Ahmed, Q.N.; Hussain, P.M.D.Z.; Othman, A.S. Comparative study on vegetative and reproductive development between weedy rice morphotypes and commercial rice varieties in Perak, Malaysia. Trop. Life Sci. Res. 2012, 23, 17–25. [Google Scholar]
- Sánchez-Olquín, E.; Arrieta-Espinoza, G.; Espinoza Esquivel, A.M. Vegetative and reproductive development of Costa Rican weedy rice compared with commercial rice (Oryza sativa). Planta Daninha 2007, 25, 13–23. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Zang, L.; Du, W.; Hou, Y.; Ruan, C.; Desneux, N. Early flowering and rapid grain filling determine early maturity and escape from harvesting in weedy rice. Pest Manag. Sci. 2018, 74, 465–476. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, W.; Meng, L.; Qiang, S.; Dai, W.; Zhang, Z.; Song, X. Rapid endosperm development promotes early maturity in weedy rice (Oryza sativa f. spontanea). Weed Sci. 2020, 68, 168–178. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, W.; Li, H.; Dai, W.; Zhang, Z.; Qiang, S.; Song, X. The rapid cytological process of grain determines early maturity in weedy rice. Front. Plant Sci. 2021, 12, 711321. [Google Scholar] [CrossRef]
- Grime, J.P. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. Am. Nat. 1977, 111, 1169–1194. [Google Scholar] [CrossRef]
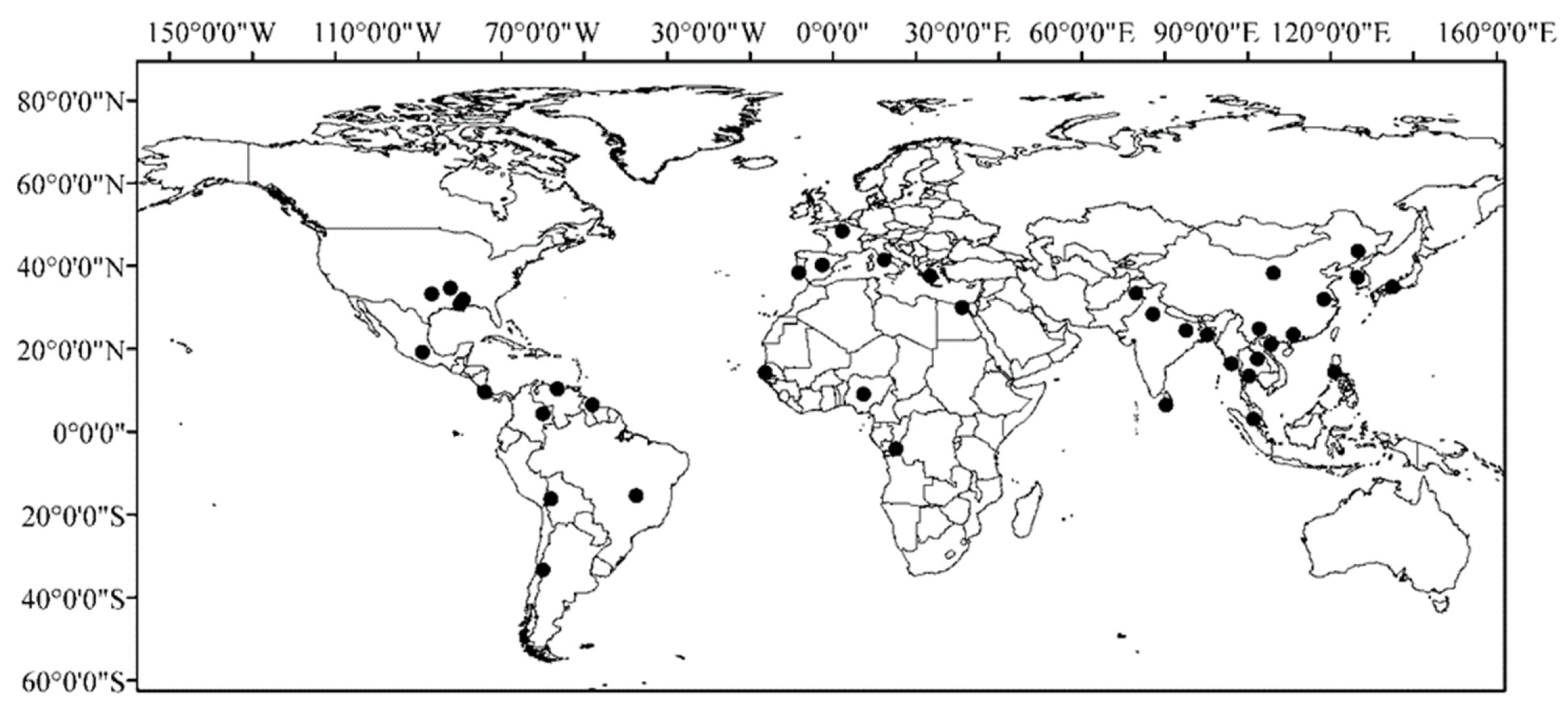

| Region | Country/Area | Occurrence Characteristics | Biological Traits | Yield Impact |
|---|---|---|---|---|
| Asia | China | Widely distributed in Northeast, Yangtze River Basin, and South China; more severe in direct-seeded fields | High genetic and phenotypic diversity; early maturity; strong seed shattering | 10–60% yield loss, quality deterioration [3,5,11] |
| India | Common across major rice-growing states; associated with direct seeding practices | Rapid seed germination, dormancy variation | Up to 60% yield loss [2,3,12,13,14] | |
| Sri Lanka | Severe in traditional paddy fields; resistant to manual weeding | Strong seed shattering, seed dormancy, mimicry with cultivated rice | 20–40% yield loss [15] | |
| Philippines | Widely present in irrigated and rainfed lowlands | Strong competitiveness, high tillering ability | 15–40% yield loss [5,10,16] | |
| Thailand | Serious problem in direct-seeded rice systems | Early heading, high tiller production | 30–60% yield loss [5,10,17] | |
| Vietnam | High infestation in Mekong Delta; worsened by direct seeding | Fast growth, strong competitiveness | 20–50% yield loss [5,10] | |
| Malaysia | Severe in Peninsular Malaysia; linked to commercial seed contamination | High shattering, mimicry with cultivated rice | 25–60% yield loss [3,4] | |
| Myanmar | Widespread in lowland rice areas | Early maturity, rapid seedling growth | 20–40% yield loss [10] | |
| Korea and Japan | Present but less severe than SE Asia | Limited genetic variation, shattering types | 10–20% yield loss [10,18,19] | |
| Americas | USA | Severe in southern rice belt (Arkansas, Louisiana, Texas, Mississippi) | Known as red rice; high shattering, red pericarp | 20–80% yield loss [1,6,7,8,9] |
| Brazil | Serious in southern states (Rio Grande do Sul) | Herbicide-resistant populations, red pericarp | 30–70% yield loss [20] | |
| Colombia, Cuba, Venezuela | Common in direct-seeded rice | Mimicry, dormancy, rapid seedling growth | 20–50% yield loss [21] | |
| Europe | Italy | Major issue in Po Valley rice areas (riso crodo) | Morphological mimicry, high shattering | 30–60% yield loss, quality decline [22,23] |
| Spain | Present in Ebro Delta and other rice regions | Mimicry, strong competitiveness | 20–40% yield loss [22,24] | |
| Africa | Nigeria | Infests irrigated and upland rice | Diverse biotypes, shattering | 20–40% yield loss [25] |
| Senegal, Mali | Serious in traditional paddy systems | Dormancy, shattering, competitiveness | 15–35% yield loss [22,25] |
| Gene Name | Pathway/Mechanism | Function and Effect in Weedy Rice | Comparative Note (vs. Cultivated Rice) |
|---|---|---|---|
| qSH1 [59,60,61] | Abscission Layer (AZ) Development | A major regulator promoting the formation of a complete and functional abscission layer. | Cultivated rice often has non-functional alleles of qSH1, leading to an underdeveloped AZ. |
| SH4 [62,63] | Abscission Layer (AZ) Development | Transcription factor that initiates the development of the abscission layer. | A key domestication gene. Mutations in SH4 are responsible for non-shattering in most cultivated rice. |
| OsCPL1 [64,65,66] | Cell Wall Degradation | Likely involved in the process of cell separation within the abscission zone. Expression is significantly higher in weedy rice. | Lower expression in cultivated rice contributes to the failure of the AZ to break down. |
| OsXTH8 and OsCel9D [64] | Cell Wall Modification and Degradation | Encode enzymes (xyloglucan endotransglucosylase/hydrolase and cellulase) that directly break down cell walls in the AZ, facilitating cell separation. | Differential expression in weedy rice is crucial for its high shattering ability. |
| SHAT1 [65,66] | Abscission Layer Development | Transcription factor that affects the formation of the abscission layer. | Participates in the regulatory network controlling AZ development. |
| ABA [67] | Hormonal Signaling | Abscisic Acid (ABA) facilitates shedding by creating a hormonal imbalance that activates the abscission process. | Demonstrates the complex hormonal control beyond genetic factors that favors shattering in weedy rice. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, X.; Zhao, C.; Liu, K.; Wang, W.; Huo, Z.; Song, X.; Qiang, S. Advances in Research on the Biological Characteristics of Weedy Rice. Plants 2025, 14, 3188. https://doi.org/10.3390/plants14203188
Liang X, Zhao C, Liu K, Wang W, Huo Z, Song X, Qiang S. Advances in Research on the Biological Characteristics of Weedy Rice. Plants. 2025; 14(20):3188. https://doi.org/10.3390/plants14203188
Chicago/Turabian StyleLiang, Xingyi, Can Zhao, Kunlun Liu, Weiling Wang, Zhongyang Huo, Xiaoling Song, and Sheng Qiang. 2025. "Advances in Research on the Biological Characteristics of Weedy Rice" Plants 14, no. 20: 3188. https://doi.org/10.3390/plants14203188
APA StyleLiang, X., Zhao, C., Liu, K., Wang, W., Huo, Z., Song, X., & Qiang, S. (2025). Advances in Research on the Biological Characteristics of Weedy Rice. Plants, 14(20), 3188. https://doi.org/10.3390/plants14203188







