Overexpression of VtF3′5′H and RhNHX Genes Alters Flower Color and Plant Morphology in Transgenic Rose ‘Red Farm’
Abstract
1. Introduction
2. Results
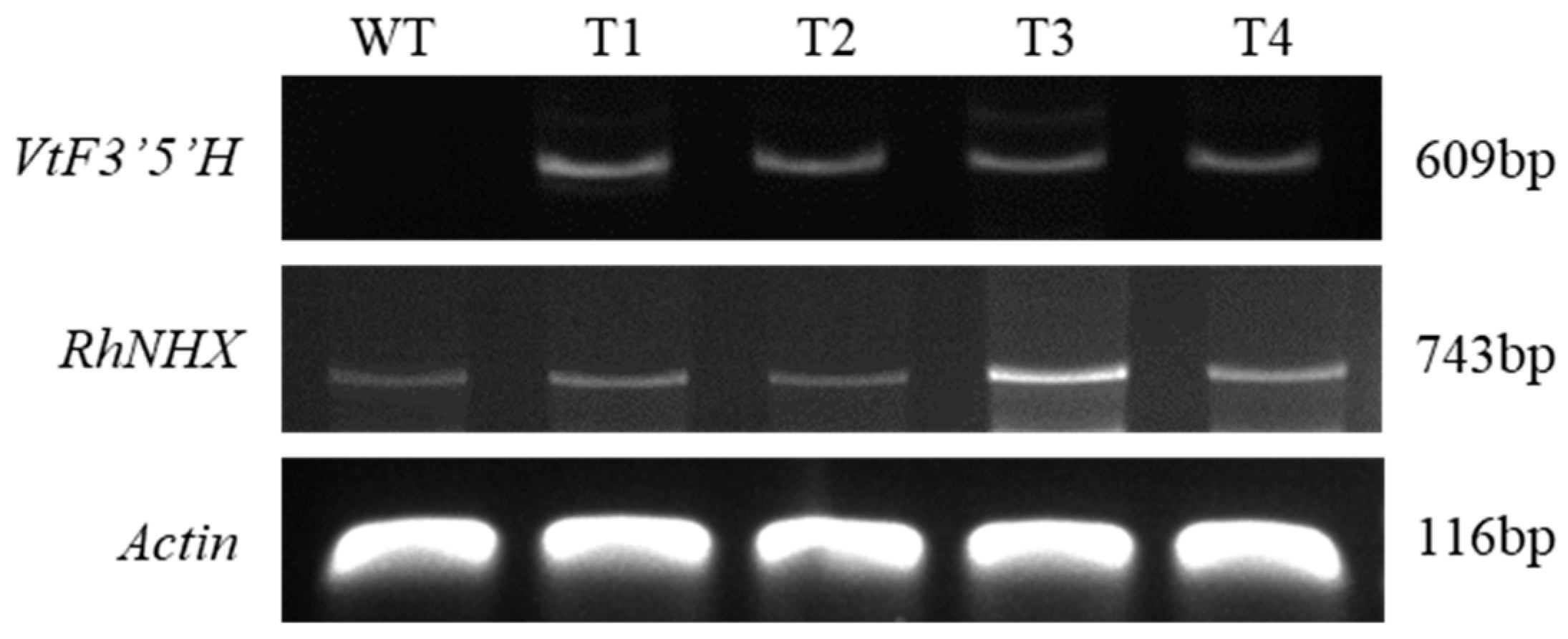
2.1. Confirmation of Gene Introduction in Transgenic Plants
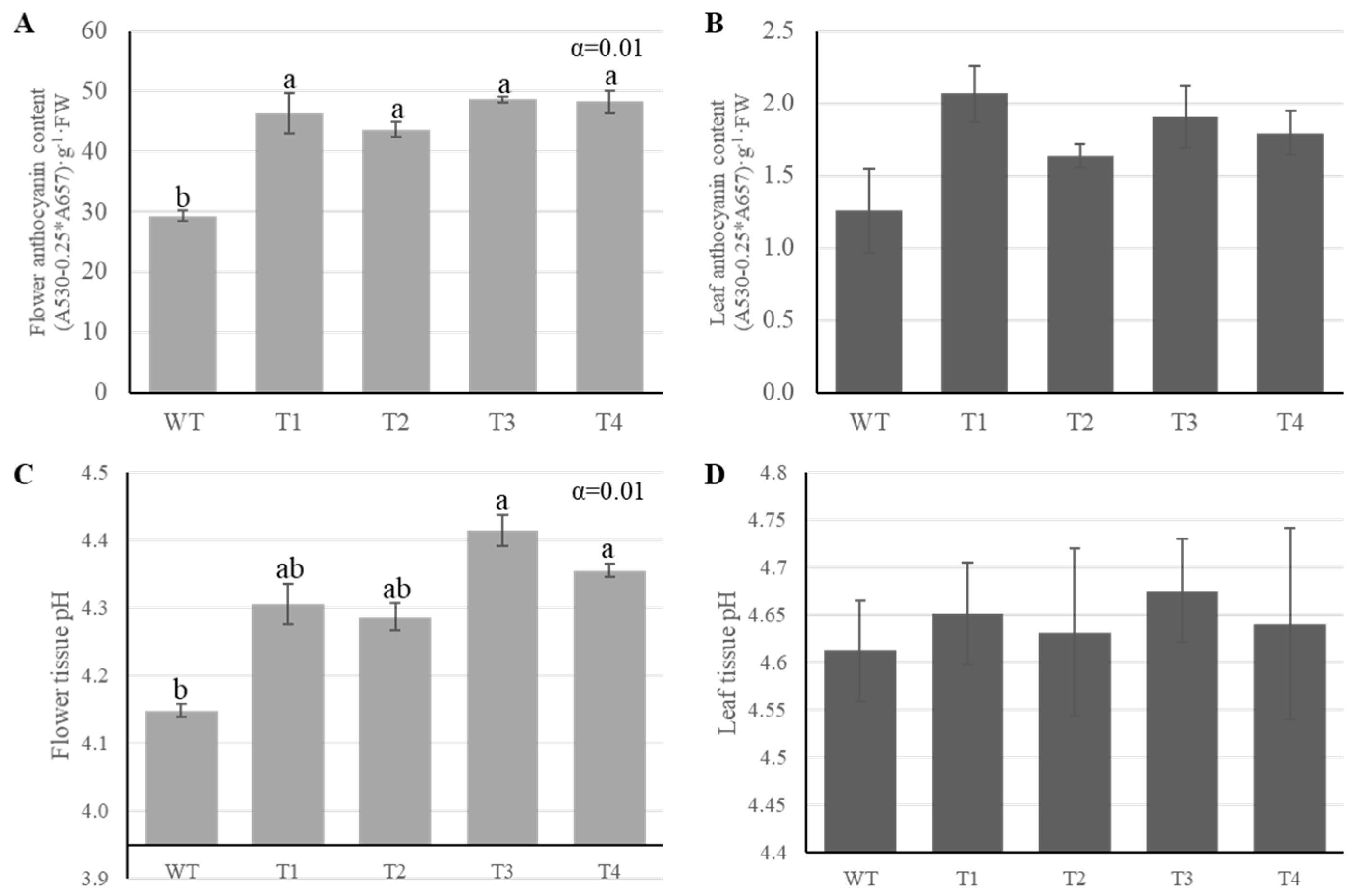
2.2. Anthocyanin Accumulation and Petal pH Changes
2.3. Growth Trait Changes in Transgenic Plant
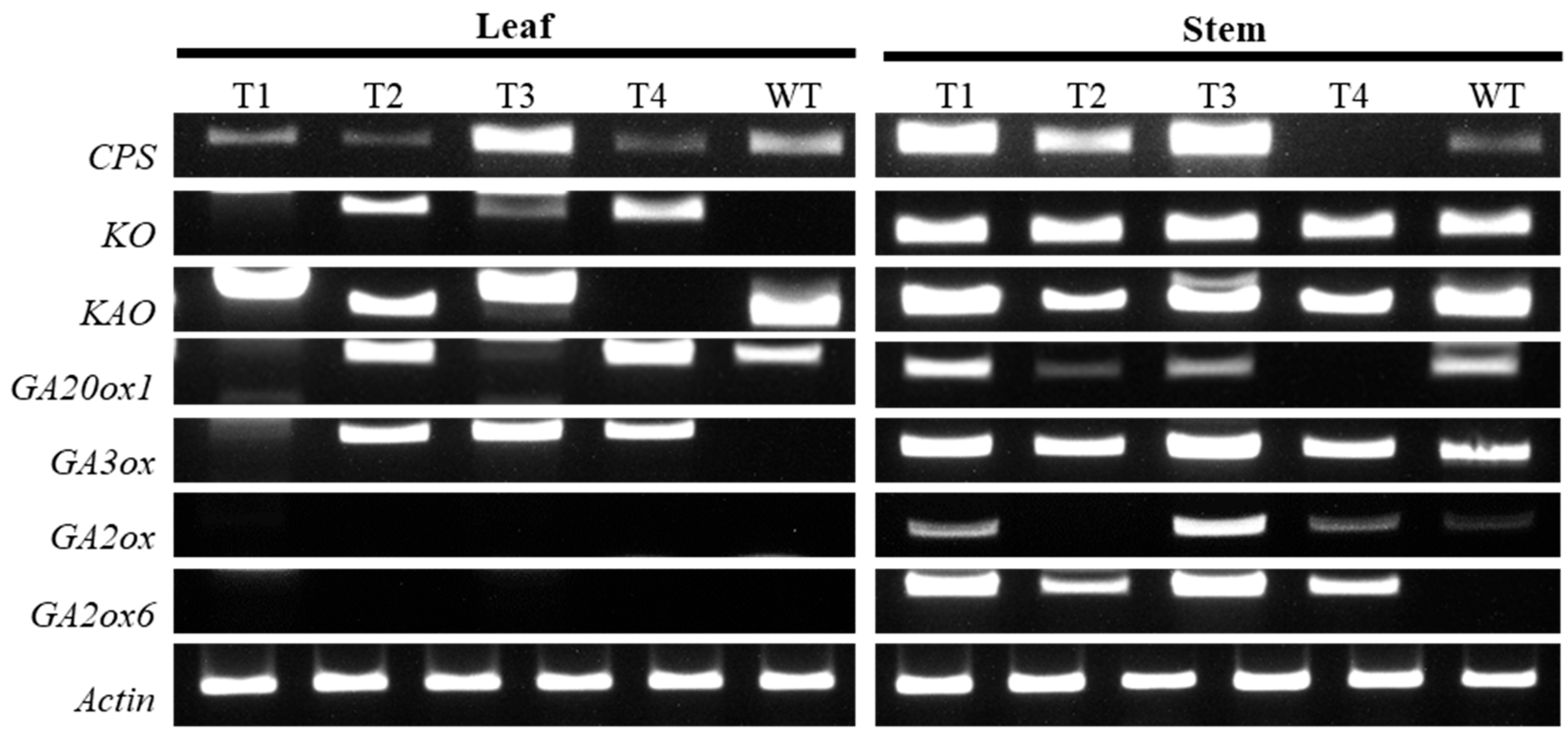
2.4. Expression of Genes Related to GA Metabolism
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Culture Conditions
4.2. Preparation of Transformation Plasmids
4.3. Agrobacterium Cultivation and Co-Culture
4.4. PCR Confirmation and Acclimatization of Transgenic Plants
4.5. Analysis of Total Anthocyanin and pH
4.6. Phenotypic Characteristics of Transgenic Plants
4.7. Gene Expression Analysis of the Gibberellin Biosynthesis Pathway
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Behe, B.K.; Nelson, R.G.; Barton, S.S.; Hall, C.R.; Safley, C.D.; Turner, S. Consumer preferences for geranium flower color, leaf variegation, and price. HortScience 1999, 34, 740–742. [Google Scholar] [CrossRef]
- Yue, C.; Behe, B.K. Consumers’ Preferences for Cut-Flower Color on Calendar and Noncalendar Occasions. HortScience 2010, 45, 78–82. [Google Scholar] [CrossRef]
- Ellis, L.; Ficek, C. Color preferences according to gender and sexual orientation. Personal. Individ. Differ. 2001, 31, 1375–1379. [Google Scholar] [CrossRef]
- Debener, T.; Mattiesch, L. Construction of a genetic linkage map for roses using RAPD and AFLP markers. Theor. Appl. Genet. 1999, 99, 891–899. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F. Flower colour and cytochromes P450. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120432. [Google Scholar] [CrossRef]
- Noda, N. Recent advances in the research and development of blue flowers. Breed. Sci. 2018, 68, 79–87. [Google Scholar] [CrossRef]
- Kong, J.M.; Chia, L.S.; Goh, N.K.; Chia, T.F.; Brouillard, R. Analysis and biological activities of anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Zhao, J.; Dixon, R.A. The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci. 2010, 15, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Ishiguro, K.; Taniguchi, M.; Tanaka, Y. Functional analysis of Antirrhinum kelloggii flavonoid 3′-hydroxylase and flavonoid 3′, 5′-hydroxylase genes; critical role in flower color and evolution in the genus Antirrhinum. J. Plant Res. 2012, 125, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y. Flower colour and cytochromes P450. Phytochem. Rev. 2006, 5, 283–291. [Google Scholar] [CrossRef]
- Katsumoto, Y.; Fukuchi-Mizutani, M.; Fukui, Y.; Brugliera, F.; Holton, T.A.; Karan, M.; Nakamura, N.; Yonekura-Sakakibara, K.; Togami, J.; Pigeaire, A.; et al. Engineering of the rose flavonoid biosynthetic pathway successfully generated blue-hued flowers accumulating delphinidin. Plant Cell Physiol. 2007, 48, 1589–1600. [Google Scholar] [CrossRef]
- Nakatsuka, T.; Mishibaa, K.I.; Abe, Y.; Kubota, A.; Kakizaki, Y.; Yamamura, S.; Nishihara, M. Flower color modification of gentian plants by RNAi-mediated gene silencing. Plant Biotechnol. 2008, 25, 61–68. [Google Scholar] [CrossRef]
- Holton, T.A.; Tanaka, Y. Blue roses—A pigment of our imagination. Trends Biotechnol. 1994, 12, 40–42. [Google Scholar] [CrossRef]
- Tanaka, Y.; Brugliera, F.; Chandler, S. Recent progress of flower colour modification by biotechnology. Int. J. Mol. Sci. 2009, 10, 5350–5369. [Google Scholar] [CrossRef]
- He, H.; Ke, H.; Keting, H.; Qiaoyan, X.; Silan, D. Flower Colour Modification of Chrysanthemum by Suppression of F3′H and Overexpression of the Exogenous Senecio cruentus F3′5′H Gene. PLoS ONE 2013, 8, e74395. [Google Scholar] [CrossRef]
- Noda, N.; Yoshioka, S.; Kishimoto, S.; Nakayama, M.; Douzono, M.; Tanaka, Y.; Aida, R. Generation of blue chrysanthemums by anthocyanin B-ring hydroxylation and glucosylation and its coloration mechanism. Sci. Adv. 2017, 3, e1602785. [Google Scholar] [CrossRef]
- Han, X.; Luo, Y.; Lin, J.; Wu, H.; Sun, H.; Zhou, L.; Chen, S.; Guan, Z.; Fang, W.; Zhang, F.; et al. Generation of purple-violet chrysanthemums via anthocyanin B-ring hydroxylation and glucosylation introduced from Osteospermum hybrid F3′5′H and Clitoria ternatea A3′5′GT. Ornam. Plant Res. 2021, 1, 4. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Fukada-Tanaka, S.; Inagaki, Y.; Saito, N.; Yonekura-Sakakibara, K.; Tanaka, Y.; Kusumi, T.; Iida, S. Genes Encoding the Vacuolar Na+/H+ Exchanger and Flower Coloration. Plant Cell Physiol. 2001, 42, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Mori, M.; Kondo, T. Blue flower color development by anthocyanins: From chemical structure to cell physiology. Nat. Prod. Rep. 2009, 26, 884–915. [Google Scholar] [CrossRef] [PubMed]
- Stavenga, D.G.; Leertouwer, H.L.; Dudek, B.; Van der Kooi, C.J. Coloration of flowers by flavonoids and consequences of pH dependent absorption. Front. Plant Sci. 2021, 11, 600124. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Ko, M.J.; Chung, M.S. Anthocyanin structure and pH dependent extraction characteristics from blueberries (Vaccinium corymbosum) and chokeberries (Aronia melanocarpa) in subcritical water state. Foods 2021, 10, 527. [Google Scholar] [CrossRef]
- Fukada-Tanaka, S.; Inagaki, Y.; Yamaguchi, T.; Saito, N.; Iida, S. Colour-enhancing protein in blue petals. Nature 2000, 407, 581. [Google Scholar] [CrossRef]
- Pardo, J.M.; Cubero, B.; Leidi, E.O.; Quintero, F.J.; Mendoza, I. Alkali cation exchangers: Roles in cellular homeostasis and stress tolerance. J. Exp. Bot. 2006, 57, 1181–1199. [Google Scholar] [CrossRef]
- Blumwald, E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef]
- Leidi, E.O.; Barragán, V.; Rubio, L.; El-Hamdaoui, A.; Ruiz, M.T.; Cubero, B.; Fernández, J.A.; Bressan, R.A.; Hasegawa, P.M.; Quintero, F.J.; et al. The AtNHX1 exchanger mediates potassium compartmentation in vacuoles of transgenic tomato. Plant J. 2010, 61, 495–506. [Google Scholar] [CrossRef]
- Rodríguez-Rosales, M.P.; Gálvez, F.J.; Huertas, R.; Aranda, M.N.; Baghour, M.; Cagnac, O.; Venema, K. Plant NHX cation/proton antiporters. Plant Signal. Behav. 2009, 4, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Bassil, E.; Tajima, H.; Liang, Y.C.; Ohto, M.A.; Ushijima, K.; Nakano, R.; Esumi, T.; Coku, A.; Belmonte, M.; Blumwald, E. The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome-associated and necessary for plant growth and development. Plant Cell 2011, 23, 224–239. [Google Scholar] [CrossRef]
- Wang, B.; Wang, H.; Liu, M.; He, G.; Ming, F. The vacuole pH-related gene RcNHX2 affects flower color shift and Na+ homeostasis in roses. Plant Sci. 2025, 357, 112476. [Google Scholar] [CrossRef]
- Xu, Q.; Xia, M.; He, G.; Zhang, Q.; Meng, Y.; Ming, F. New insights into the influence of NHX-type Cation/H+ antiporter on flower color in Phalaenopsis orchids. J. Plant Physiol. 2022, 279, 153857. [Google Scholar] [CrossRef]
- Xu, J.; Shin, J.Y.; Park, P.M.; An, H.R.; Kim, Y.J.; Kim, S.J.; Lee, S.Y. Flower color modification through co-overexpression of the VtF3′5′H and RhNHX genes in Rosa hybrida. Plant Cell Tissue Organ Cult. 2023, 153, 403–416. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem. Eng. J. 2003, 14, 217–225. [Google Scholar] [CrossRef]
- Castaneda-Ovando, A.; Pacheco-Hernandez, M.L.; Paez-Hernandez, M.E.; Rodriguez, J.A.; Galan-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P.; Sponsel, V. A century of gibberellin research. J. Plant Growth Regul. 2015, 34, 740–760. [Google Scholar] [CrossRef]
- Sakamoto, T.; Kobayashi, M.; Itoh, H.; Tagiri, A.; Kayano, T.; Tanaka, H.; Iwahori, S.; Matsuoka, M. Expression of a gibberellin 2-oxidase gene around the shoot apex is related to phase transition in rice. Plant Physiol. 2001, 125, 1508–1516. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.P. The molecular mechanism and evolution of the GA–GID1–DELLA signaling module in plants. Curr. Biol. 2011, 21, R338–R345. [Google Scholar] [CrossRef]
- Depuydt, S.; Hardtke, C.S. Hormone signalling crosstalk in plant growth regulation. Curr. Biol. 2011, 21, R365–R373. [Google Scholar] [CrossRef]
- Weiss, D.; Ori, N. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiol. 2007, 144, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signalling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Davière, J.M.; Achard, P. A pivotal role of DELLAs in regulating multiple hormone signals. Mol. Plant 2016, 9, 10–20. [Google Scholar] [CrossRef]
- Gaxiola, R.A.; Li, J.; Undurraga, S.; Dang, L.M.; Allen, G.J.; Alper, S.L.; Fink, G.R. Drought- and salt-tolerant plants result from overexpression of the AVP1 H+-pump. Proc. Natl. Acad. Sci. USA 2001, 98, 11444–11449. [Google Scholar] [CrossRef]
- Debener, T.; Linde, M. Exploring Complex Ornamental Genomes: The Rose as a Model Plant. Crit. Rev. Plant Sci. 2009, 28, 267–280. [Google Scholar] [CrossRef]
- Bendahmane, M.; Dubois, A.; Raymond, O.; Bris, M. Genetics and genomics of flower initiation and development in roses. J. Exp. Bot. 2013, 64, 847–857. [Google Scholar] [CrossRef]
- Quattrocchio, F.; Verweij, W.; Kroon, A.; Spelt, C.; Mol, J.; Koes, R. PH4 of Petunia Is an R2R3 MYB Protein That Activates Vacuolar Acidification through Interactions with Basic-Helix-Loop-Helix Transcription Factors of the Anthocyanin Pathway. Plant Cell 2006, 18, 1274–1291. [Google Scholar] [CrossRef] [PubMed]
- Pucker, B.; Kleinbölting, N.; Weisshaar, B. Large scale genomic rearrangements in selected Arabidopsis thaliana T-DNA lines are caused by T-DNA insertion mutagenesis. BMC Genom. 2021, 22, 877. [Google Scholar] [CrossRef]
- Stewart, A. Selecting compact cultivars for horticulture from wild plant populations. Acta Hortic. 2017, 1212, 13–14. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.L.; Kim, J.H.; Ko, J.Y.; Kim, S.T.; Lee, E.K.; Kim, W.H.; Kwon, O.H. Production of somatic embryo and transgenic plants derived from breeding lines of Rosa hybrida L. Hortic. Environ. Biotechnol. 2013, 54, 172–176. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cheon, K.S.; Kim, S.Y.; Kim, J.H.; Kwon, O.H.; Lee, H.J.; Kim, W.H. Expression of SOD2 enhances tolerance to drought stress in roses. Hortic. Environ. Biotechnol. 2020, 61, 569–576. [Google Scholar] [CrossRef]
- Lee, S.Y.; Shin, J.Y.; Kwon, D.H.; Xu, J.; Kim, J.H.; Ahn, C.H.; Jang, S.; Kwon, O.H.; Lee, H.J.; Kim, W.H. Preventing scattering of Tetranychus urticae in Rosa hybrida through dsCOPB2 expression. Sci. Hortic. 2022, 301, 111113. [Google Scholar] [CrossRef]
- Naing, A.H.; Ai, T.N.; Lim, K.B.; Lee, I.J.; Kim, C.K. Overexpression of Rosea1 from snapdragon enhances anthocyanin accumulation and abiotic stress tolerance in transgenic tobacco. Front. Plant. 2018, 9, 1070. [Google Scholar] [CrossRef] [PubMed]
- Manteau, S.; Abouna, S.; Lambert, B.; Legendre, L. Differential regulation by ambient pH of putative virulence factor secretion by the phytopathogenic fungus Botrytis cinerea. FEMS Microbiol. Ecol. 2003, 43, 359–366. [Google Scholar] [CrossRef]


| Stem length (cm) | Stem diameter(mm) | No. of leaves | No. of prickles | No. of branches | Fresh weight (g) | |||||||||||||||
| Wild type | 66.8 | a | 4.2 | a | 8 | a | 13.6 | a | 0.5 | b | 22.6 | a | ||||||||
| T1 | 32.7 | b | 3.3 | ab | 6 | ab | 2.1 | b | 4 | ab | 17.6 | ab | ||||||||
| T2 | 31 | b | 2.9 | b | 5.8 | b | 2.8 | b | 2.8 | ab | 14.9 | ab | ||||||||
| T3 | 36.7 | b | 3.1 | b | 6.9 | ab | 2.4 | b | 3.4 | ab | 15.2 | ab | ||||||||
| T4 | 29.5 | b | 2.9 | b | 6.9 | ab | 0.8 | b | 4.4 | a | 11.1 | b | ||||||||
| Significance z | *** | * | * | *** | *** | * | ||||||||||||||
| Peduncle length (cm) | Peduncle diameter (mm) | No. of petals | Flower width (cm) | Flower height (cm) | ||||||||||||||||
| Wild type | 13.6 | a | 3.1 | a | 27.8 | b | 6.4 | a | 4.8 | a | ||||||||||
| T1 | 8.7 | b | 2.3 | ab | 87.9 | a | 5.5 | ab | 4.4 | ab | ||||||||||
| T2 | 8.1 | b | 2.1 | b | 75.9 | a | 5.4 | ab | 4.1 | abc | ||||||||||
| T3 | 7.2 | b | 2.3 | ab | 78 | a | 5 | b | 3.7 | bc | ||||||||||
| T4 | 7.7 | b | 1.8 | b | 79.4 | a | 4.9 | b | 3.4 | c | ||||||||||
| Significance z | *** | *** | *** | * | *** | |||||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.Y.; Lee, S.Y.; Kim, Y.J.; Choi, Y.J.; Lim, S.H.; Kang, Y.-I. Overexpression of VtF3′5′H and RhNHX Genes Alters Flower Color and Plant Morphology in Transgenic Rose ‘Red Farm’. Plants 2025, 14, 3185. https://doi.org/10.3390/plants14203185
Lee KY, Lee SY, Kim YJ, Choi YJ, Lim SH, Kang Y-I. Overexpression of VtF3′5′H and RhNHX Genes Alters Flower Color and Plant Morphology in Transgenic Rose ‘Red Farm’. Plants. 2025; 14(20):3185. https://doi.org/10.3390/plants14203185
Chicago/Turabian StyleLee, Ka Youn, Su Young Lee, Yae Jin Kim, Youn Jung Choi, So Hyeon Lim, and Yun-Im Kang. 2025. "Overexpression of VtF3′5′H and RhNHX Genes Alters Flower Color and Plant Morphology in Transgenic Rose ‘Red Farm’" Plants 14, no. 20: 3185. https://doi.org/10.3390/plants14203185
APA StyleLee, K. Y., Lee, S. Y., Kim, Y. J., Choi, Y. J., Lim, S. H., & Kang, Y.-I. (2025). Overexpression of VtF3′5′H and RhNHX Genes Alters Flower Color and Plant Morphology in Transgenic Rose ‘Red Farm’. Plants, 14(20), 3185. https://doi.org/10.3390/plants14203185






