Effect of Weather Conditions on Phytochemical Profiles in Organically Grown Cowpea (Vignaunguiculata L. Walp)
Abstract
1. Introduction
2. Results
2.1. Weather Data
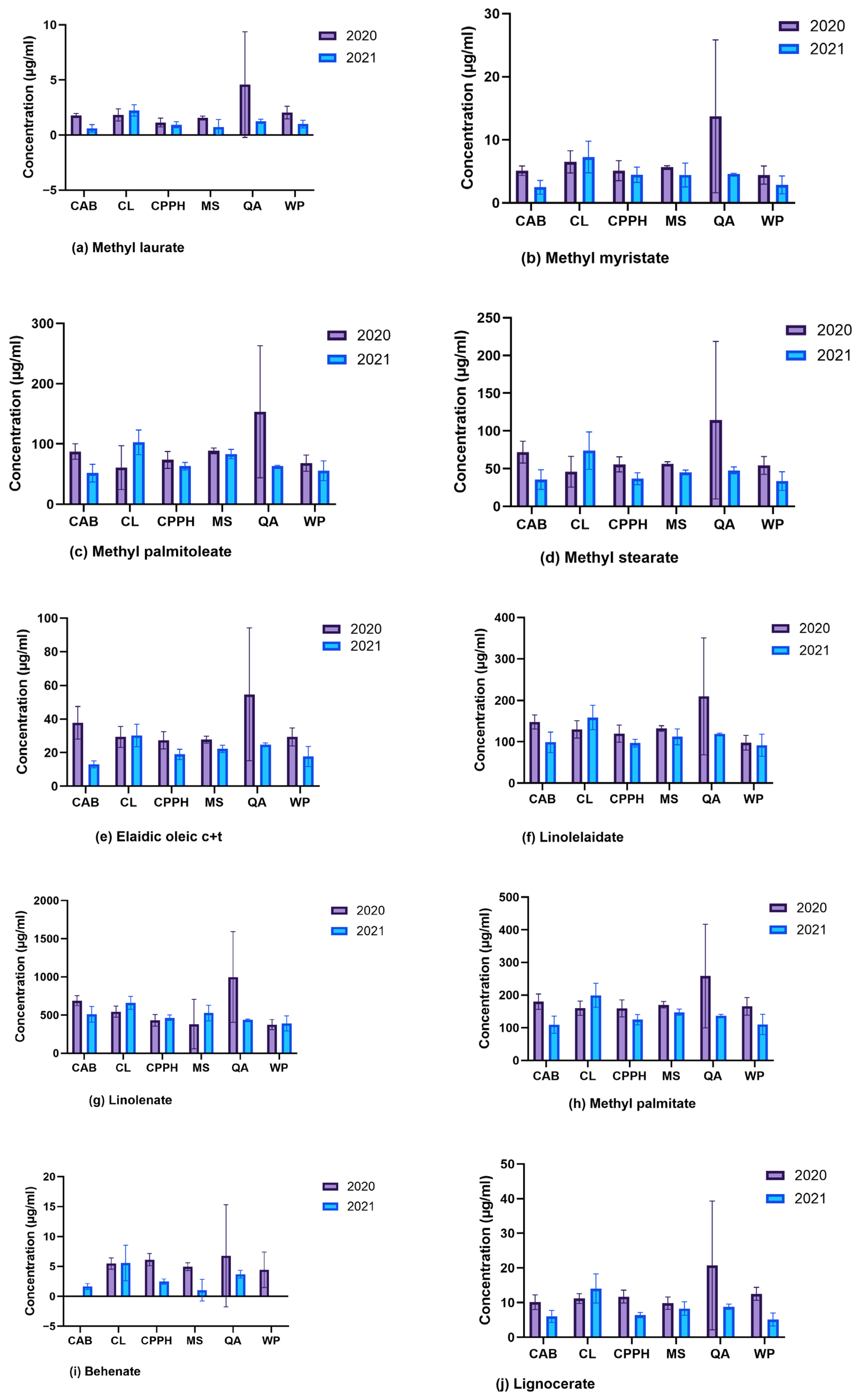
2.2. Identification of Targeted Phytochemicals Using GC-MS
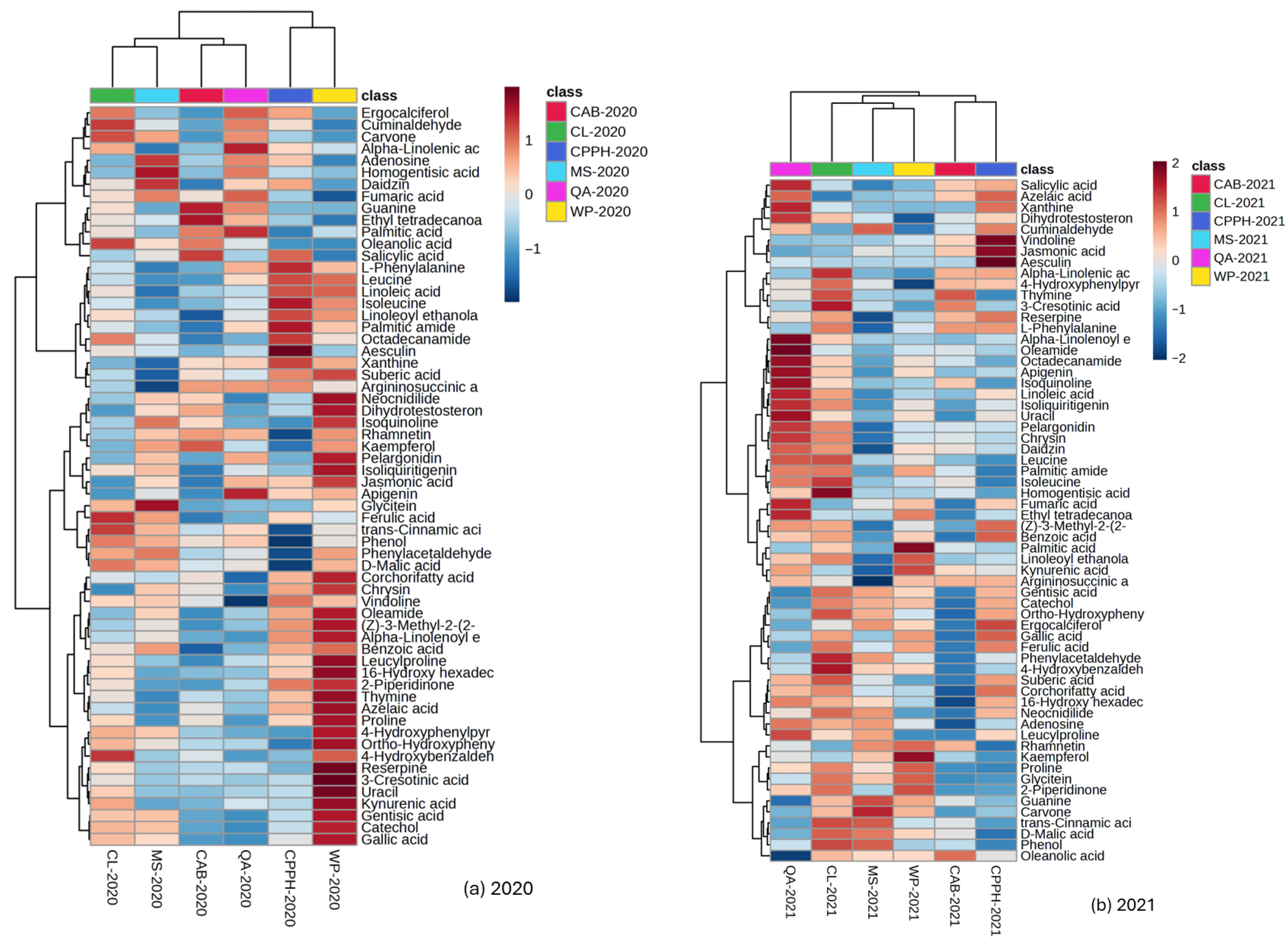
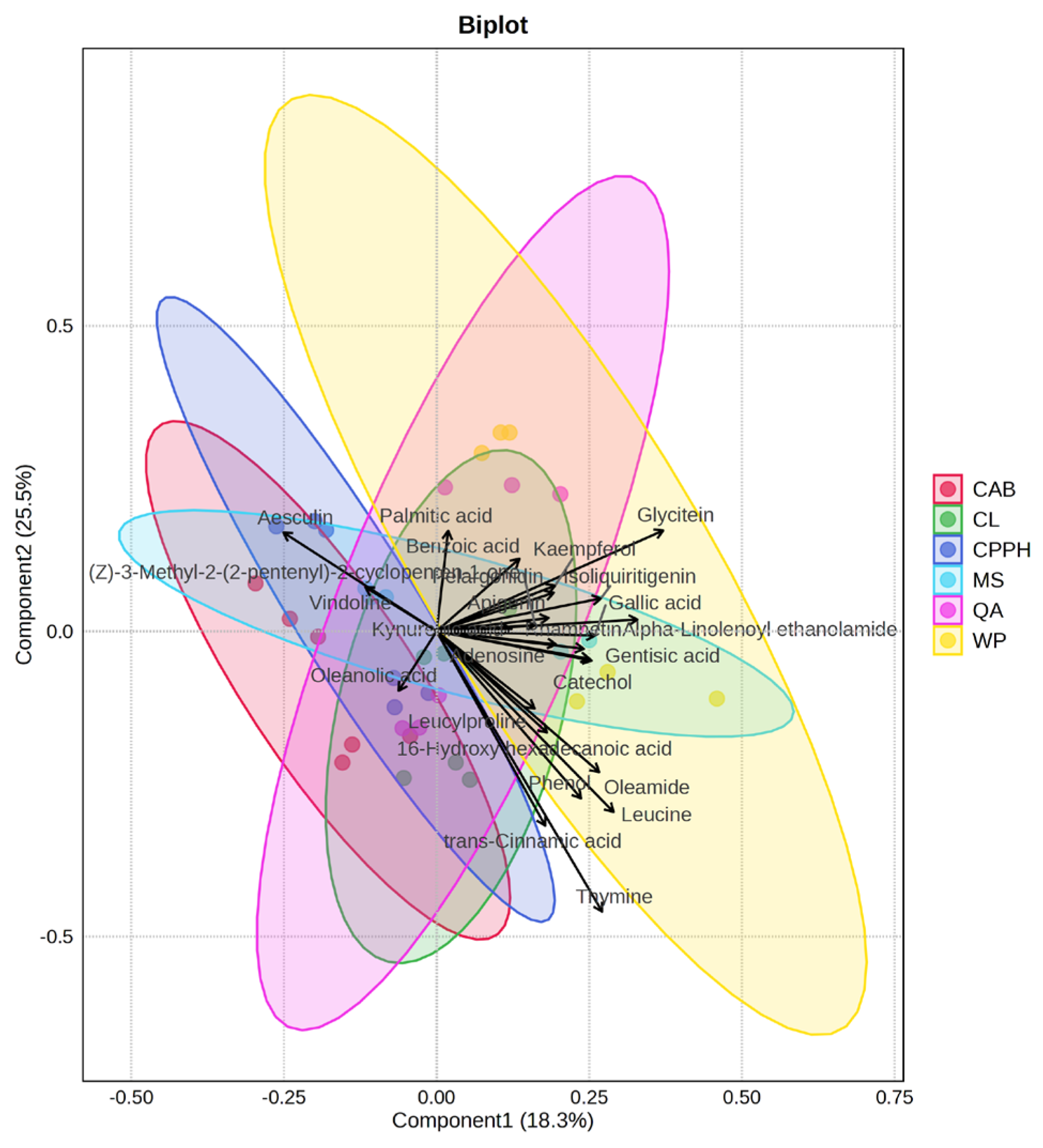
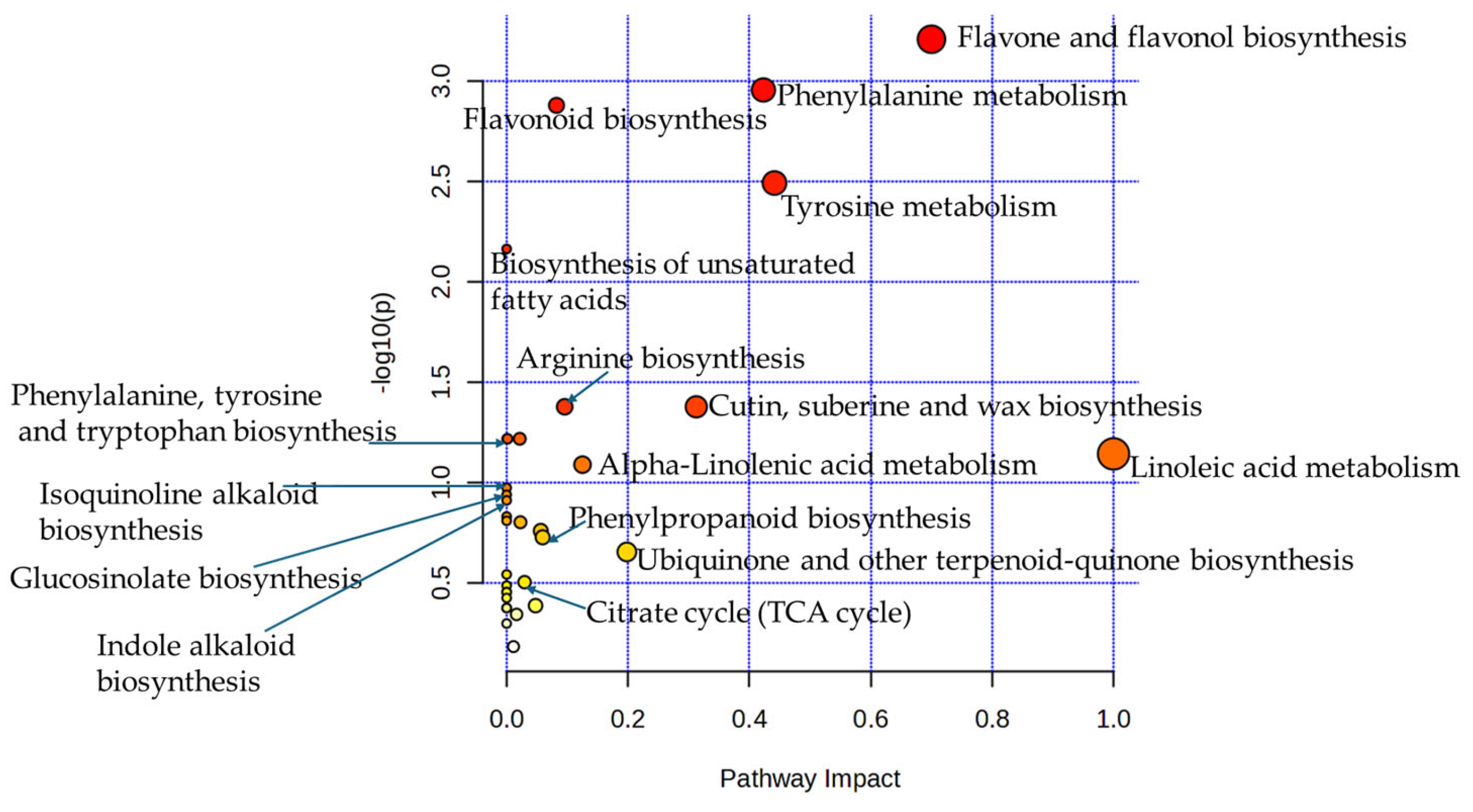
2.3. Identification of Untargeted Phytochemicals with LC-MS/MS
3. Discussion
4. Materials and Methods
4.1. Research Site
4.2. Climatic Conditions
4.3. Variety Selection and Experiment Design
4.4. Sample Collection and Preparation
4.5. Extraction of FAMEs
GC-MS Analysis
4.6. Extraction of Other Phytochemicals
Characterization of Extracts Using LC-MS/MS Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AL | Alabama |
| ANOVA | Analysis of variance |
| CAB | California Black Eye Pea |
| CAENS | College of Agriculture, Environment and Nutrition Sciences |
| CL | Colossus |
| CPPH | Coronet Pinkeye Purple Hull |
| GC-MS | Gas chromatography–mass spectrometry |
| GWCAES | George Washington Carver Agricultural Experiment Station |
| FAMEs | Fatty acid methyl esters |
| FDR | False discovery rate |
| HCl | Hydrochloric acid |
| HMDB | Human Metabolome Database |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LC-MS/MS | Liquid chromatography–tandem mass spectrometry |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| MS | Mississippi Silver |
| NIFA | National Institute of Food and Agriculture |
| NIST | National Institute of Standards and Technology |
| NWCC | National Water and Climate Center |
| QA | Queen Anne |
| RT | Retention time |
| UFA | Unsaturated fatty acids |
| USA | United States of America |
| USDA | United States Department of Agriculture |
| WP | Whippoorwill Steele’s Black |
Appendix A
| Phytochemical Name | Formula | Calc. MW | RT (min) | Cowpea Variety (F.val) | Cowpea Variety (raw.p) | Cowpea Variety (adj.p) | Seasons (F.val) | Seasons (raw.p) | Seasons (adj.p) | Interaction (F.val) | Interaction (raw.p) | Interaction (adj.p) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Glycitein | C16 H12 O5 | 284.06849 | 13.9 | 202.82 | 8.19E−19 | 5.57E−17 | 78.146 | 5.16E−09 | 1.95E−08 | 35.918 | 2.20E−10 | 2.14E−09 |
| Rhamnetin | C16 H12 O7 | 316.05832 | 13.907 | 177.8 | 3.81E−18 | 1.30E−16 | 31.796 | 8.33E−06 | 2.098E−05 | 8.119 | 0.000134 | 0.0003375 |
| Aesculin | C15 H16 O9 | 340.07931 | 11.688 | 147.1 | 3.44E−17 | 7.80E−16 | 53.3 | 1.53E−07 | 4.34E−07 | 3.46 | 0.017 | 0.02513 |
| D-Malic acid | C4 H6 O5 | 134.02148 | 12.879 | 103.13 | 2.04E−15 | 3.47E−14 | 76.56 | 6.24E−09 | 2.23E−08 | 7.397 | 0.000254 | 0.0005956 |
| Phenol | C6 H6 O | 94.04183 | 12.141 | 89.653 | 1.00E−14 | 1.36E−13 | 66.681 | 2.19E−08 | 6.77E−08 | 8.472 | 0.0000988 | 0.0002799 |
| Argininosuccinic acid | C10 H18 N4 O6 | 290.11873 | 13.23 | 70.806 | 1.42E−13 | 1.61E−12 | 26.152 | 3.12E−05 | 7.072E−05 | 2.25 | 0.082 | 0.10521 |
| Salicylic acid | C7 H6 O3 | 138.03166 | 7.727 | 69.137 | 1.85E−13 | 1.80E−12 | 196.22 | 4.77E−13 | 3.60E−12 | 49.927 | 6.62E−12 | 1.13E−10 |
| Chrysin | C15 H10 O4 | 254.05787 | 13.759 | 58.774 | 1.12E−12 | 9.52E−12 | 9.597 | 5.00E−03 | 0.0077273 | 140.59 | 5.81E−17 | 3.95E−15 |
| Isoliquiritigenin | C15 H12 O4 | 256.07359 | 15.295 | 55.159 | 2.24E−12 | 1.69E−11 | 0.313 | 0.581 | 0.61731 | 38.816 | 9.75E−11 | 1.33E−09 |
| Ferulic acid | C10 H10 O4 | 194.05786 | 12.225 | 50.908 | 5.36E−12 | 3.64E−11 | 8.653 | 7.00E−03 | 0.010348 | 6.817 | 0.000437 | 0.0009905 |
| Homogentisic acid | C8 H8 O4 | 168.04218 | 11.777 | 45.384 | 1.85E−11 | 1.14E−10 | 87.106 | 1.86E−09 | 7.91E−09 | 65.33 | 3.48E−13 | 7.89E−12 |
| Gentisic acid | C7 H6 O4 | 154.0265 | 3.746 | 40.64 | 6.00E−11 | 3.40E−10 | 18.921 | 2.17E−04 | 0.0004307 | 13.656 | 0.0000023 | 1.20E−05 |
| trans-Cinnamic acid | C9 H8 O2 | 148.05239 | 12.143 | 39.938 | 7.22E−11 | 3.78E−10 | 92.105 | 1.09E−09 | 4.94E−09 | 8.157 | 0.000129 | 0.0003374 |
| Catechol | C6 H6 O2 | 110.03672 | 3.743 | 36.419 | 1.90E−10 | 9.23E−10 | 18.839 | 2.22E−04 | 0.0004307 | 8.784 | 0.0000761 | 0.000225 |
| Daidzin | C21 H20 O9 | 416.11089 | 12.66 | 35.555 | 2.44E−10 | 1.11E−09 | 657.23 | 5.99E−19 | 2.04E−17 | 100.9 | 2.62E−15 | 8.91E−14 |
| Pelargonidin | C15 H10 O5 | 270.05284 | 13.917 | 31.263 | 9.21E−10 | 3.91E−09 | 7.368 | 1.20E−02 | 0.017362 | 36.371 | 1.93E−10 | 2.14E−09 |
| Gallic acid | C7 H6 O5 | 170.02144 | 1.565 | 24.816 | 9.34E−09 | 3.74E−08 | 5.946 | 2.30E−02 | 0.030667 | 9.613 | 0.0000389 | 0.000147 |
| L-Phenylalanine | C9 H11 N O2 | 165.07891 | 3.2 | 24.006 | 1.29E−08 | 4.87E−08 | 519.8 | 8.99E−18 | 1.53E−16 | 15.923 | 6.03E−07 | 4.10E−06 |
| Vindoline | C25 H32 N2 O6 | 456.23372 | 12.799 | 21.547 | 3.68E−08 | 1.32E−07 | 12.038 | 2.00E−03 | 0.0032381 | 8.889 | 0.0000698 | 0.000225 |
| Benzoic acid | C7 H6 O2 | 122.03674 | 13.151 | 20.144 | 6.97E−08 | 2.37E−07 | 26.147 | 3.12E−05 | 7.072E−05 | 8.808 | 0.0000746 | 0.000225 |
| 3-Cresotinic acid | C8 H8 O3 | 152.04728 | 12.353 | 15.991 | 5.8E−07 | 1.86E−06 | 956.78 | 7.52E−21 | 5.11E−19 | 28.004 | 2.81E−09 | 2.39E−08 |
| (Z)-3-Methyl-2-(2-pentenyl)-2-cyclopenten-1-one | C11 H16 O | 164.12007 | 15.306 | 15.881 | 6.17E−07 | 1.86E−06 | 7.091 | 1.40E−02 | 0.019833 | 11.029 | 0.0000134 | 0.0000536 |
| Apigenin | C15 H10 O5 | 270.05276 | 14.624 | 15.798 | 6.47E−07 | 1.86E−06 | 4.475 | 0.045 | 0.056667 | 7.972 | 0.000152 | 0.0003691 |
| Xanthine | C5 H4 N4 O2 | 152.03339 | 12.848 | 15.716 | 6.77E−07 | 1.86E−06 | 145.43 | 1.13E−11 | 7.68E−11 | 6.027 | 0.00095 | 0.0019 |
| Kynurenic acid | C10 H7 N O3 | 189.04263 | 11.745 | 15.698 | 6.84E−07 | 1.86E−06 | 4.642 | 0.041 | 0.052604 | 3.697 | 0.013 | 0.020091 |
| 16-Hydroxy hexadecanoic acid | C16 H32 O3 | 272.23477 | 20.512 | 11.744 | 8.09E−06 | 2.12E−05 | 396.62 | 1.97E−16 | 2.23E−15 | 11.425 | 0.0000101 | 4.29E−05 |
| 4-Hydroxybenzaldehyde | C7 H6 O2 | 122.03674 | 11.66 | 10.872 | 0.000015 | 3.78E−05 | 20.85 | 1.25E−04 | 0.0002576 | 4.18 | 0.007 | 0.012205 |
| Oleamide | C18 H35 N O | 264.24532 | 20.914 | 9.035 | 0.0000618 | 0.0001501 | 567.83 | 3.25E−18 | 7.37E−17 | 15.399 | 8.12E−07 | 4.68E−06 |
| Ortho-Hydroxyphenylacetic acid | C8 H8 O3 | 152.04727 | 6.521 | 7.021 | 0.00036 | 0.0008441 | 18.746 | 2.28E−04 | 0.0004307 | 6.133 | 0.000854 | 0.0018148 |
| Isoleucine | C6 H13 N O2 | 131.09454 | 1.422 | 6.968 | 0.000379 | 0.0008591 | 390.96 | 2.32E−16 | 2.25E−15 | 15.368 | 8.26E−07 | 4.68E−06 |
| Carvone | C10 H14 O | 150.1044 | 13.204 | 6.843 | 0.000426 | 0.0009345 | 0.834 | 0.37 | 0.41246 | 3.378 | 0.019 | 0.026917 |
| Oleanolic acid | C30 H48 O3 | 478.34286 | 20.361 | 6.293 | 0.000728 | 0.0015228 | 5.933 | 2.30E−02 | 0.030667 | 3.201 | 0.024 | 0.03264 |
| Isoquinoline | C9 H7 N | 129.05771 | 12.915 | 6.278 | 0.000739 | 0.0015228 | 0.06 | 0.808 | 0.83248 | 11.878 | 7.37E−06 | 3.34E−05 |
| Linoleic acid | C18 H32 O2 | 280.24024 | 21.057 | 6.135 | 0.000852 | 0.0016592 | 104.7 | 3.13E−10 | 1.64E−09 | 3.685 | 0.013 | 0.020091 |
| Suberic acid | C8 H14 O4 | 174.08913 | 12.759 | 6.132 | 0.000854 | 0.0016592 | 215.73 | 1.71E−13 | 1.45E−12 | 9.083 | 0.0000595 | 0.0002023 |
| Fumaric acid | C4 H4 O4 | 116.01098 | 1.224 | 5.798 | 0.001 | 0.0018889 | 22.762 | 7.44E−05 | 0.0001581 | 4.96 | 0.003 | 0.0055135 |
| Azelaic acid | C9 H16 O4 | 188.10468 | 13.011 | 5.238 | 0.002 | 0.0035789 | 79.984 | 4.15E−09 | 1.66E−08 | 5.142 | 0.002 | 0.0038857 |
| Ergocalciferol | C28 H44 O | 396.33939 | 21.735 | 5.127 | 0.002 | 0.0035789 | 1.006 | 0.326 | 0.37803 | 4.195 | 0.007 | 0.012205 |
| Guanine | C5 H5 N5 O | 151.04937 | 1.531 | 4.802 | 0.003 | 0.0049756 | 38.346 | 2.13E−06 | 5.57E−06 | 13.025 | 3.44E−06 | 1.67E−05 |
| Linoleoyl ethanolamide | C20 H37 N O2 | 323.28241 | 19.639 | 4.974 | 0.003 | 0.0049756 | 9.227 | 6.00E−03 | 0.0090667 | 1.938 | 0.125 | 0.15455 |
| Palmitic acid | C16 H32 O2 | 273.26681 | 16.566 | 4.803 | 0.003 | 0.0049756 | 16.219 | 4.92E−04 | 0.0009042 | 5.055 | 0.003 | 0.0055135 |
| 2-Piperidinone | C5 H9 N O | 99.06835 | 2.77 | 4.691 | 0.004 | 0.0064762 | 73.488 | 9.09E−09 | 3.09E−08 | 1.802 | 0.151 | 0.18336 |
| Ethyl tetradecanoate | C16 H32 O2 | 256.24015 | 20.074 | 4.117 | 0.008 | 0.012651 | 13.493 | 1.00E−03 | 0.0017 | 8.399 | 0.000105 | 0.0002856 |
| Kaempferol | C15 H10 O6 | 286.0478 | 12.374 | 3.905 | 0.01 | 0.015455 | 0.006 | 0.937 | 0.937 | 1.004 | 0.437 | 0.45024 |
| Adenosine | C10 H13 N5 O4 | 267.09683 | 1.398 | 3.852 | 0.011 | 0.016622 | 3.834 | 0.062 | 0.076655 | 1.654 | 0.184 | 0.21951 |
| Leucine | C6 H13 N O2 | 131.09457 | 1.629 | 3.497 | 0.016 | 0.022667 | 135.1 | 2.41E−11 | 1.49E−10 | 3.808 | 0.011 | 0.01781 |
| Thymine | C5 H6 N2 O2 | 126.04288 | 1.463 | 3.493 | 0.016 | 0.022667 | 452.79 | 4.37E−17 | 5.94E−16 | 6.027 | 0.00095 | 0.0019 |
| Alpha-Linolenic acid | C18 H30 O2 | 278.22457 | 20.324 | 3.494 | 0.016 | 0.022667 | 23.617 | 5.93E−05 | 0.0001301 | 2.325 | 0.074 | 0.096769 |
| Phenylacetaldehyde | C8 H8 O | 120.05746 | 12.563 | 3.269 | 0.022 | 0.030531 | 0.999 | 0.328 | 0.37803 | 1.257 | 0.314 | 0.33362 |
| Uracil | C4 H4 N2 O2 | 112.02722 | 1.248 | 2.715 | 0.044 | 0.05984 | 13.479 | 0.001 | 0.0017 | 2.092 | 0.101 | 0.12719 |
| Neocnidilide | C12 H18 O2 | 176.12005 | 15.839 | 2.658 | 0.048 | 0.064 | 28.482 | 1.78E−05 | 4.32E−05 | 9.197 | 0.0000542 | 0.000194 |
| Palmitic amide | C16 H33 N O | 255.25625 | 20.705 | 2.621 | 0.05 | 0.065385 | 112.62 | 1.52E−10 | 8.61E−10 | 3.398 | 0.018 | 0.026043 |
| Corchorifatty acid F | C18 H32 O5 | 328.22417 | 14.584 | 2.463 | 0.062 | 0.079547 | 15.385 | 6.41E−04 | 1.15E−03 | 3.563 | 0.015 | 0.022667 |
| Dihydrotestosterone | C19 H30 O2 | 290.22462 | 19.365 | 2.356 | 0.071 | 0.089407 | 11.999 | 2.00E−03 | 3.24E−03 | 17.225 | 2.97E−07 | 2.24E−06 |
| Reserpine | C33 H40 N2 O9 | 608.26352 | 19.8 | 2.323 | 0.074 | 0.091491 | 102.14 | 3.99E−10 | 1.94E−09 | 3.167 | 0.025 | 0.033333 |
| Alpha-Linolenoyl ethanolamide | C20 H35 N O2 | 321.26674 | 17.68 | 2.278 | 0.079 | 0.095929 | 1.237 | 0.277 | 0.33046 | 6.264 | 0.000749 | 0.001643 |
| Leucylproline | C11 H20 N2 O3 | 228.14737 | 11.6 | 1.988 | 0.117 | 0.13717 | 42.96 | 8.86E−07 | 2.41E−06 | 3.344 | 0.02 | 0.027755 |
| Octadecanamide | C18 H37 N O | 283.28756 | 22.018 | 1.931 | 0.126 | 0.14522 | 69.375 | 1.53E−08 | 4.95E−08 | 3.865 | 0.01 | 0.016585 |
| Proline | C5 H9 N O2 | 115.06327 | 1.276 | 1.743 | 0.163 | 0.1817 | 55.436 | 1.10E−07 | 3.25E−07 | 1.142 | 0.366 | 0.38289 |
| Cuminaldehyde | C10 H12 O | 148.08875 | 12.364 | 1.247 | 0.318 | 0.34324 | 6.478 | 0.018 | 0.02498 | 0.898 | 0.498 | 0.498 |
| 4-Hydroxyphenylpyruvic acid | C9 H8 O4 | 180.04219 | 13.663 | 0.956 | 0.464 | 0.493 | 9.535 | 0.005 | 0.0077273 | 3.933 | 0.01 | 0.016585 |
| Jasmonic acid | C12 H18 O3 | 210.12554 | 14.863 | 0.923 | 0.483 | 0.50529 | 4.805 | 0.038 | 0.049692 | 0.963 | 0.46 | 0.46687 |
| Pathway | Total | Expected | Hits | Raw p | −Log10(p) | Holm Adjust | FDR | Impact |
|---|---|---|---|---|---|---|---|---|
| Flavone and flavonol biosynthesis | 10 | 0.18457 | 3 | 0.00062 | 3.2073 | 0.057085 | 0.040597 | 0.7 |
| Phenylalanine metabolism | 12 | 0.22149 | 3 | 0.00111 | 2.9548 | 0.10098 | 0.040597 | 0.42308 |
| Flavonoid biosynthesis | 47 | 0.8675 | 5 | 0.001324 | 2.8782 | 0.11914 | 0.040597 | 0.08216 |
| Tyrosine metabolism | 17 | 0.31378 | 3 | 0.003223 | 2.4917 | 0.28687 | 0.074136 | 0.44134 |
| Biosynthesis of unsaturated fatty acids | 22 | 0.40606 | 3 | 0.006861 | 2.1636 | 0.60378 | 0.12625 | 0 |
| Arginine biosynthesis | 18 | 0.33223 | 2 | 0.041895 | 1.3778 | 1 | 0.55062 | 0.09568 |
| Cutin, suberine, and wax biosynthesis | 18 | 0.33223 | 2 | 0.041895 | 1.3778 | 1 | 0.55062 | 0.3125 |
| Valine, leucine, and isoleucine biosynthesis | 22 | 0.40606 | 2 | 0.060457 | 1.2186 | 1 | 0.5562 | 0 |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 22 | 0.40606 | 2 | 0.060457 | 1.2186 | 1 | 0.5562 | 0.0015 |
| Alanine, aspartate, and glutamate metabolism | 22 | 0.40606 | 2 | 0.060457 | 1.2186 | 1 | 0.5562 | 0.02159 |
| Linoleic acid metabolism | 4 | 0.07383 | 1 | 0.07188 | 1.1434 | 1 | 0.60118 | 1 |
| alpha-Linolenic acid metabolism | 26 | 0.47989 | 2 | 0.081315 | 1.0898 | 1 | 0.62341 | 0.125 |
| Isoquinoline alkaloid biosynthesis | 6 | 0.11074 | 1 | 0.10593 | 0.975 | 1 | 0.74963 | 0 |
| Glucosinolate biosynthesis | 65 | 1.1997 | 3 | 0.11486 | 0.93982 | 1 | 0.75129 | 0 |
| Indole alkaloid biosynthesis | 7 | 0.1292 | 1 | 0.12249 | 0.91189 | 1 | 0.75129 | 0 |
| Valine, leucine, and isoleucine degradation | 37 | 0.68293 | 2 | 0.14739 | 0.83154 | 1 | 0.80423 | 0 |
| Tropane, piperidine, and pyridine alkaloid biosynthesis | 9 | 0.16612 | 1 | 0.15475 | 0.81038 | 1 | 0.80423 | 0 |
| Purine metabolism | 75 | 1.3843 | 3 | 0.15735 | 0.80313 | 1 | 0.80423 | 0.02272 |
| Pyrimidine metabolism | 41 | 0.75676 | 2 | 0.17362 | 0.76041 | 1 | 0.84067 | 0.05624 |
| Phenylpropanoid biosynthesis | 43 | 0.79367 | 2 | 0.18702 | 0.72811 | 1 | 0.86031 | 0.05935 |
| Ubiquinone and other terpenoid-quinone biosynthesis | 48 | 0.88596 | 2 | 0.22116 | 0.6553 | 1 | 0.96888 | 0.19809 |
| beta-Alanine metabolism | 18 | 0.33223 | 1 | 0.28627 | 0.54322 | 1 | 1 | 0 |
| Citrate cycle (TCA cycle) | 20 | 0.36915 | 1 | 0.31269 | 0.50488 | 1 | 1 | 0.0295 |
| One carbon pool by folate | 21 | 0.38761 | 1 | 0.32555 | 0.48738 | 1 | 1 | 0 |
| Fatty acid elongation | 23 | 0.42452 | 1 | 0.35057 | 0.45523 | 1 | 1 | 0 |
| Pyruvate metabolism | 23 | 0.42452 | 1 | 0.35057 | 0.45523 | 1 | 1 | 0 |
| Pantothenate and CoA biosynthesis | 25 | 0.46144 | 1 | 0.37469 | 0.42633 | 1 | 1 | 0 |
| Glycosylphosphatidylinositol (GPI)-anchor biosynthesis | 28 | 0.51681 | 1 | 0.40926 | 0.388 | 1 | 1 | 0.04762 |
| Cyanoamino acid metabolism | 29 | 0.53527 | 1 | 0.42037 | 0.37637 | 1 | 1 | 0 |
| Biosynthesis of various plant secondary metabolites | 29 | 0.53527 | 1 | 0.42037 | 0.37637 | 1 | 1 | 0 |
| Arginine and proline metabolism | 32 | 0.59064 | 1 | 0.4525 | 0.34438 | 1 | 1 | 0.01637 |
| Fatty acid degradation | 37 | 0.68293 | 1 | 0.50227 | 0.29906 | 1 | 1 | 0 |
| Fatty acid biosynthesis | 56 | 1.0336 | 1 | 0.65453 | 0.18407 | 1 | 1 | 0.01123 |
| Compound | Target Ion and Qualifier Ions | RT Min | Quadratic Term | Linear Term | Constant Term | Correlation Coefficient (r2) |
|---|---|---|---|---|---|---|
| 10:0 methyl decanoate | 74.2, 87.2, 55.15, 186.4 | 5.2 | 1.561e−005 | 3.815e−003 | 1.598e−003 | 0.999 |
| 12:0 methyl laurate | 74.2, 87.15, 55.15, 214.40 | 5.8 | 1.548e−005 | 4.004e−003 | 3.019e−003 | 0.999 |
| 14:0 methyl myristate | 74.2, 87.15, 55.15, 242.4 | 6.7 | 1.780e−005 | 3.890e−003 | 3.610e−003 | 0.999 |
| Methyl palmitate | 74.2, 87.2, 55.15, 270.6 | 8.1 | 2.918e−005 | 5.769e−003 | 5.368e−003 | 0.999 |
| 16:1 methyl palmitoleate | 55.15, 74.2, 69.15, 268.4 | 8.3 | 2.638e−006 | 7.876e−004 | −3.739e−004 | 0.999 |
| 18:0 Methyl stearate | 74.2, 7.2, 55.15, 98.4 | 10.3 | 0.000e+000 | 4.689e−003 | −2.116e−003 | 0.998 |
| 18:1 elaidic oleic cis & trans | 55.15, 74.2, 69.15, 296.4 | 10.55 | 1.296e−005 | 2.069e−003 | 1.766e−003 | 0.999 |
| 18:2n−6 linolelaidate | 65.15, 55.15, 81.2, 294.4 | 14.0 | 5.593e−006 | 1.618e−003 | −5.778e−004 | 0.999 |
| 18:3n−6 linolenate | 67.5, 74.2, 55.15, 150 | 14.2 | 0.000e+000 | 2.673e−005 | 3.958e−004 | 0.574 |
| 18:3n−3 linolenate | 67.15, 79.15. 55.15, 292.4 | 14.7 | 1.000e−006 | 6.153e−004 | −1.126e−003 | 0.998 |
| 22:0 behenate | 74.15, 87.2, 55.2, 354.6 | 20.4 | 1.741e−005 | 3.296e−003 | −9.790e−004 | 0.999 |
| 24:0 lignocerate | 74.2, 87.2, 55.2, 382.4 | 22.9 | 2.426e−005 | 2.509e−003 | −1.856e−003 | 0.999 |
References
- Osipitan, O.A.; Fields, J.S.; Lo, S.; Cuvaca, I. Production Systems and Prospects of Cowpea (Vigna unguiculata (L.) Walp.) in the United States. Agronomy 2021, 11, 2312. [Google Scholar] [CrossRef]
- Nkhoma, N.; Shimelis, H.; Laing, M.D.; Shayanowako, A.; Mathew, I. Assessing the genetic diversity of cowpea [Vigna unguiculata (L.) Walp.] germplasm collections using phenotypic traits and SNP markers. BMC Genet. 2020, 21, 110. [Google Scholar] [CrossRef] [PubMed]
- Omomowo, O.I.; Babalola, O.O. Constraints and Prospects of Improving Cowpea Productivity to Ensure Food, Nutritional Security and Environmental Sustainability. Front. Plant Sci. 2021, 12, 751731. [Google Scholar] [CrossRef] [PubMed]
- Lyngdoh, C.; Bahadur, V.; David, A.; Prasad, V.; Jamir, T. Effect of Organic Manures, Organic Supplements and Biofertilizers on Growth and Yield of Cowpea [Vigna unguiculata (L.) Walp]. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1029–1036. [Google Scholar] [CrossRef]
- Boukar, O.; Fatokun, C.A.; Huynh, B.-L.; Roberts, P.A.; Close, T.J. Genomic tools in cowpea breeding programs: Status and perspectives. Front. Plant Sci. 2016, 7, 757. [Google Scholar] [CrossRef]
- Boukar, O.; Massawe, F.; Muranaka, S.; Franco, J.; Maziya-Dixon, B.; Singh, B.; Fatokun, C. Evaluation of cowpea germplasm lines for protein and mineral concentrations in grains. Plant Genet. Resour. Charact. Util. 2011, 9, 515–522. [Google Scholar] [CrossRef]
- Owade, J.O.; Abong’, G.; Okoth, M.; Mwang’oMbe, A.W. A review of the contribution of cowpea leaves to food and nutrition security in East Africa. Food Sci. Nutr. 2019, 8, 36–47. [Google Scholar] [CrossRef]
- Sheahan, C.M. Plant Guide for Cowpea (Vigna unguiculata) [Internet]. 2012. Available online: https://plants.usda.gov/DocumentLibrary/plantguide/pdf/pg_viun.pdf (accessed on 7 March 2023).
- Kemble, J.M.; Jennings, K.M.; Walgenbach, J.F.; Rudolph, R. Handbook Southeastern Vegetable Extension Workers; North Carolina State Extension: Raleigh, NC, USA, 2020. [Google Scholar]
- Nyaga, J.W.; Njeru, E.M. Potential of Native Rhizobia to Improve Cowpea Growth and Production in Semiarid Regions of Kenya. Front. Agron. 2020, 2, 606293. [Google Scholar] [CrossRef]
- Hill, S.L. Cowpea Adaptability to Southeastern Organic Farming Systems: Forage Productivity and Charcoal Rot Susceptibility Forage Productivity and Charcoal Rot Susceptibility [Internet]. 2015. Available online: https://trace.tennessee.edu/utk_gradthes (accessed on 13 September 2025).
- Quinn, J.; Myers, R. COWPEA A Versatile Legume for Hot, Dry Conditions [Internet]. 2002. Available online: www.jeffersoninstitute.org (accessed on 13 September 2025).
- Singh, S.R.; Allen, D.J. Cowpea Pests and Diseases; International Institute of Tropical Agriculture Ibadan Nigeria Manual Series NO. 2 Contents; IITA: Ibadan, Nigeria, 1979. [Google Scholar]
- Salam, U.; Ullah, S.; Tang, Z.-H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Altindal, D.; Altindal, N. Plant volatile compounds in growth. In Volatiles and Food Security: Role of Volatiles in Agro-Ecosystems; Springer: Singapore, 2017; pp. 1–13. [Google Scholar]
- He, M.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- Rowan, D.D. Volatile metabolites. Metabolites 2011, 1, 41–63. [Google Scholar] [CrossRef]
- Tilman, D.; Cassman, K.; Matson, P.; Naylor, R.; Polasky, S. Agricultural Sustainability and Intensive Production Practices [Internet]. 2002. Available online: www.nature.com/nature (accessed on 13 September 2025).
- Adamchak, R. Organic Farming; Encyclopedia Britannica, Inc.: Chicago, IL, USA, 2024; Available online: https://www.britannica.com/topic/organic-farming (accessed on 7 April 2024).
- Al-Khayri, J.M.; Rashmi, R.; Toppo, V.; Chole, P.B.; Banadka, A.; Sudheer, W.N.; Nagella, P.; Shehata, W.F.; Al-Mssallem, M.Q.; Alessa, F.M.; et al. Plant Secondary Metabolites: The Weapons for Biotic Stress Management. Metabolites 2023, 13, 716. [Google Scholar] [CrossRef]
- Ooko Abong’, G.; Muzhingi, T.; Wandayi Okoth, M.; Ng’ang’a, F.; Ochieng’, P.E.; Mahuga Mbogo, D.; Malavi, D.; Akhwale, M.; Ghimire, S. Phytochemicals in Leaves and Roots of Selected Kenyan Orange Fleshed Sweet Potato (OFSP) Varieties. Int. J. Food Sci. 2020, 2020, 3567972. [Google Scholar] [CrossRef]
- Zhu, F.; Shang, J.; Wong, S.-M. Editorial: Induced plant resistance against pathogens by application of bioactive molecules. Front. Plant Sci. 2023, 14, 1282909. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2019, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Jayawardhane, J.; Goyali, J.C.; Zafari, S.; Igamberdiev, A.U. The Response of Cowpea (Vigna unguiculata) Plants to Three Abiotic Stresses Applied with Increasing Intensity: Hypoxia, Salinity, and Water Deficit. Metabolites 2022, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Goufo, P.; Moutinho-Pereira, J.M.; Jorge, T.F.; Correia, C.M.; Oliveira, M.R.; Rosa, E.A.S.; António, C.; Trindade, H. Cowpea (Vigna unguiculata L. Walp.) metabolomics: Osmoprotection as a physiological strategy for drought stress resistance and improved yield. Front. Plant Sci. 2017, 8, 586. [Google Scholar] [CrossRef]
- Zhukov, A.V. Palmitic acid and its role in the structure and functions of plant cell membranes. Russ. J. Plant Physiol. 2015, 62, 706–713. [Google Scholar] [CrossRef]
- Kumar, M.; Patel, M.K.; Kumar, N.; Bajpai, A.B.; Siddique, K.H.M. Metabolomics and molecular approaches reveal drought stress tolerance in plants. Int. J. Mol. Sci. 2021, 22, 9108. [Google Scholar] [CrossRef]
- Ohlrogge, J.; Browse, J. Lipid Biosynthesis. Plant Cell Am. Soc. Plant Physiol. 1995, 7, 957. [Google Scholar]
- Ichihara, K.; Fukubayashi, Y. Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res. 2010, 51, 635–640. [Google Scholar] [CrossRef]
- Jafarihaghighi, F.; Ardjmand, M.; Hassani, M.S.; Mirzajanzadeh, M.; Bahrami, H. Effect of Fatty Acid Profiles and Molecular Structures of Nine New Source of Biodiesel on Combustion and Emission. ACS Omega 2020, 5, 16053–16063. [Google Scholar] [CrossRef]
- Gao, H.; Ma, K.; Ji, G.; Pan, L.; Zhou, Q. Lipid transfer proteins involved in plant–pathogen interactions and their molecular mechanisms. Mol. Plant Pathol. 2022, 23, 1815–1829. [Google Scholar] [CrossRef] [PubMed]
- Kuźniak, E.; Gajewska, E. Lipids and Lipid-Mediated Signaling in Plant–Pathogen Interactions. Int. J. Mol. Sci. 2024, 25, 7255. [Google Scholar] [CrossRef]
- Jin, W.; Yang, Z.; Xu, K.; Liu, Q.; Luo, Q.; Li, L.; Xiang, X. A Comprehensive Review of Plant Volatile Terpenoids, Elucidating Interactions with Surroundings, Systematic Synthesis, Regulation, and Targeted Engineering Production. Biology 2025, 14, 466. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, T.; Khan, M.U.; Sharma, V.; Gupta, K. Terpenoids in essential oils: Chemistry, classification, and potential impact on human health and industry. Phytomed. Plus 2024, 4, 100549. [Google Scholar] [CrossRef]
- Khanam, S.; Mishra, P.; Faruqui, T.; Alam, P.; Albalawi, T.; Siddiqui, F.; Rafi, Z.; Khan, S. Plant-based secondary metabolites as natural remedies: A comprehensive review on terpenes and their therapeutic applications. Front. Pharmacol. 2025, 16, 1587215. [Google Scholar] [CrossRef]
- Câmara, J.S.; Perestrelo, R.; Ferreira, R.; Berenguer, C.V.; Pereira, J.A.M.; Castilho, P.C. Plant-Derived Terpenoids: A Plethora of Bioactive Compounds with Several Health Functions and Industrial Applications—A Comprehensive Overview. Molecules 2024, 29, 3861. [Google Scholar] [CrossRef]
- Bacong, J.R.C.; Juanico, D.E.O. Predictive Chromatography of Leaf Extracts Through Encoded Environmental Forcing on Phytochemical Synthesis. Front. Plant Sci. 2021, 12, 613507. [Google Scholar] [CrossRef]
- Gitonga, H.W.; Kyamanywa, S.; Arusei, P.; Lukanda, M.M.; Edema, R.; Dramadri, I.O. Genotype × Environment Interaction Influence Secondary Metabolite in Cowpea Infested by Flower Bud Thrips. Agronomy 2022, 12, 3210. [Google Scholar] [CrossRef]
- BEN Mansour-Gueddes, S.; Saidana-Naija, D.; Bchir, A.; Braham, M. Climate change effects on phytochemical compounds and antioxidant activity of Olea europaea L. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 436–455. [Google Scholar] [CrossRef]
- Alves, A.S.D.S.C.; Acioli, A.N.S.; Ferreira, G.S.L.; Silva, N.M.D. Effect of cowpea (Vigna unguiculata L. Walp.) sowing season on population dynamics of pest insects. Arq. Do Inst. Biológico 2022, 89, e00322021. [Google Scholar]
- Jeff Whitworth, R.; Ahmad, A. MF2865 Cowpea Aphid [Internet]. Available online: www.bookstore.ksre.ksu.edu (accessed on 13 September 2025).
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Reshi, Z.A.; Ahmad, W.; Lukatkin, A.S.; Bin Javed, S. From Nature to Lab: A Review of Secondary Metabolite Biosynthetic Pathways, Environmental Influences, and In Vitro Approaches. Metabolites 2023, 13, 895. [Google Scholar] [CrossRef]
- Grigorieva, E.; Livenets, A.; Stelmakh, E. Adaptation of Agriculture to Climate Change: A Scoping Review. Climate 2023, 11, 202. [Google Scholar] [CrossRef]
- Ouédraogo, M.; Barry, S.; Zougmoré, R.B. How using weather and climate information services may impact farm productivity and technical efficiency: Evidence from cowpea and sesame producers in burkina faso. Clim. Chang. Econ. 2023, 14, 2350011. [Google Scholar] [CrossRef]
- Forero, L.E.; Grenzer, J.; Heinze, J.; Schittko, C.; Kulmatiski, A. Greenhouse- and Field-Measured Plant-Soil Feedbacks Are Not Correlated. Front. Environ. Sci. 2019, 7, 184. [Google Scholar] [CrossRef]
- Rahnama, A.; Salehi, F.; Meskarbashee, M.; Khanlou, K.M.; Ghorbanpour, M.; Harrison, M.T. High temperature perturbs physicochemical parameters and fatty acids composition of safflower (Carthamus tinctorius L.). BMC Plant Biol. 2024, 24, 1080. [Google Scholar] [CrossRef]
- Alsajri, F.A.; Wijewardana, C.; Irby, J.T.; Bellaloui, N.; Krutz, L.J.; Golden, B.; Gao, W.; Reddy, K.R. Developing functional relationships between temperature and soybean yield and seed quality. Agron. J. 2020, 112, 194–204. [Google Scholar] [CrossRef]
- Kurasiak-Popowska, D.; Graczyk, M.; Przybylska-Balcerek, A.; Stuper-Szablewska, K. Influence of variety and weather conditions on fatty acid composition of winter and spring Camelina sativa varieties in Poland. Eur. Food Res. Technol. 2020, 247, 465–473. [Google Scholar] [CrossRef]
- Niu, Y.; Xiang, Y. An overview of biomembrane functions in plant responses to high-temperature Stress. Front. Plant Sci. 2018, 9, 915. [Google Scholar] [CrossRef]
- Zhao, X.; Wei, J.; He, L.; Zhang, Y.; Zhao, Y.; Xu, X.; Wei, Y.; Ge, S.; Ding, D.; Liu, M.; et al. Identification of fatty acid desaturases in maize and their differential responses to low and high temperature. Genes 2019, 10, 445. [Google Scholar] [CrossRef]
- Zheng, G.; Tian, B.; Zhang, F.; Tao, F.; Li, W. Plant adaptation to frequent alterations between high and low temperatures: Remodelling of membrane lipids and maintenance of unsaturation levels. Plant Cell Environ. 2011, 34, 1431–1442. [Google Scholar] [CrossRef]
- Sharma, P.; Lakra, N.; Goyal, A.; Ahlawat, Y.K.; Zaid, A.; Siddique, K.H.M. Drought and heat stress mediated activation of lipid signaling in plants: A critical review. Front. Plant Sci. 2023, 14, 1216835. [Google Scholar] [CrossRef]
- Falcone, D.L.; Ogas, J.P.; Somerville, C.R. Regulation of membrane fatty acid composition by temperature in mutants of Arabidopsis with alterations in membrane lipid composition. BMC Plant Biol. 2004, 4, 17. [Google Scholar] [CrossRef]
- He, M.; Qin, C.-X.; Wang, X.; Ding, N.-Z. Plant Unsaturated Fatty Acids: Biosynthesis and Regulation. Front. Plant Sci. 2020, 11, 390. [Google Scholar] [CrossRef]
- Xia, J.; Wishart, D.S. MetPA: A web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 2010, 26, 2342–2344. [Google Scholar] [CrossRef]
- Mageney, V.; Neugart, S.; Albach, D.C. A guide to the variability of flavonoids in Brassica oleracea. Molecules 2017, 22, 252. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.-H.; Jo, I.-H.; Sebastin, R.; Jeong, W.T.; Oh, S.; Heo, T.-Y.; Sung, J.; Hyun, T.K.; So, Y.-S.; Yu, J.-K.; et al. Comparative Analysis of Polyphenolic Compounds in Different Amaranthus Species: Influence of Genotypes and Harvesting Year. Antioxidants 2024, 13, 501. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xia, E.; Fu, J.; Xu, Y.; Zhao, X.; Tong, W.; Tang, Q.; Tadege, M.; Fernie, A.R.; Zhao, J. Diverse roles of MYB transcription factors in regulating secondary metabolite biosynthesis, shoot development, and stress responses in tea plants (Camellia sinensis). Plant J. 2022, 110, 1144–1165. [Google Scholar] [CrossRef]
- Mpofu, A.; Sapirstein, H.D.; Beta, T. Genotype and environmental variation in phenolic content, phenolic acid composition, and antioxidant activity of hard spring wheat. J. Agric. Food Chem. 2006, 54, 1265–1270. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W. Effect of plant growth temperature on antioxidant capacity in strawberry. J. Agric. Food Chem. 2001, 49, 4977–4982. [Google Scholar] [CrossRef]
- Moreira, X.; Abdala-Roberts, L.; Hidalgo-Galvez, M.D.; Vázquez-González, C.; Pérez-Ramos, I.M. Micro-climatic effects on plant phenolics at the community level in a Mediterranean savanna. Sci. Rep. 2020, 10, 14757. [Google Scholar] [CrossRef]
- Shamloo, M.; Babawale, E.A.; Furtado, A.; Henry, R.J.; Eck, P.K.; Jones, P.J.H. Effects of genotype and temperature on accumulation of plant secondary metabolites in Canadian and Australian wheat grown under controlled environments. Sci. Rep. 2017, 7, 9133. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Huang, L.-J.; Sun, X.-F.; Zhao, L.-L.; Wang, P.-C. Differential Physiological, Transcriptomic, and Metabolomic Responses of Paspalum wettsteinii Under High-Temperature Stress. Front. Plant Sci. 2022, 13, 865608. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Rusalepp, L. The Impact of Environmental Drivers and Competition on Phenolic Metabolite Profiles in Hybrid Aspen and Silver Birch [Internet]. 2023. Available online: https://www.researchgate.net/publication/376414803 (accessed on 13 September 2025).
- Selinga, T.I.; Maseko, S.T.; Gabier, H.; Rafudeen, M.S.; Muasya, A.M.; Crespo, O.; Ogola, J.B.O.; Valentine, A.J.; Ottosen, C.-O.; Rosenqvist, E.; et al. Regulation and physiological function of proteins for heat tolerance in cowpea (Vigna unguiculata) genotypes under controlled and field conditions. Front. Plant Sci. 2022, 13, 954527. [Google Scholar] [CrossRef]
- Erb, M.; Kliebenstein, D.J. Plant Secondary Metabolites as Defenses, Regulators, and Primary Metabolites: The Blurred Functional Trichotomy. Plant Physiol. 2020, 184, 39–52. [Google Scholar] [CrossRef]
- Mayer, R.R.; Cherry, J.H.; Rhodes, D. Effects of Heat Shock on Amino Acid Metabolism of Cowpea Cells. Plant Physiol. 1990, 94, 796–810. [Google Scholar] [CrossRef]
- Fraire-Velázquez, S.; Emmanuel, V. Abiotic Stress in Plants and Metabolic Responses. In Abiotic Stress-Plant Responses and Applications in Agriculture; InTech: London, UK, 2013. [Google Scholar]
- Perkowski, M.C.; Warpeha, K.M. Phenylalanine roles in the seed-to-seedling stage: Not just an amino acid. Plant Sci. 2019, 289, 110223. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Widhalm, J.R.; Qian, Y.; Maeda, H.; Cooper, B.R.; Jannasch, A.S.; Gonda, I.; Lewinsohn, E.; Rhodes, D.; Dudareva, N. An alternative pathway contributes to phenylalanine biosynthesis in plants via a cytosolic tyrosine:phenylpyruvate aminotransferase. Nat. Commun. 2013, 4, 2833. [Google Scholar] [CrossRef] [PubMed]
- Timoneda, A.; Sheehan, H.; Feng, T.; Lopez-Nieves, S.; Maeda, H.A.; Brockington, S. Redirecting Primary Metabolism to Boost Production of Tyrosine-Derived Specialised Metabolites in Planta. Sci. Rep. 2018, 8, 17256. [Google Scholar] [CrossRef] [PubMed]
- Collakova, E.; DellaPenna, D. The Role of Homogentisate Phytyltransferase and Other Tocopherol Pathway Enzymes in the Regulation of Tocopherol Synthesis during Abiotic Stress. Plant Physiol. 2003, 133, 930–940. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Patil, J.R.; Mhatre, K.J.; Yadav, K.; Yadav, L.S.; Srivastava, S.; Nikalje, G.C. Flavonoids in plant-environment interactions and stress responses. Discov. Plants 2024, 1, 68. [Google Scholar] [CrossRef]
- Jan, R.; Kim, N.; Lee, S.-H.; Khan, M.A.; Asaf, S.; Lubna; Park, J.-R.; Asif, S.; Lee, I.-J.; Kim, K.-M. Enhanced Flavonoid Accumulation Reduces Combined Salt and Heat Stress Through Regulation of Transcriptional and Hormonal Mechanisms. Front. Plant Sci. 2021, 12, 796956. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Chen, A.; Liu, T.; Wang, Z.; Chen, X. Plant root suberin: A layer of defence against biotic and abiotic stresses. Front. Plant Sci. 2022, 13, 1056008. [Google Scholar] [CrossRef]
- Upchurch, R.G. Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress. Biotechnol. Lett. 2008, 30, 967–977. [Google Scholar] [CrossRef]
- Abugri, D.A.; Pritchett, G. Determination of chlorophylls, carotenoids, and fatty acid profiles of tetrapleura tetraptera seeds and their health implication. J. Herbs Spices Med. Plants 2013, 19, 391–400. [Google Scholar] [CrossRef]
- Abugri, D.; Tiimob, B.; Apalangya, V.; Pritchett, G.; McElhenney, W. Bioactive and nutritive compounds in Sorghum bicolor (Guinea corn) red leaves and their health implication. Food Chem. 2013, 138, 718–723. [Google Scholar] [CrossRef]
| Season | June | July | August | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Relative Humidity (%) | Rainfall (mm) | Max (°C) | Min (°C) | Max (°C) | Min (°C) | Max (°C) | Min (°C) | Max (°C) | Min (°C) | |
| 2020 | 97.92 ± 0.54 a | 1423 ± 13.19 a | 32.02 ± 0.21 a | 20.21 ± 0.19 a | 30.98 ± 0.33 a | 18.88 ± 0.40 a | 32.58 ± 0.33 a | 21.01 ± 0.18 a | 32.47 ± 0.36 a | 20.7 ± 0.21 a |
| 2021 | 97.66 ± 0.57 a | 774 ± 3.17 b | 29.88 ± 0.23 b | 18.80 ± 0.21 b | 29.50 ± 0.33 b | 17.91 ± 0.32 a | 28.84 ± 0.37 b | 18.14 ± 0.17 b | 31.29 ± 0.38 b | 20.32 ± 0.39 a |
| p value | 0.74 | <0.0001 | <0.0001 | <0.0001 | 0.0027 | 0.0617 | <0.0001 | <0.0001 | 0.0279 | 0.3952 |
| CV (%) | 0.055 | 0.084 | 0.069 | 0.097 | 0.06 | 0.11 | 0.064 | 0.049 | 0.065 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mweta, J.M.; Kanyairita, G.G.; Quarcoo, F.; Makwinja, F.; Abugri, D.A.; Bernard, G.; Nashar, T.; Mortley, D.G.; Boersma, M.; Bonsi, C.K. Effect of Weather Conditions on Phytochemical Profiles in Organically Grown Cowpea (Vignaunguiculata L. Walp). Plants 2025, 14, 3179. https://doi.org/10.3390/plants14203179
Mweta JM, Kanyairita GG, Quarcoo F, Makwinja F, Abugri DA, Bernard G, Nashar T, Mortley DG, Boersma M, Bonsi CK. Effect of Weather Conditions on Phytochemical Profiles in Organically Grown Cowpea (Vignaunguiculata L. Walp). Plants. 2025; 14(20):3179. https://doi.org/10.3390/plants14203179
Chicago/Turabian StyleMweta, Jamila M., Getrude G. Kanyairita, Franklin Quarcoo, Faraja Makwinja, Daniel A. Abugri, Gregory Bernard, Toufic Nashar, Desmond G. Mortley, Melissa Boersma, and Conrad K. Bonsi. 2025. "Effect of Weather Conditions on Phytochemical Profiles in Organically Grown Cowpea (Vignaunguiculata L. Walp)" Plants 14, no. 20: 3179. https://doi.org/10.3390/plants14203179
APA StyleMweta, J. M., Kanyairita, G. G., Quarcoo, F., Makwinja, F., Abugri, D. A., Bernard, G., Nashar, T., Mortley, D. G., Boersma, M., & Bonsi, C. K. (2025). Effect of Weather Conditions on Phytochemical Profiles in Organically Grown Cowpea (Vignaunguiculata L. Walp). Plants, 14(20), 3179. https://doi.org/10.3390/plants14203179






