High-Blue/Low-Red Mixed Light Modulates Photoperiodic Flowering in Chrysanthemum via Photoreceptor and Sugar Pathways
Abstract
1. Introduction
2. Results
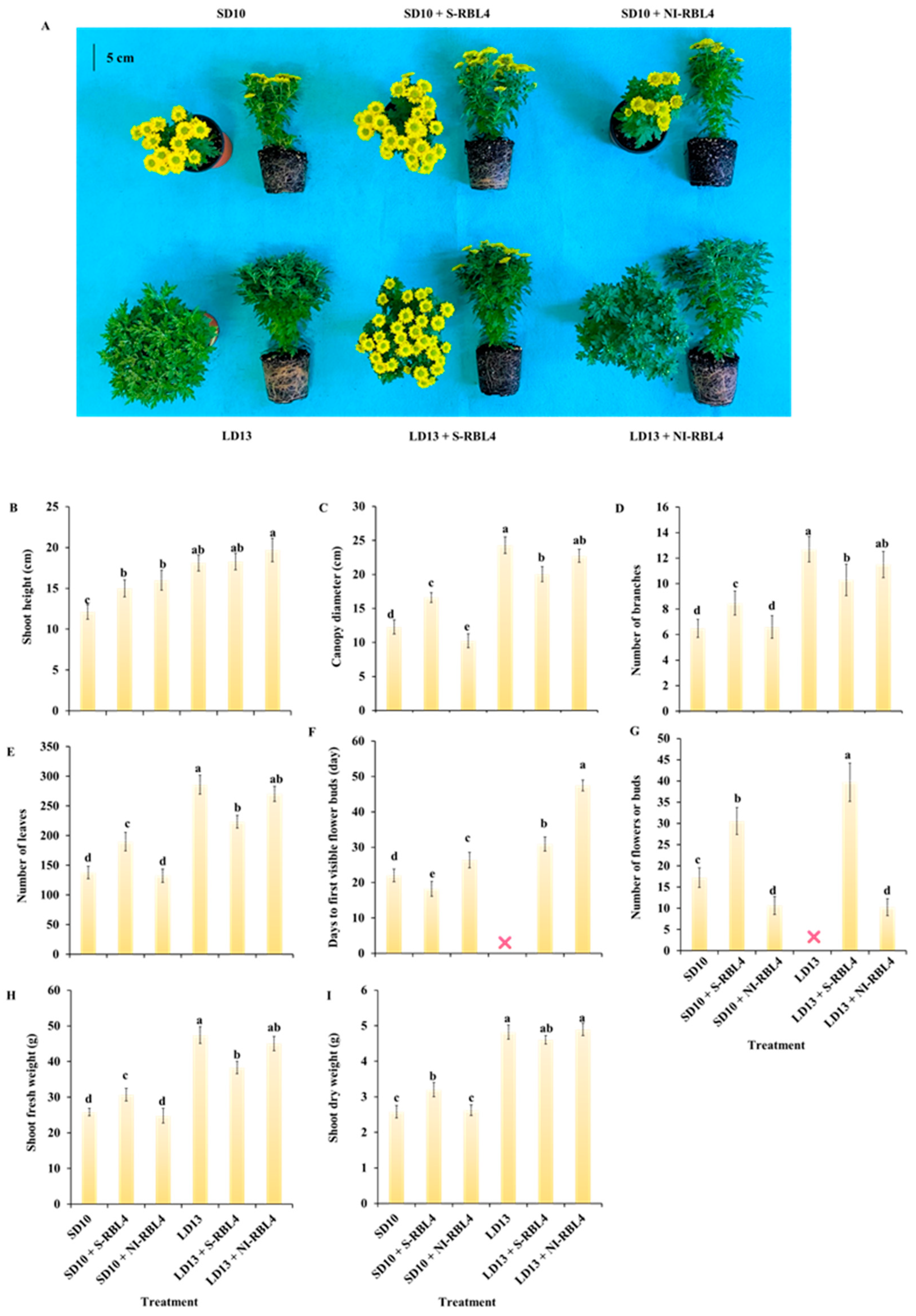
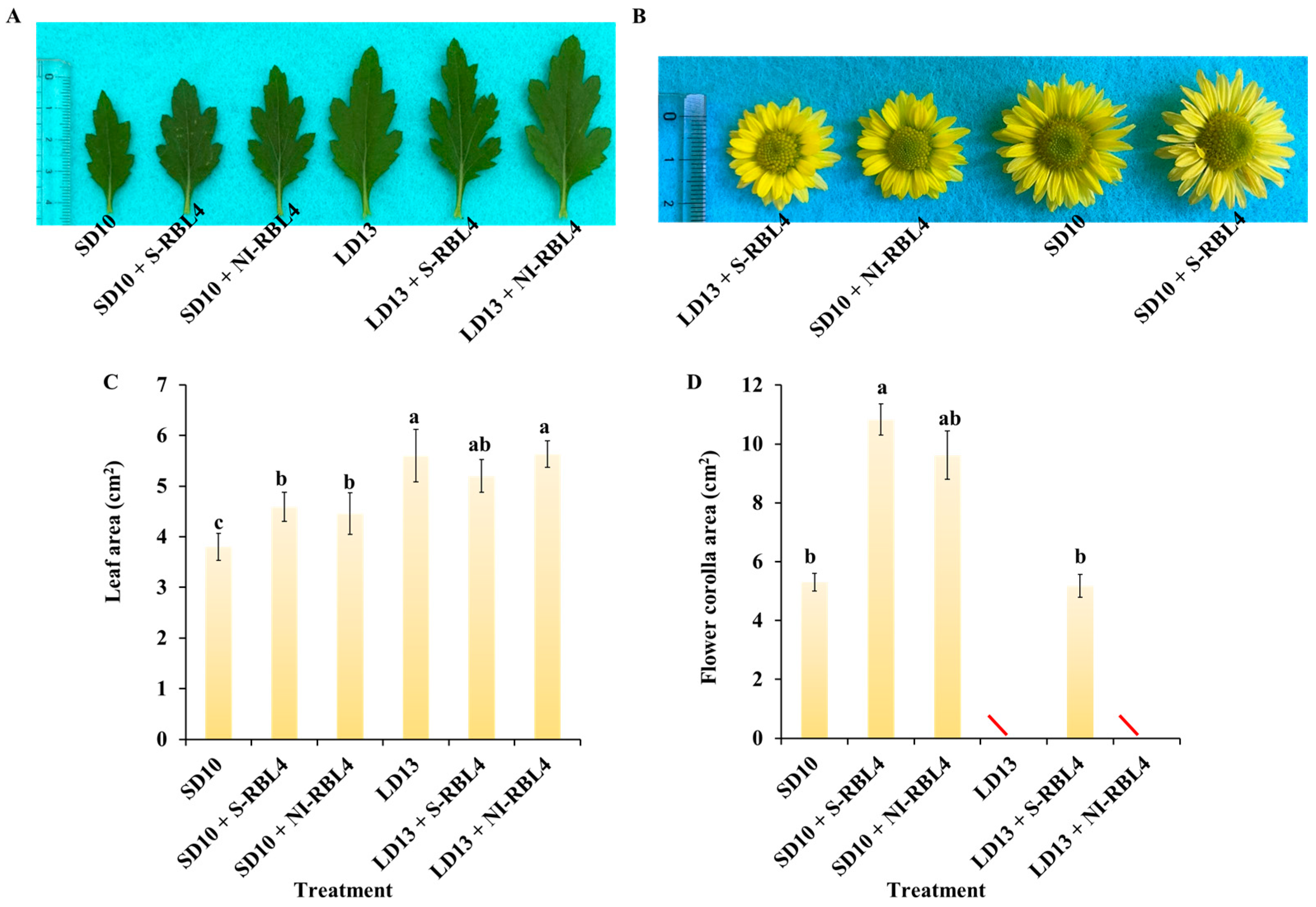
2.1. Growth and Flowering
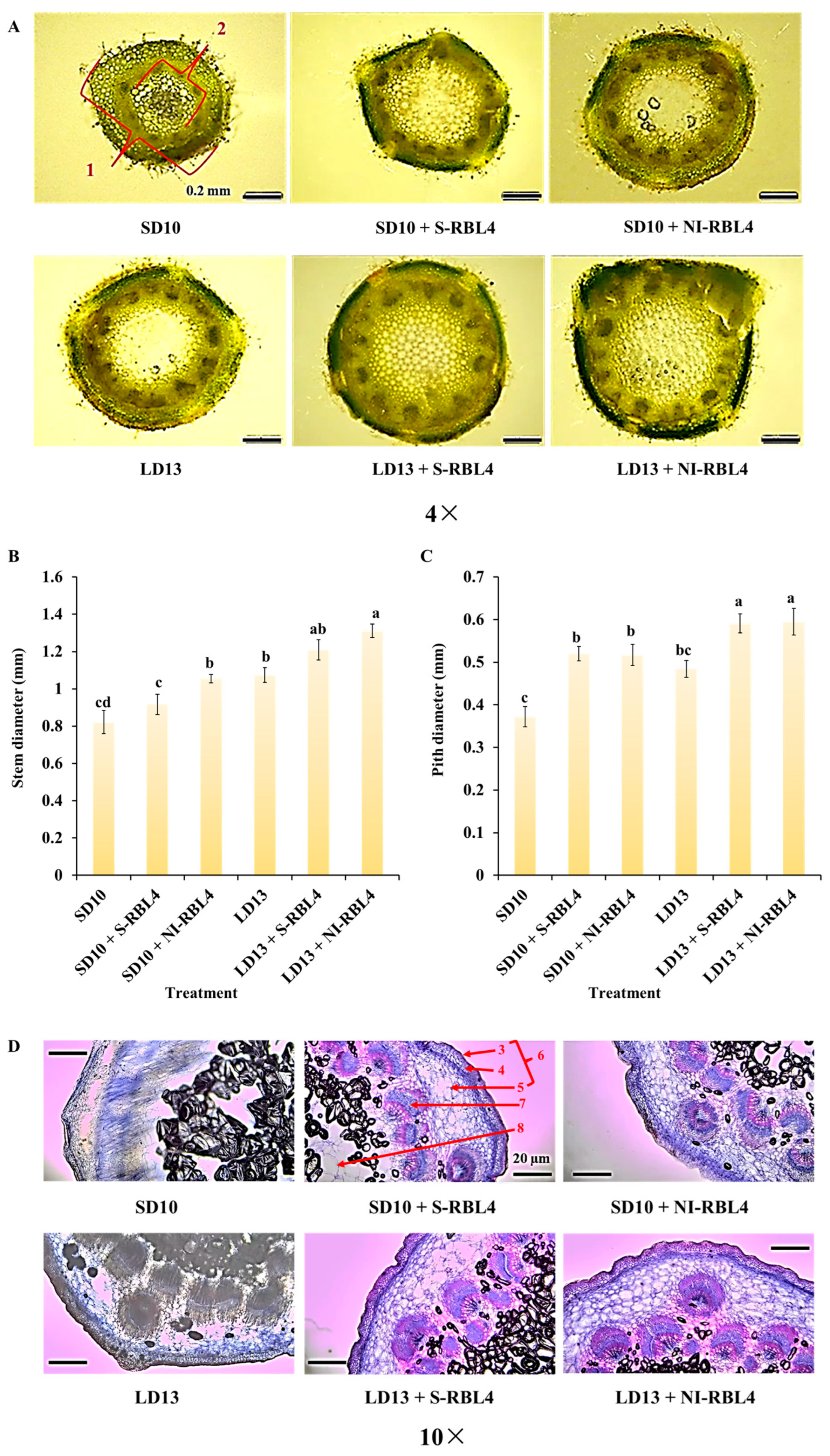
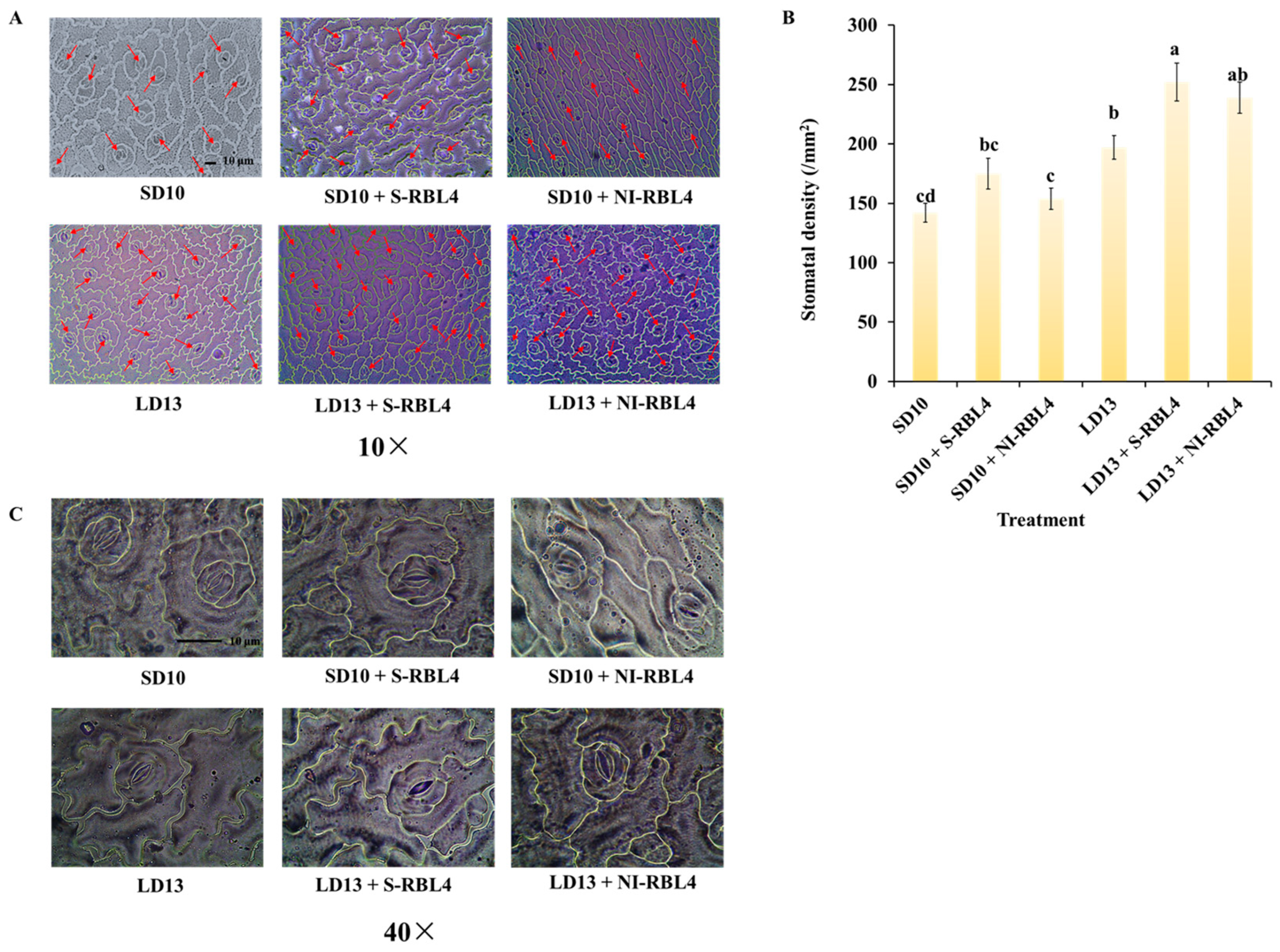
2.2. Stem Anatomical Structures and Stomatal Characteristics
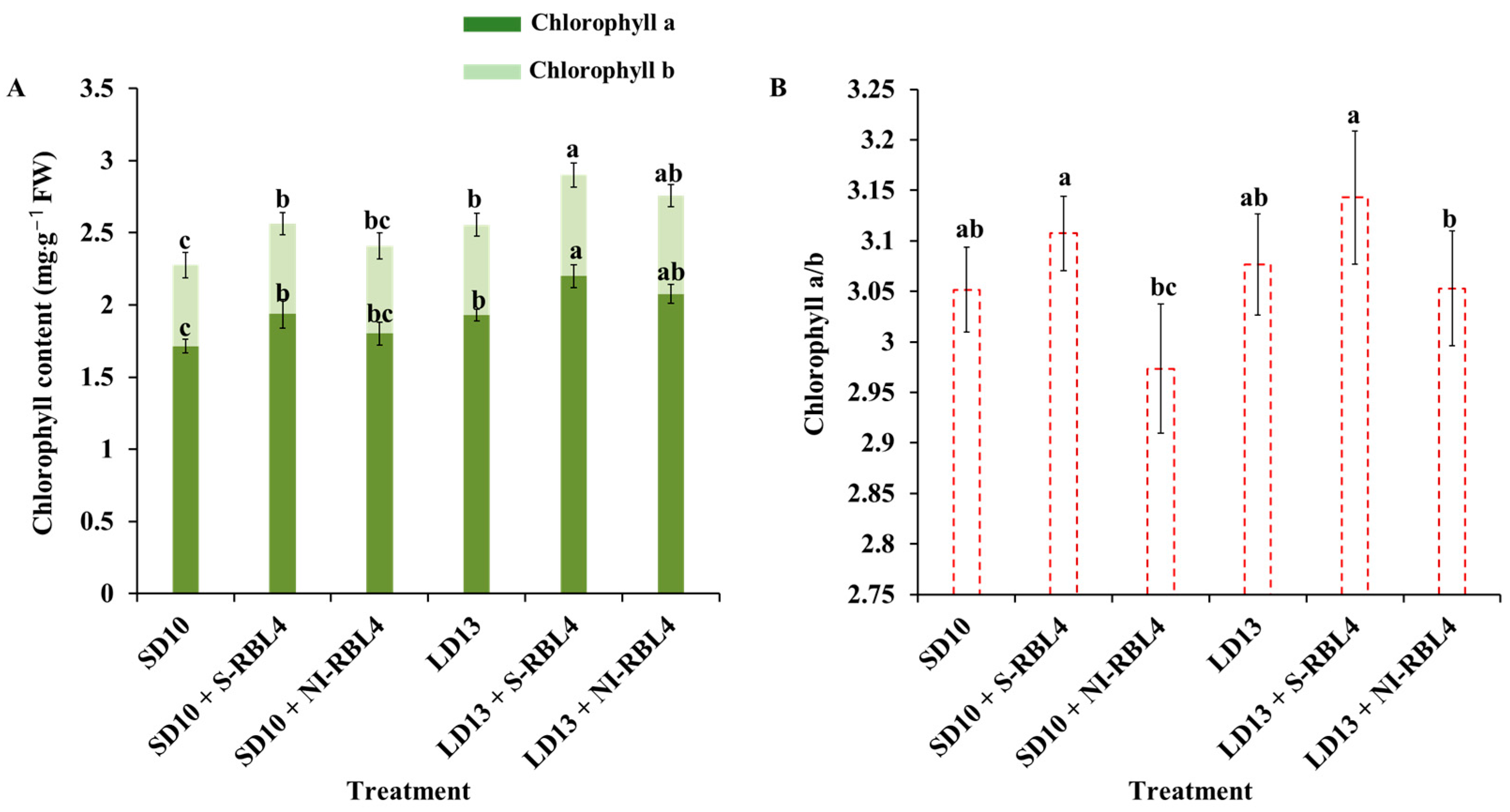
2.3. Chlorophyll Content
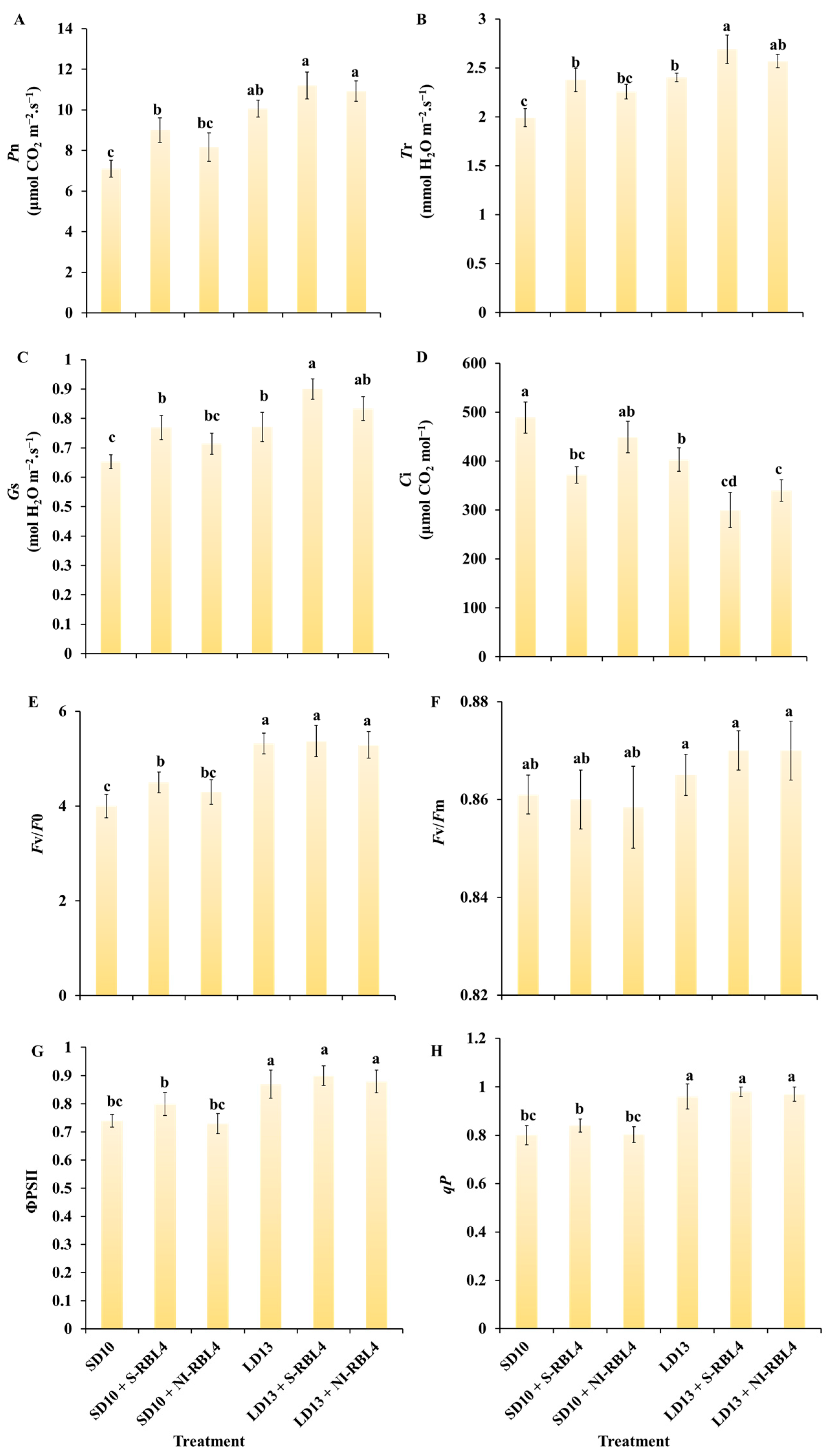
2.4. Photosynthetic and Chlorophyll Fluorescence Parameters
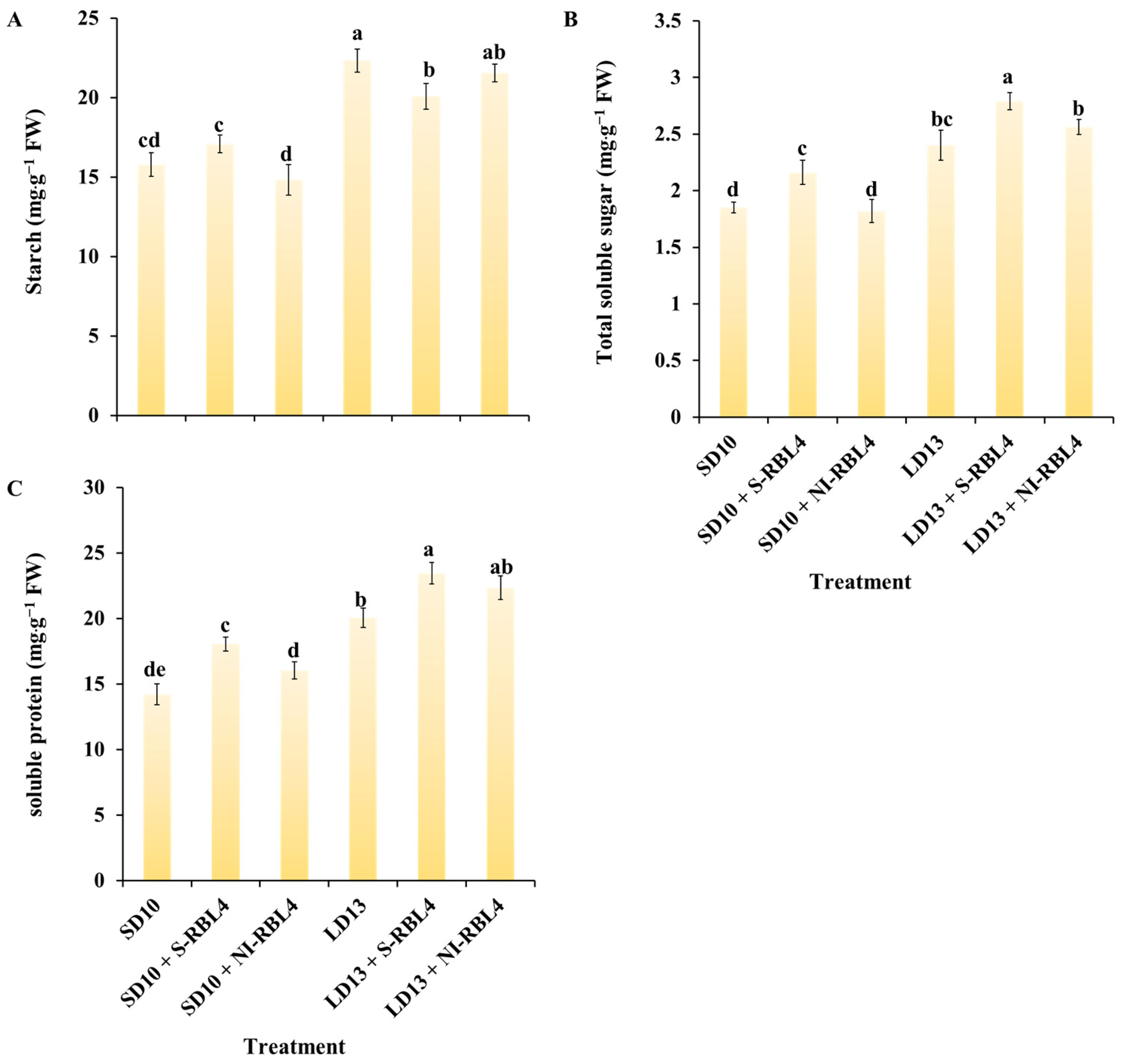
2.5. Carbohydrates and Soluble Protein
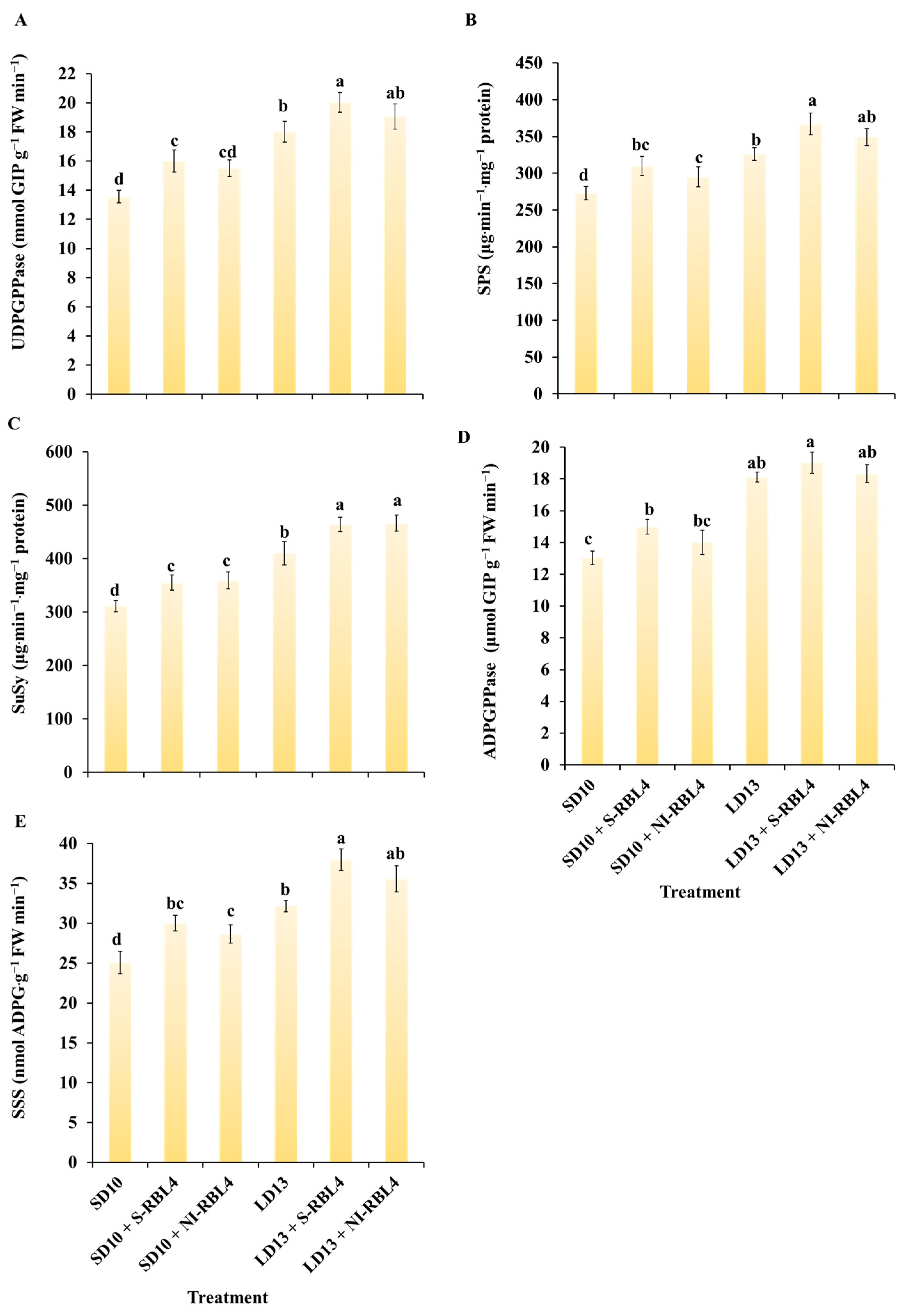
2.6. Enzyme Activities Related to Sugar Metabolism
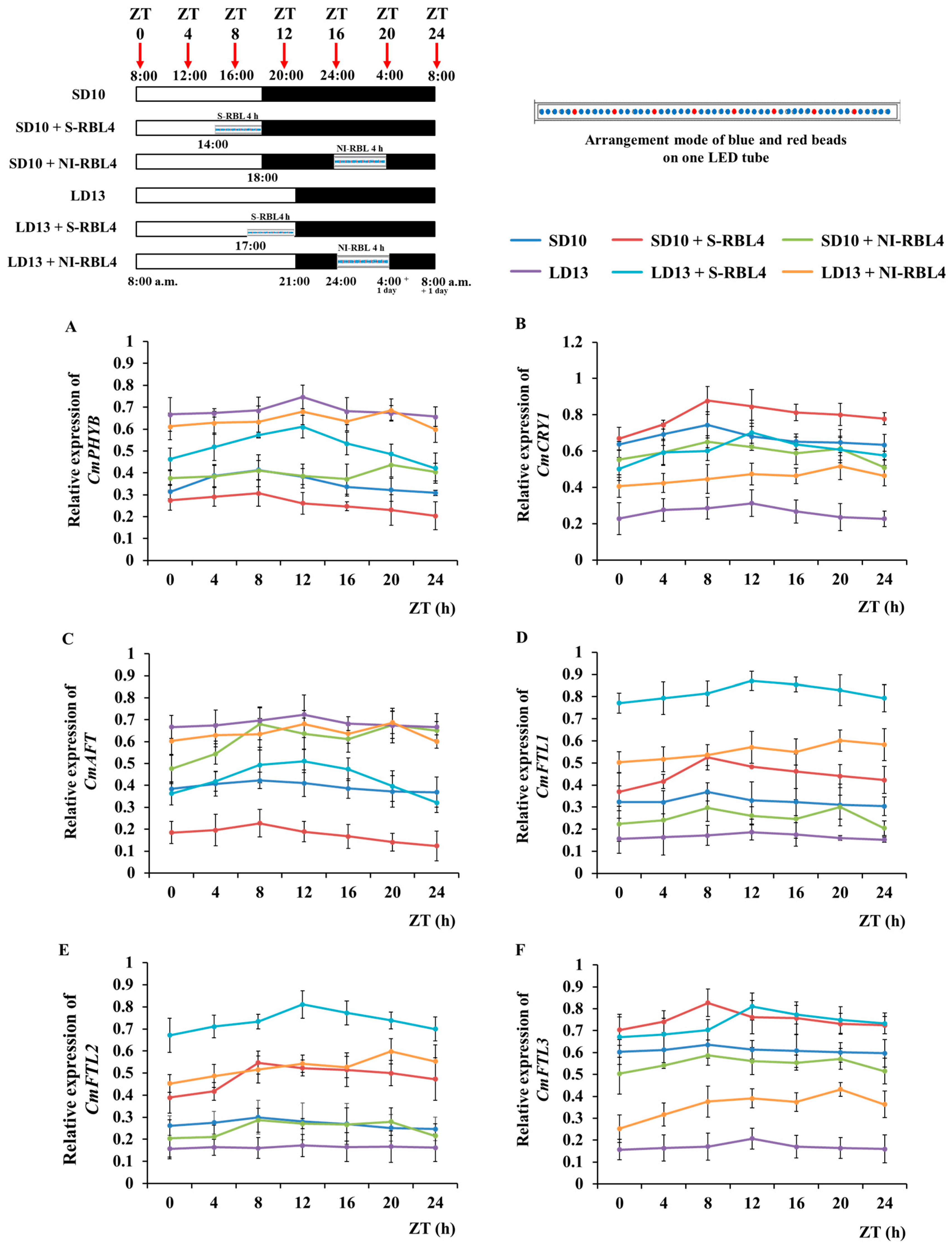
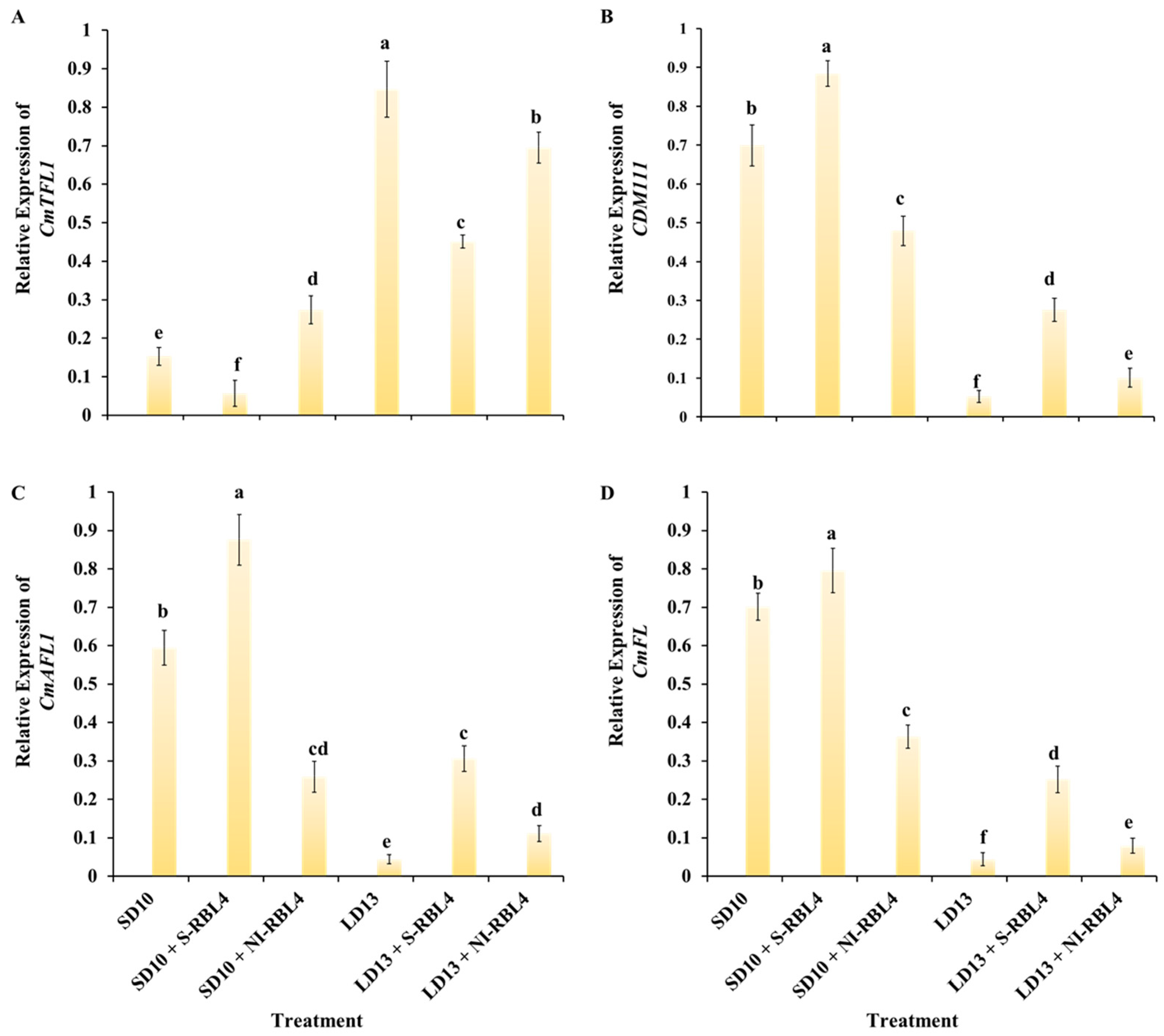
2.7. Expression Level of Flowering- or Photoreceptor-Related Genes
3. Discussion
3.1. Nutritional Growth and Physiological Characteristics in Response to “High-Blue/Low-Red” Mixed Light in Photoperiodic Treatments
3.2. Photoperiodic Flowering in Response to Photoreceptor-Mediated Florigenic and Anti-Florigenic Gene Expression Under “High-Blue/Low-Red” Mixed Light in Photoperiodic Treatments
3.3. Photoperiodic Flowering in Response to the Co-Regulation of Photoperiod- and Sucrose-Mediated Pathways Under “High-Blue/Low-Red” Mixed Light in Photoperiodic Treatment
3.4. Ornamental Traits of Flowering and Branching in Response to CmTFL1 Under “High-Blue/Low-Red” Mixed Light in Photoperiodic Treatments
4. Materials and Methods
4.1. Experimental Set-Up and Cultivation Conditions
4.2. Light Treatments
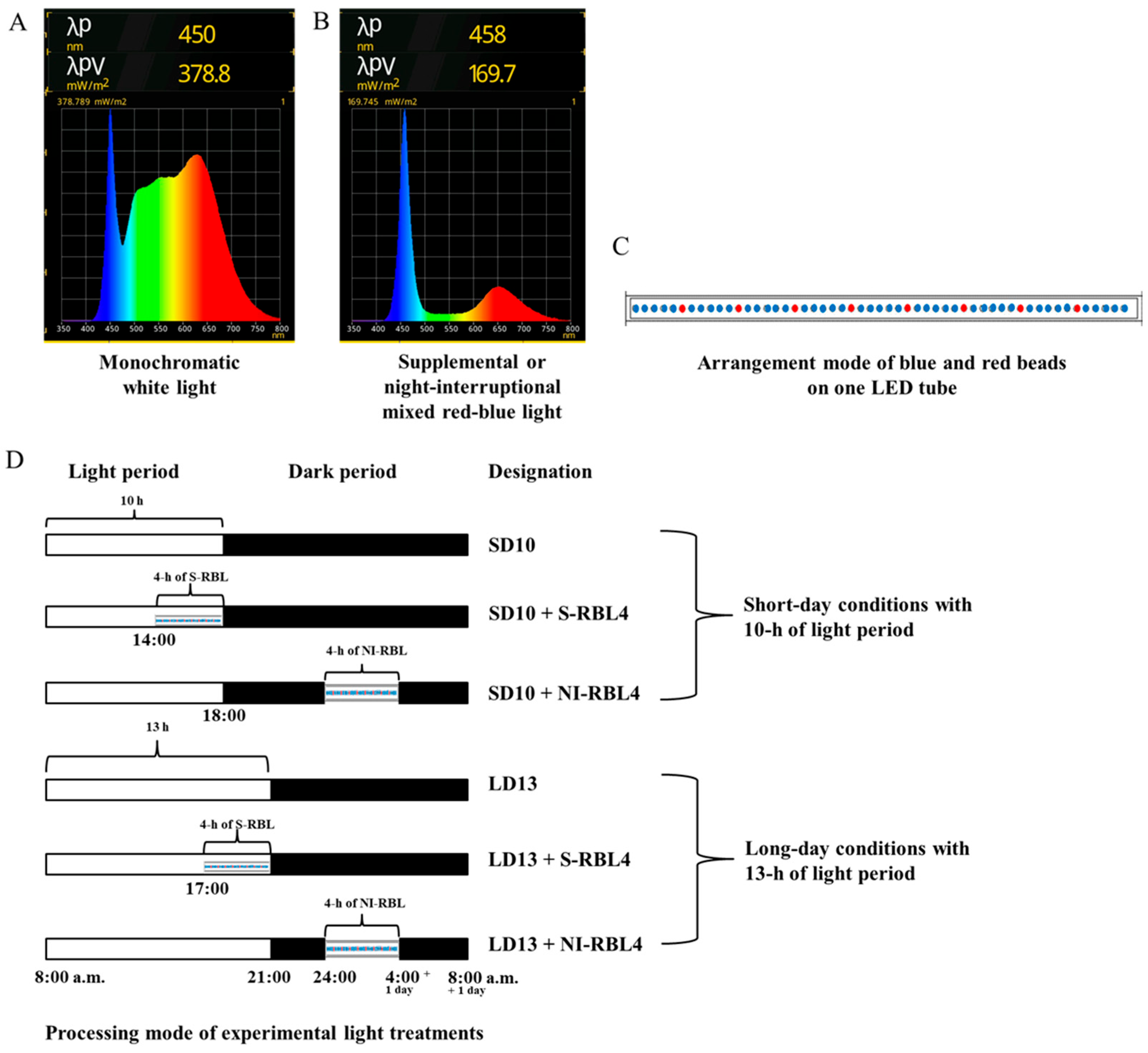
4.3. Measurement of Plant Growth Indexes
4.4. Microscopic Observation of Stem Cross Sections and Stomatal Traits
4.5. Measurement of Photosynthetic and Chlorophyll Fluorescence Parameters
4.6. Determination of Chlorophyll Content
4.7. Sample Preparation for Biochemical Analyses and Total RNA Extraction
4.8. Determination of Carbohydrates and Soluble Protein
4.9. Determination of Enzyme Activities Related to Sugar Metabolism
4.10. Verification by Real-Time Quantitative PCR
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bünning, E. Die endogene Tagesrhythmik als Grundlage der photoperiodischen Reaktion. Ber. Dtsch. Bot. Ges. 1936, 54, 590–607. [Google Scholar]
- Pittendrigh, C.S.; Minis, D.H. The entrainment of circadian oscillations by light and their role as photoperiodic clocks. Am. Nat. 1964, 98, 261–294. [Google Scholar] [CrossRef]
- Yanovsky, M.J.; Kay, S.A. Living by the calendar: How plants know when to flower. Nat. Rev. Mol. Cell Biol. 2003, 4, 265–276. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Weigel, D. Move on up, it’s time for change—Mobile signals controlling photoperiod-dependent flowering. Genes Dev. 2007, 21, 2371–2384. [Google Scholar] [CrossRef]
- Thomas, B. Light signals and flowering. J. Exp. Bot. 2006, 57, 3387–3393. [Google Scholar] [CrossRef]
- Johnson, E.; Bradley, M.; Harberd, N.P.; Whitelam, G.C. Photoresponses of light-grown phyA mutants of Arabidopsis (phytochrome a is required for the perception of daylength extensions). Plant Physiol. 1994, 105, 141–149. [Google Scholar] [CrossRef]
- Mockler, T.; Yang, H.; Yu, X.; Parikh, D.; Cheng, Y.C.; Dolan, S.; Lin, C. Regulation of photoperiodic flowering by Arabidopsis photoreceptors. Proc. Natl. Acad. Sci. USA 2003, 100, 2140–2145. [Google Scholar] [CrossRef]
- Goto, N.; Kumagai, T.; Koornneef, M. Flowering responses to light-breaks in photomorphogenic mutants of Arabidopsis thaliana, a long-day plant. Physiol. Plant. 1991, 83, 209–215. [Google Scholar] [CrossRef]
- Devlin, P.F.; Patel, S.R.; Whitelam, G.C. Phytochrome E influences internode elongation and flowering time in Arabidopsis. Plant Cell 1998, 10, 1479–1487. [Google Scholar] [CrossRef]
- Devlin, P.F.; Robson, P.R.; Patel, S.R.; Goosey, L.; Sharrock, R.A.; Whitelam, G.C. Phytochrome D acts in the shade-avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiol. 1999, 119, 909–916. [Google Scholar] [CrossRef]
- Mockler, T.C.; Guo, H.; Yang, H.; Duong, H.; Lin, C. Antagonistic actions of Arabidopsis cryptochromes and phytochrome B in the regulation of floral induction. Development 1999, 126, 2073–2082. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Praekelt, U.; Stoddart, W.M.; Billingham, O.E.; Halliday, K.J.; Whitelam, G.C. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 2003, 131, 1340–1346. [Google Scholar] [CrossRef]
- Guo, H.; Yang, H.; Mockler, T.C.; Lin, C. Regulation of flowering time by Arabidopsis photoreceptors. Science 1998, 279, 1360–1363. [Google Scholar] [CrossRef]
- Somers, D.E.; Devlin, P.F.; Kay, S.A. Phytochromes and cryptochromes in the entrainment of the Arabidopsis circadian clock. Science 1998, 282, 1488–1490. [Google Scholar] [CrossRef]
- Valverde, F.; Mouradov, A.; Soppe, W.; Ravenscroft, D.; Samach, A.; Coupland, G. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 2004, 303, 1003–1006. [Google Scholar] [CrossRef]
- Thomas, B.; Vince-Prue, D. Photoperiodism in Plants; Elsevier: San Diego, CA, USA, 1996. [Google Scholar]
- Runkle, E.S.; Heins, R.D. Specific functions of red, far red, and blue light in flowering and stem extension of long-day plants. J. Am. Soc. Hortic. Sci. 2001, 126, 275–282. [Google Scholar] [CrossRef]
- Izawa, T.; Oikawa, T.; Tokutomi, S.; Okuno, K.; Shimamoto, K. Phytochromes confer the photoperiodic control of flowering in rice (a short-day plant). Plant J. 2000, 22, 391–399. [Google Scholar] [CrossRef]
- Izawa, T.; Oikawa, T.; Sugiyama, N.; Tanisaka, T.; Yano, M.; Shimamoto, K. Phytochrome mediates the external light signal to repress FT orthologs in photoperiodic flowering of rice. Genes Dev. 2002, 16, 2006–2020. [Google Scholar] [CrossRef]
- Takano, M.; Inagaki, N.; Xie, X.; Kiyota, S.; Baba-Kasai, A.; Tanabata, T.; Shinomura, T. Phytochromes are the sole photoreceptors for perceiving red/far-red light in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 14705–14710. [Google Scholar] [CrossRef]
- Ishikawa, R.; Tamaki, S.; Yokoi, S.; Inagaki, N.; Shinomura, T.; Takano, M.; Shimamoto, K. Suppression of the floral activator Hd3a is the principal cause of the night break effect in rice. Plant Cell 2005, 17, 3326–3336. [Google Scholar] [CrossRef]
- Ishikawa, R.; Shinomura, T.; Takano, M.; Shimamoto, K. Phytochrome dependent quantitative control of Hd3a transcription is the basis of the night break effect in rice flowering. Genes Genet. Syst. 2009, 84, 179–184. [Google Scholar] [CrossRef]
- Takimoto, A.; Naito, Y. Studies on the light controlling flower initiation of pharbitis nil X. Photoperiodic responses of the seedlings grown under various light conditions. Bot. Mag. Tokyo 1962, 75, 255–263. [Google Scholar] [CrossRef]
- Salisbury, F.B. Time measurement and the light period in flowering. Planta 1965, 66, 1–26. [Google Scholar] [CrossRef]
- Ohtani, T.; Ishiguri, Y. Inhibitory action of blue and far-red light in the flowering of Lemna paucicostata. Physiol. Plant. 1979, 47, 255–259. [Google Scholar] [CrossRef]
- Cathey, H.; Borthwick, H. Photoreversibility of floral initiation in chrysanthemum. Bot. Gaz. 1957, 119, 71–76. [Google Scholar] [CrossRef]
- Kadman-Zahavi, A.; Ephrat, E. Effect of red and far-red illuminations at the end of short days and their interaction with night-break illuminations, on flowering of Chrysanthemum morifolium plants. Plant Cell Physiol. 1973, 14, 409–411. [Google Scholar]
- McMahon, M.; Kelly, J. CuSO4 filters influence flowering of chrysanthemum cv. Spears. Sci. Hortic. 1999, 79, 207–215. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. The flowering of SDP chrysanthemum in response to intensity of supplemental or night-interruptional blue light is modulated by both photosynthetic carbon assimilation and photoreceptor-mediated regulation. Front. Plant Sci. 2022, 13, 981143. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Low-intensity blue light supplemented during photoperiod in controlled environment induces flowering and antioxidant production in kalanchoe. Antioxidants 2022, 11, 811. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Blue light supplemented at intervals in long-day conditions intervenes in photoperiodic flowering, photosynthesis, and antioxidant properties in chrysanthemums. Antioxidants 2022, 11, 2310. [Google Scholar] [CrossRef]
- Yang, J.; Song, J.; Jeong, B.R. Both the positioned supplemental or night-interruptional blue light and the age of leaves (or tissues) are important for flowering and vegetative growth in chrysanthemum. Plants 2024, 13, 2874. [Google Scholar] [CrossRef]
- Tylewicz, S.; Petterle, A.; Marttila, S.; Miskolczi, P.; Azeez, A.; Singh, R.K.; Immanen, J.; Mähler, N.; Hvidsten, T.R.; Eklund, D.M.; et al. Photoperiodic control of seasonal growth is mediated by ABA acting on cell-cell communication. Science 2018, 360, 212–215. [Google Scholar] [CrossRef]
- Bauerle, W.L.; Oren, R.; Way, D.A.; Qian, S.S.; Stoy, P.C.; Thornton, P.E.; Bowden, J.D.; Hoffman, F.M.; Reynolds, R.F. Photoperiodic regulation of the seasonal pattern of photosynthetic capacity and the implications for carbon cycling. Proc. Natl. Acad. Sci. USA 2012, 109, 8612–8617. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, Y.; Zhang, Y.; Ren, S.; Wei, Y.; Zhang, Y. Short-day photoperiod effects on plant growth, flower bud differentiation, and yield formation in adzuki bean (Vigna angularis). Int. J. Agric. Biol. 2016, 18, 337–345. [Google Scholar] [CrossRef]
- Hendriks, J.H.M.; Kolbe, A.; Gibon, Y.; Stitt, M.; Geigenberger, P. ADP-glucose pyrophosphorylase is activated by posttranslational redox-modification in response to light and to sugars in leaves of Arabidopsis and other plant species. Plant Physiol. 2003, 133, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Pearcy, R.W. Radiation and Light Measurements. In Plant Physiological Ecology: Field Methods and Instrumentation; Springer: Dordrecht, The Netherlands, 1989; pp. 97–116. [Google Scholar]
- Gent, M.P. Dynamic carbohydrate supply and demand model of vegetative growth: Response to temperature, light, carbon dioxide, and day length. Agronomy 2018, 8, 21. [Google Scholar] [CrossRef]
- Wei, H.; Ren, J.; Zhou, J. Effect of exponential fertilization on growth and nutritional status in buddhist pine (Podocarpus macrophyllus [thunb.] D. Don) seedlings cultured in natural and prolonged photoperiods. Soil Sci. Plant Nutr. 2013, 59, 933–941. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, H.; Zhang, J.; Zhao, Q.; Meng, G.; Zhang, J.; Wang, H. Growth, nutrient uptake and utilization responses of buddhist pine and japanese maple seedlings to the extended photoperiod. J. Zhejiang Univ. Sci. B 2016, 42, 190–198. [Google Scholar]
- Garner, W.W. Comparative responses of long-day and short-day plants to relative length of day and night. Plant Physiol. 1933, 8, 347–356. [Google Scholar] [CrossRef]
- Stirbet, A. On the relation between the kautsky effect (chlorophyll a fluorescence induction) and photosystem II: Basics and applications of the OJIP fluorescence transient. J. Photochem. Photobiol. B 2011, 104, 236–257. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, X.; Lin, Y.; Li, J.; Chen, C.; Wu, C. Effect of salt stress on photosynthetic and chlorophyII fluorescent characteristics in Alnus formosana seedlings. J. For. Environ. 2013, 33, 193–199. [Google Scholar]
- Teng, Z.Y.; Zhang, H.H.; Dai, X.; Hu, J.W.; Zhang, X.L.; Sun, G.Y. Effects of drought stress on PS II photochemical activity in leaves of morus alba. Acta Agric. Zhejiangensis 2016, 28, 1–8. [Google Scholar]
- Yao, N.; Liu, J.F.; Jiang, Z.P.; Chang, E.M.; Zhao, X.L.; Xie, R.; Wang, Q. Effects of photoperiod and light quality on seedling growth and chlorophyll fluorescence kinetics of Quercus L. J. Nanjing For. Univ. 2022, 46, 111–120. [Google Scholar]
- Lee, H.; Han, G.; Cheong, E.J. Effect of different treatments and light quality on Ulmus pumila L. germination and seedling growth. For. Sci. Technol. 2021, 17, 162–168. [Google Scholar] [CrossRef]
- Cioć, M.; Dziurka, M.; Pawłowska, B. Changes in endogenous phytohormones of Gerbera jamesonii axillary shoots multiplied under different light emitting diodes light quality. Molecules 2022, 27, 1804. [Google Scholar] [CrossRef]
- Kurepin, L.V.; Shah, S.; Reid, D.M. Light quality regulation of endogenous levels of auxin, abscisic acid and ethylene production in petioles and leaves of wild type and ACC deaminase Transgenic brassica napus seedlings. Plant Growth Regul. 2007, 52, 53–60. [Google Scholar] [CrossRef]
- Li, W.; Liu, S.W.; Ma, J.J.; Liu, H.M.; Han, F.X.; Li, Y.; Niu, S.H. Gibberellin signaling is required for far-red light-induced shoot elongation in Pinus tabuliformis seedlings. Plant Physiol. 2020, 182, 658–668. [Google Scholar]
- Wei, H.; Hauer, R.J.; Chen, G.; Chen, X.; He, X. Growth, nutrient assimilation, and carbohydrate metabolism in korean pine (Pinus koraiensis) seedlings in response to light spectra. Forests 2019, 11, 44. [Google Scholar] [CrossRef]
- Xu, D.; Gao, W.; Ruan, J. Effects of light quality on plant growth and development. Plant Physiol. J. 2015, 51, 1217–1234. [Google Scholar]
- Thomas, B. Specific effects of blue light on plant growth and development. In Plants and the Daylight Spectrum; Academic Press: London, UK, 1981; pp. 443–459. [Google Scholar]
- Richter, G.; Wessel, K. Red light inhibits blue light-induced chloroplast development in cultured plant cells at the mRNA level. Plant Mol. Biol. 1985, 5, 175–182. [Google Scholar]
- Wang, H.; Gu, M.; Cui, J.; Shi, K.; Zhou, Y.; Yu, J. Effects of light quality on CO2 assimilation, chlorophyll-fluorescence quenching, expression of calvin cycle genes and carbohydrate accumulation in Cucumis sativus. J. Photochem. Photobiol. B 2009, 96, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Hundrieser, J.; Richter, G. Blue light-induced synthesis of ribulosebisphosphate carboxylase in cultured plant cells. Plant Cell Rep. 1982, 1, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Roscher, E.; Zetsche, K. The effects of light quality and intensity on the synthesis of ribulose-1,5-bisphosphate carboxylase and its mRNAs in the green alga chlorogonium elongatum. Planta 1986, 167, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, A.; Miyachi, S. Blue light-induced formation of phosphoenolpyruvate carboxylase in colorless chlorella mutant cells. Plant Cell Physiol. 1975, 16, 729–736. [Google Scholar] [CrossRef]
- Ruyters, G.; Conradt, W. Blue light-effects on enzymes of the carbohydrate metabolism in chlorella 1. Pyruvate kinase. In The Blue Light Syndrome; Springer: Berlin/Heidelberg, Germany, 1980; pp. 361–367. [Google Scholar]
- Ren, M.; Liu, S.; Mao, G.; Tang, C.; Gai, P.; Guo, X.; Zheng, H.; Wang, W.; Tang, Q. Simultaneous application of red and blue light regulate carbon and nitrogen metabolism, induces antioxidant defense system and promote growth in rice seedlings under low light stress. Int. J. Mol. Sci. 2023, 24, 10706. [Google Scholar] [CrossRef]
- Zheng, L.; Labeke, V.; Marie, C. Long-term effects of red-and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef]
- Centofante, A.R. Light quality on the morphoanatomy and physiology of Campomanesia pubescens (DC.) O. Berg. seedlings. Sci. Hortic. 2020, 259, 108765. [Google Scholar] [CrossRef]
- Ma, X.F.; Hall, D.; Onge, K.R.S.; Jansson, S.; Ingvarsson, P.K. Genetic differentiation, clinal variation and phenotypic associations with growth cessation across the populus tremula photoperiodic pathway. Genetics 2010, 186, 1033–1044. [Google Scholar] [CrossRef]
- Franklin, K.A. Light and temperature signal crosstalk in plant development. Curr. Opin. Plant Biol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Chiang, C.; Bånkestad, D.; Hoch, G. Reaching natural growth: Light quality effects on plant performance in indoor growth facilities. Plants 2020, 9, 1273. [Google Scholar] [CrossRef]
- Lang, A.; Melchers, G. Die photoperiodische reaktion von hyoscyamus niger. Planta 1943, 33, 653–702. [Google Scholar] [CrossRef]
- Higuchi, Y.; Narumi, T.; Oda, A.; Nakano, Y.; Sumitomo, K.; Fukai, S.; Hisamatsu, T. The gated induction system of a systemic floral inhibitor, antiflorigen, determines obligate short-day flowering in chrysanthemums. Proc. Natl. Acad. Sci. USA 2013, 110, 17137–17142. [Google Scholar] [CrossRef] [PubMed]
- Komiya, R.; Yokoi, S.; Shimamoto, K. A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development 2009, 136, 3443–3450. [Google Scholar] [CrossRef] [PubMed]
- Oda, A.; Narumi, T.; Li, T.; Kando, T.; Higuchi, Y.; Sumitomo, K.; Fukai, S.; Hisamatsu, T. CsFTL3, a chrysanthemum FLOWERING LOCUS T-like gene, is a key regulator of photoperiodic flowering in chrysanthemums. J. Exp. Bot. 2012, 63, 1461–1477. [Google Scholar] [PubMed]
- Yu, S.; Cao, L.; Zhou, C.M.; Zhang, T.Q.; Lian, H.; Sun, Y.; Wu, J.; Huang, J.; Wang, G.; Wang, J.W. Sugar is an endogenous cue for juvenile-to-adult phase transition in plants. Elife 2013, 2, e00269. [Google Scholar] [CrossRef]
- Moghaddam, M.R.B.; Van den Ende, W. Sugars, the clock and transition to flowering. Front. Plant Sci. 2013, 4, 22. [Google Scholar] [CrossRef]
- Yang, L.; Xu, M.; Koo, Y.; He, J.; Poethig, R.S. Sugar promotes vegetative phase change in Arabidopsis thaliana by repressing the expression of MIR156A and MIR156C. Elife 2013, 2, e00260. [Google Scholar] [CrossRef]
- Houssa, P.; Bernier, G.; Kinet, J. Qualitative and quantitative analysis of carbohydrates in leaf exudate of the short-day plant, Xanthium strumarium L. during floral transition. J. Plant Physiol. 1991, 138, 24–28. [Google Scholar] [CrossRef]
- Ohto, M.a.; Onai, K.; Furukawa, Y.; Aoki, E.; Araki, T.; Nakamura, K. Effects of sugar on vegetative development and floral transition in Arabidopsis. Plant Physiol. 2001, 127, 252–261. [Google Scholar] [CrossRef]
- Wahl, V.; Ponnu, J.; Schlereth, A.; Arrivault, S.; Langenecker, T.; Franke, A.; Feil, R.; Lunn, J.E.; Stitt, M.; Schmid, M. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 2013, 339, 704–707. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Ren, L.; Chen, S.; Chen, F.; Jiang, J. CmFTL2 is involved in the photoperiod-and sucrose-mediated control of flowering time in chrysanthemum. Hortic. Res. 2017, 4, 17001. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liu, T.; Cheng, Y.; Sun, J.; Gao, J.; Dong, B.; Chen, S.; Chen, F.; Jiang, J. Transcriptomic analysis of differentially expressed genes in the floral transition of the summer flowering chrysanthemum. BMC Genom. 2016, 17, 673. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, Y.; Wu, Z.; Bu, X.; Fan, M.; Zhang, Q. Characterization of TEMINAL FLOWER1 homologs CmTFL1c gene from Chrysanthemum morifolium. Plant Mol. Biol. 2019, 99, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.S.; Salchert, K.; Nielsen, K.K. A TERMINAL FLOWER1-like gene from perennial ryegrass involved in floral transition and axillary meristem identity. Plant Physiol. 2001, 125, 1517–1528. [Google Scholar] [CrossRef]
- Ratcliffe, O.J.; Amaya, I.; Vincent, C.A.; Rothstein, S.; Carpenter, R.; Coen, E.S.; Bradley, D.J. A common mechanism controls the life cycle and architecture of plants. Development 1998, 125, 1609–1615. [Google Scholar] [CrossRef]
- Wang, Y.; Pijut, P.M. Isolation and characterization of a TERMINAL FLOWER 1 homolog from Prunus serotina ehrh. Tree Physiol. 2013, 33, 855–865. [Google Scholar] [CrossRef]
- Guo, X.; Zhao, Z.; Chen, J.; Hu, X.; Luo, D. A putative CENTRORADIALIS/TERMINAL FLOWER 1-like gene, Ljcen1, plays a role in phase transition in Lotus japonicus. J. Plant Physiol. 2006, 163, 436–444. [Google Scholar] [CrossRef]
- Higuchi, Y.; Hisamatsu, T. CsTFL1, a constitutive local repressor of flowering, modulates floral initiation by antagonising florigen complex activity in chrysanthemum. Plant Sci. 2015, 237, 1–7. [Google Scholar] [CrossRef]
- Pi, N.; Tam, N.; Wu, Y.; Wong, M.H. Root anatomy and spatial pattern of radial oxygen loss of eight true mangrove species. Aquat. Bot. 2009, 90, 222–230. [Google Scholar] [CrossRef]
- Ruzin, S.E. Plant Microtechnique and Microscopy; Oxford University Press: New York, NY, USA, 1999. [Google Scholar]
- Sack, L.; Buckley, T.N. The developmental basis of stomatal density and flux. Plant Physiol. 2016, 171, 2358–2363. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar]
- Roháček, K. Chlorophyll fluorescence parameters: The definitions, photosynthetic meaning, and mutual relationships. Photosynthetica 2002, 40, 13–29. [Google Scholar] [CrossRef]
- Knight, S.L.; Mitchell, C.A. Enhancement of lettuce yield by manipulation of light and nitrogen nutrition. J. Am. Soc. Hortic. Sci. 1983, 108, 750–754. [Google Scholar] [CrossRef]
- Wang, F.; Sanz, A.; Brenner, M.L.; Smith, A. Sucrose synthase, starch accumulation, and tomato fruit sink strength. Plant Physiol. 1993, 101, 321–327. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Doehlert, D.C.; Kuo, T.M.; Felker, F.C. Enzymes of sucrose and hexose metabolism in developing kernels of two inbreds of maize. Plant Physiol. 1988, 86, 1013–1019. [Google Scholar] [CrossRef]
- Liang, J.S.; Cao, X.; Xu, S.; Zhu, Q.; Song, P. Studies on the relationship between the grain sink strength and its starch accumulation in rice (O. sativa). Acta Agron. Sin. 1994, 20, 685–691. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Higuchi, Y.; Sumitomo, K.; Oda, A.; Shimizu, H.; Hisamatsu, T. Day light quality affects the night-break response in the short-day plant chrysanthemum, suggesting differential phytochrome-mediated regulation of flowering. J. Plant Physiol. 2012, 169, 1789–1796. [Google Scholar]
- Zhao, K.; Li, S.; Jia, D.; Xing, X.; Wang, H.; Song, A.; Jiang, J.; Chen, S.; Chen, F.; Ding, L. Characterization of the MADS-box gene CmFL3 in chrysanthemum. Agronomy 2022, 12, 1716. [Google Scholar]
- Park, Y.G.; Muneer, S.; Jeong, B.R. Morphogenesis, flowering, and gene expression of Dendranthema grandiflorum in response to shift in light quality of night interruption. Int. J. Mol. Sci. 2015, 16, 16497–16513. [Google Scholar] [CrossRef] [PubMed]
- Shchennikova, A.V.; Shulga, O.A.; Immink, R.; Skryabin, K.G.; Angenent, G.C. Identification and characterization of four chrysanthemum MADS-box genes, belonging to the APETALA1/FRUITFULL and SEPALLATA3 subfamilies. Plant Physiol. 2004, 134, 1632–1641. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Niki, T.; Nishijima, T.; Douzono, M.; Koshioka, M.; Hisamatsu, T. Roles of CmFL, CmAFL1, and CmSOC1 in the transition from vegetative to reproductive growth in Chrysanthemum morifolium Ramat. J. Hortic. Sci. Biotechnol. 2009, 84, 447–453. [Google Scholar] [CrossRef]
- Gu, C.; Chen, S.; Liu, Z.; Shan, H.; Luo, H.; Guan, Z.; Chen, F. Reference gene selection for quantitative real-time PCR in Chrysanthemum subjected to biotic and abiotic stress. Mol. Biotechnol. 2011, 49, 192–197. [Google Scholar] [CrossRef]
| Gene | Accession Number | Forward Primer (5′ to 3′) | Reverse Primer (5′ to 3′) | Classification of Gene Functions |
|---|---|---|---|---|
| CmACTIN | AB205087 | GATGACGCAGATCATGTTCG | AGCATGTGGAAGTGCATACC | Reference genes |
| CmEF1α | AB548817 | CTTGTTGCTTGATGACTGTGG | CTTGTTGCTTGATGACTGTGG | |
| CmCRY1 | NM-116961 | CGTAAGGGATCACCGAGTAAAG | CTTTTAGGTGGGAGTTGTGGAG | Photoreceptor genes |
| CmPHYB | AB733630 | TCCAAGAGGGTCATTTGGAG | ACCTGGCTAACCACAGCATC | |
| CmAFT | AB839766 | CAAGCAAAAAGCAAGGCAATCA | CAACCGGTAACCCCAAGTCATT | Anti-florigenic FT/TFL1 family TFL1/CEN/BFT-like gene |
| CmFTL1 | AB679270 | AATCGTGTGCTATGAGAGCC | GCTTGTAACGTCCTCTTCATGC | FT-like genes |
| CmFTL2 | AB679271 | ATGTGTTATTCCGGCAATTGGGTCG | AAATATGCATTTGTAACGTCATGTG | |
| CmFTL3 | AB679272 | GGGAAAGTGGATTTGGTGGACG | GTCTTACAATTTGGTACTGTCG | |
| CmTFL1 | AB839767 | CCATCATCAAGGCACAATTTCA | TTTCCCTTTGGCAGTTGAAGAA | Anti-florigenic TFL1/CEN-like gene; specifically highly expressed at the shoot apex |
| CDM111 | AY173054 | GGTCTCAAGAATATTCGCAC | TCATTAGTCATCCCATCAGC | Well-characterized floral meristem identity genes APETALA1 (CDM111), FRUITFULL (CmAFL1), and LEAFY (CmFL); specifically highly expressed at the shoot apex |
| CmAFL1 | AB451218 | CAAGCTCAACCATCAATAGTC | TGCAGCACATGAACGAGTAG | |
| CmFL | AB451217 | CATTGATGCCATATTTAACTC | ACACGGATCATTCATTGTATA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Cheng, Z.; Song, J.; Jeong, B.R. High-Blue/Low-Red Mixed Light Modulates Photoperiodic Flowering in Chrysanthemum via Photoreceptor and Sugar Pathways. Plants 2025, 14, 3151. https://doi.org/10.3390/plants14203151
Yang J, Cheng Z, Song J, Jeong BR. High-Blue/Low-Red Mixed Light Modulates Photoperiodic Flowering in Chrysanthemum via Photoreceptor and Sugar Pathways. Plants. 2025; 14(20):3151. https://doi.org/10.3390/plants14203151
Chicago/Turabian StyleYang, Jingli, Zhengyang Cheng, Jinnan Song, and Byoung Ryong Jeong. 2025. "High-Blue/Low-Red Mixed Light Modulates Photoperiodic Flowering in Chrysanthemum via Photoreceptor and Sugar Pathways" Plants 14, no. 20: 3151. https://doi.org/10.3390/plants14203151
APA StyleYang, J., Cheng, Z., Song, J., & Jeong, B. R. (2025). High-Blue/Low-Red Mixed Light Modulates Photoperiodic Flowering in Chrysanthemum via Photoreceptor and Sugar Pathways. Plants, 14(20), 3151. https://doi.org/10.3390/plants14203151








