Predictions of Genes Conferring Resistance to Puccinia hordei in an International Barley Panel Using Gene-for-Gene-Based Postulations and Linked Molecular Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Sowing Procedures and Plant Establishment
2.3. Inoculations, Disease Development, and Assessment
2.4. Marker Assisted Screening
2.5. Gene Postulations
3. Results
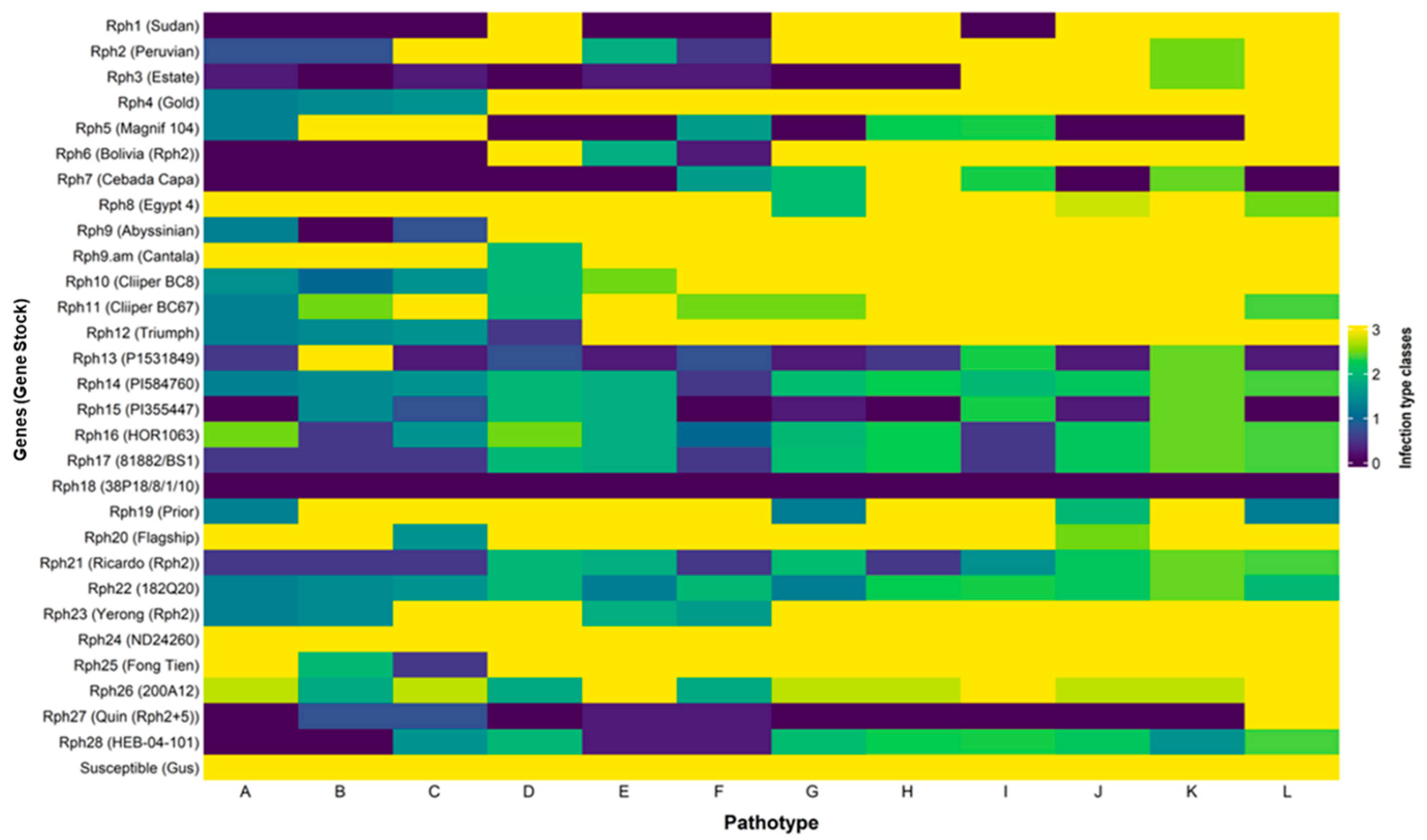
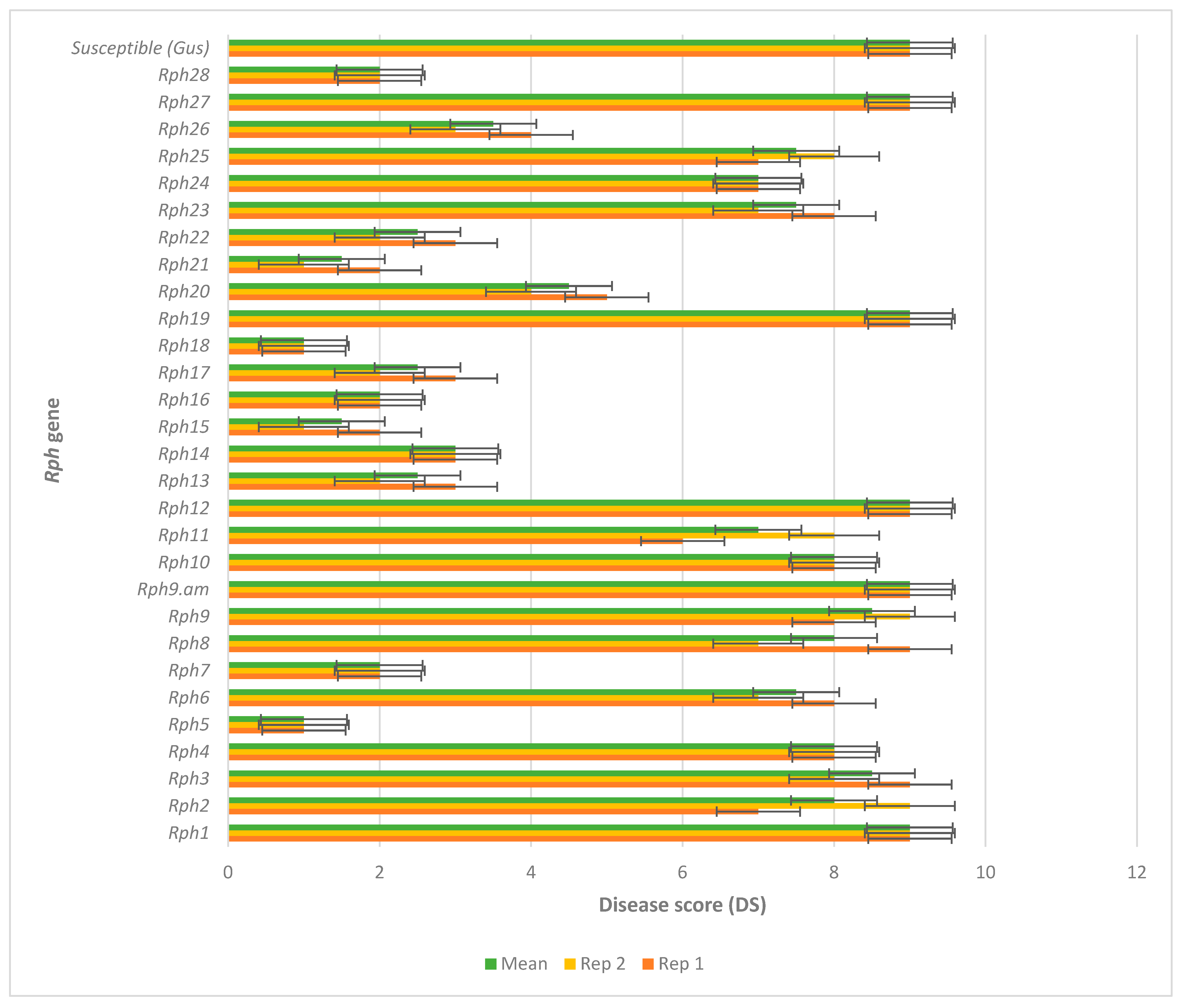
3.1. Virulence Diversity of Australian P. hordei Pathotypes
3.2. Gene-for-Gene-Based Gene Postulations
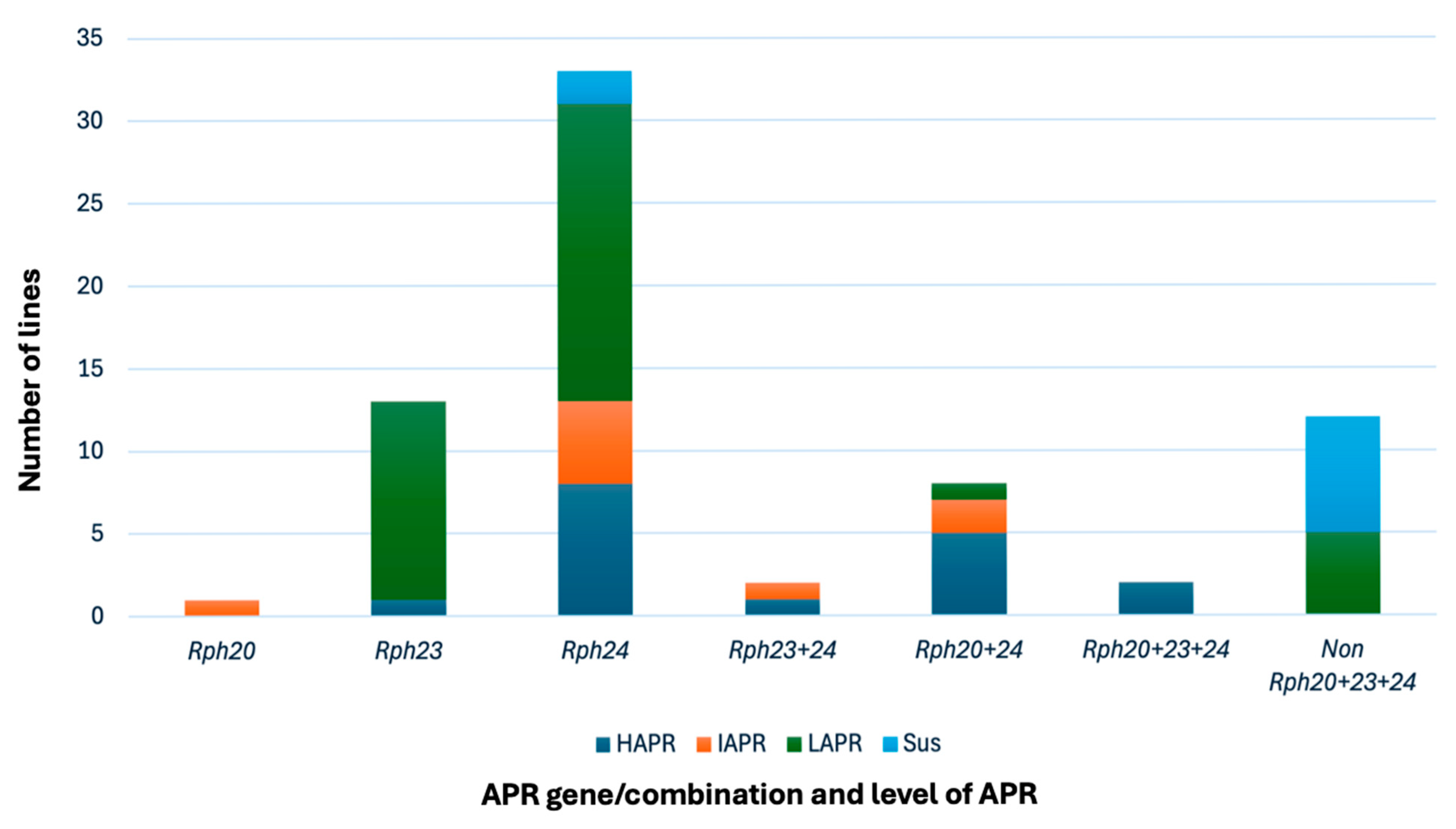
3.3. Field and Marker-Based Assessment of APR
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Park, R.F.; Golegaonkar, P.G.; Derevnina, L.; Sandhu, K.S.; Karaoglu, H.; Elmansour, H.M.; Singh, D. Leaf rust of cultivated barley pathology and control. Annu. Rev. Phytopathol. 2015, 53, 565–589. [Google Scholar] [CrossRef]
- Singh, D.; Ziems, L.A.; Dracatos, P.M.; Pourkheirandish, M.; Tshewang, S.; Czembor, P.; German, S.; Fowler, R.A.; Snyman, L.; Platz, G.J.; et al. Genome-wide association studies provide insights on genetic architecture of resistance to leaf rust in a worldwide barley collection. Mol. Breed. 2018, 38, 43. [Google Scholar] [CrossRef]
- Flor, H.H. The complementary gene systems in flax and flax rust. Adv. Genet. 1956, 8, 29–54. [Google Scholar]
- Singh, D.; Park, R.F.; McIntosh, R.A. Postulation of leaf (brown) rust resistance genes in 70 wheat cultivars grown in the United Kingdom. Euphytica 2001, 120, 205–218. [Google Scholar] [CrossRef]
- Dreiseitl, A. Postulation of specific disease resistance genes in cereals: A widely used method and its detailed description. Pathogens 2022, 11, 284. [Google Scholar] [CrossRef] [PubMed]
- Trethowan, R.M.; Nicol, J.M.; Singh, A.; Singh, R.P.; Tadesse, W.; Govidan, V.; Crespo, J.L.H.; Cullis, B.; Mazur, L.; Dieters, M.; et al. The CIMMYT Australia ICARDA Germplasm Evaluation concept: A model for international cooperation and impact. Front. Plant Sci. 2024, 15, 1435837. [Google Scholar] [CrossRef] [PubMed]
- Cotterill, P.; Park, R.F.; Rees, R. Pathogenic specialization of Puccinia hordei Otth. in Australia, 1966–1990. Aust. J. Agric. Res. 1995, 46, 127–134. [Google Scholar] [CrossRef]
- Park, R.F. Pathogenic specialization and pathotype distribution of Puccinia hordei in Australia, 1992 to 2001. Plant Dis. 2003, 87, 1311–1316. [Google Scholar] [CrossRef]
- Park, R.F.; Karakousis, A. Characterization and mapping of gene Rph19 conferring resistance to Puccinia hordei in cultivar ‘Reka I’ and several Australian barleys. Plant Breed. 2002, 121, 232–236. [Google Scholar] [CrossRef]
- Singh, D.; Dracatos, P.M.; Loughman, R.; Park, R.F. Genetic mapping of resistance to Puccinia hordei in three barley doubled-haploid populations. Euphytica 2017, 213, 16. [Google Scholar] [CrossRef]
- Mehnaz, M.; Dracatos, P.; Pham, A.; March, T.; Maurer, A.; Pillen, K.; Forrest, K.; Kulkarni, T.; Pourkheirandish, M.; Park, R.F.; et al. Discovery and fine mapping of Rph28: A new gene conferring resistance to Puccinia hordei from wild barley. Theor. Appl. Genet. 2021, 134, 2167–2179. [Google Scholar] [CrossRef]
- Fulton, T.M.; Chunwongse, J.; Tanksley, S.D. Microprep protocol for extraction of DNA from tomato and other herbaceous plants. Plant Mol. Biol. Rep. 1995, 13, 207–209. [Google Scholar] [CrossRef]
- Hickey, L.T.; Lawson, W.; Platz, G.J.; Dieters, M.; Arief, V.N.; German, S.; Fletcher, S.; Park, R.F.; Singh, D.; Pereyra, S.; et al. Mapping Rph20: A gene conferring adult plant resistance to Puccinia hordei in barley. Theor. Appl. Genet. 2011, 123, 55–68. [Google Scholar] [CrossRef]
- Dracatos, P.M.; Park, R.F.; Singh, D. Validating molecular markers for barley leaf rust resistance genes Rph20 and Rph24. Plant Dis. 2021, 105, 743–747. [Google Scholar] [CrossRef]
- Singh, D.; Dracatos, P.M.; Derevnina, L.; Zhou, M.; Park, R.F. Rph23: A new designated additive adult plant resistance gene to leaf rust in barley on chromosome 7H. Plant Breed. 2015, 134, 62–69. [Google Scholar] [CrossRef]
- Dinh, H.X.; Singh, D.; Gomez de la Cruz, D.; Hensel, G.; Kumlehn, J.; Mascher, M.; Stein, N.; Perovic, D.; Ayliffe, M.; Moscou, M.J.; et al. The barley leaf rust resistance gene Rph3 encodes a predicted membrane protein and is induced upon infection by avirulent pathotypes of Puccinia hordei. Nat. Commun. 2022, 13, 2386. [Google Scholar] [CrossRef]
- Chen, C.; Jost, M.; Outram, M.A.; Friendship, D.; Chen, J.; Wang, A.; Peryannan, S.; Bartoš, J.; Holušová, K.; Doležel, J.; et al. A pathogen-induced putative NAC transcription factor mediates leaf rust resistance in barley. Nat. Commun. 2023, 14, 5468. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Jost, M.; Clark, B.; Martin, M.; Matny, O.; Steffenson, B.J.; Franckowiak, J.D.; Mascher, M.; Singh, D.; Perovic, D.; et al. BED domain-containing NLR from wild barley confers resistance to leaf rust. Plant Biotechnol. J. 2021, 19, 1206–1215. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, J.; Bala, R.; Sharma, A.; Kumari, J.; Mavi, G.S.; Kaur, S. Postulation of leaf rust resistance genes in Indian and exotic wheat germplasm using near-isogenic lines (NILs) and molecular markers. Crop Prot. 2024, 174, 106431. [Google Scholar] [CrossRef]
- Golegaonkar, P.G.; Singh, D.; Park, R.F. Evaluation of seedling and adult plant resistance to Puccinia hordei in barley. Euphytica 2009, 166, 183–197. [Google Scholar] [CrossRef]
- Ziems, L.A.; Singh, L.; Dracatos, P.M.; Dieters, M.J.; Sanchez-Garcia, M.; Amri, A.; Verma, R.P.S.; Park, R.F.; Singh, D. Characterization of leaf rust resistance in international barley germplasm using genome-wide association studies. Plants 2023, 12, 862. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, C.T.; Singh, D.; Dracatos, P.M.; Park, R.F. Inheritance and characterization of Rph27: A third race-specific resistance gene in the barley cultivar Quinn. Phytopathology 2020, 110, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Ziems, L.A.; Hickey, L.T.; Platz, G.J.; Franckowiak, J.D.; Dracatos, P.M.; Singh, D.; Park, R.F. Characterization of Rph24: A gene conferring adult plant resistance to Puccinia hordei in barley. Phytopathology 2017, 107, 834–841. [Google Scholar] [CrossRef] [PubMed]

| Gene | Markers | Type | Forward Primer Sequence 5′-3′ | Reverse Primer Sequence 5′-3′ | Reference |
|---|---|---|---|---|---|
| Rph3 | MLOC_198 | CAPS | GCTGAGCCCCTAATACACGA | CCCATGTATGTGCTCGTTTG | Dinh et al., 2022 [16] |
| Rph7 | Hvpg4 | KASP-FAM3A1 | GAAGGTGACCAAGTTCATGCTGTCATGCTCAAAACAGGTAACCCT | Chen et al., 2023 [17] | |
| KASP-HEX3A2 | GAAGGTCGGAGTCAACGGATTGTCATGCTCAAAACAGGTAACCCA | ||||
| KASP-R3C | GTATCCCTACGCAGTTATTTGACCTATG | ||||
| Rph15 | K3 | KASP-FAMA1 | GAAGGTGACCAAGTTCATGCTGGGCTGTTATTAGCATGGTCCTC | Chen et al., 2021 [18] | |
| KASP-HEXA2 | GAAGGTCGGAGTCAACGGATTGGGCTGTTATTAGCATGGTCCTG | ||||
| KASP-K3RC | AATACCACAATGACTACCCCAGGTT | ||||
| Rph20 | bPb-0837 | DArT | GACACTTCGTGCCAGTTTG | CCTCCCTCCCTCTTCTCAAC | Hickey et al., 2011 [13] |
| Rph20 | sun690-91 | InDel | AACAAAAAGCGGCCGAAAAA | ACGGGCACATTGTGTCTATTT | Dracatos et al., 2021 [14] |
| Rph23 | Ebmac0603 | SSR | ACCGAAACTAAATGAACTACTTCG | TGCAAACTGTGCTATTAAGGG | Singh et al., 2015 [15] |
| Rph24 | sun43-44 | InDel | CTAGACACCACCACCACACC | ATACCAGAGTTTGCGTCCGG | Dracatos et al., 2021 [14] |
| Rph28 | M5 | CAPS | ATGATGTCACGGACGAGTCG | GCATCACCCTCC GTGTTGAT | Mehnaz et al., 2021 [11] |
| Postulated Gene | No. of Lines | Pathotypes ** | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | ||
| Rph2 | 8 | R | R | S | R | R | S | S | S | S |
| Rph3 | 26 | R | R | R | R | R | S | S | S | R |
| Rph5 | 1 | R | S | R | R | R | R | R | R | R |
| Rph9.am | 1 | S | S | R | S | S | S | S | S | S |
| Rph9.am+ | 2 | R | S | R | S | S | S | S | S | S |
| Rph25 | 3 | S | R | S | S | S | S | S | S | S |
| Rph25 + Rph9.am | 2 | S | R | R | S | S | S | S | S | S |
| RphUASR * | 3 | R | R | R | R | R | R | R | R | R |
| Susceptible | 22 | S | S | S | S | S | S | S | S | S |
| Line ID | Origin | Genotype | ASR | APR a | DS b | APR Level c |
|---|---|---|---|---|---|---|
| CG1 | United States | Breeding line | Rph2 | Rph24 | 8 | Susceptible |
| CG2 | Russia | Landrace | UASR | Rph24 | 3 | HAPR |
| CG3 | Armenia | Landrace | Nil | Rph24 | 7 | LAPR |
| CG4 | Pakistan | Landrace | Nil | Rph24 | 7.5 | LAPR |
| CG5 | Macedonia | Landrace | Nil | 8.5 | Susceptible | |
| CG6 | Spain | Landrace | Rph9.am+ | Rph24 | 3 | HAPR |
| CG7 | Afghanistan | Landrace | Nil | Rph24 | 3 | HAPR |
| CG8 | United States | Landrace | Nil | Rph24 | 4.5 | MAPR |
| CG9 | China | Landrace | Nil | Rph24 | 7 | LAPR |
| CG10 | Macedonia | Landrace | Nil | Rph24 | 2.5 | HAPR |
| CG11 | Macedonia | Landrace | Nil | Rph24 | 3.5 | HAPR |
| CG12 | Russia | Landrace | Nil | Rph24 | 3 | HAPR |
| CG13 | Russia | Landrace | Rph2 | Rph24 | 7.5 | LAPR |
| CG14 | Austria | Landrace | UASR | Rph24 | 6.5 | LAPR |
| CG15 | Azerbaijan | Landrace | Nil | Rph24 | 2.5 | HAPR |
| CG16 | Ethiopia | Single plant sel | Nil | Rph24 | 7 | LAPR |
| CG17 | Ethiopia | Single plant sel | Nil | Rph24 | 7 | LAPR |
| CG18 | Ethiopia | Single plant sel | Nil | Rph24 | 7 | LAPR |
| CG19 | Ethiopia | Single plant sel | Nil | Rph24 | 5 | MAPR |
| CG20 | Ethiopia | Single plant sel | Nil | Rph24 | 7 | LAPR |
| CG21 | Ethiopia | Single plant sel | Nil | Rph24 | 7.5 | LAPR |
| CG22 | Ethiopia | Single plant sel | Nil | Rph24 | 9 | Susceptible |
| CG23 | Greece | Single plant sel | Rph9.am + Rph25 | Rph24 | 7 | LAPR |
| CG24 | Greece | Single plant sel | UASR | Rph23, Rph24 | 2 | HAPR |
| CG25 | Russia | Single plant sel | Rph25 | Rph24 | 5 | MAPR |
| CG26 | Turkey | Single plant sel | Rph2 | Rph20, Rph24 | 5 | MAPR |
| CG27 | Morocco | Breeding line | Rph2 | 8 | Susceptible | |
| CG28 | Morocco | Breeding line | UASR | Rph24 | 7 | * |
| CG29 | Morocco | Breeding line | UASR | 2.5 | * | |
| CG30 | Syria | Breeding line | Rph3 | Rph24 | 5 | MAPR |
| CG31 | Syria | Breeding line | Rph3 | 8 | Susceptible | |
| CG32 | Morocco | Breeding line | Rph3 | 8 | Susceptible | |
| CG33 | Morocco | Breeding line | Rph3 | Rph24 | 5 | MAPR |
| CG34 | Morocco | Breeding line | Rph3+ | Rph20, Rph24 | 3.5 | * |
| CG35 | Morocco | Breeding line | Rph3 | Rph23 | 7.5 | LAPR |
| CG36 | Morocco | Genetic stock | Rph3 | Rph23 | 7.5 | LAPR |
| CG37 | Morocco | Genetic stock | Rph3 | Rph23 | 7.5 | LAPR |
| CG38 | Morocco | Genetic stock | Rph3 | Rph23 | 7.5 | LAPR |
| CG39 | Morocco | Genetic stock | Rph3 | Rph23 | 7 | LAPR |
| CG40 | Morocco | Genetic stock | Rph3 | Rph23 | 7.5 | LAPR |
| CG41 | Morocco | Genetic stock | Rph3 | Rph23 | 7.5 | LAPR |
| CG42 | Morocco | Unknown | Rph3 | 7 | LAPR | |
| CG43 | Morocco | Breeding line | UASR | Rph20, Rph24 | 2 | * |
| CG44 | Morocco | Breeding line | Nil | Rph20, Rph24 | 3 | HAPR |
| CG45 | Morocco | Breeding line | Rph25 | Rph20, Rph24 | 4 | MAPR |
| CG46 | Morocco | Breeding line | Nil | Rph24 | 7 | LAPR |
| CG47 | Morocco | Breeding line | Rph3 | 8 | Susceptible | |
| CG48 | Morocco | Breeding line | Rph3 | Rph24 | 7.5 | LAPR |
| CG49 | Morocco | Breeding line | Rph3 | 7.5 | LAPR | |
| CG50 | Morocco | Breeding line | Rph9.am | Rph23 | 7.5 | LAPR |
| CG51 | Morocco | Breeding line | Rph3 | 8 | Susceptible | |
| CG52 | Morocco | Breeding line | Rph3 | 8 | Susceptible | |
| CG53 | Morocco | Unknown | Rph9.am+ | 7.5 | LAPR | |
| CG54 | Morocco | Unknown | Rph5 | 3 | * | |
| CG55 | Morocco | Genetic stock | UASR | 3 | * | |
| CG56 | Morocco | Unknown | UASR | Rph23 | 2 | * |
| CG57 | Morocco | Breeding line | UASR | 2.5 | * | |
| CG58 | Morocco | Unknown | Rph3 | 7 | LAPR | |
| CG59 | Morocco | Breeding line | Rph3 | Rph23 | 6 | LAPR |
| CG60 | Morocco | Advanced line | Rph3 | Rph23 | 7 | LAPR |
| CG61 | Morocco | Breeding line | Rph3 | Rph23 | 6 | LAPR |
| CG62 | Syria | Breeding line | Rph2 | Rph20, Rph24 | 3 | HAPR |
| CG63 | Syria | Breeding line | Nil | Rph20, Rph23, Rph24 | 2.5 | HAPR |
| CG64 | Syria | Breeding line | UASR | Rph20, Rph24 | 2.5 | * |
| CG65 | Lebanon | Breeding line | UASR | Rph20, Rph24 | 4 | MAPR |
| CG66 | Lebanon | Breeding line | Nil | Rph20, Rph24 | 7 | LAPR |
| CG67 | Syria | Breeding line | Rph3 | Rph23 | 6 | LAPR |
| CG68 | Lebanon | Breeding line | Rph3 | Rph23, Rph24 | 4.5 | MAPR |
| CG69 | Lebanon | Breeding line | Rph2 | Rph24 | 7 | LAPR |
| CG70 | Lebanon | Breeding line | Rph3 | 7.5 | LAPR | |
| CG71 | Morocco | Breeding line | Rph3 | Rph24 | 6 | LAPR |
| CG72 | Syria | Breeding line | Rph2 | Rph20, Rph24 | 3 | HAPR |
| CG73 | Syria | Breeding line | Rph25 | Rph24 | 3 | HAPR |
| CG74 | Syria | Breeding line | UASR | Rph24 | 6.5 | LAPR |
| CG75 | Syria | Breeding line | Nil | Rph20 | 5 | MAPR |
| CG76 | Syria | Breeding line | Rph9.am + Rph25 | Rph20, Rph23, Rph24 | 2.5 | HAPR |
| CG77 | Syria | Breeding line | Rph2 (segregating) | Rph24 | 7 | LAPR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, D.; Ziems, L.A.; Sandhu, K.S.; Chhetri, M.; Sanchez-Garcia, M.; Amri, A.; Dieters, M.; Park, R.F. Predictions of Genes Conferring Resistance to Puccinia hordei in an International Barley Panel Using Gene-for-Gene-Based Postulations and Linked Molecular Markers. Plants 2025, 14, 3150. https://doi.org/10.3390/plants14203150
Singh D, Ziems LA, Sandhu KS, Chhetri M, Sanchez-Garcia M, Amri A, Dieters M, Park RF. Predictions of Genes Conferring Resistance to Puccinia hordei in an International Barley Panel Using Gene-for-Gene-Based Postulations and Linked Molecular Markers. Plants. 2025; 14(20):3150. https://doi.org/10.3390/plants14203150
Chicago/Turabian StyleSingh, Davinder, Laura A. Ziems, Karanjeet S. Sandhu, Mumta Chhetri, Miguel Sanchez-Garcia, Ahmed Amri, Mark Dieters, and Robert F. Park. 2025. "Predictions of Genes Conferring Resistance to Puccinia hordei in an International Barley Panel Using Gene-for-Gene-Based Postulations and Linked Molecular Markers" Plants 14, no. 20: 3150. https://doi.org/10.3390/plants14203150
APA StyleSingh, D., Ziems, L. A., Sandhu, K. S., Chhetri, M., Sanchez-Garcia, M., Amri, A., Dieters, M., & Park, R. F. (2025). Predictions of Genes Conferring Resistance to Puccinia hordei in an International Barley Panel Using Gene-for-Gene-Based Postulations and Linked Molecular Markers. Plants, 14(20), 3150. https://doi.org/10.3390/plants14203150








