Effects of Canopy Litter Removal on Canopy Structure, Understory Light and Vegetation Dynamics in Cunninghamia lanceolata Plantations of Varying Densities
Abstract
1. Introduction
2. Results
2.1. Effects of Canopy Litter Removal on Overstory Canopy Structure
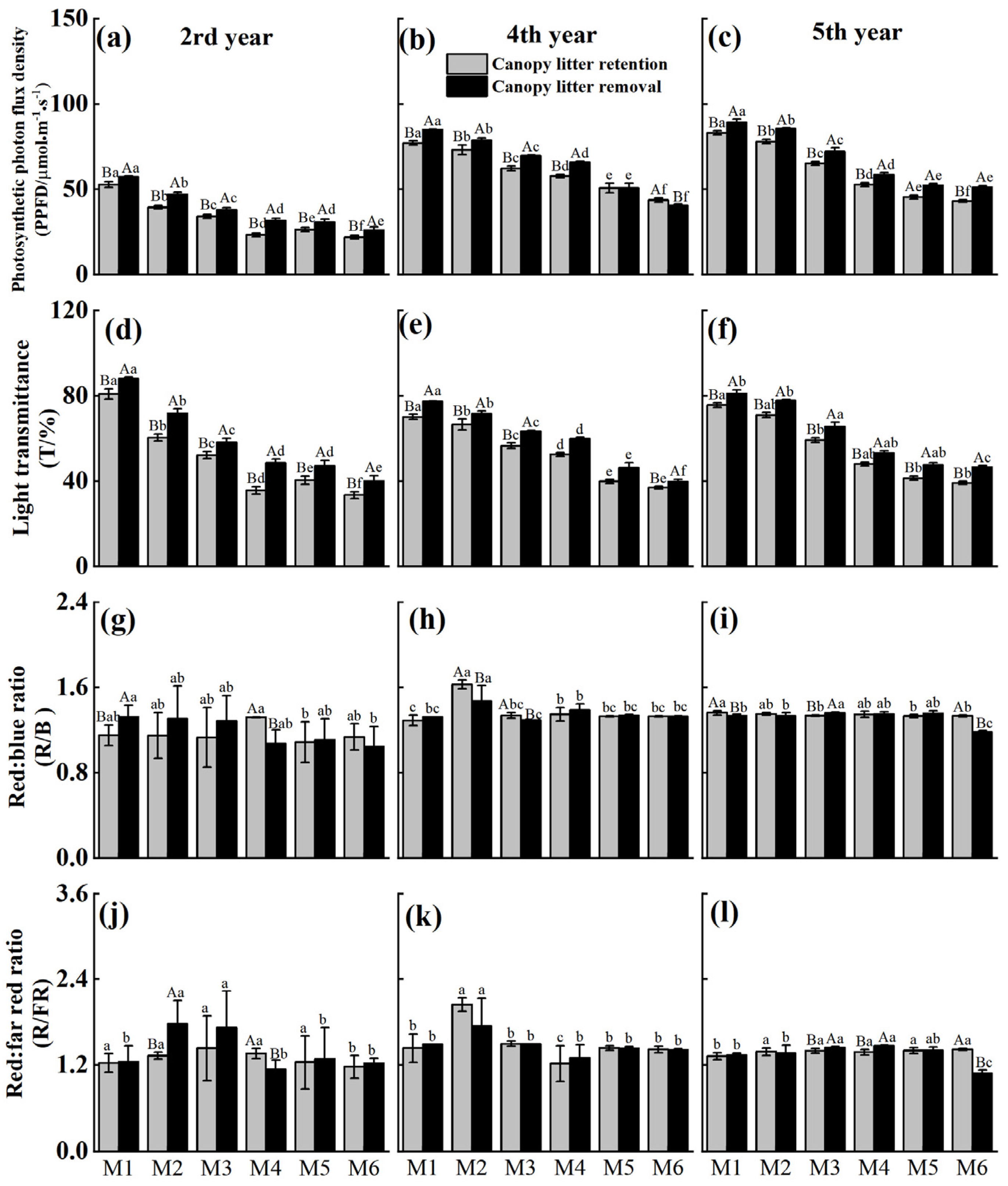
2.2. Effects of Canopy Litter Removal on Understory Light
2.3. Effects of Canopy Litter Removal on Understory Vegetation
2.3.1. Understory Composition and IVI
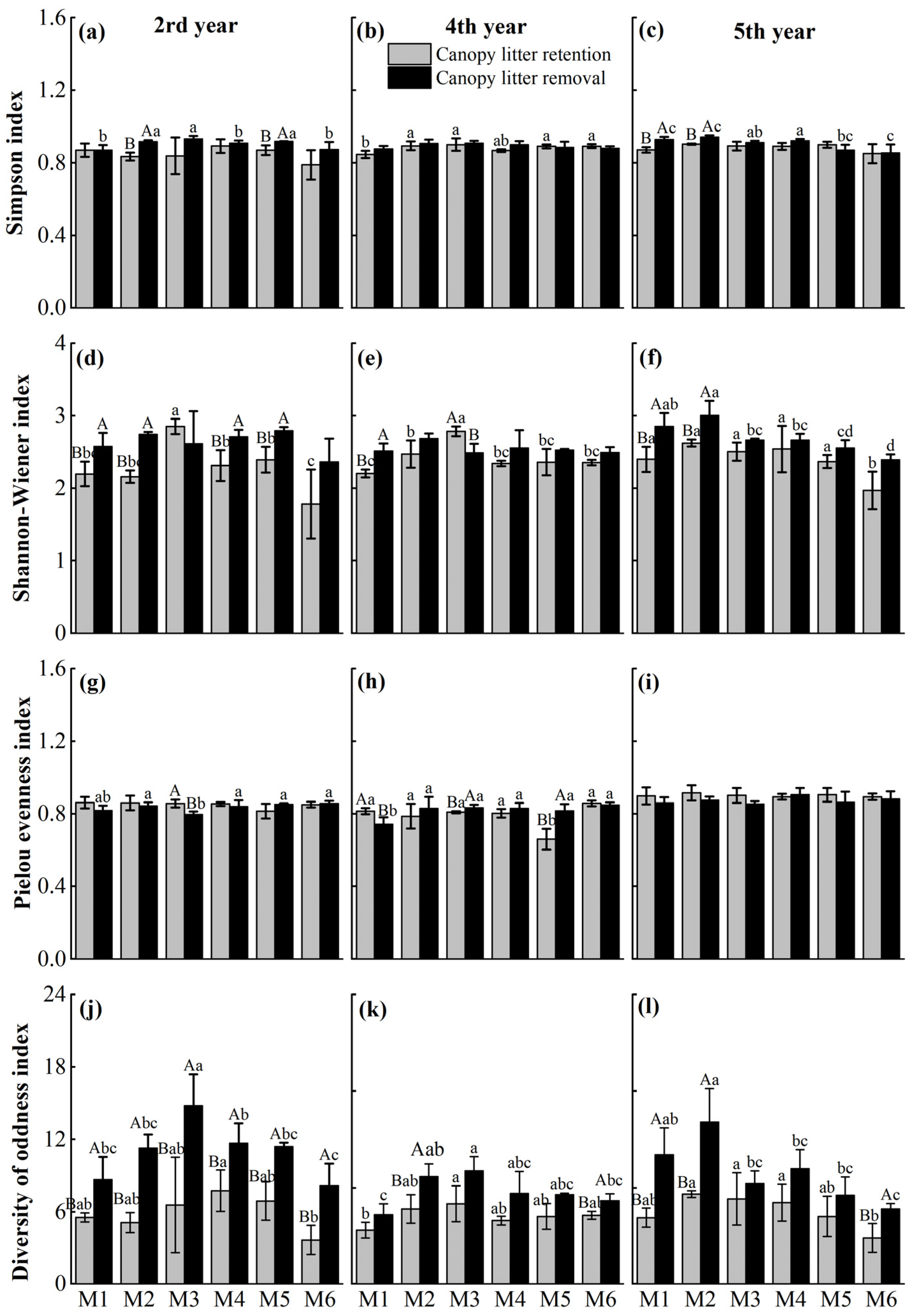
2.3.2. Understory Vegetation Diversity
2.4. Relationships Between Overstory Canopy Structure, Understory Light and Understory Vegetation
2.4.1. Relationships Between Overstory Canopy Structure and Understory Light
2.4.2. Effects of Overstory Canopy Structure, Understory Light on Understory Vegetation
3. Discussion
3.1. Effects of Canopy Litter Removal on Overstory Structure and Understory Light Environment
3.2. Effects of Canopy Litter Removal on Understory Vegetation Composition and Diversity
3.3. Implications for Forest Management
4. Materials and Methods
4.1. Study Site and Experimental Design
4.2. Measurements of Overstory Canopy Structure and Understory Light
4.3. Understory Surveys and Diversity Calculations
4.4. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Payn, T.; Carnus, J.M.; Freer-Smith, P.; Kimberley, M.; Kollert, W.; Liu, S.; Orazio, C.; Rodriguez, L.; Silva, L.N.; Wingfield, M.J. Changes in planted forests and future global implications. For. Ecol. Manag. 2015, 352, 57–67. [Google Scholar] [CrossRef]
- FAO. Global Forest Resources Assessment 2020: Main Report. Rome. 2021. Available online: https://www.fao.org/3/ca9825en/ca9825en.pdf (accessed on 10 September 2025).
- Yang, Y.S.; Wang, L.X.; Yang, Z.J.; Xu, C.; Xie, J.S.; Chen, G.S.; Lin, C.F.; Guo, J.F.; Liu, X.F.; Xiong, D.C.; et al. Large ecosystem service benefits of assisted natural regeneration. J. Geophys. Res.-Biogeo. 2018, 123, 676–687. [Google Scholar] [CrossRef]
- Zhou, L.L.; Cai, L.P.; He, Z.M.; Wang, R.W.; Wu, P.F.; Ma, X.Q. Thinning increases understory diversity and biomass, and improves soil properties without decreasing growth of Chinese fir in southern China. Environ. Sci. Pollut. Res. 2016, 23, 24135–24150. [Google Scholar] [CrossRef]
- Zhang, H.M.; Pan, F.Y.; Wen, Z.M.; Chen, W.W.; Zhou, C.F. Impacts of successive Chinese fir plantations on soil carbon and nitrogen dynamics: Conclusive insights from metagenomic analysis. J. Environ. Manag. 2025, 376, 124510. [Google Scholar] [CrossRef]
- Yu, X.T. Research progress of Chinese fir in 1990s. J. Fujian For. College 2000, 20, 179–188. (In Chinese) [Google Scholar] [CrossRef]
- State Forestry Administration (SFA) of the People’s Republic of China. Forest Resources in China (2014): The 8th National Forest Survey; State Forestry Administration (SFA) of the People’s Republic of China: Beijing, China, 2014. [Google Scholar]
- Zhou, L.L.; Li, S.B.; Jia, Y.Y.; Heal, K.V.; He, Z.M.; Wu, P.F.; Ma, X.Q. Spatiotemporal distribution of canopy litter and nutrient resorption in a chronosequence of different development stages of Cunninghamia lanceolata in southeast China. Sci. Total Environ. 2021, 762, 143153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.C.; Sheng, W.T. The study on decay of dead branches and leaves on living tress taken from crown into litter environment in a Chinese fir plantation, compared with decay in canopy. Sci. Silvae Sin. 2001, 37, 2–10. (In Chinese) [Google Scholar] [CrossRef]
- Liao, Z.H.; Fan, S.H.; Yu, X.T. Study on the biomass among different-aged Chinse fir plantations at different site conditions. Ⅳ-Biomass of dead branches and leaves in the canopy. For. Res. 1996, 9, 96–99. (In Chinese) [Google Scholar]
- Sheng, W.T.; Fan, S.H. Impact of growth and development characters of Chinese fir and its plantation on the long-term site productivity. For. Res. 2002, 15, 629–639. (In Chinese) [Google Scholar] [CrossRef]
- Gao, S.L.; He, Z.M.; Huang, Z.Q.; Lin, S.Z.; Liu, Z.M. Decomposition, carbon and nitrogen stable isotope and chemical composition of dead leaves clinging in a Chinese fir (Cunninghamia lanceolata) plantations. Chin. J. Ecol. 2015, 34, 2457–2463. (In Chinese) [Google Scholar] [CrossRef]
- Wu, X.J.; Li, J.F.; Jiang, Y.; Sun, L.J.; Wu, P.F.; Ma, X.Q. Effect of simulated warming on nutrient release during the decomposition of leaf litter and canopy litter of Chinese fir based on displacement test. Acta Ecol. Sinca 2024, 44, 7713–7724. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, Y.B.; Duan, B.L.; Xian, J.R.; Korpelainen, H.; Li, C.Y. Links between plant diversity, carbon stocks and environmental factors along a successional gradient in a subalpine coniferous forest in Southwest China. Forest Ecol. Manag. 2011, 262, 361–369. [Google Scholar] [CrossRef]
- Méndez-Dewar, G.; González-Espinosa, M.; Equihua, M. From seedling to sapling: Tree species responses to spatial and temporal understory light heterogeneity in disturbed tropical montane forests. Bot. Sci. 2015, 93, 719–729. [Google Scholar] [CrossRef]
- Valerio, M.; Ibáñez, R.; Gazol, A. The role of canopy cover dynamics over a decade of changes in the understory of an Atlantic Beech-Oak forest. Forests 2021, 12, 938. [Google Scholar] [CrossRef]
- Pelt, R.V.; Franklin, J.F. Influence of canopy structure on the understory environment in tall, old-growth, conifer forests. Can. J. For. Res. 2000, 30, 1231–1245. [Google Scholar] [CrossRef]
- Utsugi, H.; Araki, M.; Kawasaki, T.; Ishizuka, M. Vertical distributions of leaf area and inclination angle, and their relationship in a 46-year-old Chamaecyparis obtusa stand. For. Ecol. Manag. 2006, 225, 104–112. [Google Scholar] [CrossRef]
- Parker, G.G. Tamm review: Leaf Area Index (LAI) is both a determinant and a consequence of important processes in vegetation canopies. For. Eco. Manag. 2020, 477, 118496. [Google Scholar] [CrossRef]
- Valiente-Banuet, A.; Verdú, M.; Valladares, F.; García-Fayos, P. Functional and evolutionary correlations of steep leaf angles in the mexical shrubland. Oecologia 2010, 163, 25–33. [Google Scholar] [CrossRef]
- Yang, X.; Li, R.; Jablonski, A.; Stovall, A.; Kim, J.; Yi, K.; Ma, Y.X.; Beverly, D.; Phillips, R.; Novick, K.; et al. Leaf angle as a leaf and canopy trait: Rejuvenating its role in ecology with new technology. Ecol. Lett. 2023, 26, 1005–1020. [Google Scholar] [CrossRef] [PubMed]
- Valladares, F.; Skillman, J.B.; Pearcy, R.W. Convergence in light capture efficiencies among tropical forest understory plants with contrasting crown architectures: A case of morphological compensation. Am. J. Bot. 2002, 89, 1275–1284. [Google Scholar] [CrossRef] [PubMed]
- Plue, J.; Van Gils, B.; De Schrijver, A.; Peppler-Lisbach, C.; Verheyen, K. Forest herb layer response to long-term light deficit along a forest developmental series. Acta Oecol. 2013, 53, 63–72. [Google Scholar] [CrossRef]
- Brenes-Arguedas, T.; Roddy, A.B.; Coley, P.D.; Kuesar, T.A. Do differences in understory light contribute to species distributions along a tropical rainfall gradient? Oecologia 2011, 166, 443–456. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J.G. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Jankowska-Blaszczuk, M.; Daws, M.I. Impact of red: Far red ratios on germination of temperate forest herbs in relation to shade tolerance, seed mass and persistence in the soil. Funct. Ecol. 2007, 21, 1055–1062. [Google Scholar] [CrossRef]
- Wan, P.; He, R. Canopy structure and understory light characteristics of a natural Quercus aliena var. acuteserrata forest in China northwest: Influence of different forest management methods. Ecol. Eng. 2020, 153, 105901. [Google Scholar] [CrossRef]
- de Mattos, E.M.; Binkley, D.; Campoe, O.C.; Alvares, C.A.; Stape, J.L. Variation in canopy structure, leaf area, light interception and light use efficiency among Eucalyptus clones. For. Ecol. Manag. 2020, 463, 118038. [Google Scholar] [CrossRef]
- Raabe, K.; Písek, J.; Sonnentag, O.; Annuk, K. Variations of leaf inclination angle distribution with height over the growing season and light exposure for eight broadleaf tree species. Agr. Forest Meteorol. 2015, 214–215, 2–11. [Google Scholar] [CrossRef]
- Navrátil, M.; Špunda, V.; Marková, I.; Janouš, D. Spectral composition of photosynthetically active radiation penetrating into a Norway spruce canopy: The opposite dynamics of the blue/red spectral ratio during clear and overcast days. Trees 2007, 21, 311–320. [Google Scholar] [CrossRef]
- Leuchner, M.; Menzel, A.; Werner, H. Quantifying the relationship between light quality and light availability at different phenological stages within a mature mixed forest. Agr. For. Meteorol. 2007, 142, 35–44. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved—A critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between leaf pigment content and spectral reflectance across a wide range of species, leaf structures and developmental stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- McDowell, N.G.; Adams, H.D.; Bailey, J.D.; Kolb, T.S. The role of stand density on growth efficiency, leaf area index, and resin flow in southwestern ponderosa pine forests. Can. J. For. Res. 2007, 37, 343–355. [Google Scholar] [CrossRef]
- Akers, M.K.; Kane, M.; Zhao, D.H.; Teskey, R.O.; Daniels, R.F. Effects of planting density and cultural intensity on stand and crown attributes of mid-rotation loblolly pine plantations. For. Eco. Manag. 2013, 310, 468–475. [Google Scholar] [CrossRef]
- Farooq, T.H.; Yan, W.; Chen, X.Y.; Shakoor, A.; Rashid, M.H.U.; Gilani, M.M.; He, Z.M.; Wu, P.F. Dynamics of canopy development of Cunninghamia lanceolata mid-age plantation in relation to foliar nitrogen and soil quality influenced by stand density. Glob. Ecol. Conserv. 2020, 24, e01209. [Google Scholar] [CrossRef]
- Valladares, F.; Niinemets, Ü. Shade tolerance, a key plant feature of complex nature and consequences. Annu. Rev. Ecol. Evol. S. 2008, 39, 237–257. [Google Scholar] [CrossRef]
- Brelsford, C.C.; Trasser, M.; Paris, T.; Hartikainen, S.M.; Robson, M. Understory light quality affects leaf pigments and leaf phenology in different plant functional types. Physiol. Plantarum. 2022, 174, e13723. [Google Scholar] [CrossRef]
- Liu, L.Q.; Zhou, G.Y.; Zhao, H.B.; Li, L.; Qiu, Z.J. Impact of simulated forest canopy damage on understory biodiversity of natural Castanopsis fissa community. For. Res. 2013, 26, 305–311. (In Chinese) [Google Scholar] [CrossRef]
- Crouzeilles, R.; Curran, M.; Ferreira, M.S.; Lindenmayer, D.B.; Grelle, C.E.V.; Rey Benayas, J.M. A global meta-analysis on the ecological drivers of forest restoration success. Nat Commun. 2016, 7, 11666. [Google Scholar] [CrossRef]
- Skarpe, C. Shrub layer dynamics under different herbivore densities in an arid savanna, Botswana. J. Appl. Ecol. 1990, 27, 873–885. [Google Scholar] [CrossRef]
- Nicotra, A.B.; Chazdon, R.L.; Iriarte, S.V.B. Spatial heterogeneity of light and woody seedling regeneration in tropical wet forests. Ecology 1999, 80, 1908–1926. [Google Scholar] [CrossRef]
- Majasalmi, T.; Rautiainen, M. The impact of tree canopy structure on understory variation in a boreal forest. Forest. Ecol. Manag. 2020, 466, 118100. [Google Scholar] [CrossRef]
- Chen, Y.M.; Cao, Y. Response of tree regeneration and understory plant species diversity to stand density in mature Pinus tabulaeformis plantations in the hilly area of the Loess Plateau, China. Ecol. Eng. 2014, 73, 238–245. [Google Scholar] [CrossRef]
- Ali, A.; Dai, D.; Akhtar, K.; Teng, M.J.; Yan, Z.G.; Urbina-Cardona, N.; Mukkerova, J.; Zhou, Z.X. Response of understory vegetation, tree regeneration, and soil quality to manipulated stand density in a Pinus massoniana plantation. Glob. Ecol. Conserv. 2019, 20, e00775. [Google Scholar] [CrossRef]
- Helbach, J.; Frey, J.; Messier, C.; Mörsdorf, M.; Scherer-Lorenzen, M. Light heterogeneity affects understory plant species richness in temperate forests supporting the heterogeneity-diversity hypothesis. Ecol. Evol. 2022, 12, e8534. [Google Scholar] [CrossRef]
- Ampoorter, E.; Selvi, F.; Auge, H.; Lander, B.; Sigrid, B.; Elisa, C.; Andrea, C.; Mariangela, F.; Kalliopi, R.; Nurlaila, S.N.; et al. Driving mechanisms of overstorey-understory diversity relationships in European forests. Perspect. Plant Ecol. 2016, 19, 21–29. [Google Scholar] [CrossRef]
- Cazzolla Gatti, R.; Di Paola, A.; Bombelli, A.; Noce, S.; Valentini, R. Exploring the relationship between canopy height and terrestrial plant diversity. Plant Ecol. 2017, 218, 899–908. [Google Scholar] [CrossRef]
- Comita, L.S.; Queenborough, S.A.; Murphy, S.J.; Eck, J.L.; Xu, K.Y.; Krishnadas, M.; Beckman, N.; Zhu, Y. Testing predictions of the Janzen-Connell hypothesis: A meta-analysis of experimental evidence for distance- and density-dependent seed and seedling survival. J. Ecol. 2014, 102, 845–856. [Google Scholar] [CrossRef]
- Su, S.C.; Jin, N.Q.; Wei, X.L. Effects of thinning on the understory light environment of different stands and the photosynthetic performance and growth of the reforestation species Phoebe bournei. J. For. Res. 2024, 35, 6. [Google Scholar] [CrossRef]
- Farooq, T.H.; Wu, W.J.; Tigabu, M.; Ma, X.Q.; He, Z.M.; Rashid, M.H.U.; Gilani, M.M.; Wu, P.F. Growth, biomass production and root development of Chinese fir in relation to initial planting density. Forests 2019, 10, 236. [Google Scholar] [CrossRef]
- Yirdaw, E.; Luukkanen, O. Photosynthetically active radiation transmittance of forest plantation canopies in the Ethiopian highlands. For. Ecol. Manag. 2004, 188, 17–24. [Google Scholar] [CrossRef]
- DeJong, T.M. A comparison of three diversity indices based on their components of richness and evenness. Oikos 1975, 26, 222–227. [Google Scholar] [CrossRef]
- Kvålseth, T.O. Note on biological diversity, evenness, and homogeneity measures. Oikos 1991, 62, 123–127. [Google Scholar] [CrossRef]



| Values | LAI | MTA | ||
|---|---|---|---|---|
| F | P | F | P | |
| Treatment | 125.36 | <0.001 | 1.3 | 0.255 |
| Density | 1074.46 | <0.001 | 257.05 | <0.001 |
| Time | 647.84 | <0.001 | 3838.22 | <0.001 |
| Treatment × density | 2.16 | 0.060 | 3.59 | <0.01 |
| Treatment × Time | 6.04 | <0.01 | 9.51 | <0.001 |
| Density × Time | 77.53 | <0.001 | 52.76 | <0.001 |
| Treatment × density × Time | 14.63 | <0.001 | 8.25 | <0.001 |
| Values | PPFD | T | R/B | R/FR | ||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |
| Treatment | 859.28 | <0.001 | 857.34 | <0.001 | 0.14 | 0.144 | 0.22 | 0.222 |
| Density | 3704.44 | <0.001 | 3446.56 | <0.001 | 6.65 | <0.001 | 14.64 | <0.001 |
| Time | 9781.54 | <0.001 | 128.63 | <0.001 | 55.68 | <0.001 | 11.99 | <0.001 |
| Treatment × density | 13.98 | <0.001 | 13.06 | <0.001 | 2.28 | <0.05 | 1.09 | 0.368 |
| Treatment × time | 14.96 | <0.001 | 38.03 | <0.001 | 1.19 | 0.305 | 2.95 | 0.055 |
| Density × time | 109.1 | <0.001 | 94.45 | <0.001 | 3.02 | <0.01 | 5.15 | <0.001 |
| Treatment × density × Time | 13.69 | <0.001 | 10.69 | <0.001 | 3.21 | <0.001 | 3.25 | <0.001 |
| Composition | Years After Removal | Herb | Shrub | Total | |||
|---|---|---|---|---|---|---|---|
| Retention | Removal | Retention | Removal | Retention | Removal | ||
| Family | 2 | 36 | 38 | 11 | 10 | 39 | 39 |
| 4 | 32 | 37 | 7 | 12 | 33 | 38 | |
| 5 | 31 | 33 | 16 | 6 | 34 | 33 | |
| Genera | 2 | 54 | 60 | 13 | 13 | 57 | 61 |
| 4 | 48 | 59 | 9 | 14 | 50 | 60 | |
| 5 | 46 | 52 | 19 | 8 | 53 | 52 | |
| Species | 2 | 65 | 67 | 13 | 14 | 78 | 81 |
| 4 | 55 | 71 | 9 | 14 | 64 | 85 | |
| 5 | 53 | 63 | 20 | 9 | 73 | 72 | |
| Values | D | H | J | OD | ||||
|---|---|---|---|---|---|---|---|---|
| F | P | F | P | F | P | F | P | |
| Treatment | 21.02 | <0.001 | 52.13 | <0.001 | 0.58 | 0.448 | 133.41 | <0.001 |
| Density | 4.83 | <0.001 | 12.11 | <0.001 | 4.39 | <0.01 | 11.24 | <0.001 |
| Time | 3.11 | 0.051 | 2.16 | 0.123 | 56.49 | <0.001 | 5.7 | <0.01 |
| Treatment × Density | 0.89 | 0.495 | 5.26 | <0.001 | 4.64 | <0.001 | 1.53 | 0.190 |
| Treatment × Time | 4.64 | <0.01 | 3.67 | <0.05 | 6.83 | <0.01 | 5.1 | <0.01 |
| Density × Time | 1.7 | 0.097 | 3.37 | <0.01 | 2.38 | <0.05 | 4.21 | <0.01 |
| Treatment × Density × Time | 1.6 | 0.123 | 0.96 | 0.488 | 2.49 | <0.05 | 1.65 | 0.110 |
| Index | LAI | MTA | PPFD | T | R/B | R/FR |
|---|---|---|---|---|---|---|
| LAI | 1 | 0.19 ** | −0.59 ** | −0.76 ** | −0.30 ** | 0.31 ** |
| MTA | 1 | 0.35 ** | −0.21 ** | 0.39 ** | 0.04 | |
| PPFD | 1 | 0.77 ** | 0.48 ** | 0.23 ** | ||
| T | 1 | 0.25 ** | 0.20 ** | |||
| R/B | 1 | 0.68 ** | ||||
| R/FR | 1 |
| Index | D | H | J | OD |
|---|---|---|---|---|
| PPFD | 0.270 ** | 0.309 ** | −0.015 | 0.282 ** |
| T | 0.216 * | 0.337 ** | −0.046 | 0.299 ** |
| R/B | 0.330 ** | 0.132 | −0.064 | 0.265 ** |
| R/FR | 0.253 ** | 0.17 | −0.157 | 0.265 ** |
| LAI | −0.270 ** | −0.377 ** | 0.275 ** | −0.268 ** |
| MTA | 0.129 | 0.022 | 0.159 | 0.015 |
| Block Number | Stand Density (Stems·hm−2) | Latitude (N) | Longitude (E) | Slope and Aspect | Elevation (m a.s.l) | Mean DBH (cm) | Mean Height (m) |
|---|---|---|---|---|---|---|---|
| Ⅰ | 1800 (M1) | 26°9′30.60″ | 117°27′36.54″ | 17° NW | 180.07 | 13.72 | 11.59 |
| Ⅰ | 2400 (M2) | 26°9′31.38″ | 117°27′35.40″ | 28° SW | 186.41 | 13.67 | 11.92 |
| Ⅰ | 3000 (M3) | 26°9′25.80″ | 117°27′36.60″ | 20° SW | 183.36 | 12.20 | 11.93 |
| Ⅰ | 3600 (M4) | 26°9′34.02″ | 117°27′35.46″ | 24° SW | 178.52 | 12.20 | 10.66 |
| Ⅰ | 4200 (M5) | 26°9′35.04″ | 117°27′35.34″ | 28° SW | 179.79 | 11.71 | 11.40 |
| Ⅰ | 4800 (M6) | 26°9′31.86″ | 117°27′36.84″ | 19° SW | 182.19 | 11.86 | 11.85 |
| Ⅱ | 1800 (M1) | 26°9′33.12″ | 117°27′36.48″ | 17° NW | 176.46 | 14.15 | 12.84 |
| Ⅱ | 2400 (M2) | 26°9′31.14″ | 117°27′32.48″ | 23° SW | 167.59 | 13.86 | 11.42 |
| Ⅱ | 3000 (M3) | 26°9′27.35″ | 117°27′37.34″ | 24° NW | 178.85 | 13.02 | 13.26 |
| Ⅱ | 3600 (M4) | 26°9′31.98″ | 117°27′34.38″ | 20° NW | 173.09 | 13.72 | 12.09 |
| Ⅱ | 4200 (M5) | 26°9′30.30″ | 117°27′31.80″ | 22° SW | 166.56 | 11.88 | 11.05 |
| Ⅱ | 4800 (M6) | 26°9′31.62″ | 117°27′33.30″ | 22° SW | 169.24 | 11.48 | 11.64 |
| Ⅲ | 1800 (M1) | 26°9′31.44″ | 117°27′36.96″ | 24° W | 178.14 | 14.52 | 13.59 |
| Ⅲ | 2400 (M2) | 26°9′30.90″ | 117°27′35.34″ | 20° SW | 187.23 | 15.31 | 13.67 |
| Ⅲ | 3000 (M3) | 26°9′27.60″ | 117°27′36.90″ | 25° SW | 182.24 | 13.87 | 12.94 |
| Ⅲ | 3600 (M4) | 26°9′28.80″ | 117°27′36.78″ | 23° SW | 183.48 | 12.40 | 11.46 |
| Ⅲ | 4200 (M5) | 26°9′28.40″ | 117°27′36.72″ | 17° SW | 181.52 | 11.99 | 11.78 |
| Ⅲ | 4800 (M6) | 26°9′26.46″ | 117°27′36.66″ | 30° W | 179.22 | 13.18 | 13.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Zhang, L.; Liu, Q.; Chen, Y.; He, Z.; Li, S.; Ma, X. Effects of Canopy Litter Removal on Canopy Structure, Understory Light and Vegetation Dynamics in Cunninghamia lanceolata Plantations of Varying Densities. Plants 2025, 14, 3144. https://doi.org/10.3390/plants14203144
Zhou L, Zhang L, Liu Q, Chen Y, He Z, Li S, Ma X. Effects of Canopy Litter Removal on Canopy Structure, Understory Light and Vegetation Dynamics in Cunninghamia lanceolata Plantations of Varying Densities. Plants. 2025; 14(20):3144. https://doi.org/10.3390/plants14203144
Chicago/Turabian StyleZhou, Lili, Lixian Zhang, Qi Liu, Yulong Chen, Zongming He, Shubin Li, and Xiangqing Ma. 2025. "Effects of Canopy Litter Removal on Canopy Structure, Understory Light and Vegetation Dynamics in Cunninghamia lanceolata Plantations of Varying Densities" Plants 14, no. 20: 3144. https://doi.org/10.3390/plants14203144
APA StyleZhou, L., Zhang, L., Liu, Q., Chen, Y., He, Z., Li, S., & Ma, X. (2025). Effects of Canopy Litter Removal on Canopy Structure, Understory Light and Vegetation Dynamics in Cunninghamia lanceolata Plantations of Varying Densities. Plants, 14(20), 3144. https://doi.org/10.3390/plants14203144






