Physiological Responses of Grapevine Leaves to High Temperature at Different Senescence Periods
Abstract
1. Introduction
2. Results
2.1. Temperature Dynamics in the Field
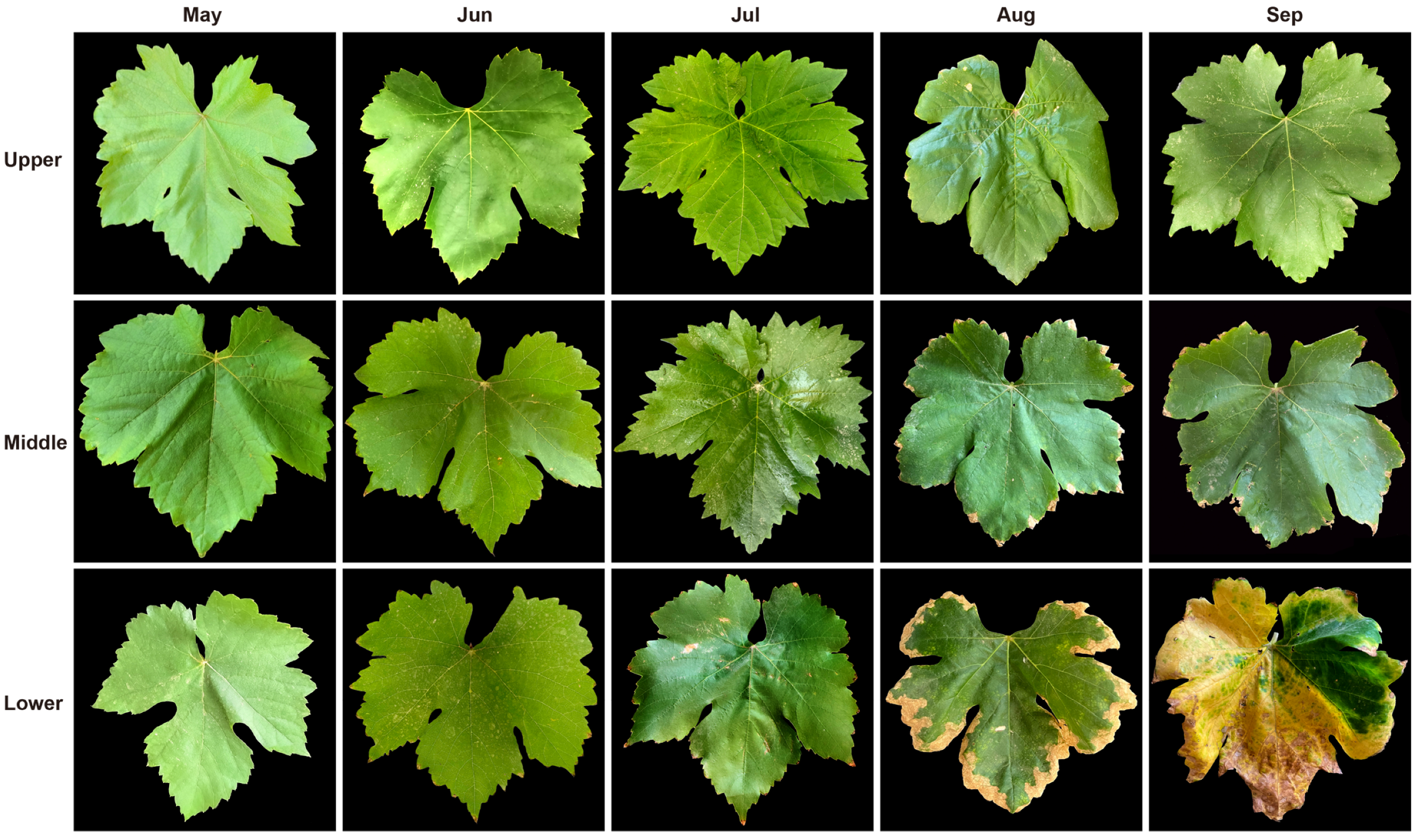
2.2. Phenotypic Observation of Leaves at Different Senescence Periods
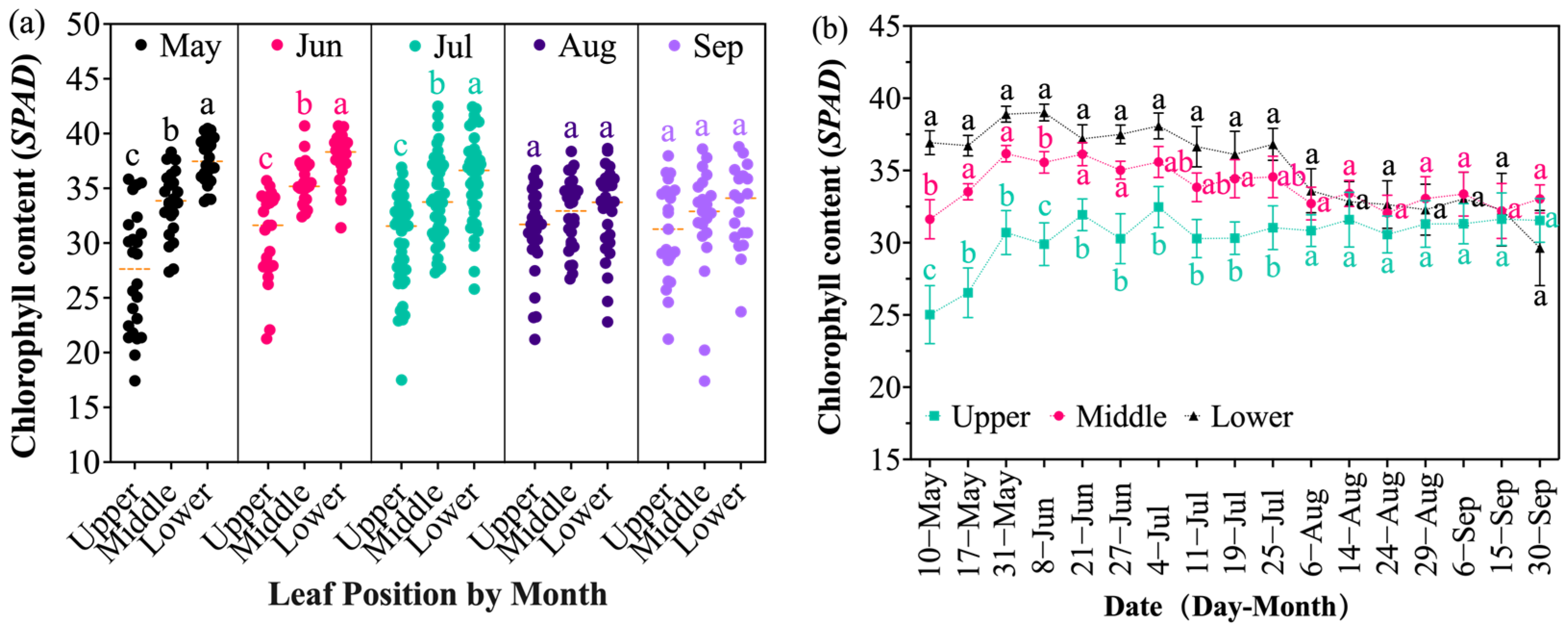
2.3. Chlorophyll Content of Grapevine Leaves at Different Senescence Stages
2.4. Effects of High Temperature on the OJIP Curve of Leaves at Different Senescence Stages
2.5. Chlorophyll Fluorescence Parameters of Grapevine Leaves at Different Senescence Stages
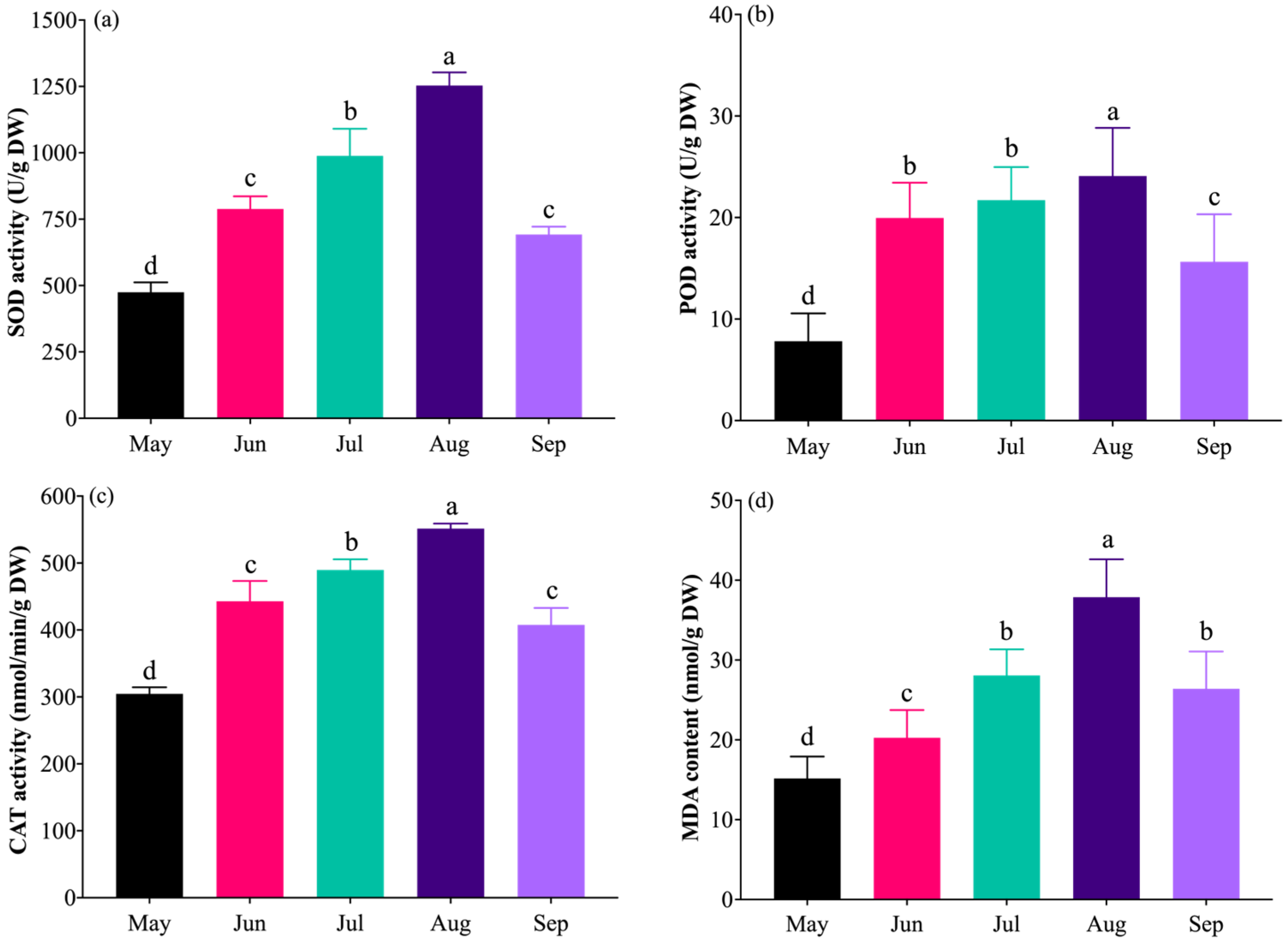
2.6. Effects of High Temperature on Antioxidant Enzyme Activities and MDA Content
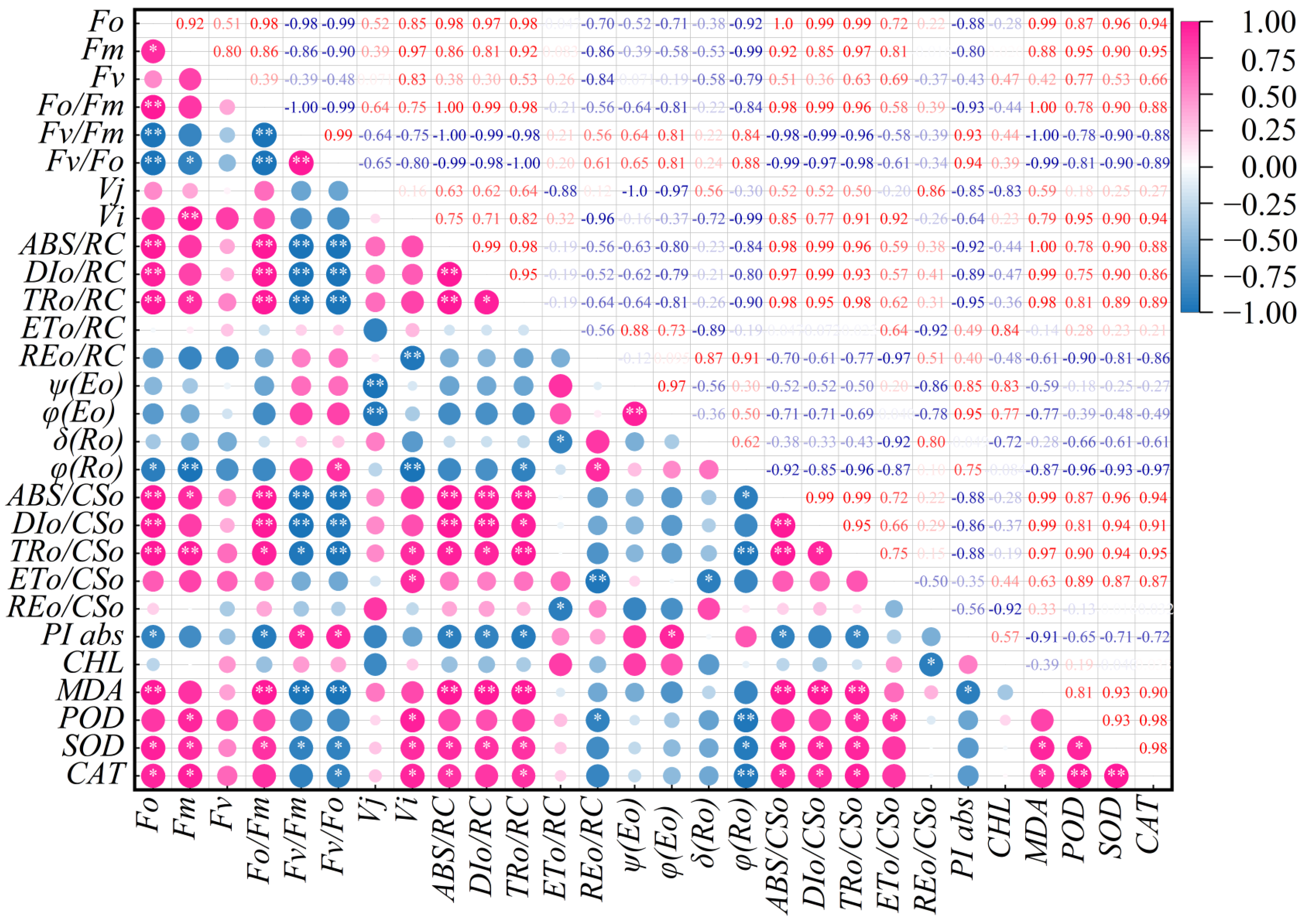
2.7. Correlation Analysis of Various Indicators
3. Discussion
4. Materials and Methods
4.1. Description of the Study Area
4.2. Plant Materials
4.3. Measurements and Methods
4.3.1. Temperature Monitoring
4.3.2. Chlorophyll Content Measurement
4.3.3. Chlorophyll Fluorescence Parameters
4.3.4. Antioxidant Enzyme Activities and MDA Content
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Liu, Z.; Liu, W.; Wu, Q.; Xie, Z.; Qi, K.; Zhang, S.; Wu, J.; Wang, P. Dual roles of pear EARLY FLOWERING 4 -like genes in regulating flowering and leaf senescence. BMC Plant Biol. 2024, 24, 1117. [Google Scholar] [CrossRef]
- Kim, C.; Kim, S.J.; Jeong, J.; Park, E.; Oh, E.; Park, Y.I.; Lim, P.O.; Choi, G. High ambient temperature accelerates leaf senescence via PHYTOCHROME-INTERACTING FACTOR 4 and 5 in Arabidopsis. Mol. Cells 2020, 43, 645–661. [Google Scholar] [CrossRef]
- Liu, H.; Shen, G.Z.; Fang, X.P.; Fu, Q.J.; Huang, K.-K.; Chen, Y.; Yu, H.; Zhao, Y.; Zhang, L.; Jin, L.; et al. Heat stress-induced response of the proteomes of leaves from Salvia splendens Vista and King. Proteome Sci. 2013, 11, 25. [Google Scholar] [CrossRef]
- Wang, X.; Pan, Y.; Liu, H.; Meng, H.; Cheng, Z. Physiological responses of cucumber seedlings to combined high-temperature and high-humidity stress at different leaf stages. Horticulturae 2024, 10, 1369. [Google Scholar] [CrossRef]
- Yang, K.; Sun, H.; Liu, M.; Zhu, L.; Zhang, K.; Zhang, Y.; Li, A.; Zhang, H.; Zhu, J.; Liu, X.; et al. Morphological and physiological mechanisms of melatonin on delaying drought-induced leaf senescence in cotton. Int. J. Mol. Sci. 2023, 24, 7269. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Zhang, Y.; Xu, M.; Zhao, M.; Chen, H.; Lu, Y.; Pang, S.; Xu, W. Characterization of low-temperature sensitivity and chlorophyll fluorescence in yellow leaf mutants of tomato. Agronomy 2024, 14, 2382. [Google Scholar] [CrossRef]
- Nakashima, S.; Yamakita, E. In Situ Visible Spectroscopic Daily Monitoring of Senescence of Japanese Maple (Acer palmatum) Leaves. Life 2023, 13, 2030. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Z.; Li, J.; Li, R.; Zhang, X.; Yao, X.; Xie, F. Comparative transcriptome-based analysis of the regulation of leaf senescence in the upper and middle canopy of different soybean cultivars. Agronomy 2024, 14, 1250. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine responses to heat stress and global warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef]
- Gong, Z.; Xiong, L.; Shi, H.; Yang, S.; Herrera-Estrella, L.R.; Xu, G.; Chao, D.Y.; Li, J.; Wang, P.Y.; Qin, F. Plant abiotic stress response and nutrient use efficiency. Sci. China Life Sci 2020, 63, 635–674. [Google Scholar] [CrossRef]
- Zha, Q.; Yin, X.; Xi, X.; Jiang, A. Colored shade nets can relieve abnormal fruit softening and premature leaf senescence of ‘Jumeigui’ grapes during ripening under greenhouse conditions. Plants 2022, 11, 1227. [Google Scholar] [CrossRef] [PubMed]
- Asproudi, A.; Petrozziello, M.; Cavalletto, S.; Guidoni, S. Grape aroma precursors in cv. Nebbiolo as affected by vine microclimate. Food Chem. 2016, 211, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Abudureheman, R.; Zhong, H.; Yadav, V.; Zhang, C.; Ma, Y.; Liu, X.; Zhang, F.; Zha, Q.; Wang, X. The impact of high temperatures in the field on leaf tissue structure in different grape cultivars. Horticulturae 2023, 9, 731. [Google Scholar] [CrossRef]
- Cataldo, E.; Puccioni, S.; Eichmeier, A.; Natale, R.; Gori, M.; Biricolti, S. Mattii GB: Effect of zeolite and irrigation treatments on grapevine leaves, an interdisciplinary approach. Plant Soil 2025, 508, 1007–1026. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, J.; Luo, W.; Niu, S.; Qu, L.; Li, J.; Chen, Y.; Li, G.; Yang, H.; Lu, D. Salicylic acid cooperates with lignin and sucrose signals to alleviate waxy maize leaf senescence under heat stress. Plant Cell Environ. 2025, 48, 4341–4355. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.J.; He, Y.N.; Jiang, A.L. High temperature in field: Effect on the leaf tissue structure of grape varieties. Chin. Agric. Sci. Bull. 2019, 35, 74–77. [Google Scholar]
- Jiang, J.F.; Zhang, Y.; Tian, Z.S.; Fan, X.C.; Liu, C.H. Observation on microstructure and ultrastucture of leaves in Vitis L. Acta Bot. Boreali-Occident. Sin. 2012, 32, 1365–1371. [Google Scholar]
- Li, Z.H.; Guo, Y.F.; Ren, G.D.; Zhang, K.W.; Miao, Y.; Guo, H.W. Advances in leaf senescence research. Plant Physiol. J. 2023, 59, 1627–1656. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, T.; Cao, J.; Wang, H.L.; Tan, S.; Zhang, Y.; Park, S.; Park, H.; Woo, H.R.; Li, X.; et al. Histone variant HTB4 delays leaf senescence by epigenetic control of Ib bHLH transcription factor-mediated iron homeostasis. New Phytol. 2023, 240, 694–709. [Google Scholar] [CrossRef]
- Sun, Y.J.; Wang, Q.; Shao, Q.W.; Xin, Z.M.; Xiao, H.J.; Cheng, J. Progress in the study of the effect of high temperature stress on plant photosynthesis. Bull. Bot. 2023, 58, 486–498. [Google Scholar]
- Kim, J.S.; Jeon, B.W.; Kim, J. Signaling peptides regulating abiotic stress responses in plants. Front. Plant Sci. 2021, 12, 704490. [Google Scholar] [CrossRef] [PubMed]
- Posch, B.C.; Kariyawasam, B.C.; Bramley, H.; Coast, O.; Richards, R.A.; Reynolds, M.P.; Trethowan, R.; Atkin, O.K. Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. J. Exp. Bot. 2019, 70, 5051–5069. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhong, H.; Ma, Y.; Bai, S.; Yadav, V.; Zhang, C.; Zhang, F.; Shi, W.; Abudureheman, R.; Wang, X. Effects of Different Biostimulants on growth and development of grapevine seedlings under high-temperature stress. Horticulturae 2024, 10, 269. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Hasan, M.M.; El-Yazied, A.A.; Alabdallah, N.M.; Hajjar, D.; Altaf, M.A.; Sun, J.; Guo, S. Melatonin pretreatment confers heat tolerance and repression of heat-induced senescence in tomato through the modulation of ABA- and GA-mediated pathways. Front. Plant Sci. 2021, 12, 650955. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, X.; Gu, L.; Liu, P.; Zhao, B.; Zhang, J.; Ren, B. The effects of high temperature, drought, and their combined stresses on the photosynthesis and senescence of summer maize. Agric. Water Manag. 2023, 289, 108525. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.; Jiang, A.; Tian, Y. Comparison of the activities of photosystem II of four table grapevine cultivars during high-temperature stress. Hortic. Environ. Biotechnol. 2018, 59, 363–371. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, Y.; Goltsev, V.; Strasser, R.J.; Kalaji, H.M.; Wang, H.; Wang, X.; Chen, S.; Qiang, S. Comparative effect of tenuazonic acid, diuron, bentazone, dibromothymoquinone and methyl viologen on the kinetics of Chl a fluorescence rise OJlP and the MR820 signal. Plant Physiol. Biochem. 2020, 156, 39–48. [Google Scholar] [CrossRef]
- Raihan, M.R.H.; Antala, M.; Stróżecki, M.; Haque, M.I.; Hasanuzzaman, M.; Juszczak, R.; Rastogi, A. Silicon-induced photosynthetic adaptations in common buckwheat under salt stress revealed by prompt chlorophyll a fluorescence analysis. Sci. Rep. 2025, 15, 19343. [Google Scholar] [CrossRef]
- Su, Y.; Li, X.; Cao, Z.; Gao, Z.; Du, Y. Effects of long-term high temperatures in the root zone on the physiological characteristics of grapevine leaves and roots: Implications for viticulture practices. Horticulturae 2024, 10, 245. [Google Scholar] [CrossRef]
- Zha, Q.; Xi, X.; He, Y.; Yin, X.; Jiang, A. Effect of short-time high-temperature treatment on the photosynthetic performance of different heat-tolerant grapevine cultivars. Photochem. Photobiol. 2021, 97, 763–769. [Google Scholar] [CrossRef]
- Oukarroum, A.; Madidi, S.E.; Schansker, G.; Strasser, R.J. Probing the responses of barley cultivars (Hordeum vulgare L.) by chlorophyll a fluorescence OLKJIP under drought stress and re-watering. Environ. Exp. Bot. 2007, 60, 438–446. [Google Scholar] [CrossRef]
- Arief, M.A.A.; Kim, H.; Kurniawan, H.; Nugroho, A.P.; Kim, T.; Cho, B.K. Chlorophyll Fluorescence Imaging for Early Detection of Drought and Heat Stress in Strawberry Plants. Plants 2023, 12, 1387. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Tang, M.; Jin, X.Q.; Li, H.; Chen, L.S.; Wang, Q.L.; Sun, A.Z.; Yi, Y.; Guo, F.Q. Regulation of Calvin-Benson cycle enzymes under high temperature stress. Abiotech 2022, 3, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Chen, S.; Yang, J.; Zhang, M.; Strasser, R.; Qiang, S. Classification and characteristics of heat tolerance in Ageratinaadenophora populations using fast chlorophyll a fluorescence rise O-J-I-P. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, C.; Yao, S. The impact of high-temperature stress on rice: Challenges and solutions. Crop J. 2021, 9, 963–976. [Google Scholar] [CrossRef]
- Yu, B.; Ming, F.; Liang, Y.; Wang, Y.; Gan, Y.; Qiu, Z.; Yan, S.; Cao, B. Heat Stress Resistance Mechanisms of Two Cucumber Varieties from Different Regions. Int. J. Mol. Sci. 2022, 23, 1817. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Wan, Z.; Huang, S.; Di, H.; He, Y.; Jin, S. Physiological and transcriptome analyses provide new insights into the mechanism mediating the enhanced tolerance of melatonin-treated rhododendron plants to heat stress. J. Integr. Agric. 2023, 22, 2397–2411. [Google Scholar] [CrossRef]
- Wu, J.Y.; Liu, G.H.; Xu, G.X.; Abudureheman, R.; Zhong, H.X.; Liu, Y.X.; Bai, S.J.; Liu, C.H.; Jiang, J.F.; Zha, Q. Research advances on heat tolerance of grapevine. Mol. Plant Breed. 2023. in press. Available online: https://link.cnki.net/urlid/46.1068.S.20230306.1552.002 (accessed on 12 June 2025).
- Wang, Y.; Liu, H. Transcriptomic and physiological analyses reveal different grape varieties response to high temperature stress. Front. Plant Sci. 2024, 15, 1313832. [Google Scholar] [CrossRef]
- Wu, J.Y.; Lian, W.J.; Zeng, X.Y.; Liu, Z.G.; Mao, L.; Liu, Y.X.; Jiang, J.F. Physiological response to high temperature and evaluation of heat tolerance of different grape varieties. Acta Bot. Boreal.-Occident. Sin. 2019, 39, 1075–1084. [Google Scholar] [CrossRef]
- Zha, Q.; Zhong, H.; Tang, M.; Yin, X.; Sun, P.; Jiang, A.; Xi, X.; Wu, J. Effects of temperature and light during the veraison period on grape berry growth. Plant Stress 2024, 14, 100642. [Google Scholar] [CrossRef]
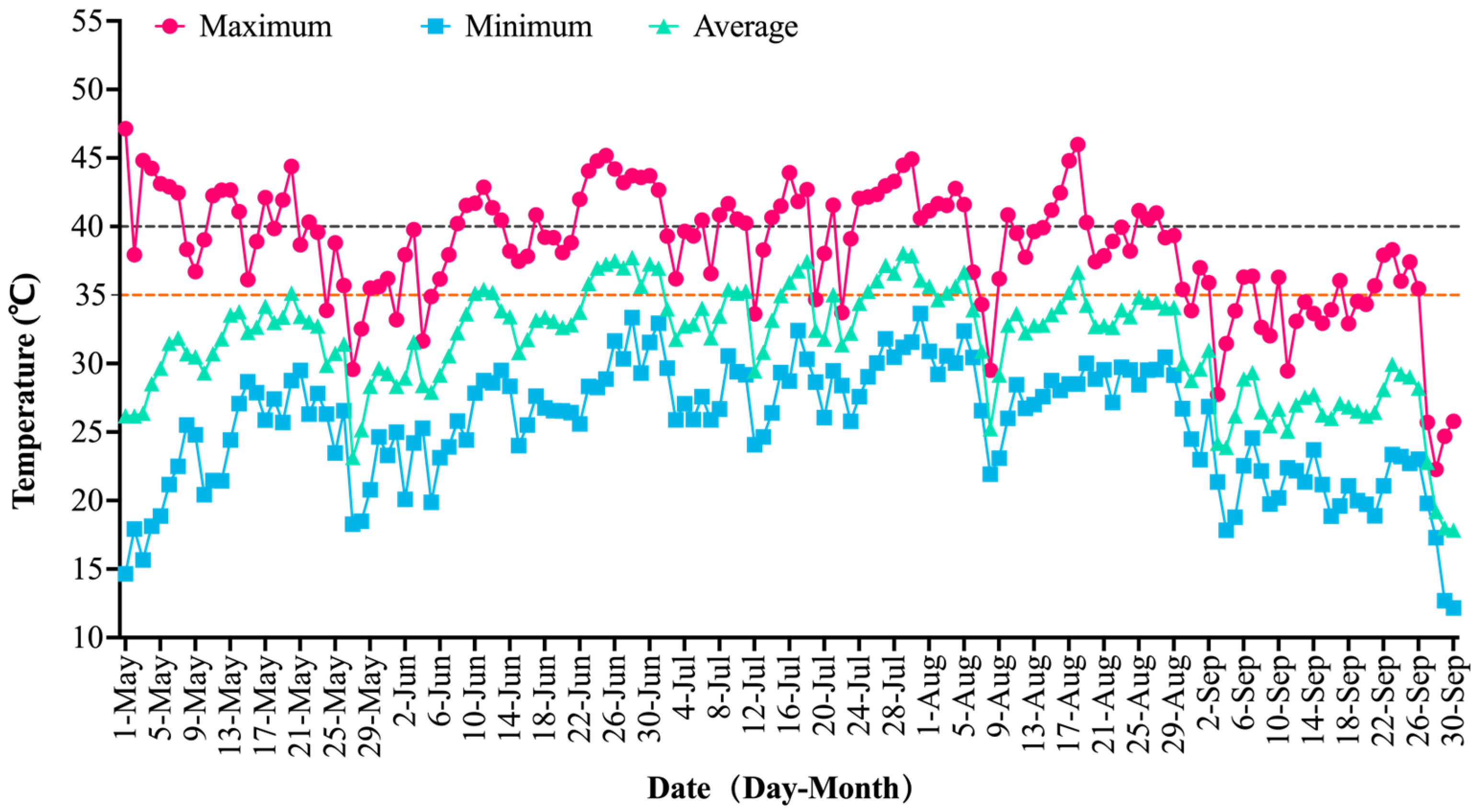


| Temperature | May | Jun | Jul | Aug | Sep |
|---|---|---|---|---|---|
| Maximum Temperature/°C | 47.13 | 45.17 | 44.93 | 45.99 | 38.30 |
| Minimum Temperature/°C | 14.65 | 19.87 | 24.08 | 21.93 | 12.15 |
| Average Temperature/°C | 30.59 | 33.35 | 34.4 | 33.27 | 26.22 |
| ≥35.00 °C/Day | 28 | 27 | 28 | 28 | 12 |
| ≥40.00 °C/Day | 14 | 16 | 20 | 15 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, S.; Abudureheman, R.; Zhang, Z.; Zhong, H.; Zhang, F.; Wang, X.; Nasir, M.; Wu, J. Physiological Responses of Grapevine Leaves to High Temperature at Different Senescence Periods. Plants 2025, 14, 3142. https://doi.org/10.3390/plants14203142
Guo S, Abudureheman R, Zhang Z, Zhong H, Zhang F, Wang X, Nasir M, Wu J. Physiological Responses of Grapevine Leaves to High Temperature at Different Senescence Periods. Plants. 2025; 14(20):3142. https://doi.org/10.3390/plants14203142
Chicago/Turabian StyleGuo, Shiwei, Riziwangguli Abudureheman, Zekai Zhang, Haixia Zhong, Fuchun Zhang, Xiping Wang, Mansur Nasir, and Jiuyun Wu. 2025. "Physiological Responses of Grapevine Leaves to High Temperature at Different Senescence Periods" Plants 14, no. 20: 3142. https://doi.org/10.3390/plants14203142
APA StyleGuo, S., Abudureheman, R., Zhang, Z., Zhong, H., Zhang, F., Wang, X., Nasir, M., & Wu, J. (2025). Physiological Responses of Grapevine Leaves to High Temperature at Different Senescence Periods. Plants, 14(20), 3142. https://doi.org/10.3390/plants14203142






