Understory Dwarf Bamboo Modulates Leaf Litter Decomposition via Interception-Induced Litter Redistribution and Space-Dependent Decomposition Dynamics: A Case Study from Jinfo Mountain, China
Abstract
1. Introduction
2. Materials and Methods
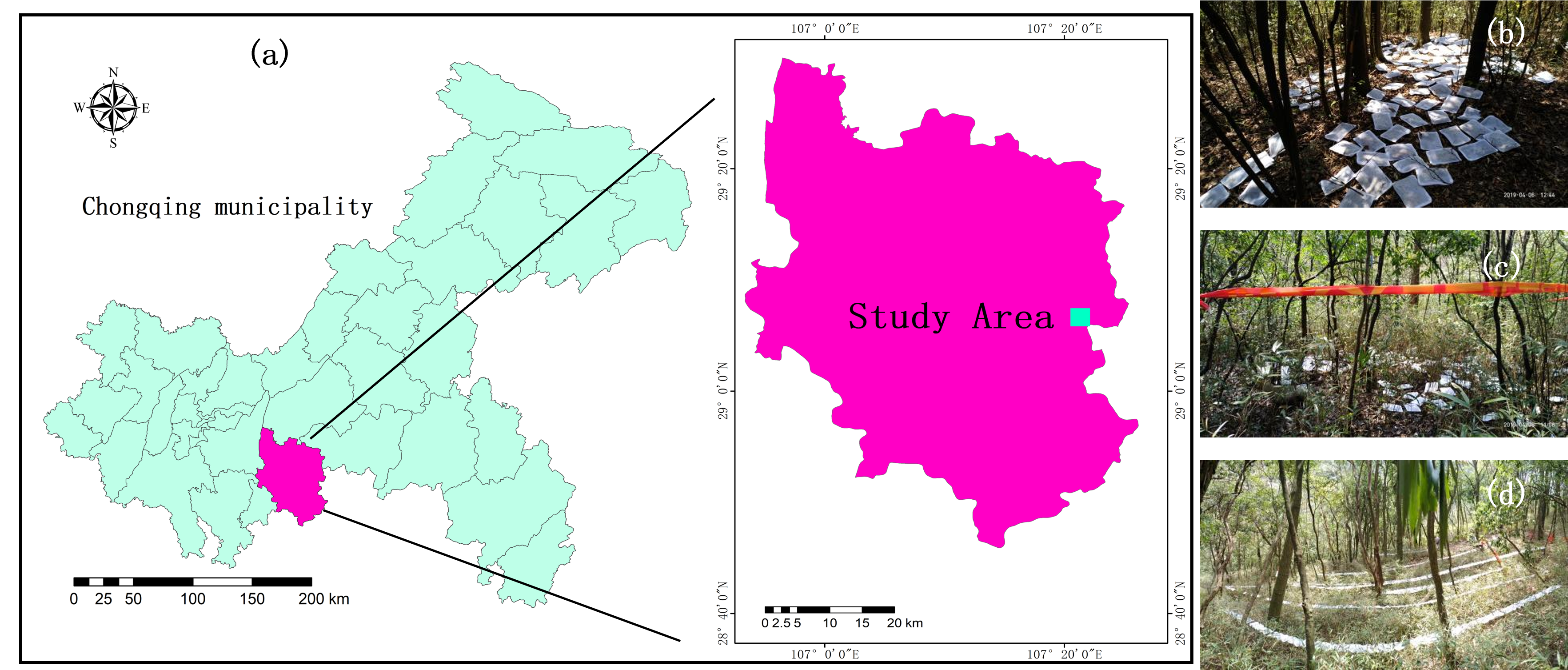
2.1. Study Site
2.2. Litterfall Collection and Litter Interception Evaluation
2.3. Litter Decomposition Experiment Design
2.3.1. Litter Decomposition Plot Setup
2.3.2. Species Selection and Experimental Operation
2.3.3. Decomposition Index
2.4. Statistical Analysis
3. Result
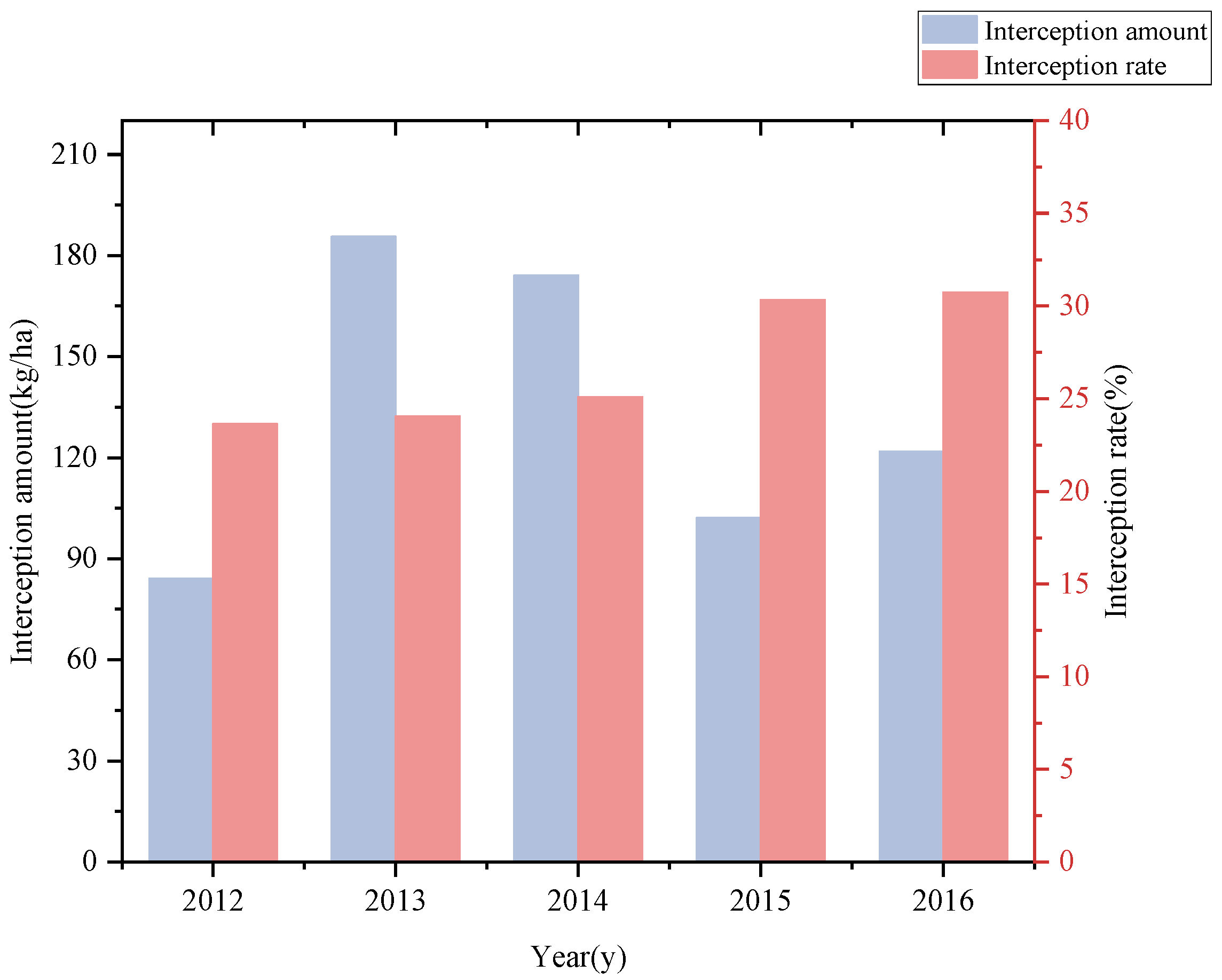
3.1. Interception of Canopy Litterfall by Understory Dwarf Bamboo
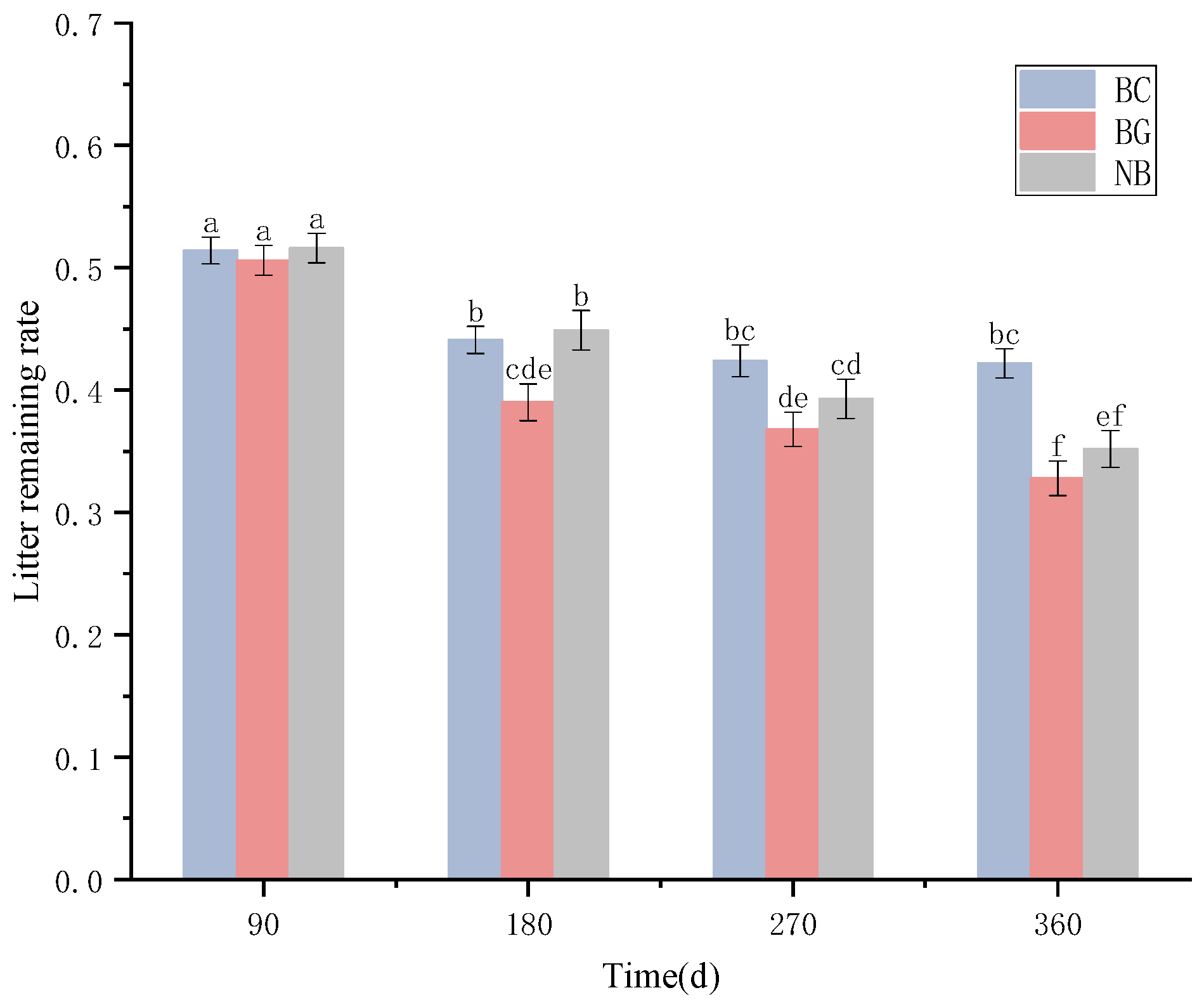
3.2. Leaf Litter Decomposition Dynamics and Decomposition Rates in Different Habitats
3.3. Litter Leaves Element Release Dynamics in Different Habitats
3.4. Factors Influencing Litter Decomposition Across Different Habitats
4. Discussion
4.1. Interception Effect of Dwarf Bamboo on Litterfall of Forest Canopy
4.2. Effect of Understory Dwarf Bamboo on Litter Decomposition
4.3. Litter Decomposition Dynamics and Nutrient Release
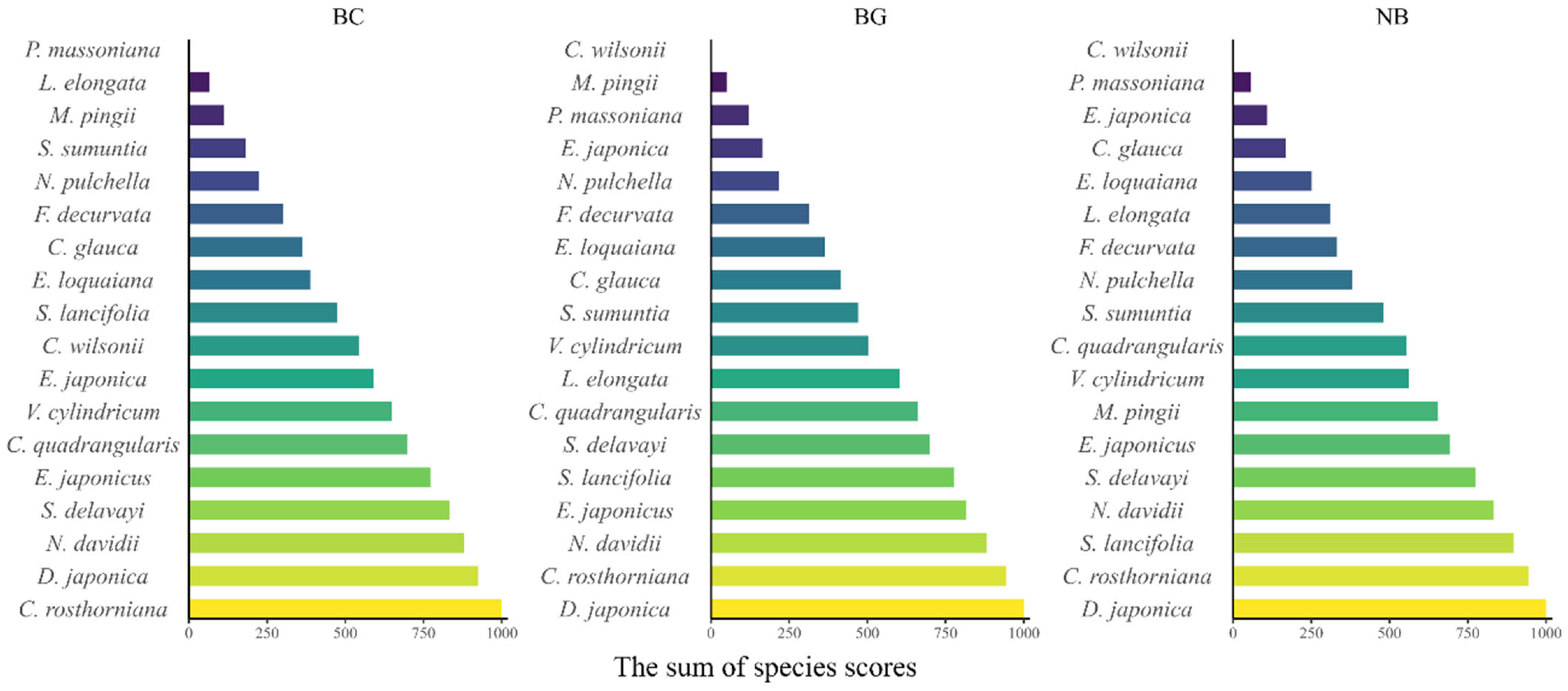
4.4. Interspecific Variation in Litter Decomposition
4.5. Limitations and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, Y.; Liu, C.; Qian, S.S.; Luo, Y.; Zhou, R.; Tang, J.; Bao, W. Large-Scale Patterns of Understory Biomass and Its Allocation across China’s Forests. Sci. Total Environ. 2022, 804, 150169. [Google Scholar] [CrossRef] [PubMed]
- Balandier, P.; Gobin, R.; Prévosto, B.; Korboulewsky, N. The Contribution of Understorey Vegetation to Ecosystem Evapotranspiration in Boreal and Temperate Forests: A Literature Review and Analysis. Eur. J. For. Res. 2022, 141, 979–997. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Plant Litter: Decomposition, Humus Formation, Carbon Sequestration, 2nd ed.; Springer: Berlin, Germany, 2008; ISBN 978-3-540-74922-6. [Google Scholar]
- Kawakami, E.; Katayama, A.; Hishi, T. Effects of Declining Understory Vegetation on Leaf Litter Decomposition in a Japanese Cool-Temperate Forest. J. For. Res. 2020, 25, 260–268. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Huang, Y.; Hui, D.; Wen, M. Effects of the Interception of Litterfall by the Understory on Carbon Cycling in Eucalyptus Plantations of South China. PLoS ONE 2014, 9, e100464. [Google Scholar] [CrossRef]
- García-Palacios, P.; Shaw, E.A.; Wall, D.H.; Hättenschwiler, S. Temporal Dynamics of Biotic and Abiotic Drivers of Litter Decomposition. Ecol. Lett. 2016, 19, 554–563. [Google Scholar] [CrossRef]
- Sykes, A.J.; Macleod, M.; Eory, V.; Rees, R.M.; Payen, F.; Myrgiotis, V.; Williams, M.; Sohi, S.; Hillier, J.; Moran, D.; et al. Characterising the Biophysical, Economic and Social Impacts of Soil Carbon Sequestration as a Greenhouse Gas Removal Technology. Glob. Change Biol. 2020, 26, 1085–1108. [Google Scholar] [CrossRef]
- Montti, L.; Villagra, M.; Campanello, P.I.; Gatti, M.G.; Goldstein, G. Functional Traits Enhance Invasiveness of Bamboos over Co-Occurring Tree Saplings in the Semideciduous Atlantic Forest. Acta Oecologica 2014, 54, 36–44. [Google Scholar] [CrossRef]
- Kudo, G.; Amagai, Y.; Hoshino, B.; Kaneko, M. Invasion of Dwarf Bamboo into Alpine Snow-meadows in Northern Japan: Pattern of Expansion and Impact on Species Diversity. Ecol. Evol. 2011, 1, 85–96. [Google Scholar] [CrossRef]
- Kudo, G.; Kawai, Y.; Amagai, Y.; Winkler, D.E. Degradation and Recovery of an Alpine Plant Community: Experimental Removal of an Encroaching Dwarf Bamboo. Alp. Bot. 2017, 127, 75–83. [Google Scholar] [CrossRef]
- De Pauw, K.; Sanczuk, P.; Meeussen, C.; Depauw, L.; De Lombaerde, E.; Govaert, S.; Vanneste, T.; Brunet, J.; Cousins, S.A.O.; Gasperini, C.; et al. Forest Understorey Communities Respond Strongly to Light in Interaction with Forest Structure, but Not to Microclimate Warming. New Phytol. 2022, 233, 219–235. [Google Scholar] [CrossRef]
- Qian, F.; Song, H.; Chen, M.; Zeng, J.; Dang, C.; Tao, J. Multivariate Path Analysis of the Relationships between Seedling Regeneration and Environmental Factors beneath a Dwarf Bamboo Understory. Ecol. Evol. 2019, 9, 10277–10290. [Google Scholar] [CrossRef]
- Dearden, F.M.; Wardle, D.A. The Potential for Forest Canopy Litterfall Interception by a Dense Fern Understorey, and the Consequences for Litter Decomposition. Oikos 2008, 117, 83–92. [Google Scholar] [CrossRef]
- Zheng, Y.; Guan, F.; Fan, S.; Yan, X.; Huang, L. Dynamics of Leaf-Litter Biomass, Nutrient Resorption Efficiency and Decomposition in a Moso Bamboo Forest After Strip Clearcutting. Front. Plant Sci. 2022, 12, 799424. [Google Scholar] [CrossRef]
- Cai, M.; Chen, H.; Tan, H.; Chen, J.; He, S.; Long, M. Temporal Dynamics of Nutrient Release from Mulching of Legume Roots and Shoots Litter Driven by Microbial Community during Decomposition in Organic Orchards. BMC Plant Biol. 2025, 25, 374. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.S. Energy Storage and the Balance of Producers and Decomposers in Ecological Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Q.; Dong, Y.; Guo, Y. The Influence of Increased Precipitation and Nitrogen Deposition on the Litter Decomposition and Soil Microbial Community Structure in a Semiarid Grassland. Sci. Total Environ. 2022, 844, 157115. [Google Scholar] [CrossRef] [PubMed]
- Yue, K. Dynamics of Calcium, Magnesium, and Manganese During Litter Decomposition in Alpine Forest Aquatic and Terrestrial Ecosystems. Ecosystems 2020, 24, 516–529. [Google Scholar] [CrossRef]
- Ge, X.; Wang, C.; Wang, L.; Zhou, B.; Cao, Y.; Xiao, W.; Li, M.-H. Drought Changes Litter Quantity and Quality, and Soil Microbial Activities to Affect Soil Nutrients in Moso Bamboo Forest. Sci. Total Environ. 2022, 838, 156351. [Google Scholar] [CrossRef]
- Bogdziewicz, M.; Kelly, D.; Thomas, P.A.; Lageard, J.G.A.; Hacket-Pain, A. Climate Warming Disrupts Mast Seeding and Its Fitness Benefits in European Beech. Nat. Plants 2020, 6, 88–94. [Google Scholar] [CrossRef]
- Ma, T.; Nan, X.; Wu, R.; Yan, H.; Wu, N.; She, J.; Bao, Z. Quantifying the Impact of Canopy Structural Characteristics on Soil Temperature Variations in Different Bamboo Communities. Atmosphere 2023, 14, 445. [Google Scholar] [CrossRef]
- Onozawa, Y.; Chiwa, M.; Komatsu, H.; Otsuki, K. Rainfall Interception in a Moso Bamboo (Phyllostachys pubescens) Forest. J. For. Res. 2009, 14, 111–116. [Google Scholar] [CrossRef]
- Xu, X.; Du, H.; Zhou, G.; Mao, F.; Li, X.; Zhu, D.; Li, Y.; Cui, L. Remote Estimation of Canopy Leaf Area Index and Chlorophyll Content in Moso Bamboo (Phyllostachys edulis (Carrière) J. Houz.) Forest Using MODIS Reflectance Data. Ann. For. Sci. 2018, 75, 33. [Google Scholar] [CrossRef]
- Yan, T.; Wang, Z.; Liao, C.; Xu, W.; Wan, L. Effects of the Morphological Characteristics of Plants on Rainfall Interception and Kinetic Energy. J. Hydrol. 2021, 592, 125807. [Google Scholar] [CrossRef]
- Li, W.; Liu, Z.; Zhao, J.; Ma, L.; Wu, J.; Qi, J.; Wang, H. Leaf Mechanical Properties as Potential Predictors of Leaf-Litter Decomposability. For. Res. 2023, 3, 21. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Li, Q.Y.; Shi, X.-P.; Tao, J.-P. Effects of Dwarf Bamboo, Fargesia Nitida (Mitford) Keng f. Ex Yi, on Bark Stripping by Ungulates in a Subalpine Abies Faxoniana Rehder & E. H. Wilson Forest, Southwest China. Contemp. Probl. Ecol. 2013, 6, 578–582. [Google Scholar] [CrossRef]
- Hirobe, M.; Miyamoto, S.; Sakamoto, K.; Kondo, J.; Otoda, T.; Akaji, Y.; Yamanaka, N. The Spatial Distributions of Understory Trees in Relation to Dwarf Bamboo Cover in a Cool-Temperate Deciduous Broadleaf Forest in Japan. J. For. Res. 2015, 20, 357–362. [Google Scholar] [CrossRef]
- Watanabe, T.; Fukuzawa, K.; Shibata, H. Temporal Changes in Litterfall, Litter Decomposition and Their Chemical Composition in Sasa Dwarf Bamboo in a Natural Forest Ecosystem of Northern Japan. J. For. Res. 2013, 18, 129–138. [Google Scholar] [CrossRef]
- Takahashi, K.; Uemura, S.; Hara, T. A Forest-Structure-Based Analysis of Rain Flow into Soil in a Dense Deciduous Betula Ermanii Forest with Understory Dwarf Bamboo. Landsc. Ecol. Eng. 2011, 7, 101–108. [Google Scholar] [CrossRef]
- Takahashi, K.; Uemura, S.; Suzuki, J.-I.; Hara, T. Effects of Understory Dwarf Bamboo on Soil Water and the Growth of Overstory Trees in a Dense Secondary Betula Ermanii Forest, Northern Japan. Ecol. Res. 2003, 18, 767–774. [Google Scholar] [CrossRef]
- Sun, H.; Hu, W.; Dai, Y.; Ai, L.; Wu, M.; Hu, J.; Zuo, Z.; Li, M.; Yang, H.; Ma, J. Moso Bamboo (Phyllostachys edulis (Carrière) J. Houzeau) Invasion Affects Soil Microbial Communities in Adjacent Planted Forests in the Lijiang River Basin, China. Front. Microbiol. 2023, 14, 1111498. [Google Scholar] [CrossRef]
- Luan, J.; Li, S.; Dong, W.; Liu, Y.; Wang, Y.; Liu, S. Litter Decomposition Affected by Bamboo Expansion Is Modulated by Litter-mixing and Microbial Composition. Funct. Ecol. 2021, 35, 2562–2574. [Google Scholar] [CrossRef]
- Santos, M.; Santos, E.; Wagner-Riddle, C.; Brown, S.; Stropes, K.; Staebler, R.; Nippert, J. Evaluating a Lagrangian Inverse Model for Inferring Isotope CO2 Exchange in Plant Canopies. Agric. For. Meteorol. 2019, 276–277, 107651. [Google Scholar] [CrossRef]
- Orrego, M.; Ugawa, S.; Inoue, A.; Laplace, S.; Kume, T.; Koga, S.; Hishi, T.; Enoki, T. Climate, Soil, and Plant Controls on Early-Stage Litter Decomposition in Moso Bamboo Stands at a Regional Scale. Front. For. Glob. Change 2022, 5, 921028. [Google Scholar] [CrossRef]
- Zhou, G.; Wan, J.; Gu, Z.; Ding, W.; Hu, S.; Du, Q.; Meng, S.; Yang, C. Functional Diversity Accelerates the Decomposition of Litter Recalcitrant Carbon but Reduces the Decomposition of Labile Carbon in Subtropical Forests. Forests 2023, 14, 2258. [Google Scholar] [CrossRef]
- Hall, S.J.; Huang, W.; Timokhin, V.I.; Hammel, K.E. Lignin Lags, Leads, or Limits the Decomposition of Litter and Soil Organic Carbon. Ecology 2020, 101, e03113. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Ma, K.; Mi, X.; Ma, Y.; Pang, M.; Lin, D.; Dou, P. Response of Litter Carbon, Nitrogen and Phosphorus to Simulated Leaching. Chin. Sci. Bull. 2018, 63, 3114–3123. [Google Scholar] [CrossRef]
- Liu, W.; Fox, J.E.D.; Xu, Z. Leaf Litter Decomposition of Canopy Trees, Bamboo and Moss in a Montane Moist Evergreen Broad-leaved Forest on Ailao Mountain, Yunnan, South-west China. Ecol. Res. 2000, 15, 435–447. [Google Scholar] [CrossRef]
- Lusk, M.G. Throughfall as an understudied biogeochemical subsidy of nutrients and carbon in the urban water cycle: Perspective and a research agenda. Discov. Water 2024, 4, 124. Available online: https://link.springer.com/article/10.1007/s43832-024-00181-y (accessed on 25 September 2025). [CrossRef]
- Bortolazzi, A.; Da Ros, L.; Rodeghiero, M.; Tognetti, R.; Tonon, G.; Ventura, M. The Canopy Layer, a Biogeochemical Actor in the Forest N-cycle. Sci. Total Environ. 2021, 776, 146024. [Google Scholar] [CrossRef]
- Robbins, C.J.; Manning, D.W.P.; Halvorson, H.M.; Norman, B.C.; Eckert, R.A.; Pastor, A.; Dodd, A.K.; Jabiol, J.; Bastias, E.; Gossiaux, A.; et al. Nutrient and Stoichiometry Dynamics of Decomposing Litter in Stream Ecosystems: A Global Synthesis. Ecology 2023, 104, e4060. [Google Scholar] [CrossRef]
- Chi, G.; Zeng, F.; Wang, Y.; Chen, X. Phosphorus Dynamics in Litter–Soil Systems during Litter Decomposition in Larch Plantations across the Chronosequence. Front. Plant Sci. 2022, 13, 1010458. [Google Scholar] [CrossRef]
- Zheng-Hu, Z.; Chuan-Kuan, W. Center for Ecological Research, Northeast Forestry University, Harbin 150040, China Responses and Regulation Mechanisms of Microbial Decomposers to Substrate Carbon, Nitro-Gen, and Phosphorus Stoichiometry. Chin. J. Plant Ecol. 2016, 40, 620–630. [Google Scholar] [CrossRef]
- Tu, L.-H.; Hu, H.-L.; Hu, T.-X.; Zhang, J.; Li, X.-W.; Liu, L.; Xiao, Y.-L.; Chen, G.; Li, R.-H. Litterfall, Litter Decomposition, and Nutrient Dynamics in Two Subtropical Bamboo Plantations of China. Pedosphere 2014, 24, 84–97. [Google Scholar] [CrossRef]
- Tao, J.; Zuo, J.; He, Z.; Wang, Y.; Liu, J.; Liu, W.; Cornelissen, J.H.C. Traits Including Leaf Dry Matter Content and Leaf pH Dominate over Forest Soil pH as Drivers of Litter Decomposition among 60 Species. Funct. Ecol. 2019, 33, 1798–1810. [Google Scholar] [CrossRef]

| Nutrient Release Rates (%) | Decomposition Time (d) | BC | BG | NB |
|---|---|---|---|---|
| C | 90 | 47.148 ± 1.000 a | 48.808 ± 1.080 a | 46.551 ± 0.972 a |
| 180 | 54.586 ± 1.005 b | 58.863 ± 1.234 a | 53.449 ± 1.119 b | |
| 270 | 57.763 ± 1.052 b | 63.564 ± 1.229 a | 58.946 ± 1.259 b | |
| 360 | 60.184 ± 1.108 b | 67.831 ± 1.246 a | 62.534 ± 1.252 b | |
| N | 90 | −45.641 ± 3.447 a | −49.034 ± 3.848 a | −46.553 ± 3.295 a |
| 180 | −52.450 ± 3.539 a | −59.682 ± 3.620 a | −51.813 ± 3.301 a | |
| 270 | −47.162 ± 3.031 a | −43.911 ± 3.04 a | −43.830 ± 3.074 a | |
| 360 | −44.592 ± 3.121 a | −43.776 ± 3.417 a | −42.562 ± 3.058 a | |
| P | 90 | −11.491 ± 3.894 a | −37.128 ± 4.519 b | −36.979 ± 4.789 b |
| 180 | −41.122 ± 5.202 a | −43.061 ± 5.631 a | −34.042 ± 4.876 a | |
| 270 | −27.086 ± 4.937 a | −14.387 ± 4.425 a | −25.328 ± 4.677 a | |
| 360 | −6.967 ± 4.071 a | −16.454 ± 5.080 a | −8.598 ± 3.899 a | |
| K | 90 | 86.302 ± 0.483 a | 83.340 ± 0.667 b | 84.591 ± 0.581 b |
| 180 | 83.503 ± 0.549 a | 72.895 ± 1.023 c | 77.996 ± 1.113 b | |
| 270 | 84.466 ± 0.697 a | 84.757 ± 0.442 a | 81.897 ± 0.767 b | |
| 360 | 86.393 ± 0.456 a | 83.019 ± 0.724 b | 83.154 ± 0.629 b | |
| C/N | 90 | 9.495 ± 0.353 a | 9.478 ± 0.395 a | 9.740 ± 0.403 a |
| 180 | 7.893 ± 0.291 a | 6.959 ± 0.326 b | 8.266 ± 0.338 a | |
| 270 | 7.522 ± 0.309 a | 6.864 ± 0.320 a | 7.549 ± 0.350 a | |
| 360 | 7.237 ± 0.302 a | 5.909 ± 0.288 b | 7.024 ± 0.349 a | |
| N/P | 90 | 25.653 ± 0.874 a | 20.827 ± 0.577 b | 20.708 ± 0.529 b |
| 180 | 21.025 ± 0.656 a | 21.101 ± 0.634 a | 21.645 ± 0.839 a | |
| 270 | 23.219 ± 0.815 a | 25.144 ± 1.348 a | 22.484 ± 0.646 a | |
| 360 | 26.546 ± 0.749 a | 24.667 ± 0.983 a | 25.139 ± 0.663 a |
| Source of Variation | F-Value | ||||||
|---|---|---|---|---|---|---|---|
| MR | C | N | P | K | C/N | N/P | |
| Species (SP) | 205.05 *** | 499.85 *** | 150.31 *** | 5.09 *** | 19.69 *** | 889.98 *** | 127.92 *** |
| Habitat (H) | 148.31 *** | 78.48 *** | 0.28 ns | 2.41 ns | 8.38 *** | 213.45 *** | 25.93 *** |
| Time (T) | 12,078.53 *** | 344.34 *** | 917.61 *** | 6.99 ** | 1637.87 *** | 2307.08 *** | 55.09 *** |
| SP × H | 7.23 *** | 4.23 *** | 6.62 *** | 1.04 ns | 0.815 ns | 57.06 *** | 8.51 *** |
| SP × T | 15.52 *** | 9.95 *** | 10.99 *** | 1.44 ns | 14.71 *** | 20.64 *** | 2.99 *** |
| H × T | 30.06 *** | 17.21 *** | 5.22 *** | 0.61 ns | 1.576 ns | 49.78 *** | 15.73 *** |
| SP × H × T | 1.94 *** | 2.83 *** | 2.63 *** | 1.02 ns | 0.86 ns | 10.73 *** | 3.01 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.-Y.; Qian, F.; Xia, C.-Y.; Xia, H.; Liu, J.-C.; Luo, W.-X.; Tao, J.-P. Understory Dwarf Bamboo Modulates Leaf Litter Decomposition via Interception-Induced Litter Redistribution and Space-Dependent Decomposition Dynamics: A Case Study from Jinfo Mountain, China. Plants 2025, 14, 3135. https://doi.org/10.3390/plants14203135
Song H-Y, Qian F, Xia C-Y, Xia H, Liu J-C, Luo W-X, Tao J-P. Understory Dwarf Bamboo Modulates Leaf Litter Decomposition via Interception-Induced Litter Redistribution and Space-Dependent Decomposition Dynamics: A Case Study from Jinfo Mountain, China. Plants. 2025; 14(20):3135. https://doi.org/10.3390/plants14203135
Chicago/Turabian StyleSong, Hai-Yan, Feng Qian, Chun-Yan Xia, Hong Xia, Jin-Chun Liu, Wei-Xue Luo, and Jian-Ping Tao. 2025. "Understory Dwarf Bamboo Modulates Leaf Litter Decomposition via Interception-Induced Litter Redistribution and Space-Dependent Decomposition Dynamics: A Case Study from Jinfo Mountain, China" Plants 14, no. 20: 3135. https://doi.org/10.3390/plants14203135
APA StyleSong, H.-Y., Qian, F., Xia, C.-Y., Xia, H., Liu, J.-C., Luo, W.-X., & Tao, J.-P. (2025). Understory Dwarf Bamboo Modulates Leaf Litter Decomposition via Interception-Induced Litter Redistribution and Space-Dependent Decomposition Dynamics: A Case Study from Jinfo Mountain, China. Plants, 14(20), 3135. https://doi.org/10.3390/plants14203135






