First Phenotypic Characterization of the Edible Fruits of Lardizabala biternata: A Baseline for Conservation and Domestication of a Neglected and Endemic Vine
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sampling Sites
2.2. Fruit Collection and Handling
2.3. Morphological Characterisation
2.4. Statistical Analysis
2.4.1. Hypothesis 1: Spatial Variation (StCr16 vs. Vald16)
2.4.2. Hypothesis 2: Seasonal Variation (Vald16 vs. Vald18)
2.5. Supporting Analyses
3. Results
3.1. Climatic Conditions at Study Sites
3.2. General Fruit Characteristics
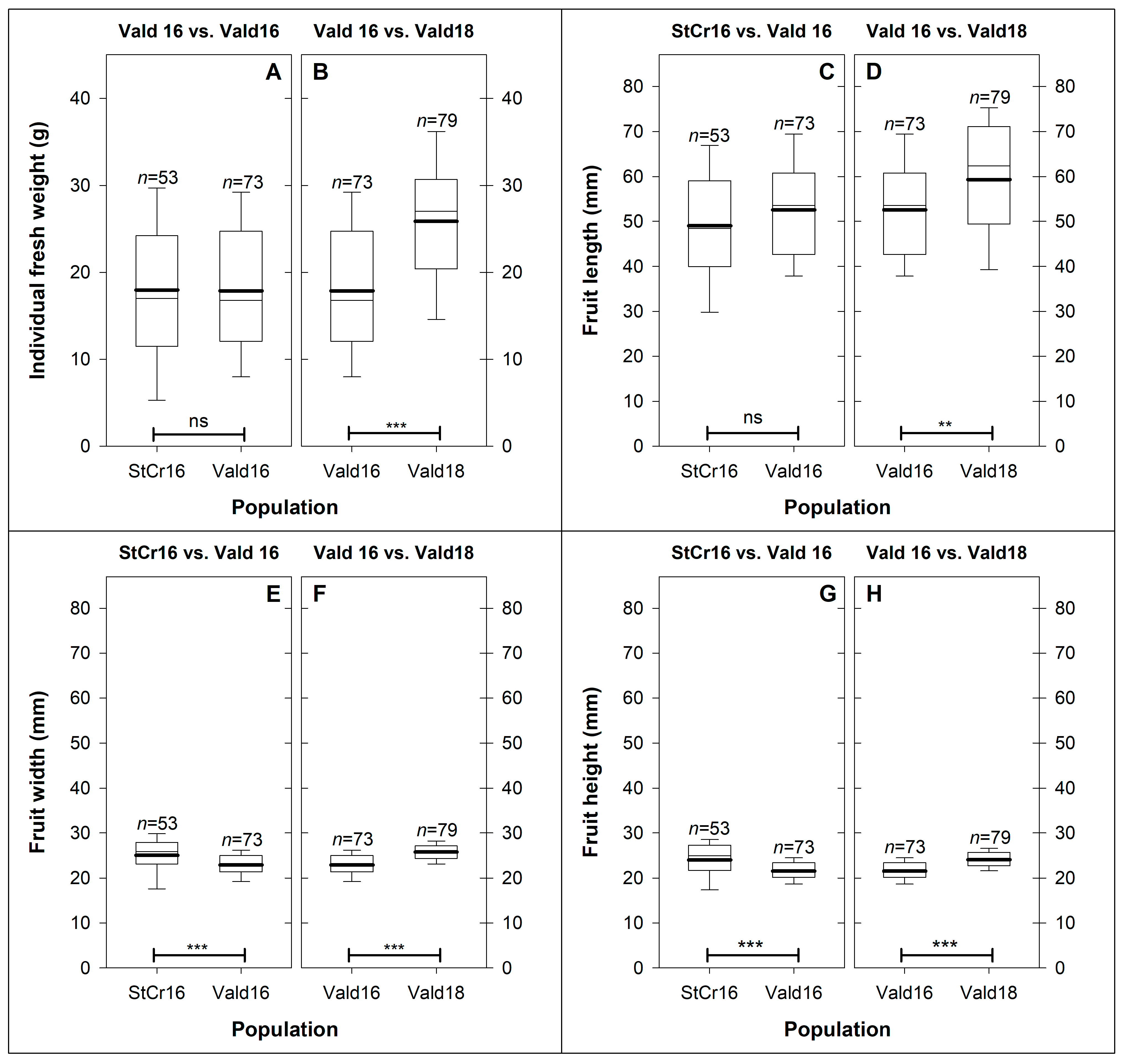
3.3. Fruit Weight and Size
3.3.1. Hypothesis 1: Spatial (StCr16 vs. Vald16) Variation in Fruit Morphology (Weight and Size)
3.3.2. Hypothesis 2: Seasonal (Vald16 vs. Vald16) Variation in Fruit Morphology (Weight and Size)
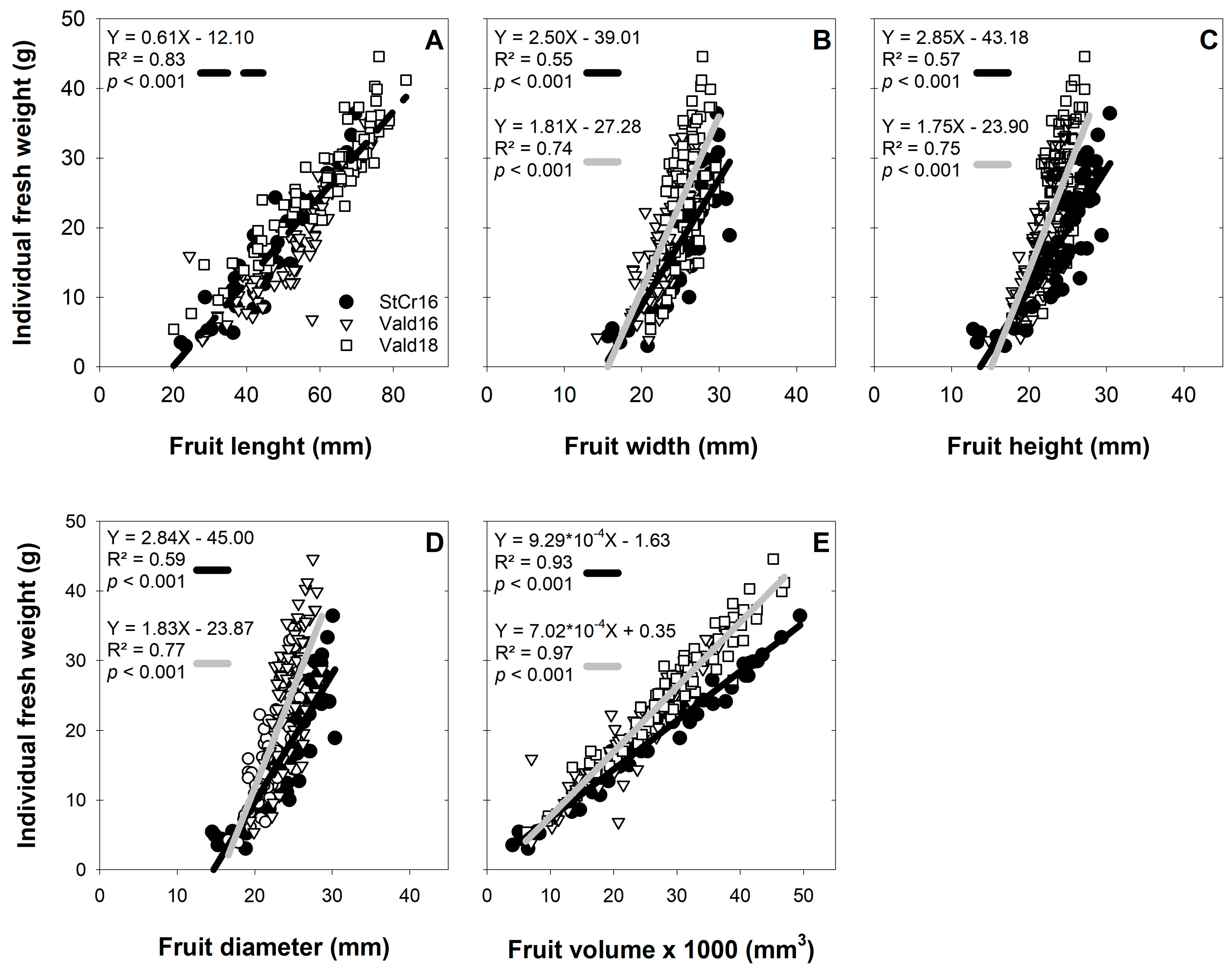
3.4. Weight and Size Associations in L. biternata Fruits
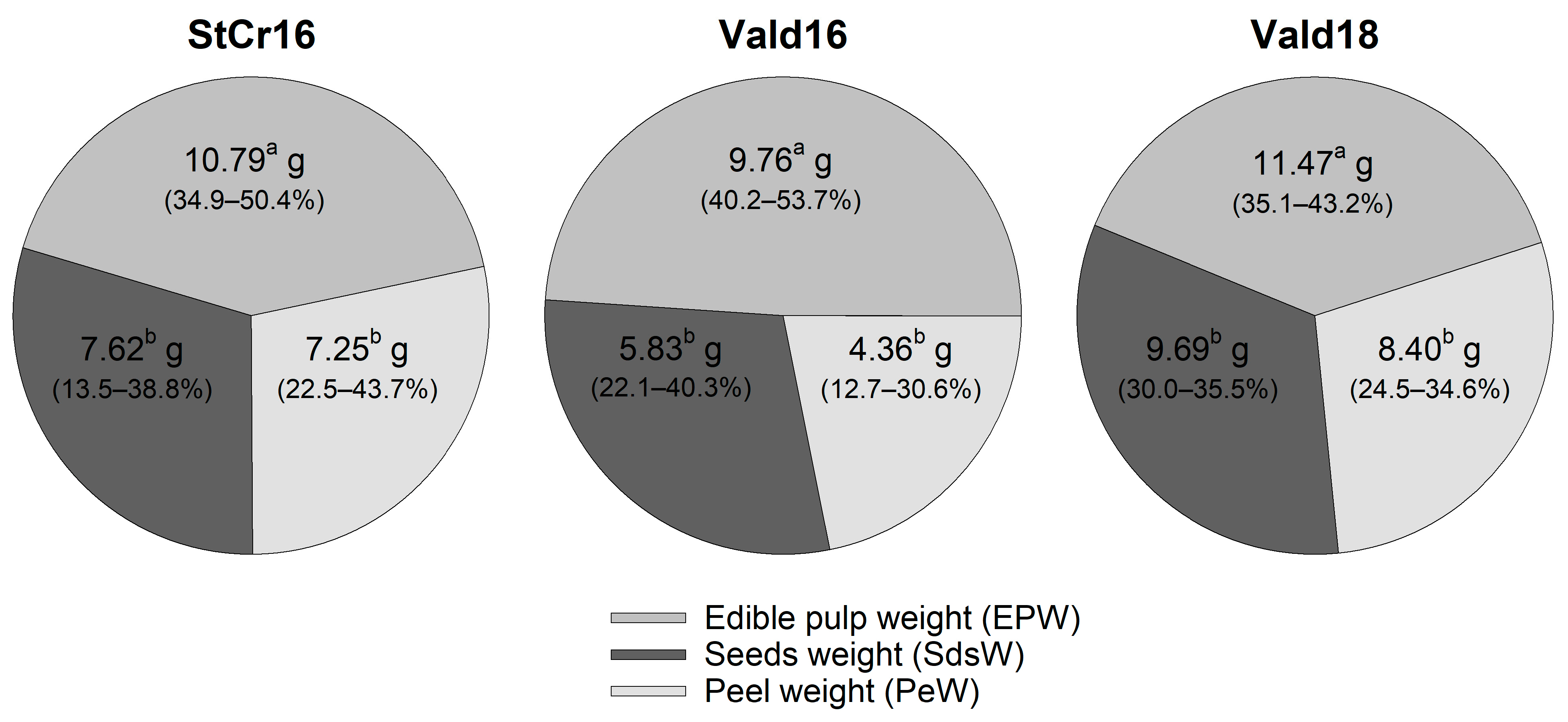
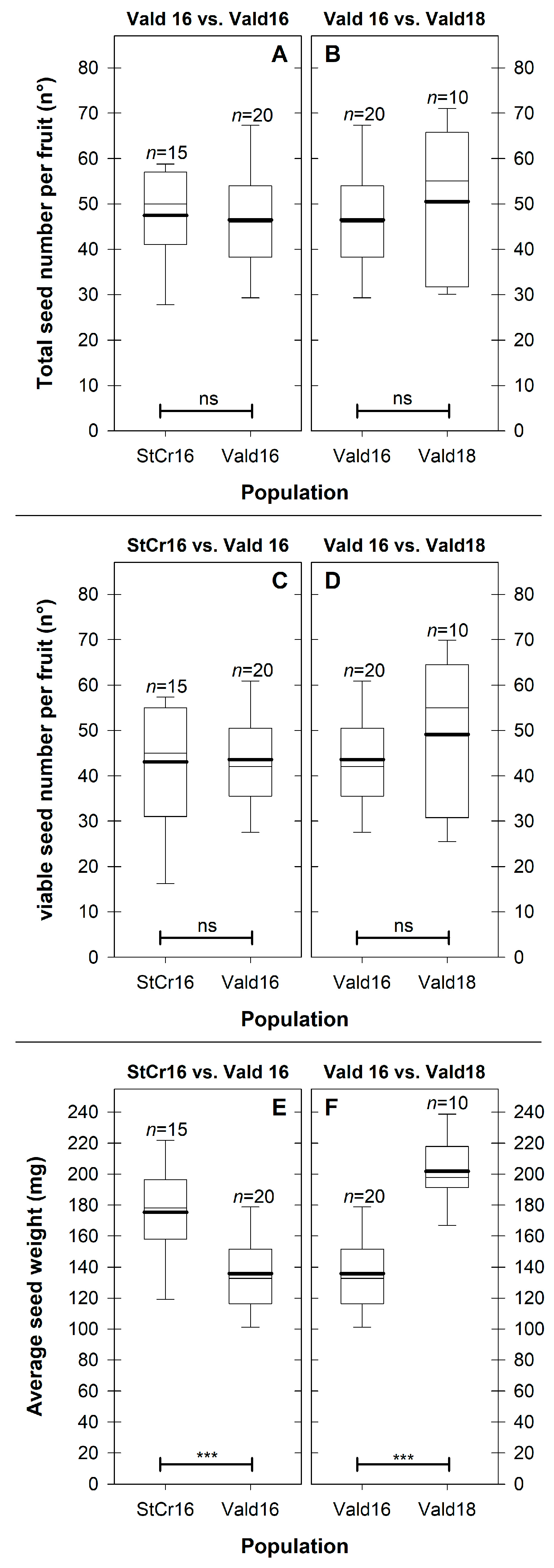
3.5. Internal Fruit Composition
3.5.1. Hypothesis 1: Spatial (Stcr16 vs. Vald16) Variation in Internal Fruit Components (Edible Pulp, Seed and Peel)
3.5.2. Hypothesis 2: Seasonal (Vald16 vs. Vald18) Variation in Internal Fruit Components (Edible Pulp, Seed and Peel)
3.6. Edible and Structural Components and Seed Characteristics Associations in L. biternata Fruits
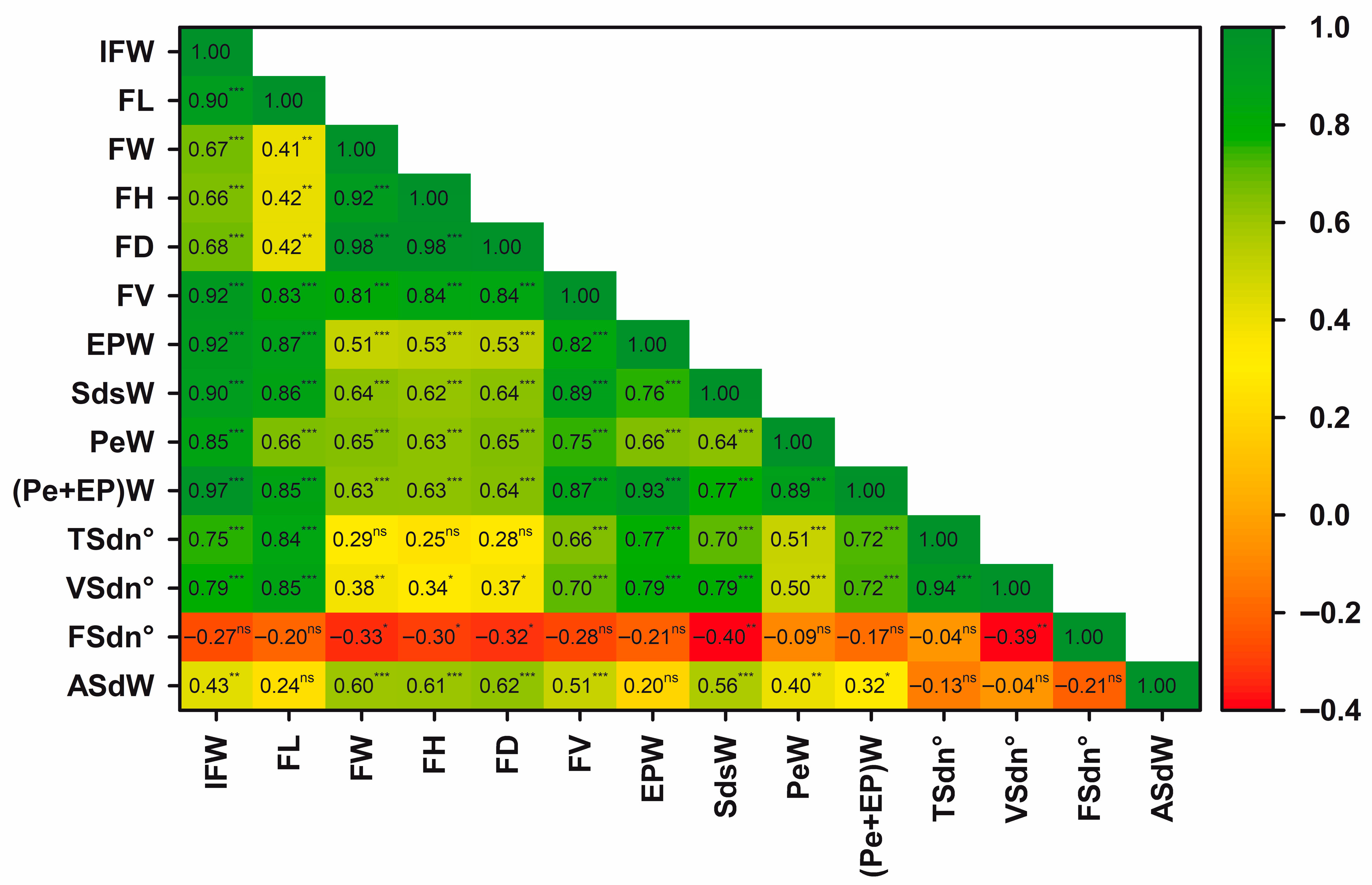
3.7. Correlations Between the Characters and Principal Components Analysis
4. Discussion
4.1. Weight of Lardizabala biternata Fruits
4.2. Edible Proportion and Nutritional Potential
4.3. Seeds as a Resource
4.4. Fruit Size of Lardizabala biternata
4.5. Impact of Climatic Conditions
4.6. Evaluation of Hypotheses
4.7. Correlations Between Phenotypic Traits
4.8. Correlations Between Phenotypic Traits Morphological Traits as Physiological and Agricultural Indicators in Lardizabala biternata
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| StCr | Santa Cruz city |
| Vald | Valdivia city |
| StCr16 | Santa Cruz sampling, season 2016 |
| Vald16 | Valdivia sampling, season 2016 |
| Vald18 | Valdivia sampling, season 2018 |
| IFW | Individual fresh weight |
| FL | Fruit length |
| FW | Fruit width |
| FH | Fruit height |
| FD | Fruit diameter |
| FV | Fruit volume |
| EPW | Edible pulp weight |
| PeW | Peel weight |
| (EP + Pe)W | edible pulp plus peel weight |
| SdsW | Total seed weight |
| TSdn° | Total seed number |
| VSdn° | Viable seed number |
| VSdn° | Nom-viable seed number |
| ASdW | Average seed weight |
References
- Ruiz, H.l.; Pavón, J. Florae Peruvianae, et Chilensis Prodromus, Sive Novorum Generum Plantarum Peruvianarum, et Chilensium Descriptiones, et Icones. = Descripciones y Láminas de los Nuevos Géneros de Plantas de la Flora del Perú y Chile; De Órden del Rey; En la Imprenta de Sancha: Madrid, Spain, 1794; p. 143, ilustration XXXVII. [Google Scholar]
- Villagrán, C. Etnobotánica indígena de los bosques de Chile: Sistema de clasificación de un recurso de uso múltiple. Rev. Chil. Hist. Nat. 1998, 71, 245–268. [Google Scholar]
- Marticorena, A.; Alarcón, D.; Abello, L.; Atala, C. Plantas Trepadoras, Epífitas y Parásitas Nativas de Chile. Guía de Campo; Corporación Chilena de la Madera: Concepción, Chile, 2010; pp. 31, 60, 61. [Google Scholar] [CrossRef]
- Cordero, S.; Abello, L.; Gálvez, F. Plantas Silvestres Comestibles y Medicinales de Chile y otras Partes del Mundo; Corporación Chilena de la Madera: Concepción, Chile, 2017; p. 269. [Google Scholar]
- Wilhelm de Moesbach, E. Botánica Indígena de Chile; Aldunate, C., Villagrán, C., Eds.; Museo Chileno de Arte Precolombino: Santiago, Chile, 1992; pp. 29, 78. [Google Scholar]
- Baeza, V.M. Los Nombres Vulgares de las Plantas Silvestres de Chile y su Concordancia con los Nombres Científicos, y Observaciones Sobre la Aplicación Técnica y Medicinal de Algunas Especies, 2nd ed.; Imprenta El Globo: Santiago, Chile, 1930; p. 51. [Google Scholar]
- Harrison, S.; Noss, R. Endemism hotspots are linked to stable climatic refugia. Ann. Bot. 2017, 119, 207–214. [Google Scholar] [CrossRef]
- Pérez-Schultheiss, J.; Fernández, L.D.; Ribeiro, F.B. Two new genera of coastal Talitridae (Amphipoda: Senticaudata) from Chile, with the first record of Platorchestia Bousfield, 1982 in the southeastern Pacific coast. Zootaxa 2024, 5477, 195–218. [Google Scholar] [CrossRef]
- Guzmán Marín, B.C.; Garrido Hernández, J.A.; Olmos de Aguilera, N.; Fuentes Molina, F.; Fernández, L.D.; Dumont Viollaz, A. Roadkill as the primary cause of death of the kodkod, pampas cat and puma in the biodiversity hotspot of central Chile. Mammalia 2025, 89, 495–503. [Google Scholar] [CrossRef]
- Parra-Gómez, A.; Fernández, L.D. Filling gaps in the diversity and biogeography of Chilean millipedes (Myriapoda: Diplopoda). Arthropod Syst. Phylogeny 2022, 80, 561–573. [Google Scholar] [CrossRef]
- Campello-Nunes, P.H.; Woelfl, S.; da Silva-Neto, I.D.; Paiva, T.D.S.; Fernández, L.D. Checklist, diversity and biogeography of ciliates (Ciliophora) from Chile. Eur. J. Protistol. 2022, 84, 125892. [Google Scholar] [CrossRef]
- Fernández, L.D.; Fournier, B.; Rivera, R.; Lara, E.; Mitchell, E.A.D.; Hernández, C.E. Water–energy balance, past ecological perturbations and evolutionary constraints shape the latitudinal diversity gradient of soil testate amoebae in south-western South America. Glob. Ecol. Biogeogr. 2016, 25, 1216–1227. [Google Scholar] [CrossRef]
- Fernández, L.D.; Lara, E.; Mitchell, E.A.D. Checklist, diversity and distribution of testate amoebae in Chile. Eur. J. Protistol. 2015, 51, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Christenhusz, M.J.M. An overview of Lardizabalaceae. Curtis’s Bot. Mag. 2012, 29, 235–276. [Google Scholar] [CrossRef]
- Rodríguez, R.; Grau, J.; Baeza, C.; Davies, A. Lista comentada de las plantas vasculares de los Nevados de Chillan, Chile. Gayana Botánica 2008, 65, 153–197. [Google Scholar] [CrossRef]
- Sánchez, A.; San Martín, J. Las comunidades relictas de Gomortega keule (Gomortegaceae, Magnoliopsida) en Chile central. An. Jardín Botánico Madr. 1999, 57, 317–326. [Google Scholar]
- Arroyo, M.T.; Matthei, O.; Marticorena, C.; Munoz, M.; Perez, F.; Humana, A.M. The vascular plant flora of the Bellotos del Melado National Reserve, VII Region, Chile: A documented checklist. Gayana 2000, 57, 117–139. [Google Scholar]
- Hauenstein, E.; Muñoz-Pedreros, A.; Yánez, J.; Sánchez, P.; Möller, P.; Guiñez, B.; Gil, C. Flora y vegetación de la Reserva Nacional Lago Peñuelas, Reserva de la Biósfera, Región de Valparaíso, Chile. Bosque 2009, 30, 159–179. [Google Scholar] [CrossRef]
- Danton, P. Rapport de la première expédition botanique aux îles Juan Fernandez (Chili) du 16 novembre 1997 au 9 février 1998 effectuée par Philippe Danton, Emmanuel Breteau et Michel Baffray. Publ. Société Linnéenne Lyon 1999, 68, 103–124. [Google Scholar]
- Gay, C. Historia Física y Política de Chile: Botánica: Tomo Primero; Fain y Thunot: Paris, France, 1845; p. 67. [Google Scholar] [CrossRef]
- Le Maout, E.; Decaisne, J. Traité Général de Botanique Descriptive et Analytique; Firmin Didot frères, fils et cie.: Paris, France, 1868; p. 347. [Google Scholar]
- Feuillée, L. Journal des Observations Physiques, Mathematiques et Botaniques, Faites par l’ordre du Roy sur les Côtes Orientales de l’Amerique Meridionale, & dans les Indes Occidentales, depuis l’année 1707. jusques en 1712./Par le R. P. Louis Feuillée, Religieux Minime, Mathematicien, Botaniste de Sa Majesté, & Correspondant de l’Académie Royale des Sciences; Tome premier; A Paris: Rue de S. Jacques, Chez Pierre Giffart, Libraire, Graveur du Roy, & de l’Academie Royale de Peinture & de Sculpture: Paris, France, 1714; p. 60. [Google Scholar] [CrossRef]
- Molina, J.I. Saggio Sulla Storia Naturale del Chili/del Signor Abate Giovanni Ignazio Molina; Nella Stamperia di S. Tommaso d’Aquino: Bologna, Italy, 1782; pp. 155–157. [Google Scholar]
- Consejo Nacional de la Cultura y las Artes. Arca del Gusto: Catálogo Alimentario Patrimonial, Productos Tradicionales y en Riesgo de Extinción en Chile; Consejo Nacional de la Cultura y las Artes: Santiago, Chile, 2014; p. 122. [Google Scholar]
- Manzur, M.; FIA. Patrimonio Alimentario de Chile: Productos y Preparaciones de la Región del BioBío; Fundación para la Innovación Agraria: Santiago, Chile, 2016; pp. 32, 180. [Google Scholar]
- Riedemann, P.; Aldunate, G. Flora Nativa de Valor Ornamental: Identificación y Propagación; Editorial Andres Bello: Santiago, Chile, 2003; p. 232. [Google Scholar]
- Silva, M.; Mancinelli, P. Oleanolic Acid in Lardizabala biternata. J. Pharm. Sci. 1961, 50, 975. [Google Scholar] [CrossRef] [PubMed]
- Carlquist, S. Wood and stem anatomy of Lardizabalaceae, with comments on the vining habit, ecology and systematics. Bot. J. Linn. Soc. 1984, 88, 257–277. [Google Scholar] [CrossRef]
- Kofuji, R.; Ueda, K.; Yamaguchi, K.; Shimizu, T. Molecular phylogeny in the Lardizabalaceae. J. Plant Res. 1994, 107, 339–348. [Google Scholar] [CrossRef]
- Muñoz-Concha, D.; Mundaca, E.; Alarcón, D.; Machuca, J.; Crisol-Martínez, E.; Loayza, A. Could foxes be surrogate seed dispersers of a megafaunal fruit vine in southern South America? Ecosphere 2022, 13, e4186. [Google Scholar] [CrossRef]
- Zou, S.; Yao, X.; Zhong, C.; Gao, P.; Wang, Z.; Huang, H. Phenotypic characterization of Stauntonia obovatifoliola Hayata subsp. urophylla germplasm: A potential new fruit crop. Genet. Resour. Crop Evol. 2020, 67, 1037–1050. [Google Scholar] [CrossRef]
- Vidaković, A.; Radunić, M.; Poljak, I. Variation in chemical composition and fruit morphometric traits of almond-leaved pear (Pyrus spinosa Forssk.) natural populations. Genet. Resour. Crop Evol. 2025, 72, 1495–1510. [Google Scholar] [CrossRef]
- Abiri, K.; Rezaei, M.; Tahanian, H.; Heidari, P.; Khadivi, A. Morphological and pomological variability of a grape (Vitis vinifera L.) germplasm collection. Sci. Hortic. 2020, 266, 109285. [Google Scholar] [CrossRef]
- Alba, V.; Bergamini, C.; Cardone, M.F.; Gasparro, M.; Perniola, R.; Genghi, R.; Antonacci, D. Morphological Variability in Leaves and Molecular Characterization of Novel Table Grape Candidate Cultivars (Vitis vinifera L.). Mol. Biotechnol. 2014, 56, 557–570. [Google Scholar] [CrossRef]
- Amir Sohail, A.I.H.R.; Manzoor, S.A.; Hussain, Q. Genetic variability and correlation studies for morphological and yield traits in maize (Zea mays L.). Pure Appl. Biol. (PAB) 2017, 6, 1234–1243. [Google Scholar] [CrossRef]
- Islam, S.; Ferdausi, A.; Sweety, A.; Das, A.; Ferdoush, A.; Haque, M. Morphological characterization and genetic diversity analyses of plant traits contributed to grain yield in maize (Zea mays L.). J. Biosci. Agric. Res. 2020, 25, 2047–2059. [Google Scholar] [CrossRef]
- Qi, W.-Z.; Liu, H.-H.; Liu, P.; Dong, S.-T.; Zhao, B.-Q.; So, H.B.; Li, G.; Liu, H.-D.; Zhang, J.-W.; Zhao, B. Morphological and physiological characteristics of corn (Zea mays L.) roots from cultivars with different yield potentials. Eur. J. Agron. 2012, 38, 54–63. [Google Scholar] [CrossRef]
- Vafaee, Y.; Ghaderi, N.; Khadivi, A. Morphological variation and marker-fruit trait associations in a collection of grape (Vitis vinifera L.). Sci. Hortic. 2017, 225, 771–782. [Google Scholar] [CrossRef]
- Li, L.; Yao, X.; Zhong, C.; Chen, X.; Huang, H. Akebia: A Potential New Fruit Crop in China. HortScience 2010, 45, 4–10. [Google Scholar] [CrossRef]
- Nazir, M.F.; Jia, T.; Xu, J.; Dai, L.; Zhao, Y.; Zou, S. Metabolomic profiling of Akebia species: Comparative analysis of bioactive compounds in the pulp of A. trifoliata, A. trifoliata ssp. australis, and A. quinata. Food Chem. X 2025, 28, 102531. [Google Scholar] [CrossRef] [PubMed]
- Ochmian, I.; Guan, T.; Kubus, M. Description and assessment of chemical properties of fruits of the chocolate vine (five-leaf Akebia) Akebia quinata (Houtt.) Decne and dead man’s fingers Decaisnea insignis (Griff.) Hokk.f. & Thomson, grown in Szczecin and in the Arboretum in Glinna (northwestern Poland). J. Elemntol. 2014, 19, 1073–1084. [Google Scholar] [CrossRef]
- Herrera, J.; Calderini, D.F. Pericarp growth dynamics associate with final grain weight in wheat under contrasting plant densities and increased night temperature. Ann. Bot. 2020, 126, 1063–1076. [Google Scholar] [CrossRef]
- Zou, S.; Yao, X.; Zhong, C.; Zhao, T.; Huang, H. Genetic analysis of fruit traits and selection of superior clonal lines in Akebia trifoliate (Lardizabalaceae). Euphytica 2018, 214, 111. [Google Scholar] [CrossRef]
- Zou, S.; Yao, X.; Zhong, C.; Zhao, T.; Huang, H. Effectiveness of recurrent selection in Akebia trifoliata (Lardizabalaceae) breeding. Sci. Hortic. 2019, 246, 79–85. [Google Scholar] [CrossRef]
- Zou, S.; Gao, P.; Jia, T.; Huang, H. Physicochemical Characteristics and Nutritional Composition during Fruit Ripening of Akebia trifoliata (Lardizabalaceae). Horticulturae 2022, 8, 326. [Google Scholar] [CrossRef]
- Callahan, A.M. Breeding for Fruit Quality. Acta Hortic. 2003, 622, 295–302. [Google Scholar] [CrossRef]
- Cellon, C.; Amadeu, R.R.; Olmstead, J.W.; Mattia, M.R.; Ferrao, L.F.V.; Munoz, P.R. Estimation of genetic parameters and prediction of breeding values in an autotetraploid blueberry breeding population with extensive pedigree data. Euphytica 2018, 214, 87. [Google Scholar] [CrossRef]
- Cockerton, H.M.; Karlström, A.; Johnson, A.W.; Li, B.; Stavridou, E.; Hopson, K.J.; Whitehouse, A.B.; Harrison, R.J. Genomic Informed Breeding Strategies for Strawberry Yield and Fruit Quality Traits. Front. Plant Sci. 2021, 12, 724847. [Google Scholar] [CrossRef]
- Hernández-Bautista, A.; Lobato-Ortiz, R.; García-Zavala, J.J.; Chávez-Servia, J.L.; Mejía-Contreras, J.A.; García-Velazquez, J.A. Breeding potential of raspberry primocane selections based on their combining abilities. Can. J. Plant Sci. 2017, 98, 28–37. [Google Scholar] [CrossRef]
- Janick, J. History of Fruit Breeding. Fruit Veg. Cereal Sci. Biotechnol. 2011, 5, 1–7. [Google Scholar]
- Masny, A.; Pruski, K.; Żurawicz, E.; Mądry, W. Breeding value of selected dessert strawberry (Fragaria × ananassa Duch.) cultivars for ripening time, fruit yield and quality. Euphytica 2016, 207, 225–243. [Google Scholar] [CrossRef]
- Stephens, M.J.; Alspach, P.A.; Beatson, R.A.; Winefield, C.; Buck, E.J. Genetic Parameters and Development of a Selection Index for Breeding Red Raspberries for Processing. J. Am. Soc. Hortic. Sci. 2012, 137, 236–242. [Google Scholar] [CrossRef]
- Verichev, K.; Carpio, M. Climatic zoning for building construction in a temperate climate of Chile. Sustain. Cities Soc. 2018, 40, 352–364. [Google Scholar] [CrossRef]
- Feron, S.; Cordero, R.R.; Damiani, A.; Llanillo, P.J.; Jorquera, J.; Sepulveda, E.; Asencio, V.; Laroze, D.; Labbe, F.; Carrasco, J.; et al. Observations and Projections of Heat Waves in South America. Sci. Rep. 2019, 9, 8173. [Google Scholar] [CrossRef]
- Feron, S.; Cordero, R.R.; Damiani, A.; MacDonell, S.; Pizarro, J.; Goubanova, K.; Valenzuela, R.; Wang, C.; Rester, L.; Beaulieu, A. South America is becoming warmer, drier, and more flammable. Commun. Earth Environ. 2024, 5, 501. [Google Scholar] [CrossRef]
- Garreaud, R.; Boisier, J.P.; Rondanelli, R.; Montecinos, A.; Sepúlveda, H.; Veloso, D. The Central Chile Mega Drought (2010–2018): A Climate dynamics perspective. Int. J. Climatol. 2019, 40, 421–439. [Google Scholar] [CrossRef]
- Fernández, L.; Rau, J.; Arriagada, A. Calidad de la vegetación ribereña del río Maullín (41°28′ S; 72°59′ O) utilizando el índice QBR. Gayana Bot. 2009, 66, 269–278. [Google Scholar] [CrossRef]
- Huang, P.; Zang, F.; Li, C.; Lin, F.; Zang, D.; Li, B.; Zheng, Y. The Akebia Genus as a Novel Forest Crop: A Review of Its Genetic Resources, Nutritional Components, Biosynthesis, and Biological Studies. Front. Plant Sci. 2022, 13, 936571. [Google Scholar] [CrossRef] [PubMed]
- Uribe, H.; Catalán, A. Caracterización Hidroclimatológica y del Uso de Suelo del Secano de la Región de O’Higgins; Instituto de Investigaciones Agropecuarias: Rengo, Chile, 2016; p. 110. [Google Scholar]
- INFODEP. Elaboración de una Base Digital del Clima Comunal de Chile: Línea Base (1980–2010) y Proyección al Año 2050; Ministerio del Medio Ambiente: Bogotá, Colombia, 2016; p. 99. [Google Scholar]
- Rozas, V.; Le Quesne, C.; Rojas-Badilla, M. Factores climáticos que controlan el crecimiento radial y la formación de fluctuaciones de densidad en la madera de Austrocedrus chilensis en Valdivia, Chile. Bosque 2016, 37, 461–471. [Google Scholar] [CrossRef]
- Hasan, A.K.; Herrera, J.; Lizana, C.; Calderini, D.F. Carpel weight, grain length and stabilized grain water content are physiological drivers of grain weight determination of wheat. Field Crops Res. 2011, 123, 241–247. [Google Scholar] [CrossRef]
- Di Rienzo, J.; Casanoves, F.; Balzarini, M.; Gonzalez, L.; Tablada, M.; Robledo, C. InfoStat, versión 2011; Facultad de Ciencias Agropecuarias, Universidad Nacional de Córdoba: Córdoba, Argentina, 2008. [Google Scholar]
- Lin, Y.-C.; Weng, Y.; Fei, Z.; Grumet, R. Mining the cucumber core collection: Phenotypic and genetic characterization of morphological diversity for fruit quality characteristics. Hortic. Res. 2025, 12, uhae340. [Google Scholar] [CrossRef]
- Khadivi, A.; Mirheidari, F.; Saeidifar, A.; Moradi, Y.; Tunç, Y. Morphological and pomological characterizations of pomegranate (Punica granatum L.) to select superior genotypes. Sci. Rep. 2025, 15, 7038. [Google Scholar] [CrossRef]
- Farnia, A.; Mansouri, M.; Farnia, A.; Branch, B. Study on morphological characteristics of maize (Zea mays L.) cultivars under different plant densities. Indian J. Nat. Sci. 2015, 5, 34–56. [Google Scholar]
- Guan, S.H.; Xia, J.M.; Lu, Z.Q.; Chen, G.T.; Jiang, B.H.; Liu, X.; Guo, D. A Structure elucidation and NMR spectral assignments of three new lignan glycosides from Akebia trifoliata. Magn. Reson. Chem. 2008, 46, 186–190. [Google Scholar] [CrossRef]
- Du, Y.; Jiang, Y.; Zhu, X.; Xiong, H.; Shi, S.; Hu, J.; Peng, H.; Zhou, Q.; Sun, W. Physicochemical and functional properties of the protein isolate and major fractions prepared from Akebia trifoliata var. australis seed. Food Chem. 2012, 133, 923–929. [Google Scholar] [CrossRef]
- Kawata, J.; Kameda, M.; Miyazawa, M. Constituents of essential oil from the dried fruits and stems of Akebia quinata (THUNB.) DECNE. J. Oleo Sci. 2007, 56, 59–63. [Google Scholar] [CrossRef]
- Wang, R.; Sun, L.; Xie, X.; Ma, L.; Liu, Z.; Liu, X.; Ji, N.; Xie, G. Biodiesel production from Stauntonia chinensis seed oil (waste from food processing): Heterogeneous catalysis by modified calcite, biodiesel purification, and fuel properties. Ind. Crops Prod. 2014, 62, 8–13. [Google Scholar] [CrossRef]
- Salvador, F.R.D.; Fisichella, M.; Fontanari, M. Correlations between fruit size and fruit quality in apple trees with high and standard crop load levels. J. Fruit Ornam. Plant Res. 2006, 14, 113–122. [Google Scholar]
- Abud, H.F.; Gonçalves, N.R.; Pereira, M.d.S.; Pereira, D.d.S.; Reis, R.d.G.E.; Bezerra, A.M.E. Germination and morphological characterization of the fruits, seeds, and seedlings of Pilosocereus gounellei. Braz. J. Bot. 2012, 35, 11–16. [Google Scholar] [CrossRef]
- Šuklje, K.; Lisjak, K.; Baša Česnik, H.; Janeš, L.; Du Toit, W.; Coetzee, Z.; Vanzo, A.; Deloire, A. Classification of Grape Berries According to Diameter and Total Soluble Solids to Study the Effect of Light and Temperature on Methoxypyrazine, Glutathione, and Hydroxycinnamate Evolution during Ripening of Sauvignon blanc (Vitis vinifera L.). J. Agric. Food Chem. 2012, 60, 9454–9461. [Google Scholar] [CrossRef] [PubMed]
- Naderiboldaji, M.; Khadivi, A.; Tabatabaeefar, A.; Varnamkhasti, M.; Zamani, Z. Some Physical Properties of Sweet Cherry (Prunus avium L.) Fruit. Am.-Eurasian J. Agric. Environ. Sci. 2008, 3, 513–520. [Google Scholar]
- Li, M.; Chen, M.; Zhang, Y.; Fu, C.; Xing, B.; Li, W.; Qian, J.; Li, S.; Wang, H.; Fan, X.; et al. Apple Fruit Diameter and Length Estimation by Using the Thermal and Sunshine Hours Approach and Its Application to the Digital Orchard Management Information System. PLoS ONE 2015, 10, e0120124. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; McGregor, C.; Liu, S.; Luan, F.; Gao, M.; Weng, Y. Genetic architecture of fruit size and shape variation in cucurbits: A comparative perspective. Theor. Appl. Genet. 2020, 133, 1–21. [Google Scholar] [CrossRef]
- Łangowski, Ł.; Stacey, N.; Østergaard, L. Diversification of fruit shape in the Brassicaceae family. Plant Reprod. 2016, 29, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xu, Y.; Fang, P.; Guo, Q.; Huang, W.; Hou, J.; Wan, H.; Zhang, S. Genetic Regulation of Fruit Shape in Horticultural Crops: A Review. Horticulturae 2024, 10, 1151. [Google Scholar] [CrossRef]
- Delgado, V.; Garay, O.J.; Arévalo-Galarza, M.D.L.; Gautier, H. Increased Temperature Affects Tomato Fruit Physicochemical Traits at Harvest Depending on Fruit Developmental Stage and Genotype. Horticulturae 2023, 9, 212. [Google Scholar] [CrossRef]
- Sarricolea, P.; Herrera-Ossandon, M.; Meseguer-Ruiz, Ó. Climatic regionalisation of continental Chile. J. Maps 2017, 13, 66–73. [Google Scholar] [CrossRef]
- Warrington, I.; Fulton, T.; Halligan, E.; Silva, H. Apple Fruit Growth and Maturity are Affected by Early Season Temperatures. Am. Soc. Hortic. Sci. 1999, 124, 468. [Google Scholar] [CrossRef]
- Zonia, L.; Munnik, T. Life under pressure: Hydrostatic pressure in cell growth and function. Trends Plant Sci. 2007, 12, 90–97. [Google Scholar] [CrossRef]
- Bátori, Z.; Erdős, L.; Gajdács, M.; Barta, K.; Tobak, Z.; Frei, K.; Tölgyesi, C. Managing climate change microrefugia for vascular plants in forested karst landscapes. For. Ecol. Manag. 2021, 496, 119446. [Google Scholar] [CrossRef]
- Muñoz-Sáez, A.; Choe, H.; Boynton, R.M.; Elsen, P.R.; Thorne, J.H. Climate exposure shows high risk and few climate refugia for Chilean native vegetation. Sci. Total Environ. 2021, 785, 147399. [Google Scholar] [CrossRef]
- Vergara, P.M.; Zúñiga, A.H.; Alaniz, A.J.; Fierro, A.; Quiroz, M.; Hidalgo-Corrotea, C.M.; Carvajal, M.A.; Carlos, C.-C.; Moreira-Arce, D.; Borquez, C. Disentangling the ecological value of tree hollows to wildlife along elevation gradients: The case of southern temperate forests. For. Ecol. Manag. 2024, 571, 122236. [Google Scholar] [CrossRef]
- Cheng-Yih, W.; Kubitzki, K. Lardizabalaceae. In Flowering Plants·Dicotyledons: Magnoliid, Hamamelid and Caryophyllid Families; Kubitzki, K., Rohwer, J.G., Bittrich, V., Eds.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 361–365. [Google Scholar]
- Majidi, M.M.; Mirlohi, A.; Amini, F. Genetic variation, heritability and correlations of agro-morphological traits in tall fescue (Festuca arundinacea Schreb.). Euphytica 2009, 167, 323–331. [Google Scholar] [CrossRef]
- Donoso, I.; Schleuning, M.; García, D.; Fründ, J. Defaunation effects on plant recruitment depend on size matching and size trade-offs in seed-dispersal networks. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162664. [Google Scholar] [CrossRef]
- Qiu, T.; Andrus, R.; Aravena, M.-C.; Ascoli, D.; Bergeron, Y.; Berretti, R.; Berveiller, D.; Bogdziewicz, M.; Boivin, T.; Bonal, R.; et al. Limits to reproduction and seed size-number trade-offs that shape forest dominance and future recovery. Nat. Commun. 2022, 13, 2381. [Google Scholar] [CrossRef]
- Sadras, V.O. Evolutionary aspects of the trade-off between seed size and number in crops. Field Crops Res. 2007, 100, 125–138. [Google Scholar] [CrossRef]
- Iezzoni, A.F.; Pritts, M.P. Applications of Principal Component Analysis to Horticultural Research. Hortscience 1991, 26, 334–338. [Google Scholar] [CrossRef]
- Jia, T.; Feng, C.; Zou, S.; Gao, P. The Main Physicochemical Characteristics and Nutrient Composition during Fruit Ripening of Stauntonia obovatifoliola Subsp. Urophylla (Lardizabalaceae). Horticulturae 2023, 9, 29. [Google Scholar] [CrossRef]
- Lee, S.G.; Kim, S.K.; Lee, H.J.; Lee, H.S.; Lee, J.H. Impact of moderate and extreme climate change scenarios on growth, morphological features, photosynthesis, and fruit production of hot pepper. Ecol. Evol. 2018, 8, 197–206. [Google Scholar] [CrossRef]
- Ramirez, N.; Barrios, Y.; Briceño, H. Correlations between morphological fruit types, fruit and seed colors, and functional groups. Biota Neotrop. 2021, 21, e20211238. [Google Scholar] [CrossRef]
- Naor, A.; Klein, I.; Doron, I.; Gal, Y.; Ben-David, Z.; Bravdo, B. Irrigation and Crop Load Interactions in Relation to Apple Yield and Fruit Size Distribution. J. Am. Soc. Hortic. Sci. 1997, 122, 411–414. [Google Scholar] [CrossRef]
- Rapoport, H.; Costagli, G.; Gucci, R. The Effect of Water Deficit during Early Fruit Development on Olive Fruit Morphogenesis. Am. Soc. Hortic. Sci. 2004, 129, 121–127. [Google Scholar] [CrossRef]
- Bertin, N. Analysis of the Tomato Fruit Growth Response to Temperature and Plant Fruit Load in Relation to Cell Division, Cell Expansion and DNA Endoreduplication. Ann. Bot. 2005, 95, 439–447. [Google Scholar] [CrossRef]
- Sharma, G.; Khan, Z.; Das, D.; Singh, S.; Singh, S.; Kumar, M.; Tiwari, R.R.; Sarma, D.K. Thermal influence on development and morphological traits of Aedes aegypti in central India and its relevance to climate change. Parasites Vectors 2025, 18, 279. [Google Scholar] [CrossRef]
- Talukder, M.R.; Bappy, N.A.H.; Haque, M.M.; Molla, M.A.H.; Alam, M.Z.; Mosharaf, M.K.; Limon, G.S.; Rafiquzzaman, S.M. Fluctuation of ambient day-night temperature influences morphological traits, floral characters, fruit yield and quality of summer tomato genotypes grown in hydroponics. New Zealand J. Crop Hortic. Sci. 2025, 53, 2731–2754. [Google Scholar] [CrossRef]
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Z.; Wang, Y.; Bao, R.; Chen, X.; Fu, S.; Tian, M.; Zhang, Y. Research on Flexible End-Effectors with Humanoid Grasp Function for Small Spherical Fruit Picking. Agriculture 2023, 13, 123. [Google Scholar] [CrossRef]
- Fernández-Serrano, P.; Tarancón, P.; Besada, C. Consumer Information Needs and Sensory Label Design for Fresh Fruit Packaging. An Exploratory Study in Spain. Foods 2020, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Dewi, T.; Risma, P.; Oktarina, Y. Fruit sorting robot based on color and size for an agricultural product packaging system. Bull. Electr. Eng. Inform. 2020, 9, 1438–1445. [Google Scholar] [CrossRef]
- Jung, H.J.; Lee, C.O.; Lee, K.T.; Choi, J.; Park, H.J. Structure-activity relationship of oleanane disaccharides isolated from Akebia quinata versus cytotoxicity against cancer cells and NO inhibition. Biol. Pharm. Bull. 2004, 27, 744–747. [Google Scholar] [CrossRef]
- Koo, H.J.; Sung, Y.Y.; Kim, H.K. Inhibitory effects of Akebia quinata ethanol extract on TNF-α-mediated vascular inflammation in human aortic smooth muscle cells. Mol. Med. Rep. 2013, 7, 379–383. [Google Scholar] [CrossRef]
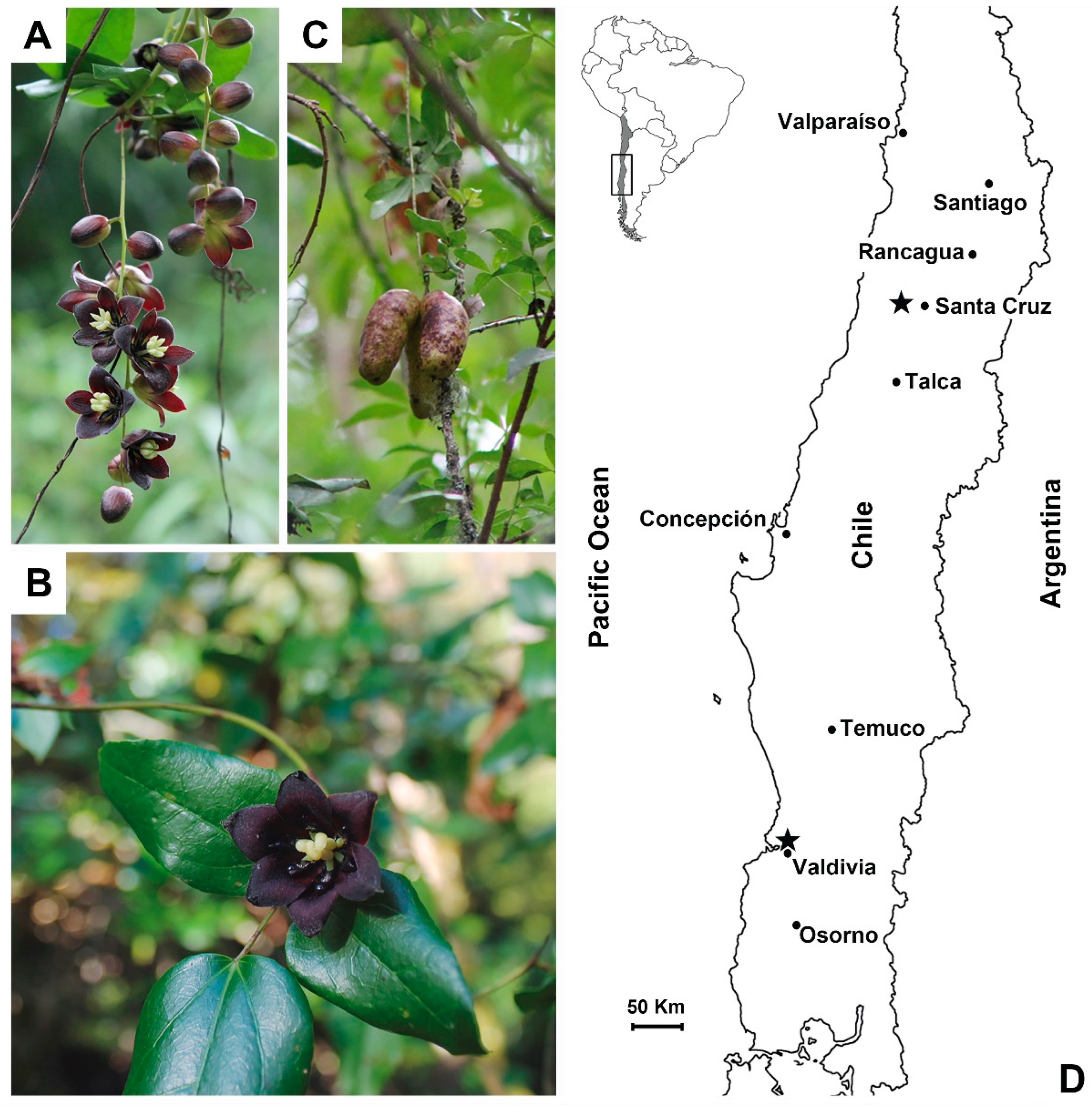
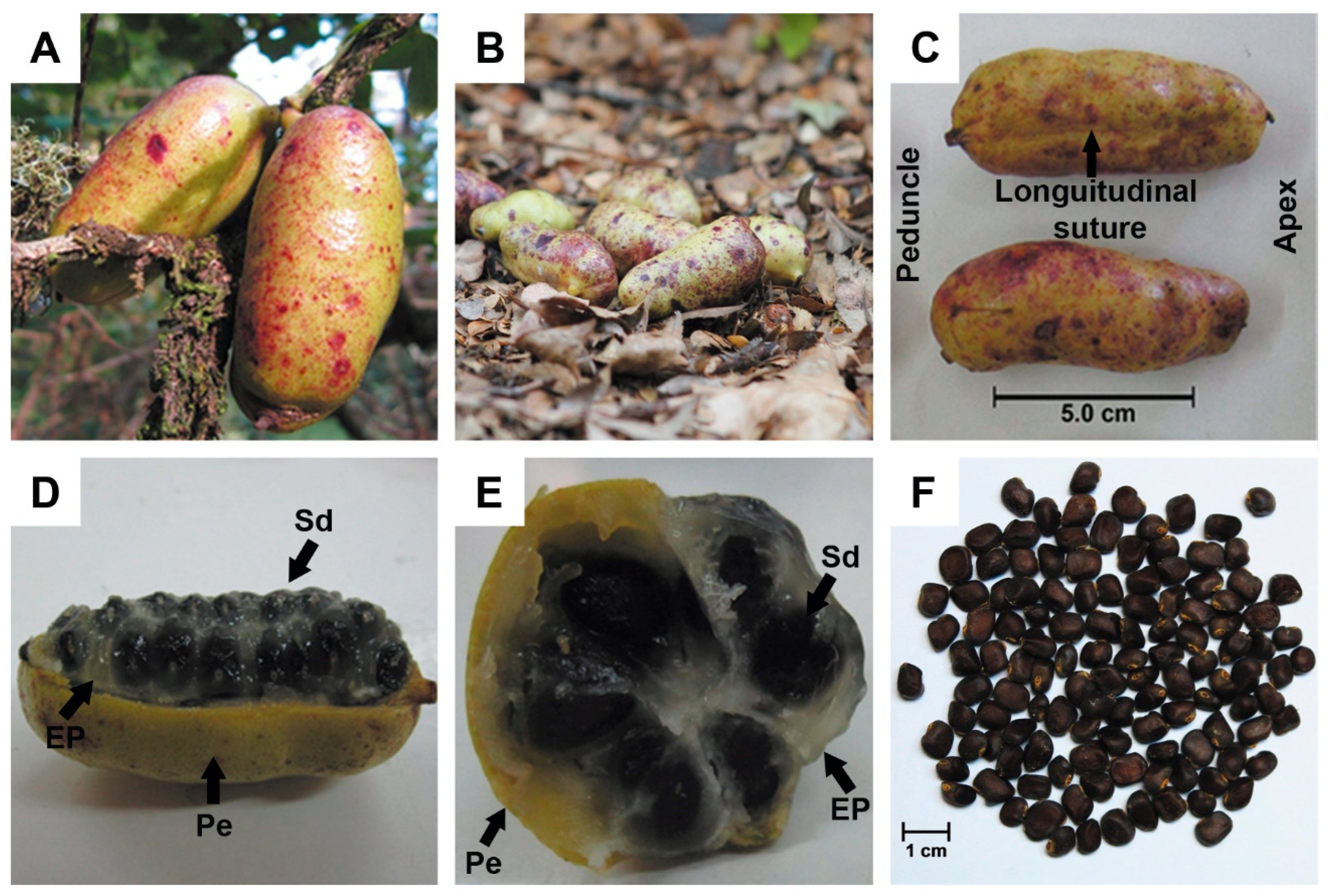



| Season | Location | Season Time | Data Harvest | T°max (°C) | T°min (°C) | T°mean (°C) | Rainfall (mm) |
|---|---|---|---|---|---|---|---|
| StCr16 | Santa Cruz | 2015/16 | February | 23.1 | 8.8 | 16.0 | 586 |
| Vald16 | Valdivia | 2015/16 | March | 17.5 | 7.0 | 12.2 | 1869 |
| Vald18 | Valdivia | 2017/18 | March | 16.6 | 6.9 | 11.7 | 2119 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, J.; Fernández, L.D. First Phenotypic Characterization of the Edible Fruits of Lardizabala biternata: A Baseline for Conservation and Domestication of a Neglected and Endemic Vine. Plants 2025, 14, 3126. https://doi.org/10.3390/plants14203126
Herrera J, Fernández LD. First Phenotypic Characterization of the Edible Fruits of Lardizabala biternata: A Baseline for Conservation and Domestication of a Neglected and Endemic Vine. Plants. 2025; 14(20):3126. https://doi.org/10.3390/plants14203126
Chicago/Turabian StyleHerrera, Jaime, and Leonardo D. Fernández. 2025. "First Phenotypic Characterization of the Edible Fruits of Lardizabala biternata: A Baseline for Conservation and Domestication of a Neglected and Endemic Vine" Plants 14, no. 20: 3126. https://doi.org/10.3390/plants14203126
APA StyleHerrera, J., & Fernández, L. D. (2025). First Phenotypic Characterization of the Edible Fruits of Lardizabala biternata: A Baseline for Conservation and Domestication of a Neglected and Endemic Vine. Plants, 14(20), 3126. https://doi.org/10.3390/plants14203126







