The Construction of a Standard Karyotype of Intermediate Wheatgrass and Its Potential Progenitor Species
Abstract
1. Introduction
2. Results
2.1. Oligo-FISH of IWG and Its Ancestral Diploid Species
2.2. Karyotype Analysis of Th. intermedium
2.3. Karyotype Analysis of Tetraploid Species of Thinopyrum
2.4. Karyotype Analysis of Three Diploid Species That Were Implicated as Progenitors of IWG
2.5. Karyotype Evolution Analysis of Different Species
3. Discussion
3.1. Progress in the Study of Th. intermedium and Related Species
3.2. Karyotype Analysis on Th. intermedium and Related Species
3.3. Karyotype Evolution on Th. intermedium and Related Species
4. Materials and Methods
4.1. Plant Materials
4.2. Probe Preparation
4.3. Chromosome Preparation and GISH, FISH Protocol
4.4. Karyotype Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jungers, J.M.; DeHaan, L.R.; Betts, K.J.; Sheaffer, C.C.; Wyse, D.L. Intermediate Wheatgrass Grain and Forage Yield Responses to Nitrogen Fertilization. Agron. J. 2017, 109, 462–472. [Google Scholar] [CrossRef]
- Tyl, C.; Ismail, B.P. Compositional evaluation of perennial wheatgrass (Thinopyrum intermedium) breeding populations. Int. J. Food Sci. Technol. 2019, 54, 660–669. [Google Scholar] [CrossRef]
- Pototskaya, I.V.; Shamanin, V.P.; Aydarov, A.N.; Morgounov, A.I. The use of wheatgrass (Thinopyrum intermedium) in breeding. Vavilovskii Zhurnal Genet. Sel. 2022, 26, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.T.; Zhang, N.; Boshoff, W.H.P.; Li, H.W.; Li, B.; Li, Z.S.; Zheng, Q. Identification and introgression of a novel leaf rust resistance gene from Thinopyrum intermedium chromosome 7Js into wheat. Theor. Appl. Genet. 2023, 136, 231. [Google Scholar] [CrossRef]
- Nie, L.M.; Yang, Y.N.; Zhang, J.; Fu, T.H. Disomic chromosome addition from Thinopyrum intermedium to bread wheat appears to confer stripe rust resistance. Euphytica 2019, 215, 56. [Google Scholar] [CrossRef]
- Zheng, X.W.; Tang, C.G.; Han, R.; Zhao, J.J.; Qiao, L.; Zhang, S.W.; Qiao, L.Y.; Ge, C.; Zheng, J.; Liu, C. Identification, Characterization, and Evaluation of Novel Stripe Rust-Resistant Wheat-Thinopyrum intermedium Chromosome Translocation Lines. Plant Dis. 2020, 104, 875–881. [Google Scholar] [CrossRef]
- Walls, J.; Rajotte, E.; Rosa, C. The Past, Present, and Future of Barley Yellow Dwarf Management. Agriculture 2019, 9, 23. [Google Scholar] [CrossRef]
- Ali, N. Wheat–Thinopyrum intermedium introgression lines enhancing wheat streak mosaic virus (WSMV) resistance. Clim. Change Food Secur. Emphas. Wheat 2020, 243–255. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Feng, X.; Zhao, J.; Deng, P.; Wang, Y.; Zhang, H.; Liu, X.; Li, T.; Chen, C.; et al. Molecular cytogenetics and development of St-chromosome-specific molecular markers of novel stripe rust resistant wheat-Thinopyrum intermedium and wheat-Thinopyrum ponticum substitution lines. BMC Plant Biol. 2022, 22, 111. [Google Scholar] [CrossRef]
- Bajgain, P.; Zhang, X.F.; Anderson, J.A. Genome-Wide Association Study of Yield Component Traits in Intermediate Wheatgrass and Implications in Genomic Selection and Breeding. G3-Genes Genomes Genet. 2019, 9, 2429–2439. [Google Scholar] [CrossRef]
- Crain, J.; Bajgain, P.; Anderson, J.; Zhang, X.F.; DeHaan, L.; Poland, J. Enhancing Crop Domestication Through Genomic Selection, a Case Study of Intermediate Wheatgrass. Front. Plant Sci. 2020, 11, 319. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Choudhary, A.; Kaur, H.; Mehta, S. A walk towards Wild grasses to unlock the clandestine of gene pools for wheat improvement: A review. Plant Stress 2022, 3, 100048. [Google Scholar] [CrossRef]
- Zhang, X.; Ohm, J.-B.; Haring, S.; DeHaan, L.R.; Anderson, J.A. Towards the understanding of end-use quality in intermediate wheatgrass (Thinopyrum intermedium): High-molecular-weight glutenin subunits, protein polymerization, and mixing characteristics. J. Cereal Sci. 2015, 66, 81–88. [Google Scholar] [CrossRef]
- de Oliveira, G.; Brunsell, N.A.; Crews, T.E.; DeHaan, L.R.; Vico, G. Carbon and water relations in perennial Kernza (Thinopyrum intermedium): An overview. Plant Sci. 2020, 295, 110279. [Google Scholar] [CrossRef]
- Craine, E.B.; DeHaan, L.R. Nutritional Quality of Early-Generation Kernza Perennial Grain. Agriculture 2024, 14, 919. [Google Scholar] [CrossRef]
- de Oliveira, G.; Brunsell, N.A.; Sutherlin, C.E.; Crews, T.E.; DeHaan, L.R. Energy, water and carbon exchange over a perennial Kernza wheatgrass crop. Agric. For. Meteorol. 2018, 249, 120–137. [Google Scholar] [CrossRef]
- Crain, J.; Wagoner, P.; Larson, S.; DeHaan, L. Origin of current intermediate wheatgrass germplasm being developed for Kernza grain production. Genet. Resour. Crop Evol. 2024, 71, 4963–4978. [Google Scholar] [CrossRef]
- Dewey, D.R. The Genomic System of Classification as a Guide to Intergeneric Hybridization with the Perennial Triticeae. In Gene Manipulation in Plant Improvement: 16th Stadler Genetics Symposium; Gustafson, J.P., Ed.; Springer: Boston, MA, USA, 1984; pp. 209–279. [Google Scholar]
- Dvořák, J. Genome relationships among Elytrigia (=Agropyron) elongata, E. stipifolia, “E. elongata 4x”, E. caespitita, E. intermedia, and “E. elongata 10x”. Can. J. Genet. Cytol. 1981, 23, 481–492. [Google Scholar] [CrossRef]
- Qiao, L.; Liu, S.; Li, J.; Li, S.; Yu, Z.; Liu, C.; Li, X.; Liu, J.; Ren, Y.; Zhang, P.; et al. Development of Sequence-Tagged Site Marker Set for Identification of J, JS, and St Sub-genomes of Thinopyrum intermedium in Wheat Background. Front. Plant Sci. 2021, 12, 685216. [Google Scholar] [CrossRef]
- Li, G.-R.; Liu, C.; Li, C.-H.; Zhao, J.-M.; Zhou, L.; Dai, G.; Yang, E.-N.; Yang, Z.-J. Introgression of a novel Thinopyrum intermedium St-chromosome-specific HMW-GS gene into wheat. Mol. Breed. 2013, 31, 843–853. [Google Scholar] [CrossRef]
- Muramatsu, M. Cytogenetics of decaploid Agropyron elongatum (Elytrigia elongata) (2n = 70). I. Frequency of decavalent formation. Genome 1990, 33, 811–817. [Google Scholar] [CrossRef]
- Löve, Á. Conspectus of the Triticeae. Feddes Repert. 1984, 95, 425–521. [Google Scholar] [CrossRef]
- Liu, Z.W.; Wang, R.R.-C. Genome analysis of Elytrigia caespitosa, Lophopyrum nodosum, Pseudoroegneria geniculata ssp. scythica, and Thinopyrum intermedium (Triticeae: Gramineae). Genome 1993, 36, 102–111. [Google Scholar] [CrossRef]
- Liu, Z.W.; Wang, R.R.-C. Genome constitutions of Thinopyrum curvifolium, T. scirpeum, T. distichum, and T. junceum (Triticeae: Gramineae). Genome 1993, 36, 641–651. [Google Scholar] [CrossRef]
- Wang, R.R.-C.; von Bothmer, R.; Dvorák, J.; Fedak, G.; Linde-Laursen, I.; Muramatsu, M. Genome Symbols in theae (Poaceae). In Proceedings of the 2nd International Triticeae Symposium, Logan, UT, USA, 20–24 June 1994; pp. 29–34. [Google Scholar]
- Cseh, A.; Yang, C.; Hubbart-Edwards, S.; Scholefield, D.; Ashling, S.S.; Burridge, A.J.; Wilkinson, P.A.; King, I.P.; King, J.; Grewal, S. Development and validation of an exome-based SNP marker set for identification of the St, Jr and Jvs genomes of Thinopyrym intermedium in a wheat background. Theor. Appl. Genet. 2019, 132, 1555–1570. [Google Scholar] [CrossRef]
- Kruppa, K.; Molnár-Láng, M. Simultaneous visualization of different genomes (J, JSt and St) in a Thinopyrum intermedium × Thinopyrum ponticum synthetic hybrid (Poaceae) and in its parental species by multicolour genomic in situ hybridization (mcGISH). Comp. Cytogenet. 2016, 10, 283–293. [Google Scholar] [CrossRef]
- Mahelka, V.; Kopecký, D.; Baum, B.R. Contrasting Patterns of Evolution of 45S and 5S rDNA Families Uncover New Aspects in the Genome Constitution of the Agronomically Important Grass Thinopyrum intermedium (Triticeae). Mol. Biol. Evol. 2013, 30, 2065–2086. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Wang, L.L.; Xin, Z.Y.; Lin, Z.S. Development of new PCR markers specific to a Thinopyrum intermedium chromosome 2Ai-2 and cloning of the St-specific sequences. Yi Chuan Xue Bao 2002, 29, 627–633. [Google Scholar]
- Chen, Q.; Conner, R.L.; Laroche, A.; Thomas, J.B. Genome analysis of Thinopyrum intermedium and Thinopyrum ponticum using genomic in situ hybridization. Genome 1998, 41, 580–586. [Google Scholar] [CrossRef]
- Qi, F.; Liang, S.; Xing, P.; Bao, Y.; Wang, R.R.; Li, X. Genome Analysis of Thinopyrum intermedium and Its Potential Progenitor Species Using Oligo-FISH. Plants 2023, 12, 3705. [Google Scholar] [CrossRef]
- Liu, Z.; Li, D.; Zhang, X. Genetic Relationships Among Five Basic Genomes St, E, A, B and D in Triticeae Revealed by Genomic Southern and in situ Hybridization. J. Integr. Plant Biol. 2007, 49, 1080–1086. [Google Scholar] [CrossRef]
- Mahelka, V.; Kopecký, D.; Paštová, L. On the genome constitution and evolution of intermediate wheatgrass (Thinopyrum intermedium: Poaceae, Triticeae). BMC Evol. Biol. 2011, 11, 127. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ge, S.; Tang, H.; Zhang, X.; Zhu, G.; Lu, B.-R. Phylogenetic relationships in Elymus (Poaceae: Triticeae) based on the nuclear ribosomal internal transcribed spacer and chloroplast trnL-F sequences. New Phytol. 2006, 170, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Chen, W.-J.; Yan, H.; Wang, Y.; Kang, H.-Y.; Zhang, H.-Q.; Zhou, Y.-H.; Sun, G.-L.; Sha, L.-N.; Fan, X. Evolutionary patterns of plastome uncover diploid-polyploid maternal relationships in Triticeae. Mol. Phylogenetics Evol. 2020, 149, 106838. [Google Scholar] [CrossRef]
- Tang, Z.X.; Yang, Z.J.; Fu, S.L.; Yang, M.Y.; Li, G.R.; Zhang, H.Q.; Tan, F.Q.; Ren, Z. A new long terminal repeat (LTR) sequence allows to identify J genome from JS and St genomes of Thinopyrum intermedium. J. Appl. Genet. 2011, 52, 31–33. [Google Scholar] [CrossRef]
- Wang, R.R.-C.; Larson, S.R.; Jensen, K.B.; Bushman, B.S.; DeHaan, L.R.; Wang, S.; Yan, X. Genome evolution of intermediate wheatgrass as revealed by EST-SSR markers developed from its three progenitor diploid species. Genome 2015, 58, 63–70. [Google Scholar] [CrossRef]
- Wang, L.; Shi, Q.; Su, H.; Wang, Y.; Sha, L.; Fan, X.; Kang, H.; Zhang, H.; Zhou, Y. St2-80: A new FISH marker for St genome and genome analysis in Triticeae. Genome 2017, 60, 553–563. [Google Scholar] [CrossRef]
- Yang, Z.J.; Liu, C.; Feng, J.; Li, G.R.; Zhou, J.P.; Deng, K.J.; Ren, Z.L. Studies on genome relationship and species-specific PCR marker for Dasypyrum breviaristatum in Triticeae. Hereditas 2006, 143, 47–54. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Z.J.; Jia, J.Q.; Li, G.R.; Zhou, J.P.; Ren, Z.L. Genomic distribution of a long terminal repeat (LTR) Sabrina-like retrotransposon in Triticeae species. Cereal Res. Commun. 2009, 37, 363–372. [Google Scholar] [CrossRef]
- Grewal, S.; Yang, C.; Edwards, S.H.; Scholefield, D.; Ashling, S.; Burridge, A.J.; King, I.P.; King, J. Characterisation of Thinopyrum bessarabicum chromosomes through genome-wide introgressions into wheat. Theor. Appl. Genet. 2018, 131, 389–406. [Google Scholar] [CrossRef]
- Arano, H. Cytological Studies in Subfamily Carduoideae (Compositae) of Japan IX. The Karyotype Analysis and Phylogenic Considerations on Pertya and Ainsliaea (2). Shokubutsugaku Zasshi 1963, 76, 32–39. [Google Scholar] [CrossRef]
- Paszko, B. A critical review and a new proposal of karyotype asymmetry indices. Plant Syst. Evol. 2006, 258, 39–48. [Google Scholar] [CrossRef]
- Stebbins, G.L. Taxonomy and the evolution of genera, with special reference to the family gramineae. Evolution 1956, 10, 235–245. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Q.; Zhang, Z.; He, X.; Yu, Y. MATO: An updated tool for capturing and analyzing cytotaxonomic and morphological data. Innov. Life 2023, 1, 100010. [Google Scholar] [CrossRef]
- Jensen, K.B.; Dewey, D.R.; Zhang, Y.F. Mode of pollination of perennial species of the Triticeae in relation to genomically defined genera. Can. J. Plant Sci. 1990, 70, 215–225. [Google Scholar] [CrossRef]
- Hsiao, C.; Wang, R.R.-C.; Dewey, D.R. Karyotype analysis and genome relationships of 22 diploid species in the tribe Triticeae. Can. J. Genet. Cytol. 1986, 28, 109–120. [Google Scholar] [CrossRef]
- Oinuma, T. Karyomorphology of cereals. Jpn. J. Genet. 1953, 28, 219–226. [Google Scholar] [CrossRef]
- Du, P.; Zhuang, L.; Wang, Y.; Yuan, L.; Wang, Q.; Wang, D.; Dawadondup; Tan, L.; Shen, J.; Xu, H.; et al. Development of oligonucleotides and multiplex probes for quick and accurate identification of wheat and Thinopyrum bessarabicum chromosomes. Genome 2017, 60, 93–103. [Google Scholar] [CrossRef]
- Kato, A. Air drying method using nitrous oxide for chromosome counting in maize. Biotech. Histochem. 1999, 74, 160–166. [Google Scholar] [CrossRef]
- Han, F.; Liu, B.; Fedak, G.; Liu, Z. Genomic constitution and variation in five partial amphiploids of wheat--Thinopyrum intermedium as revealed by GISH, multicolor GISH and seed storage protein analysis. Theor. Appl. Genet. 2004, 109, 1070–1076. [Google Scholar] [CrossRef]
- He, F.; Xing, P.; Bao, Y.; Ren, M.; Liu, S.; Wang, Y.; Li, X.; Wang, H. Chromosome Pairing in Hybrid Progeny between Triticum aestivum and Elytrigia elongata. Front. Plant Sci. 2017, 8, 2161. [Google Scholar] [CrossRef]
- Cui, Y.; Xing, P.; Qi, X.; Bao, Y.; Wang, H.; Wang, R.R.C.; Li, X. Characterization of chromosome constitution in three wheat—Thinopyrum intermedium amphiploids revealed frequent rearrangement of alien and wheat chromosomes. BMC Plant Biol. 2021, 21, 129. [Google Scholar] [CrossRef]
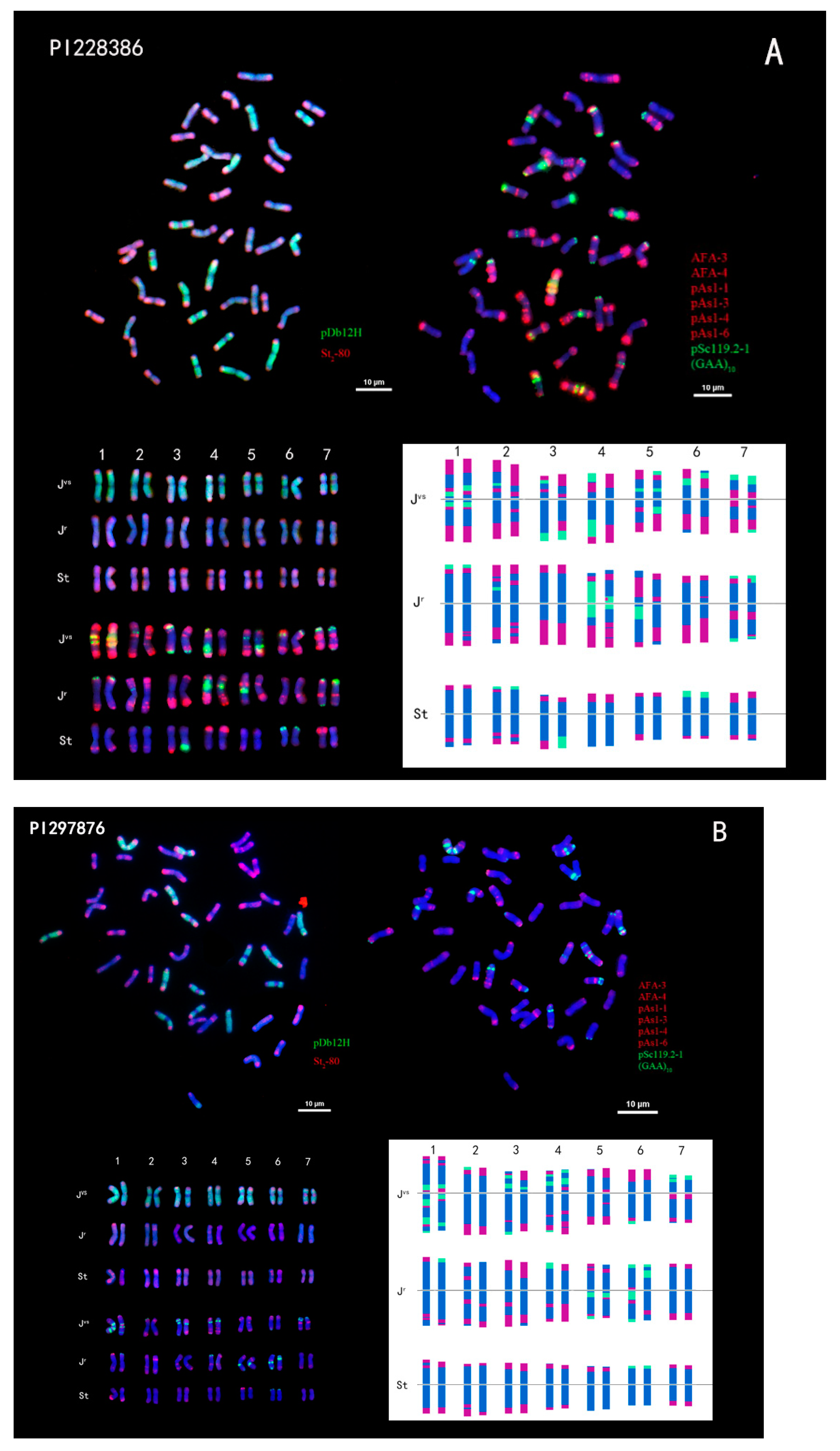
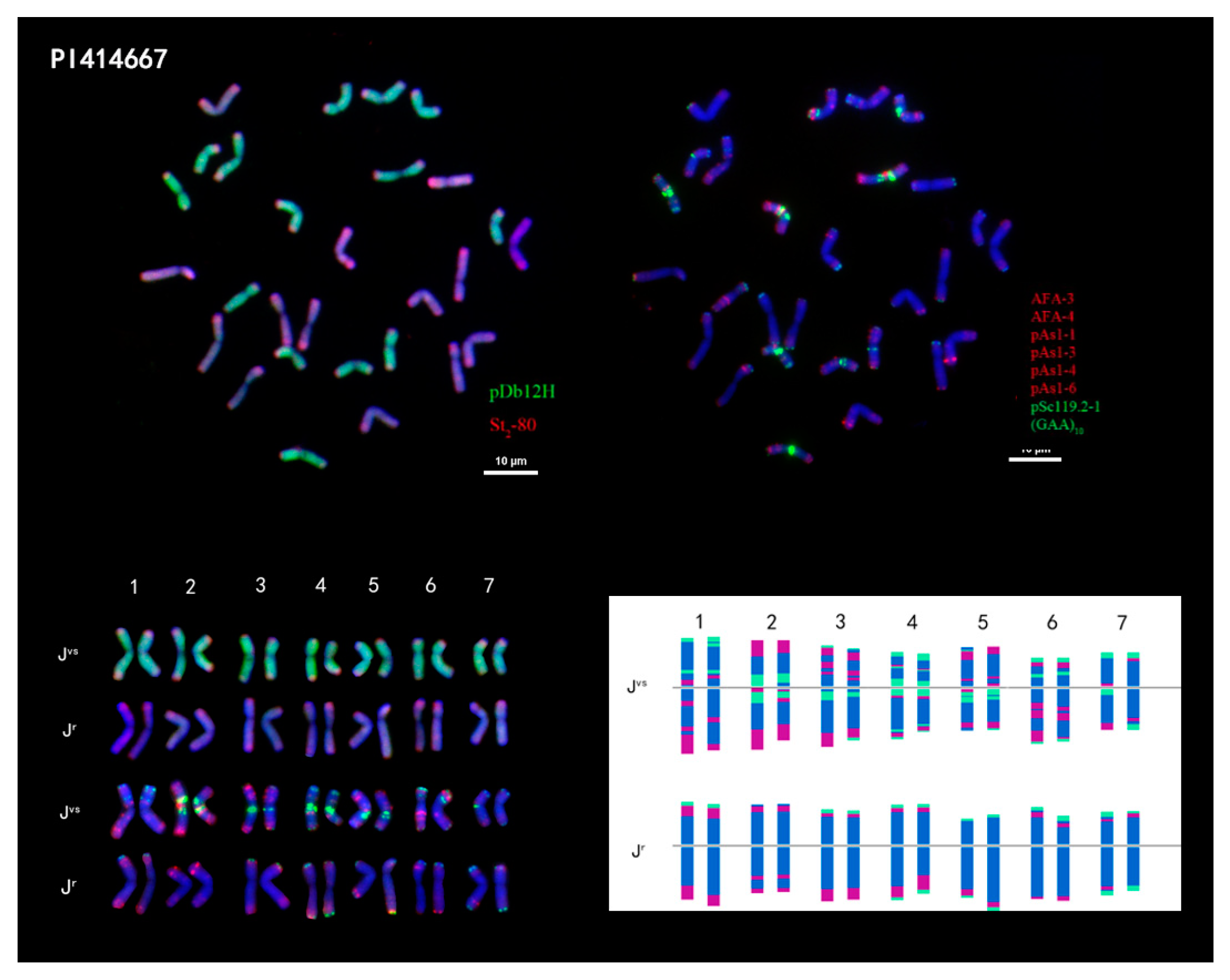
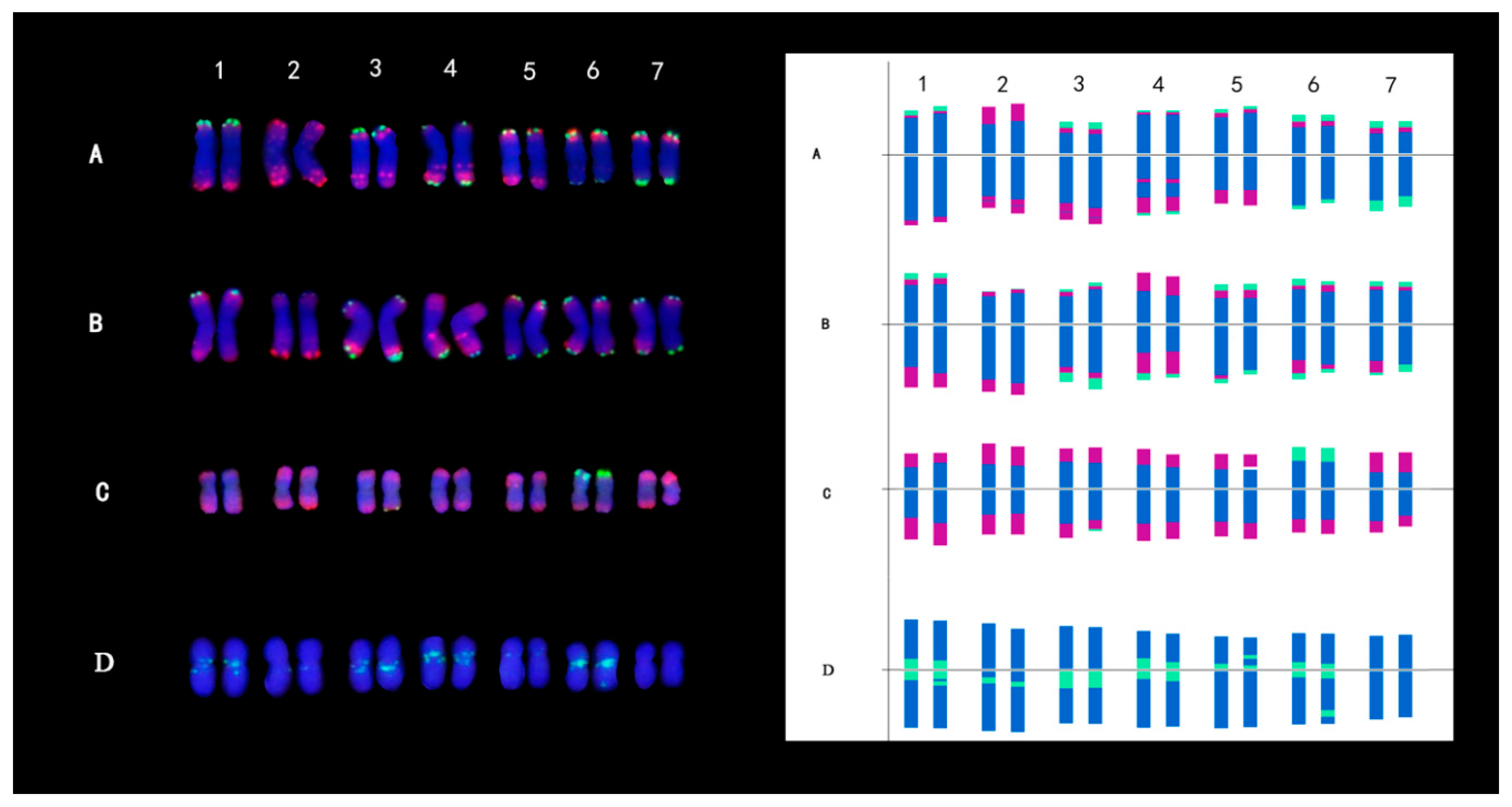
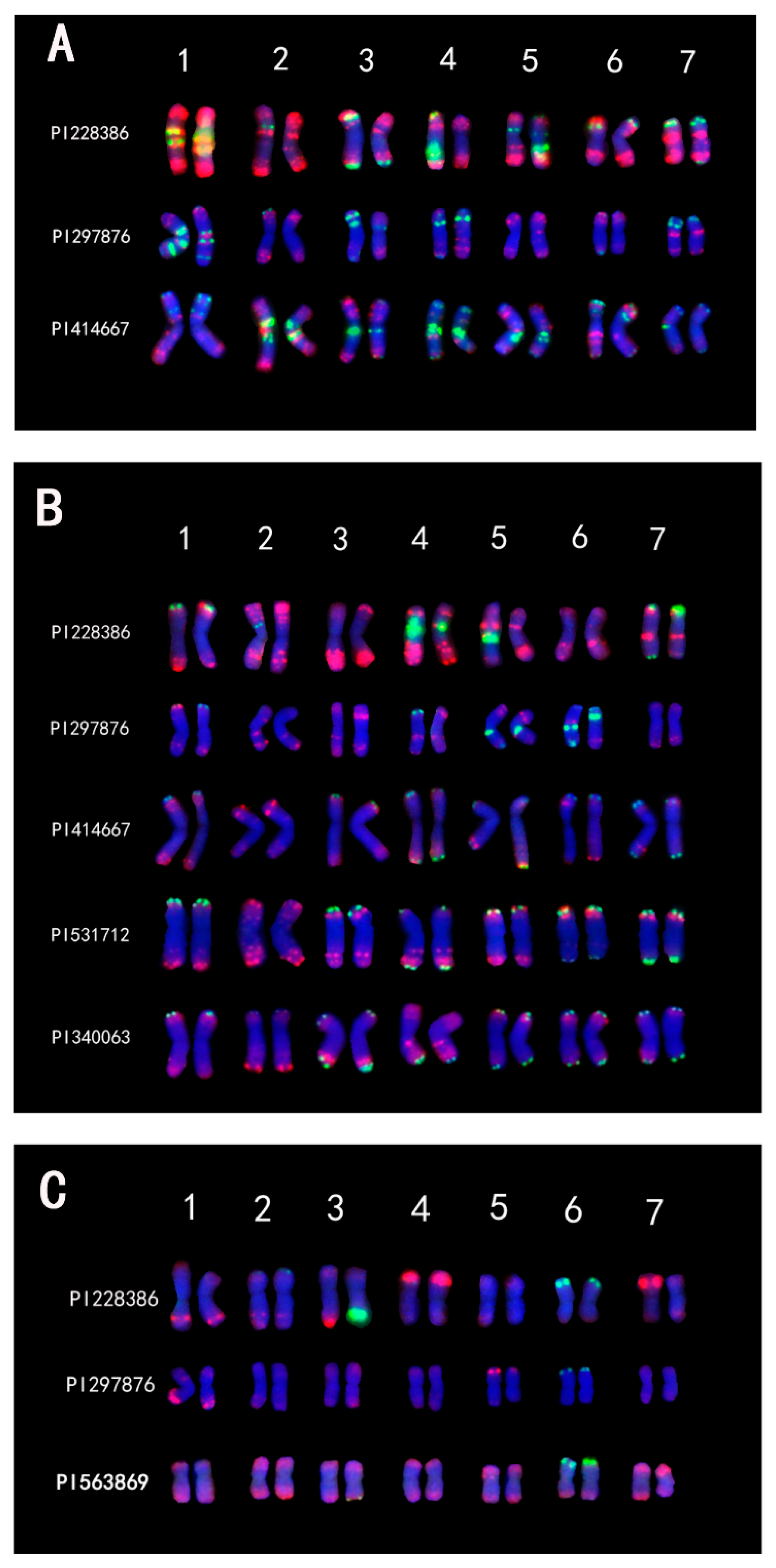
| Species | ID | Chr Number | Origin | Note |
|---|---|---|---|---|
| Thinopyrum intermedium (Host) Barkworth and D. R. Dewey | PI 228386 PI 297876 | 42 42 | Iran Former, Soviet Union | |
| Th. junceiforme (A. and D. Löve) A. Löve | PI 414667 | 28 | Greece | listed as Thinopyrum junceum (L.) Á. Löve |
| Th. bessarabicum (Savul. and Rayss) A. Löve | PI 531712 | 14 | Estonia | |
| Th. elongatum (Host) D. R. Dewey | PI 340063 | 14 | Turkey | |
| Pseudoroegneria spicata (Pursh) Á. Löve | PI 563869 | 14 | Oregon, USA | |
| Dasypyrum villosum (L.) Candargy | 14 | From X-F Li’s collection |
| Species | Genome | Chromosome No. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| 228286 | Jvs | L + S (%) | 17.74 | 15.41 | 14.43 | 14.03 | 12.79 | 13.64 | 11.97 |
| L/S | 1.14 | 1.07 | 1.78 | 1.96 | 1.22 | 1.21 | 1.42 | ||
| Type | m | m | sm | sm | m | m | m | ||
| Jr | L + S (%) | 15.78 | 15.29 | 15.26 | 14.26 | 14.18 | 12.81 | 12.41 | |
| L/S | 1.07 | 1.05 | 1.08 | 1.35 | 1.36 | 1.41 | 1.33 | ||
| Type | m | m | m | m | m | m | m | ||
| St | L + S (%) | 16.94 | 15.71 | 14.78 | 13.66 | 13.28 | 13.03 | 12.59 | |
| L/S | 1.15 | 1.11 | 1.97 | 1.64 | 1.14 | 1.06 | 1.15 | ||
| Type | m | m | sm | m | m | m | m | ||
| 297876 | Jvs | L + S (%) | 17.91 | 15.95 | 14.6 | 14.88 | 13.12 | 12.41 | 11.12 |
| L/S | 1.05 | 1.66 | 1.85 | 1.8 | 1.32 | 1.27 | 1.71 | ||
| Type | m | m | sm | sm | m | m | sm | ||
| Jr | L + S (%) | 16.22 | 16.2 | 15.31 | 13.69 | 12.71 | 12.54 | 13.32 | |
| L/S | 1.15 | 1.09 | 1.26 | 1.17 | 1.08 | 1.18 | 1.31 | ||
| Type | m | m | m | m | m | m | m | ||
| St | L + S (%) | 16.44 | 16.09 | 15.44 | 14.38 | 13.21 | 11.97 | 12.47 | |
| L/S | 1.19 | 1.52 | 1.36 | 1.36 | 1.44 | 1.1 | 1.13 | ||
| Type | m | m | m | m | m | m | m | ||
| Species | Genome | Chromosome No. | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| 414667 | Jvs | L + S (%) | 17.89 | 16.33 | 15.15 | 12.73 | 12.82 | 13.24 | 11.84 |
| L/S | 1.29 | 1.23 | 1.43 | 1.41 | 1.03 | 1.83 | 1.21 | ||
| Type | m | m | m | m | M | sm | m | ||
| Jr | L + S (%) | 15.94 | 14.72 | 14.36 | 14.03 | 14.01 | 14.02 | 12.92 | |
| L/S | 1.36 | 1.25 | 1.56 | 1.15 | 2.11 | 1.61 | 1.4 | ||
| Type | m | m | m | m | sm | sm | m | ||
| Species | Chromosome No. | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Thinopyrum bessarabicum | L + S (%) | 16.58 | 15.12 | 14.92 | 14.21 | 13.82 | 12.93 | 12.42 |
| L/S | 1.5 | 1.13 | 1.37 | 2.11 | 1.05 | 1.3 | 1.63 | |
| Type | m | m | m | sm | m | m | m | |
| Th. elongatum | L + S (%) | 16.29 | 14.84 | 14.69 | 14.15 | 13.45 | 13.56 | 13.02 |
| L/S | 1.23 | 1.1 | 2.06 | 1.61 | 1.36 | 1.17 | 1.15 | |
| Type | m | m | sm | m | m | m | m | |
| Pseudoroegneria spicata | L + S (%) | 14.88 | 14.93 | 14.46 | 14.73 | 14.31 | 13.9 | 12.78 |
| L/S | 1.51 | 1.04 | 1.11 | 1.39 | 1.07 | 1.42 | 1.13 | |
| Type | m | m | M | m | m | m | m | |
| Dasypyrum villosum | L + S (%) | 16.24 | 15.79 | 14.48 | 14.13 | 13.57 | 13.48 | 12.32 |
| L/S | 1.17 | 1.42 | 1.24 | 1.54 | 1.79 | 1.51 | 1.42 | |
| Type | m | m | m | m | sm | m | m | |
| Type | Genome | ID | |||||
|---|---|---|---|---|---|---|---|
| PI 228386 | PI 297876 | PI 414667 | PI 340036 | PI 531712 | PI 563869 | ||
| LC/SC | Jvs | 1.51 | 1.64 | 1.53 | - | - | - |
| Jr | 1.30 | 1.36 | 1.30 | 1.28 | 1.38 | - | |
| St | 1.45 | 1.42 | - | - | - | 1.26 | |
| AsK% | Jvs | 57.49% | 59.60% | 56.99% | - | - | - |
| Jr | 54.84% | 53.99% | 59.29% | 57.40% | 58.32% | - | |
| St | 56.17% | 56.52% | - | - | - | 55.09% | |
| AI | Jvs | 1.73 | 2.06 | 1.56 | |||
| Jr | 0.65 | 0.42 | 0.75 | 0.99 | 1.33 | ||
| St | 1.56 | 0.87 | 0.44 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liang, S.; Qi, F.; Bao, Y.; Wang, R.R.-C.; Li, X. The Construction of a Standard Karyotype of Intermediate Wheatgrass and Its Potential Progenitor Species. Plants 2025, 14, 196. https://doi.org/10.3390/plants14020196
Wang L, Liang S, Qi F, Bao Y, Wang RR-C, Li X. The Construction of a Standard Karyotype of Intermediate Wheatgrass and Its Potential Progenitor Species. Plants. 2025; 14(2):196. https://doi.org/10.3390/plants14020196
Chicago/Turabian StyleWang, Lin, Shuang Liang, Fei Qi, Yinguang Bao, Richard R.-C. Wang, and Xingfeng Li. 2025. "The Construction of a Standard Karyotype of Intermediate Wheatgrass and Its Potential Progenitor Species" Plants 14, no. 2: 196. https://doi.org/10.3390/plants14020196
APA StyleWang, L., Liang, S., Qi, F., Bao, Y., Wang, R. R.-C., & Li, X. (2025). The Construction of a Standard Karyotype of Intermediate Wheatgrass and Its Potential Progenitor Species. Plants, 14(2), 196. https://doi.org/10.3390/plants14020196








