Spatial Distribution Characteristics of Micronutrients and Their Deficiency Effect on the Root Morphology and Architecture in Citrus Rootstock
Abstract
1. Introduction
2. Results
2.1. Mineral Nutrient Distribution in Different Root Parts
2.2. Principal Component Analysis of Nutrient Distribution
2.3. Root Morphology Under Micronutrient Deficiency Conditions
2.4. RSA Assay
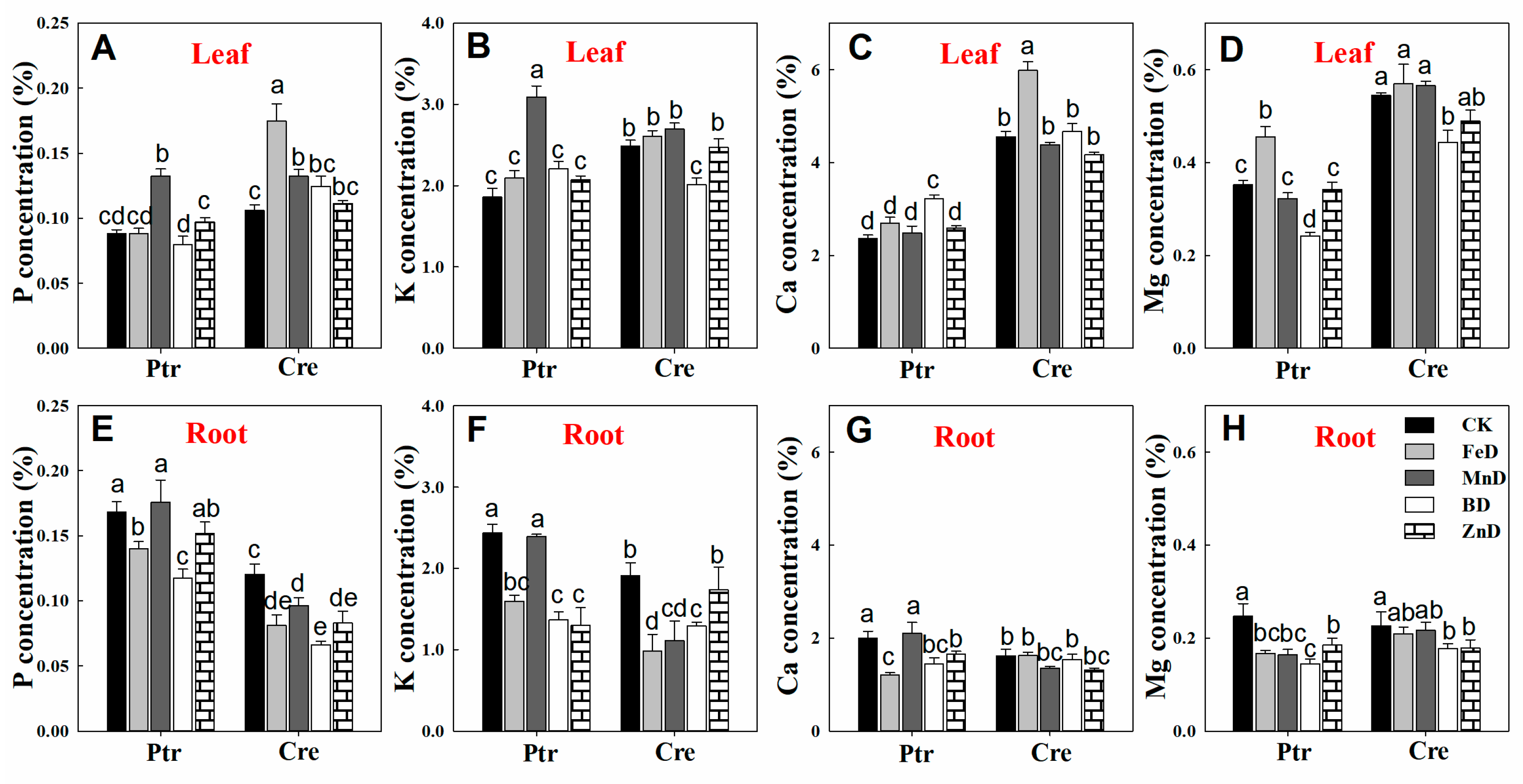
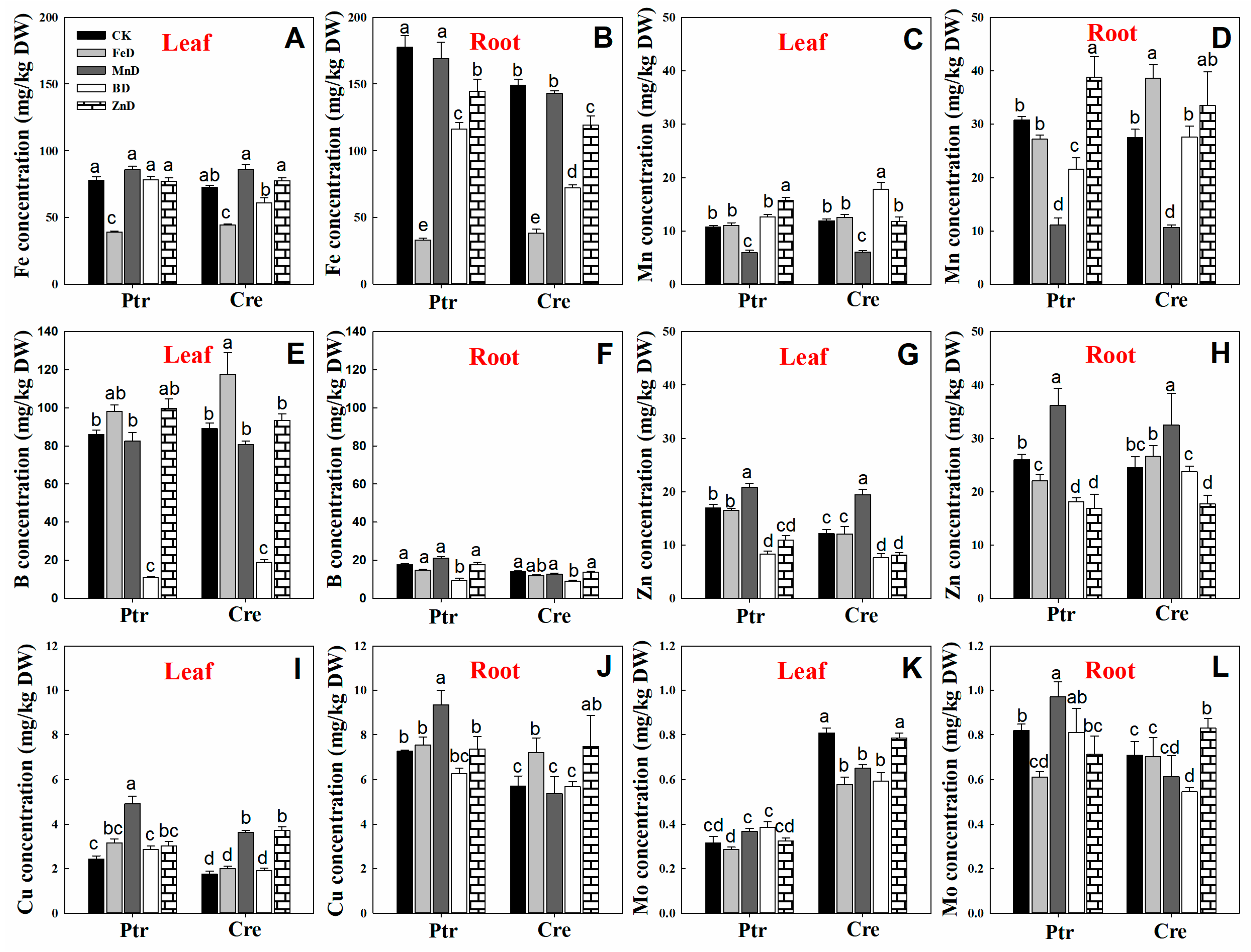
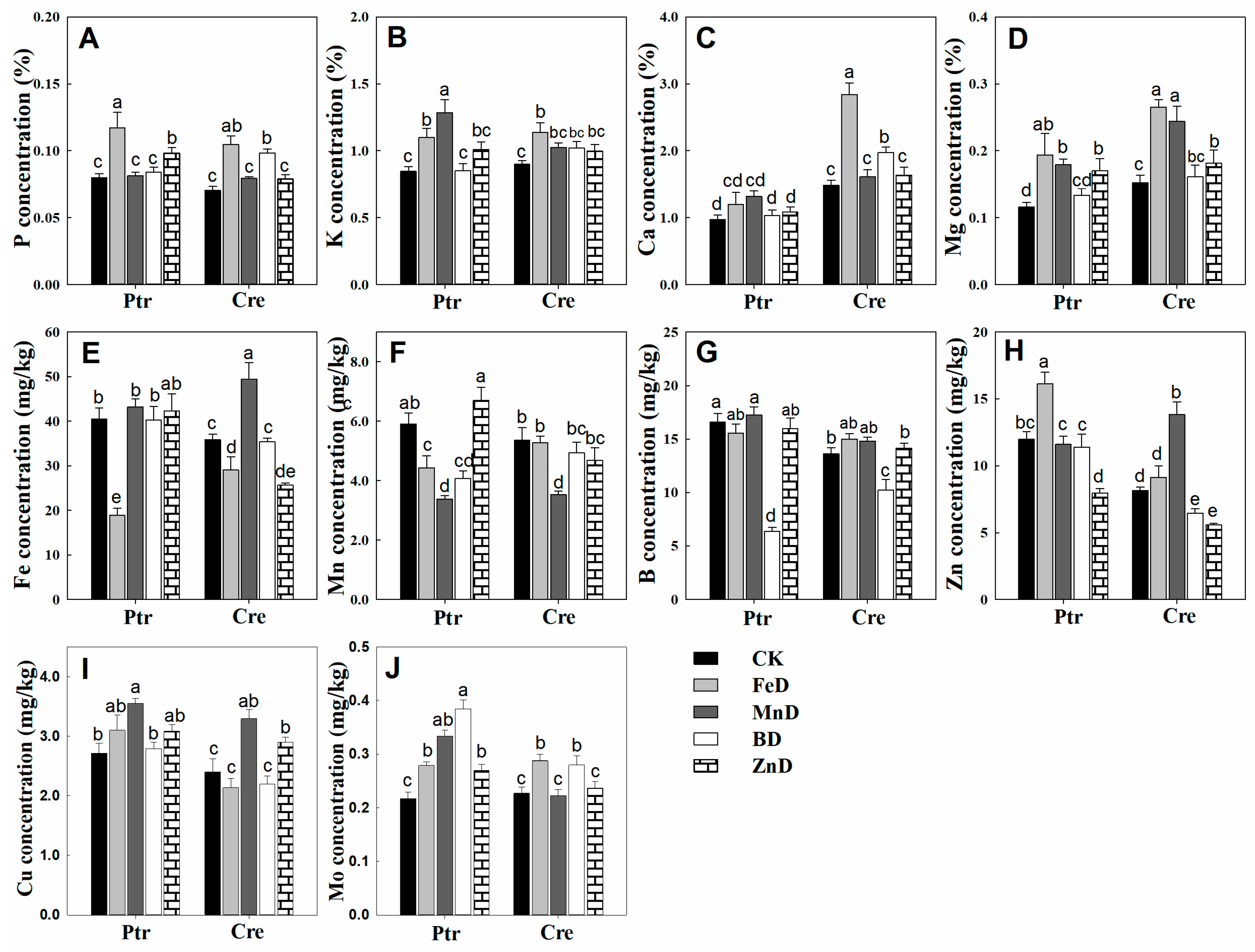
2.5. Mineral Nutrient Concentration Analysis
2.6. Dynamic Analysis of Lateral Roots Development
3. Discussion
3.1. Mineral Nutrient Distribution in Citrus Root
3.2. Adaptive Growth of Root and Stem Under Micronutrient Deficiency Conditions
3.3. Micronutrient Deficiency Affects the Formation and Growth of LR
4. Materials and Methods
4.1. Plant Materials and Treatment
4.2. Sampling and Measurement of Plant Growth Parameters
4.3. Root Morphology and Analysis
4.4. Dynamic Analysis of LR Development
4.5. Determination of Mineral Nutrients
4.6. Experimental Design and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- López-Bucio, J.; Cruz-Ramírez, A.; Herrera-Estrella, L. The role of nutrient availability in regulating root architecture. Curr. Opin. Plant Biol. 2003, 6, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Meister, R.; Rajani, M.S.; Ruzicka, D.; Schachtman, D.P. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar] [CrossRef]
- White, P.J. Root traits benefitting crop production in environments with limited water and nutrient availability. Ann. Bot. 2019, 124, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Shahbaz, M.; Koirala, M.; Blagodatskaya, E.; Seidel, S.J.; Kuzyakov, Y.; Pausch, J. Root trait plasticity and plant nutrient acquisition in phosphorus limited soil. J. Plant Nutr. Soil Sci. 2019, 182, 945–952. [Google Scholar] [CrossRef]
- Péret, B.; Clément, M.; Nussaume, L.; Desnos, T. Root developmental adaptation to phosphate starvation: Better safe than sorry. Trends Plant Sci. 2011, 16, 442–450. [Google Scholar] [CrossRef]
- Williamson, L.C. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef]
- Tyburski, J.; Dunajska, K.; Tretyn, A. A role for redox factors in shaping root architecture under phosphorus deficiency. Plant Signal. Behav. 2010, 5, 64–66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tian, H.Y.; De Smet, I.; Ding, Z.J. Shaping a root system: Regulating lateral versus primary root growth. Trends Plant Sci. 2014, 19, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Barlow, P.W. Dual pathways for regulation of root branching by nitrate. Proc. Natl. Acad. Sci. USA 1999, 96, 6529–6534. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.A.; Araus, V.; Lu, C.; Parry, G.; Green, P.J.; Coruzzi, G.M.; Gutiérrez, R.A. Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 4477–4482. [Google Scholar] [CrossRef]
- Perez-Torres, C.A.; López-Bucio, J.; Cruz-Ramírez, A.; Ibarra-Laclette, E.; Dharmasiri, S.; Estelle, M.; Herrera-Estrella, L. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. Plant Cell 2008, 20, 3258–3272. [Google Scholar] [CrossRef]
- Reymond, M.; Svistoonoff, S.; Loudet, O.; Nussaume, L.; Desnos, T. Identification of QTL controlling root growth response to phosphate starvation in Arabidopsis thaliana. Plant Cell Environ. 2010, 29, 115–125. [Google Scholar] [CrossRef]
- Krouk, G.; Lacombe, B.; Bielach, A.; Perrine-Walker, F.; Malinska, K.; Mounier, E.; Hoyerova, K.; Tillard, P.; Leon, S.; Ljung, K. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev. Cell 2010, 18, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.S.; Han, S.; Qi, Y.P.; Yang, L.T. Boron stresses and tolerance in citrus. Afr. J. Biotechnol. 2012, 11, 5961–5969. [Google Scholar]
- Fu, X.Z.; Peng, L.Z.; Xing, F.; Ling, L.L.; Chun, C.P.; Jing, C.L.; Cao, L. Zinc deficiency in citrus: Current studies and future perspectives. J. Fruit Sci. 2014, 31, 132–139. [Google Scholar]
- Fu, L.N.; Zhu, Q.Q.; Sun, Y.Y.; Du, W.; Pan, Z.Y.; Peng, S.A. Physiological and transcriptional changes of three citrus rootstock seedlings under iron deficiency. Front. Plant Sci. 2017, 8, 01104. [Google Scholar] [CrossRef] [PubMed]
- Forner-Giner, M.A.; Alcaide, A.; Primo-Millo, E.; Forner, J.B. Performance of ‘Navelina’ orange on 14 rootstocks in Northern Valencia (Spain). Sci. Hortic. 2003, 98, 223–232. [Google Scholar] [CrossRef]
- Sharma, R.M.; Dubey, A.K.; Awasthi, O.P.; Kaur, C. Growth, yield, fruit quality and leaf nutrient status of grapefruit (Citrus paradisi Macf.): Variation from rootstocks. Sci. Hortic. 2016, 210, 41–48. [Google Scholar] [CrossRef]
- Li, Q.H.; Liu, Y.Z.; Pan, Z.Y.; Xie, S.; Peng, S.A. Boron deficiency alters root growth and development and interacts with auxin metabolism by influencing the expression of auxin synthesis and transport genes. Biotechnol. Biotechnol. Equip. 2016, 30, 661–668. [Google Scholar] [CrossRef]
- Mei, L.; Li, Q.H.; Wang, H.; Sheng, O.; Peng, S.A. Boron deficiency affects root vessel anatomy and mineral nutrient allocation of Poncirus trifoliata (L.) raf. Acta Physiol. Plant. 2016, 38, 86. [Google Scholar] [CrossRef]
- Wu, X.W.; Riaz, M.; Yan, L.; Du, C.Q.; Liu, Y.L.; Jiang, C.C. Boron deficiency in trifoliate orange induces changes in pectin composition and architecture of components in root cell walls. Front. Plant Sci. 2017, 8, 1882. [Google Scholar] [CrossRef]
- Zhou, G.F.; Peng, S.A.; Liu, Y.Z.; Wei, Q.J.; Han, J.; Islam, M.Z. The physiological and nutritional responses of seven different citrus rootstock seedlings to boron deficiency. Trees 2014, 28, 295–307. [Google Scholar] [CrossRef]
- Marschner, H. Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1995. [Google Scholar]
- Chapman, N.; Miller, A.J.; Lindsey, K.; Whalley, W.R. Roots, water, and nutrient acquisition: Let’s get physical. Trends Plant Sci. 2012, 17, 701–710. [Google Scholar] [CrossRef]
- Liu, G.D.; Hu, P.; Zeng, Y.; Zeng, Z.J.; Guan, G.; Yao, F.X.; Zhou, G.F. Mineral nutrients distribution in leaf different parts in navel orange plant. Acta Hortic. Sin. 2019, 46, 47–56. [Google Scholar]
- Callahan, D.L.; Hare, D.J.; Bishop, D.P.; Doble, P.A.; Roessner, U. Elemental imaging of leaves from the metal hyperaccumulating plant Noccaea caerulescens shows different spatial distribution of Ni, Zn and Cd. RSC Adv. 2016, 6, 2337. [Google Scholar] [CrossRef]
- Vogel-Mikuš, K.; Simcic, J.; Pelicon, P.; Budnar, M.; Kump, P.; Necemer, M.; Mesjasz-Przybylowicz, J.; Przybylowicz, W.J.; Regvar, M. Comparison of essential and non-essential element distribution in leaves of the Cd/Zn hyperaccumulator Thlaspi praecox as revealed by micro-PIXE. Plant Cell Environ. 2008, 31, 1484–1496. [Google Scholar] [CrossRef]
- McClure, G.W. Nutrient distribution in root zones. III. Further studies of the effects of phosphorus distribution on corn and wheat. Can. J. Bot. 1972, 50, 2275–2282. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, Z.; Chen, X.; Jia, Z.; Su, Y. Responses of root morphology and architecture to phosphorus deficiency at seedling stage of tobacco (nicotiana tabacum) growth. Aust. J. Crop Sci. 2013, 7, 1967–1972. [Google Scholar]
- Péret, B.; Desnos, T.; Jost, R.; Kanno, S.; Berkowitz, O.; Nussaume, L. Root architecture responses: In search of phosphate. Plant Physiol. 2014, 166, 1713–1723. [Google Scholar] [CrossRef]
- Silva, A.D.; Bruno, I.P.; Franzini, V.I.; Marcante, N.C.; Benitiz, L.; Muraoka, T. Phosphorus uptake efficiency, root morphology and architecture in Brazilian wheat cultivars. J. Radioanal. Nucl. Chem. 2016, 307, 1055–1063. [Google Scholar] [CrossRef]
- Qiao, H.T.; Yang, H.Q.; Sheng, E.B.; Jiang, Q.Q.; You, S.Z.; Zhang, L.; Ran, K.; Zhang, X.R. Effect of nitrogen deficient and iron deficient on root architecture of young seedlings of Malus hupehensis (Pamp) Rehd. Acta Hortic. Sin. 2009, 36, 321–326. [Google Scholar]
- Chen, Y.Y.; Hu, C.Y.; Xiao, J.X. Effects of arbuscular mycorrhizal inoculation on the growth, zinc distribution and photosynthesis of two citrus cultivars grown in low-zinc soil. Trees 2014, 28, 1427–1436. [Google Scholar] [CrossRef]
- Fan, W.G.; Yang, H.Q.; Han, X.J. Changes of root architecture and phosphorus uptake by roots of Malus hupehensis (Pamp) Rehd. under the condition of phosphorus deficiency. Acta Hortic. Sin. 2007, 34, 1341–1346. [Google Scholar]
- Plaxton, W.C.; Tran, H.T. Metabolic adaptations of phosphate-starved plants. Plant Physiol. 2011, 156, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Fabiańska, I.; Gerlach, N.; Almario, J.; Bucher, M. Plant-mediated effects of soil phosphorus on the root-associated fungal microbiota in Arabidopsis thaliana. New Phytol. 2019, 221, 2123–2137. [Google Scholar] [CrossRef]
- Luo, G.T.; Liu, X.N.; Zhang, M.M.; Yu, H.; Hu, Z.; Wang, F.S.; Zhu, S.P.; Zhao, X.C. Root morphology of citrus rootstocks and drought tolerance evaluation of their grafted plants. J. Fruit Sci. 2020, 37, 1314–1325. [Google Scholar]
- Zhu, S.P.; Huang, T.J.; Yu, X.; Hong, Q.B.; Xiang, J.S.; Zeng, A.Z.; Gong, G.Z.; Zhao, X.C. The effects of rootstocks on performances of three late-ripening navel orange varieties. J. Integr. Agric. 2020, 19, 1802–1812. [Google Scholar] [CrossRef]
- He, M.; Tian, Y.; Yu, Y.; Pu, J.N.; Zhang, C.Y.; He, R.J.; Zhang, Y.Q.; Lyu, Q.; Xie, R.J.; Ma, Y.Y.; et al. Effect of different root stocks on nutrient absorption and utilization of vegetative organs in ‘Ehime 28’. J. Southwest Univ. 2022, 44, 80–87. [Google Scholar]
- Song, F.; Qin, W.; Kuang, B.; Sun, H.; Chen, Q.X.; Jiang, Y.C.; Liu, J.H.; Wu, L.M. Effects of approach-grafting rootstock combinations on nutrition, growth and development, and fruit quality of Lane Late navel orange. J. Huazhong Agric. Univ. 2022, 41, 116–121. [Google Scholar]
- Malamy, J.E. Intrinsic and environmental response pathways that regulate root system architecture. Plant Cell Environ. 2005, 28, 67–77. [Google Scholar] [CrossRef]
- Jourquina, J.; Fukakic, H.; Beeckman, T. Peptide-receptor signaling controls lateral root development. Plant Physiol. 2020, 182, 1645–1656. [Google Scholar] [CrossRef]
- Huang, R.; Zheng, R.; He, J.; Zhou, Z.; Xu, T. Noncanonical auxin signaling regulates cell division pattern during lateral root development. Proc. Natl. Acad. Sci. USA 2019, 116, 21285–21290. [Google Scholar] [CrossRef]
- Zhu, Q.; Shao, Y.; Ge, S.; Zhang, M.; Zhang, T.; Hu, X.; Liu, Y.; Walker, J.; Zhang, S.; Xu, J. A MAPK cascade downstream of IDA–HAE/HSL2 ligand–receptor pair in lateral root emergence. Nat. Plants 2019, 5, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.F.; Wei, Q.J.; Li, B.X.; Zeng, X.L.; Liu, G.D. Establishment and optimization of a hydroponic system for root morphological and nutritional analysis of citrus. Sci. Agric. 2020, 77, e20180261. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Dimassi, N.; Bosabalidis, A.M.; Therios, I.N.; Patakas, A.; Giannakoula, A. Boron toxicity in ‘Clementine’ mandarin plants grafted on two rootstocks. Plant Sci. 2004, 166, 539–547. [Google Scholar] [CrossRef]
- Sheng, O.; Song, S.W.; Peng, S.A.; Deng, X.X. The effects of low boron on growth, gas exchange, boron concentration and distribution of ‘Newhall’ navel orange (Citrus sinensis Osb.) plants grafted on two rootstocks. Sci. Hortic. 2009, 121, 278–283. [Google Scholar] [CrossRef]
- Storey, R.; Treeby, M.T. Nutrient uptake into navel orange during fruit development. J. Hortic. Sci. Biotechnol. 2000, 77, 91–99. [Google Scholar] [CrossRef]

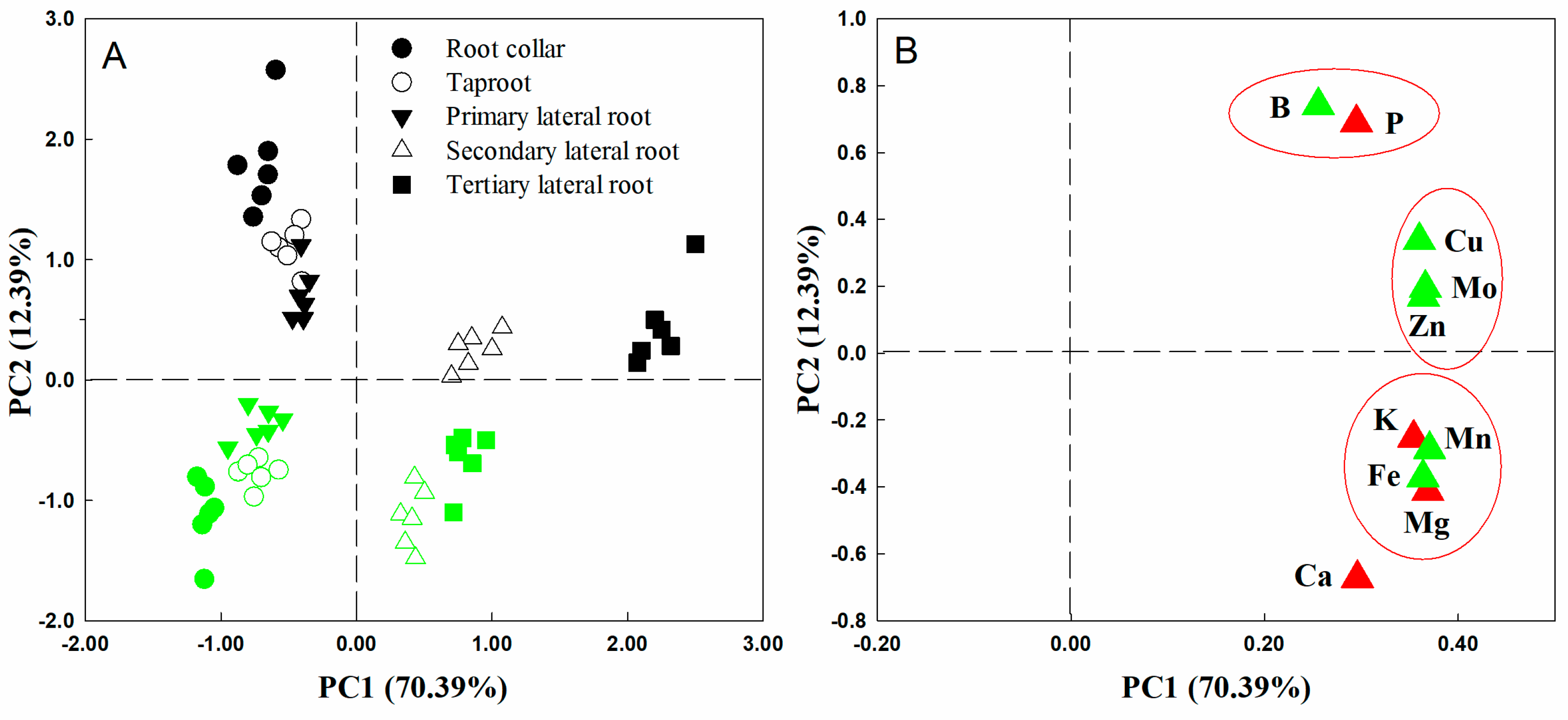

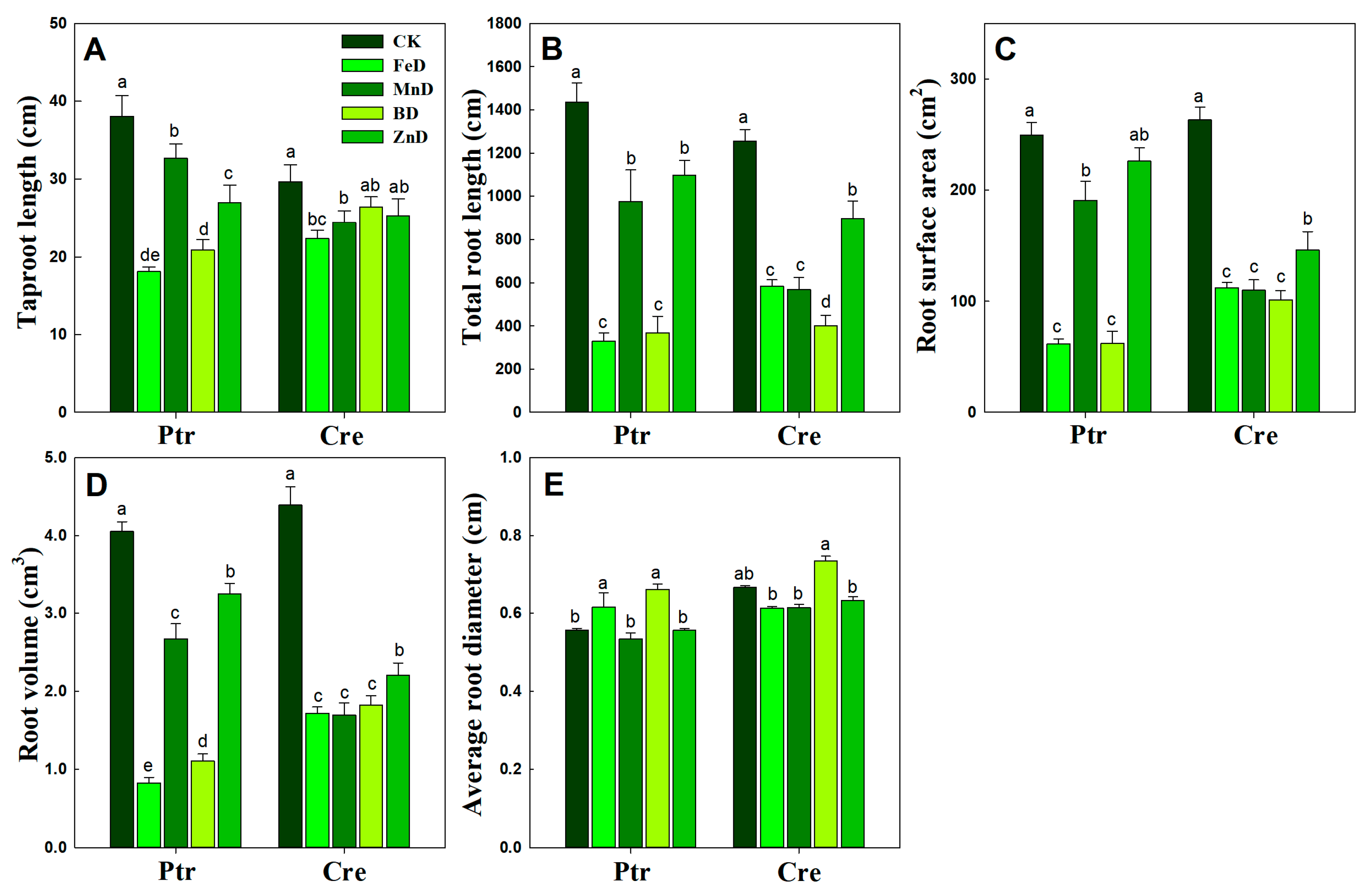
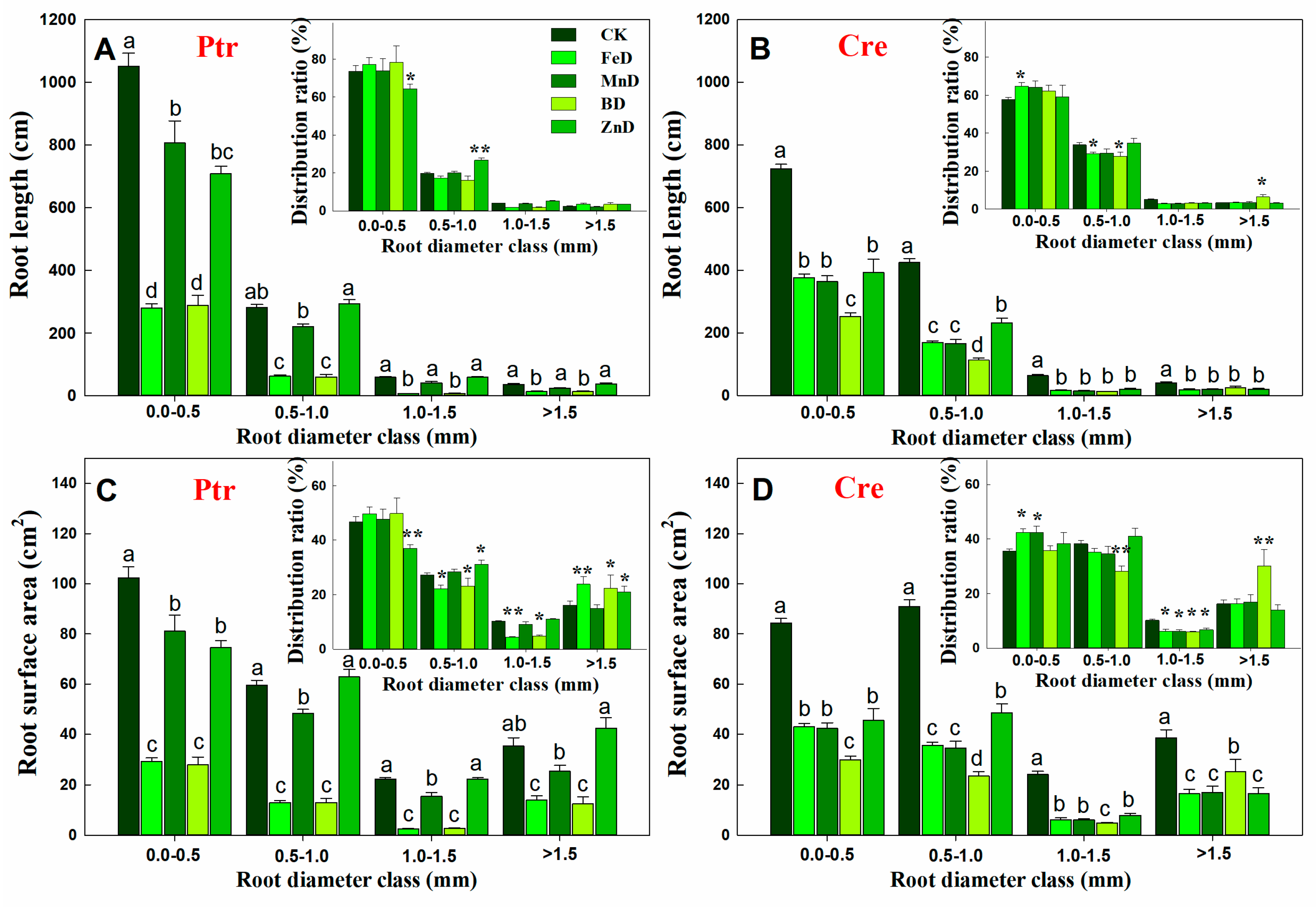
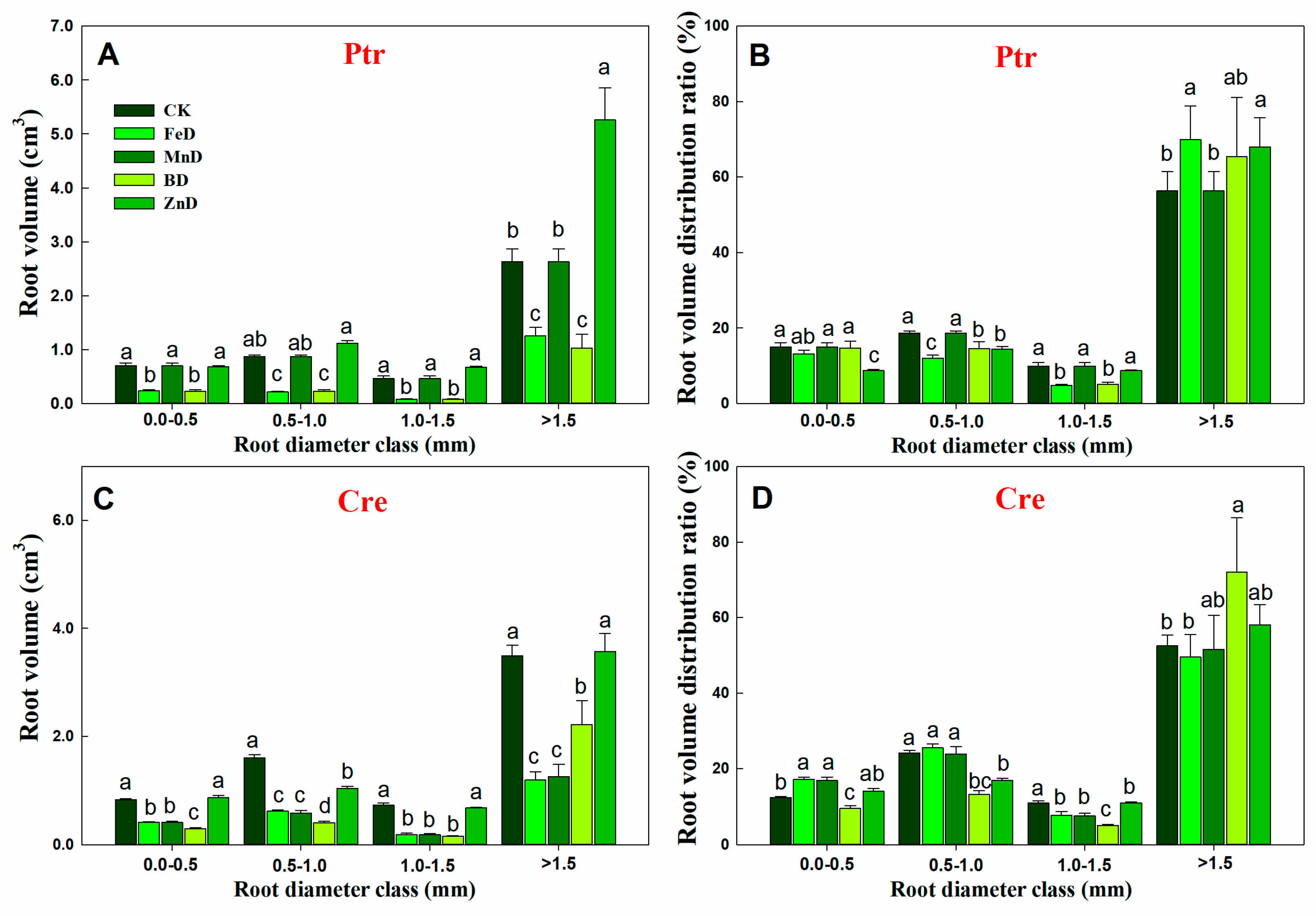

| Species | Growth Parameters | CK | FeD | MnD | BD | ZnD |
|---|---|---|---|---|---|---|
| Ptr | Stem length (cm) | 83.64 ± 3.37 b | 29.21 ± 3.50 d | 34.60 ± 6.35 d | 74.50 ± 6.37 c | 95.98 ± 4.47 a |
| Stem diameter (mm) | 4.57 ± 0.08 b | 4.56 ± 0.12 b | 3.12 ± 0.23 c | 5.94 ± 0.11 a | 5.13 ± 0.14 ab | |
| Stem dry weight (g) | 2.39 ± 0.25 b | 0.89 ± 0.10 c | 0.50 ± 0.15 c | 3.18 ± 0.50 a | 3.32 ± 0.21 a | |
| Root dry weight (g) | 1.58 ± 0.21 a | 0.34 ± 0.10 c | 1.07 ± 0.09 b | 0.58 ± 0.04 c | 1.40 ± 0.18 a | |
| Cre | Stem length (cm) | 76.92 ± 2.60 a | 27.76 ± 2.08 c | 50.32 ± 5.13 b | 30.62 ± 5.38 c | 30.54 ± 5.98 c |
| Stem diameter (mm) | 4.81 ± 0.17 a | 3.26 ± 0.07 b | 3.41 ± 0.21 b | 4.15 ± 0.33 ab | 3.46 ± 0.20 b | |
| Stem dry weight (g) | 2.49 ± 0.20 a | 0.52 ± 0.04 b | 0.97 ± 0.16 b | 0.87 ± 0.28 b | 0.87 ± 0.20 b | |
| Root dry weight (g) | 1.39 ± 0.06 a | 0.57 ± 0.02 c | 0.90 ± 0.08 b | 0.52 ± 0.05 c | 0.60 ± 0.07 c |
| Species | Treatment | Primary Lateral Roots | Secondary Lateral Roots | |||
|---|---|---|---|---|---|---|
| Number | Length (cm) | Number | Length (cm) | Density (Number/cm) | ||
| Ptr | CK | 19.56 ± 1.17 a | 8.23 ± 0.55 a | 24.44 ± 2.30 a | 2.90 ± 0.08 a | 2.96 ± 0.21 b |
| FeD | 13.22 ± 1.11 c | 4.85. ± 0.31 c | 14.33 ± 0.74 c | 1.92 ± 0.47 c | 3.02 ± 0.32 b | |
| MnD | 16.78 ± 1.29 b | 7.07 ± 0.63 b | 19.44 ± 2.85 b | 2.48 ± 0.23 b | 2.90 ± 0.38 b | |
| BD | 20.67 ± 1.61 a | 4.93 ± 0.34 d | 23.89 ± 2.68 a | 1.15 ± 0.13 d | 4.93 ± 0.61 a | |
| ZnD | 18.11 ± 2.42 ab | 7.66 ± 0.60 ab | 22.89 ± 4.74 ab | 2.62 ± 0.76 ab | 3.14 ± 0.48 b | |
| Cre | CK | 22.33 ± 1.61 a | 7.67 ± 0.74 a | 19.83 ± 1.74 a | 2.66 ± 0.13 a | 2.67 ± 0.26 b |
| FeD | 16.00 ± 1.18 b | 6.18 ± 0.33 cd | 15.83 ± 0.95 b | 2.05 ± 0.14 b | 2.57 ± 0.09 b | |
| MnD | 18.17 ± 1.30 ab | 6.54 ± 0.44 c | 13.83 ± 1.08 c | 1.78 ± 0.14 c | 2.14 ± 0.17 c | |
| BD | 21.17 ± 1.17 a | 5.51 ± 0.57 d | 20.67 ± 1.94 a | 1.44 ± 0.17 d | 3.96 ± 0.53 a | |
| ZnD | 20.33 ± 2.09 a | 6.88 ± 0.43 b | 13.33 ± 2.54 c | 1.79 ± 0.19 c | 1.98 ± 0.20 c | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, G.; Fu, Y.; Yang, M.; Li, Y.; Zhang, J. Spatial Distribution Characteristics of Micronutrients and Their Deficiency Effect on the Root Morphology and Architecture in Citrus Rootstock. Plants 2025, 14, 158. https://doi.org/10.3390/plants14020158
Zhou G, Fu Y, Yang M, Li Y, Zhang J. Spatial Distribution Characteristics of Micronutrients and Their Deficiency Effect on the Root Morphology and Architecture in Citrus Rootstock. Plants. 2025; 14(2):158. https://doi.org/10.3390/plants14020158
Chicago/Turabian StyleZhou, Gaofeng, Yiping Fu, Mei Yang, Yanhong Li, and Jing Zhang. 2025. "Spatial Distribution Characteristics of Micronutrients and Their Deficiency Effect on the Root Morphology and Architecture in Citrus Rootstock" Plants 14, no. 2: 158. https://doi.org/10.3390/plants14020158
APA StyleZhou, G., Fu, Y., Yang, M., Li, Y., & Zhang, J. (2025). Spatial Distribution Characteristics of Micronutrients and Their Deficiency Effect on the Root Morphology and Architecture in Citrus Rootstock. Plants, 14(2), 158. https://doi.org/10.3390/plants14020158






