Leaf and Root Functional Traits of Woody and Herbaceous Halophytes and Their Adaptations in the Yellow River Delta
Abstract
1. Introduction
2. Results
2.1. Morphology Trait Variation
2.2. Anatomy Trait Variations
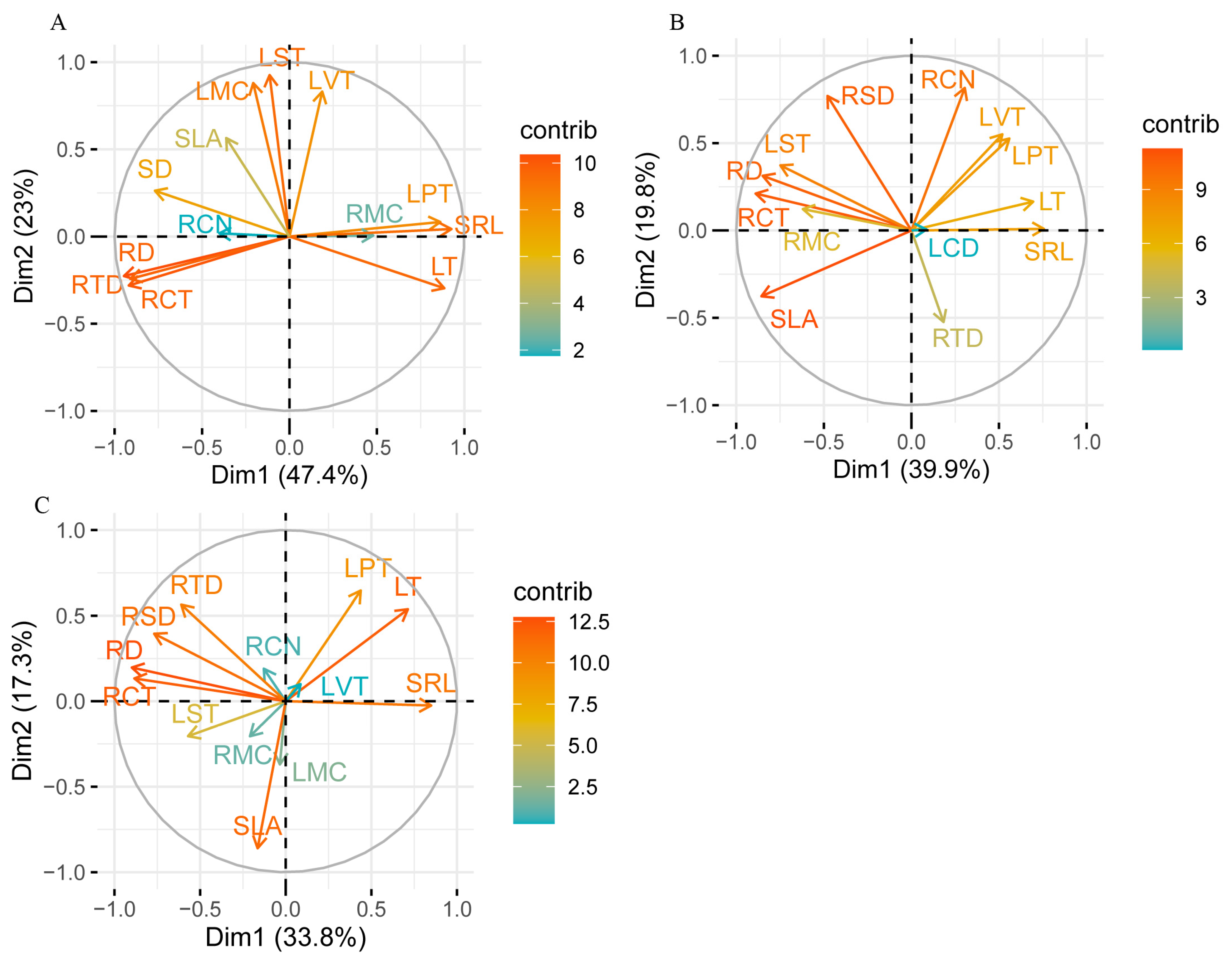
2.3. Trait Associations Within/Between Above- and Below-Ground Organs
3. Discussion
3.1. Leaf and Root Trait Variations Between Two Groups
3.2. Trait Correlations
4. Materials and Methods
4.1. Study Site
4.2. Sample Collection
4.3. Trait Measurement
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Basak, N.; Rai, A.K.; Sundha, P.; Meena, R.L.; Bedwal, S.; Yadav, R.K.; Sharma, P.C. Assessing soil quality for rehabilitation of salt-affected agroecosystem: A comprehensive review. Front. Environ. Sci. 2022, 10, 935785. [Google Scholar] [CrossRef]
- Rewald, B.; Shelef, O.; Ephrath, J.E.; Rachmilevitch, S. Adaptive plasticity of salt-stressed root systems. In Ecophysiology and Responses of Plants under Salt Stress; Ahmad, P., Azooz, M.M., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 169–201. [Google Scholar]
- Grigore, M. Handbook of Halophytes-From Molecules to Ecosystems Towards Biosaline Agriculture; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Freschet, G.T.; Pagès, L.; Iversen, C.M.; Comas, L.H.; Rewald, B.; Roumet, C.; Klimešová, J.; Zadworny, M.; Poorter, H.; Postma, J.A.; et al. A starting guide to root ecology: Strengthening ecological concepts and standardizing root classification, sampling, processing and trait measurements. New Phytol. 2021, 232, 973–1122. [Google Scholar] [CrossRef] [PubMed]
- Violle, C.; Navas, M.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Uchiya, P.; Escaray, F.J.; Bilenca, D.; Pieckenstain, F.; Ruiz, O.A.; Menendez, A.B. Salt effects on functional traits in model and in economically important Lotus species. Plant Biol. 2016, 18, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Bona, C.; Moryel Pellanda, R.; Bergmann Carlucci, M.; De Paula Machado, R.G.; Ciccarelli, D. Functional traits reveal coastal vegetation assembly patterns in a short edaphic gradient in southern Brazil. Flora 2020, 271, 151661. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, J.A.; Mancera Pineda, J.E.; Melgarejo, L.M.; Medina Calderón, J.H. Functional traits of leaves and forest structure of neotropical mangroves under different salinity and nitrogen regimes. Flora 2018, 239, 52–61. [Google Scholar] [CrossRef]
- Chang, Y.; Fan, Y.; Li, Z.; Lv, G. Relationship between photosynthetic characteristics, anatomical structure, and physiological indexes of two halophytes in different habitats. Forests 2022, 13, 2189. [Google Scholar] [CrossRef]
- Xia, C.; Liu, G.; Xia, H.; Jiang, F.; Meng, Y. Influence of saline intrusion on the wetland ecosystem revealed by isotopic and hydrochemical indicators in the Yellow River Delta, China. Ecol. Indic. 2021, 133, 108422. [Google Scholar] [CrossRef]
- Mazhar, S.; Pellegrini, E.; Contin, M.; Bravo, C.; de Nobili, M. Impacts of salinization caused by sea level rise on the biological processes of coastal soils—A review. Front. Environ. Sci. 2022, 10, 909415. [Google Scholar] [CrossRef]
- Liu, S.; Hou, X.; Yang, M.; Cheng, F.; Coxixo, A.; Wu, X.; Zhang, Y. Factors driving the relationships between vegetation and soil properties in the Yellow River Delta, China. Catena 2018, 165, 279–285. [Google Scholar] [CrossRef]
- Zhou, D.; Ni, Y.; Yu, X.; Lin, K.; Du, N.; Liu, L.; Guo, X.; Guo, W. Trait-based adaptability of Phragmites australis to the effects of soil water and salinity in the Yellow River Delta. Ecol. Evol. 2021, 11, 11352–11361. [Google Scholar] [CrossRef]
- Ma, X.; Yan, J.; Wang, F.; Qiu, D.; Jiang, X.; Liu, Z.; Sui, H.; Bai, J.; Cui, B. Trait and density responses of Spartina alterniflora to inundation in the Yellow River Delta, China. Mar. Pollut. Bull. 2019, 146, 857–864. [Google Scholar] [CrossRef]
- Fan, X.; Xu, Z.; Liu, X.; Tang, C.; Wang, L.; Han, X. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Lockhart, B.R.; Gardiner, E.S.; Stautz, T.; Leininger, T.D. Development and plasticity of endangered ShrubLindera melissifolia (Lauraceae) seedlings under contrasting light regimes. Plant Spec. Biol. 2012, 27, 30–45. [Google Scholar] [CrossRef]
- Stanton, K.M.; Weeks, S.S.; Dana, M.N.; Mickelbart, M.V. Light Exposure and shade effects on growth, flowering, and leaf morphology of Spiraea alba Du Roi and Spiraea tomentosa L. HortScience 2010, 45, 1912–1916. [Google Scholar] [CrossRef]
- Samuelson, L.J.; Stokes, T.A. Leaf physiological and morphological responses to shade in grass-stage seedlings and young trees of longleaf pine. Forests 2012, 3, 684–699. [Google Scholar] [CrossRef]
- Wang, G.G.; Bauerle, W.L. Effects of light intensity on the growth and energy balance of photosystem II electron transport in Quercus alba seedlings. Ann. Forest Sci. 2006, 63, 111–118. [Google Scholar] [CrossRef]
- Xia, J.; Zhao, X.; Ren, J.; Lang, Y.; Qu, F.; Xu, H. Photosynthetic and water physiological characteristics of Tamarix chinensis under different groundwater salinity conditions. Environ. Exp. Bot. 2017, 138, 173–183. [Google Scholar] [CrossRef]
- Tayir, M.; Dai, Y.; Shi, Q.; Abdureyim, A.; Erkin, F.; Huang, W. Distinct leaf functional traits of Tamarix chinensis at different habitats in the hinterland of the Taklimakan desert. Front. Plant Sci. 2023, 13, 1094049. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hussain, T.; Feng, X.; Guo, K.; Chen, H.; Yang, C.; Liu, X. Comparative study on the resistance of Suaeda glauca and Suaeda salsa to drought, salt, and alkali stresses. Ecol. Eng. 2019, 140, 105593. [Google Scholar] [CrossRef]
- Song, J.; Wang, B. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 2015, 115, 541–553. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, Z.; Dong, X. Anatomical structures of fine roots of 91 vascular plant species from four groups in a temperate forest in Northeast China. PLoS ONE 2019, 14, e0215126. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Gao, G.; Wang, Y.; Wang, D.; Wang, Z.; Gu, J. Coordinated responses of leaf and absorptive root traits under elevated CO2 concentration in temperate woody and herbaceous species. Environ. Exp. Bot. 2020, 179, 104199. [Google Scholar] [CrossRef]
- Guo, D.; Li, H.; Mitchell, R.J.; Han, W.; Hendricks, J.J.; Fahey, T.J.; Hendrick, R.L. Fine root heterogeneity by branch order: Exploring the discrepancy in root turnover estimates between minirhizotron and carbon isotopic methods. New Phytol. 2008, 177, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhao, X.; Zhao, H.; Huang, Y.; Zuo, X. Linking root morphology, longevity and function to root branch order: A case study in three shrubs. Plant Soil. 2010, 336, 197–208. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, Y.; Zhang, J.; Wu, J.; Tang, Y.; Cao, G.; Zhang, F.; Cui, X. Root size and soil environments determine root lifespan: Evidence from an alpine meadow on the Tibetan Plateau. Ecol. Res. 2013, 28, 493–501. [Google Scholar] [CrossRef]
- Eissenstat, D.M. Costs and benefits of constructing roots of small diameter. J. Plant Nutr. 1992, 15, 763–782. [Google Scholar] [CrossRef]
- Eissenstat, D.M.; Yanai, R.D. The ecology of root lifespan. Adv. Ecol. Res. 1997, 27, 1–60. [Google Scholar]
- Chen, X.; Chen, D.; Liu, H.; Zhao, C.; Zhao, W.; Dong, Z.; Zhang, Y.; Wang, Y. Responses of fine root anatomical traits of eleven tree species to the soil conditions of coastal saline-alkali stand sites of the Yellow River delta. Acta Ecol. Sin. 2021, 41, 4150–4159. (In Chinese) [Google Scholar]
- Rewald, B.; Raveh, E.; Gendler, T.; Ephrath, J.E.; Rachmilevitch, S. Phenotypic plasticity and water flux rates of Citrus root orders under salinity. J. Exp. Bot. 2012, 63, 2717–2727. [Google Scholar] [CrossRef]
- Kong, D.; Ma, C.; Zhang, Q.; Li, L.; Chen, X.; Zeng, H.; Guo, D. Leading dimensions in absorptive root trait variation across 96 subtropical forest species. New Phytol. 2014, 203, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Xu, Y.; Dong, X.; Wang, H.; Wang, Z. Root diameter variations explained by anatomy and phylogeny of 50 tropical and temperate tree species. Tree Physiol. 2014, 34, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zeng, H.; Eissenstat, D.; Guo, D. Variation of first order root traits across climatic gradients and evolutionary trends in geological time. Glob. Ecol. Biogeogr. 2013, 22, 846–856. [Google Scholar] [CrossRef]
- Comas, L.H.; Mueller, K.E.; Taylor, L.L.; Midford, P.E.; Callahan, H.S.; Beerlingz, D.H. Evolutionary patterns and biogeochemical significance of angiosperm root traits. Int. J. Plant Sci. 2012, 173, 584–595. [Google Scholar] [CrossRef]
- Guo, D.; Xia, M.; Wei, X.; Chang, W.; Liu, Y.; Wang, Z. Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol. 2008, 180, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary history resolves global organization of root functional traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef]
- Kong, D.; Wang, J.; Zeng, H.; Liu, M.; Miao, Y.; Wu, H.; Kardol, P. The nutrient absorption-transportation hypothesis: Optimizing structural traits in absorptive roots. New Phytol. 2017, 213, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Gambetta, G.A.; Fei, J.; Rost, T.L.; Knipfer, T.; Mattews, M.A.; Shackel, K.E.; Walker, M.A.; McElrone, A.J. Water uptake along the length of grapevine fine roots: Developmental anatomy, tissue-specific aquaporin expression, and pathways of water transport. Plant Physiol. 2015, 163, 1254–1265. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, Z.; Gu, J. Functional trait plasticity but not coordination differs in absorptive and transport fine roots in response to soil depth. Forests 2020, 11, 42. [Google Scholar] [CrossRef]
- Kembel, S.W.; Cahill, J.F. Independent evolution of leaf and root traits within and among temperate grassland plant communities. PLoS ONE 2011, 6, e19992. [Google Scholar] [CrossRef] [PubMed]
- Tjoelker, M.G.; Craine, J.M.; Wedin, D.; Reich, P.B.; Tilman, D. Linking leaf and root trait syndromes among 39 grassland and savannah species. New Phytol. 2005, 167, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Withington, J.M.; Reich, P.B.; Oleksyn, J.; Eissenstat, D.M. Comparisons of structure and life span in roots and leaves among temperate trees. Ecol. Monogr. 2006, 76, 381–397. [Google Scholar] [CrossRef]
- Valverde-Barrantes, O.J.; Authier, L.; Schimann, H.; Baraloto, C. Root anatomy helps to reconcile observed root trait syndromes in tropical tree species. Am. J. Bot. 2021, 108, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, D.; Wang, X.; Gu, J.; Mei, L. Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 2006, 288, 155–171. [Google Scholar] [CrossRef]
- Wickham, H.; Chang, W.; Henry, L.; Pederson, T.L.; Takahashi, K.; Wikle, C.; Woo, K. Package ‘ggplot2’. 2018. Available online: https://cran.r-project.org/web/packages/ggplot2/index.html (accessed on 1 October 2024).
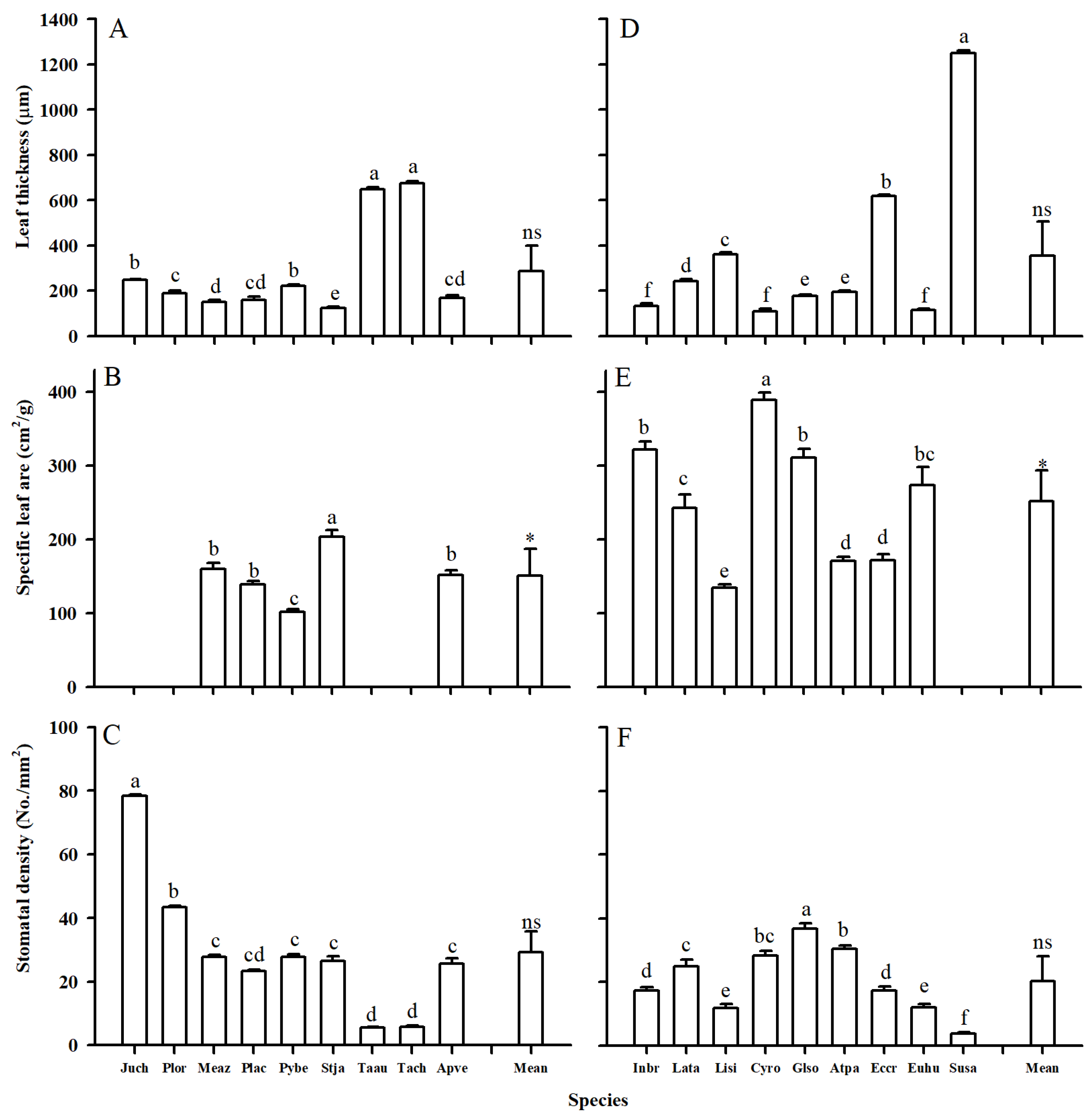


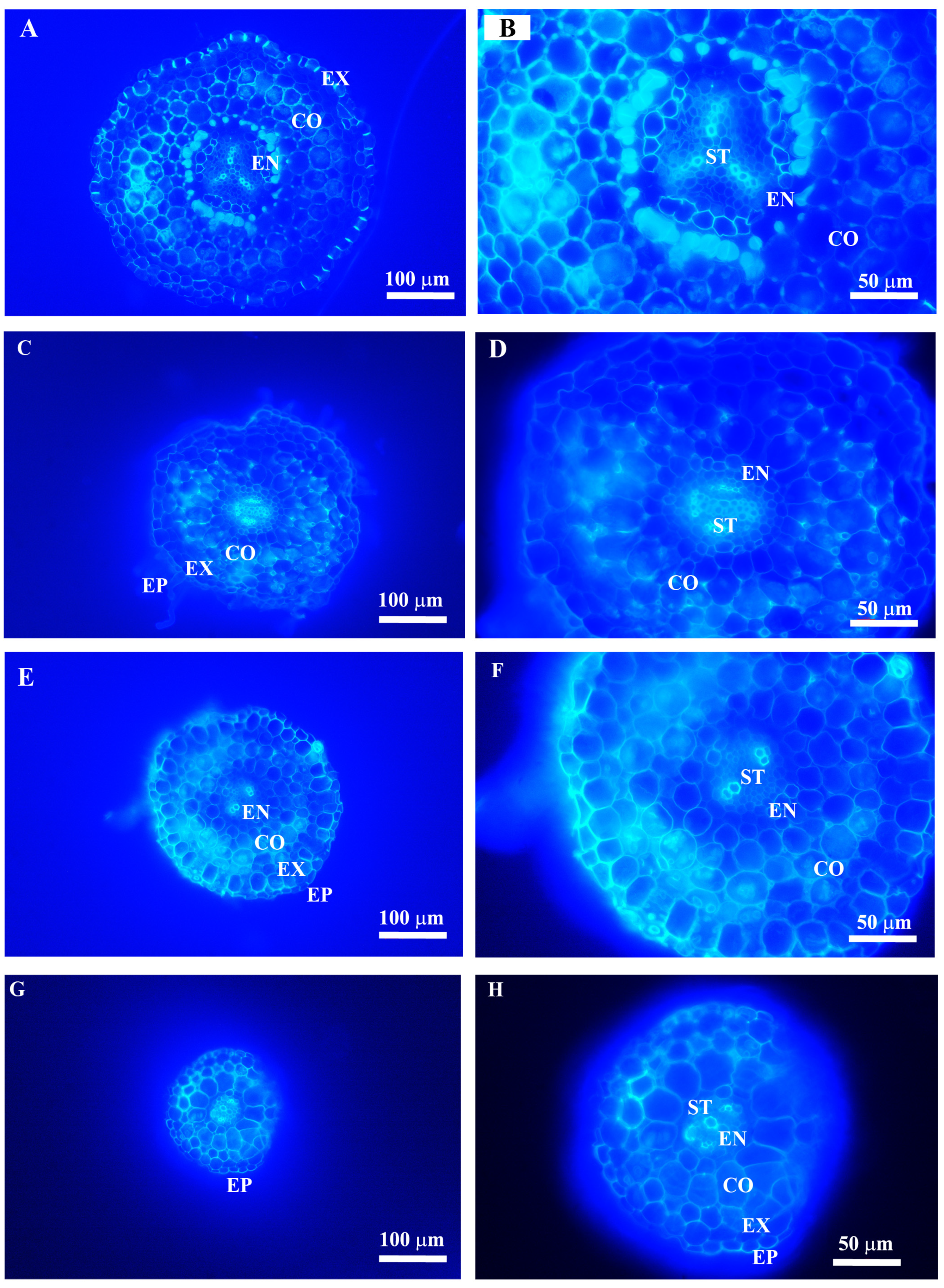
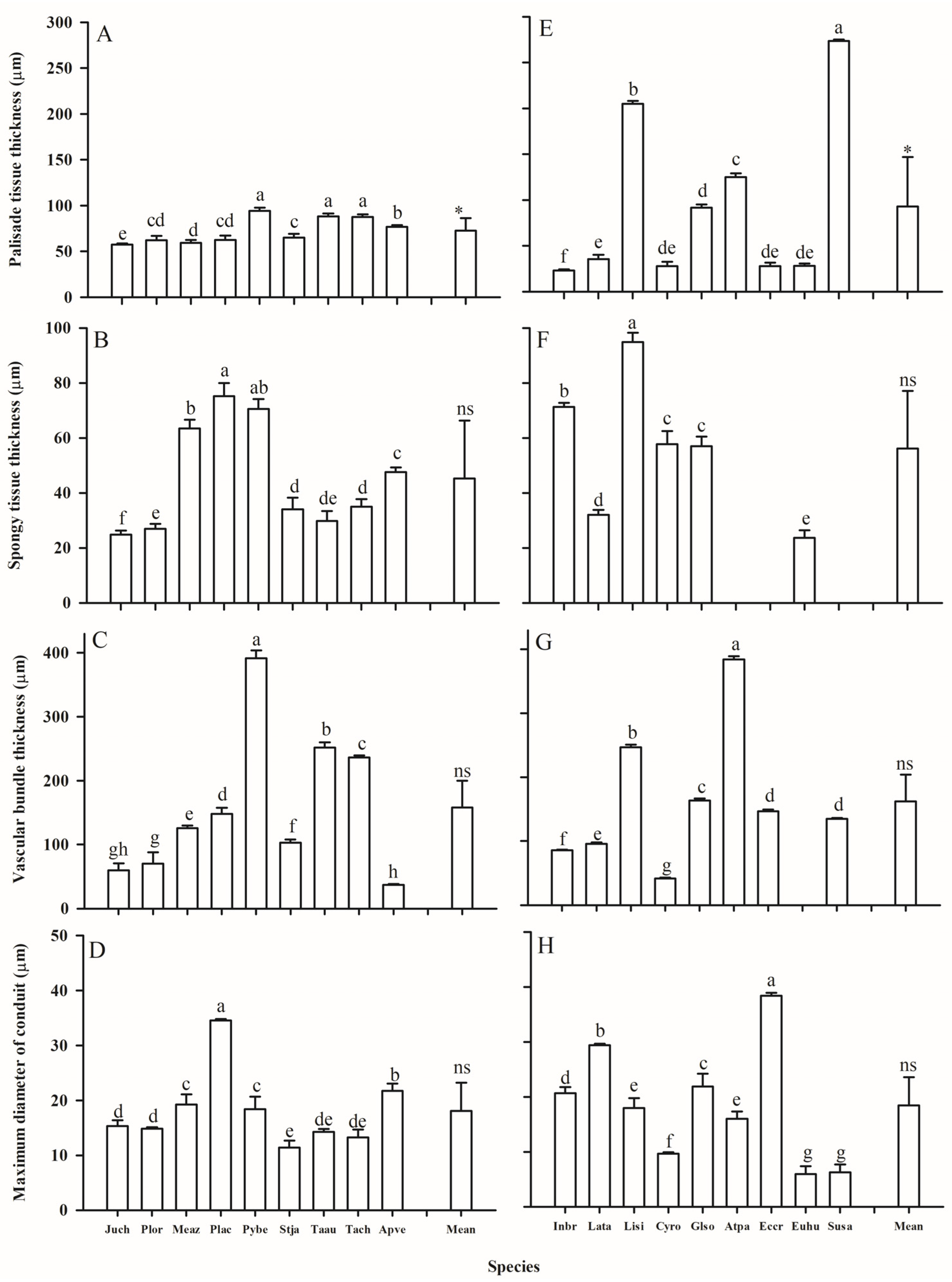



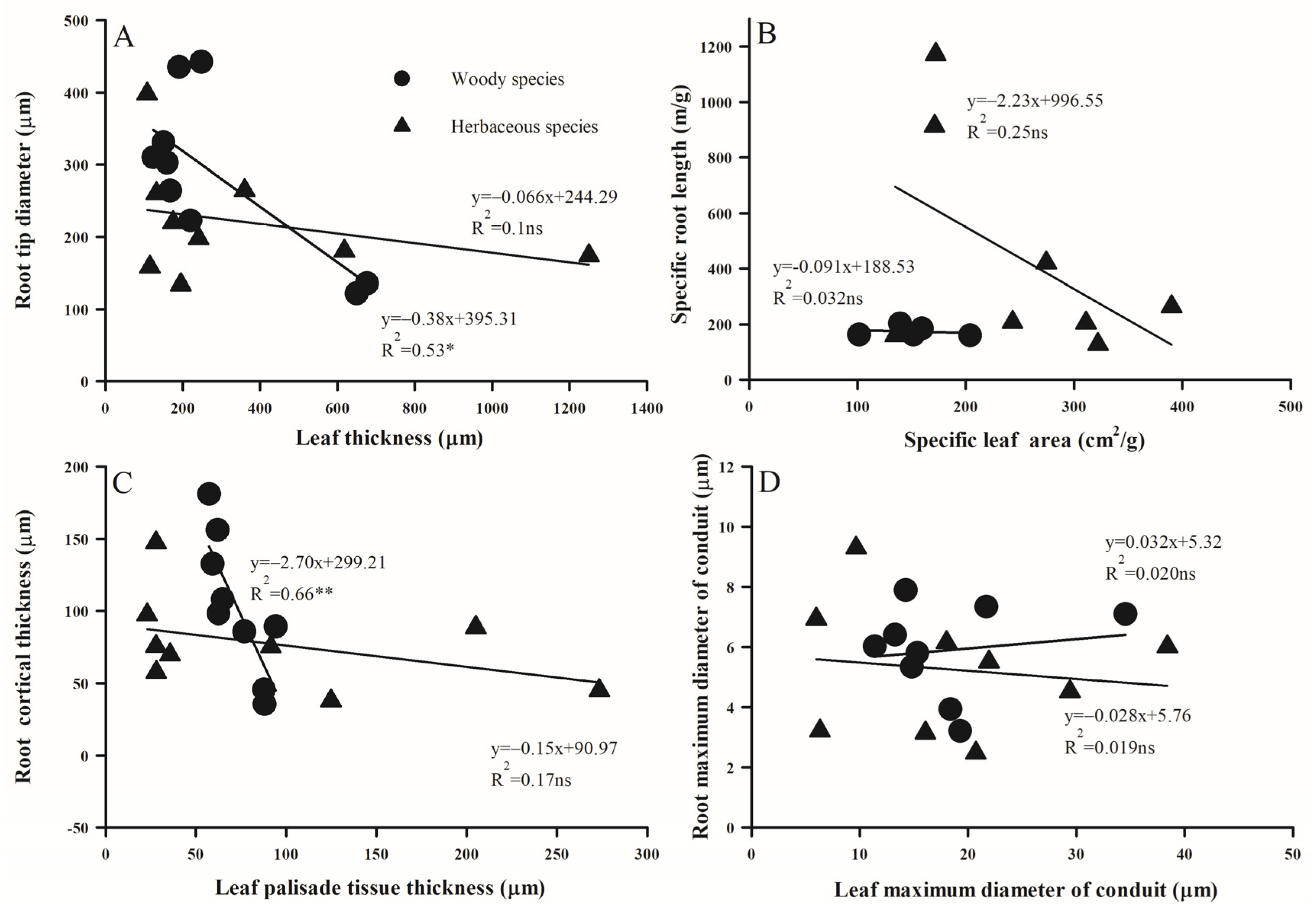
| Woody Species | Herbaceous Species | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Minimum | Maximum | Mean | CV | Minimum | Maximum | Mean | CV | ||
| RD | 0.12 | 0.44 | 0.30 | 0.34 | 0.13 | 0.40 | 0.22 | 0.36 | 0.050 |
| SRL | 33.25 | 526.47 | 220.97 | 0.82 | 88.69 | 1172.04 | 473.24 | 0.81 | 0.025 |
| RTD | 0.15 | 0.31 | 0.24 | 0.27 | 0.07 | 0.16 | 0.11 | 0.37 | 0.001 |
| LT | 150.15 | 675.87 | 286.99 | 0.75 | 107.63 | 1250.46 | 372.55 | 1.05 | 0.326 |
| SLA | 101.43 | 203.86 | 151.10 | 0.25 | 134.70 | 390.06 | 252.35 | 0.35 | 0.018 |
| RCT | 35.38 | 180.83 | 103.44 | 0.46 | 37.90 | 147.40 | 77.14 | 0.42 | 0.054 |
| RST | 40.82 | 111.20 | 76.75 | 0.31 | 28.46 | 89.85 | 50.78 | 0.39 | 0.005 |
| RNC | 4.44 | 30.33 | 9.22 | 0.89 | 4.22 | 16.27 | 8.41 | 0.52 | 0.366 |
| RMC | 3.21 | 7.33 | 5.89 | 0.26 | 2.49 | 9.30 | 5.25 | 0.41 | 0.238 |
| SD | 5.41 | 78.32 | 29.31 | 0.74 | 3.76 | 36.82 | 20.28 | 0.52 | 0.139 |
| GCL | 20.34 | 38.24 | 24.89 | 0.22 | 13.92 | 35.70 | 23.65 | 0.35 | 0.302 |
| VD | 1.37 | 21.91 | 12.46 | 0.68 | 0.90 | 1.71 | 1.25 | 0.22 | 0.001 |
| LPT | 57.29 | 94.22 | 72.52 | 0.20 | 22.89 | 205.10 | 93.03 | 0.98 | 0.265 |
| LST | 24.81 | 75.19 | 45.24 | 0.44 | 23.65 | 94.92 | 56.09 | 0.46 | 0.359 |
| LVT | 37.05 | 747.95 | 224.80 | 1.01 | 41.41 | 246.67 | 162.50 | 0.67 | 0.032 |
| LMC | 11.37 | 21.69 | 18.09 | 0.38 | 5.98 | 29.42 | 18.50 | 0.58 | 0.469 |
| Species | Abbreviation | Life Form | Family |
|---|---|---|---|
| Juniperus chinensis L. | Juch | evergreen tree | Cupressaceae |
| Platycladus orientalis (L.) Franco | Plor | evergreen tree | Cupressaceae |
| Melia azedarach L. | Meaz | deciduous tree | Meliaceae |
| Platanus × acerifolia (Aiton) Willd. | Plac | deciduous tree | Platanaceae |
| Pyrus betulifolia Bunge | Pybe | deciduous tree | Rosaceae |
| Styphnolobium japonicum (L.) Schott | Stja | deciduous tree or shrub | Leguminosae |
| Tamarix austromongolica Nakai | Taau | deciduous tree or shrub | Tamaricaceae |
| Tamarix chinensis Lour. | Tach | deciduous tree or shrub | Tamaricaceae |
| Apocynum venetum L. | Apve | perennial erect subshrub | Apocynaceae |
| Inula britannica L. | Inbr | perennial herb | Asteraceae |
| Lactuca tatarica (L.) C. A. Mey. | Lata | perennial herb | Asteraceae |
| Limonium sinense (Girard) Kuntze | Lisi | perennial herb | Plumbaginaceae |
| Cynanchum rostellatum (Turcz.) Liede & Khanum | Cyro | perennial vine herb | Apocynaceae |
| Glycine soja Siebold & Zucc. | Glso | annual vine herb | Leguminosae |
| Atriplex patens (Litv.) Iljin | Atpa | annual herb | Amaranthaceae |
| Echinochloa crus-galli (L.) P. Beauv. | Eccr | annual herb | Poaceae |
| Euphorbia humifusa Willd. | Euhu | annual herb | Euphorbiaceae |
| Suaeda salsa (L.) Pall. | Susa | annual herb | Amaranthaceae |
| Functional Trait | Abbreviation | Units | Description |
|---|---|---|---|
| Morphology | |||
| Root tip diameter | RD | mm | Average diameter of root tip |
| Specific root length | SRL | m/g | Length per unit dry mass of root tip |
| Root tissue density | RTD | g/cm3 | Mass per unit root volume of root tip |
| Leaf thickness | LT | μm | Average thickness of leaf |
| Specific leaf area | SLA | cm2/g | Area per unit dry mass of leaf |
| Anatomy | |||
| Root cortical thickness | RCT | μm | Average thickness of cortex containing exodermis, endodermis, and cortical parenchyma cells |
| Root stele diameter | RST | μm | Average diameter of vascular cylinder |
| Number of conduits in root | RNC | No. | Number of conduits in the stele |
| Maximum diameter of root conduit | RMC | μm | Mean value of first three largest conduits per root stele |
| Stomatal density | SD | No./mm2 | Total number of stomata per leaf area |
| Guard cell length | GCL | μm | Average length of guard cell in lower leaf epidermis |
| Vein density | VD | mm/mm2 | Total vein length number of stomata per leaf area |
| Palisade tissue thickness | LPT | μm | Average thickness of palisade tissue in leaf cross-section |
| Spongy tissue thickness | LST | μm | Average thickness of spongy tissue in leaf cross-section |
| Leaf vascular tissue thickness | LVT | μm | Average thickness of leaf vascular bundle along the main vein |
| Maximum diameter of leaf conduit | LMC | μm | Mean value of first three largest conduits per leaf vascular bundle |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, H.; Wang, J.; Mu, L.; Li, Z. Leaf and Root Functional Traits of Woody and Herbaceous Halophytes and Their Adaptations in the Yellow River Delta. Plants 2025, 14, 159. https://doi.org/10.3390/plants14020159
Wang Y, Wu H, Wang J, Mu L, Li Z. Leaf and Root Functional Traits of Woody and Herbaceous Halophytes and Their Adaptations in the Yellow River Delta. Plants. 2025; 14(2):159. https://doi.org/10.3390/plants14020159
Chicago/Turabian StyleWang, Yan, Hao Wu, Jian Wang, Liqiang Mu, and Zhongyue Li. 2025. "Leaf and Root Functional Traits of Woody and Herbaceous Halophytes and Their Adaptations in the Yellow River Delta" Plants 14, no. 2: 159. https://doi.org/10.3390/plants14020159
APA StyleWang, Y., Wu, H., Wang, J., Mu, L., & Li, Z. (2025). Leaf and Root Functional Traits of Woody and Herbaceous Halophytes and Their Adaptations in the Yellow River Delta. Plants, 14(2), 159. https://doi.org/10.3390/plants14020159





