Sida L.: Ethnobotany, Pharmacology, and Phytochemistry: A Review
Abstract
1. Introduction
1.1. Malvaceae Family
1.2. Genus Sida L.
1.3. Botanical Description
1.4. Uses
2. Materials and Methods
2.1. Bibliographic Search
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Data Presentation
3. Results
3.1. Botanical Description of Species from the Genus Sida
3.1.1. Sida acuta Burm.f.
3.1.2. Sida ciliaris L.
3.1.3. Sida cordata (Burm f.) Borss. Waalk.
3.1.4. Sida cordifolia L.
3.1.5. Sida cordifolia L. subsp. maculata (Cav.) Marais
3.1.6. Sida glutinosa Comm. ex Cav.
3.1.7. Sida hyssopifolia C. Presl
3.1.8. Sida javensis Cav.
3.1.9. Sida linifolia Juss. ex Cav.
3.1.10. Sida mysorensis Wight & Arn.
3.1.11. Sida planicaulis Cav.
3.1.12. Sida rhombifolia L.
3.1.13. Sida rhombifolia L. var. rhombifolia
3.1.14. Sida rhombifolia subsp. retusa (L.) Borss. Waalk.
3.1.15. Sida santaremensis Monteiro
3.1.16. Sida spinosa L.
3.1.17. Sida tuberculata R.E. Fr.
3.2. Sida Genus Species Names, Uses and Descriptions
3.3. Pharmacological Activities of Some Sida Species
3.4. Phytochemistry
| Species | Scientific Synonym | Common Name | Commercial Uses | Traditional Medicinal Uses and Application Methods | References |
|---|---|---|---|---|---|
| Sida acuta Burm.f. | Sida carpinifolia L.f. Sida carpinifolia Bourg. ex Griseb. Sida planicaulis Cav. | -Vavalisin de Filipinas -Malva del Brasil -Malva de caballo (en Cuba) -Ancoacha del Perú -Pickna del Perú. | N/R | Different parts of the plant are used for: Dandruff Rheumatism Hepatic disorders Kidney stones Nervous disorders Leaves prepared in “juice” are used to: Helminth management Vomiting Gastric problems The roots are employed as a treatment for: Respiratory diseases, such as tuberculosis and cough. Hemorrhoids Kidney inflammation Decrease fever Heart disease To cure the empacho | [32,47,48] |
| Sida ciliaris L. | Sida fulva A.St.-Hil. Sida involucrata A.Rich. Sida muricata Cav. Sida longistipula Merr. Sida plumosa Cav. Sida tridentata Cav. Sida ononidifolia Gand. Sida microtricha Gand. Sida minutifolia Gand. Sida avicularioides Gand. Sida bellidifolia Gand. | -Fan petals with fringes -Fan petals with bangs -Sida with fringes -Sida with bangs -Salmon Sida -Bishop’s cord -Huinar | N/R | This plant is used as a remedy for: Stomach and toothache Antiseptic agent Cure sores and general wounds Pharyngitis | [34,49] |
| Sida cordata (Burm f.) Borss. Waalk. | Sida cordata var. cordata Sida cordata var. nasirii Abedin Lamarkia morifolia Medik. Melochia cordata Burm.f. Sida humilis Cav. Sida humilis var. veronicifolia (Lam.) Mast. Sida mathewsii Turcz. Sida morifolia Cav. Sida multicaulis Cav. Sida pilosa Retz. Sida radicans Cav. Sida retzii J.F.Gmel. Sida unilocularis L’Hér. Sida veronicifolia Lam. Sida veronicifolia var. humulis (Cav.) K.Schum. Sida veronicifolia var. multicaulis (Cav.) Baker f. | In India: -Farid buti -Rajbala -Bhumibala -Shaktibala In Pakistan -Simak | N/R | Root and stem paste of this species is used: To remove pus from boils by external application. In Hindu medicine, is known as Siddha or Ayurveda and the roots are used as: Diuretic Astringent Remedy for stomach problems Febrifuge Demulcent Seeds are administered to serve as: Laxative Aphrodisiac Demulcent It is recommended against: Cystitis Colic Gonorrhea Hemorrhoids Other medicinal uses include: Neurological disorders such as hemiplegia, facial paralysis, sciatica, general weakness, and headache. Urinary problems Tuberculosis Diabetes Fever and rheumatism Uterine disorders | [14,50] |
| Sida cordifolia L. | Malvinda cordifolia (L.) Medik | -Bala -Country mallow -Flannel Sida -Flannel grass -Heart leaf Sida -Sida-White burr | As a forage plant, insulation raw material and cellulose source. | The plant is used as a curative for: Dysentery, diarrhea Bronchial asthma Cold Chills Fever Headache Cough, nasal congestion or wheezing Nasal congestion Edema Weight loss Malaria Urinary problems | [27,51] |
| Sida cordifolia L. subsp. maculata (Cav.) Marais | Sida maculata Cav. | Flannel weed | N/R | It is used in Indian, American, and Chinese medicinal systems to treat conditions such as asthma, gonorrhea, nasal congestion, stomatitis, and inflammatory disorders. | [17,52] |
| Sida hyssopifolia C.Presl | Sida callifera Griseb. Sida collina Schltdl. Sida corymbosa R.E.Fr. Sida costata Schltdl. | Hyssop-leaved sida. Hyssop-leaf sida. Hyssop sida. | Used as animal food. | It is used in traditional medicine for treating diarrhea, dysentery, fevers and inflammations. | [23,39] |
| Sida glutinosa Comm. ex Cav. | S. mysorensis Wt. & Arn. S. glabra | -Smooth fanpetals -Sticky fanpetals -Cuban mallow -“Escobita dulce” | Stems provide textile fiber. | Roots and aerial parts of this plant are useful to treat: Pulmonary tuberculosis Rheumatism | [38] |
| Sida javensis Cav. | Sida pilosa Retz. | -Java golden flower noon (Taiwan) -Java yellow ripening (China) | N/R | This plant is utilized for its medicinal properties, the roots are the key component in treating conditions such as snake bites, rheumatic pains, tuberculosis, and malaria. Also, it is employed as a remedy for boils, fevers, heart disease, and hemorrhoids. Certain regions also use it to manage respiratory illnesses like asthma, pneumonia, and bronchitis | [38,39,53] |
| Sida linifolia Juss. ex Cav. | Malva hirsuta Aubl. Sida angustissima Miq. Sida campii Vell. Sida graminifolia Rich. Sida linearifolia Thonn. Sida longifolia Brandegee. Sida viminea Fisch. ex Link. | -Trebol sabanero (savannah clover) -Tongue of bird -Lancet leaf -Flaxleaf Fanpetals | Remediation of contaminated soils by metals due to their capacity to store nickel. | The infusion made from flowers and leaves is used as: Laxative Stomach reliever | [32,34,54] |
| Sida mysorensis Wight & Arn. | Sida glutinosa Roxb. Sida urticifolia Wight & Arn. Sida wightiana D.Dietr. | -Fan petals from Mysore (India) -Chinese muntjac (species of barking deer, Muntiacus reevesi) | Their fibers serve to reinforce polymeric compounds. | In Ayurvedic medicine, seeds are used to: Improve appetite Maharastra tribes (Indian western peninsular region) use leaves powder to: Heal wounds. | [41] |
| Sida planicaulis Cav. | Malvastrum carpinifolium (Medik.) A.Gray Malvinda carpinifolia Medik. Sida acuta subsp. carpinifolia (Medik.) Borss.Waalk. Sida acuta var. carpinifolia (Medik.) K.Schum. Sida carpinifolia L.f. | In Brazil: guanxuma, chá da Índia, guaxima, malva brava, relógio de vaqueiro, vassoura, vassourinha, douradinha do campo, vassoura tupixá, and tupitixa | It is considered an invasive weed in some areas, as it can negatively affect livestock due to its toxicity. | In Brazil, it is used to treat body pain. In India, it is traditionally used as a tonic, for urinary and blood disorders, and for liver and nervous system issues. | [22] |
| Sida rhombifolia L. | Diadesma rhombifolia Raf. Malva rhombifolia (L.) E.H.L.Krause Napaea rhombifolia (L.) Moench Sida adusta Marais. Sida andicola Gand. Sida arbuscula Zipp. ex Span. Sida canariensis Cav. Sida canescens Cav. Sida compressa DC. Sida forsteri Montrouz. Sida fryxellii Sivar. & Pradeep Sida grata Gand. Sida hondensis Kunth Sida incerta A.St.-Hil. & Naudin Sida insularis Hatus. Sida kohautiana C.Presl. Sida nudata Gand. Sida philippica DC. Sida praelonga Gand. Sida pringlei Gand. Sida recisa Link Sida retusifolia Stokes | -Arrowleaf sida -Big Jack -Cuban jute -Indian Hemp -Paddy lucerne -Queensland hemp -Rhombus-leaved sida -Sida Hemp -Escobilla -Malvilla -Malva amarilla -Tlalamate -Malvavisco -Naranjillo -Oreja de burro | Sourcing of high-quality natural fibers to produce textiles and handicrafts. | In Mexico, it is traditionally used in different states to treat: Oaxaca Rectal baths “Susto” in English scare Epilepsy Nerves Fatigue and weakness in children Hungry Courage or muin Toothache Veracruz The “latido” (heartbeat) Gingivitis Inflamed belly ”Empacho”, diarrhea Cough Hair loss Weakness Morelos Gingivitis Puebla A dough made with leaves are used for: Aphthas (Postemillas) Infusions made from the branches are used for: Stomach pain, gastritis and/or stomach ulcers With the root “agua de tiempo” (water at room temperature) is prepared for: Dysentery A decoction made from branches or leaves for: Washes Macerated plant for: Emplasts Nayarit Macerated with beef fat is placed on a wipe for: Pimples or “born” (boils) Cure wounds as a disinfectant Prepared in other ways, it is used for: Animal bites Anti-crotalic Fever | [34,48,55] |
| Sida rhombifolia subsp. retusa (L.) Borss.Waalk. | Sida rhombifolia var. retusa (L.) Griseb. Sida retusa L. Meximalva retusa (L.) F.C.Ho | Janglimedhi (Hindi); Atibata, Kallam gadale (Kannada); Bala (Sanskrit); Athibala, Bala, Jangli methi (Marathi) | N/R | A decoction derived from the plant’s roots is highly employed by local Ayurvedic practitioners. They use it to treat rheumatism and a range of neurological conditions, such as epilepsy. Furthermore, it acts as a diuretic for urinary calculus issues and as an antipyretic for fevers that involve shivering and convulsions. | [56] |
| Sida rhombifolia L. var. rhombifolia | Sida rhombifolia L. var. rhombifolia Sida rhomboidea Roxb. Sida rhombifolia var. rhomboidea (Roxb.) Mast Sida adjusta Marais Sida alba Cav. Sida canariensis Cav. | Arrow leaf sida, Cuban jute, Indian hemp, Broom weed | N/R | Pounded leaves of the plant are applied as a paste to reduce swelling and as a cure for boils and headaches. Root decoction is taken as tea to treat diarrhea. In India, the plant is used in the treatment of gonorrhea. In Europe it is used as antitubercular agent. Decoction of the plant is used to treat rheumatic pain, cardiac problems and biliary problems in children. Fresh plant juice is used as demulcent and diuretic. | [57,58] |
| Sida santaremensis Monteiro | Sida glaziovii Sida rhombifolia var. Subtomentosa Sida santaremensis var. krapovickasiana | -Moth fanpetals -Brazilian Sida -Guanxuma | N/R | This species is useful to treat: Cough Fever Vascular diseases Hypertension | [45] |
| Sida spinosa L. | Sida angustifolia Lam. Sida spinosa var. angustifolia Sida escobilla | -Escoba dura (Hard broom) -Huinar -Malva de caballo (Horse mallow) -Quesillo | It is used to feed animals as well as for medicinal purposes. | In India it is used to treat: Ulcers Urinary and skin diseases Asthma Snake bites Arthritis Bronchitis Burning sensation Hemorrhoids Fever Weaknesses Decoction made from leaves is given for: Calming bladder and genitourinary tract irritation | [20,27] |
| Sida tuberculata R.E. Fr. | Sida hyssopifolia C. Presl | -Escoba | N/R | Commonly used to treat: Inflammation Diabetes Vascular disorders. Wound healing Against insect bites Analgesic Healing | [34,59,60] |
| Species | Pharmacological Activity | In Vitro Assay | In Vivo Assay | Extract and Plant Tissue | Dose or Concentration | Statistical Significance | Control | Reference |
|---|---|---|---|---|---|---|---|---|
| S. acuta Burm.f. | Antioxidant | Antioxidant enzymes glutathione reductase, superoxide dismutase, catalase and malonylaldehyde quantification to estimate lipid peroxidation in Wistar rats’ plasma. | Liver and kidney damage induced with carbon tetrachloride (3 mL/kg bw) and rifampicn (250 mg/kg bw). | Leaves ethanolic extract, administered o.p. | 50 and 100 mg/kg | p < 0.05. | Silymarin (100 mg/kg bw.). | [61] |
| Antimicrobial | P. falciparum drug sensitivity in vitro assay. | Oleanolic acid and cryptolepine isolated from the EtOAc-soluble fraction of whole-plant hydroalcoholic extract. | 3 µg/mL | N/R | Artemisinin (250 μg/mL). | [62] | ||
| NCCLS microdilution method. | Methanolic from roots. | 1800 μg/mL | p < 0.05 | N/R | [63] | |||
| Standard inhibition zone method against Gram-positive and Gram-negative pathogens. | Aqueous leaves extract. Alkaloid fraction obtained from aqueous leaves extract. | 20, 40 and 60 µg/mL 2000 µg/mL | N/R N/R | Amoxicilin. Penicillin 10 UI, sulfadiazin 0.25 mg and spectinomycin 100 µg. | [63] [64] | |||
| Minimal inhibitory concentration test against Gram-negative bacilli strains. | Leaf ethanolic extract. | 8 mg/mL | p < 0.05 | Gentamicin. | [65] | |||
| Anticarcinogenic | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay against human breast cancer cells, ATCC® HTB-26™ | Methanolic and aqueous aerial parts extracts. | 25, 50, 75, 100 and 1000 µg/mL (MeOH); 100, 250, 500, 750 and 1000 µg/mL (Water). | p < 0.05 | DMSO (0.1%). | [66] | ||
| Anti-lipooxygenase | In vitro enzyme inhibition as well as molecular docking studies. | Crude ethanolic extract from whole plant. | 40 mg/mL. | N/R | Nordihydroguaiaretic acid (100–600 mg/mL) | [67] | ||
| Antiviral | MTT assay against MCF-7 tumor cells compared to a cisplatin reference drug, as well as against the normal LLC-Mk2 cell line. | Methanolic extract from whole plant. | 100 mg/mL | N/R | Cells with no virus (SV, herpes simplex virus; SINV, Sindbis virus or poliovirus type 1), and cells infected with untreated virus. | [68] | ||
| Antihyperglycemic | Mammalian and yeast α-glucosidase inhibition assay. | Acetonic from aerial parts. | 100 µg/mL−1 | p ≤ 0.05 and p ≤ 0.01. | Acarbose and quercetin. | [69] | ||
| Anti-inflammatory | TPA-induced ear edema assay in mice. | Acetonic from aerial parts. | 1 mg/ear | p ≤ 0.05 and p ≤ 0.01. | Indomethacin (0.10 mg/ear). | [69] | ||
| Analgesic | Acetic-acid-induced writhing test and the formalin test in mice. | Aqueous and acetone extract. | 200, 400 y 600 mg/kg. | p ≤ 0.05. | Paracetamol (100 mg/kg). | [70] | ||
| Antinociceptive | Formalin-induced nociception (plantar surface) in mice. | Aqueous and acetone extract. | 200, 400 y 600 mg/kg. | p ≤ 0.05. | Paracetamol (100 mg/kg). | [70] | ||
| Anxiolytic | Elevated plus maze (EPM) and open-field tests (OFT) in CD1 mice. | Ethanolic extract from leaves and stems. | 50, 100, 300, and 500 mg/kg. | p < 0.05. | Diazepam at 1 mg/kg (EPM) as well as 1 and 5 mg/kg (OFT). | [71] | ||
| Antionvulsivant | PTZ-induced seizures (75 mg/kg) in CD1 mice. | Ethanolic extract from leaves and stems. | 500 mg/kg. | p < 0.05. | Diazepam (1 mg/kg). | [71] | ||
| Sedative | Sodium pentobarbital-induced sleeping time test in CD1 mice. | Ethanolic from leaves and stems. | 500 and 1000 mg/kg. | p < 0.05. | Diazepam (1 mg/kg). | [71] | ||
| Antipyretic | Yeast suspension-induced pyrexia in Wistar rats. | Petroleum ether, acetonic, ethanolic, and aqueous from leaves. | 500 mg/kg. | p < 0.05. | Paracetamol (30 mg/kg). | [72] | ||
| S. ciliaris L. | Photoprotective | In vitro solar photoprotection assays to quantify UV radiation. | Hexane, ethanol, and methanol fractions from aerial parts. | 500 and 1000 µg/mL. | N/R | Combination of the p-aminobenzoic acid ester (7% octyl dimethyl p-aminobenzoic acid) and the benzophenone derivative (3% oxybenzone). | [73] | |
| S. cordata (Burm f.) Borss. Waalk. | Antioxidant | In vitro antioxidant assays in blood samples. | STZ-induced diabetes (55 mg/kg−1) in Wistar rats. | Ethanolic extract from aerial parts. | 200 and 400 mg/kg. | p < 0.05. | Glibenclamide (5 mg/kg). | [74] |
| In vitro antioxidant assays. | Methanolic extract from roots. | 10% w/v | N/R | Ascorbic acid and/or Trolox (10–1000 µg/mL). | [75] | |||
| In vitro antioxidant, anti-lipid peroxidation, and phosphomolybdate assays in blood, pancreas, liver, and testes homogenates. | Alloxan-induced diabetes in Sprague–Dawley rats | Methanolic extract from the entire plant and its hexane, ethyl acetate, n-butanol, and aqueous fractions. | 150 and 300 mg/kg. | p < 0.05. | Glibenclamide (5 mg/kg). | [76] | ||
| Nephroprotective | In vitro antioxidant enzymes activities quantification and lipid peroxidation (TBARS) in renal homogenates. | CCl4-induced toxicity model (1 mL/kg) in Sprague–Dawley rats. | Ethyl acetate fraction derived from the whole plant methanolic extract. | 150 and 300 mg/kg. | p < 0.05. | Silymarin | [77] | |
| Antidiabetic | Alloxan-induced diabetes in Sprague–Dawley rats. | Ethyl acetate fraction isolated from the whole-plant methanolic extract. | 150 and 300 mg/kg. | p < 0.05. | Glibenclamide (5 mg/kg). | [76] | ||
| Anti-lipoxygenase | In vitro enzyme inhibition as well as molecular docking studies. | Crude ethanolic extract from whole plant. | 40 mg/mL. | N/R | Nordihydroguaiaretic acid (100–600 mg/mL). | [67] | ||
| Gastroprotective | Indomethacin-induced gastric ulcer in rats. | Ethanolic extract from leaves. | 200 y 400 mg/kg. | p < 0.001. | N/R | [78] | ||
| Anti-asthmatic | Isolated goat tracheal chain preparation. | Clonidine-induced catalepsy in mice. | Ethanolic extract from leaves. | 100, 200 and 400 mg/kg. | p < 0.001. | Histamine at 100 µg/mL (in vitro) and chlorpheniramine maleate (in vivo). | [78] | |
| Antibacterial | S. aureus, E. coli and P. aeruginosa growth inhibition assays in cultures. | Ethanolic, aqueous and chloroformic from roots. | 200 y 400 mg/kg. | p < 0.001/p < 0.01. | Ampicillin | [79] | ||
| Hepatoprotective | In vitro assessment of liver markers. Lipid peroxidation, and estimation of serum enzymes and bilirubin. | CCl4-induced acute liver toxicity in rats | Hydroalcoholic extract from leaves. | 100, 200, and 400 mg/kg. | p < 0.05 or p < 0.001. | Silymarin (100 mg/kg). | [80] | |
| S. cordifolia L. | Antioxidant | DPPH and FRAP (ferric reducing antioxidant power) free radical scavenging in vitro assays. | Methanolic extract from roots. | 10% w/v. | N/R | Ascorbic acid and/or Trolox (10–1000 µg/mL). | [75] | |
| In vitro DPPH assay. | In vivo methods in wild-type S. cerevisiae BY 4743 (WT) and knock-out strain (Δtrx2) against H2O2-induced stress mediated damages. | Ethyl acetate, methanol, and water extracts from aerial parts. | 100 µL (in vitro) and (0.4, 0.8, 1.6 mg/m (in vivo). | p < 0.01 and p < 0.001. | Ascorbic acid (10 mM in vivo and 0.2–1.0 mg/mL in vitro). | [81] | ||
| Antidiarrheal | Castor oil-induced diarrhea, magnesium sulphate-induced diarrhea in Wister albino rats. | Hydroalcoholic extracts from roots. | 100, 200, and 400 mg/kg. | p < 0.001. | Loperamide (5 mg/kg). | [82] | ||
| Wound healing | Dexamethasone-induced (1 mg/kg) retardation of wound healing in rats. | Different hydrogel formulation mixed with ethyl acetate, methanol, or aqueous extracts from aerial parts. | 2.5% | p < 0.01 and p < 0.05. | SSDeeUltra (silver sulphadiazine/chlorhexidine gluconate/Aloe vera) | [81] | ||
| Analgesic | Acetic acid-induced pain model and hot plate method in mice. | Ethyl acetate and methanol extracts from aerial parts. | 150, 300, and 600 mg/kg. | p < 0.001. | Aspirin (100 mg/kg) and morphine (4 mg/kg). | [51] | ||
| Acetic-acid-induced writhing test and the formalin test in mice. | Aqueous and acetone extract. | 200, 400, and 600 mg/kg. | p ≤ 0.05. | Paracetamol (100 mg/kg). | [70] | |||
| Anti-lipoxygenase | In vitro enzyme inhibition and molecular docking studies. | Crude ethanolic extract from whole plant. | 40 mg/mL. | N/R | Nordihydroguaiaretic acid (100–600 mg/mL). | [67] | ||
| Anti-inflammatory | Carrageenan-induced paw edema in rats. | Ethyl acetate and methanol extracts from aerial parts and roots. | 150, 300, 600 mg/kg. | p < 0.001. | Indomethacin (6 mg/kg). | [51] | ||
| Quinolinic-acid-induced neurotoxicity (55 µg/100 g bwt/day) in rats. | Ethanolic extract from roots. | 50 mg/100 g bwt/day. | p < 0.05. | Deprenyl (100 µg/100 g bwt/day). | [83] | |||
| Antibacterial | Minimum inhibitory/bactericidal concentrations (MIC/MBCs) for Gram-positive and negative bacterial strains assay. | Hexane, chloroform, methanol, and aqueous crude extracts from roots. | 8000 to 0.003 μg/mL | N/R | Methicillin-resistant S. aureus and S. epidermidis. | [84] | ||
| Hypoglycemic | Glucose tolerance test in 18 h fasted rats. | Methanol extract from aerial parts and roots. | 600 mg/kg | p < 0.05. | Normal rats. | [51] | ||
| Antidiabetic | STZ-induced diabetes (55 mg/kg−1) in Wistar rats. | Ethanol extract from aerial parts. | 200 and 400 mg/kg. | p < 0.05. | Glibenclamide (5 mg/kg). | [74] | ||
| Antiperoxidative | In vitro hydroperoxide estimation by Mair and Hall’s method in quinolinic-acid-induced neurotoxicity in rats. | Ethanolic extract from roots. | 50 mg/100 g bwt/day. | p < 0.05. | Deprenyl (100 µg/100 g bwt/day). | [83] | ||
| Anti-arthritic | Patients with knee osteoarthritis (joints) administered with Ayurvedic preparation for 30 days (o.p). | Bala moola churna ksheerapaka (medicated milk) from roots Ayurvedic medicine. | 80 mL | p < 0.05. | N/R | [85] | ||
| Antihyperlipidemic | Tissue damage biomarker estimation (total cholesterol, triglycerides, low density lipids, plasma creatinine, plasma urea nitrogen). | Ethanol extract from aerial parts. | 200 y 400 mg/kg. | p < 0.05. | Glibenclamide (5 mg/kg). | [74] | ||
| Immunomodulatory | LPS-induced cytokine expression estimation on splenocytes, macrophages and RAW 264.7. | Aqueous extract from roots. | 100 ng/mL. | p < 0.001. | LPS-untreated cells. | [86] | ||
| Cytotoxic | MTT assay on human breast cancer (MCF7), ovarian cancer (PA1), colon cancer (HT29), melanoma (A375), liver cancer (HepG2), and normal mouse embryonic fibroblast (NIH3T3) cell lines. | Ethanolic extract. | 3.125, 6.25, 12.5, 25, 50 and 100 μg/mL | p < 0.05. | Cisplatin and 5-Fluorouracil for HT29 cells, both at (3.125, 6.25, 12.5, 25, 50, and 100 μg/mL). | [86] | ||
| S. cordifolia L. subsp. maculata (Cav.) Marais | Antioxidant | Free radical scavenging (DPPH) assay. | Ethanol extract from whole plant and its hexane, chloroform, ethyl acetate, butanol fractions isolated. | Different concentrations within the range of 24.0 to 143.0 μg/mL. | p < 0.05. | Ascorbic acid and BHT with EC50 of 14.08 and 20.26 µM, respectively. | [87] | |
| S. glutinosa Comm. ex Cav. | Antioxidant | Free radical scavenging (DPPH) assay. | Glutinoside, 24(28)-dehydromakisterone A and chrysin isolated from the butanolic fraction obtained from the aerial parts. | 1, 5, 10, 25, 50, 75 µg/mL. | N/R | BHT (di-tert-butylhydroxytoluene) at 50 and 100 µg/mL. | [88] | |
| Antifungal | Growth inhibition test on F. oxysporum strain medium microdilution in 96-well plates. | Pentyl-10,12 dimethyl-11-hydroxyoleate and kaempferol-5-O-β-D-(6”-O-trans coumaroyl)- glucopiranoside isolated from methanolic aerial parts extract. | 100 μg/disc. | N/R | N/R | [89] | ||
| Hepatoprotective | Estimation of glutamic-oxaloacetic transaminase, glutamic-pyruvic transaminase, alkaline phos-phatase, as well as glycerol kinase enzymes inhibition. | Glutinoside and 24(28)-dehydromakisterone A isolated from the aerial parts methanolic extract. | 5, 10, 15, 20, and 25 µg/µL. | p ≤ 0.05. | Atorvastatin (10 µg/mL). | [88] | ||
| Antibacterial | Kirby–Bauer technique against E. coli and B. subtilis strains. | Glutinoside and 24(28)-dehydromakisterone A isolated from the aerial parts methanolic extract. | 10 µg/µL. | N/R | Gentamicin (10 µg/µL). | [90] | ||
| S. hyssopifolia C. Presl | Antihemorrhagic | Tail cut bleeding induction model in rats. | Methanolic of leaves. | 250, 500, and 1000 mg/kg. | p ≤ 0.05/p < 0.01. | N/R | [91] | |
| Uterotonic | In vitro model of collagen-induced contraction in human uterine cells. | Aqueous from the whole plant | 100–400 µg/mL. | p < 0.05. | Oxytocin (100 nM). | [92] | ||
| Anti-ulcerogenic | Ethanol- and Diclofenac-induced gastric ulcer models in rats. | Aqueous leaf extract. | 250, 500, and 1000 mg/kg. | p < 0.05. | Omeprazole (20 mg/kg). | [93] | ||
| Wound healing | Wound excision model in Wistar rats. | Cream formulates with aqueous leaf extract. | 2.5%, 5%, and 10%. | p < 0.05, p < 0.01, and p < 0.001. | N/R | [93] | ||
| Anti-inflammatory | Albumin-induced paw edema in rats. | Aqueous leaf extract. | 250, 500, 1000 mg/kg. | p < 0.05, p < 0.01, and p < 0.001. | Naprozen (5 mg/kg). | [93] | ||
| S. linifolia Juss. ex Cav | Anti-inflammatory | Protease, platelet aggregation, phospho-lipase A2 inhibition assays, albumin denaturation, membrane stabilization and heat-induced hemolysis in human red blood cells. | Ethanolic extract from leaf. | 0.1, 0.2, 0.4, 0.6, and 0.8 mg/mL | p < 0.05. | Diclofenac, aspirin, and prednisolone. | [94] | |
| Antimalarial | Malaria-infected mouse model. | Ethanolic leaf extract. | 100, 200, and 400 mg/kg. | p < 0.05. | Artesunate (80 mg/kg). | [94] | ||
| Carrageenan and albumin-induced paw edema, formalin-induced arthritis tests in mice. | Ethanolic leaf extract. | 200, 400, and 600 mg/kg. | p < 0.05. | Aspirin (100 mg/kg). | [95] | |||
| Antinociceptive | Formalin-induced arthritis and acetic acid-induced writhing tests in mice. | Ethanolic leaf extract. | 200, 400, and 600 mg/kg. | p < 0.05 | Aspirin (100 mg/kg). | [95] | ||
| Antioxidant | DPPH, ferric reducing power (FRAP), and total antioxidant capacity (TAC) assays; nitric oxide. | Ethanolic leaf fraction extract. | 0.2, 0.4, 0.6, 0.8, and 1.0 mg/mL | p < 0.05. | Gallic acid, butylated hydroxytoluene, and ascorbic acid. | [95] | ||
| Sida javensis Cav. | Schistosomicidal | Schistosoma mansoni-infected mice model. | Aqueous from the whole plant. | 40, 80, and 160 mg/kg. | p < 0.05. | Praziquantel (100 mg/kg). | [96] | |
| n-Butanol fraction from aerial aqueous extract. | 8, 4, 2, 1, 0.5, and 0.25 mg/mL. | p < 0.05. | Penicilin (10,000 U/mL), streptomicyn (10,000 µg/mL), and amphotericin (25 µg/mL). | [97] | ||||
| Toxicological safety | Hematological and biochemical analysis on mice blood samples. | Schistosoma mansoni-infected mice model. | Aqueous from the whole plant. | Unique dose of 4, 8,12, 16, or 20 g/kg (oral acute); 400, 800, or 1600 mg/kg/8 d (sub-acute oral). | p < 0.05. | N/R | [96] | |
| Antioxidant | Radical scavenging DPPH assay. | Aqueous aerial extract and the ethyl acetate fraction obtained from it. | Five concentrations in the range of 25 to 200 μg/mL. | p < 0.05. | Rutin (2.5, 10, 15, 20, and 25 μg/mL). | [97] | ||
| Hepatoprotective | Biochemical analysis to estimate the levels of malondialdehyde, lipid hydroperoxides, eosinophil peroxidase, myeloperoxidase, CAT, and SOD in mice liver homogenates. | Schistosoma mansoni-infected mice model. | Aqueous aerial extract (SpAE) and the n-butanol fraction (SpBF) obtained from it. | 100, 200, and 400 mg/kg (SpAE), and 50, 100, and 200 mg/kg (SpBF). | p < 0.001. | Praziquantel (100 mg/kg). | [98] | |
| Antifibrotic | Determination of hepatic hydroxyproline and γ-interferon. | Schistosoma mansoni-infected mice model. | SpAE and SpBF. | 100, 200, and 400 mg/kg (SpAE), and 50, 100, and 200 mg/kg (SpBF). | p < 0.05 and p < 0.001. | Praziquantel (100 mg/kg). | [98] | |
| S. mysorensis Wight & Arn. | Antioxidant | DPPH and FRAP (ferric reducing antioxidant power) free radical scavenging in vitro assays. | Methanolic from roots. | 10% w/v | N/R | Ascorbic acid and/or Trolox (10–1000 µg/mL). | [75] | |
| Lipoxygenase inhibition | In vitro enzymatic inhibition assays. | Whole-plant crude ethanolic extract. | 40 mg/mL. | N/R | Nordihydroguaiaretic acid (100–600 mg/mL). | [67] | ||
| S. planicaulis Cav | Cytotoxic and genotoxic | MTT cell viability test in SH-5YSY cells. | Ethanolic leaf extracts. | 4000, 2000, 1000, 500, 250, 125, 62.5, 31.25, 15.62, or 7 μg/mL. | p < 0.05. | DMSO (20%) and H2O2 (2 mM). | [99] | |
| S. rhombifolia L. | Antioxidant | DPPH and FRAP in vitro assays. | Methanolic from roots. | 10% w/v | N/R | Ascorbic acid and/or Trolox (10–1000 µg/mL). | [75] | |
| TLC-Bioautography method with DPPH reagent. | Methanol from leaves, stems, and roots. | 10 mg/mL. | N/R | N/R | [100] | |||
| DPPH, ABTS•+ and FRAP in vitro assays. | Volatile organic compounds isolated from leaves and stem of the plant. | 8 mg/mL. | N/R | BHT and Trolox. | [101] | |||
| DPPH in vitro assays. | Ethanolic from aerial parts. | 200 mg/kg. | N/R | Ascorbic acid. | [102] | |||
| Antihyperglycemic | Quantification of mammalian and yeast α-glucosidase enzyme activity. | Acetonic from aerial parts, trans-ferulate p-hydroxyphenethyl, and β-sitosteryl gluco-pyranosyl isolated from it. | 100 µg/mL−1 | p ≤ 0.05 and p ≤ 0.01. | Acarbose and quercetin. | [69] | ||
| Anti-inflammatory | TPA-induced ear edema in mice. | p-hydroxyphenethyl trans-ferulate isolated from the hexanic aerial parts extract. | 1 µmol/ear | p ≤ 0.05 and p ≤ 0.01. | Indomethacin (1 mg/ear). | [69] | ||
| LPS-induced periapical inflammation in rats. | Ethanolic from roots. | 0.6, 1.2, and 2.4 g/kg. | p < 0.05. | Diclofenac (1.2 g/kg). | [103] | |||
| Nitric oxide inhibition in LPS-treated RAW 264.7 cell cultures. | Hexanic from the whole plant. | 100 μg/mL. | p < 0.05. | A fresh culture medium. | [104] | |||
| Carrageenin-induced paw edema in rat. | Ethanolic from aerial parts. | 200 mg/kg. | p ≤ 0.05. | Indomethacin (10 mg/kg). | [102] | |||
| Antinociceptive | Acetic acid-induced writhing and hot plate tests in mice. | Ethanolic and acetonic from aerial parts. | 200 mg/kg. | p ≤ 0.05, p ≤ 0.01, or p ≤ 0.001. | Indomethacin (5 mg/kg) and morphine (10 mg/kg). | [102] | ||
| Anti- cholinesterase | Acetylcholinesterase enzyme activity quantification assay. | Hexanic of the whole plant. | 100 μg/mL. | p < 0.05. | Tacrine. | [104] | ||
| Cytotoxic | Methyl thiazole tetrazolium (MTT) reduction assay against cancer cell lines. | Hexanic of the whole plant. | 100 μg/mL. | p < 0.05. | N/R | [104] | ||
| Vasorelaxant | Percent relaxation assay in rodent mesenteric arteries precontracted with phenylephrine. | Quindolinone and the salt of cryptolepine isolated from aerial parts ethanolic extract. | 10−12–10−3 M. | p < 0.05. | N/R | [105] | ||
| Antifungal | Determination of minimum inhibitory concentration. | 10-methylcryptolepinone and 10-ethylcryptolepinone isolated from the ethanol crude extract. | 64, 256 and 512 μg/mL−1. | N/R | Amphotericin B (32 μg/mL−1). | [106] | ||
| Proapoptotic | Flow cytometry analysis and real-time PCR assay in HepG2 cells. | EtOAc extract of the leaf. | 300 μg/mL. | p < 0.01 and p < 0.001. | Cells without extract treatment. | [107] | ||
| Antiproliferative | MTT assay against HepG2 cells. | EtOAc extract of the leaf. | 364.3 μg/mL. | p < 0.01 and p < 0.001. | Cells without extract treatment. | [107] | ||
| Antidiarrheal | Castor oil-induced diarrhoea, Castor oil-induced enteropooling and gastrointestinal motility tests in rat. | Methanolic root extract. | 200 and 400 mg/kg. | p < 0.05, p < 0.01, and p < 0.001. | Diphenoxylate (50 mg/kg) and Atropine sulphate (0.1 mg/kg). | [108] | ||
| Antibacterial | Determination of minimum inhibitory concentration. | 10-methylcryptolepinone and 10-ethylcryptolepinone isolated from the ethanol crude extract. | N/R | N/R | Gentamicin (64 μg/mL−1). | [106] | ||
| Disk diffusion antimicrobial assay. | Methanolic root extract. | 50 mg/mL. | N/R | Ciprofloxacin. | [109] | |||
| Determination of zone of inhibition. | Aqueous-methanol aerial part extract. | 250 and 500 mg/mL. | p < 0.05. | Chloroamphinicole (30 μg/mL). | [110] | |||
| Anxiolytic | Elevatedplus maze model in mice. | Ethanolic extract of the whole plant. | 300 mg/kg. | p < 0.01. | Diazepam (2 mg/kg). | [111] | ||
| Nephroprotective | Biochemical analysis of diabetic rat blood samples. | NAD and STZ-induced diabetic nephropathy in rats. | Aerial parts hydroalcoholic extract. | 200 mg/kg. | p < 0.05. | Pioglitazone (10 mg/kg). | [112] | |
| S. rhombifolia subsp. retusa (L.) Borss.Waalk. | Antioxidant | DPPH and FRAP (ferric reducing antioxidant power) free radical scavenging in vitro assays. | Methanolic from roots. | 10% w/v | N/R | Ascorbic acid and/or Trolox (10–1000 µg/mL). | [75] | |
| Hypnotic/sedative | Pentobarbital-induced hypnosis in mice. | Crude aqueous extract from roots. | 3, 10, and 15 g/kg. | p < 0.01 and p < 0.001. | Chlorpromazine (10 mg/kg). | [76] | ||
| Antipyretic | Yeast-induced pyrexia in rat. | Crude aqueous extract from roots. | 5 and 10 g/kg. | p < 0.01 and p < 0.001. | Acetyl salicylic acid (150 mg/kg). | [76] | ||
| Hypoglycemic | Streptozotocin (STZ)-induced diabetes in rats. | Aqueous extract of leaves. | 200 and 300 mg/kg. | p < 0.05. | Glibenclamide (10 mg/kg). | [113] | ||
| Hypolipidemic | Biochemical analysis of blood samples from mice receiving treatment for 14 days. | Aqueous extract of leaves. | 200 mg/kg. | p < 0.05. | Fenofibrate (100 mg/kg). | [113] | ||
| Anticancer | Diethylnitrosamine-induced preneoplasia in rats. | Methanolic seed extract | 50 and 100 mg/kg. | p < 0.05 and p < 0.001. | N/R | [114] | ||
| S. rhombifolia L. var. rhombifolia | Antioxidant | Metal iron chelating, DPPH, TEAC, H2O2, O2, HO· and NO· radical scavenging activities in vitro assays. | Methanol from aerial parts. | 50, 100, 200, 500, and 800 µg/mL. | p < 0.05. | Ascorbic acid | [115] | |
| Antinociceptive | Acetic acid-induced writhing test in rats. | Ethyl acetate from leaves. | 200 mg/kg. | p < 0.01. | Acetylsalicylic acid (100 mg/kg). | [116] | ||
| Anti-inflammatory | Carrageenin-induced edema in rat paw. | Butanolic leaves extract. | 200 mg/kg. | p < 0.001. | Phenylbutazone (100 mg/kg), | [116] | ||
| S. santaremensis Monteiro | Immunomodulatory | LPS-stimulated mice macrophages. | kaempferol 3-O-β-D-glucosyl-6’’-α-L-rhamnoside isolated from ethanolic aerial parts extract. | 3.125 and 100 μM | p < 0.01 and p < 0.001. | Non LPS-stimulated macrophages. | [117] | |
| Antileishmania | Evaluation of the leishmanicidal activity in vitro. | kaempferol 3-O-β-D-glucosyl-6’’-α-L-rhamnoside isolated from ethanolic aerial parts extract. | 6.25–800 μg/mL−1 | p < 0.01 and p < 0.001. | Amphotericin B (2 μg/mL). | [117] | ||
| Vasorelaxant | Precontractions induced with L-phenylephrine hydrochloride (Phe) or KCl and in vitro evaluation of endothelial-derived factors associated with vasorelaxation on rat superior mesenteric artery rings. | Ethanolic aerial parts extract. | (10−9 to 10−5 mol/L). | p < 0.01 and p < 0.001. | L-NAME (100 µmol/L); indomethacin (10 µmol/L) and atropine (1 nmol/L). | [45] | ||
| S. spinosa L. | Antipyretic | Yeast-induced pyrexia in rats. | Aqueous from root. | 400 mg/kg. | p < 0.01. | Aspirin. | [118] | |
| Antimicrobial | Antimicrobial activity through agar diffusion technique. | Aqueous from root. | 50 and 75 μL. | N/R | N/R | [118] | ||
| Antimicrobial activity through agar diffusion technique. | Ethanolic extract of whole plant. | 100, 200, 300, 400, and 500 μg/disc. | N/R | Ciprofloxacin (5 μg/disc). | [119] | |||
| Determination of minimum inhibitory concentration. | Ethanolic leaf extract | 50, 100, 200, 300, 400, and 500 μg/disc. | N/R | Ciprofloxacin (5 µg/disc) and Amphotericin B (30 µg/disc). | [120] | |||
| Anthelmintic | Anthelmintic screening using Indian adult earthworm (Pheretima posthuma). | Ethanolic extracts of leaves. | 25, 50, 100 mg/mL. | p < 0.05. | Mebendazole (25, 50,100 mg/mL). | [121] | ||
| S. tuberculata R.E. Fr. | Anti-inflammatory | Carrageenan-induced peritonitis model. | Methanolic extract from leaves. | 10–300 mg/kg−1 | p < 0.05, p < 0.01, and p < 0.001. | Dexamethasone (0.5 mg/kg−1). | [122] | |
| In vitro assays to measure cytokine levels on rats’ knees with monosodium iodoacetate (MIA)-induced knee osteoarthritis. | Aqueous from leaves and roots plus photobiomodulation therapy (PBMT; 904 nm, 18 J/cm2). | 5 mg/mL. | p < 0.05, p < 0.01, and p < 0.001. | Diclofenac (10 mg/kg). | [123] | |||
| Antitumor | Cell viability assay against HepG2 and MCF-7 (tumor cell lines). | Methanolic extract from leaves and roots. | 543.6–593.4 μg/mL−1. | p < 0.05. | Human leukocytes (non-malignant cell line). | [122] | ||
| Anti-nociceptive | Acetic acid-induced abdominal writhes and formalin model in mice. | Methanolic extract from leaves. | 100 mg kg−1. | p < 0.001. | Naloxone (1 mg kg−1). | [59] | ||
| Antioxidant | DPPH, ABTS+, Nitrogen derivative species radical scavenging activities, TBARS, Deoxyribose and FRAP in vitro assays. | Hydroethanolic extracts from leaves. | 0.015 mg/mL−1. | p < 0.05. | N/R | [59] | ||
| In vitro biochemical analyses of samples from rats with osteoarthritis induced by monosodium iodoacetate (MIA). | Infusion from leaves. | 30 mg/mL. | p < 0.05. | Diclofenac (10 mg/kg). | [124] |
| Isolated Compound | Sida Species | Extract and Plant Part | Reference | Pharmacological Activities | Reference |
|---|---|---|---|---|---|
| Alkaloids | |||||
| Cryptolepine | S. acuta Burm.f. and S. rhombifolia L. | Whole-plant hydroethanolic extract. | [62] | BACE1 and Aβ inhibition, anti- Alzheimer, antitumor, decreases topoisomerase I and II activities, vasorelaxant, antiproliferative, proapoptotic, antibacterial, among others. | [105,125] |
| S. rhombifolia L. | Total alkaloidal fraction obtained from a crude ethanolic extract of aerial parts. | [106] | |||
| Raw material and derived herbal preparations. | [126] | ||||
| S. spinosa L. | Petroleum-ether and ethanol extracts of aerial parts and roots. | [127,128] | |||
| Vasicine | S. cordata (Burm f.) Borss. Waalk. | Ethyl acetate, ethanol, aqueous, and chloroform extracts from leaves and stems. | [129] | Anticholinesterase, anti-allergic, antibacterial, anti-inflammatory, uterotonic, cardioprotective, antiasthmatic, antioxidant, among others. | [130] |
| S. cordifolia L. | Water-soluble alkaloid fraction from roots. | [131] | |||
| S. rhombifolia L. | Raw material and derived herbal preparations. | [126] | |||
| S. spinosa L. | Petroleum-ether and ethanol extracts of aerial parts and roots. | [127,128] | |||
| S. tuberculata R.E.Fr. | Methanolic extracts of leaves and roots. | [60] | |||
| S. acuta, S. rhombifolia subsp. retusa, S. spinosa L., S. cordata (Burm f.) Borss, S. cordifolia L. | Methanolic root extracts | [132] | |||
| Vasicinone | S. cordata (Burm f.) Borss. Waalk. | Ethyl acetate, ethanol, aqueous, and chloroform extracts from leaves and stems. | [129] | Antioxidant, anti-inflammatory, neuroprotective, cytotoxic, anticancer, and proapoptotic. | [130] |
| S. cordifolia L. | Methanolic root extracts. | [132] | |||
| S. rhombifolia L. | Raw material and derived herbal preparations. | [128] | |||
| S. spinosa L. | Petroleum-ether and ethanol extracts of aerial parts and roots. | [127,128] | |||
| Vasicinol | Antibacterial, anti-inflammatory, antioxidant, anticholinesterase, and sucrase-inhibitory effects. | [130,133] | |||
| S. rhombifolia L. | Raw material and derived herbal preparations. | [126] | |||
| S. cordifolia L. | Benzene extract of the air-dried powdered root. | [17] | |||
| 10 methylcryptolepinone 10-ethylcryptolepinone | Sida rhombifolia L. | Total alkaloidal fraction obtained from a crude ethanolic extract of aerial parts. | [106] | Antifungal. | [106] |
| Ephedrine | S. cordata (Burm f.) Borss. Waalk. | Ethyl acetate, ethanol, aqueous, and chloroform extracts from leaves and stems. | [129] | Stimulates alpha- and beta-adrenergic receptors; bronchodilator; decongestant; increases arterial blood pressure, cardiac index, stroke volume, and systemic vascular resistance. | [134] |
| S. cordifolia L. | Water-soluble alkaloid fraction from roots. | [131,135,136] | |||
| Methanolic root and aerial extracts. | |||||
| Hydroalcoholic aerial parts extracts. | |||||
| S. rhombifolia L. | Hydroalcoholic aerial parts extracts. | [136] | |||
| S. spinosa L. | Ethanolic extract of the whole plant. | [137] | |||
| Ψ-ephedrine | S. cordifolia L. | Water-soluble alkaloid fraction from roots. | [131] | Stimulates alpha-adrenergic receptors; increases breathing rate and blood pressure; accelerates heart rate; causes bronchodilatation; raises blood glucose levels; stimulates the CNS; and produces a sense of increased energy and improved mood. | [138] |
| S. rhombifolia L. | Hydroalcoholic aerial parts extracts. | [139] | |||
| S. spinosa L. | Ethanolic extract of the whole plant. | [137] | |||
| Petroleum-ether and ethanol extracts of aerial parts and roots. | [128] | ||||
| N-methyl ephedrine and N-methyl pseudoephedrine | S. cordata (Burm f.) Borss. Waalk. | Ethyl acetate, ethanol, aqueous, and chloroform extracts from leaves and stems. | [131] | ||
| Hypaphorine | S. cordifolia L. | Benzene extract of the air-dried powdered root. | [17] | Increases non-rapid-eye-movement sleep time; anti-inflammatory. | [140] |
| Crude extract from the leaves. | [141] | ||||
| S. spinosa L. | Aqueous and ethanolic extracts of leaves. | [127,128] | |||
| Flavonoids | |||||
| Quercetin 3-(2Gxylosylrutinoside) | S. rhombifolia L. S. acuta Burm.f. | Petroleum ether, chloroform, acetone, ethanolic, and aqueous extracts from leaves. | [142] | Anti-neuroinflammatory effects against LPS-induced damage in N9 cells in vitro. | [143] |
| Manghaslin | S. rhombifolia L. and S. acuta Burm.f. | Cytotoxic activity on T24 and MRC5 cells. | [144] | ||
| Myricetin 7-Rhamnoside | S. rhombifolia L. S. acuta Burm.f. | Anti-photoaging, anticancer, antihypertensive, immunomodulatory, anti-inflammatory, antiallergic, and analgesic. | [145] | ||
| Isorhamnetin 3-O-[b-D-glucopyranosyl-(1->2)-a L-rhamnopyranoside] | S. rhombifolia L. S. acuta Burm.f. | Inhibition of AChE, α-amylase, and α-glycosidase enzymes; antioxidant, anti-Alzheimer, antidiabetic, and cytotoxic effects. | [146] | ||
| Peltatoside | S. rhombifolia L. S. acuta Burm.f. | Anti-inflammatory and antinociceptive activities. | [147] | ||
| Quercimeritrin | S. rhombifolia L. S. acuta Burm.f. | Antioxidant, vasorelaxant, and α-glucosidase enzyme inhibition. | [144] | ||
| Rutin | S. rhombifolia L. S. acuta Burm.f. | Antioxidant, anti-inflammatory, antidiabetic, antiapoptotic, neuroprotective, nephroprotective, and hepatoprotective, among others | [148] | ||
| S. rhombifolia L. | Hydroalcoholic crude extract from leaves. | [149] | |||
| S. acuta Burm.f. | Ethanolic leaf extract. | [150] | |||
| Kaempferol | S. acuta Burm.f. | Ethanolic leaf extract. | [150] | Antioxidant, anti-inflammatory cardioprotective, neuroprotective, hepatoprotective, antidiabetic; promotes eye, skin, and respiratory system health. | [151] |
| S. rhombifolia L. | Crude ethanolic extract from aerial parts. | [105] | |||
| Kaempferol-3-(6-p-Coumaroyl) glucopyranoside. | S. tuberculata R.E.Fr. | Methanolic extracts of leaves and roots. | [60] | Antioxidant and proapoptotic activities. | [152] |
| Kaempferol-3-O-β-D-glucose-6″-α-D-rhamnose | S. rhombifolia L. | Crude ethanolic extract from aerial parts. | [105] | ||
| Tiliroside | S. rhombifolia L. | Whol-plant hydroethanolic extract. | [62] | Antioxidant, anti-obesity, anti-diabetic, anti-inflammatory, and analgesic. | [152] |
| Quercetin | S. cordifolia L. | Petroleum ether and ethanol extracts from leaves. | [152] | Antioxidant, vasorelaxant, anti-inflammatory, neuroprotective, and inhibits the α-glucosidase enzyme. | [153] |
| S. acuta Burm.f. | Ethanolic leaf extract | [150] | |||
| S. linifolia Juss. ex Cav | Ethanolic leaf fraction. | [150] | |||
| S. rhombifolia L. | Ethanolic leaves and stem extracts | [153] | |||
| Isorhamnetin 3-O-[b-D-glucopyranosyl-(1->2)-a L-rhamnopyranoside] | S. rhombifolia L. and S. acuta Burm.f. | Petroleum ether, chloroform, acetone, ethanolic, and aqueous extracts from leaves. | [142] | Cardiovascular and cerebrovascular protection, antitumor, anti-inflammatory, antioxidant, organ protection, anti-obesity. | [154] |
| Terpenes | |||||
| Oleanolic acid | S. acuta Burm.f. and S. rhombifolia L. | Whole-plant hydroethanolic extract. | [62] | Antidyslipidemic, antidiabetic, antiviral, anti-HIV, antibacterial, antifungal, anticancer, anti-inflammatory, hepatoprotective, gastroprotective, antiatherosclerotic, and antiplasmodial. | [155] |
| Ursolic acid | Anticancer, anti-inflammatory, antimicrobial, antidiabetic, cardiovascular protection, an-tihyperlipidemic, antifungal, antihyperuricemic, anti-obesity, antibacterial, antiviral, antiestrogenic, and other properties. | [155] | |||
| β-amyrin glucoside | S. acuta Burm.f. and S. rhombifolia L. | Whole-plant hydroethanolic extract. | [62] | Cytotoxic and antiplasmodial. | [156] |
| S. rhombifolia subsp. retusa (L.) Borss.Waalk | Methanolic extract of leaves. | [157] | |||
| Phytol | S. cordata (Burm f.) Borss | Whole-plant ethanolic extract. | [158] | Antimicrobial, anticarcinogenic, anti-teratogenic, cytotoxic, antitumor, anticonvulsant, anxiolytic, antidepressant, antinociceptive, and anti-inflammatory. | [159] |
| S. rhombifolia subsp. retusa (L.) Borss.Waalk | Methanolic extract of leaves. | [157] | |||
| Squalene | S. rhombifolia subsp. retusa (L.) Borss.Waalk | [157] | Antitumor, antioxidant, and emollient. | [160] | |
| Pheophytins | |||||
| 132-hydroxypheophytin α | S. acuta Burm.f. | Hexane, acetone, and methanol from aerial parts. | [69] | Antibacterial activity against S. aureus strains and induces NQO-1 enzyme activity in liver cell lines. | [161] |
| Phytosterols | |||||
| β-sitosterol glucoside | S. acuta Burm.f. and S. rhombifolia L. | Hexane, acetone, and methanol from aerial parts. | [69] | Inhibits the mammalian enzyme α-glucosidase. | [69] |
| S. linifolia Juss. ex Cav | Alcoholic root extracts. | [54,95] | |||
| S. rhombifolia L. | Whole-plant hydroethanolic extract. | [62] | |||
| Daucosterol | S. rhombifolia L. var. Rhombifolia. | n-hexane soluble fraction of methanolic stem extract. | [162] | Chemopreventive, neuroprotective, antioxidant, anti-inflammatory, antidiabetic, immunoregulatory, and anticancer. | [163] |
| Stigmasterol | S. rhombifolia subsp. retusa (L.) Borss.Waalk. | Methanolic extract of leaves. | [114] | Antibacterial, anticancer, anti-inflammatory, neuroprotective. | [164] |
| S. cordata (Burm f.) Borss. Waalk. | Petroleum ether, ethanol, chloroform, and acetone leaf extracts. | [165] | |||
| β-sitosterol | S. acuta Burm.f. and S. rhombifolia L. | Hexane, acetone, and methanol from aerial parts. | [69] | Anti-inflammatory, anticancer, hepatoprotective, antioxidant, cardioprotective, antidiabetic, and mitigates cognitive impairment. | [166] |
| S. cordifolia L. | Seed oil. | [131] | |||
| S. rhombifolia L. | Aerial hexane extract. | [69] | |||
| S. rhombifolia L. | Ethanolic extract of aerial parts. | [105] | |||
| S. acuta Burm.f. and S. rhombifolia L. | Whole-plant hydroethanolic extract. | [62] | |||
| S. rhombifolia subsp. retusa (L.) Borss.Waalk | Alcoholic root extracts. | [114] | |||
| γ-sitosterol | S. rhombifolia subsp. retusa (L.) Borss.Waalk | Methanolic extract of leaves. | [157] | Antidiabetic, antiapoptotic, antihyperglycemic, anti-inflammatory; inhibits glucogenesis, among others. | [167] |
| S. cordata (Burm f.) Borss. Waalk. | Petroleum ether, ethanol, chloroform, and acetone leaf extracts. | [165] | |||
| Ecdysteroids | |||||
| 20-hydroxyecdysone, 20-Hydroxyecdysone-3-O-b-D-xylose, and 20-Hydroxyecdysone-3-O-b-D-glycopyranoside | Sida tuberculata R.E.Fr. | Methanolic extracts of leaves and roots. | [60,168] | Antioxidant, hypoglycemic, cardioprotective, hepatoprotective, neuroprotective, anticancer, anti-inflammatory, vasorelaxant, among others. | [60,168] |
| 20-hydroxyecdisone 20,22-monoacetonide. | S. acuta Burm.f. and S. rhombifolia L. | Hexane, acetone, and methanol from aerial parts. | [69] | ||
| 20-Hydroxy-24-hydroxymethylecdysone | |||||
| 25-acetoxy-20-hydroxyecdysone 3-O-β-D-glucopyranoside | |||||
| Phthalates | |||||
| Di(2-etilhexil)phtalate | S. acuta Burm.f., S. cordifolia L. | Whole plant methanolic extract. | [67] | Antimicrobial, cytotoxic, anti-inflammatory, and anti-lipoxigenase. | [67] |
| Coumarins | |||||
| (E)-suberenol | S. acuta Burm.f. and S. rhombifolia L. | Whole-plant hydroethanolic extract. | [62] | Antiplasmodial, anticoagulant, antifungal, anti-inflammatory, and antioxidant. | [62,169] |
| Thamnosmonin | S. acuta Burm.f. and S. rhombifolia L. | Whole-plant or aerial-parts hydroethanolic extract and EtOAc-soluble fractions. | [62] | Antiplasmodial and cercaricidal; antioxidant, anti-ulcer, antimalarial, antidiabetic, and anticancer. | [62,169] |
| Xanthyletin | S. acuta Burm.f. and S. rhombifolia L. | Whole-plant or aerial-parts hydroethanolic extract and EtOAc-soluble fractions. | [62] | Cytotoxic, anti-inflammatory, antitumor, anti α–glucosidase, phytotoxic, and antibacterial. | [62,170] |
| Scopoletin | S. rhombifolia L. | Ethanolic extract of aerial parts. | [104] | Anti-cancer, antidiabetic, anti-inflammatory, cardioprotective, anti-neuroinflammatory, anti-AChE, hepatoprotective, among others. | [171] |
| S. acuta Burm.f. | Ethyl acetate-soluble extract of the whole plant. | [172] | |||
| Scoparone | S. rhombifolia L. | Crude ethanolic extract from aerial parts. | [104] | Anti-inflammatory, antioxidant, anti-apoptotic, antimicrobial, anticancer, anti-depressive, antinociceptive, anti-cholinesterase, anti-hypertensive, and anxiolytic. | [170] |
| Fatty acids | |||||
| Palmitic acid | S. rhombifolia L. | Aqueous extract from leaves and stems. | [101] | Anti-inflammatory. | [173] |
| Sida cordifolia L. | Hydroalcoholic extract from leaves and roots. | [25] | |||
| Oleic acid | S. cordata (Burm f.) Borss. Waalk. | Whole-plant ethanolic extract. | [158] | Antioxidant, improves endothelial dysfunction, hypocholesterolemic, and anti-inflammatory. | [174] |
| Ethyl acetate, ethanol, aqueous, and chloroform extracts from leaves and stems. | [126] | ||||
| S. rhombifolia L. | Aqueous extract from leaves and stems. | [101] | |||
| Stearic acid | Sida cordifolia L. | Hydroalcoholic extract from leaves and roots. | [25] | Immunomodulatory. | [174] |
| Malvalic acid | S. rhombifolia L. | Leaves and stems. | [101] | Anti-inflammatory, antimicrobial, and hypotensive. | [25] |
| Linoleic acid | S. rhombifolia L. | Leaves and stems. | [101] | Promotes oxidative stress, cytotoxicity, and lipid peroxidation. | [175] |
| Myristic acid | |||||
| Nonanoic acid | S. cordata (Burm f.) Borss. Waalk. | Whole-plant ethanolic extract. | [158] | Antimicrobial, energy modulator. | [175] |
| Octadecanoic acid, ethyl ester. | Antiproliferative and proapoptotic. | [175] | |||
| Octadecadienoic acid 9,12,15-Octadecadienoic acid, methyl ester, (Z,Z,Z)- 9,12-Octadecadienoic acid, methyl ester, (E,E)- | Inhibits glucose production, immunomodulator, and anti-inflammatory. | [175] | |||
| Caffeic acid | S. rhombifolia L. | Hydroalcoholic extract from leaves. | [149] | Anti-inflammatory, antioxidant, and neuroprotective. | [176] |
| Xanthones | |||||
| 1,6-dihydroxyxanthone | S. acuta Burm.f. and S. rhombifolia L. | Whole-plant hydroethanolic extract. | [63] | Antioxidant and anticancer. | [177] |
| Ceramides | |||||
| Rhombifoliamide | S. acuta Burm.f. and S. rhombifolia L. | Whole-plant hydroethanolic extract. | [62] | Antiplasmodial, antioxidant, anti-inflammatory, and antidiabetic. | [62] |
| Phenolic compounds | |||||
| Rosmarinic acid | S. cordifolia L. | Methanolic root extract. | [84] | Antioxidant, anti-inflammatory, neuroprotective, antibacterial, and anti-neuroinflammatory. | [178] |
| Ferulic acid derivatives | S. acuta Burm.f. | Leaf ethanolic extract. | [65] | Anticarcinogenic, anti-neuroinflammatory, hepatoprotective, antioxidant, and antidiabetic. | [179] |
| Leaf aqueous extract. | [180] | ||||
| Methanolic root extract. | [181] | ||||
| S. linifolia Juss. ex Cav | Ethanolic leaf fraction. | [182] | |||
| Gallic acid | S. acuta Burm.f. | Leaf ethanolic extract. | [65] | Anticancer, antiinflammatory, antiobesity, antioxidant, anti-arthritic, anti-asthmatic. | [183] |
| Chlorogenic acid | S. linifolia Juss. ex Cav | Ethanolic leaf fraction. | [182] | Hypolipidemic, antioxidant, neuroprotective, antiviral, antibacterial, and antifungal. | [183] |
| 4-methoxy cinnamic acid Vanillic acid Ellagic acid Sinapic acid | S. linifolia Juss. ex Cav | Ethanolic leaf fraction. | [182] | ||
| p-hydroxybenzoic acid | S. acuta Burm.f. | Leaf aqueous extract. | [180] | Antimicrobial, antialgal, antimutagenic, antiestrogenic, hypoglycemic, anti-inflammatory, anti-platelet aggregating, nematicidal, antiviral, antioxidant, etc. | [184] |
| Resveratrol | S. acuta Burm.f. | Leaf ethanolic extract. Leaf aqueous extract. | [65] [180] | Anti-obesity, cardioprotective neuroprotective, antitumor, antidiabetic, antioxidants, anti-age, anticancer, anti-inflammatory, vasculoprotective, antiobesity, among others. | [185] |
| p-hydroxyphenethyl trans-ferulate | S. rhombifolia L. and S. acuta Burm.f. | Aerial hexane extract. | [69] | Antioxidant, yeast and mammalian α-glucosidase inhibition. | [69] |
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lara, E.A.; Fernández, E.; Zepeda-del-Valle, J.M.; Lara, D.J.; Aguilar, A.; VanDamme, P. Etnomedicina en los altos de Chiapas, México. Bol. Latinoam. Caribe Plant Med. Aromat. 2019, 18, 42–57. [Google Scholar] [CrossRef]
- Das, U.; Islam, S. A review study on different plants in Malvaceae family and their medicinal uses. Am. J. Biomed. Sci. Res. 2019, 3, 94–97. [Google Scholar] [CrossRef]
- Hardy, K. Paleomedicine and the Evolutionary Context of Medicinal Plant Use. Rev. Bras. Farm. 2021, 31, 1–15. [Google Scholar] [CrossRef]
- Ortega-Cala, L.L.; Monroy-Ortiz, C.; Monroy-Martínez, R.; Colín-Bahena, O.; Flores-Franco, G.; Luna-Cavazos, M.; Monroy-Ortiz, R. Plantas medicinales utilizadas para enfermedades del sistema digestivo en Tetela del Volcán, Estado de Morelos, México. Bol. Latinoam. Caribe Plant Med. Aromat. 2019, 18, 106–129. [Google Scholar]
- Reeves, H.M. Sahagún’s “Florentine codex,” a little known Aztecan natural history of the Valley of Mexico. Arch. Nat. Hist. 2006, 33, 302–321. [Google Scholar] [CrossRef]
- Reimers, E.A.L.; Fernández, E.C.; Reimers, D.J.L.; Chaloupkova, P.; Del Valle, J.M.Z.; Milella, L.; Russo, D. An Ethnobotanical Survey of Medicinal Plants Used in Papantla, Veracruz, Mexico. Plants 2019, 8, 246. [Google Scholar] [CrossRef]
- Navarrete-Linares, F. Los Pueblos Indígenas de México, 1st ed.; Comisión Nacional para el Desarrollo de los Pueblos Indígenas, Programa de las Naciones Unidas para el Desarrollo: Mexico, Mexico, 2008; pp. 70–89. [Google Scholar]
- WHO. 2023. Available online: https://www.who.int/es/news-room/questions-and-answers/item/traditional-medicine (accessed on 13 February 2025).
- Osuna-Torres, L.; Tapia-Pérez, M.E.; Aguilar-Contreras, A. Plantas Medicinales de la Medicina Tradicional Mexicana para Tratar Afecciones Gastrointestinales. Estudio Etnobotánico, Fitoquímico y Farmacológico; Publicacions i Edicions Universitat de Barcelona: Barcelona, España, 2005; pp. 16–17. [Google Scholar]
- Orozco-Martínez, J.; Lira-Saade, R.; Jiménez-Estrada, M.; Ávila-Acevedo, J.G.; Serrano-Parrales, R.; Hernández-Delgado, T. Medicinal plants of Oaxaca, Mexico: Ethnobotany and antibacterial activity. Bol. Latinoam. Caribe Plant Med. Aromat. 2020, 19, 221–235. [Google Scholar]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Karade, D.; Vijayasarathi, D.; Kadoo, N.; Vyas, R.; Ingle, P.K.; Karthikeyan, M. Design of novel drug-like molecules using informatics rich secondary metabolites analysis of Indian medicinal and aromatic plants. Comb. Chem. High. Throughput Screen. 2020, 23, 1113–1131. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar] [CrossRef]
- Aminah, N.S.; Laili, E.R.; Rafi, M.; Rochman, A.; Insanu, M.; Tun, K.N.W. Secondary metabolite compounds from Sida genus and their bioactivity. Heliyon 2021, 7, e06682. [Google Scholar] [CrossRef]
- Walker, C.C. Malvaceae. In Dicotyledons: Rosids (Illustrated Handbook of Succulent Plants), 2nd ed.; Eggli, U., Nyffeler, R., Eds.; Springer: Cham, Switzerland, 2023; pp. 827–833. [Google Scholar] [CrossRef]
- Paul, T.K.; Nayar, M.P. Malvaceae. In Fascicles of Flora of India, Fascicle 19; Nayar, M.P., Thothathri, K., Sanjappa, M., Eds.; Botanical Survey of India: Calcutta, India, 1988; pp. 64–233. [Google Scholar]
- Kumar, S.; Kumar, S.; Vishnoi, V.K.; Kumar, P.; Maheshwari, D.K. Sida cordifolia L.: Ethnobotany, Phytochemistry, Phytonanotechnology, and Commercial Application. Curr. Pharm. Biotechnol. 2023, 25, 838–859. [Google Scholar] [CrossRef]
- Linnaeus, C.V. Species Plantarum, 1st ed.; Salvius: Stockholm, Suecia, 1753; Volume 2, pp. 683–686. [Google Scholar]
- Krapovickas, A. Las especies de Sida Secc. Malacroideae (Malvaceae) del Cono Sur de Sudamérica. Bonplandia 2007, 16, 209–253. [Google Scholar] [CrossRef]
- Fryxell, P.A. Familia Malvaceae Fascicle 16 In Flora del Bajío y de Regiones Adyacentes; Rzedowski, J., de Rzedowski, G.C., Eds.; Instituto de Ecología A.C. Centro Regional del Bajío: Pátzcuaro, México, 1993; pp. 137–158. [Google Scholar] [CrossRef]
- Flores-Franco, G.; Rangel-Altamirano, M.G.; Wehncke-Rodríguez, E.V.; Bonilla-Barbosa, J.; Cruz-Durán, R.; Valencia-A, S. Flora nativa y vegetación de la Reserva de la Biosfera Sierra de Huautla, Morelos, México. Acta Botánica Mex. 2024, 131, e2388. [Google Scholar] [CrossRef]
- Brandão, J.L.; Baracho, G.S.; Sales, M.F.; Viegas Filho, M.P. Synopsis of Sida (Malvaceae, Malvoideae, Malveae) in the state of Pernambuco, Brazil. Phytotaxa 2017, 307, 205–227. [Google Scholar] [CrossRef]
- Rodrigues, F.C.; Morais de Oliveira, A.F. The genus Sida L. (Malvaceae): An update of its ethnomedicinal use, pharmacology and phytochemistry. S. Afr. J. Bot. 2020, 132, 432–462. [Google Scholar] [CrossRef]
- Rodrigo, A.P. Las especies argentinas y uruguayas del género Sida. (Malvaceae). Rev. Mus. La Plata Nva Ser. Secc. Bot. 1944, 6, 81–212. Available online: http://sedici.unlp.edu.ar/handle/10915/144022 (accessed on 14 July 2025).
- Ahmed, H.; Juraimi, A.S.; Hamdani, M.S.; Rafii, Y.M.; Aslani, F.; Omar, D. Comparative phytotoxic effects of aerial and root aqueous extracts of Sida cordifolia L. on germination and seedling vigour performance of lettuce, tomato and carrot. Bangladesh J. Bot. 2017, 46, 323–328. [Google Scholar]
- Fernandes de Oliveira, A.M.; Sousa-Pinheiro, L.; Souto-Pereira, C.K.; Neves-Matias, W.; Albuquerque-Gomes, R.; Souza-Chaves, O.; Vanderlei-de Souza, M.D.F.; Nóbrega de Almeida, R.; Simões-de Assis, T. Total Phenolic content and antioxidant activity of some Malvaceae family species. Antioxidants 2012, 1, 33–43. [Google Scholar] [CrossRef]
- Dinda, B.; Das, N.; Dinda, S.; Dinda, M.; SilSarma, I. The genus Sida L.—A traditional medicine: Its ethnopharmacological, phytochemical and pharmacological data for commercial exploitation in herbal drugs industry. J. Ethnopharmacol. 2015, 24, 135–176. [Google Scholar] [CrossRef]
- Dorado, O. Inventario Florístico de la Sierra de Huautla, Morelos; Universidad Autónoma del Estado de Morelos, Centro de Educación Ambiental e Investigación Sierra de Huautla: México, México, 1997; p. 34. [Google Scholar]
- Dorado, O.; Maldonado, B.; Arias, D.; Sorani, V.; Ramírez, R.; Leyva, E.; Valenzuela, D. Programa de Conservación y Manejo Reserva de la Biosfera Sierra de Huautla; Comisión Nacional de Áreas Naturales Protegidas (CONANP)—Secretaría de Medio Ambiente y Recursos Naturales (SEMARNAT): México, México, 2005; pp. 15–16. [Google Scholar]
- Chaachouay, N.; Zidane, L. Plant-derived natural products: A source for drug discovery and development. Drugs Drug Candidates 2024, 3, 184–207. [Google Scholar] [CrossRef]
- Marhold, K.; Kučera, J.; Akopian, J.A.; Alves, L.I.F.; Alves, W.S.; Baracho, G.S.; Barros e Silva, A.E.; Batista, F.R.C.; Calado, L.L.; Cordeiro, J.M.P.; et al. IAPT chromosome data 39—Extended version. Taxon 2023, 72, 1189–1192. [Google Scholar] [CrossRef]
- Fryxell, P.A. Malvaceae of Mexico. Syst. Bot. Monogr. 1988, 25, 1–522. [Google Scholar] [CrossRef]
- Lavia, G.I.; Fernández, A.; Krapovickas, A. Cromosomas de especies americanas de Sida (MALVACEAE). Bonplandia 2007, 16, 255–258. Available online: https://www.redalyc.org/articulo.oa?id=685773827003 (accessed on 4 April 2025). [CrossRef]
- Rondón, J.B. La subfamilia Malvoideae (Malvaceae s.l.) en el occidente del estado Sucre, Venezuela. UDO Agric. 2009, 9, 599–621. [Google Scholar]
- Kumar, S.; Kumari, S.; Chand-Gupta, R. Cytological investigations of some polypetalous plants from District Sirmaur of Himachal Pradesh in the Western Himalayas, India. Chromosom. Bot. 2012, 7, 87–96. [Google Scholar] [CrossRef]
- Exell, A.W. Malvaceae. In Flora Zambesiaca; Crown Agents: London, UK, 1961; pp. 420–483. [Google Scholar]
- Venkatesh, K.H.; Dinesh, B.; Venu, N.; Munirajappa. Chromosome numbers and karyotype studies of few members of Malvales. Am. J. Phytomed Clin. Ther. 2015, 3, 178–184. [Google Scholar]
- Areces-Berazaín, F.; Fryxell, P.A. Malvaceae. In Flora de la República de Cuba, 13th Fascículo; Greuter, W., Rankin Rodríguez, R., Eds.; Botanischer Garten und Berlin: Berlin, Germany, 2007; pp. 1–229. [Google Scholar]
- García-Mendoza, A.; Meave, J. Diversidad Florística de Oaxaca: De Musgos a Angiospermas, 1st ed.; Instituto de Biología, Universidad Nacional Autónoma de México-CONABIO: Ciudad de México, Mexico, 2011; pp. 154–196. [Google Scholar]
- Singh, A.K.; Sahu, R.K. Seedling Morphology and its Systematic Implications Within the Genus Sida L. (Malvaceae). Indian J. For. 2014, 37, 73–80. [Google Scholar] [CrossRef]
- Huang-Hua, R.S. Sida Linnaeus, Sp. Fl. China 2007, 12, 270–275. [Google Scholar]
- Rao, K.S.; Swamy, R.K.; Kumar, D.; R., A.S.; Bhat, K.G. Flora of Peninsular India. 2019. Available online: https://indiaflora-ces.iisc.ac.in/FloraPeninsular/herbsheet.php?id=0&cat=7 (accessed on 14 July 2025).
- Sivarajan, V.V.; Pradeep, A.K. Taxonomy of the Sida rhombifolia (Malvaceae) complex in India. SIDA Contrib. Bot. 1994, 16, 63–78. Available online: https://www.jstor.org/stable/41967083 (accessed on 14 July 2025).
- Sharif-Shamima, N.; Sadia-Sultana, D.; Lubna, A.; Rabeya, B.; Zaman, M.A.; Sheikh-Shamimul, A. Differential Fluorescent Chromosome Banding of Four Sida spp. (Malvaceae). Cytologia 2003, 68, 25–30. [Google Scholar]
- Rufino-Arcanjo, D.D.; Muniz-Oliveira, N.N.P.; Ferreira-Filho, E.S.; Albuquerque da Costa, D.; Chaves, M.H.; Romão-Borges, A.C.; Pereira de Oliveira, A.; Meneses-Oliveira, R.C. Vasorelaxant response induced by Sida santaremnensis H. Monteiro ethanol extract on rat superior mesenteric artery. AJB 2011, 10, 14587–14597. [Google Scholar] [CrossRef]
- Fontes de Sousa, V.; Soares de Oliveira, V. Sida santaremensis (MALVACEAE): A new record for Paraíbastate, in caatinga domain, northeastern Brazil. Pesqui. BOTÂNICA 2019, 73, 47–54. [Google Scholar]
- Andrade-Cetto, A.; Heinrich, M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J. Ethnopharmacol. 2005, 99, 325–348. [Google Scholar] [CrossRef]
- Monroy-Ortiz, C.; Castillo-España, P. Plantas Medicinales Utilizadas en el Estado de Morelos, 2nd ed.; Universidad Autónoma del Estado de Morelos: Cuernavaca, Mexico, 2007; p. 186. [Google Scholar]
- Alarcón-Aguilar, F.J.; Román-Ramos, R. Antidiabetic plants in Mexico and Central America. In Traditional Medicines for Modern Times: Antidiabetic Plants, 1st ed.; Soumyanath, A., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2006; pp. 179–193. [Google Scholar]
- Adhikari, D.C.; Das, B.D. Some medicinal plants uses in ethnical group from Biratnagar, Eastern, Nepal. Am. Sci. Res. J. Eng. Tech. Sci. 2018, 41, 233–239. [Google Scholar]
- Kanth, V.R.; Diwan, P.V. Analgesic, anti-inflammatory and hypoglycaemic activities of Sida cordifolia. Phytother. Res. 1999, 13, 75–77. [Google Scholar] [CrossRef]
- Baracho, G.S.; Agra, M.D.F. New synonyms and lectotypifications in Sida (Malvaceae-Malveae) reveal the first record and extension of the distribution area of Sida maculata to Brazil. Phytotaxa 2024, 660, 287–293. [Google Scholar] [CrossRef]
- Felayati, T.; Rustiami, H.; Susandarini, R. Sida penambangensis (Malvaceae), a new Sida species from East Java, Indonesia. Reinwardtia 2024, 23, 33–38. [Google Scholar] [CrossRef]
- Nwankwo, N.E.; Ezeako, E.C.; Nworah, N.E.; Ogara, L.O.; Oka, S.A.; Aham, E.C.; Joshua, P.E.; Nwiloh, B.I.; Ezike, T.C.; Ashiakpa, N.P.; et al. Bioactive compounds anti-infammatory anti-nociceptive and antioxidant potentials of ethanolic leaf fraction of Sida linifolia L (Malvaceae). Arab. J. Chem. 2023, 16, 104398. [Google Scholar] [CrossRef]
- García-Regalado, G. Plantas Medicinales de Aguascalientes, 1st ed.; Universidad Autónoma de Aguascalientes: Aguascalientes, Mexico, 2014; p. 50. [Google Scholar]
- Thangam, J.; Shanthakumari, G. Central nervous system effects of Sida retusa root. Jpn. J. Pharmacol. 1971, 21, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Digital Flora of Eastern Ghats. Available online: https://indiaflora-ces.iisc.ac.in/EasternGhats/herbsheet.php?id=2320&cat=4 (accessed on 14 July 2025).
- Plants of the World Online (POWO). Royal Botanic Gardens, Kew: Kew, UK, 2024. Available online: https://www.plantsoftheworldonline.org/ (accessed on 22 April 2025).
- Bai, M.D.A.; Rani, S.P.S.; Balachandran, S.; Jayakumar, G. The use of Sida plant in the preparation of Nayapayam Kashayam. IJRAP 2012, 3, 99–104. [Google Scholar]
- da Rosa, H.S.; Coelho, I.S.; da Silva, M.D.; Fernandes, M.S.; Bertelli, P.R.; Minetto, L.; Moura, S.; de Paula, F.; Santos, A.R.; Mendez, A.S.L.; et al. Sida tuberculata extract reduces the nociceptive response by chemical noxious stimuli in mice: Implications for mechanism of action, relation to chemical composition and molecular docking. Phytother. Res. 2018, 33, 224–233. [Google Scholar] [CrossRef]
- Ogunmoyole, T.; Falusi, O.O.; Oderinde, F. Sida acuta leaf extract attenuates oxidants-induced animal model of nephrotoxicity and hepatotoxicity. Clin. Phytosci. 2022, 8, 5. [Google Scholar] [CrossRef]
- Kamdoum, B.C.; Simo, I.; Wouamba, S.C.N.; Tchatat-Tali, B.M.; Ngameni, B.; Fotso, G.W.; Ambassa, P.; Fabrice, F.B.; Lenta, B.N.; Sewald, N.; et al. Chemical constituents of two Cameroonian medicinal plants: Sida rhombifolia L. and Sida acuta Burm. f. (Malvaceae) and their antiplasmodial activity. Nat. Prod. Res. 2022, 36, 5311–5318. [Google Scholar] [CrossRef]
- Karou, S.D.; Tchacondo, T.; Tchibozo, M.A.; Anani, K.; Ouattara, L.; Simpore, J.; de Souza, C. Screening Togolese medicinal plants for few pharmacological properties. Pharmacogn. Res. 2012, 4, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, R.P.; Bhuvaneshwari, V.; Malayaman, V.; Ranjithkumar, R.; Sathiyavimal, S. Phytochemical screening of aqueous leaf extract of Sida acuta Burm.f. and its antibacterial activity. J. Eme Tech. Innov. Res. 2018, 5, 474–478. Available online: http://www.jetir.org/papers/JETIR1808069.pdf (accessed on 25 November 2024).
- Smanthong, N.; Tavichakorntrakool, R.; Tippayawat, P.; Lulitanond, A.; Pinlaor, P.; Daduang, J.; Sae-Ung, N.; Chaveerach, A.; Phetcharaburanin, J.; Boonsiri, P. Anti-proteus activity, anti-struvite crystal, and phytochemical analysis of Sida acuta Burm. F. ethanolic leaf extract. Molecules 2022, 27, 1092. [Google Scholar] [CrossRef]
- Uysal, S.; Gevrenova, R.; Sinan, K.I.; Bayarslan, A.U.; Altunoglu, Y.C.; Zheleva-Dimitrova, D.; Ak, G.; Cengiz-Baloglu, M.; Etienne, O.K.; Lobine, D.; et al. New perspectives into the chemical characterization of Sida acuta Burm. f. extracts with respect to its anti-cancer, antioxidant and enzyme inhibitory effects. Process Biochem. 2021, 105, 91–101. [Google Scholar] [CrossRef]
- Preethidan, D.S.; Arun, G.; Surendran, M.P.; Prasanth, S.; Sabu, A.; Sadasivan, C.; Haridas, M. Lipoxygenase inhibitory activity of some Sida species due to di(2-ethylhexyl) phthalate. Curr. Sci. 2013, 105, 232–237. Available online: http://www.jstor.org/stable/24092643 (accessed on 13 September 2024).
- Anani, K.; Hudson, J.B.; de Souza, C.; Akpagana, K.; Tower, G.H.; Arnason, J.T.; Gbeassor, M. Investigation of medicinal plants of togo for antiviral and antimicrobial activities. Pharm. Biol. 2000, 38, 40–45. [Google Scholar] [CrossRef]
- Arciniegas, A.; Pérez-Castorena, A.L.; Nieto-Camacho, A.; Kita, Y.; Vivar, A.R.D. Anti-hyperglycemic, antioxidant, and anti-inflammatory activities of extracts and metabolites from Sida acuta and Sida rhombifolia. Quim. Nova 2017, 40, 176–181. [Google Scholar] [CrossRef]
- Konaté, K.; Bassolé, I.H.; Hilou, A.; Aworet-Samseny, R.R.; Souza, A.; Barro, N.; Dicko, M.H.; Datté, J.Y.; M’Batchi, B. Toxicity assessment and analgesic activity investigation of aqueous acetone extracts of Sida acuta Burn f. and Sida cordifolia L. (Malvaceae), medicinal plants of Burkina Faso. BMC Complement. Altern. Med. 2012, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- Benjumea, D.; Gómez-Betancur, I.; Vásquez, J. Neuropharmacological effects of the ethanolic extract of Sida acuta. Rev. Bras. Farm. 2016, 26, 209–215. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, S.; Shrama, D. Antipyretic efficacy of various extracts of Sida acuta leaves. Res. J. Pharm. Biol. Chem. Sci. 2012, 3, 515–518. [Google Scholar]
- Machado-De Queiroz, W.A. Estudo Fitoquímico e Avaliação Biológica Pioneiros da Espécie Sida ciliaris L. (Malvaceae Sensu lato). Master’s Thesis, Paraíba Federal University, Paraíba, Brazil, 2022. [Google Scholar]
- Ahmad, M.; Prawez, S.; Sultana-Raina, R.; Pankaj, N.K.; Kumar-Verma, P.; Rahman, S. Anti-hyperglycemic, anti-hyperlipidemic and antioxidant potential of alcoholic-extract of Sida cordifolia (Areal Part) in streptozotocin-induced-diabetes in Wistar-Rats. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 397–405. [Google Scholar] [CrossRef]
- Subramanya, M.D.; Pai, S.R.; Upadhya, V.; Ankad, G.M.; Bhagwat, S.S.; Hegde, H.V. Total polyphenolic contents and in vitro antioxidant properties of eight Sida species from Western Ghats, India. J. Ayurveda Integr. Med. 2015, 6, 24–28. [Google Scholar] [CrossRef]
- Shah, N.A.; Khan, M.R. Antidiabetic effect of Sida cordata in alloxan induced diabetic rats. Biomed. Res. Int. 2014, 2014, 671294. [Google Scholar] [CrossRef]
- Shah, N.A.; Khan, M.R.; Nigussie, D. Phytochemical investigation and nephroprotective potential of Sida cordata in rat. BMC Complement. Altern. Med. 2017, 17, 388–397. [Google Scholar] [CrossRef]
- Kamble, S.D.; Purane, L.M.; Devade, O.; Redasani, V. In Vitro and In Vivo evalution of anti-asthmatic activity of leaves of Sida veronicafolia (Lam). RJPPD 2024, 16, 269–273. [Google Scholar] [CrossRef]
- Gulnaz, A.R.; Thabassum, S.; Salahuddin, M.; Savitha, G. Biological activity and phytochemical screening of different extracts of Sida cordata (Burm.F.) borssum root. Indian. J. Clin. Anat. Physiol. 2018, 3, 15–18. [Google Scholar] [CrossRef]
- Mistry, S.; Dutt, K.R.; Jena, J. Protective effect of Sida cordata leaf extract against CCl(4) induced acute liver toxicity in rats. Asian Pac. J. Trop. Med. 2013, 6, 280–284. [Google Scholar] [CrossRef]
- Kumar, S.; Lakshmi, P.K.; Sahi, C.; Pawar, R.S. Sida cordifolia accelerates wound healing process delayed by dexamethasone in rats: Effect on ROS and probable mechanism of action. J. Ethnopharmacol. 2019, 235, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Shahed-Al-Mahmud, M.; Jahan, T.; Towhidul-Islam, M. Antidiarrheal activities of hydroalcoholic extract of Sida cordifolia roots in Wister albino rats. Orient. Pharm. Exp. Med. 2018, 18, 51–58. [Google Scholar] [CrossRef]
- Swathy, S.S.; Panicker, S.; Nithya, R.S.; Anuja, M.M.; Rejitha, S.; Indira, M. Antiperoxidative and antiinflammatory effect of Sida cordifolia Linn. on quinolinic acid induced neurotoxicity. Neurochem. Res. 2010, 35, 1361–1367. [Google Scholar] [CrossRef]
- Iqbal, H.; Wright, C.L.; Jones, S.; da Silva, G.R.; McKillen, J.; Gilmore, B.F.; Kavanagh, O.; Green, B.D. Extracts of Sida cordifolia contain polysaccharides possessing immunomodulatory activity and rosmarinic acid compounds with antibacterial activity. BMC Complement. Med. Ther. 2022, 22, 27–43. [Google Scholar] [CrossRef]
- Mathew, M.; Jayshree, C.; Jibi, V.; Nilima, G. A phytochemical study of bala dwayam (Sida cordifolia & Abutilon indicum Linn.) And clinical evaluation of its moola churna ksheerapaka in Sandhigata vata with special reference to janu sandhi. IAMJ 2021, 12, 292–295. [Google Scholar] [CrossRef]
- Pratima, M.B.; Sunganthi, V.; Milind, V.B.; Kothai, R. In vitro antiproliferative activity of ethanolic extract of Sida cordifolia Linn against various cancer cell lines. Al Ameen J. Med. Sci. 2020, 13, 234–241. Available online: http://ajms.alameenmedical.org/ArticlePDFs/4%20AJMS%20V13.N4.2020%20p%20234-241.pdf (accessed on 20 November 2024).
- Silva, D.A.; da Silva, T.M.S.; da Silva-Lins, A.C.; da Costa, D.A.; Cavalcante-Sobral, J.M.; Matias, W.N.; Vanderlei de Souza, M.F.; Braz-Filho, R. Chemical constituents and antioxidant activity of Sida galheirensis Ulbr. (Malvaceae). Quim. Nova 2006, 29, 1250–1254. [Google Scholar] [CrossRef]
- Das, N.; Nath, J.; Dinda, B. Antioxidant Phytochemicals from Sida glutinosa. J. Pharm. Res. 2012, 5, 4845–4848. Available online: https://www.researchgate.net/publication/285598598_Antioxidant_Phytochemicals_from_Sida_glutinosa (accessed on 16 December 2024).
- Das, N.; Saha, T.; Dinda, B. A new antifungal aliphatic fatty acid ester from the aerial parts of Sida glutinosa. Chem. Nat. Compd. 2016, 52, 388–390. [Google Scholar] [CrossRef]
- Das, N.; Das, M.C.; Dinda, B. Enzyme inhibitor and antimicrobial phytochemicals from aerial parts of Sida glutinosa. IJPPR 2014, 6, 91–96. Available online: https://impactfactor.org/PDF/IJPPR/6/IJPPR,Vol6,Issue1,Article15.pdf (accessed on 5 January 2025).
- John-Africa, L.B.; Aboh, M. Evaluation of the haemostatic activities of Sida corymbosa in rats. Br. J. Pharm. Res. 2015, 5, 431–436. [Google Scholar] [CrossRef]
- Attah, A.F.; O’Brien, M.; Koehbach, J.; Sonibare, M.A.; Moody, J.O.; Smith, T.J.; Gruber, C.W. Uterine contractility of plants used to facilitate childbirth in Nigeria ethnomedicine. J. Ethnopharmacol. 2012, 143, 377–382. [Google Scholar] [CrossRef]
- John-Africa, L.B.; Yahaya, T.A.; Isimi, C.Y. Antiulcer and wound healing activities of Sida corymbosa in rats. Afr. J. Tradit. Complement. Altern. Med. 2014, 11, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Nwankwo, N.E.; Ashiakpa, P.N. Antimalarial potential of ethanol extract, and anti-inflammatory properties of flavonoid-, terpenoid-, and alkaloid-rich fractions of Sida linifolia L. Food Mater. Res. 2024, 4, e018. [Google Scholar] [CrossRef]
- Nwankwo, N.E.; Okeke, E.S.; Nworah, F.N.; Ezeako, E.C. Phytochemical composition and potential anti-inflammatory and antioxidant mechanisms of leaf extracts of Sida linifolia L. (Malvaceae). J. Herb. Med. 2023, 38, 100630. [Google Scholar] [CrossRef]
- Jatsa, H.B.; Endougou, A.M.E.; Kemeta, D.R.A.; Kenfack, C.M.; Tchuem-Tchuente, L.A.; Kamtchouing, P. In Vivo antischistosomal and toxicological evaluation of Sida pilosa Retz on mice BALB/c. Pharmacologyonline 2009, 3, 531–538. Available online: https://www.researchgate.net/publication/283212550_In_vivo_antischistosomal_and_toxicological_evaluat (accessed on 5 January 2025).
- Jatsa, H.B.; Pereira, C.A.J.; Pereira, A.B.D.; Negrão-Corrêa, D.A.; Braga, F.C.; Maciel, G.M.; Castilho, R.O.; Kamtchouing, P.; Teixeira, M.M. In vitro evaluation of Sida pilosa Retz (malvaceae) aqueous extract and derived fractions on Schistosoma mansoni. Pharmacol. Pharm. 2015, 6, 380–390. [Google Scholar] [CrossRef]
- Jatsa, H.B.; Russo, R.C.; Pereira, C.A.; Aguilar, E.C.; Garcia, C.C.; Araújo, E.S.; Oliveira, J.L.; Rodrigues, V.F.; de Oliveira, V.G.; Alvarez-Leite, J.I.; et al. Improvement of the liver pathology by the aqueous extract and the n-butanol fraction of Sida pilosa Retz in Schistosoma mansoni-infected mice. J. Ethnopharmacol. 2016, 180, 114–123. [Google Scholar] [CrossRef]
- Selbach, M.T.; Scotti, A.S.; Feistel, C.C.; Nicolau, C.C.; Dalberto, D.; Dos Santos, N.G.; Borsoi, G.; Ferraz, A.B.F.; Grivicich, I.; de Souza, G.M.S.; et al. Evaluation of the cytotoxic and genotoxic effects of Sida planicaulis Cav extract using human neuroblastoma cell line SH-SY5Y. J. Toxicol. Env. Health A 2021, 84, 345–355. [Google Scholar] [CrossRef]
- Ratna-Sari, E.; Suhatri, N.; Ismed, F.; Prima-Putra, D. Inventory, morphological and antioxidant profile of the Sumatera Sidaguri (Sida Spp.) plants. Adv. Health Sci. Res. 2021, 40, 65–74. [Google Scholar] [CrossRef]
- Xu, Z.; Gao, P.; Liu, D.; Song, W.; Zhu, L.; Liu, X. Chemical composition and In Vitro antioxidant activity of Sida rhombifolia L. volatile organic compounds. Molecules 2022, 27, 7067. [Google Scholar] [CrossRef]
- Kumar, A.; Mishra, P.G.; Tomar, S.D.; Pathak, M.; Pandey, K.R.; Singh, L. Phyto-therapeutic potential of aerial part of Sida rhombifolia for antiinflammatory, antinociceptive, and antioxidant activity. Int. J. Pharm. Qual. Assur. 2023, 14, 91–95. [Google Scholar] [CrossRef]
- Tanumihardja, M.; Natsir, N.; Mattulata, I.K.; Lukman, M. Potent anti-inflammatory effect of root of sidaguri (Sida rhombifolia L) on rat periapical lesion model. IJTPR 2016, 8, 412–415. Available online: http://impactfactor.org/PDF/IJTPR/8/IJTPR,Vol8,Issue6,Article1.pdf (accessed on 5 January 2025).
- Mah, S.H.; Teh, S.S.; Ee, G.C.L. Anti-inflammatory, anti-cholinergic and cytotoxic effects of Sida rhombifolia. Pharm. Biol. 2017, 55, 920–928. [Google Scholar] [CrossRef]
- Chaves, O.S.; Teles, Y.C.; Monteiro, M.M.; Mendes-Junior, L.D.; Agra, M.F.; Braga, V.A.; Silva, T.M.; Souza, M.F. Alkaloids and phenolic compounds from Sida rhombifolia L. (Malvaceae) and vasorelaxant activity of two indoquinoline alkaloids. Molecules 2017, 22, 94. [Google Scholar] [CrossRef]
- Oliveira, M.S.; Chaves, O.S.; Cordeiro, L.V.; Gomes, A.N.P.; Fernandes, D.A.; Teles, Y.C.F.; da Silva, T.M.S.; Freire, K.R.L.; Lima, E.O.; Agra, M.F.; et al. Indoquinoline alkaloids from Sida rhombifolia (L.) (Malvaceae) and antimicrobial evaluation of cryptolepinone derivatives. J. Braz. Chem. Soc. 2023, 34, 220–227. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ebrahimzadeh, M.A.; Rafiei, A.; Kardan, M.; Ebrahimi, M.A. Sida rhombifolia exerts anti-proliferative and pro-apoptotic effects in human liver cancer HepG2 cells In Vitro. Asian Pac. J. Cancer Prev. 2022, 23, 3677–3684. [Google Scholar] [CrossRef]
- Ranjan, S.R.; Shankar, M.U.; Kumar, P.S.; Saiprasanna, B. Evaluation of antidiarrhoeal activity of Sida rhombifolia Linn. root. Int. Res. J. Pharm. 2011, 2, 157–160. [Google Scholar]
- Saini, A.K.; Velumani, A.N.; Sakarkar, S.N.; Singh, B.K.; Rizwan, M.; Gupta, A.A. Antibacterial activity of compounds extracted from Sida rhombifolia Linn. Bull. Environ. Pharmacol. Life Sci. 2023, 2023, 495–500. [Google Scholar]
- Debalke, D.; Birhan, M.; Kinubeh, A.; Yayeh, M. Assessments of Antibacterial effects of aqueous-ethanolic extracts of Sida rhombifolia’s aerial part. Sci. World J. 2018, 2018, 8429809. [Google Scholar] [CrossRef]
- Sundaraganapathy, R.; Niraimathi, V.; Thangadurai, A.; Jambulingam, M.; Narasimhan, B.; Aakash, D. Phytochemical studies and pharmacological screening of Sida rhombifolia Linn. Hygeia J. D Med. 2013, 5, 19–22. [Google Scholar]
- Imran, M.; Robert, S.M.J.; Sharma, M.; Aeri, V. Evaluation of Sida cordifolia and Sida rhombifolia extracts in a rat model of streptozotocin-induced diabetic nephropathy. Polim. Med. 2023, 53, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Dhalwal, K.; Shinde, V.M.; Singh, B.; Mahadik, K.R. Hypoglycemic and hypolipidemic effect of Sida rhombifolia ssp. retusa in diabetic induced animals. Int. J. Phytomed 2010, 2, 160–165. [Google Scholar] [CrossRef]
- Poojari, R.; Gupta, S.; Maru, G.; Khade, B.; Bhagwat, S. Sida rhombifolia ssp. retusa seed extract inhibits DEN-induced murine hepatic preneoplasia and carbon tetrachloride hepatotoxicity. Asian Pac. J. Cancer Prev. 2009, 10, 1107–1112. [Google Scholar]
- Thounaojam, M.C.; Jadeja, N.; Devkar, R.V.; Ramachandran, A.V. Antioxidant and free radical scavenging activity of Sida rhomboidea Roxb methanolic extract determined using different in vitro models. Bol. Latinoam. Caribe Plantas Med. Aromát 2010, 9, 191–198. Available online: http://www.redalyc.org/articulo.oa?id=85615232005 (accessed on 10 January 2025).
- Venkatesh, S.; Reddy, Y.S.; Suresh, B.; Reddy, B.M.; Ramesh, M. Antinociceptive and anti-inflammatory activity of Sida rhomboidea leaves. J. Ethnopharmacol. 1999, 67, 229–232. [Google Scholar] [CrossRef]
- Marques, A.E.F.; Monteiro, T.M.; dos Santos, C.R.B.; Piuzevan, M.R.; de Souza, M.d.F.V.; Mororó, G.T.; Alves, M.M.d.M.; Carvalho, F.A.d.A.; Arcanjo, D.D.R.; Gonçalves, J.C.R.; et al. Flavonoids isolated from Sida santaremnensis H. Monteiro (“Guanxuma”) and evaluation of biological activities. Ciênc. E Nat. 2021, 43, e58. [Google Scholar] [CrossRef]
- Sangreskopp, M.A.; Kulkarni, P.; Mannasaheb, B.A. Antipyretic and antimicrobial potential of Sida spinosa Linn. aqueous root extract in rats. Int. J. Phytopharm. 2013, 3, 50–55. Available online: https://www.researchgate.net/publication/307798107_Antipyretic_and_antimicrobial_potential_of_Sida_spinosa_linn_aqueous_root_extract (accessed on 18 December 2024).
- Selvadurai, S.; Senthamarai, R.; Sri-Vijaya-Kirubha, T.; Vasuki, K. Antimicrobial activity of ethanolic extract of the whole plant of Sida spinosa Linn. (Malvaceae). J. Nat. Prod. Plant Resour. 2011, 1, 36–40. Available online: https://www.researchgate.net/publication/267296468_Anitimicrobial_activity_of_ethanolic_extract_of_the_whole_plant_of_Sida_Spinosa_Linn_Malvaceae (accessed on 10 January 2025).
- Navaneethakrishnan, S.; Suresh, K.; Satyanarayana, T.; Mohideen, S.; Kiran-Kumar, G. Antimicrobial activity of ethanolic leaf extract of Sida spinosa Linn. (Malvaceae). Asian J. Plant Sci. Res. 2011, 1, 65–67. Available online: https://www.imedpub.com/abstract/antimicrobial-activity-of-ethanolic-leaf-extract-of-sida-spinosa-linnmalvaceae-12507.html (accessed on 12 January 2025).
- Tirkey, R. Screening for phytochemical, flavonoid content, antioxidant and anthelmintic potential of Sida spinosa. IJPSR 2019, 10, 5587–5591. [Google Scholar]
- Silva da Rosa, H.; Santos, M.C.; Costa, M.T.; Salgueiro, A.; Duarte-da Silva, M.; Nogueira-Librelotto, D.R.; Jesse, C.; Machado, M.M.; Souza-de Oliveira, L.F.; Folmer, V.; et al. Sida tuberculata: In vitro cytotoxicity and in vivo anti-inflammatory effect. J. Ethnopharmacol. 2022, 287, 114956. [Google Scholar] [CrossRef] [PubMed]
- Yamada, E.F.; Dos Santos-Stein, C.; Moresco, R.N.; Bobinski, F.; Palandi, J.; Fernandes, P.F.; Folmer, V.; da Silva, M.D. Photobiomodulation and Sida tuberculata combination declines the inflammation’s markers in knee-induced osteoarthritis. Lasers Med. Sci. 2022, 37, 193–204. [Google Scholar] [CrossRef]
- Yamada, E.F.; Olin, L.C.; Pontel, C.L.; da Rosa, H.S.; Folmer, V.; da Silva, M.D. Sida tuberculata reduces oxidative stress and pain caused by the knee osteoarthritis. J. Ethnopharmacol. 2020, 248, 112277. [Google Scholar] [CrossRef] [PubMed]
- Priya, M.; Arumugam, K.; Chakaravarthy, C.; Chandran, K.; Bahadur-Sultan, A.; Zochedh, A. Graph theory network, molecular docking, quantum chemicals and pharmacokinetics-based investigation on phytochemicals from Sida rhombifolia against Alzheimer’s disease. PACs 2024, 44, 1947–1970. [Google Scholar] [CrossRef]
- Rahate, S.P.; Singh, M.; Verma, A.K.; Kumar, N.; Tiwari, N.; Shanker, K. Densitometric method for assessment of six specialized metabolites in four Sida sp. and its congener Abutilon indicum: Targeted metabolomics, greenness assessment, and chemometrics analysis. J. Pharm. Biomed. Anal. 2024, 240, 115945. [Google Scholar] [CrossRef]
- Sharma, D.; Saluja, M.S.; Mishra, R. A brief overview of the ethnomedical, pharmacological, and phytochemical uses of Sida spinosa. Int. Med. Pharm. Drug Res. 2023, 7, 15–19. [Google Scholar] [CrossRef]
- Prakash, A.; Varma, R.K.; Ghosal, S. Alkaloid constituents of Sida acuta, S. humilis, S. rhombifolia and S. spinosa. Planta Med. 1981, 43, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Mani, S.; Punnoose, A.M.; Mani, E.; Mahin, R.R.N. Plant description, phytochemical constituents and pharmacological attributes of the plant Sida cordata: A Perspective Review. J. Nat. Remedies 2025, 25, 483–497. [Google Scholar] [CrossRef]
- Khandelwal, P.; Wadhwani, B.D.; Rao, R.S.; Mali, D.; Vyas, P.; Kumar, T.; Nair, R. Exploring the pharmacological and chemical aspects of pyrrolo-quinazoline derivatives in Adhatoda vasica. Heliyon 2024, 10, e25727. [Google Scholar] [CrossRef] [PubMed]
- Sutradhar, R.K.; Rahman, A.M.; Ahmad, M.; Bachar, S.C.; Saha, A.; Kumar-Guha, S. Bioactive alkaloid from Sida cordifolia Linn. with analgesic and anti-inflammatory activities. IJPT 2006, 5, 175–178. [Google Scholar]
- Subramanya, M.D.; Pai, S.R.; Ankad, G.M.; Hegde, H.V.; Roy, S.; Hoti, S.L. Simultaneous determination of vasicine and vasicinone by High-performance liquid chromatography in roots of eight Sida species. AYU 2016, 37, 135–139. [Google Scholar] [CrossRef]
- Dey, T.; Dutta, P.; Manna, P.; Kalita, J.; Boruah, H.P.D.; Buragohain, A.K.; Unni, B. Anti-proliferative activities of vasicinone on lung carcinoma cells mediated via activation of both mitochondria-dependent and independent pathways. Biomol. Ther. 2018, 26, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.Z.; Azab, S.S.; Khattab, A.R.; Farag, M.A. Over a century since ephedrine discovery: An updated revisit to its pharmacological aspects, functionality and toxicity in comparison to its herbal extracts. Food Funct. 2021, 12, 9563–9582. [Google Scholar] [CrossRef]
- Karmvir-Jat, R.K. Extraction, isolation and standardization of herbal species Sida. J. Drug Deliv. Ther. 2022, 12, 62–75. Available online: https://jddtonline.info/index.php/jddt/article/view/5175 (accessed on 8 April 2025). [CrossRef]
- Imran, M.; Patil, S.P.; Abourehab, M.A.S.; Aeri, V.; Kesharwani, P. Quality by design-based optimization of Soxhlet extraction and identification of ephedrine by a HPTLC method for Sida rhombifolia and Sida Cordifolia. Biomed. Chromatogr. 2022, 36, e5479. [Google Scholar] [CrossRef]
- Selvadurai, S.; Senthamarai, R.; Kiruba, T.; Nagarajan, G.; Gayasuddin, M. Antidiabetic activity of whole plant of Sida spinosa Linn. (Malvaceae) on diabetic induced rats. IJRPP 2012, 1, 224–229. [Google Scholar]
- Głowacka, K.; Wiela-Hojeńska, A. Pseudoephedrine-Benefits and Risks. Int. J. Mol. Sci. 2021, 22, 5146. [Google Scholar] [CrossRef]
- Tanumihadja, M.; Mattulada, I.K.; Natsir, N.; Subehan, S.; Mandey, F.; Muslimin, L. Structural assessment of chemical constituent of Sidaguri (Sida rhombifolia Linn) and its ability to inhibit cyclooxygenase. Pesqui. Bras. Odontopediatria Clin. Integr. 2019, 19, e4773. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, X.; Cai, W.; Qiu, L. Hypaphorine attenuates lipopolysaccharide-induced endothelial inflammation via regulation of TLR4 and PPAR-γ dependent on PI3K/Akt/mTOR signal pathway. Int. J. Mol. Sci. 2017, 18, 844. [Google Scholar] [CrossRef]
- Joseph, B.; Ajisha, A.U.; Kumari, S.; Sujatha, S. Effect of bioactive compounds and its pharmaceutical activities of Sida cordifolia (Linn.). Int. J. Biol. Med. Res. 2011, 2, 1038–1042. [Google Scholar]
- Selvadurai, S.; Shanmugapandiyan, P. Preliminary phytochemical analysis on the leaves extracts of Sida acuta Burm.f. and Sida rhombifolia Linn. family Malvaceae. RJPT 2022, 15, 1512–1516. [Google Scholar] [CrossRef]
- Zhou, D.; Wei, H.; Jiang, Z.; Li, X.; Jiao, K.; Jia, X.; Hou, Y.; Li, N. Natural potential neuroinflammatory inhibitors from Alhagi sparsifolia Shap. Bioorg Med. Chem. Lett. 2017, 27, 973–978. [Google Scholar] [CrossRef]
- da Silva, R.G.; Almeida, T.C.; Reis, A.C.C.; Filho, S.A.V.; Brandão, G.C.; da Silva, G.N.; de Sousa, H.C.; de Almeida, V.L.; Lopes, J.C.D.; de Souza, G.H.B. In silico pharmacological prediction and cytotoxicity of flavonoids glycosides identified by UPLC-DAD-ESI-MS/MS in extracts of Humulus lupulus leaves cultivated in Brazil. Nat. Prod. Res. 2021, 35, 5918–5923. [Google Scholar] [CrossRef]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Myricetin: A Multifunctional Flavonol in Biomedicine. Curr. Pharmacol. Rep. 2022, 8, 48–61. [Google Scholar] [CrossRef]
- Kiziltaş, H. Comprehensive evaluation of Reseda lutea L. (Wild Mignonette) and 7 isolated flavonol glycosides: Determination of antioxidant activity, anti-Alzheimer, antidiabetic and cytotoxic effects with in vitro and in silico methods. Turk. J. Chem. 2022, 46, 1185–1198. [Google Scholar] [CrossRef]
- Prata, M.N.L.; Charlie-Silva, I.; Gomes, J.M.M.; Barra, A.; Berg, B.B.; Paiva, I.R.; Melo, D.C.; Klein, A.; Romero, M.G.M.C.; Oliveira, C.C.; et al. Anti-inflammatory and immune properties of the peltatoside, isolated from the leaves of Annona crassiflora Mart., in a new experimental model zebrafish. Fish. Shellfish. Immunol. 2020, 101, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Cristina da Costa-Araldi, I.; Piber-de Souza, T.; de Souza-Vencato, M.; de Andrade-Fortes, T.; Emanuelli-Mello, C.B.; Sorraila-de Oliveira, J.; Dornelles, G.L.; Melazzo-de Andrade, C.; Maciel, R.M.; Danesi, C.C.; et al. Preclinical safety assessment of the crude extract from Sida rhombifolia L. aerial parts in experimental models of acute and repeated-dose 28 days toxicity in rats. Regul. Toxicol. Pharmacol. 2021, 124, 104974. [Google Scholar] [CrossRef] [PubMed]
- Muneeswari, P.; Kunnathupara-Bhaskaran, S.; Poornima, K. Identification of active pharmaceuticals of Sida acuta Burm. f. leaves using GC-MS and HPTLC fingerprinting. IJPSR 2019, 10, 1194–1207. [Google Scholar] [CrossRef]
- Alrumaihi, F.; Almatroodi, S.A.; Alharbi, H.O.A.; Alwanian, W.M.; Alharbi, F.A.; Almatroudi, A.; Rahmani, A.H. Pharmacological potential of kaempferol, a flavonoid in the management of pathogenesis via modulation of inflammation and other biological activities. Molecules 2024, 29, 2007. [Google Scholar] [CrossRef]
- Li, X.; Tian, Y.; Wang, T.; Lin, Q.; Feng, X.; Jiang, Q.; Liu, Y.; Chen, D. Role of the p-Coumaroyl Moiety in the Antioxidant and Cytoprotective Effects of Flavonoid Glycosides: Comparison of Astragalin and Tiliroside. Molecules 2017, 22, 1165. [Google Scholar] [CrossRef]
- Padhy, I.; Dash, P.; Sahu, R.K.; Saha, G. Anti-inflammatory activity of isolated flavonoid from Sida cordifolia leaves extract. Int. J. Med. Res. Health Sci. 2019, 8, 75–81. [Google Scholar]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. Biomed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef] [PubMed]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul-Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-derived bioactive compounds in colon cancer treatment: An updated review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef]
- Maiyo, F.; Moodley, R.; Singh, M. Phytochemistry, cytotoxicity and apoptosis studies of β-sitosterol-3-O-glucoside and β-amyrin from Prunus africana. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Pooja, J.; Vivek, K.; Deepti, T.; Hritika, G.; Aman, A.; Vijai, M. Phytochemical composition of Sida rhombifolia ssp. retusa (L.) Bross.: A comprehensive GC/MS analysis. Xi’an Shiyou Daxue Xuebao (Ziran Kexue Ban) 2024, 67, 103–123. [Google Scholar] [CrossRef]
- Ganesh, M.; Mohankumar, M. Extraction and identification of bioactive components in Sida cordata (Burm.f.) using gas chromatography-mass spectrometry. J. Food Sci. Technol. 2017, 54, 3082–3091. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra-Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Huang, Z.R.; Lin, Y.K.; Fang, J.Y. Biological and pharmacological activities of squalene and related compounds: Potential uses in cosmetic dermatology. Molecules 2009, 14, 540–554. [Google Scholar] [CrossRef]
- Moeurng, S.; Posridee, K.; Kamkaew, A.; Thaiudom, S.; Oonsivilai, A.; Oonsivilai, R. Identification of Pheophytin a and Hydroxy Pheophytin a from Rang Chuet (Thunbergia laurifolia Linn.) as Potent NQO-1 Inducers in Liver Cells. Foods 2024, 13, 1443. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Ashraful-Alam, M.; Rahman, M.; Rashid, M. Phytochemical and Biological Investigations of Sida rhomboidea Linn. IJAM 2008, 7, 10–13. [Google Scholar]
- El Omari, N.; Jaouadi, I.; Lahyaoui, M.; Benali, T.; Taha, D.; Bakrim, S.; El Menyiy, N.; El Kamari, F.; Zengin, G.; Bangar, S.P.; et al. Natural sources, pharmacological properties, and health benefits of daucosterol: Versatility of actions. Appl. Sci. 2022, 12, 5779. [Google Scholar] [CrossRef]
- Si, W.; Chen, Z.; Bei, J.; Chang, S.; Zheng, Y.; Gao, L.; Zhao, G.; Li, X.; Zhang, D. Stigmasterol alleviates neuropathic pain by reducing Schwann cell-macrophage cascade in DRG by modulating IL-34/CSF1R. CNS Neurosci. Ther. 2024, 30, e14657. [Google Scholar] [CrossRef]
- Banni, M.; Jayaraj, M. Identification of bioactive compounds of leaf extracts of Sida cordata (Burm.f.) Borss. Waalk. by GC/MS analysis. Appl. Biochem. Biotechnol. 2023, 195, 556–572. [Google Scholar] [CrossRef]
- Yadav, S.; Aggarwal, P.; Khan, F.; Khodve, G.; Padhy, D.S.; Yadav, P.; Banerjee, S. β-sitosterol protects against aluminium chloride-mediated neurotoxicity. Curr. Alzheimer Res. 2023, 20, 29–37. [Google Scholar] [CrossRef]
- Tripathi, N.; Kumar, S.; Singh, R.; Singh, C.J.; Singh, P.; Varshney, V. Isolation and identification of gamma-sitosterol by GC-MS from roots of Girardinia heterophylla. Orient. J. Chem. 2013, 29, 705–707. [Google Scholar] [CrossRef]
- da Rosa, H.S.; Salgueiro, A.C.; Colpo, A.Z.; Paula, F.R.; Mendez, A.S.; Folmer, V. Sida tuberculata (Malvaceae): A study based on development of extractive system and in silico and in vitro properties. Braz. J. Med. Biol. Res. 2016, 49, e5282. [Google Scholar] [CrossRef]
- Gong, X.; Bai, M.; Lu, G.; Yang, B.; Lei, T.; Shizhi, J. Total synthesis of murraol, (E)-Suberenol and toward the collective total synthesis of exotines A, cnidimonins A-Cetc. Tetrahedron 2022, 126, 133061. [Google Scholar] [CrossRef]
- Tavakoli, S.; Delnavazi, M.R.; Hadjiaghaee, R.; Jafari-Nodooshan, S.; Khalighi-Sigaroodi, F.; Akhbari, M.; Hadjiakhoondi, A.; Yassa, N. Bioactive coumarins from the roots and fruits of Ferulago trifida Boiss., an endemic species to Iran. Nat. Prod. Res. 2018, 32, 2724–2728. [Google Scholar] [CrossRef]
- Parama, D.; Girisa, S.; Khatoon, E.; Kumar, A.; Alqahtani, M.S.; Abbas, M.; Sethi, G.; Kunnumakkara, A.B. An overview of the pharmacological activities of scopoletin against different chronic diseases. Pharmacol. Res. 2022, 179, 106202. [Google Scholar] [CrossRef]
- Nagarajan, S.; Murali, R. An overview of the traditional importance, phytochemistry, and pharmacological properties of Sida acuta Burm.f. Ann. Phytomed 2022, 11, 245–254. [Google Scholar] [CrossRef]
- Amine, H.; Benomar, Y.; Taouis, M. Publisher Correction: Palmitic acid promotes resistin induced insulin resistance and inflammation in SH SY5Y human neuroblastoma. Sci. Rep. 2021, 11, 12935. [Google Scholar] [CrossRef]
- Santa-María, C.; López-Enríquez, S.; Montserrat-de la Paz, S.; Geniz, I.; Reyes-Quiroz, M.E.; Moreno, M.; Palomares, F.; Sobrino, F.; Alba, G. Update on anti-inflammatory molecular mechanisms induced by Oleic Acid. Nutrients 2023, 15, 224. [Google Scholar] [CrossRef] [PubMed]
- Nava-Lauson, C.B.; Tiberti, S.; Corsetto, P.A.; Conte, F.; Tyagi, P.; Machwirth, M.; Ebert, S.; Loffreda, A.; Scheller, L.; Sheta, D.; et al. Linoleic acid potentiates CD8+ T cell metabolic fitness and antitumor immunity. Cell Metab. 2023, 35, 633–650.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Q.; Zhang, L.; Wang, Q.; Yang, Z.; Liu, J.; Feng, L. Caffeic acid reduces A53T α-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol. Res. 2019, 150, 104538. [Google Scholar] [CrossRef]
- Kurniawan, Y.S.; Priyangga, K.T.A.; Jumina; Pranowo, H.D.; Sholikhah, E.N.; Zulkarnain, A.K.; Fatimi, H.A.; Julianus, J. An update on the anticancer activity of xanthone derivatives: A review. Pharmaceuticals 2021, 14, 1144. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, Z.; Chen, L.; Peng, X.; Luan, F.; Hu, J.; Xie, H.; Liu, R.; Zeng, N. Rosmarinic acid alleviate CORT-induced depressive-like behavior by promoting neurogenesis and regulating BDNF/TrkB/PI3K signaling axis. Biomed. Pharmacother. 2024, 170, 115994. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Goldbeck, R.; dos Santos-Dantas, W.; Squina, F. Ferulic acid and derivatives: Molecules with potential application in the pharmaceutical field. Braz. J. Pharm. Sci. 2013, 49, 395–411. [Google Scholar] [CrossRef]
- Chumpol, W.; Tavichakorntrakool, R.; Lulitanond, A.; Daduang, J.; Saisud, P.; Sribenjalux, P.; Prasongwatana, V.; Boonsiri, P. The antibacterial activity of the aqueous extract of Sida acuta Burm. F. Southeast. Asian J. Trop. Med. Public. Health 2018, 49, 285–291. [Google Scholar]
- Sreedevi, C.D.; Latha, P.G.; Ancy, P.; Suja, S.R.; Shyamal, S.; Shine, V.J.; Sini, S.; Anuja, G.I.; Rajasekharan, S. Hepatoprotective studies on Sida acuta Burm. f. J. Ethnopharmacol. 2009, 124, 171–175. [Google Scholar] [CrossRef]
- Ezeako, E.C.; Nworah, F.N.; Osuji, D.O. Phytocompounds, antioxidant potential, and inhibitory actions of ethanolic leaf fraction of Sida linifolia Linn. (Malvaceae) on enzymes linked to infammation, diabetes, and neurological disorders. Futur. J. Pharm. Sci. 2023, 9, 73. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Herbal beverages: Bioactive compounds and their role in disease risk reduction—A review. J. Tradit. Complement. Med. 2018, 8, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and Its Effects on the Vascular System. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef]
- Pubmed. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=Sida+rhombifolia&size=50 (accessed on 13 May 2025).
- Hui, Y.; Wang, X.; Yu, Z.; Fan, X.; Cui, B.; Zhao, T.; Mao, L.; Feng, H.; Lin, L.; Yu, Q.; et al. Scoparone as a therapeutic drug in liver diseases: Pharmacology, pharmacokinetics and molecular mechanisms of action. Pharmacol. Res. 2020, 160, 105170. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, D.; Kong, L.; Du, W.; Qu, L.; Yang, Y.; Rao, G.; Huang, F.; Tong, X. Vasicine attenuates atherosclerosis via lipid regulation, inflammation inhibition, and autophagy activation in ApoE-/- mice. Int. Immunopharmacol. 2024, 142, 112996. [Google Scholar] [CrossRef]
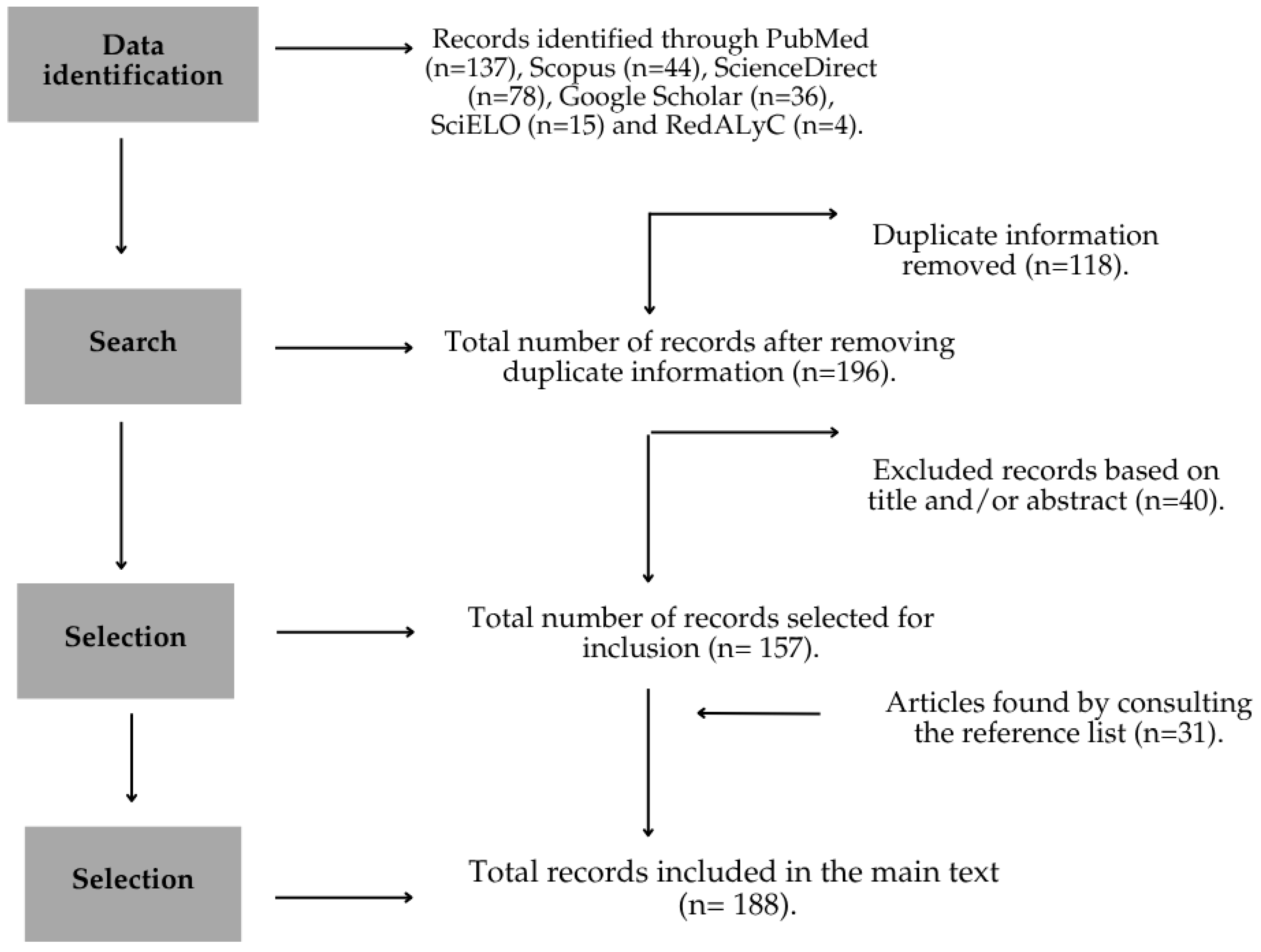
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Ferrer, E.; Herrera-Ruiz, M.; Campos-Vidal, Y.; Flores-Franco, G.; Monterrosas-Brisson, N. Sida L.: Ethnobotany, Pharmacology, and Phytochemistry: A Review. Plants 2025, 14, 3115. https://doi.org/10.3390/plants14193115
Jiménez-Ferrer E, Herrera-Ruiz M, Campos-Vidal Y, Flores-Franco G, Monterrosas-Brisson N. Sida L.: Ethnobotany, Pharmacology, and Phytochemistry: A Review. Plants. 2025; 14(19):3115. https://doi.org/10.3390/plants14193115
Chicago/Turabian StyleJiménez-Ferrer, Enrique, Maribel Herrera-Ruiz, Yrvinn Campos-Vidal, Gabriel Flores-Franco, and Nayeli Monterrosas-Brisson. 2025. "Sida L.: Ethnobotany, Pharmacology, and Phytochemistry: A Review" Plants 14, no. 19: 3115. https://doi.org/10.3390/plants14193115
APA StyleJiménez-Ferrer, E., Herrera-Ruiz, M., Campos-Vidal, Y., Flores-Franco, G., & Monterrosas-Brisson, N. (2025). Sida L.: Ethnobotany, Pharmacology, and Phytochemistry: A Review. Plants, 14(19), 3115. https://doi.org/10.3390/plants14193115







