Abstract
Salix suchowensis is an ideal model organism for investigating nitrogen (N) transport mechanisms due to its low N-input requirements. Accurate quantification of gene expression is essential for elucidating these processes, with quantitative real-time PCR (RT-qPCR) being the preferred method. However, the identification of stable reference genes for normalization in Salix suchowensis under varying N conditions remains unresolved. In this study, thirteen commonly employed candidate reference genes were evaluated across root, stem, and leaf tissues, under four N treatments (NH4NO3, NH4+, NO3−, and N deficiency). Five genes (UBQ1, UBQ3, 18S, H2A2, and H2B2) were excluded due to poor amplification efficiency or irregular melting curves. The remaining eight genes were further assessed for expression stability using the geNorm, NormFinder, and BestKeeper algorithms. Integrated ranking via RefFinder identified EF1α, EFβ, and αTUB as the most stable reference genes. GeNorm analysis suggested that two reference genes were sufficient for reliable normalization. Validation using the N-responsive gene SsAMT1 and SsNRT2 confirmed the stability of EF1α, EFβ, and αTUB as suitable reference genes. Based on comprehensive stability assessments and experimental validation, we recommended EF1α + αTUB as optimal reference gene pairs for RT-qPCR normalization under varying N conditions. Furthermore, the consistent expression of EF1α and αTUB across nine willow genotypes highlighted their broader applicability within Salix species. This study provides valuable methodological guidance for advancing molecular research on N transport in woody perennial plants.
1. Introduction
Nitrogen (N) is a macronutrient for plant growth and development [1]. In soils, N exists in both inorganic and organic forms, with inorganic ammonium (NH4+) and nitrate (NO3−) representing the primary inorganic N sources taken up by plants. Typically, NO3− is dominate in well-aerated soils, whereas NH4+ tends to accumulate in acidic or anaerobic environments where nitrification is inhibited [2,3,4]. The preference for NH4+ or NO3− as a primary N source is fundamentally dependent on plant genotype, which reflects the species’ evolutionary adaptation to its native soil environment. For instance, conifers from boreal forests exhibited a marked preference for NH4+, having evolved on acidic, NH4+-rich soils where nitrification is low [5]. Meanwhile, species like Populus spp. were often assumed to prefer NO3− due to their association with floodplains and high nitrate reductase activity [6]. Moreover, acid-tolerant species such as Oxalis acetosella, adapted to high-acid environments, grew equally well with NH4+ alone. In contrast, Urtica dioica, a species from less acidic soils, strongly favored the presence of NO3− [7]. Different N forms distinctly influence plant physiological responses, especially affecting root system architecture [8]. As the principal site for N uptake, roots exhibit high morphological plasticity in response to environmental cues [9]. Studies have demonstrated that NO3− supply stimulates the elongation of primary and secondary lateral roots, whereas NH4+ availability enhances the density of secondary and tertiary lateral roots, resulting in shorter but densely branched root systems [10,11]. A high concentration of NH4+ negatively impacts leaf development by inhibiting cell expansion, impairing osmotic adjustment, and disrupting hormonal signaling. Ammonium-preferring plants usually exhibit smaller leaf area and lower leaf biomass compared to nitrate-preferring plants, ultimately resulting in reduced carbon accumulation [12]. In contrast, NO3−, apart from a portion stored in vacuoles, needs to be reduced to NH4+ before being assimilated into amino acids for protein synthesis, a process that is an energetically demanding [13,14]. Despite this energy requirement, NO3− as a sole N source does not exert toxic effects on plant growth, whereas NH4+ can be toxic and often results in growth inhibition [15,16].
Salix suchowensis is a compact shrub willow predominantly found in temperate regions of East and Central China [17,18]. Typically reaching heights of up to 3 m, it forms dense stands that contribute significantly to ecosystem stability. Remarkably, Salix suchowensis achieves sexual maturity within one year, allowing multiple harvests within short timeframes [19]. This trait makes it particularly suitable for short-rotation coppice (SRC) systems, in which cyclical harvesting occurs at intervals of 2–3 years [20]. The species’ natural ability to redistribute nutrients during its perennial growth cycle supported its classification as a low-N-input crop [21], enhancing its values for urban landscaping, as well as for bioenergy and industrial applications [19]. Physiological studies have further revealed that Salix suchowensis possessed exceptional N-use traits. It was noted that even minimal N levels (10 μM NH4+) were sufficient to stimulate the growth. A series of 15N-labeling assays revealed that within 1 min of NH4+ exposure, the process of uptake, assimilation into amino-N, and transport to aerial tissues were completed. This rapid N uptake and transport abilities underlined the species’ adaptation to environments with extremely low N availability [22], a trait not reported in other willow species. Despite these insights, the molecular mechanisms underlying N uptake and source-to-sink transport in Salix remained largely unknown. Importantly, the completion of the Salix suchowensis genome provides a valuable resource for exploring the genetic mechanisms of N transport processes in perennial woody species [23]. Investigating the spatial and temporal expression patterns of candidate genes will be essential for elucidating their functional roles.
Quantitative real-time PCR (RT-qPCR) has been widely applied for analysis of gene expression patterns, owing to its high accuracy, reproducibility, sensitivity, and high-throughput capability [24,25]. To ensure the accuracy and reliability of RT-qPCR results and control for variations between and within samples, standardization using one or more internal reference genes was essential [26]. These reference genes serve as internal controls to normalize gene expression data and minimize experimental errors [27]. However, no single reference gene exhibits universally stable expression among different plant species, tissues, and experimental conditions [28]. For example, in peach, TEF2 (Translation elongation factor 2), UBQ10 (Ubiquitin protein 10), and RPII (RNA polymerase II) were the most stable reference genes [29], whereas in rice, UBQ5 and eEF-1α (Elongation factor-1α) were suitable [30]. In pepper, UBI-3 (Ubiquitin-conjugating protein), β-TUB (Beta tubulin), and GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) were identified as the most stable reference genes [31]. In addition, even within the same species, different tissues or treatment conditions can influence the stability of internal reference genes. In Salix species, ACT (Actin) and DnaJ49 (Chaperone protein DnaJ49) were found to be the most stably expressed reference genes in male and female flowers of Salix suchowensis at different stages of development [32]. In Salix matsudana, α-TUB2 and DnaJ49 were stably expressed across different tissues under various stress conditions [33], while in Salix viminalis leaves, TIP41 (Type 2A phosphatase Activator) exhibited the highest stability [34]. In Salix psammophila, the optimal reference genes selected for gene expression analysis were UBC (Ubiquitin-conjugating enzyme E2) and LTA4H (Leukotriene A-4 hydrolase homolog) for heat or drought treatment, HIS (Histone superfamily protein H3) and ARF2 (ADP-ribosylation factor 2) for cold treatment, OTU (OTU-like cysteine protease family protein) and Actin 7 for salt treatment [35]. Although reference gene studies existed in other willows, suitable reference genes for RT-qPCR normalization under variable N conditions and across diverse tissues in willow species have not been identified. Given the genomic resources of Salix suchowensis and its unique N physiology, identifying and validating stable reference genes is essential for supporting accurate gene expression studies in this system.
To address this gap, the present study aimed to identify stable reference genes for accurate gene expression analysis in high N-use efficiency Salix suchowensis exposed to different N forms across root, stem, and leaf tissues. Thirteen candidate reference genes (EF1α, EFβ, αTUB, βTUB, GAPDH, 18s, Actin1, Actin2, H2A1, H2A2, UBQ1, UBQ3, and H2B2) were selected for evaluation. The stability of these candidate genes was systematically assessed using geNorm, NormFinder and BestKeeper. Comprehensive analysis identified the αTUB/EF1α or αTUB/EFβ combinations as the most suitable reference gene pairs for RT-qPCR normalization in Salix suchowensis tissues under varying N treatments. Furthermore, the stability and suitability of the selected reference genes were validated under long-term N treatments and across diverse Salix genotypes, with N-responsive target genes further confirming their reliability. The findings provide a crucial foundation for future molecular studies on N uptake and transport mechanisms in Salix suchowensis and related woody plants.
2. Results
2.1. Verification of Primer Specificity and Amplification Efficiency
Thirteen commonly used candidate reference genes (EF1α, EFβ, αTUB, βTUB, GAPDH, 18s, Actin1, Actin2, H2A1, H2A2, UBQ1, UBQ3, and H2B2) were initially selected for analysis in Salix suchowensis (Table 1, File S1). Primer amplification efficiency and specificity were assessed using PCR and RT-qPCR. The amplification products of these candidates were 100–200 bp in length (Figure S1A). Among these, UBQ1, UBQ3, and H2B2 were minimally detectable and the RT-qPCR melting curve revealed that UBQ1 and H2B2 did not yield a single melting peak (Figures S1 and S2). Consequently, UBQ1, UBQ3, and H2B2 were excluded from further analysis. Analysis of PCR gel electrophoresis, RT-qPCR cycle threshold (Ct) values, and melting curves indicated that the remaining ten candidate reference genes produced distinct single bands, Ct values below 30, and single-peaked melting curves (Figures S1 and S2). Standard curves were further constructed for other ten candidates, showing that the amplification efficiencies of 18S and H2A2 were 237% and 155%, respectively, exceeding acceptable ranges (Figure S3). The remaining eight primer pairs demonstrated amplification efficiencies within the acceptable range of 90–110% and exhibited linear regression correlation coefficients (R2) greater than 0.99 (Figure S3). Based on their amplification efficiency and product specificity, these eight candidate reference genes were selected for further stability evaluation.

Table 1.
General information of the thirteen candidate reference genes in Salix suchowensis.
2.2. Expression Profiles of Reference Genes in Roots, Stems, and Leaves Under N Treatments
In gene expression analysis, the Ct value is a critical parameter reflecting the expression level of a gene, with lower Ct values indicating higher gene expression. Ideally, the Ct values of a reference gene should fall within the range of 15–30. In this study, Ct values of eight candidate reference genes were measured across various experimental conditions, including different tissues (roots, stems, and leaves) and N treatments (NH4NO3, NH4+, NO3−, and −N) (Figure 1). Among the candidate genes, EF1α exhibited the highest average expression level, with an average Ct value of 20.59, while Actin2 showed the lowest average expression level, with an average Ct value of 28.21. The Ct values for βTUB ranged from 23.44 to 28.71, displaying the least variation in the expression levels among the eight candidates (Figure 1). In contrast, H2A1 exhibited the most variability, with Ct values being 23.72–37.21 (Figure 1). These findings highlighted significant differences in the expression levels of these candidate reference genes across tissues and treatments.
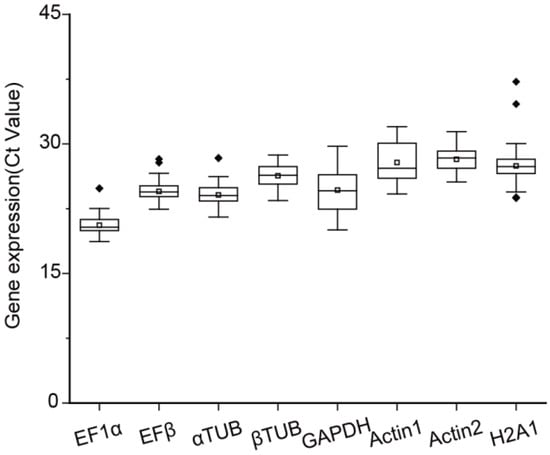
Figure 1.
Expression profile of the eight candidate reference genes in root, stem, and leaf tissues of Salix suchowensis seedlings under different N treatments for 24 h. The boxplot presented the concentrated range of the cycle threshold (Ct) values, with the horizontal line inside representing the median, the white box indicating the mean, and the dots (◆) denoting outliers.
2.3. Expression Stability Analysis of the Selected Eight Candidate Reference Genes
The expression stability of eight candidate reference genes was assessed using geNorm, NormFinder, and BestKeeper tools. GeNorm analysis was conducted to calculate the M values of these genes, where the M value serves as an indicator of expression stability. Genes with M values greater than 1.5 were considered unsuitable as reference genes due to their lower stability, whereas those with M values below 1.5 were deemed appropriate for use [26]. Under N treatments, all candidates, except GAPDH, had M values below 1.5, signifying their suitability as reference genes (Figure 2A, Table 2). Among these, EF1α/EFβ and αTUB/βTUB exhibited the smallest M values, making them the most stable reference genes under N treatments (Figure 2A, Table 2). Additionally, the M values of all reference genes across different organs (roots, stems, and leaves) were below 1.5, highlighting their relatively stable expression patterns (Figure 2B, Table 2). Specifically, EF1α and αTUB were the most stable reference genes in roots, stems, and leaves, respectively (Figure 2B, Table 2). Under all experimental conditions, EFβ and EF1α had an M value of 0.35, indicating they were the most stable internal reference genes overall (Figure 2C, Table 2). GeNorm was further used to calculate the paired variation (V) values, which determine the optimal number of reference genes required for normalization (Figure 3). The paired variation threshold was set at 0.15, with Vn/n + 1 < 0.15 indicating the optimal number of reference genes. Across all experimental conditions, the variation value V2/3 was 0.148, which is less than 0.15, suggesting that two reference genes were sufficient for accurate normalization (Figure 3).
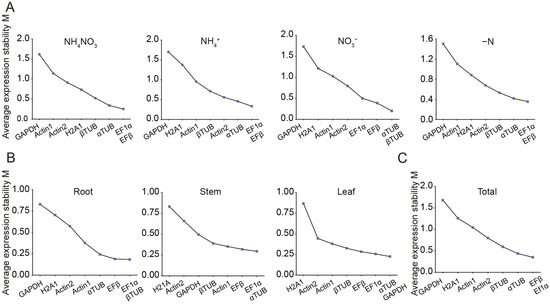
Figure 2.
The average expression stability values (M) of the eight candidate reference genes based on geNorm algorithm. M values of candidate reference genes across different nitrogen treatments (A), organs (B), and the entire sample set (C). Genes with lower M values exhibited higher stability. The least stable genes were shown on the left, while the most stable ones were displayed on the right.

Table 2.
Stability analysis of eight candidate reference genes using the geNorm calculation method. EF1α, EFβ, and αTUB, identified as the most stable reference genes, were marked in dusty pink, aqua, and grey-purple, respectively.
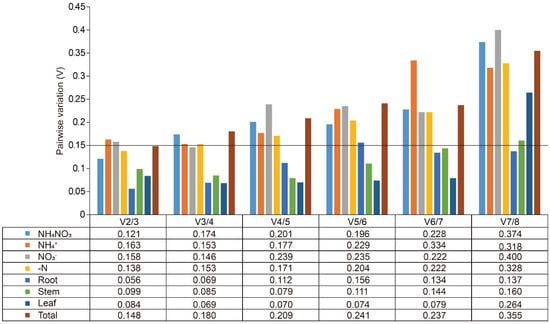
Figure 3.
Pairwise variation (V) of the eight candidate reference genes in Salix suchowensis. V was calculated using geNorm to identify the optimal number of reference genes required for accurate normalization under different treatments or various tissues, using a threshold of 0.15.
The NormFinder algorithm evaluated gene expression stability by employing an analysis of variance to calculate the stability value (S value) [36]. A lower S value indicated greater stability of gene expression. In the experimental samples subjected to various N treatments, αTUB emerged as the most stable reference gene under NH4NO3 and NO3− treatments, whereas EFβ exhibited the highest stability under NH4+ and −N treatment (Table 3). When analyzing different plant organs, αTUB was identified as the most stable reference gene in both roots and leaves, while EF1α demonstrated the highest stability in stem tissues (Table 3). Overall, EFβ proved to be the most consistently stable reference gene, displaying superior stability across both N treatments and different organs (Table 3). This suggests EFβ’s suitability as a reliable internal control for normalizing gene expression data in a wide range of experimental conditions.

Table 3.
Stability analysis of eight candidate reference genes using the NormFinder calculation method. EF1α, EFβ, and αTUB, identified as the most stable reference genes, were marked in dusty pink, aqua, and grey-purple, respectively.
The Bestkeeper software evaluated the raw Ct values of each candidate reference gene obtained through RT-qPCR and ranked their stability based the standard deviation (SD) and coefficient of variance (CV). A lower SD value indicated higher stability in gene expression. When analyzed across both N treatments and different organs, the stability ranking of the eight candidate reference genes was as follows: EFβ > EF1α > αTUB > βTUB > Actin2 > H2A1 > Actin1 > GAPDH (Table 4). This ranking highlighted EFβ as the most stable reference gene, while GAPDH exhibited the lowest stability.

Table 4.
Calculation of the SD and CV of the Ct values of eight candidate reference genes using the BestKeeper tool. EF1α, EFβ, and αTUB, identified as the most stable reference genes, were marked in dusty pink, aqua, and grey-purple, respectively.
The results obtained from geNorm, NormFinder, and BestKeeper showed some variations in the stability rankings of the candidate reference genes. To address these differences and ensure a more comprehensive and reliable evaluation, RefFinder was employed to integrate and rank the results from these algorithms. This approach provided a more robust analysis of the stability of the eight candidate reference genes. Based on the RefFinder analysis, αTUB, EFβ and EF1α were identified as the most stable reference genes (Table 5). To further explore the overlap of candidate reference genes under different conditions, a Venn diagram was generated to illustrate the shared stable reference genes across both N treatments and various plant organs. The results revealed that EFβ, αTUB, EF1α, and βTUB were stable under N treatments, while αTUB, EF1α, and EFβ were consistently stable across different organs (Figure 4).

Table 5.
Comprehensive ranking of the expression stability of the eight candidate reference genes based on the RefFinder tool. EF1α, EFβ, and αTUB, identified as the most stable reference genes, were marked in dusty pink, aqua, and grey-purple, respectively.
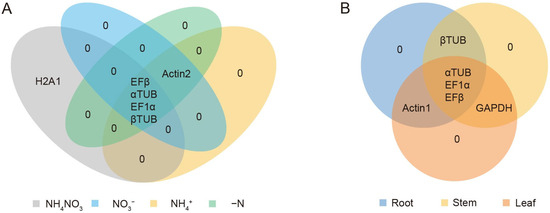
Figure 4.
Venn diagram analysis of the top five most stable candidate reference genes for different nitrogen treatment (A) or various tissues (B). The top five most stable genes were generated by RefFinder: https://blooge.cn/RefFinder/ (accessed on 3 January 2024).
2.4. Validation of the Stability of the Selected Candidate Reference Genes
To evaluate the stability of the selected reference genes under prolonged N regimes, we assessed the expression of the most stable (EF1α, EFβ, αTUB) and the least stable (GAPDH, H2A1) reference genes in the roots, stems, and leaves of Salix suchowensis subjected to different N treatments for 5 days. The Ct values for EF1α, EFβ, and αTUB remained within narrow ranges (17.12–22.55, 21.03–25.71, and 19.17–25.93, respectively), indicating stable expression, whereas GAPDH and H2A1 exhibited greater variability, with Ct values ranging from 18.01 to 29.74 and from 23.18 to 34.26, respectively (Figure 5A).
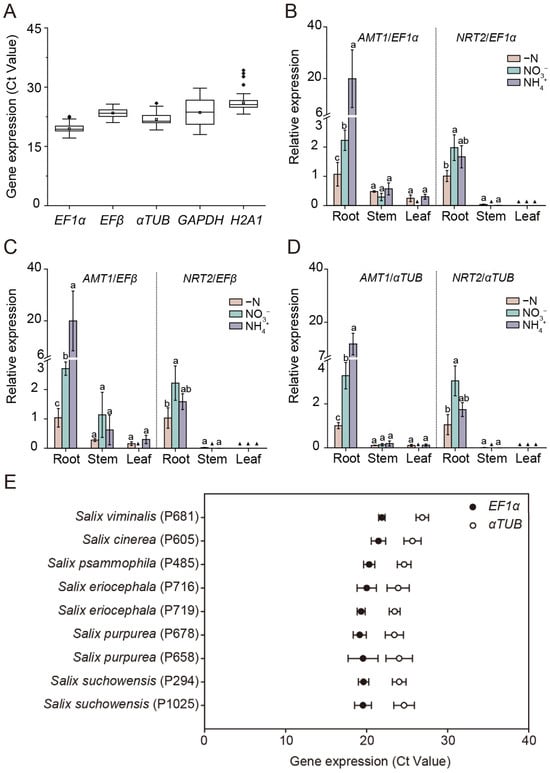
Figure 5.
Validation of the stable reference genes under long-term nitrogen regimes, with target genes, and across different Salix genotypes. (A) Expression profiles of the most stable (EF1α, EFβ, αTUB) and the least stable (GAPDH, H2A1) reference genes in roots, stems, and leaves of Salix suchowensis seedlings under different nitrogen (N) treatments for 5 days. Boxplots show cycle threshold (Ct) distributions, with the horizontal line indicating the median and the white box the mean. (B–D) Expression of ammonium transporter 1 (SsAMT1) and nitrate transporter 2 (SsNRT2) in roots, stems, and leaves of Salix suchowensis seedlings exposed to different N treatments for 24 h. Relative expression levels were normalized to EF1α (B), EFβ (C), and αTUB (D), and calculated using the 2−ΔΔCt method, with gene expression in roots under −N treatment serving as the control. Different letters indicate significant differences at p < 0.05 of the same gene among different N treatments. (▲) Value lower than 0.001. (E) Expression profile of EF1α and αTUB in roots of diverse Salix genotypes. Values in panels (B–E) are means ± SD (n = 3–4 biological replicates).
To further verify reference gene performance under N-responsive conditions, we examined the expression patterns of ammonium transporter 1 (SsAMT1) and nitrate transporter 2 (SsNRT2), two well-established marker genes expressed in root cells that mediated the uptake and transport of NH4+ and NO3− from the soil [37,38,39,40]. Normalization using EF1α, EFβ, αTUB or their combination yielded consistent expression profiles (Figure 5B–D and Figure S4). Specifically, SsAMT1 exhibited higher expression levels in roots and was upregulated by NH4+ treatment, while the transcript abundance of SsNRT2 was much higher in roots than that in stems or leaves, and its expression was also strongly induced by NO3− supply (Figure 5B–D and Figure S4). The results confirmed that EF1α, EFβ and αTUB were highly stable reference genes for normalizing gene expression in the roots, stems and leaves of Salix suchowensis under various N treatments. Considering that EF1α and EFβ belong to the same gene family, it was recommended to select EF1α + αTUB or EFβ + αTUB as the optimal combination of reference genes for normalization in Salix Suchowensis.
To extend validation across genetic backgrounds, we further examined the Ct values of EF1α and αTUB in the roots of nine willow genotypes. Both genes displayed narrow Ct ranges (17.51–22.42 and 22.23–27.69, respectively) with minimal variation among genotypes (Figure 5E), supporting their robustness and broader applicability as reference genes across Salix species.
3. Discussion
RT-qPCR remains a cornerstone method for gene expression analysis due to its sensitivity, specificity, and reproducibility [41]. However, its accuracy highly depends on the stability of internal reference genes, which serve as critical normalization controls to account for technical variability. Indeed, these genes should exhibit stable expression across diverse tissue types and experimental conditions [42]. However, expression stability is often context-dependent. For example, a gene stable in floral tissues may vary significantly in vegetative organs due to differences in metabolic activity and gene regulation. In Salix suchowensis, ACT displayed consistent expression in male and female flowers at varying developmental stages but fluctuated across roots, stems, and leaves (Figure 2, Table 4) [32]. The results aligned with the findings that many reference genes commonly used in vegetative tissues are not stably expressed in seeds and pollen [43,44]. Given its potential as a model for studying N transport in woody plants [22], validating suitable reference genes in Salix suchowensis under varying N conditions is essential.
Studies across plant species have consistently shown that no single reference gene maintains universal stability under N treatments. For instance, CACS (Clathrin adaptor complex subunit), TIP41, F-box protein and EFα were most stable in cucumbers [45], while ELF1B (Eukaryotic elongation factor 1β) and ACT11 (Actin) performed best in soybean [46]. Similarly, Actin and α-Tub (α-tubulin) were most reliable in salt-tolerant plant Salicornia europaea [47]. These examples underscore the need for species- and condition-specific validation, as gene stability is influenced by both genetic background and environmental conditions. In this study, EF1α, EFβ, and αTUB were identified as the most stable reference genes in Salix suchowensis across tissues and varying N treatment conditions (Figure 4, Table 5). Their stability likely reflected their roles in fundamental cellular processes less influenced by N fluctuations. In contrast, Actin, despite its widespread use, exhibited relatively low expression stability under the tested conditions (Figure 2, Table 5), reinforcing the importance of empirical validation over traditional assumptions.
Plant EF1α and EF1β are involved in protein synthesis, during which the nascent polypeptide chain extends by one amino acid residue during one elongation cycle. Therein, EF1α delivered aminoacyl-tRNA to the ribosomal A-site during elongation [48]. It has been reported as one of the most stable internal references under diverse abiotic stress conditions in pearl millet [49], in different tissues of rice [50], and In Populus euphratica under cold stress [51]. Similarly, EFβ has been shown to maintain stable expression during soybean development [52] and was also identified as a universally reliable reference gene in Fucus distichus [53]. αTUB, a key regulator of microtubule form and function, enables plants to dynamically sculpt their cytoskeleton for development, stress adaptation, and cellular innovation [54,55]. It has demonstrated high stability in cucumber treated with multiple hormones [56] and across various experimental conditions in Toona ciliata [57]. Stability assessments using geNorm, NormFinder, and BestKeeper revealed both overlaps and discrepancies in gene rankings. GeNorm identified EF1α, EFβ, and αTUB as the most stable genes across all conditions (Figure 2). NormFinder supported αTUB and EFβ as top candidates under N treatments and EF1α as the most stable in stems (Table 3). BestKeeper, using a different statistical approach, also ranked EFβ, EF1α, and αTUB among the top candidates (Table 4). These consistent results across multiple algorithms enhance the reliability of the selected reference genes. Notably, geNorm’s paired variation value confirmed that two reference genes are sufficient for reliable normalization, reducing experimental burden without compromising accuracy (Figure 3). Normalizing data with two or more reference genes provides greater reliability compared to using a single gene [36,58]. Importantly, functional validation using SsAMT1 and SsNRT2 confirmed that normalization with EF1α, EFβ, and αTUB produced biologically meaningful expression patterns (Figure 5B–D), consistent with the roles of AMT1 in NH4+ uptake and NRT2 in NO3− transport [40]. This demonstrated that appropriate reference gene selection not only improves data reliability but also ensures correct interpretation of nutrient-responsive expression profiles. The observed stability of EF1α and αTUB across nine willow genotypes further suggested their utility in natural populations (Figure 5E). Nevertheless, the present evaluation was restricted to root tissues. Reference gene stability can be influenced by multiple factors, including primer efficiency, experimental treatments, and tissue specificity. Thus, while our results provided valuable preliminary evidence, the utility of EF1α and αTUB as stable reference genes across diverse willow genotypes requires further validation. Comprehensive assessments under a wider range of conditions and tissues would strengthen their application in future gene expression studies of natural willow populations. In Salix suchowensis, EF1α and αTUB are evidently stable reference gene pairs for RT-qPCR-based gene expression analyses under N treatments.
In conclusion, our findings emphasized that reference gene stability is not universal and that commonly used genes such as Actin may not be appropriate in all contexts. Therefore, the selection of reference genes should be guided by empirical validation rather than convention. In this study, the identification of EF1α, EFβ, and αTUB as robust reference genes was valuable for Salix research. Their applicability across tissues, N treatments, and genotypes enhanced the reproducibility of RT-qPCR studies and established a methodological foundation for future work on N metabolism and broader physiological processes in woody plants. Further studies evaluating these genes under additional stress conditions would help to establish even broader applicability and enhance the reliability of gene expression normalization in forest genetics research.
4. Materials and Methods
4.1. Growth of Salix suchowensis and Harvest
Cuttings (1 cm in diameter and 12 cm in length) taken from annual branches of willow species were cultivated hydroponically under natural light in a greenhouse at Yangzhou University, following the method described by Guo et al. [59]. Briefly, uniform cuttings were initially grown in plastic containers with 14 L of water for three weeks, then transferred to 1/4-strength modified Hoagland nutrient solutions for one week. After four weeks, the seedlings of Salix suchowensis (ecotype NANJING) were moved to N-free nutrient solutions for one week. Subsequently, they were treated with solutions containing 0.3 mM NH4NO3, 0.3 mM NH4+, 0.3 mM NO3−, or without N for 24 h and 5 days. After the treatment, roots, stems, and leaves of each plant were harvested and immediately frozen in liquid N for RNA extraction. For the validation assay across different willow genotypes, seedlings of nine genotypes of willows were grown for five weeks in Hoagland nutrient solutions. Subsequently, root tissues from each genotype were then harvested for RNA extraction. The solutions were refreshed every three days. Each treatment was performed with four biological replicates.
4.2. RNA Extraction, First-Strand cDNA Synthesis, and RT-qPCR Analysis
Total RNA of willow tissues was isolated using the FastPure Universal Plant Total RNA Isolation Kit (Vazyme, Nanjing, China) and reverse transcribed into cDNA using the HiScript II Q RT SuperMix with a gDNA wiper (Vazyme, Nanjing, China). RT-qPCR was conducted with the ChamQ SYBR qPCR Master Mix (Vazyme, Nanjing, China) on a BioRad CFX Connect Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Each reaction was carried out in a 20-μL mixture containing 10 μL of ChamQ SYBR qPCR Master Mix, 0.4 μL of forward primer, 0.4 μL of reverse primer, 1 μL of cDNA, and 8.2 μL of distilled water. Each cDNA sample was analyzed in two technical replicates.
4.3. Selection of Candidate Reference Genes and Primer Design
The full-length sequences of thirteen candidate reference genes (EF1α, EFβ, αTUB, βTUB, GAPDH, 18s, Actin1, Actin2, H2A1, H2A2, UBQ1, UBQ3, and H2B2) were identified using a manual BLAST search: “https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 23 August 2023)” in the S. suchowensis genome (Table 1, File S1). Quantitative primers for these genes were designed using the Genscript online tool: “https://www.genscript.com.cn/tools/real-time-pcr-taqman-primer-design-tool (accessed on 7 September 2023)” (Table S1, File S1). The primer design parameters included a GC content of 45–65%, an optimal melting temperature (Tm) of 55–65 °C, primer lengths between 18 and 25 bp, and amplicon lengths ranging from 80 to 200 bp. Primer specificity was confirmed through BLAST analysis against S. suchowensis genome. Primers were then synthesized by Tsingke Biotech Co., Ltd. (Nanjing, China), and their amplicon size and specificity were verified by PCR followed by 3% agarose gel electrophoresis and RT-qPCR.
4.4. Establishment of Reference Gene Primer Standard Curve
The standard curves were established using a gradient dilution of cDNA (50, 5−1, 5−2, 5−3, 5−4, 5−5, 5−6, and 5−7), with the dilution factors plotted on the x-axis and the corresponding Ct values on the y-axis [60]. Slope analysis was conducted using linear regression models. RT-qPCR efficiency (E) was calculated using the formula: E = (10[−1/slope] − 1) × 100 [61]. Internal reference genes with E ranging from 90% to 110% and r2 > 0.99 were selected.
4.5. Statistical Analysis
The stability and optimal number of candidate endogenous reference genes under a range of experimental conditions (different N treatments and tissues) were analyzed using three software packages: geNorm, NormFinder, and Bestkeeper. Additionally, the stability rankings of selected reference genes were comprehensively evaluated using RefFinder [62].
The geNorm tool assessing gene expression stability (M-values) requires the conversion of raw Ct values to 2−ΔCt values (ΔCt = original Ct − lowest Ct in each group). The geNorm algorithm also determined the optimal number of reference genes by evaluating Vn/n + 1 value, where the default threshold was 0.15. If Vn/n + 1 < 0.15, the optimal number of reference genes was n; otherwise, it was n + 1 [26].
The NormFinder algorithm employed an ANOVA-based model to assess the stability of reference gene expression by analyzing variations both within and between groups [36]. A lower stability value indicated a more stable reference gene, guiding the selection of the most appropriate internal control.
The BestKeeper tool calculated the expression stability of candidate reference genes by calculating the standard deviation (SD) and coefficient of variation (CV) from raw Ct values. Genes with lower SD and CV were considered more stable and suitable as reference genes [63].
RefFinder integrates the results from geNorm, NormFinder, and BestKeeper to rank the stability of all candidate reference genes, identifying the most consistently expressed gene [64].
4.6. Gene Expression Level Analysis Using Various Reference Genes
The sequences of SsAMT1 (KAG5224131.1) and SsNRT2 (KAG5238048.1) were identified through a BLAST search in the Salix suchowensis genome and selected as a target gene to validate the reliability of the reference genes. RT-qPCR was conducted to measure the expression levels of SsAMT1 and SsNRT2 under different N treatments for 24 h and across various tissues using specific primers (Table S1). Relative expression levels were normalized to the reference genes (EF1α, EFβ, αTUB) and calculated using the 2−ΔΔCt. Data were presented as mean ± standard deviation (SD) of four biological replicates. Significant differences were analyzed using one-way ANOVA followed by Tukey’s test in IBM SPSS Statistics version 21. Diagrams were created using Origin Pro 2025 (64-bit) SR1 10.2.0.196.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants14193101/s1: Figure S1. Analysis of thirteen candidate reference gene products using agarose gel electrophoresis (A) and RT-qPCR (B); Figure S2. Melting curve analysis of thirteen candidate reference genes in Salix suchowensis; Figure S3. The amplification efficiency of PCR primers of thirteen candidate reference genes in Salix suchowensis; Figure S4. Expression of ammonium transporter 1 (SsAMT1) and nitrate transporter 2 (SsNRT2) in roots, stems, and leaves of Salix suchowensis seedlings exposed to different N treatments for 24 h. Table S1. Primers used for RT-qPCR analysis. File S1. The sequences of the 13 candidate reference genes in Salix suchowensis.
Author Contributions
Conceptualization, L.H., Y.Z., X.H. and N.G.; methodology, L.H., Y.Z., F.G. and Y.F.; validation, L.H. and N.G.; formal analysis, L.H.; investigation, X.H. and N.G.; resources, J.T. and X.H.; data curation, L.H., J.Z., J.S. and N.G.; writing—original draft preparation, L.H.; writing—review and editing, X.H. and N.G.; visualization, L.H. and Y.Z.; supervision, X.H., J.T. and N.G.; project administration, X.H. and N.G.; funding acquisition, X.H. and N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Jiangsu Province Innovation and Extension Project of Forestry Science and Technology of China (grant number LYKJ[2023]16), the National Natural Science Foundation of China (grant number 42207001), and the Postgraduate Research and Practice Innovation Program of Jiangsu Province of China (grant number SJCX24_2285).
Data Availability Statement
Raw data will be made available upon request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Engels, C.; Kirkby, E.; White, P. Chapter 5—Mineral nutrition, yield and source–sink relationships. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012; pp. 85–133. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol. 2009, 12, 250–258. [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root nitrogen acquisition and assimilation. Plant Soil 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2016, 68, 2501–2512. [Google Scholar] [CrossRef]
- Kronzucker, H.J.; Siddiqi, M.Y.; Glass, A. Kinetics of NH4+ influx in spruce. Plant Physiol. 1996, 110, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Rennenberg, H.; Wildhagen, H.; Ehlting, B. Nitrogen nutrition of poplar trees. Plant Biol. 2010, 12, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Falkengren-Grerup, U. Interspecies differences in the preference of ammonium and nitrate in vascular plants. Oecologia 1995, 102, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Giehl, R.F.H.; von Wiren, N. Root Nutrient Foraging. Plant Physiol. 2014, 166, 509–517. [Google Scholar] [CrossRef]
- Giehl, R.F.H.; Gruber, B.D.; von Wirén, N. It’s time to make changes: Modulation of root system architecture by nutrient signals. J. Exp. Bot. 2014, 65, 769–778. [Google Scholar] [CrossRef]
- Meier, M.; Liu, Y.; Lay-Pruitt, K.S.; Takahashi, H.; von Wirén, N. Auxin-mediated root branching is determined by the form of available nitrogen. Nat. Plants 2020, 6, 1136–1145. [Google Scholar] [CrossRef]
- Lima, J.E.; Kojima, S.; Takahashi, H.; von Wirén, N. Ammonium triggers lateral root branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-dependent manner. Plant Cell 2010, 22, 3621–3633. [Google Scholar] [CrossRef]
- Guo, S.; Brück, H.; Sattelmacher, B. Effects of supplied nitrogen form on growth and water uptake of French bean (Phaseolus vulgaris L.) plants. Plant Soil 2002, 239, 267–275. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Miao, Y.-F.; Li, S.-X. Effect of ammonium and nitrate nitrogen fertilizers on wheat yield in relation to accumulated nitrate at different depths of soil in drylands of China. Field Crops Res. 2015, 183, 211–224. [Google Scholar] [CrossRef]
- Hou, R.; Xu, X.; Zhu, O. Effect of experimental warming on nitrogen uptake by winter wheat under conventional tillage versus no-till systems. Soil Tillage Res. 2018, 180, 116–125. [Google Scholar] [CrossRef]
- Britto, D.T.; Siddiqi, M.Y.; Glass, A.D.M.; Kronzucker, H.J. Futile transmembrane NH4+ cycling: A cellular hypothesis to explain ammonium toxicity in plants. Proc. Natl. Acad. Sci. USA 2001, 98, 4255–4258. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Fu, W.; Niu, C.; Hu, C.; Zhang, P.; Chen, Y. Constructing and validating estimation models for individual-tree aboveground biomass of Salix suchowensis in China. Forests 2024, 15, 1371. [Google Scholar] [CrossRef]
- Yang, G.; Li, X.; Xu, Q.; Ling, J.; Yin, T. Effects of cutting size on the growth and wood property traits of short-rotation coppice willows. Can. J. For. Res. 2020, 50, 1365–1372. [Google Scholar] [CrossRef]
- Jia, H.; Wang, L.; Li, J.; Sun, P.; Lu, M.; Hu, J. Physiological and metabolic responses of Salix sinopurpurea and Salix suchowensis to drought stress. Trees 2020, 34, 563–577. [Google Scholar] [CrossRef]
- Karp, A.; Hanley, S.J.; Trybush, S.O.; Macalpine, W.; Pei, M.; Shield, I. Genetic improvement of willow for bioenergy and biofuels. J. Integr. Plant Biol. 2011, 53, 151–165. [Google Scholar] [CrossRef]
- Fabio, E.S.; Smart, L.B. Effects of nitrogen fertilization in shrub willow short rotation coppice production—A quantitative review. Glob. Change Biol. Bioenergy 2018, 10, 548–564. [Google Scholar] [CrossRef]
- Guo, N.; Gao, F.; Huang, L.; Li, S.; Huang, R.; Cao, Y.; Liu, W.-S.; Xu, G.; Tao, J. Where there is nitrogen, there is growth: Willows’ fast uptake and transport abilities facilitate their thriving in extremely low-nitrogen environments. Ind. Crops Prod. 2025, 230, 121088. [Google Scholar] [CrossRef]
- Dai, X.; Hu, Q.; Cai, Q.; Feng, K.; Ye, N.; Tuskan, G.A.; Milne, R.; Chen, Y.; Wan, Z.; Wang, Z.; et al. The willow genome and divergent evolution from poplar after the common genome duplication. Cell Res. 2014, 24, 1274–1277. [Google Scholar] [CrossRef]
- VanGuilder, H.D.; Vrana, K.E.; Freeman, W.M. Twenty-five years of quantitative PCR for gene expression analysis. BioTechniques 2018, 44, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Kubista, M.; Andrade, J.M.; Bengtsson, M.; Forootan, A.; Jonák, J.; Lind, K.; Sindelka, R.; Sjöback, R.; Sjögreen, B.; Strömbom, L.; et al. The real-time polymerase chain reaction. Mol. Asp. Med. 2006, 27, 95–125. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef] [PubMed]
- Mafra, V.; Kubo, K.S.; Alves-Ferreira, M.; Ribeiro-Alves, M.; Stuart, R.M.; Boava, L.P.; Rodrigues, C.M.; Machado, M.A. Reference genes for accurate transcript normalization in citrus genotypes under different experimental conditions. PLoS ONE 2012, 7, e31263. [Google Scholar] [CrossRef]
- Volkov, R.A. Heat-stress-dependency and developmental modulation of gene expression: The potential of house-keeping genes as internal standards in mRNA expression profiling using real-time RT-PCR. J. Exp. Bot. 2003, 54, 2343–2349. [Google Scholar] [CrossRef]
- Tong, Z.; Gao, Z.; Wang, F.; Zhou, J.; Zhang, Z. Selection of reliable reference genes for gene expression studies in peach using real-time PCR. BMC Mol. Biol. 2009, 10, 71. [Google Scholar] [CrossRef]
- Jain, M. Genome-wide identification of novel internal control genes for normalization of gene expression during various stages of development in rice. Plant Sci. 2009, 176, 702–706. [Google Scholar] [CrossRef]
- Wan, H.; Yuan, W.; Ruan, M.; Ye, Q.; Wang, R.; Li, Z.; Zhou, G.; Yao, Z.; Zhao, J.; Liu, S.; et al. Identification of reference genes for reverse transcription quantitative real-time PCR normalization in pepper (Capsicum annuum L.). Biochem. Biophys. Res. Commun. 2011, 416, 24–30. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, Y.; Wu, H.; Yin, T. A selection of reliable reference genes for gene expression analysis in the female and male flowers of Salix suchowensis. Plants 2022, 11, 647. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, X.; Chen, S.; Zheng, L.; He, X.; Liu, M.; Qiao, G.; Wang, Y.; Zhuo, R. Selection of suitable reference genes for quantitative real-time PCR gene expression analysis in Salix matsudana under different abiotic stresses. Sci. Rep. 2017, 7, 40290. [Google Scholar] [CrossRef] [PubMed]
- Ambroise, V.; Legay, S.; Guerriero, G.; Hausman, J.-F.; Cuypers, A.; Sergeant, K. Selection of appropriate reference genes for gene expression analysis under abiotic stresses in Salix viminalis. Int. J. Mol. Sci. 2019, 20, 4210. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jia, H.; Han, X.; Zhang, J.; Sun, P.; Lu, M.; Hu, J. Selection of reliable reference genes for gene expression analysis under abiotic stresses in the desert biomass willow, Salix psammophila. Front. Plant Sci. 2016, 7, 1505. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Cao, H.; Liu, Q.; Liu, X.; Ma, Z.; Zhang, J.; Li, X.; Shen, L.; Yuan, J.; Zhang, Q. Phosphatidic acid regulates ammonium uptake by interacting with AMMONIUM TRANSPORTER 1;1 in Arabidopsis. Plant Physiol. 2023, 193, 1954–1969. [Google Scholar] [CrossRef]
- Kawai, M.; Tabata, R.; Ohashi, M.; Honda, H.; Kamiya, T.; Kojima, M.; Takebayashi, Y.; Oishi, S.; Okamoto, S.; Hachiya, T.; et al. Regulation of ammonium acquisition and use in Oryza longistaminata ramets under nitrogen source heterogeneity. Plant Physiol. 2022, 188, 2364–2376. [Google Scholar] [CrossRef]
- Konishi, N.; Ma, J.F. Three polarly localized ammonium transporter 1 members are cooperatively responsible for ammonium uptake in rice under low ammonium condition. New Phytol. 2021, 232, 1778–1792. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Nolan, T.; Pfaffl, M.W. Quantitative real-time RT-PCR—A perspective. J. Mol. Endocrinol. 2005, 34, 597–601. [Google Scholar] [CrossRef]
- Udvardi, M.K.; Czechowski, T.; Scheible, W.-R.D. Eleven golden rules of quantitative RT-PCR. Plant Cell 2008, 20, 1736–1737. [Google Scholar] [CrossRef]
- Paolacci, A.R.; Tanzarella, O.A.; Porceddu, E.; Ciaffi, M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol. Biol. 2009, 10, 11. [Google Scholar] [CrossRef]
- Wei, L.Q.; Xu, W.Y.; Deng, Z.Y.; Su, Z.; Xue, Y.; Wang, T. Genome-scale analysis and comparison of gene expression profiles in developing and germinated pollen in Oryza sativa. BMC Genom. 2010, 11, 338. [Google Scholar] [CrossRef] [PubMed]
- Warzybok, A.; Migocka, M. Reliable reference genes for normalization of gene expression in cucumber grown under different nitrogen nutrition. PLoS ONE 2013, 8, e72887. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Chen, S.; Shan, Z.; Yang, Z.; Chen, L.; Zhang, C.; Yuan, S.; Hao, Q.; Zhang, X.; Qiu, D.; et al. Stability evaluation of reference genes for gene expression analysis by RT-qPCR in soybean under different conditions. PLoS ONE 2017, 12, e0189405. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Ma, J.; Wang, J.; Wu, X.; Li, P.; Yao, Y. Validation of suitable reference genes for gene expression analysis in the halophyte Salicornia europaea by real-time quantitative PCR. Front. Plant Sci. 2014, 5, 788. [Google Scholar] [CrossRef]
- Xu, B.; Liu, L.; Song, G. Functions and regulation of translation elongation factors. Front. Mol. Biosci. 2022, 8, 816398. [Google Scholar] [CrossRef]
- Shivhare, R.; Lata, C. Selection of suitable reference genes for assessing gene expression in pearl millet under different abiotic stresses and their combinations. Sci. Rep. 2016, 6, 23036. [Google Scholar] [CrossRef]
- Jain, M.; Nijhawan, A.; Tyagi, A.K.; Khurana, J.P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2006, 345, 646–651. [Google Scholar] [CrossRef]
- Wang, H.L.; Chen, J.; Tian, Q.; Wang, S.; Xia, X.; Yin, W. Identification and validation of reference genes for Populus euphratica gene expression analysis during abiotic stresses by quantitative real-time PCR. Physiol. Plant. 2014, 152, 529–545. [Google Scholar] [CrossRef]
- Jian, B.; Liu, B.; Bi, Y.; Hou, W.; Wu, C.; Han, T. Validation of internal control for gene expression study in soybean by quantitative real-time PCR. BMC Mol. Biol. 2008, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Linardić, M.; Braybrook, S.A. Identification and selection of optimal reference genes for qPCR-based gene expression analysis in Fucus distichus under various abiotic stresses. PLoS ONE 2021, 16, e0233249. [Google Scholar] [CrossRef] [PubMed]
- McKean, P.G.; Vaughan, S.; Gull, K. The extended tubulin superfamily. J. Cell Sci. 2001, 114, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Ludueña, R.F. Multiple forms of tubulin: Different gene products and covalent modifications. Int. Rev. Cytol. 1998, 178, 207–275. [Google Scholar] [CrossRef]
- Wan, H.; Zhao, Z.; Qian, C.; Sui, Y.; Malik, A.A.; Chen, J. Selection of appropriate reference genes for gene expression studies by quantitative real-time polymerase chain reaction in cucumber. Anal. Biochem. 2010, 399, 257–261. [Google Scholar] [CrossRef]
- Song, H.; Mao, W.; Duan, Z.; Que, Q.; Zhou, W.; Chen, X.; Li, P. Selection and validation of reference genes for measuring gene expression in Toona ciliata under different experimental conditions by quantitative real-time PCR analysis. BMC Plant Biol. 2020, 20, 450. [Google Scholar] [CrossRef]
- Li, L.; Li, N.; Fang, H.; Qi, X.; Zhou, Y. Selection and validation of reference genes for normalisation of gene expression in Glehnia littoralis. Sci. Rep. 2020, 10, 7374. [Google Scholar] [CrossRef]
- Guo, N.; Fan, L.; Cao, Y.; Ling, H.; Xu, G.; Zhou, J.; Chen, Q.; Tao, J. Comparison of two willow genotypes reveals potential roles of iron-regulated transporter 9 and heavy-metal ATPase 1 in cadmium accumulation and resistance in Salix suchowensis. Ecotoxicol. Environ. Saf. 2022, 244, 114065. [Google Scholar] [CrossRef]
- Fu, H.; Huang, T.; Yin, C.; Xu, Z.; Li, C.; Liu, C.; Wu, T.; Song, F.; Feng, F.; Yang, F. Selection and validation of reference genes for RT-qPCR normalization in Bradysia odoriphaga (Diptera: Sciaridae) Under Insecticides Stress. Front. Physiol. 2022, 12, 818210. [Google Scholar] [CrossRef]
- Radonić, A.; Thulke, S.; Mackay, I.M.; Landt, O.; Siegert, W.; Nitsche, A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004, 313, 856–862. [Google Scholar] [CrossRef]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. miRDeepFinder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Xie, F.; Wang, J.; Zhang, B. RefFinder: A web-based tool for comprehensively analyzing and identifying reference genes. Funct. Integr. Genom. 2023, 23, 125. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).