Effects of Biochar Combined with Nitrogen Fertilizer Application on Pepper Yield, Quality and Rhizosphere Soil Microbial Community Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Determination Indicators and Methods
2.2.2. Soil Sample Collection
2.2.3. Determination of Soil Physicochemical Properties
2.2.4. Soil Total DNA Extraction and High-Throughput Sequencing
2.2.5. Data Processing and Analysis
3. Analysis
3.1. The Synergistic Effect of Biochar and Nitrogen Fertilizer on the Regulation of Rhizosphere Soil Properties of Pepper
3.1.1. Regulation of Biochar and Nitrogen Fertilizer on Soil Physical and Chemical Characteristics
3.1.2. Effects of Different Biochar and Nitrogen Fertilizer Combined Application on Soil Nutrient Effectiveness
3.1.3. Effects of Different Biochar and Nitrogen Fertilizer Combined Application on Soluble Salt Ion Content in Pepper Rhizosphere Soil
3.2. Effects of Different Biochar and Nitrogen Fertilizer Combined Applications on Pepper Fruit Yield and Quality
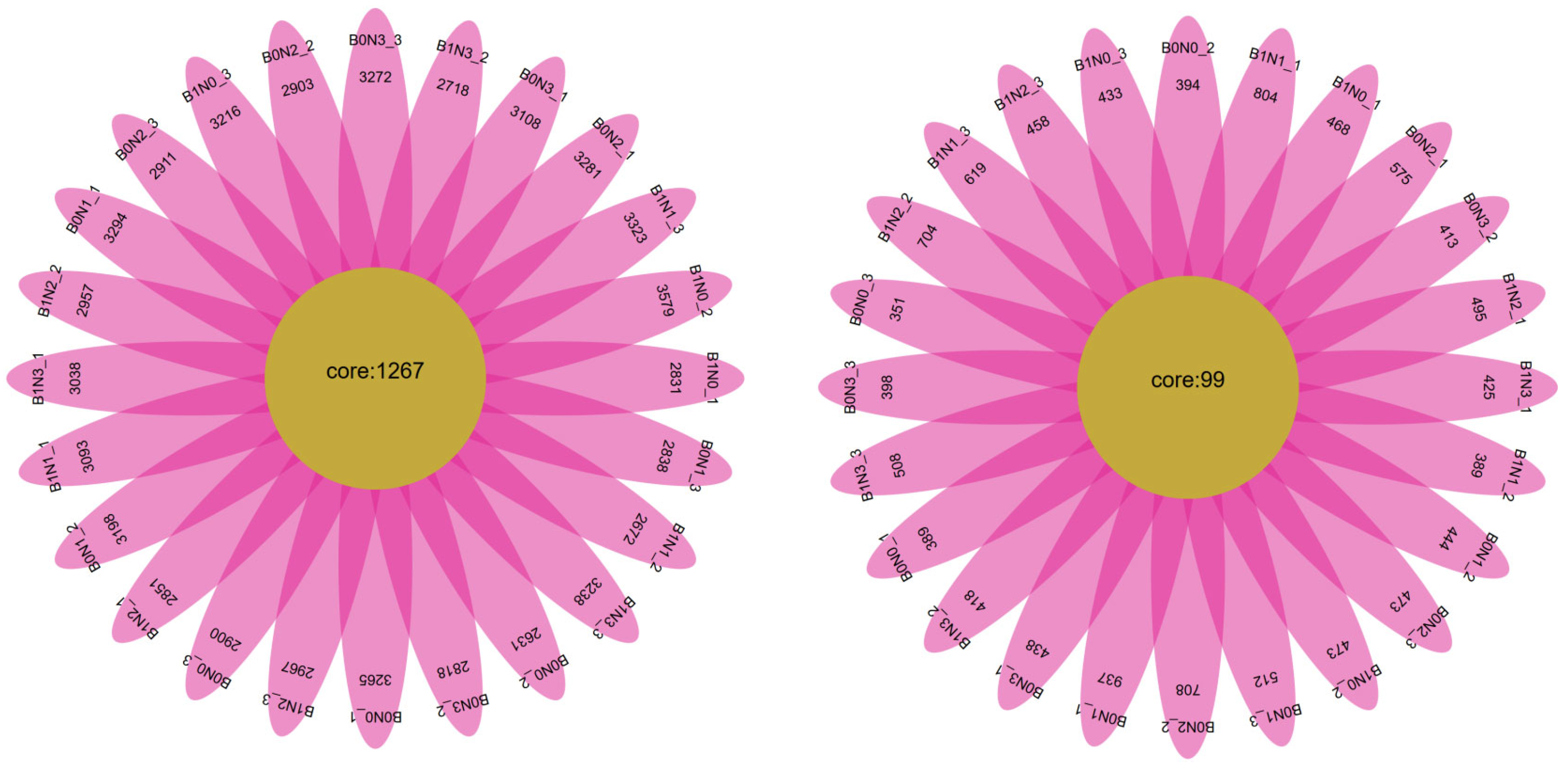
3.3. OTU Cluster Analysis of Pepper Rhizosphere Soil Under Combined Application of Different Biochar and Nitrogen Fertilizer
3.4. Analysis of the Effects of Different Biochar and Nitrogen Fertilizer Combined Applications on Microbial Diversity in Pepper Rhizosphere Soil
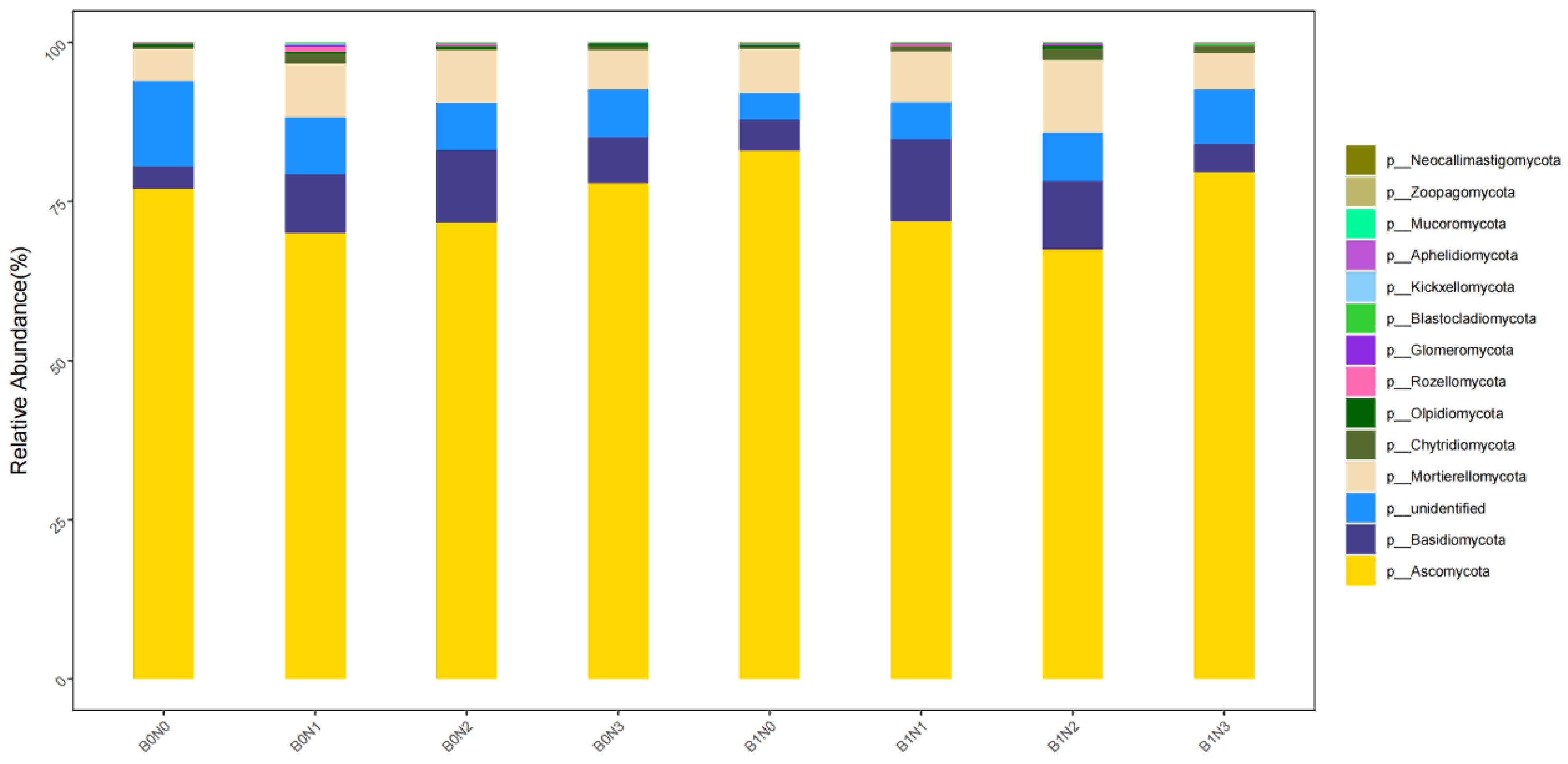
3.5. Analysis of Microbial Community Composition in Pepper Rhizosphere Soil Under Combined Application of Different Biochar and Nitrogen Fertilizer
3.6. Correlation Analysis Between Microbial Community Composition and Soil Physicochemical Properties
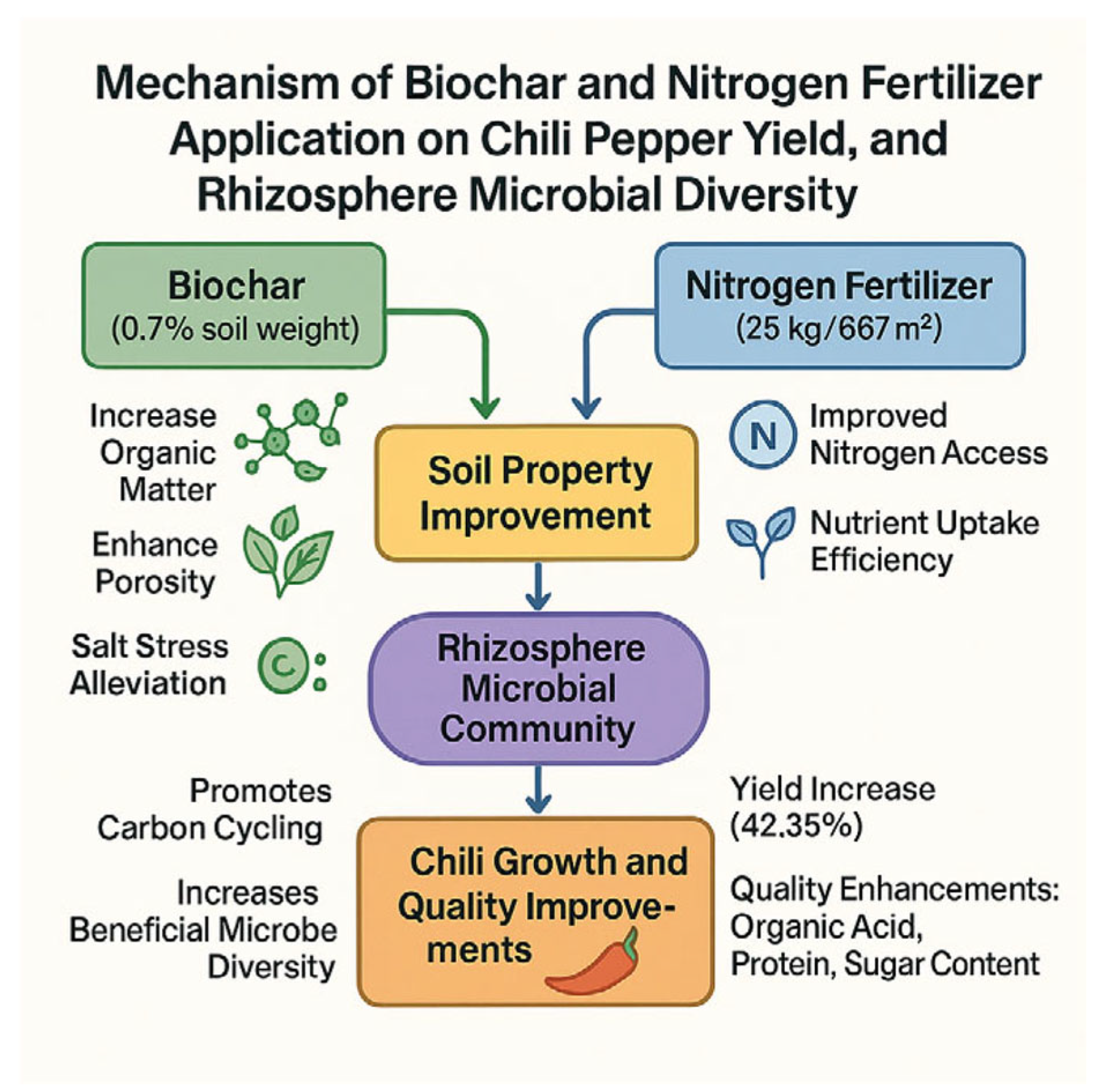
4. Discussion
5. Results
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Yilmaz, E.; Arsunar, E.S.; Aydeniz, B.; Güneşer, O. Cold pressed capia pepper seed (Capsicum annuum L.) oils: Composition, aroma and sensory properties. Eur. J. Lipid Sci. Technol. 2015, 117, 1016–1026. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, H.; Zhang, Z.; Li, X.; Yu, Z.; Fan, Y.; Yan, L. SLAF-seq technology-based genome-wide association and population structure analyses of hot pepper and sweet pepper. BMC Genom. 2025, 26, 258. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chems. 2005, 53, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Yang, T.; Zhang, G.R.; Guo, H.Y.; Tang, Y.P.; Yang, S.B. Research Progress on Capsaicin in Pepper Fruits. China Cucurbits Veg. 2021, 34, 1–9. [Google Scholar]
- Zou, X.X.; Yang, S.; Zhu, F.; Yuan, F. Development and Future Trends of the High-Quality Fresh Pepper Industry in China. Acta Hortic. Sin. 2024, 51, 27–38. [Google Scholar]
- Yang, Z.Z. Progress and Prospects in the Breeding of Chili Pepper Varieties in China. China Veg. 2017, 30, 1–6. [Google Scholar]
- Zhang, D.M.; Lei, F.; Xiao, T.B.; Zeng, J.H.; Wu, Y.J.; Xie, L.S. Effects of applying potassium sulfate calcium-magnesium fertilizer on peppers. China Cucurbits Veg. 2018, 31, 30–33. [Google Scholar]
- Yasuor, H.; Ben-Gal, A.; Yermiyahu, U.; Beit-Yannai, E.; Cohen, S. Nitrogen Management of Greenhouse Pepper Production: Agronomic, Nutritional, and Environmental Implications. HortScience 2013, 48, 1241–1249. [Google Scholar] [CrossRef]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Elia, A.; Conversa, G. Agronomic and physiological responses of a tomato crop to nitrogen input. Eur. J. Agron. 2012, 40, 64–74. [Google Scholar] [CrossRef]
- Zhang, M.M.; Dong, B.D.; Qiao, Y.Z.; Shi, C.H.; Yang, H.; Wang, Y.K.; Liu, M.Y. Yield and water use responses of winter wheat to irrigation and nitrogen application in the North China Plain. J. Agric. Sci. 2018, 17, 1194–1206. [Google Scholar] [CrossRef]
- Zhang, C.; Shen, L.; Yang, S.; Chang, T.; Luo, M.; Zhen, S.; Ji, X. Effect of Nitrogen Fertilizer on Capsaicinoids and Related Metabolic Substances of Dried Chili Pepper Fruit. Horticulturae 2024, 10, 831. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 year trends in nitrogen use efficiency of world cropping systems: The relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Sun, L.; Lu, Y.; Yu, F.; Kronzucker, H.J.; Shi, W. Biological nitrification inhibition by rice root exudates and its relationship with nitrogen-use efficiency. New Phytol. 2016, 212, 646–656. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, Z.; Van Zwieten, L.; Bolan, N.; Dong, D.; Quin, B.F.; Meng, J.; Li, F.; Wu, F.; Wang, H.; et al. A critical review of biochar-based nitrogen fertilizers and their effects on crop production and the environment. Biochar 2022, 4, 36. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Neupane, A.; Abdoulmoumine, N.; DeBruyn, J.M.; Walker, F.R.; Jagadamma, S. Co-application of biochar and nitrogen fertilizer reduced nitrogen losses from soil. PLoS ONE 2021, 16, e0248100. [Google Scholar] [CrossRef]
- Demirbas, A.; Arin, G. An overview of biomass pyrolysis. Energy Sources 2002, 24, 471–482. [Google Scholar] [CrossRef]
- Dumroese, R.K.; Heiskanen, J.; Englund, K.; Tervahauta, A. Pelleted biochar: Chemical and physical properties show potential use as a substrate in container nurseries. Biomass Bioenergy 2011, 35, 2018–2027. [Google Scholar] [CrossRef]
- Fedeli, R.; Alexandrov, D.; Celletti, S.; Nafikova, E.; Loppi, S. Biochar improves the performance of Avena sativa L. grown in gasoline-polluted soils. Environ. Sci. Pollut. Res. 2023, 30, 28791–28802. [Google Scholar] [CrossRef]
- Sheng, Y.Q.; Zhu, L.Z. Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci. Total Environ. 2018, 622–623, 1391–1399. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Suliman, W.; Harsh, J.B.; Abu-Lail, N.I.; Fortuna, A.M.; Dallmeyer, I.; Garcia-Pérez, M. The role of biochar porosity and surface functionality in augmenting hydrologic properties of a sandy soil. Sci. Total Environ. 2017, 574, 139–147. [Google Scholar] [CrossRef]
- Olmo, M.; Villar, R.; Salazar, P.; Alburquerque, J.A. Changes in soil nutrient availability explain biochar’s impact on wheat root development. Plant Soil 2016, 399, 333–343. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; Van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Aust. J. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Downie, A.; Morris, S.; Petty, S.; Rust, J.; Chan, K.Y. A glasshouse study on the interaction of low mineral ash biochar with nitrogen in a sandy soil. Aust. J. Soil Res. 2010, 48, 569–576. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, Y.; Wei, Q.; Liu, L.; Gu, X.; Gou, J.; Wang, M. Chemical Fertilizer Reduction Combined with Biochar Application Ameliorates the Biological Property and Fertilizer Utilization of Pod Pepper. Agronomy 2023, 13, 1616. [Google Scholar] [CrossRef]
- Sarfraz, R.; Shakoor, A.; Abdullah, M.; Arooj, A.; Hussain, A.; Xing, S. Impact of integrated application of biochar and nitrogen fertilizers on maize growth and nitrogen recovery in alkaline calcareous soil. Soil Sci. Plant Nutr. 2017, 63, 488–498. [Google Scholar] [CrossRef]
- Khan, Z.; Zhang, K.; Khan, M.N.; Fahad, S.; Xu, Z.; Hu, L. Coupling of Biochar with Nitrogen Supplements Improve Soil Fertility, Nitrogen Utilization Efficiency and Rapeseed Growth. Agronomy 2020, 10, 1661. [Google Scholar] [CrossRef]
- Khan, Z.; Khan, M.N.; Luo, T.; Zhang, K.; Zhu, K.; Rana, M.S.; Hu, L.; Jiang, Y. Compensation of high nitrogen toxicity and nitrogen deficiency with biochar amendment through enhancement of soil fertility and nitrogen use efficiency promoted rice growth and yield. GCB Bioenergy 2021, 13, 1765–1784. [Google Scholar] [CrossRef]
- Bao, S.D. Agrochemical Analysis of Soil; China Agriculture Press: Beijing, China, 2005. [Google Scholar]
- Wu, C.; Sun, Q.; Ren, Z.; Xia, N.; Wang, Z.; Sun, H.; Wang, W. Combined effects of nitrogen fertilizer and biochar on the growth, yield, and quality of pepper. Open Life Sci. 2024, 19, 20220882. [Google Scholar] [CrossRef]
- Zhang, Z.A.; Chen, Z.Y. Experimental Techniques in Plant Physiology; Jilin University Press: Changchun, China, 2008; pp. 60–176. [Google Scholar]
- Busschei, W.J.; Novak, J.; Evans, D.E.; Watts, D.W. Influences of pecan biochar on physical properties of a Norfolk loamy sand. Soil Sci. 2010, 175, 10–14. [Google Scholar] [CrossRef]
- Pan, S.Y.; Dong, C.D.; Su, J.F.; Wang, P.Y.; Chen, C.W.; Chang, J.S.; Kim, H.; Huang, C.P.; Hung, C.M. The Role of Biochar in Regulating the Carbon, Phosphorus, and Nitrogen Cycles Exemplified by Soil Systems. Sustainability 2021, 13, 5612. [Google Scholar] [CrossRef]
- Šimanský, V.; Horák, J.; Igaz, D.; Jonczak, J.; Markiewicz, M.; Felber, R.; Rizhiya, E.Y.; Lukac, M. How dose of biochar and biochar with nitrogen can improve the parameters of soil organic matter and soil structure? Biologia 2016, 71, 989–995. [Google Scholar] [CrossRef]
- Jiang, Y.X.; Liu, J.; Li, Y.Y.; Xiao, P.; Liu, S.; Shao, J.H.; Cai, Y.X.; Yan, X.Q.; Fan, L. Biochar amendment reduces biological nitrogen fixation and nitrogen use efficiency in cadmium-contaminated paddy fields. J. Environ. Manag. 2023, 344, 118404. [Google Scholar] [CrossRef]
- Duan, Y.; Gao, S.; Hanson, B. Effects of biochar and fertilizer sources on nitrogen uptake by chilli pepper plants under Mediterranean climate. Soil Use Manag. 2022, 38, 714–728. [Google Scholar] [CrossRef]
- Mohawesh, O.; Albalasmeh, A.; Gharaibeh, M.; Deb, S.; Simpson, C.; Singh, S.; Al-Soub, B.; Hanandeh, A.E. Potential Use of Biochar as an Amendment to Improve Soil Fertility and Tomato and Bell Pepper Growth Performance Under Arid Conditions. J. Soil Sci. Plant Nutr. 2021, 21, 2946–2956. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Wang, Z.J.; Chen, Y.Z.; Wang, Y.Q.; Zhu, Q.L.; Hu, T.Y.; Hu, Y.J.; Wu, Y.Z.; Meng, L.; Tang, S.R. Effects of biochar on N2O emissions and related functional genes in chili pepper cultivation soil in tropical regions. Environ. Sci. 2023, 44, 3418–3425. [Google Scholar]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Nelson, P.F. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersicon esculentum). Chemosphere 2010, 78, 1167–1171. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Li, H.; Zhang, A.; Rengel, Z. Long-term biochar application promotes rice productivity by regulating root dynamic development and reducing nitrogen leaching. GCB Bioenergy 2021, 13, 257–268. [Google Scholar] [CrossRef]
- Gaskin, J.; Steiner, C.; Harris, K.; Das, K.C.; Preston, B. Effect of low-temperature pyrolysis conditions on biochar for agricultural use. Trans. ASABE 2008, 51, 2061–2069. [Google Scholar] [CrossRef]
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Chen, Y.; Shinogi, Y. Influence of biochar use on sugarcane growth, matrix parameters, and groundwater quality. Aust. J. Soil Res. 2010, 48, 526–530. [Google Scholar] [CrossRef]
- Da Silva Mendes, J.; Fernandes, J.D.; Chaves, L.H.G.; Guerra, H.O.C.; Tito, G.A.; de Brito Chaves, I. Chemical and Physical Changes of Soil Amended with Biochar. Water Air Soil Pollut. 2021, 232, 338. [Google Scholar] [CrossRef]
- Chaganti, V.N.; Crohn, D.M. Evaluating the relative contribution of physiochemical and biological factors in ameliorating a saline–sodic soil amended with composts and biochar and leached with reclaimed water. Geoderma 2015, 259–260, 45–55. [Google Scholar] [CrossRef]
- Siedt, M.; Schäffer, A.; Smith, K.E.C.; Nabel, M.; Roß-Nickoll, M.; van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Mavi, M.S.; Choudhary, O.P.; Gupta, N.; Singh, Y. Rice straw biochar application to soil irrigated with saline water in a cotton-wheat system improves crop performance and soil functionality in north-west India. J. Environ. Manag. 2021, 295, 113277. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, J.; Han, L.; Tan, J.; Ge, X.; Nan, Q. Biochar addition reduces salinity in salt-affected soils with no impact on soil pH: A meta-analysis. Geoderma 2024, 443, 116829. [Google Scholar] [CrossRef]
- Mao, Q.; He, B.; Ma, M.; Wang, L.; Koike, T.; Agathokleous, E. Effects of biochar on karst lime soil nutrients, soil microbial communities and physiology of Sichuan pepper plants. Ann. Appl. Biol. 2022, 181, 357–366. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Feng, Y. The effects of biochar addition on soil physicochemical properties: A review. CATENA 2021, 202, 105284. [Google Scholar] [CrossRef]
- Duan, M.; Liu, G.; Zhou, B.; Chen, X.; Wang, Q.; Zhu, H.; Li, Z. Effects of modified biochar on water and salt distribution and water-stable macro-aggregates in saline-alkaline soil. J. Soils Sediments 2021, 21, 2192–2202. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, L.; Chen, Y.; Li, W.; Li, Z.; Luo, Z. Characteristics of biochar produced from yak manure at different pyrolysis temperatures and its effects on the yield and growth of highland barley. Chem. Speciat. Bioavailab. 2018, 30, 57–67. [Google Scholar] [CrossRef]
- Hammer, E.C.; Forstreuter, M.; Rillig, M.C.; Kohler, J. Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl. Soil Ecol. 2015, 96, 114–121. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Hussain, B.; Ashraf, M.N.; Rahman, S.U.; Abbas, A.; Li, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Singh, P.C.; Mishra, A.; Chauhan, P.S.; Dwivedi, S.; Bais, R.T.; Tripathi, R.D. Trichoderma: A potential bioremediator for environmental clean up. Clean Technol. Environ. Policy 2013, 15, 541–550. [Google Scholar] [CrossRef]
- Haider, F.U.; Coulter, J.A.; Cheema, S.A.; Farooq, M.; Wu, J.; Zhang, R.; Shuaijie, G.; Liqun, C.; Fan, L. Co-application of biochar and microorganisms improves soybean performance and remediate cadmium-contaminated soil. Ecotoxicol. Environ. Saf. 2021, 214, 112112. [Google Scholar] [CrossRef]
- Zhao, S.; Qiu, S.; Xu, X.; Ciampitti, I.A.; Zhang, S.; He, P. Change in straw decomposition rate and soil microbial community composition after straw addition in different long-term fertilization soils. Appl. Soil Ecol. 2019, 138, 123–133. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Q.; Zhang, F.; Wang, W.; Wu, C. Effects of Biochar on the Yield of Melon and the Diversity of Rhizosphere Soil Microbial Communities Under Saline–Alkali Stress. Plants 2025, 14, 1423. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Shang, J.; Liu, K.; He, Y.; Shao, X. The Synergistic Effect of Biochar and Microorganisms Greatly Improves Vegetation and Microbial Structure of Degraded Alpine Grassland on Qinghai–Tibet Plateau. Agronomy 2023, 13, 2203. [Google Scholar] [CrossRef]


| Handling Names | Proportion of Biochar Application (%) | The Ratio of Pure Nitrogen Application (kg/hm2) |
|---|---|---|
| B0N0 (control) | 0 | 0 |
| B0N1 | 0 | 75 |
| B0N2 | 0 | 375 |
| B0N3 | 0 | 675 |
| B1N0 | 0.7 | 0 |
| B1N1 | 0.7 | 75 |
| B1N2 | 0.7 | 375 |
| B1N3 | 0.7 | 675 |
| Treatments | pH | Electrical Conductivity (μS/m) | Porosity of Substrate (%) | Volume Weight of Soil (g/cm3) | Salt Content (g/kg) | Organic Carbon (g/kg) |
|---|---|---|---|---|---|---|
| B0N0 | 6.05 ± 0.002 d | 315.03 ± 2.65 d | 50.07 ± 0.58 f | 1.15 ± 0.01 c | 4.15 ± 0.03 b | 14.43 ± 0.03 g |
| B0N1 | 6.22 ± 0.02 c | 296.01 ± 1.16 f | 53.27 ± 0.56 e | 1.28 ± 0.02 b | 3.05 ± 0.02 f | 14.65 ± 0.02 f |
| B0N2 | 6.07 ± 0.02 d | 282.34 ± 1.86 g | 59.14 ± 0.71 c | 1.21 ± 0.02 c | 4.42 ± 0.03 a | 14.89 ± 0.02 e |
| B0N3 | 6.28 ± 0.02 b | 282.34 ± 0.88 g | 54.54 ± 0.41 e | 1.36 ± 0.05 a | 3.77 ± 0.18 c | 14.98 ± 0.02 d |
| B1N0 | 6.10 ± 0.01 d | 329.02 ± 1.73 b | 57.04 ± 0.29 d | 0.92 ± 0.01 f | 4.56 ± 0.03 a | 15.03 ± 0.01 d |
| B1N1 | 6.31 ± 0.02 b | 308.67 ± 1.74 e | 60.14 ± 0.38 c | 0.99 ± 0.02 e | 3.57 ± 0.02 d | 15.23 ± 0.02 b |
| B1N2 | 6.60 ± 0.02 a | 334.67 ± 2.85 a | 65.6 ± 0.38 a | 1.08 ± 0.02 d | 3.35 ± 0.02 e | 15.11 ± 0.05 c |
| B1N3 | 6.29 ± 0.02 b | 322.67 ± 1.21 c | 62.47 ± 0.47 b | 1.20 ± 0.01 c | 2.99 ± 0.02 f | 15.49 ± 0.01 a |
| ANOVA | ||||||
| B | ** | ** | ** | ** | ** | ** |
| N | ** | ** | ** | ** | ** | ** |
| B × N | ** | ** | ns | * | ** | ** |
| Treatments | Total Nitrogen (g/kg) | Alkaline Hydrolyzable Nitrogen (mg/kg) | Available Phosphorus (mg/kg) | Fast-Acting Potassium (mg/kg) |
|---|---|---|---|---|
| B0N0 | 1.95 ± 0.01 e | 137.06 ± 0.35 f | 231.14 ± 0.59 c | 13.27 ± 0.02 d |
| B0N1 | 1.98 ± 0.01 bc | 145.72 ± 0.32 c | 220.70 ± 0.40 d | 13.07 ± 0.03 e |
| B0N2 | 1.96 ± 0.01 de | 148.81 ± 0.30 b | 231.17 ± 0.62 c | 13.57 ± 0.02 c |
| B0N3 | 2.02 ± 0.01 a | 149.97 ± 0.61 ab | 210.70 ± 0.38 e | 14.06 ± 0.02 a |
| B1N0 | 1.96 ± 0.01 de | 137.6 ± 0.28 f | 221.67 ± 0.91 d | 13.34 ± 0.04 d |
| B1N1 | 1.98 ± 0.01 cd | 143.34 ± 0.52 d | 201.10 ± 0.59 f | 13.61 ± 0.01 c |
| B1N2 | 1.99 ± 0.01 bc | 141.07 ± 0.46 e | 241.44 ± 0.84 b | 13.73 ± 0.01 b |
| B1N3 | 2.00 ± 0.01 bc | 150.67 ± 0.35 a | 251.64 ± 0.89 a | 14.15 ± 0.04 a |
| ANOVA | ||||
| B | ns | ** | ** | ** |
| N | ** | ** | ** | ** |
| B × N | ** | ** | ns | * |
| Treatments | K+ (g/kg) | Na+ (g/kg) | Ca2+ (g/kg) | Mg2+ (g/kg) | Cl− (g/kg) |
|---|---|---|---|---|---|
| B0N0 | 4.26 ± 0.12 g | 0.75 ± 0.01 a | 0.58 ± 0.01 e | 0.35 ± 0.01 g | 1.60 ± 0.07 a |
| B0N1 | 4.54 ± 0.08 f | 0.69 ± 0.02 b | 0.67 ± 0.01 c | 0.72 ± 0.02 e | 1.38 ± 0.04 b |
| B0N2 | 4.89 ± 0.08 e | 0.58 ± 0.02 d | 0.68 ± 0.03 b | 0.79 ± 0.01 d | 1.21 ± 0.18 d |
| B0N3 | 5.67 ± 0.12 c | 0.46 ± 0.01 f | 0.71 ± 0.02 a | 0.99 ± 0.03 a | 0.96 ± 0.14 f |
| B1N0 | 4.89 ± 0.12 e | 0.63 ± 0.03 c | 0.55 ± 0.01 f | 0.34 ± 0.01 f | 1.31 ± 0.07 c |
| B1N1 | 5.32 ± 0.16 d | 0.52 ± 0.03 e | 0.62 ± 0.02 d | 0.45 ± 0.01 d | 1.13 ± 0.04 e |
| B1N2 | 6.18 ± 0.12 b | 0.45 ± 0.02 f | 0.67 ± 0.01 c | 0.86 ± 0.02 c | 0.96 ± 0.04 f |
| B1N3 | 6.65 ± 0.08 a | 0.34 ± 0.01 g | 0.72 ± 0.03 a | 0.9 ± 0.03 a | 0.78 ± 0.14 g |
| ANOVA | |||||
| B | ** | ** | ** | ** | ** |
| N | ** | ** | ** | ** | ** |
| B × N | ** | ** | ** | ** | ** |
| Treatments | Yield (kg/hm2) | PFPN (kg/kg) | Titratable Acid (%) | Soluble Protein (mg/100 g) | Soluble Sugar (%) | Vitamin C Content (mg/100 g) | Free Amino Acid Content (μg/100 g) |
|---|---|---|---|---|---|---|---|
| B0N0 | 17,459.7 ± 535.35 e | N/A | 0.18 ± 0.01 d | 14.26 ± 0.24 f | 1.56 ± 0.04 d | 37.90 ± 0.71 e | 607.52 ± 3.16 d |
| B0N1 | 19,769.7 ± 204.3 d | 263.6 ± 2.7 c | 0.22 ± 0.01 c | 16.75 ± 0.25 e | 1.79 ± 0.04 c | 40.35 ± 0.40 d | 627.75 ± 3.34 cd |
| B0N2 | 21,850.5 ± 258.45 c | 58.3 ± 0.7 d | 0.24 ± 0.01 c | 21.72 ± 0.65 d | 2.05 ± 0.05 b | 47.63 ± 0.45 bc | 634.92 ± 2.12 c |
| B0N3 | 22,993.2 ± 262.65 c | 34.1 ± 0.4 e | 0.29 ± 0.02 b | 27.52 ± 0.40 b | 2.01 ± 0.05 b | 47.96 ± 0.82 b | 649.09 ± 4.85 b |
| B1N0 | 19,546.35 ± 442.35 d | N/A | 0.21 ± 0.02 cd | 17.02 ± 0.80 e | 1.61 ± 0.01 d | 39.83 ± 0.12 d | 616.58 ± 1.20 de |
| B1N1 | 22,572.45 ± 193.8 bc | 301.0 ± 2.6 b | 0.27 ± 0.02 b | 24.88 ± 0.58 c | 1.83 ± 0.05 c | 46.14 ± 0.85 c | 628.91 ± 2.09 cd |
| B1N2 | 24,854.1 ± 458.4 a | 66.3 ± 1.2 c | 0.37 ± 0.02 a | 33.98 ± 0.74 a | 2.35 ± 0.09 a | 52.74 ± 0.77 a | 655.17 ± 5.53 b |
| B1N3 | 24,341.55 ± 335.85 a | 36.1 ± 0.5 e | 0.34 ± 0.04 a | 32.87 ± 0.90 a | 2.24 ± 0.07 a | 51.39 ± 0.60 a | 685.39 ± 6.58 a |
| ANOVA | |||||||
| B | ** | ** | * | ** | ** | ** | ** |
| N | ** | ** | ** | ** | ** | ** | ** |
| B × N | ** | ** | ** | ** | ** | ** | ** |
| Microbiome | Sample Name | Observed Species Index | Chao1 Index | Simpson Index | Shannon Index |
|---|---|---|---|---|---|
| Bacteria | B0N0 | 3865.61 ± 184.02 b | 4039.15 ± 368.03 b | 1 a | 9.92 ± 0.17 a |
| B0N1 | 4376.8 ± 138.9 a | 4557.92 ± 136.49 ab | 1 a | 10.02 ± 0.05 a | |
| B0N2 | 4298.63 ± 124.7 ab | 4393.58 ± 221.18 ab | 1 a | 10.02 ± 0.07 a | |
| B0N3 | 4199.52 ± 89.08 ab | 4472.32 ± 167.64 ab | 1 a | 9.99 ± 0.03 a | |
| B1N0 | 4308.7 ± 112.65 ab | 4264.01 ± 348.85 b | 1 a | 10.05 ± 0.13 a | |
| B1N1 | 4429.69 ± 80.38 a | 4919.83 ± 190.77 ab | 0.99 a | 9.83 ± 0.17 a | |
| B1N2 | 4592.02 ± 181.05 a | 5529.79 ± 224.89 a | 1 a | 9.92 ± 0.07 a | |
| B1N3 | 4398.23 ± 58.14 a | 5116.11 ± 174.67 ab | 1 a | 9.92 ± 0.13 a | |
| Fungi | B0N0 | 477.02 ± 13.58 b | 575.08 ± 17.42 b | 0.79 ± 0.03 bc | 4.17 ± 0.15 b |
| B0N1 | 563.33 ± 23.92 ab | 658.29 ± 20.42 ab | 0.94 ± 0.02 a | 5.92 ± 0.48 a | |
| B0N2 | 607.67 ± 33.19 ab | 766.69 ± 57.33 a | 0.94 ± 0.01 a | 5.76 ± 0.23 a | |
| B0N3 | 515.33 ± 11.67 b | 598.64 ± 9.98 c | 0.89 ± 0.04 ab | 5.02 ± 0.43 ab | |
| B1N0 | 556.97 ± 12.62 ab | 677.85 ± 14.72 ab | 0.79 ± 0.06 c | 4.32 ± 0.37 b | |
| B1N1 | 593.14 ± 64.49 ab | 569.41 ± 25.57 b | 0.88 ± 0.02 abc | 5.16 ± 0.19 ab | |
| B1N2 | 718.09 ± 43.82 a | 750.79 ± 99.96 a | 0.95 ± 0.01 a | 5.83 ± 0.08 a | |
| B1N3 | 582.67 ± 33.19 ab | 628.06 ± 14.301 ab | 0.91 ± 0.02 a | 5.16 ± 0.39 ab | |
| ANOVA | |||||
| Bacteria | B | * | * | ns | ns |
| N | ns | ** | ns | ns | |
| B × N | * | ** | ns | ns | |
| Fungi | B | ** | ns | ns | ns |
| N | ** | ** | ** | ** | |
| B × N | ** | * | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Sun, Q.; Wang, W. Effects of Biochar Combined with Nitrogen Fertilizer Application on Pepper Yield, Quality and Rhizosphere Soil Microbial Community Diversity. Plants 2025, 14, 3082. https://doi.org/10.3390/plants14193082
Wu C, Sun Q, Wang W. Effects of Biochar Combined with Nitrogen Fertilizer Application on Pepper Yield, Quality and Rhizosphere Soil Microbial Community Diversity. Plants. 2025; 14(19):3082. https://doi.org/10.3390/plants14193082
Chicago/Turabian StyleWu, Chunyan, Qiyuan Sun, and Wei Wang. 2025. "Effects of Biochar Combined with Nitrogen Fertilizer Application on Pepper Yield, Quality and Rhizosphere Soil Microbial Community Diversity" Plants 14, no. 19: 3082. https://doi.org/10.3390/plants14193082
APA StyleWu, C., Sun, Q., & Wang, W. (2025). Effects of Biochar Combined with Nitrogen Fertilizer Application on Pepper Yield, Quality and Rhizosphere Soil Microbial Community Diversity. Plants, 14(19), 3082. https://doi.org/10.3390/plants14193082




