Abstract
The cowpea weevil, Callosobruchus maculatus (F.), stands out as one of the most destructive field-to-storage pests of leguminous crops. This study investigates the potential of essential oils derived from two Satureja species, Satureja hortensis L. and Satureja khuzistanica Jamzad, for managing C. maculatus. Bioassay results revealed that both S. hortensis (72 h LC50 = 0.20 µL/g) and S. khuzistanica (72 h LC50 = 0.19 µL/g) essential oils exhibited significant toxicity against C. maculatus adults. The essential oils extended development time, reduced adult longevity, and decreased fecundity of the pest. Key population parameters, including intrinsic growth rate (r) and net reproductive rate (R0), were significantly lowered, particularly by S. hortensis essential oil. Age-specific survival (lx) and fecundity (mx) rates were also declined in treated groups, with delayed reproductive peaks compared to controls. Chemical analyses of S. hortensis and S. khuzistanica essential oils indicated that carvacrol (30.9% and 62.9%, respectively), γ-terpinene (25.5% and 4.3%), p-cymene (9.7% and 7.9%), and thymol (3.7% and 9.3%) were the major components. Hierarchical cluster analysis (HCA) was carried out to compare and contrast the compositions with previous works. The results demonstrated that S. hortensis and S. khuzistanica essential oils, given their lethal and sublethal effects against C. maculatus, can be introduced as natural alternatives to hazardous chemical insecticides, highlighting the need for further research in this field.
1. Introduction
The cowpea weevil, Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae), is a widely distributed insect pest that infests legumes, especially cowpea (Vigna unguiculata (L.) Walpers) seeds, both in the field and during storage [1]. As a cosmopolitan insect pest, C. maculatus is found in tropical and subtropical regions around the world and is originally native to Africa and Asia [2]. The feeding and activities of C. maculatus can cause significant quantitative and qualitative damage to crops, resulting in seed perforation, reduced marketability, decreased weight, and lower germination capacity [3].
The widespread use of chemical insecticides in the management of insect pests has resulted in several negative consequences, including potential risks to human health, environmental pollution, harm to non-target organisms, and the emergence of pest resistance [4,5]. For example, the resistance of C. maculatus to conventional pyrethroid and organophosphate insecticides, such as cypermethrin, permethrin, and pirimiphos-methyl has been reported [6]. Thus, the development of pest control agents that are both highly effective and environmentally benign, with minimal impact on non-target species, is crucial [7].
The genus Satureja, part of the Lamiaceae family, includes numerous species commonly known as savory [8]. Along with application in food industries, different biological effects of Satureja essential oils, from antioxidant to antibacterial and anticancer activities, were documented in the recent research [9,10,11]. Recent research has also shown that essential oils extracted from various species within this genus, which are rich in terpenes and phenylpropanoids, possess significant insecticidal properties for controlling insect pests [12,13,14]. For example, the essential oil from Satureja hortensis L., which is rich in the phenylpropanoid estragole (82.1%), demonstrated toxicity against adults of the lesser grain borer (Rhyzopertha dominica F.) and the red flour beetle (Tribolium castaneum Herbst), with 24 h LC50 values of 27.21 and 38.91 µL/L, respectively [15]. Additionally, the essential oil of Satureja khuzistanica Jamzad showed considerable insecticidal potential against fourth-instar larvae and adult Colorado potato beetles (Leptinotarsa decemlineata (Say)), with 24 h LC50 values of 23.36 ppm and 17.96 ppm, respectively [16]. The primary constituents of this essential oil were carvacrol (81.1%), p-cymene (3.3%), and γ-terpinene (3.2%).
The susceptibility of C. maculatus to plant-derived essential oils has been revealed in recent years [17,18]. More specifically, studies have examined not only acute lethal effects but also sublethal impacts of essential oils on biological and population parameters of C. maculatus [19,20,21,22]. Aimad et al. [21] reported substantial fumigant toxicity for three additional species, including wormwood (Artemisia herba-alba Asso), chamomile (Matricaria recutita L.), and yellow fleabane (Dittrichia viscosa L.). These treatments also reduced oviposition and emergence of adults. Notably, the essential oil of fennel (Foeniculum vulgare Mill.) significantly reduced biological (longevity, fecundity and oviposition period of adults) and key life table parameters (intrinsic rate of increase (r), net reproductive rate (R0), gross reproductive rate (GRR), and finite rate of increase (λ)) of the pest [14]. More precisely, the susceptibility of C. maculatus to the essential oils manifests not only in rapid pest mortality but also in significant alterations to its biological parameters and life table analysis. The evaluation of such parameters is a cornerstone of modern Integrated Pest Management (IPM), as it provides the essential quantitative data required to understand population dynamics, predict outbreak potential, and design effective, targeted control strategies [23,24]. Therefore, as part of eco-friendly management strategies for the C. maculatus, the present study was conducted to investigate the efficacy of essential oils extracted from two Iranian Satureja species, namely S. hortensis and S. khuzistanica, on the mortality and biological and demographic parameters of the pest. It should be emphasized that the toxicity of S. khuzistanica essential oil against C. maculatus has been investigated for the first time in the present study. Furthermore, the chemical composition of the studied essential oils was analyzed, and the potential relationship between the identified compounds and the observed insecticidal properties has been discussed.
2. Results
2.1. Chemical Profile of Commercial Essential Oils
A total of 46 compounds were identified in the essential oil of S. hortensis, accounting for 98.1% of the total composition, while 55 compounds were identified in S. khuzistanica essential oil, 97.5% of the total (Table 1). The major components in S. hortensis essential oil were carvacrol (30.9%), γ-terpinene (25.5%), and p-cymene (9.7%), while S. khuzistanica essential oil was dominated by carvacrol (62.9%), along with thymol (9.3%), and p-cymene (7.9%).

Table 1.
Chemical profile and percent composition of essential oils isolated from Satureja hortensis and S. khuzistanica.
2.2. Acute Toxicity of Essential Oils
The results demonstrated that the tested concentrations of both S. hortensis (F = 30.79; df = 5, 71; p < 0.001) and S. khuzistanica (F = 59.72; df = 5, 71; p < 0.001) essential oils had significant effects on C. maculatus mortality. Similarly, exposure time significantly influenced mortality for both S. hortensis (F = 15.58; df = 2, 71; p < 0.001) and S. khuzistanica (F = 19.27; df = 2, 71; p < 0.001) essential oils. However, the interaction between exposure time and concentration was not significant for either S. hortensis (F = 0.26; df = 10, 71; p = 0.99) or S. khuzistanica (F = 0.35; df = 10, 71; p = 0.96).
The results of Probit analyses from lethality bioassays are presented in Table 2. The LC50 value for S. hortensis essential oil was 0.44 μL/g at 24 h, decreasing to 0.20 μL/g by 72 h. A similar trend was observed for S. khuzistanica essential oil, with LC50 values declining from 0.36 μL/g (24 h) to 0.19 μL/g (72 h). The proximity of the LC50 and LC90 values for all exposure times arises from the steep dose–response curve and the variability observed at high mortality levels. The lower LC50 values over time, coupled with higher relative potency, indicate that the toxicity of both essential oils increased significantly with prolonged exposure. Furthermore, R-squared (R2) values demonstrated a positive correlation between essential oil concentration and pest mortality, confirming concentration-dependent efficacy against C. maculatus.

Table 2.
Probit analyses of the mortality of Callosobruchus maculatus adults exposed to Satureja hortensis and S. khuzistanica essential oils after 24 and 48, and 72 h.
2.3. Effects on Biological and Life Table Parameters
Effects of 24 h LC30 and LC50 values of S. hortensis (0.13 and 0.44 μL/g, respectively) and S. khuzistanica essential oils (0.15 and 0.36 μL/g, respectively) on the biological and life table parameters of C. maculatus are indicated at Table 3 and Table 4. The immature development (egg, larva and pupa period) of the pest was enlarged by the LC50 of S. khuzistanica essential oil. The immature survival has not shown a significant reduction with essential oils. The male adult longevity was shortened by S. hortensis essential oil, while the longevity of females was decreased by both essential oils and all treatments. Fecundity of the pest significantly decreased by both essential oils compared to the control group. The lowest fecundity was observed for adults treated with LC50 of S. hortensis. Total pre-ovipositional period (TPOP) was increased by the LC50 of S. khuzistanica essential oil (Table 3).

Table 3.
Biological parameters (mean ± SE) of Callosobruchus maculatus treated with Satureja hortensis and S. khuzistanica essential oils and on untreated control group.

Table 4.
Life-table parameters (mean ± SE) of Callosobruchus maculatus treated with Satureja hortensis and S. khuzistanica essential oils and on untreated control group.
Intrinsic rate of population increase (r) and net reproductive rate (R0) were significantly decreased by the essential oil of S. hortensis. The r was also decreased by LC50 of S. khuzistanica. The gross reproductive rate (GRR) of the pest was significantly decreased by both essential oils compared to the control group. The finite rate of increase (λ) was also significantly decreased by the essential oil of S. hortensis. A diverse outcome was achieved for mean generation time (T): increasing by LC50 of S. khuzistanica and decreasing by LC30 of S. hortensis compared to the control group.
Based on the age-specific survival rate (lx), the interval from first oviposition to death of the last female was 32 days in the control group. This duration was reduced to 30 days under treatment with LC30 and LC50 of S. hortensis essential oil, as well as the LC30 of S. khuzistanica essential oil. This reduction was accompanied by a noticeably smaller area under the lx curve, indicating decreased overall survival in treated populations compared to controls. The highest age-stage fecundity (mx) was observed in the control group (13.9 eggs at 25 days). Sublethal concentrations of S. hortensis essential oil significantly reduced mx to 5.25 eggs (at 24 days) for the LC30 treatment and 3.25 eggs (at 28 days) for the LC50 treatment. Similarly, exposure to S. khuzistanica essential oil resulted in mx of 6.25 eggs (at 25 days) and 7.50 eggs (at 27 days) for the LC30 and LC50 treatments, respectively. In general, mx of C. maculatus treated by both Satureja essential oils at all treatments was significantly decreased compared to the control group. These results indicate a concentration-dependent reduction in reproductive output in C. maculatus, with both essential oils demonstrating delayed peak fecundity compared to the control group (Figure 1).
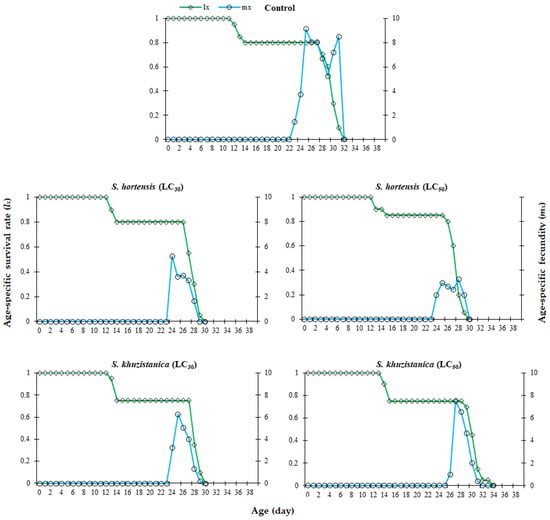
Figure 1.
Age-specific survival rate (lx) and age-specific fecundity (mx) of Callosobruchus maculatus exposed to two different lethal concentration (LC) values of Satureja hortensis and S. khuzistanica essential oils compared to control responses.
3. Discussion
The phytochemical profiles of S. hortensis were reviewed in 2018 [30], and there have been numerous publications on the essential oil compositions. The essential oils of S. hortensis are generally dominated by carvacrol, p-cymene, and γ-terpinene. In an analysis of 30 accessions of S. hortensis from Iran, two major clusters were identified: (1) carvacrol (42.0–58.2%), γ-terpinene (18.3–28.5%), and p-cymene (4.3–14.9%), and (2) dominated by carvacrol (76.0–83.3%) [31]. The chemical composition of an essential oil can have profound effects on biological activity. In order to place the composition of the essential oil used in this study with previous compositions, a hierarchical cluster analysis (HCA) was carried out. The HCA, based on the major essential oil components of essential oils from this work and those reported in the literature [9,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57], reveals five major clusters (Figure 2): Group 1 is a carvacrol/γ-terpinene cluster, Group 2 is a carvacrol/p-cymene cluster. Group 3 has nearly equivalent concentrations of γ-terpinene and carvacrol. Group 4 has a high concentration of carvacrol. Group 5 has a high concentration of thymol. The essential oil of S. hortensis from this study is found in Group 3.
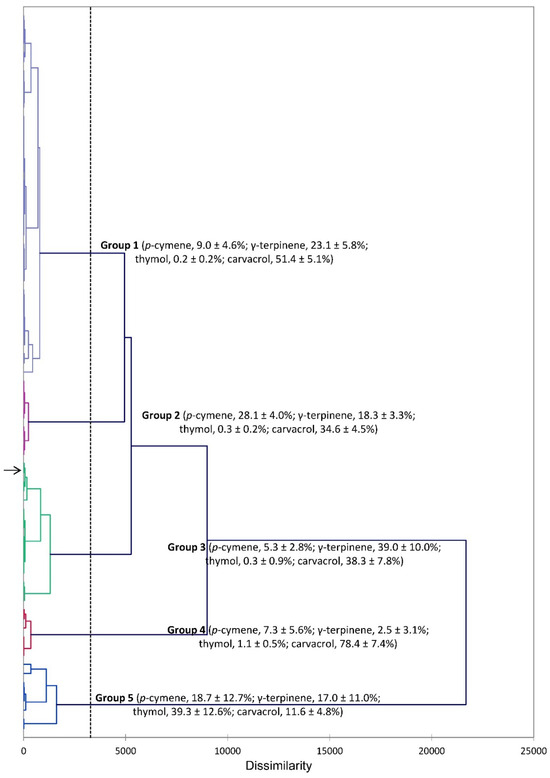
Figure 2.
Dendrogram obtained from hierarchical cluster analysis (HCA) of Satureja hortensis essential oil compositions (major components). The essential oil sample from this study is indicated by the arrow (→).
The essential oils of S. khuzistanica are generally dominated by carvacrol. However, there is some variation with some samples showing lower levels of carvacrol. An HCA treatment based on the compositions of the essential oils from this study and those previously reported [38,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86] illustrates these variations (Figure 3). The HCA shows three groups, which are separated based on the concentrations of carvacrol. Group 1 shows relatively low concentrations of carvacrol (40.9 ± 11.7%), Group 2 has moderate levels of carvacrol (66.2 ± 3.0%), and Group 3, the largest group, has high concentrations of carvacrol (91.4 ± 4.9%). The S. khuzistanica sample from this present study is found in Group 2.
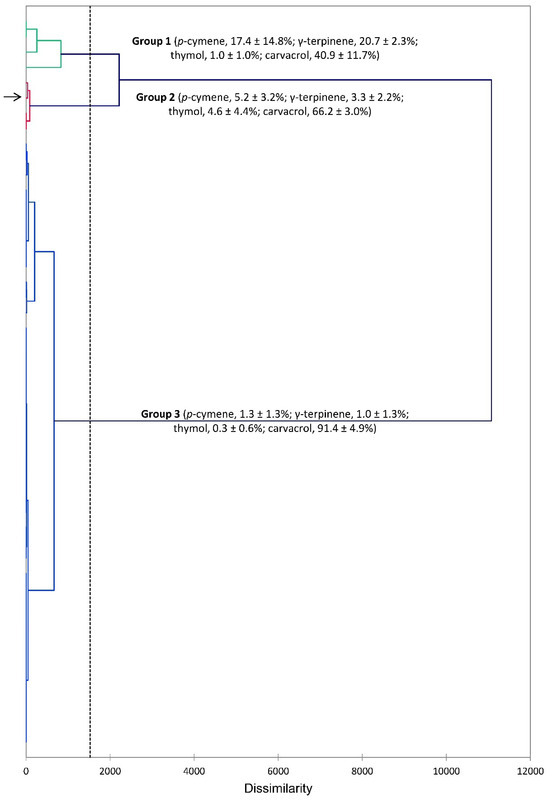
Figure 3.
Dendrogram obtained from hierarchical cluster analysis (HCA) of Satureja khuzistanica essential oil compositions (major components). The essential oil sample from this study is indicated by the arrow (→).
The essential oils of S. hortensis and S. khuzistanica demonstrated significant efficacy against C. maculatus, with mortality rates increasing over time. While the insecticidal potential of S. khuzistanica essential oil against C. maculatus is reported here for the first time, the toxicity of S. hortensis essential oil has been documented in previous studies, which corroborate our findings. For instance, Heydarzade and Moravvej [12] reported contact toxicity of S. hortensis oil (sourced from the Mashhad province of Iran), with LC50 values of 535.7 and 641.0 μL/m2 against male and female adults of C. maculatus, respectively. The major constituents of essential oil were carvacrol (50.1%), thymol (27.8%), γ-terpinene (4.7%), and p-cymene (4.3%). Although these compounds were also identified in our study, their quantity differed considerably: 30.9%, 3.7%, 25.5%, and 9.7%, respectively. In another study, Zandi-Sohani [87] found that S. hortensis essential oil (from the Khuzestan province of Iran) had an LC50 value of 1.50 μL/L against C. maculatus adults via fumigation, with a concentration of 60 μL/L causing 91.1% mortality after 24 h. Zandi-Sohani [87] used filter paper fumigation bioassays, while our treatment involved impregnating cowpea seeds with the essential oil, a method that combines contact and fumigant actions and is highly relevant for practical pest management in field and storage conditions. Accordingly, the disparity in LC50 values between our study (0.44 μL/g) and those mentioned above can be attributed to the geographical origin of the plant material, variations in essential oil chemical composition, and differences in bioassay methodologies. Furthermore, the insecticidal effects of dominant terpenes identified in S. hortensis and S. khuzistanica essential oils, including carvacrol, p-cymene, thymol, and γ-terpinene, are well-documented in previous studies [47,88,89]. Additionally, recent studies have elucidated the multi-faceted modes of action exhibited by plant-derived compounds such as carvacrol, thymol, and γ-terpinene against insect pests. For instance, thymol application in the cotton bollworm (Helicoverpa armigera (Hübner)), was found to suppress key detoxification enzymes, including general esterases, glutathione S-transferase, and cytochrome P450, as well as inhibit acetylcholinesterase activity [90]. A similar inhibitory effect was observed in the red flour beetle (Tribolium castaneum Herbest), where carvacrol exposure led to decreased activity of both glutathione S-transferase and acetylcholinesterase [91]. Immunosuppressive properties and potential for genome damage of γ-terpinene against the melon fruit fly (Zeugodacus cucurbitae (Coquillett)) were also revealed [92]. Interestingly, the essential oil of S. hortensis (γ-terpinene/carvacrol-rich chemotype) was generally more active than the essential oil of S. khuzistanica (4.3% γ-terpinene, 62.9% carvacrol). The dual activities of γ-terpinene and carvacrol may be responsible for the more pronounced activity of S. hortensis essential oil. It can therefore be concluded that the insecticidal efficacy of S. hortensis and S. khuzistanica essential oils is likely due to the presence and multiple action of these compounds in their essential oils.
Along with lethality, the essential oils of S. hortensis and S. khuzistanica disrupted the life history parameters of C. maculatus. Treatment with S. khuzistanica essential oil resulted in increased developmental duration, a longer total pre-ovipositional period, and decreased immature survival. In general, exposure to sublethal stressors similar insecticide treatments can prolong developmental time and reduce survival rates [14,93]. Both essential oils significantly reduced the female longevity and fecundity of C. maculatus compared to the control. The lowest fecundity, representing a 78% reduction relative to the control, was observed in adults treated with the S. hortensis essential oil.
The reduction in fecundity may be attributed to several factors, including adult female mortality, decreased oviposition, and disruption of the vitellogenesis process [14,94,95]. The disruption of C. maculatus biological parameters by essential oils is consistent with previous research. For instance, Elhourri et al. [95] reported that the essential oil of Chenopodium ambrosioïdes L. decreased the survival, adult longevity, and fecundity of C. maculatus. Similarly, essential oils from Majorana hortensis Moench., Rosmarinus officinalis L., Syzygium aromaticum, and Thymus vulgaris L. negatively affected its fecundity [96]. A recent study by Naseri et al. [14] aligns with our findings, showing that essential oils of Foeniculum vulgare Mill. and Pimpinella anisum L. reduced immature survival, adult longevity, fecundity, and oviposition period. Furthermore, the essential oil of S. hortensis and its main component, carvacrol (the dominant compound in both essential oils studied here), reduced the emergence and longevity of the gray knot-horn (Acrobasis advenella (Zinck.)) [47]. Collectively, these results confirm that plant essential oils can alter the biological parameters of C. maculatus, an effect likely mediated by their specific chemical constituents.
The present study demonstrated that the essential oils of S. hortensis and S. khuzistanica significantly affected key life table parameters of C. maculatus, including the intrinsic rate of increase (r), net reproductive rate (R0), gross reproductive rate (GRR), finite rate of increase (λ), and mean generation time (T). Although reductions in these demographic indices have been previously documented for other essential oils [14,97,98], the effectiveness of S. hortensis and S. khuzistanica essential oils is reported here for the first time. These findings show that, besides their acute lethal effects, both essential oils can significantly reduce the population growth of C. maculatus in next generations, validating their potential as effective natural insecticides.
4. Materials and Methods
4.1. Essential Oils and Their Chemical Profile
The essential oils of S. hortensis and S. khuzistanica (100% purity) were obtained from Barij Essence Company (Kashan, Esfahan, Iran). The essential oils were stored at 4 °C in a refrigerator until use. The chemical profile of essential oils was analyzed using gas chromatography (Agilent 7890B, Santa Clara, CA, USA) coupled with a mass spectrometer (Agilent 5977A, Santa Clara, CA, USA). The evaluation was performed using an HP-5 ms capillary column (30 m × 0.25 mm × 0.25 µm). The carrier gas was helium with a column head pressure of 8.23 psi (56.8 kPa) and a flow rate of 1.0 mL/min. Inlet temperature was 280 °C and interface temperature was 280 °C. The GC oven temperature program was used as follows: 60 °C initial temperature, hold for 5 min; increased at 6 °C/min to 240 °C, then 10 °C/min to 310 °C. A 1% w/v solution of the sample in methanol was prepared and 1 μL was injected using a split ratio of 100:1. Identification of the oil components was based on their retention indices, determined by reference to a homologous series of n-alkanes [99], and by comparison of their mass spectral fragmentation patterns with those reported in the databases [25,26,27,28].
4.2. Insect Pest Rearing
The initial population of C. maculatus was sourced from a laboratory colony maintained on mung bean (Vigna radiata (L.) at the University of Mohaghegh Ardabili, Iran. For colony maintenance, 200 g of mung bean seeds (Vigna radiata (L.) ‘Parto’ cultivar) were placed in wide-mouthed cylindrical five-glass jars (18.5 cm diameter × 8 cm height) covered with mesh fabric to ensure proper ventilation. Each jar was infested with 50 randomly selected adult insects of mixed sex, and the jars were kept in a growth chamber under controlled conditions of 28 ± 1 °C, 60 ± 5% relative humidity, and a photoperiod of 14 h of light and 10 h of darkness. Newly emerged adults, 24 h old, from the colony were used for all bioassays [100].
4.3. Acute Toxicity of Essential Oils
Initial experiments were conducted to determine the minimum and maximum concentrations of essential oils needed to achieve mortality rates of approximately 25% to 75%. Based on logarithmic intervals, six concentrations were selected for the main bioassays, along with a control: 1.0–17.0 and 1.0–12.0 μL/7 g for S. hortensis and S. khuzistanica corresponding to 0.14–2.43 and 0.14–1.71 μL/g, respectively. For each treatment, the concentration was mixed with 2 mL of acetone and applied to 7 g of green gram seeds in a glass Petri dish (8 cm in diameter and 2 cm in height). The mixture was stirred thoroughly with a metal spoon for 2 min at room temperature until it was completely dry. The control treatment consisted of seeds treated with acetone alone, without any essential oil. After drying, the treated seeds were transferred into 6 cm Petri dishes, where 10 adults (5 males and 5 females) were introduced into each dish. Each treatment was replicated four times, and mortality was assessed at 24, 48, and 72 h post-treatment. Insects were considered dead if they showed no movement in their legs or antennae when gently prodded with a brush [14].
4.4. Effects on Biological and Life Table Parameters
The effects of 24 h LC30 and LC50 values of S. hortensis (0.13 and 0.44 μL/g, respectively) and S. khuzistanica (0.15 and 0.36 μL/g, respectively) essential oils on life history parameters of C. maculatus were evaluated. Eighty 1-day-old weevils (40 males + 40 females) of were exposed essential oils, while the control group was treated only with the solvent acetone. After 24 h, adult insects were removed from the rearing environment, and individual seeds containing single eggs were transferred to 6 cm diameter Petri dishes covered with mesh fabric. The seeds were monitored daily, and the emergence time of each adult insect was recorded. Upon female emergence, they were separately paired with male insects from the same treatment group. Adult longevity was recorded until the death of the last surviving male and female individuals. All procedures, except for the treatment of adult insects with the essential oils, were also performed on the control group and the collected data were subsequently analyzed to evaluate key biological parameters of the pest population, including pre-adults longevity and survival and adult longevity and fecundity. The age-stage-specific survival rate (lx; probability of surviving to age x) and age-stage-specific fecundity (mx; mean number of eggs laid per individual at age x) were calculated to estimate life table parameters [101,102]. These variables were computed using the following equations (where x = age, j = stage, and n = total number of developmental stages):
The intrinsic rate of increase (r), net reproductive rate (R0), gross reproductive rate (GRR), finite rate of increase (λ), and mean generation time (T) were also computed by the following formula:
λ = er
T = (Ln R0)/r
4.5. Statistical Analyses
The acute toxicity data were subjected to analysis of variance (ANOVA), with mean comparisons performed using the Tukey HSD test (p < 0.05). Bioassay data for lethal concentration estimation and regression line analysis were analyzed using Probit analysis in SPSS software (Version 16). Life table parameters were analyzed using the TWOSEX-MSChart software (Version 21/10/2023) [103], with 100,000 bootstrap repetitions employed. Statistical differences between biological parameters were determined using paired bootstrap tests (p < 0.05). Agglomerative hierarchical cluster analyses (HCA) were carried using XLSTAT v. 2018.1.1.62926 (Addinsoft, Paris, France). In both S. hortensis and S. khuzistanica, the percentages of four components (p-cymene, γ-terpinene, thymol, and carvacrol) were used for the analysis. Dissimilarity was used to determine clustering based on Euclidean distance, and Ward’s method was used to define agglomeration.
5. Conclusions
Based on the present findings, the essential oils of S. hortensis and S. khuzistanica exhibited significant toxicity against adult C. maculatus, with lethality increasing over time. In addition to these acute lethal effects, application of the oils at their LC30 and LC50 caused significant disruption to several biological parameters of the pest, including prolonged larval and pupal development, reduced immature survival, shortened adult longevity, a decreased oviposition period, and a 54–78% reduction in fecundity. Furthermore, key population growth parameters r, R0, GRR, and λ were all significantly reduced compared to the control group. This indicates a strong inhibitory effect of the essential oils on the population growth of the subsequent generation. Chemical analyses identified terpenes as the dominant compounds in both oils, primarily monoterpene hydrocarbons and oxygenated monoterpenoids such as carvacrol, γ-terpinene, p-cymene, and thymol. In conclusion, the terpene-rich essential oils of S. hortensis and S. khuzistanica show great potential as accessible, effective, and eco-friendly botanical insecticides for the management of C. maculatus. In the present study, both contact and fumigant effects of the essential oils contributed concurrently to the observed mortality of the pest. Given that C. maculatus is a field-to-storage pest, separate investigations into contact and fumigant toxicity would be highly beneficial for potential field applications and direct protection of stored grains, respectively. To facilitate their practical application, further research is highly recommended in the following areas: evaluation of insecticidal activities of essential oils from other S. hortensis and S. khuzistanica chemotypes, investigating the side effects on non-target organisms (particularly beneficial predators and parasitoids), enhancing stability and persistence through advanced formulation technologies like micro- and nano-emulsions, and evaluating the residual effects of these essential oils on treated stored seeds.
Author Contributions
Conceptualization, A.E., B.N. and A.A.; methodology, A.E., B.N. and A.A.; software, A.E. and A.A.; validation, A.E., B.N. and W.N.S.; formal analysis, A.E., A.A. and W.N.S.; investigation, A.E. and A.A.; resources, A.E.; writing—original draft preparation, A.E., B.N., A.A. and W.N.S.; writing—review and editing, A.E., B.N., A.A. and W.N.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
This research was supported by the University of Mohaghegh Ardabili, which is greatly appreciated.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Phillips, T.W.; Throne, J.E. Biorational approaches to managing stored-product insects. Annu. Rev. Entomol. 2010, 55, 375–397. [Google Scholar] [CrossRef]
- Kébé, K.; Alvarez, N.; Tuda, M.; Arnqvist, G.; Fox, C.W.; Sembène, M.; Espíndola, A. Global phylogeography of the insect pest Callosobruchus maculatus (Coleoptera: Bruchinae) relates to the history of its main host, Vigna unguiculata. J. Biogeogr. 2017, 44, 2515–2526. [Google Scholar] [CrossRef]
- Kalpna; Hajam, Y.A.; Kumar, R. Management of stored grain pest with special reference to Callosobruchus maculatus, a major pest of cowpea: A review. Heliyon 2022, 8, e08703. [Google Scholar] [CrossRef]
- Daraban, G.M.; Hlihor, R.; Suteu, D. Pesticides vs. biopesticides: From pest management to toxicity and impacts on the environment and human health. Toxics 2023, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakas, C.A.; Tom, J.; Sreejith, S. Impact of insecticides on man and environment. In Biomedical Applications and Toxicity of Nanomaterials; Mohanan, P.V., Kappalli, S., Eds.; Springer: Singapore, 2023; pp. 783–801. [Google Scholar] [CrossRef]
- Zongo, S.; Coulibaly, A.Y.; Drabo, S.F.; Gnankiné, O.; Kiendrebeogo, M.; Doumma, A.; Sembene, M.; Sanon, A. Metabolic resistance to pyrethroids (Py) and organophosphates (Op) in Callosobruchus maculatus (Fab.) (Coleoptera: Chrysomelidae: Bruchinae) a major pest of stored cowpeas in West Africa. Int. J. Pest Manag. 2020, 67, 338–345. [Google Scholar] [CrossRef]
- Seiber, J.N.; Coats, J.; Duke, S.O.; Gross, A.D. Biopesticides: State of the art and future opportunities. J. Agric. Food Chem. 2014, 62, 11613–11619. [Google Scholar] [CrossRef]
- Nooshkam, A.; Mumivand, H.; Hadian, J.; Alemardan, A.; Morshedloo, M.R. Drug yield and essential oil and carvacrol contents of two species of Satureja (S. khuzistanica Jamzad and S. rechingeri Jamzad) cultivated in two different locations. J. Appl. Res. Med. Aromat. Plants 2017, 6, 126–130. [Google Scholar] [CrossRef]
- Chambre, D.R.; Moisa, C.; Lupitu, A.; Copolovici, L.; Pop, G.; Copolovici, D.-M. Chemical composition, antioxidant capacity, and thermal behavior of Satureja hortensis essential oil. Sci. Rep. 2020, 10, 21322. [Google Scholar] [CrossRef] [PubMed]
- Rashidipour, M.; Ashrafi, B.; Nikbakht, M.R.; Veiskarami, S.; Taherikalani, M.; Soroush, S. Encapsulation of Satureja khuzistanica Jamzad essential oil in chitosan nanoparticles with enhanced antibacterial and anticancer activities. Prep. Biochem. Biotechnol. 2021, 51, 971–978. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, S.R.; Ebrahimi, S.N.; Rezadoost, H. Simultaneous ultrasound-assisted hydrodistillation of essential oil from aerial parts of the Satureja khuzistanica Jamzad and its antibacterial activity. J. Med. Plants 2021, 20, 47–59. [Google Scholar] [CrossRef]
- Heydarzade, A.; Moravvej, G. Contact toxicity and persistence of essential oils from Foeniculum vulgare, Teucrium polium and Satureja hortensis against Callosobruchus maculatus (Fabricius) adults (Coleoptera: Bruchidae). Türk. Entomol. Derg. 2012, 36, 507–519. [Google Scholar]
- Ebadollahi, A.; Jalali Sendi, J.; Ziaee, M.; Krutmuang, P. Acaricidal, insecticidal, and nematicidal efficiency of essential oils isolated from the Satureja genus. Int. J. Environ. Res. Public Health 2021, 18, 6050. [Google Scholar] [CrossRef] [PubMed]
- Naseri, B.; Ebadollahi, A.; Ebadi, A.; Elahi, M.; Afshari, F.; Pourabad, R.F. The possibility of using plant essential oils to enhance the insecticidal properties of spinosad against Callosobruchus maculatus (F.). J. Stored Prod. Res. 2025, 114, 102748. [Google Scholar] [CrossRef]
- Ebadollahi, A. Estragole-rich essential oil of summer savory (Satureja hortensis L.) as an eco-friendly alternative to the synthetic insecticides in management of two stored-products insect pests. Acta Agric. Slov. 2020, 115, 307–314. [Google Scholar] [CrossRef]
- Taghizadeh Saroukolai, A.; Nouri-Ganbalani, G.; Rafiee-Dastjerdi, H.; Hadian, J. Antifeedant activity and toxicity of some plant essential oils to Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Plant Prot. Sci. 2014, 50, 207–216. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Jalali Sendi, J.; Setzer, W.N.; Changbunjong, T. Encapsulation of Eucalyptus largiflorens essential oil by mesoporous silicates for effective control of the cowpea weevil, Callosobruchus maculatus (Fabricius) (Coleoptera: Chrysomelidae). Molecules 2022, 27, 3531. [Google Scholar] [CrossRef]
- Mssillou, I.; Saghrouchni, H.; Saber, M.; Zannou, A.J.; Balahbib, A.; Bouyahya, A.; Allali, A.; Lyoussi, B.; Derwich, E. Efficacy and role of essential oils as bio-insecticide against the pulse beetle Callosobruchus maculatus (F.) in post-harvest crops. Ind. Crops Prod. 2022, 189, 115786. [Google Scholar] [CrossRef]
- Izakmehri, K.; Saber, M.; Mehrvar, A.; Hassanpouraghdam, M.B.; Vojoudi, S. Lethal and sublethal effects of essential oils from Eucalyptus camaldulensis and Heracleum persicum against the adults of Callosobruchus maculatus. J. Insect Sci. 2013, 13, 152. [Google Scholar] [CrossRef]
- Matos, L.F.; Barbosa, D.R.E.S.; Lima, E.D.C.; Dutra, K.D.A.; Navarro, D.M.D.A.F.; Alves, J.L.R.; Silva, G.N. Chemical composition and insecticidal effect of essential oils from Illicium verum and Eugenia caryophyllus on Callosobruchus maculatus in cowpea. Ind. Crops Prod. 2020, 145, 112088. [Google Scholar] [CrossRef]
- Aimad, A.; Bourhia, M.; Hana, H.; Sanae, R.; Salamatullah, A.M.; Soufan, W.; Rihan, H.Z.; Ouahmane, L.; Youness, E.A.; Noureddine, E.; et al. Essential oils from Artemisia herba-alba Asso., Matricaria recutita L., and Dittrichia viscosa L. (Asteraceae): A promising source of eco-friendly agents to control Callosobruchus maculatus Fab. warehouse pest. J. Chem. 2022, 2022, 2373460. [Google Scholar] [CrossRef]
- Et-tazy, L.; Lamiri, A.; Krimi Bencheqroun, S.; Errati, H.; Hashem, A.; Dolores, G.; Fathi, E.; Satrani, B.; Essahli, M.; Satia, L. Exploring synergistic insecticidal effects of binary mixtures of major compounds from six essential oils against Callosobruchus maculatus. Sci. Rep. 2025, 15, 98566. [Google Scholar] [CrossRef]
- Stark, J.D.; Banks, J.E. Population-level effects of pesticides and other toxicants on arthropods. Annu. Rev. Entomol. 2003, 48, 505–519. [Google Scholar] [CrossRef]
- Pedigo, L.P.; Rice, M.E. Entomology and Pest Management, 6th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2009. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007. [Google Scholar]
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Dissertation, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]
- Mondello, L. FFNSC 3; Shimadzu Scientific Instruments: Columbia, MD, USA, 2016. [Google Scholar]
- NIST20; NIST/EPA/NIH Mass Spectral Library (NIST20). National Institute of Standards and Technology: Gaithersburg, MD, USA, 2020.
- Valizadeh, B.; Jalali Sendi, J.; Oftadeh, M.; Ebadollahi, A.; Krutmuang, P. Ovicidal and physiological effects of essential oils extracted from six medicinal plants on the elm leaf beetle, Xanthogaleruca luteola (Mull.). Agronomy 2021, 11, 2015. [Google Scholar] [CrossRef]
- Fierascu, I.; Dinu-Pirvu, C.E.; Fierascu, R.C.; Velescu, B.S.; Anuta, V.; Ortan, A.; Jinga, V. Phytochemical profile and biological activities of Satureja hortensis L.: A review of the last decade. Molecules 2018, 23, 2458. [Google Scholar] [CrossRef]
- Hadian, J.; Ebrahimi, S.N.; Salehi, P. Variability of morphological and phytochemical characteristics among Satureja hortensis L. accessions of Iran. Ind. Crops Prod. 2010, 32, 62–69. [Google Scholar] [CrossRef]
- Azaz, A.D.; Kürkcüoglu, M.; Satil, F.; Baser, K.H.C.; Tümen, G. In vitro antimicrobial activity and chemical composition of some Satureja essential oils. Flavour Fragr. J. 2005, 20, 587–591. [Google Scholar] [CrossRef]
- Alizadeh, A.; Khoshkhui, M.; Javidnia, K.; Firuzi, O.; Tafazoli, E.; Khalighi, A. Effects of fertilizer on yield, essential oil composition, total phenolic content and antioxidant activity in Satureja hortensis L. (Lamiaceae) cultivated in Iran. J. Med. Plants Res. 2010, 4, 33–40. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Al-Moghazy, M.; ElSayed, A.A.A. The in vitro cytotoxicity, antioxidant and antibacterial potential of Satureja hortensis L. essential oil cultivated in Egypt. Bioorg. Chem. 2020, 95, 103559. [Google Scholar] [CrossRef] [PubMed]
- Baher, Z.F.; Mirza, M.; Ghorbanli, M.; Rezaii, M.B. The influence of water stress on plant height, herbal and essential oil yield and composition in Satureja hortensis L. Flavour Fragr. J. 2002, 17, 275–277. [Google Scholar] [CrossRef]
- Deans, S.G.; Svoboda, K.P. Antibacterial activity of summer savory (Satureja hortensis L.) essential oil and its constituents. J. Hortic. Sci. 1989, 64, 205–210. [Google Scholar] [CrossRef]
- Estaji, A.; Roosta, H.R.; Rezaei, S.A.; Hosseini, S.S.; Niknam, F. Morphological, physiological and phytochemical response of different Satureja hortensis L. accessions to salinity in a greenhouse experiment. J. Appl. Res. Med. Aromat. Plants 2018, 10, 25–33. [Google Scholar] [CrossRef]
- Farzaneh, M.; Kiani, H.; Sharifi, R.; Reisi, M.; Hadian, J. Chemical composition and antifungal effects of three species of Satureja (S. hortensis, S. spicigera, and S. khuzistanica) essential oils on the main pathogens of strawberry fruit. Postharvest Biol. Technol. 2015, 109, 145–151. [Google Scholar] [CrossRef]
- Gursoy, U.K.; Gursoy, M.; Gursoy, O.V.; Cakmakci, L.; Könönen, E.; Uitto, V.J. Anti-biofilm properties of Satureja hortensis L. essential oil against periodontal pathogens. Anaerobe 2009, 15, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Sadraei, H.; Ghannadi, A.R.; Mohseni, M. Antispasmodic and anti-diarrhoeal effect of Satureja hortensis L. essential oil. J. Ethnopharmacol. 2000, 71, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Hazrati, H.; Saharkhiz, M.J.; Niakousari, M.; Moein, M. Natural herbicide activity of Satureja hortensis L. essential oil nanoemulsion on the seed germination and morphophysiological features of two important weed species. Ecotoxicol. Environ. Saf. 2017, 142, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Heidarpour, O.; Esmaielpour, B.; Soltani, A.A.; Khorramdel, S. Effect of vermicompost on essential oil composition of (Satureja hortensis L.) under water stress condition. J. Essent. Oil-Bear. Plants 2019, 22, 484–492. [Google Scholar] [CrossRef]
- Ilić, Z.S.; Milenković, L.; Stanojević, L.; Danilović, B.; Šunić, L.; Milenković, A.; Kevrešan, Ž.; Stanojević, J.; Cvetković, D. Phytochemical composition and antimicrobial activities of the essential oils from summer savory (Satureja hortensis L.) growing in shading condition. J. Essent. Oil-Bear. Plants 2023, 26, 1397–1409. [Google Scholar] [CrossRef]
- Karimi, N.; Yari, M.; Ghasmpour, H.R. Identification and comparison of essential oil composition and mineral changes in different phenological stages of Satureja hortensis L. Iran. J. Plant Physiol. 2012, 3, 577–582. [Google Scholar]
- Katar, D.; Kacar, O.; Kara, N.; Aytaç, Z.; Göksu, E.; Kara, S.; Katar, N.; Erbaş, S.; Telci, İ.; Elmastaş, M. Ecological variation of yield and aroma components of summer savory (Satureja hortensis L.). J. Appl. Res. Med. Aromat. Plants 2017, 7, 131–135. [Google Scholar] [CrossRef]
- Kizil, S.; Turk, M.; Özguven, M.; Khawar, K.M. Full blooming stage is suitable for herbage yield and essential oil content of summer savory (Satureja hortensis L.). J. Essent. Oil-Bear. Plants 2009, 12, 620–629. [Google Scholar] [CrossRef]
- Magierowicz, K.; Górska-Drabik, E.; Sempruch, C. The insecticidal activity of Satureja hortensis essential oil and its active ingredient -carvacrol against Acrobasis advenella (Zinck.) (Lepidoptera, Pyralidae). Pestic. Biochem. Physiol. 2019, 153, 122–128. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N. Chemical composition and antimicrobial activity of Satureja hortensis and Trachyspermum copticum essential oil. Iran. J. Microbiol. 2011, 3, 194–200. [Google Scholar]
- Mihajilov-Krstev, T.; Radnović, D.; Kitić, D.; Zlatković, B.; Ristić, M.; Branković, S. Chemical composition and antimicrobial activity of Satureja hortensis L. essential oil. Cent. Eur. J. Biol. 2009, 4, 411–416. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Beiranvand, M. Chemical composition of the essential oil from the aerial parts of Satureja hortensis as a potent medical plant using traditional hydrodistillation. J. Chem. Health Risks 2013, 3, 43–54. [Google Scholar]
- Omidbaigi, R.; Hejazi, M. Essential oil content and compositions of Satureja hortensis of two different origins. J. Essent. Oil-Bear. Plants 2004, 7, 175–178. [Google Scholar] [CrossRef]
- Pfefferkorn, A.; Krüger, H.; Pank, F. Chemical composition of Satureja hortensis L essential oils depending on ontogenetic stage and season. J. Essent. Oil Res. 2008, 20, 303–305. [Google Scholar] [CrossRef]
- Popovici, R.; Vaduva, D.; Pinzaru, I.; Dehelean, C.; Farcas, C.; Coricovac, D.; Danciu, C.; Popescu, I.; Alexa, E.; Lazureanu, V.; et al. A comparative study on the biological activity of essential oil and total hydro-alcoholic extract of Satureja hortensis L. Exp. Ther. Med. 2019, 18, 932–942. [Google Scholar] [CrossRef]
- Saharkhiz, M.J.; Zomorodian, K.; Rezaei, M.R.; Saadat, F.; Rahimi, M.J. Influence of growth phase on the essential oil composition and antimicrobial activities of Satureja hortensis. Nat. Prod. Commun. 2011, 6, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Sefidkon, F.; Jamzad, Z. Chemical composition of the essential oil of three Iranian Satureja species (S. mutica, S. macrantha and S. intermedia). Food Chem. 2005, 91, 1–4. [Google Scholar] [CrossRef]
- Sharifzadeh, A.; Khosravi, A.R.; Ahmadian, S. Chemical composition and antifungal activity of Satureja hortensis L. essentiall oil against planktonic and biofilm growth of Candida albicans isolates from buccal lesions of HIV+ individuals. Microb. Pathog. 2016, 96, 1–9. [Google Scholar] [CrossRef]
- Wesolowska, A.; Grzeszczuk, M.; Jadczak, D. Influence of distillation method on the content and composition of essential oil isolated from summer savory (Satureja hortensis L.). J. Essent. Oil-Bear. Plants 2015, 18, 215–221. [Google Scholar] [CrossRef]
- Abbasloo, E.; Amiresmaili, S.; Shirazpour, S.; Khaksari, M.; Kobeissy, F.; Thomas, T.C. Satureja khuzistanica Jamzad essential oil and pure carvacrol attenuate TBI-induced inflammation and apoptosis via NF-κB and caspase-3 regulation in the male rat brain. Sci. Rep. 2023, 13, 4780. [Google Scholar] [CrossRef] [PubMed]
- Abbasloo, E.; Dehghan, F.; Khaksari, M.; Najafipour, H.; Vahidi, R.; Dabiri, S.; Sepehri, G.; Asadikaram, G. The anti-inflammatory properties of Satureja khuzistanica Jamzad essential oil attenuate the effects of traumatic brain injuries in rats. Sci. Rep. 2016, 6, 31866. [Google Scholar] [CrossRef] [PubMed]
- Davazdahemami, S.; Sefidcon, F.; Rezaei, M.; Naderi, M. The effect of drought stress on quantitative and qualitative characters of essential oil and carvacrol yield in two endemic species of savory (Satureja bachtiarica and S. khuzistarica) in Iran. Tech. J. Eng. Appl. Sci. 2014, 4, 143–146. [Google Scholar]
- Davazdahemami, S.; Sefidcon, F.; Rezaei, M.; Naderi, M. Chemical composition of the essential oils of five cultivated savory species in Iran: Satureja bachtiarica, S. khuzistanica, S. sahandica, S. spicigera and S. hortensis. Int. J. Biosci. 2014, 5, 47–50. [Google Scholar] [CrossRef]
- Darabad, A.H.; Rahimi, M.; Rafati, H. Continuous hydrodistillation-ultrasonication flow batch-mode operation system to enhance essential oil extraction from Satureja khuzistanica and evaluation of in vitro anti-inflammatory activity. Ind. Crops Prod. 2022, 188, 115540. [Google Scholar] [CrossRef]
- Alizadeh, A. Essential oil constituents, phenolic content and antioxidant activity of two endemic Satureja species from Iran. Bangladesh J. Bot. 2017, 46, 925–931. [Google Scholar]
- Eftekhar, F.; Ashoori, N.; Yousefzadi, M. In-vitro antimicrobial activity and chemical composition of Satureja khuzistanica Jamzad essential oils against multidrug-resistant Acinetobacter baumannii. Avicenna J. Clin. Microbiol. Infect. 2017, 4, e45601. [Google Scholar] [CrossRef]
- Ezatpour, B.; Dorosti, N.; Rezaee, E.; Ghaziani, F. Comparison of the chemical composition and biological activities of essential oils from two Satureja species: Molecular docking studies. J. Mex. Chem. Soc. 2023, 67, 70–81. [Google Scholar] [CrossRef]
- Fallahi, S.; Beyranvand, M.; Mahmoudvand, H.; Nayebzadeh, H.; Kheirandish, F.; Jahanbakhsh, S. Chemical composition, acute and sub-acute toxicity of Satureja khuzistanica essential oil in mice. Marmara Pharm. J. 2017, 21, 515–523. [Google Scholar] [CrossRef]
- Farsam, H.; Amanlou, M.; Radpour, M.R.; Salehinia, A.N.; Shafiee, A. Composition of the essential oils of wild and cultivated Satureja khuzistanica Jamzad from Iran. Flavour Fragr. J. 2004, 19, 308–310. [Google Scholar] [CrossRef]
- Ghodrati, L.; Alizadeh, A.; Ketabchi, S. Essential oil constituents and antimicrobial activities of Iranian Satureja khuzistanica Jamzad. Int. J. Biosci. 2015, 6, 249–257. [Google Scholar] [CrossRef]
- Hadian, J.; Mirjalili, M.H.; Reza Kanani, M.; Salehnia, A.; Ganjipoor, P. Phytochemical and morphological characterization of Satureja khuzistanica Jamzad populations from Iran. Chem. Biodivers. 2011, 8, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Hadian, J.; Akramian, M.; Heydari, H.; Mumivand, H.; Asghari, B. Composition and in vitro antibacterial activity of essential oils from four Satureja species growing in Iran. Nat. Prod. Res. 2012, 26, 98–108. [Google Scholar] [CrossRef]
- Khakzad, S.; Rahmani, F.; Hojjati, M.; Tabandeh, M.R. Anti-carcinogenic effects of Satureja khuzistanica and Zataria multiflora essential oils on K562 cell line proliferation. J. Food Bioprocess Eng. 2019, 2, 127–132. [Google Scholar]
- Kheirandish, F.; Chegeni, R.; Delfan, B.; Jabari, M.; Ebrahimzadeh, F.; Rashidipour, M. The cytotoxic and antileishmanial effects of Satureja khuzistanica essential oil. Herbal Med. J. 2016, 1, 11–17. [Google Scholar]
- Khosravinia, H.; Ghasemi, S.; Alavi, E.R. The effect of savory (Satureja khuzistanica) essential oils on performance, liver and kidney functions in broiler chickens. J. Anim. Feed Sci. 2013, 22, 50–55. [Google Scholar] [CrossRef]
- Kouravand, F.; Jooyandeh, H.; Barzegar, H.; Hojjati, M. Characterization of cross-linked whey protein isolate-based films containing Satureja khuzistanica Jamzad essential oil. J. Food Process. Preserv. 2017, 42, e13557. [Google Scholar] [CrossRef]
- Mahboubi, M.; Attaran, B. Satureja khuzistanica Jamzad essential oil and its anti-candidal activities against clinical isolates of Candida albicans isolated from women with candidiasis. Rev. Infectio. 2019, 23, 16–21. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N. The antibacterial activity of Satureja khuzistanica essential oil against clinical isolates of E. coli. Jundishapur J. Nat. Pharm. Prod. 2016, 11, e30034. [Google Scholar] [CrossRef]
- Mazarei, Z.; Rafati, H. Nanoemulsification of Satureja khuzistanica essential oil and pure carvacrol; comparison of physicochemical properties and antimicrobial activity against food pathogens. LWT 2019, 100, 328–334. [Google Scholar] [CrossRef]
- Rahimifard, M.; Sefidkon, F.; Fekri, S.; Azimi, R.; Ahmadi, S. Comparison of chemical composition and physical properties of Satureja khuzistanica essential oil in different growing conditions (wild and cultivated) in Lorestan Province. J. Med. Plants By-Products 2024, 13, 577–581. [Google Scholar] [CrossRef]
- Sadeghian, F.; Hadian, J.; Hadavi, M.; Mohamadi, A.; Ghorbanpour, M.; Ghafarzadegan, R. Effects of exogenous salicylic acid application on growth, metabolic activities and essential oil composition of Satureja khuzistanica Jamzad. J. Med. Plants 2013, 12, 70–82. [Google Scholar]
- Saharkhiz, M.J.; Zomorodian, K.; Taban, A.; Pakshir, K.; Heshmati, K.; Rahimi, M.J. Chemical composition and antimicrobial activities of three Satureja species against food-borne pathogens. J. Essent. Oil-Bear. Plants 2016, 19, 1984–1992. [Google Scholar] [CrossRef]
- Saidi, M. Antioxidant activities and chemical composition of essential oils from Satureja khuzistanica, Oliveria decumbens and Thymus daenensis. J. Essent. Oil-Bear. Plants 2014, 17, 513–521. [Google Scholar] [CrossRef]
- Sefidkon, F.; Ahmadi, S. Essential oil of Satureja khuzistanica Jamzad. J. Essent. Oil Res. 2000, 12, 427–428. [Google Scholar] [CrossRef]
- Shariat, A.; Sefidkon, F. Tetraploid induction in savory (Satureja khuzistanica): Cytological, morphological, phytochemical and physiological changes. Plant Cell Tissue Organ Cult. 2021, 146, 137–148. [Google Scholar] [CrossRef]
- Taban, A.; Rahimi, M.J.; Saharkhiz, M.J.; Hadian, J.; Zomorodian, K. The efficacy of Satureja khuzistanica essential oil treatment in reducing Escherichia coli O157: H7 load on alfalfa seeds prior to sprouting. J. Food Saf. 2013, 33, 121–127. [Google Scholar] [CrossRef]
- Vozhdehnazari, M.S.; Shirazi, Z.; Samavat, S. Differentiative assessment between Satureja rechingeri Jamzad and Satureja khuzistanica Jamzad using morphological, phytochemical, and molecular traits. Iran. J. Med. Aromat. Plants Res. 2023, 39, 163–174. [Google Scholar]
- Yousefzadi, M.; Riahi-Madvar, A.; Hadian, J.; Rezaee, F.; Rafiee, R.; Biniaz, M. Toxicity of essential oil of Satureja khuzistanica: In vitro cytotoxicity and anti-microbial activity. J. Immunotoxicol. 2014, 11, 50–55. [Google Scholar] [CrossRef]
- Zandi-Sohani, N. A comparative study on fumigant toxicity of Zataria multiflora and Satureja hortensis (Lamiaceae) to Callosobruchus maculatus (Coleoptera: Chrysomelidae). Int. J. Trop. Insect Sci. 2012, 32, 142–147. [Google Scholar] [CrossRef]
- Park, J.; Jeon, Y.; Lee, C.; Chung, N.; Lee, H. Insecticidal toxicities of carvacrol and thymol derived from Thymus vulgaris Lin. Against Pochazia shantungensis Chou & Lu., newly recorded pest. Sci. Rep. 2017, 7, 40902. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Han, C.; Zhang, C.; Kuchkarova, N.; Wei, C.; Zhang, C.; Shao, H. Allelopathic, phytotoxic, and insecticidal effects of Thymus proximus Serg. essential oil and its major constituents. Front. Plant Sci. 2021, 12, 689875. [Google Scholar] [CrossRef] [PubMed]
- Afrazeh, Z.; Sendi, J.J. Eco-friendly control of Helicoverpa armigera using synergistic mixtures of thymol and eucalyptol. Sci. Rep. 2025, 15, 1–11. [Google Scholar] [CrossRef]
- Eltalawy, H.M.; El-Fayoumi, H.; Aboelhadid, S.M.; Al-Quraishy, S.; El-Mallah, A.M.; Abo El-Ela, F.I.; Awad, E.M.; Abdel-Baki, A.S. Insecticidal activity and systematic insights of carvacrol against Tribolium castaneum: Acetylcholinesterase inhibition, oxidative stress, and molecular docking. J. Stored Prod. Res. 2025, 112, 102638. [Google Scholar] [CrossRef]
- Diksh Singh, S.; Mahajan, E.; Sohal, S.K. Growth inhibitory, immunosuppressive, cytotoxic, and genotoxic effects of γ-terpinene on Zeugodacus cucurbitae (Coquillett) (Diptera: Tephritidae). Sci. Rep. 2023, 13, 16472. [Google Scholar] [CrossRef]
- Naseri, B.; Abedi, Z.; Abdolmaleki, A.; Jafary-Jahed, M.; Borzoui, E.; Mansouri, S.M. Fumigant toxicity and sublethal effects of Artemisia khorassanica and Artemisia sieberi on Sitotroga cerealella (Lepidoptera: Gelechiidae). J. Insect Sci. 2017, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Fatiha, R.A.; Kada, R.; Khelil, M.A.; Pujade-Villar, J. Biological control against the cowpea weevil (Callosobruchus chinensis L., Coleoptera: Bruchidae) using essential oils of some medicinal plants. J. Plant Prot. Res. 2014, 54, 211–217. [Google Scholar] [CrossRef]
- Elhourri, M.; Amechrouq, A.; Riffi, O.; Fliou, J.; Sabiri, M. Study of the impact of the essential oil of Chenopodium ambrosioides (L.) (Chenopodiaceae) on Callosobruchus maculatus (Coleoptera: Bruchidae). Int. J. Environ. Stud. 2021, 78, 1044–1057. [Google Scholar] [CrossRef]
- El-Enine, A.; Habeba, M.O.; Metwally, E.M.; El-Deeb, M.A.; Mohamed, Z.A. Biological and lethal effects of some volatile plant oils, aloe aqueous extract and spinosad on cowpea beetle, Callosobruchus maculatus (F.). Zagazig J. Agric. Res. 2016, 43, 977–988. [Google Scholar] [CrossRef]
- Nouri Ganbalani, G.; Abedi, Z.; Mottaghinia, L.; Nouri, A. Lethal and sublethal effects of essential oils of ajwain (Carum copticum L.) and fennel (Foeniculum vulgare Mill) along with diatomaceous earth on some life table parameters of the lesser grain borer (Rhyzopertha dominica F.). Iran. J. Plant Prot. Sci. 2023, 53, 209–224. [Google Scholar] [CrossRef]
- El Kasimi, R.; Douiri, F.; Haddi, K.; Boughdad, A. Bioactivity of essential oil from Citrus aurantium peel against the pulse beetle Callosobruchus maculatus F. on chickpea. Agriculture 2023, 13, 232. [Google Scholar] [CrossRef]
- van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Ebadi, A.; Naseri, B.; Besheli, B.A.; Razmjou, J.; Ebadollahi, A.; Pourabad, R.F.; Elahi, M.; Afshari, F. Resistance of some lentil cultivars against the cowpea beetle, Callosobruchus maculatus (F.) (Coleoptera: Chrysomelidae). J. Stored Prod. Res. 2025, 111, 102546. [Google Scholar] [CrossRef]
- Chi, H. TIMING-MSChart: A Computer Program for Population Project Based on Age-Stage, Two-Sex Life Table; National Chung Hsing University: Taichung, Taiwan, 2020. [Google Scholar]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H.; Kavousi, A.; Gharekhani, G.; Atlihan, R.; Ozgokçe, M.S.; Güncan, A.; Desneux, N. Advances in theory, data analysis, and application of the age-stage, two-sex life table for demographic research, biological control, and pest management. Entomol. Gen. 2023, 43, 705–735. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).