Leaf Blight in Ilex verticillata Caused by Alternaria alternata: Mechanisms of Antioxidant Defense, Phytohormone Crosstalk, and Oxidative Stress Responses
Abstract
1. Introduction
2. Results
2.1. Isolation and Purification of the Pathogen Causing Holly Leaf Blight
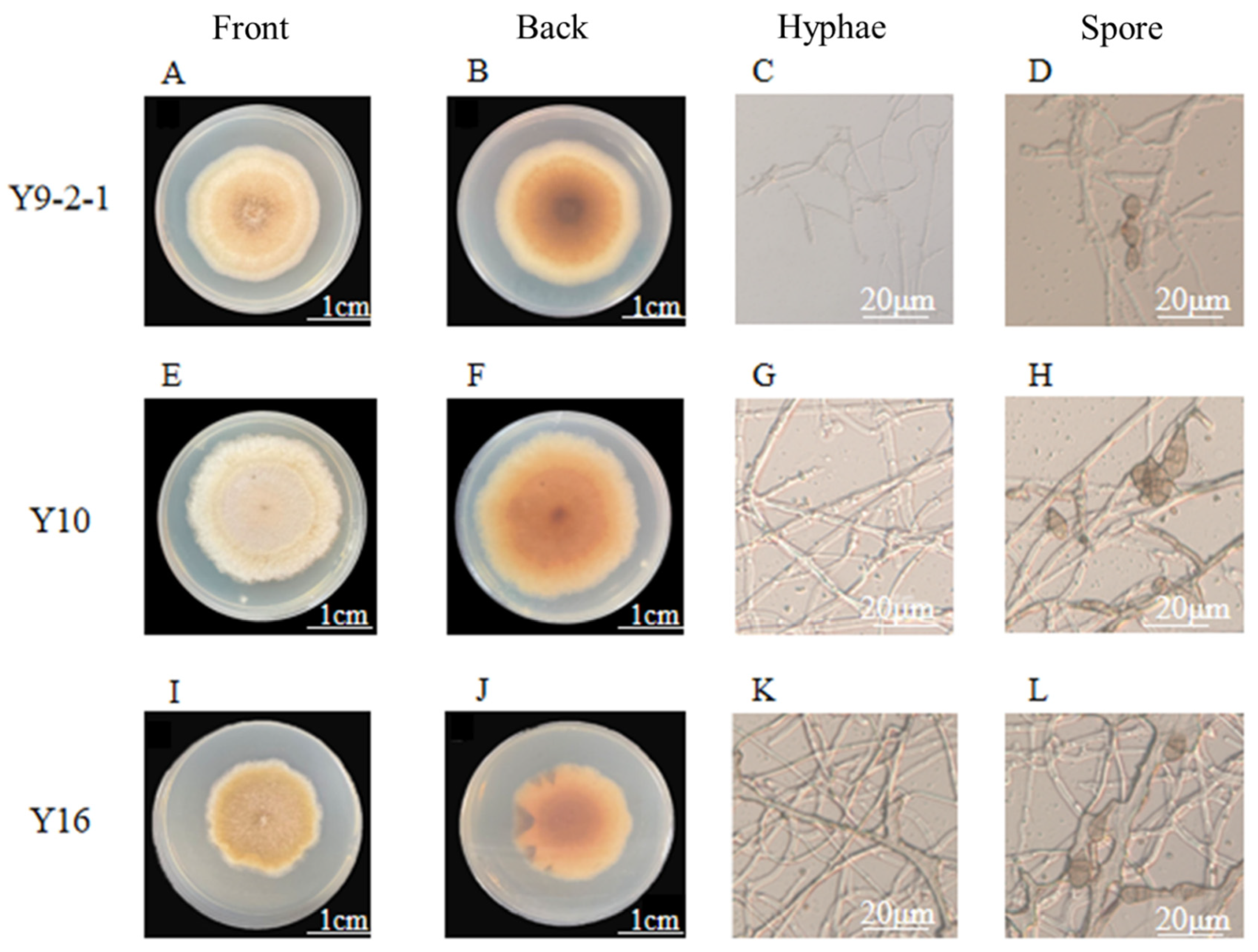
2.2. Morphological Characteristics and Phylogenetic Analysis of Whorled Holly Leaf Blight Pathogen
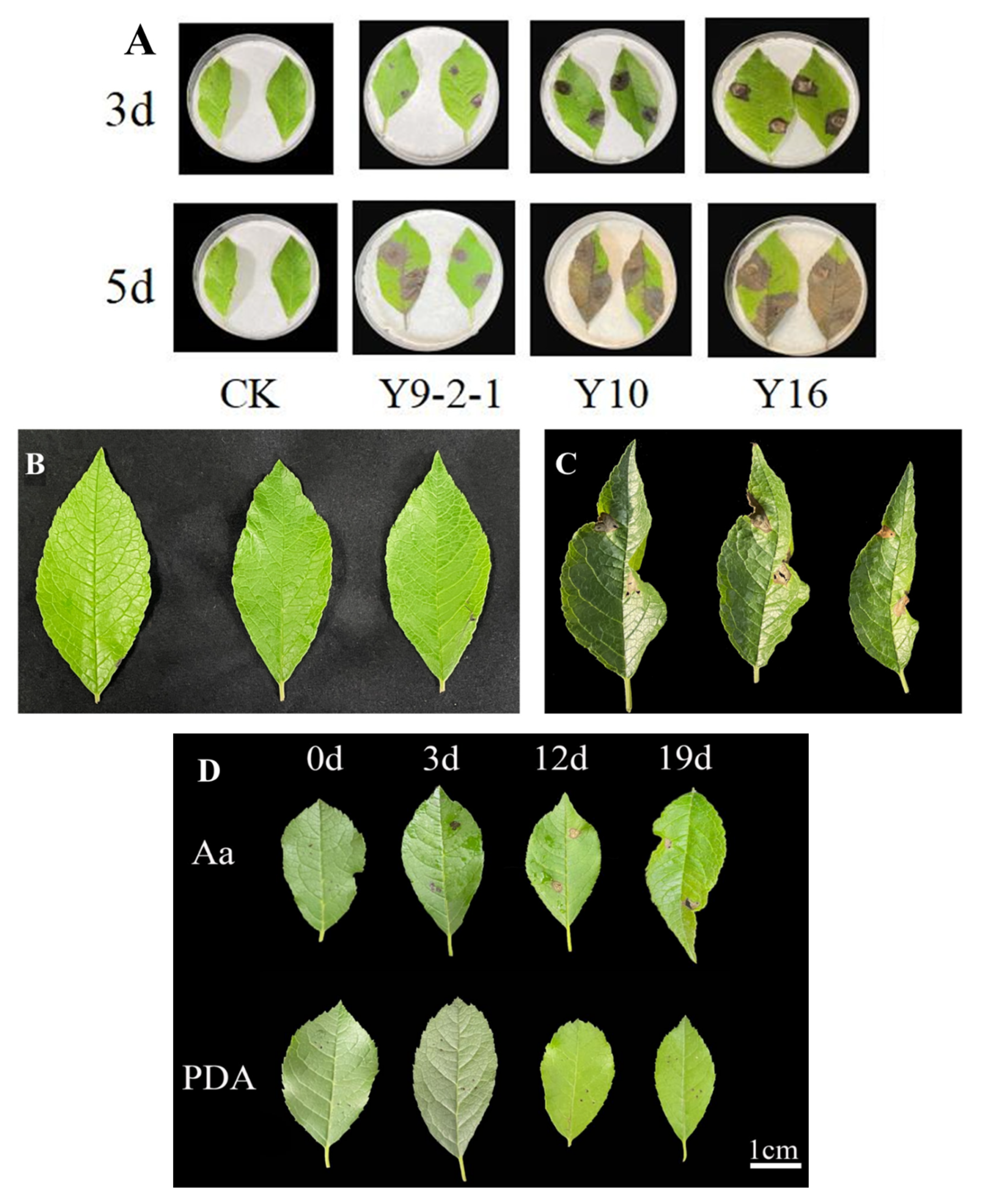
2.3. Pathogenicity Assessment of Alternaria alternata on Ilex verticillata
2.4. Transcriptomic Profiling of Host Responses During Alternaria alternata Infection
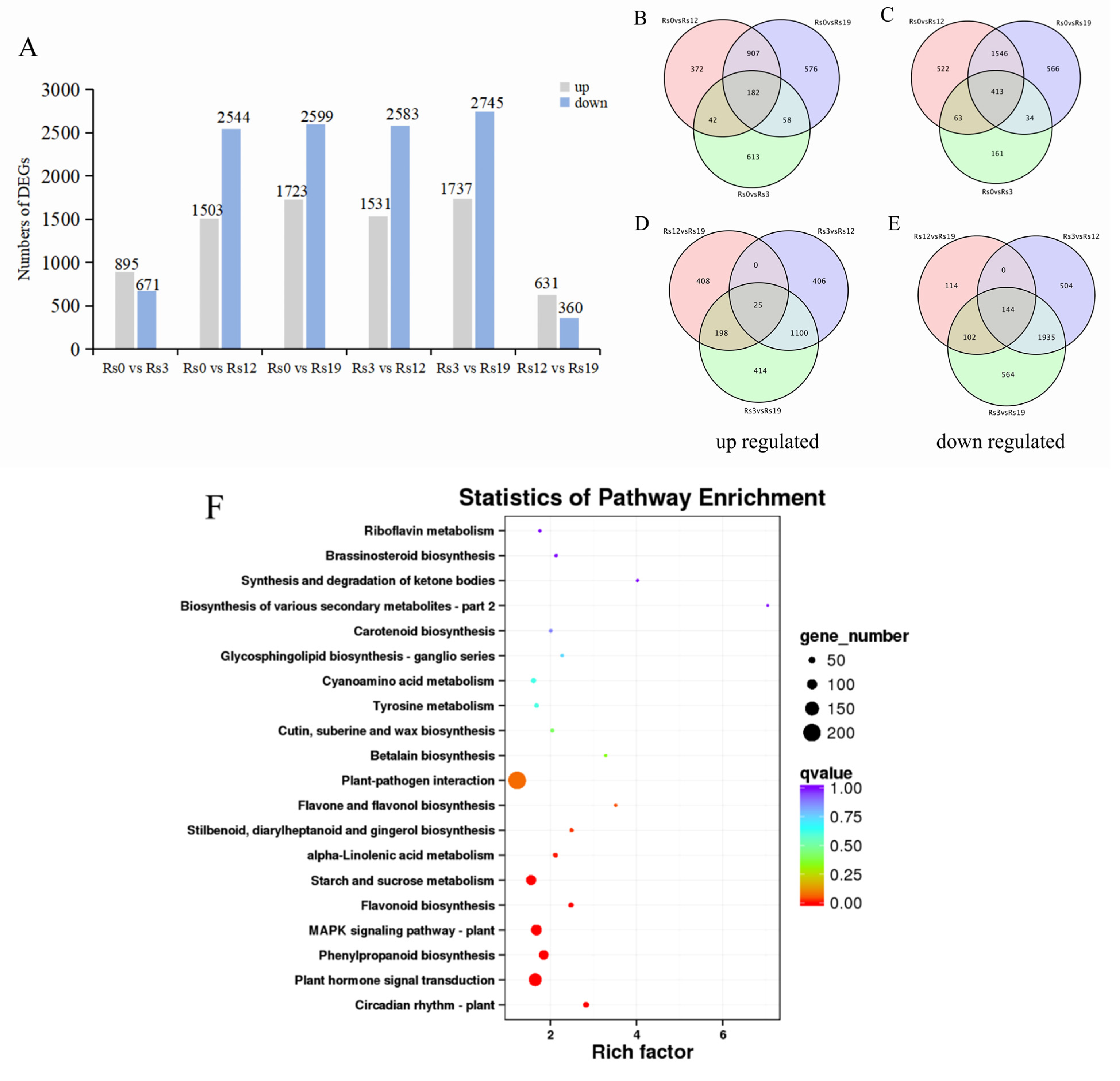
2.4.1. Global Differential Gene Expression Patterns
2.4.2. Stage-Specific Transcriptional Signatures
2.4.3. KEGG Enrichment Analysis of Differential Gene Expression
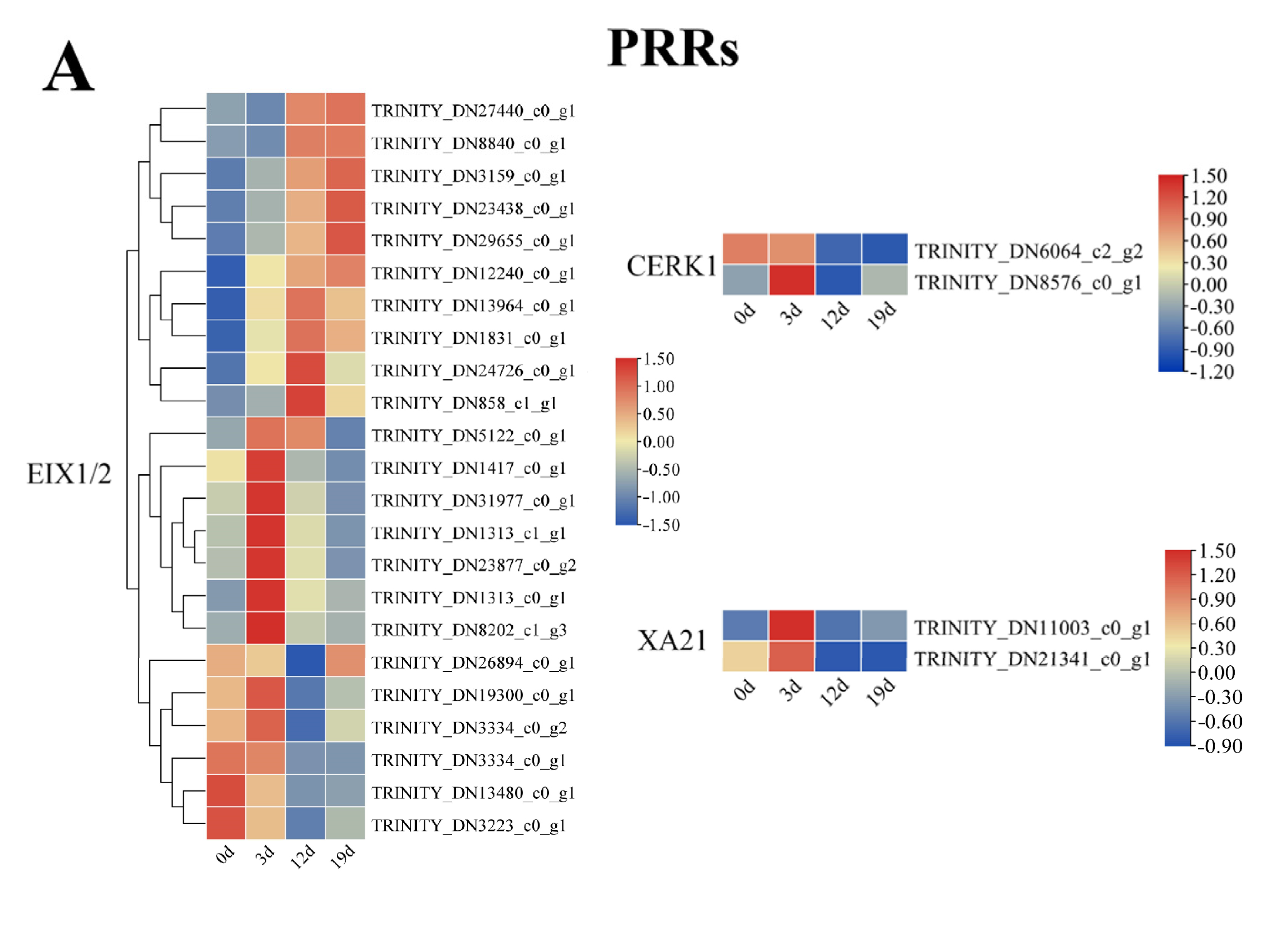
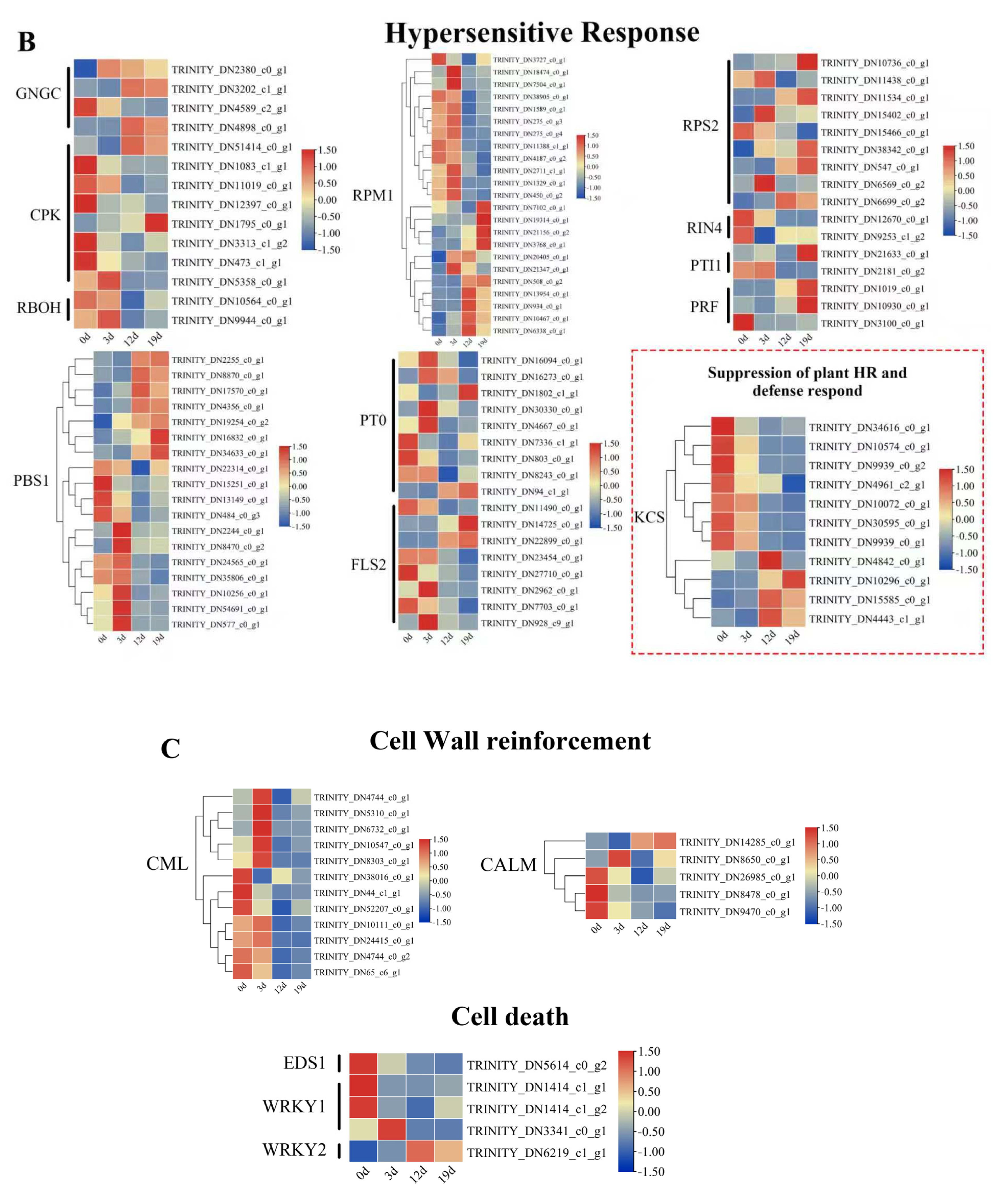
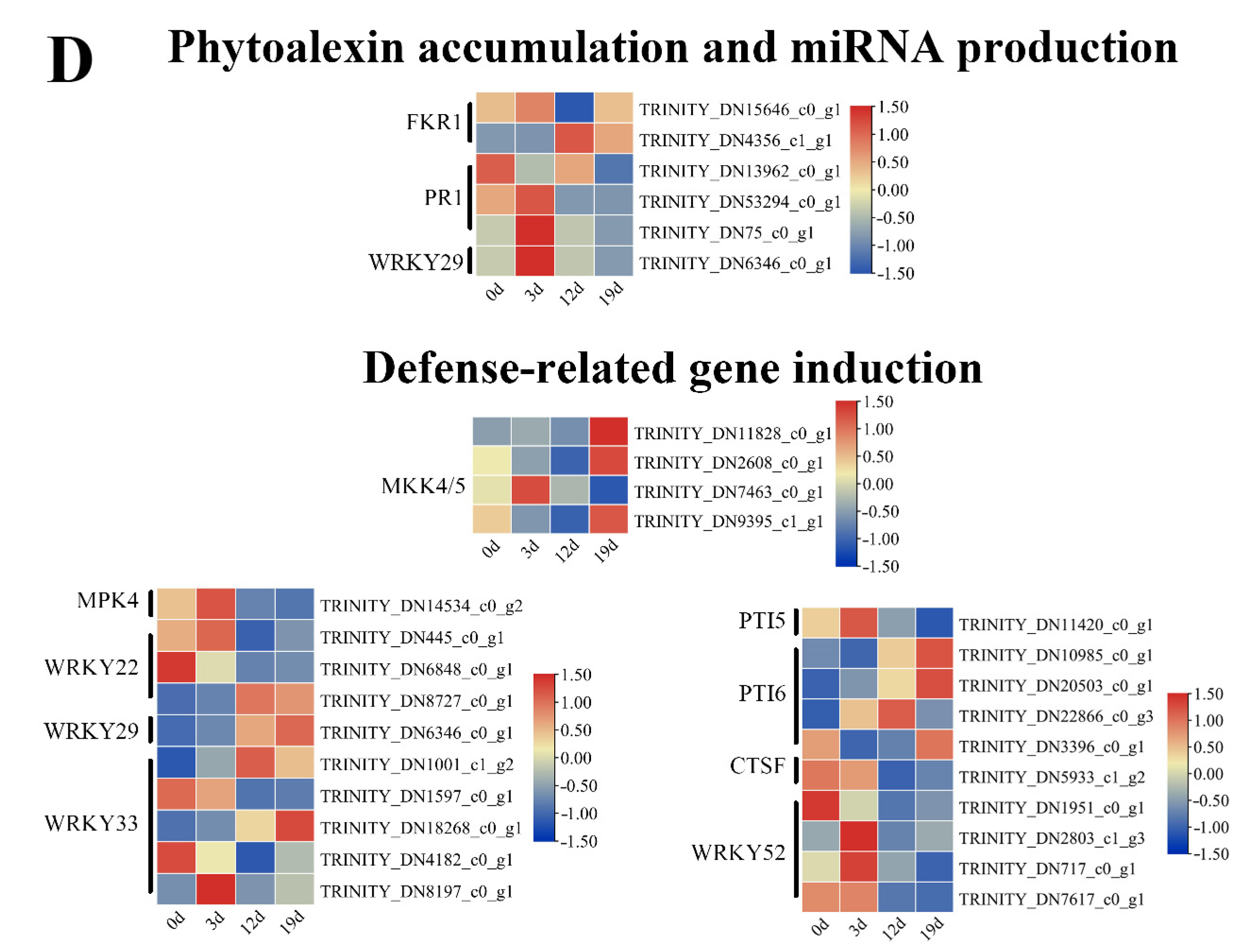
2.5. Transcriptional Dynamics of Plant-Pathogen Interaction Genes During Infection
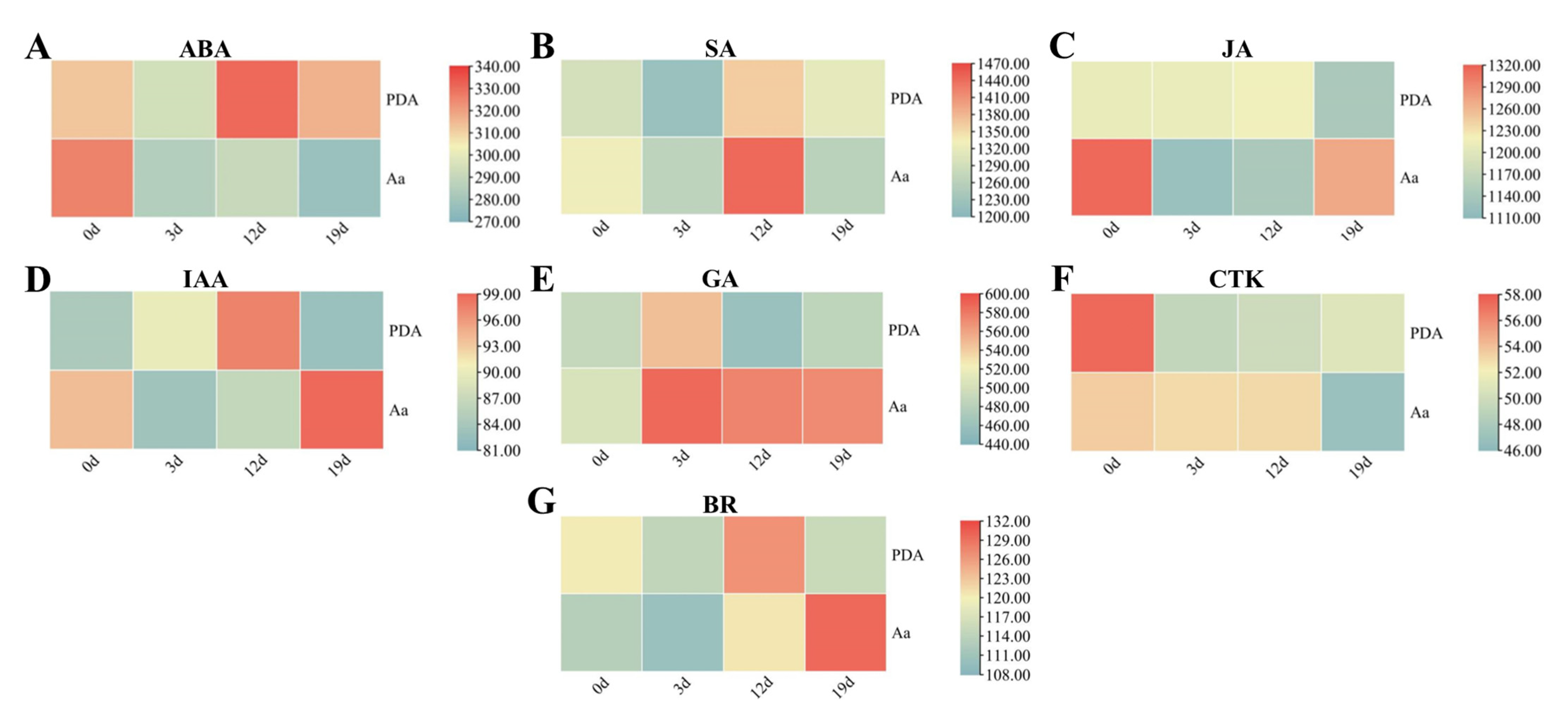
2.6. Dynamic Changes in Endogenous Hormone Levels During Pathogen Infection
2.6.1. Variation in Stress-Related Phytohormones During Pathogenesis
2.6.2. Modulation of Growth-Related Hormones During Pathogenesis
2.7. Oxidative Stress Responses During Pathogen Infection
3. Discussion
4. Materials and Methods
4.1. Plant Material Collection and Preparation
4.2. Experimental Design and Growth Conditions
4.3. Pathogen Isolation and Culture
4.4. Morphological Characterization and Molecular Identification
4.5. Pathogenicity Assays
4.6. Transcriptome Sequencing and Bioinformatics Analysis
4.7. Determination of Endogenous Plant Hormones
4.8. Extraction of Crude Enzyme Solution
4.8.1. Superoxide Dismutase (SOD) Activity Assay
4.8.2. Catalase (CAT) Activity Assay
4.8.3. Peroxidase (POD) Activity Assay
4.9. Malondialdehyde (MDA) Content Determination
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yao, X.; Zhang, F.; Corlett, R.T. Utilization of the Hollies (Ilex L. spp.): A Review. Forests 2022, 13, 94. [Google Scholar] [CrossRef]
- Yin, Y.; Hou, Z.; Sun, Q.; Zhu, B.; Liu, J.; Zou, Y.; Hao, M. Predicting the Habitat Suitability of Ilex verticillata (Aquifoliaceae) in China with Field-Test Validations. PLoS ONE 2025, 20, e0315908. [Google Scholar] [CrossRef] [PubMed]
- Piassetta, R.d.R.L.; Mikos, A.P.; Auer, C.G. Fungal Diseases of Yerba-Mate Culture (Ilex paraguariensis) in Brazil. BIOFIX Sci. J. 2021, 6, 153–159. [Google Scholar] [CrossRef]
- Bregant, C.; Carloni, F.; Linaldeddu, B.T.; Maddau, L.; Marcolongo, M.; Montecchio, L.; Murolo, S.; Piškur, B.; Ogris, N. First Report of Phytophthora ilicis Causing Leaf Spot, Shoot Blight and Bleeding Canker on Ilex aquifolium in Slovenia. New Dis. Rep. 2024, 50, e70005. [Google Scholar] [CrossRef]
- Deng, W.; Zhang, W. Application of Ilex Plants in Urban Greening. J. Landsc. Res. 2021, 13, 121–123. [Google Scholar]
- Kumar, N.; Dutta, R.; Ajay, B.C.; Radhakrishnan, T. Alternaria Leaf Blight (Alternaria spp.)–an Emerging Foliar Fungal Disease of Winter-Summer Groundnut (Arachis hypogaea): A Review. Indian. J. Agric. Sci. 2022, 92, 1043–1050. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Yang, X.; Cao, H.; Li, J.; Cao, P.; Guo, L.; Wang, X.; Zhao, J.; Xiang, W. Alternaria spp. Associated with Leaf Blight of Maize in Heilongjiang Province, China. Plant Dis. 2022, 106, 572–584. [Google Scholar] [CrossRef]
- Ma, G.; Bao, S.; Zhao, J.; Sui, Y.; Wu, X. Morphological and Molecular Characterization of Alternaria Species Causing Leaf Blight on Watermelon in China. Plant Dis. 2021, 105, 60–70. [Google Scholar] [CrossRef]
- Ahmad, T.; Nie, C.; Cao, C.; Xiao, Y.; Yu, X.; Liu, Y. First Record of Alternaria tenuissima Causing Aloe Barbadensis Leaf Blight and Leaf Spot Disease in Beijing, China. Crop Prot. 2024, 175, 106447. [Google Scholar] [CrossRef]
- Schmey, T.; Tominello-Ramirez, C.S.; Brune, C.; Stam, R. Alternaria Diseases on Potato and Tomato. Mol. Plant Pathol. 2024, 25, e13435. [Google Scholar] [CrossRef]
- Cao, C.; Gong, S.; Li, Y.; Tang, J.; Li, T.; Zhang, Q. Pathogenic Factors and Mechanisms of the Alternaria Leaf Spot Pathogen in Apple. Horticulturae 2024, 10, 212. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, H.; Yue, Y.; Zhou, T.; Zhu, Z.; Wang, C. Integrated Transcriptomic and Transgenic Analyses Reveal Potential Mechanisms of Poplar Resistance to Alternaria alternata Infection. BMC Plant Biol. 2022, 22, 413. [Google Scholar] [CrossRef] [PubMed]
- Ping, H.; Rao, W.; Dong, Z.; He, X.; Zhao, J.; Chen, Z.; Liu, L. Physiological and Biochemical Responses of Tobacco at Different Growth Stages to Alternaria alternata. J. Phytopathol. 2024, 172, e13431. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Chen, W.; Mao, B. Colletotrichum Siamense Strain Lvy 9 Causing Spot Anthracnose on Winterberry Holly in China. Microorganisms 2023, 11, 976. [Google Scholar] [CrossRef]
- Rao, M.J.; Zheng, B. The Role of Polyphenols in Abiotic Stress Tolerance and Their Antioxidant Properties to Scavenge Reactive Oxygen Species and Free Radicals. Antioxidants 2025, 14, 74. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Zhou, C.; Jiao, J.; Cheng, P.; Yang, L.; Wei, W.; Shen, Q.; Ji, P.; Yang, Y.; et al. Antioxidant Defense System in Plants: Reactive Oxygen Species Production, Signaling, and Scavenging During Abiotic Stress-Induced Oxidative Damage. Horticulturae 2025, 11, 477. [Google Scholar] [CrossRef]
- Rao, M.J.; Duan, M.; Ikram, M.; Zheng, B. ROS Regulation and Antioxidant Responses in Plants Under Air Pollution: Molecular Signaling, Metabolic Adaptation, and Biotechnological Solutions. Antioxidants 2025, 14, 907. [Google Scholar] [CrossRef]
- Schmid-Siegert, E.; Stepushenko, O.; Glauser, G.; Farmer, E.E. Membranes as Structural Antioxidants: Recycling of Malondialdehyde to Its Source in Oxidation-Sensitive Chloroplast Fatty Acids. J. Biol. Chem. 2016, 291, 13005–13013. [Google Scholar] [CrossRef]
- Shanthi, K.C. Biochemical defense mechanisms in medicinal and aromatic plants: Early-stage responses to fungal infections. Biochem. Cell Arch. 2024, 24, 1759. [Google Scholar] [CrossRef]
- Deng, B.; Jia, B.; Liu, G.; Zhang, X. Transcriptome Analysis to Elucidate the Enhanced Cold Resistance of Phoebe zhennan Pretreated with Exogenous Calcium. Int. J. Agric. Biol. 2021, 25, 151–159. [Google Scholar] [CrossRef]
- Duan, M.; Bao, L.; Eman, M.; Han, D.; Zhang, Y.; Zheng, B.; Yang, S.; Rao, M.J. The Ectopic Expression of the MpDIR1(t) Gene Enhances the Response of Plants from Arabidopsis thaliana to Biotic Stress by Regulating the Defense Genes and Antioxidant Flavonoids. Plants 2024, 13, 2692. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.J.; Duan, M.; Eman, M.; Yuan, H.; Sharma, A.; Zheng, B. Comparative Analysis of Citrus Species’ Flavonoid Metabolism, Gene Expression Profiling, and Their Antioxidant Capacity under Drought Stress. Antioxidants 2024, 13, 1149. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, C.; Zhou, Q.; Liao, M.; Feng, Z.; Zhu, P.; Wang, M.; Luo, Y.; Luo, G. Transcriptome Analysis of Gardenia Jasminoides Ellis in Response to Botryosphaeria dothidea. J. Plant Biochem. Biotechnol. 2023, 34, 179–190. [Google Scholar] [CrossRef]
- Appu, M.; Ramalingam, P.; Sathiyanarayanan, A.; Huang, J. An Overview of Plant Defense-Related Enzymes Responses to Biotic Stresses. Plant Gene 2021, 27, 100302. [Google Scholar] [CrossRef]
- Bora, S.; Borah, R.; Giri, K. Role of Proteins and Enzymes in Plant Disease Control. In Plant Protection: From Chemicals to Biologicals; Walter de Gruyter GmbH & Co KG: Berlin, Germany, 2022; p. 395. [Google Scholar]
- McCombe, C.L.; Greenwood, J.R.; Solomon, P.S.; Williams, S.J. Molecular Plant Immunity against Biotrophic, Hemibiotrophic, and Necrotrophic Fungi. Essays Biochem. 2022, 66, 581–593. [Google Scholar] [CrossRef]
- Fang, X.; Xie, Y.; Yuan, Y.; Long, Q.; Zhang, L.; Abid, G.; Zhang, W. The Role of Salicylic Acid in Plant Defense Responses against Biotic Stresses. Plant Horm. 2025, 1, e004. [Google Scholar] [CrossRef]
- Roychowdhury, R.; Hada, A.; Biswas, S.; Mishra, S.; Prusty, M.R.; Das, S.P.; Ray, S.; Kumar, A.; Sarker, U. Jasmonic Acid (JA) in Plant Immune Response: Unravelling Complex Molecular Mechanisms and Networking of Defence Signalling against Pathogens. J. Plant Growth Regul. 2025, 44, 89–114. [Google Scholar] [CrossRef]
- Li, H.; Lu, Y.; Liu, Z.; Ren, Q.; Liu, Z.; Liu, S.; Ren, R.; Wang, F.; Liu, Y.; Zhang, Y. Transcriptomic Analysis Unveils Alterations in the Genetic Expression Profile of Tree Peony (Paeonia suffruticosa Andrews) Infected by Alternaria alternata. BMC Genom. 2024, 25, 861. [Google Scholar] [CrossRef]
- Zhang, S.; Miao, W.; Liu, Y.; Jiang, J.; Chen, S.; Chen, F.; Guan, Z. Jasmonate Signaling Drives Defense Responses against Alternaria alternata in Chrysanthemum. BMC Genom. 2023, 24, 553. [Google Scholar] [CrossRef]
- Ma, L.; Li, R.; Ma, L.; Song, N.; Xu, Z.; Wu, J. Involvement of NAC Transcription Factor NaNAC29 in Alternaria alternata Resistance and Leaf Senescence in Nicotiana attenuata. Plant Divers. 2021, 43, 502–509. [Google Scholar] [CrossRef]
- Wang, W.; Bai, X.-D.; Chen, K.; Gu, C.-R.; Yu, Q.-B.; Jiang, J.; Liu, G.-F. Role of PsnWRKY70 in Regulatory Network Response to Infection with Alternaria alternata (Fr.) Keissl in Populus. Int. J. Mol. Sci. 2022, 23, 7537. [Google Scholar] [CrossRef]
- Liu, W.; Xu, S.; Ou, C.; Liu, X.; Zhuang, F.; Deng, X.W. T2T Genomes of Carrot and Alternaria dauci and Their Utility for Understanding Host–Pathogen Interactions during Carrot Leaf Blight Disease. Plant J. 2024, 120, 1643–1661. [Google Scholar] [CrossRef] [PubMed]
- Rajarammohan, S. Redefining Plant-Necrotroph Interactions: The Thin Line between Hemibiotrophs and Necrotrophs. Front. Microbiol. 2021, 12, 673518. [Google Scholar] [CrossRef] [PubMed]
- Afzal, K.; Mujtaba, M.; Wang, Y.; Zhou, B. Effectors of Plants Pathogenic Fungi and Fungal like Microbes: A Comprehensive Review on Mechanisms, Roles, and Host Interactions. Front. Plant Sci. 2025, 16, 1626960. [Google Scholar] [CrossRef] [PubMed]
- Devanna, B.N.; Jain, P.; Solanke, A.U.; Das, A.; Thakur, S.; Singh, P.K.; Kumari, M.; Dubey, H.; Jaswal, R.; Pawar, D. Understanding the Dynamics of Blast Resistance in Rice-Magnaporthe Oryzae Interactions. J. Fungi 2022, 8, 584. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Z. A Double--edged Sword: Reactive Oxygen Species (ROS) during the Rice Blast Fungus and Host Interaction. FEBS J. 2022, 289, 5505–5515. [Google Scholar] [CrossRef]
- Malvestiti, M.C.; Steentjes, M.B.F.; Beenen, H.G.; Boeren, S.; van Kan, J.A.L.; Shi-Kunne, X. Analysis of Plant Cell Death-Inducing Proteins of the Necrotrophic Fungal Pathogens Botrytis squamosa and Botrytis elliptica. Front. Plant Sci. 2022, 13, 993325. [Google Scholar] [CrossRef]
- Nadarajah, K.K. Defensive Strategies of ROS in Plant–Pathogen Interactions. In Plant Pathogen Interaction; Springer: Berlin/Heidelberg, Germany, 2024; pp. 163–183. [Google Scholar]
- Huang, Y.; Li, J.; Nong, C.; Lin, T.; Fang, L.; Feng, X.; Chen, Y.; Lin, Y.; Lai, Z.; Miao, L. Piriformospora indica Enhances Resistance to Fusarium wilt in Strawberry by Increasing the Activity of Superoxide Dismutase, Peroxidase, and Catalase, While Reducing the Content of Malondialdehyde in the Roots. Horticulturae 2024, 10, 240. [Google Scholar] [CrossRef]
- Narsai, R.; Wang, C.; Chen, J.; Wu, J.; Shou, H.; Whelan, J. Antagonistic, Overlapping and Distinct Responses to Biotic Stress in Rice (Oryza sativa) and Interactions with Abiotic Stress. BMC Genom. 2013, 14, 1–21. [Google Scholar] [CrossRef]
- Gupta, R.; Anand, G.; Bar, M. Developmental Phytohormones: Key Players in Host-Microbe Interactions. J. Plant Growth Regul. 2023, 42, 7330–7351. [Google Scholar] [CrossRef]
- Wang, X.; Cui, J.; Li, X.; Wang, G.; Zheng, G.; Li, Y.; Li, X.; Ma, L.; Wang, L. Comparative Study on Growth, Physiological and Biotic Stress Resistance of Cucumber (Cucumis sativus L.) by Exogenous Application of Jasmonic Acid and Methyl Jasmonate under Greenhouse Conditions. Russ. J. Plant Physiol. 2022, 72, 176. [Google Scholar] [CrossRef]
- Bharath, P.; Gahir, S.; Raghavendra, A.S. Abscisic Acid-Induced Stomatal Closure: An Important Component of Plant Defense against Abiotic and Biotic Stress. Front. Plant Sci. 2021, 12, 615114. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, X.; Zhang, S.; Yang, J.; Liu, F.; Fan, J. Comprehensive Transcriptome Analysis of Grafting onto Artemisia scoparia W. to Affect the Aphid Resistance of Chrysanthemum (Chrysanthemum morifolium T.). BMC Genom. 2019, 20, 776. [Google Scholar] [CrossRef]
- Kochba, J.; Lavee, S.; Spiegel-Roy, P. Differences in Peroxidase Activity and Isoenzymes in Embryogenic Ane Non-Embryogenic ‘Shamouti’ Orange Ovular Callus Lines. Plant Cell Physiol. 1977, 18, 463–467. [Google Scholar] [CrossRef]
- Zhang, R.; Peng, F.; Li, Y. Pecan Production in China. Sci. Hortic. 2015, 197, 719–727. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Chanana, S.; Thomas, C.S.; Zhang, F.; Rajski, S.R.; Bugni, T.S. HCAPCA: Automated Hierarchical Clustering and Principal Component Analysis of Large Metabolomic Datasets in R. Metabolites 2020, 10, 297. [Google Scholar] [CrossRef] [PubMed]



| Gene | Primer Sequence (5′–3′) |
|---|---|
| ITS | ITS1: TCCGTAGGTGAACCTGCGG |
| ITS4: TCCTCCGCTTATTGATATGC | |
| TEF1-α | EF1-728F: CATCGAGAAGTTCGAGAAGG |
| EF1-986R: TACTTGAAGGAACCCTACC | |
| G3PDH | F: ATTGACATCGTCGCTGTCAACGA |
| R: ACCCCACTCGTTGTCGTACCA | |
| RPB2 | F: GATGATCGTGATCATTTCGG |
| R: CCCATAGCTTGCTTACCCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Zhou, C.; Cheng, P.; Huang, L.; Shen, Q.; Zheng, Y.; Li, Y.; Dai, W.; Zhang, J.; Shen, D.; et al. Leaf Blight in Ilex verticillata Caused by Alternaria alternata: Mechanisms of Antioxidant Defense, Phytohormone Crosstalk, and Oxidative Stress Responses. Plants 2025, 14, 3057. https://doi.org/10.3390/plants14193057
Lu H, Zhou C, Cheng P, Huang L, Shen Q, Zheng Y, Li Y, Dai W, Zhang J, Shen D, et al. Leaf Blight in Ilex verticillata Caused by Alternaria alternata: Mechanisms of Antioxidant Defense, Phytohormone Crosstalk, and Oxidative Stress Responses. Plants. 2025; 14(19):3057. https://doi.org/10.3390/plants14193057
Chicago/Turabian StyleLu, Huijie, Caixia Zhou, Peiwen Cheng, Liangye Huang, Qinyuan Shen, Ye Zheng, Yihui Li, Wenjun Dai, Jianhong Zhang, Dengfeng Shen, and et al. 2025. "Leaf Blight in Ilex verticillata Caused by Alternaria alternata: Mechanisms of Antioxidant Defense, Phytohormone Crosstalk, and Oxidative Stress Responses" Plants 14, no. 19: 3057. https://doi.org/10.3390/plants14193057
APA StyleLu, H., Zhou, C., Cheng, P., Huang, L., Shen, Q., Zheng, Y., Li, Y., Dai, W., Zhang, J., Shen, D., Sharma, A., Rao, M. J., Zheng, B., & Yuan, H. (2025). Leaf Blight in Ilex verticillata Caused by Alternaria alternata: Mechanisms of Antioxidant Defense, Phytohormone Crosstalk, and Oxidative Stress Responses. Plants, 14(19), 3057. https://doi.org/10.3390/plants14193057









