Decoding Salinity Tolerance in Salicornia europaea L.: Image-Based Oxidative Phenotyping and Histochemical Mapping of Pectin and Lignin
Abstract
1. Introduction
2. Results
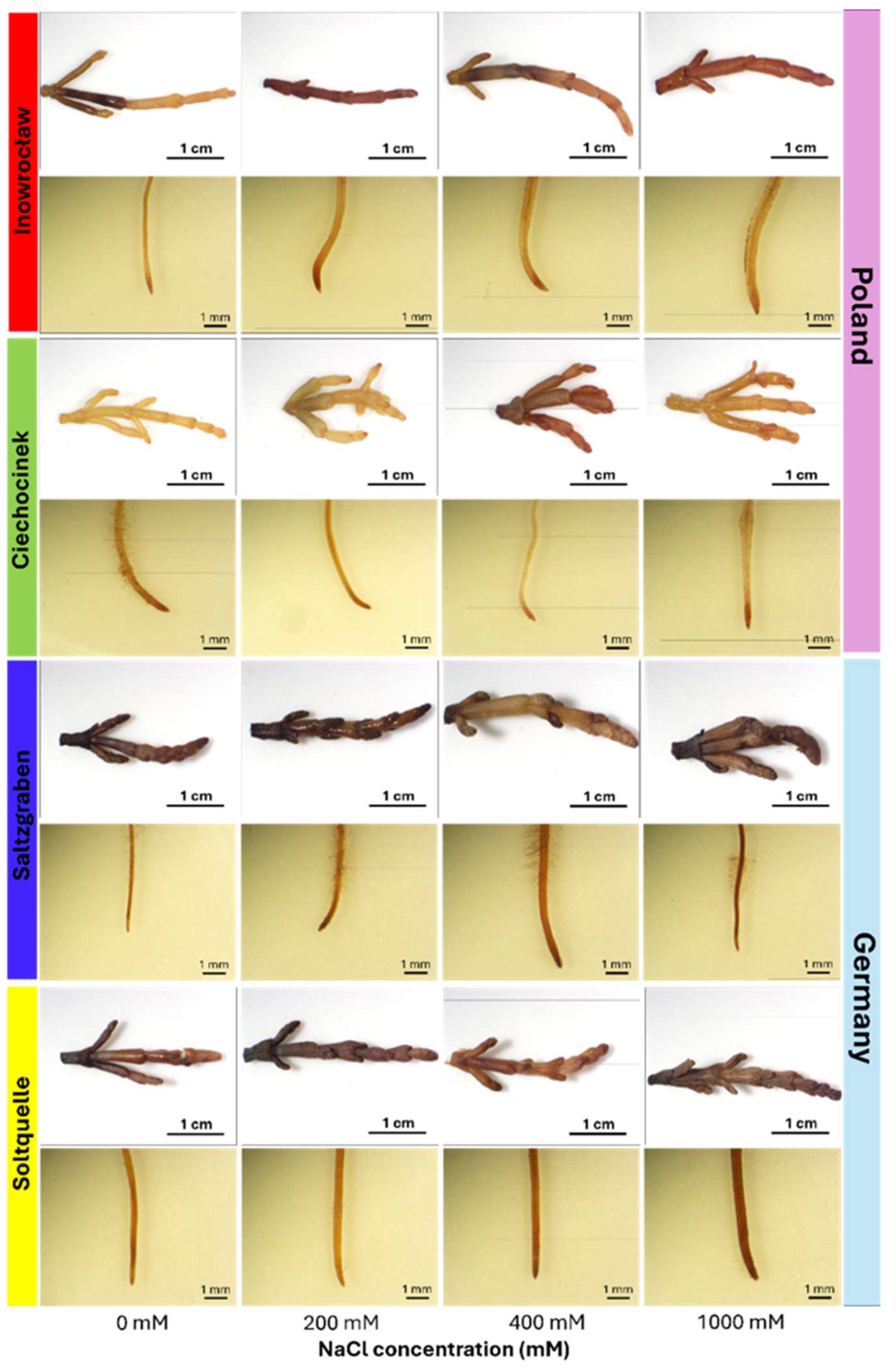
2.1. Lipid Peroxidation Visualization
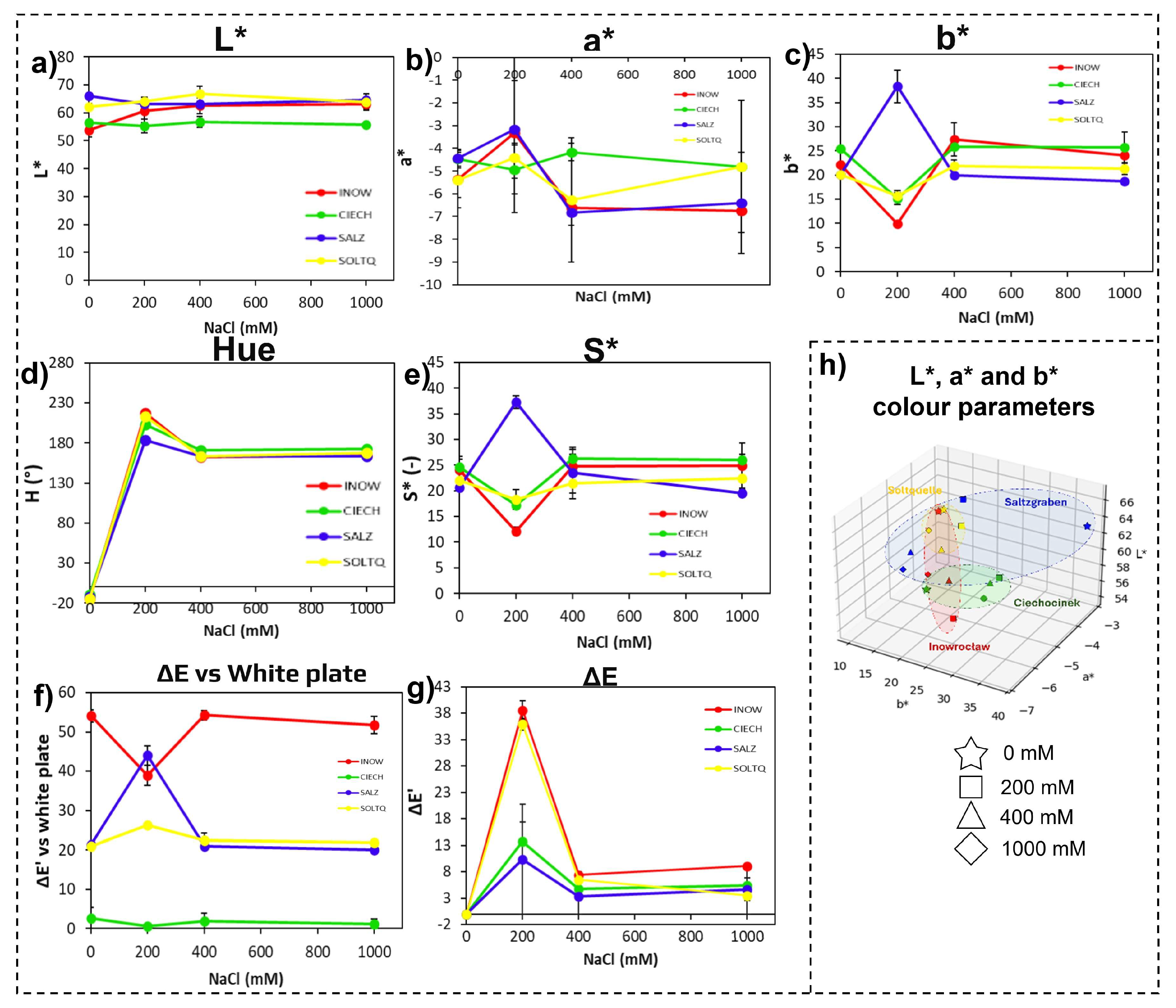
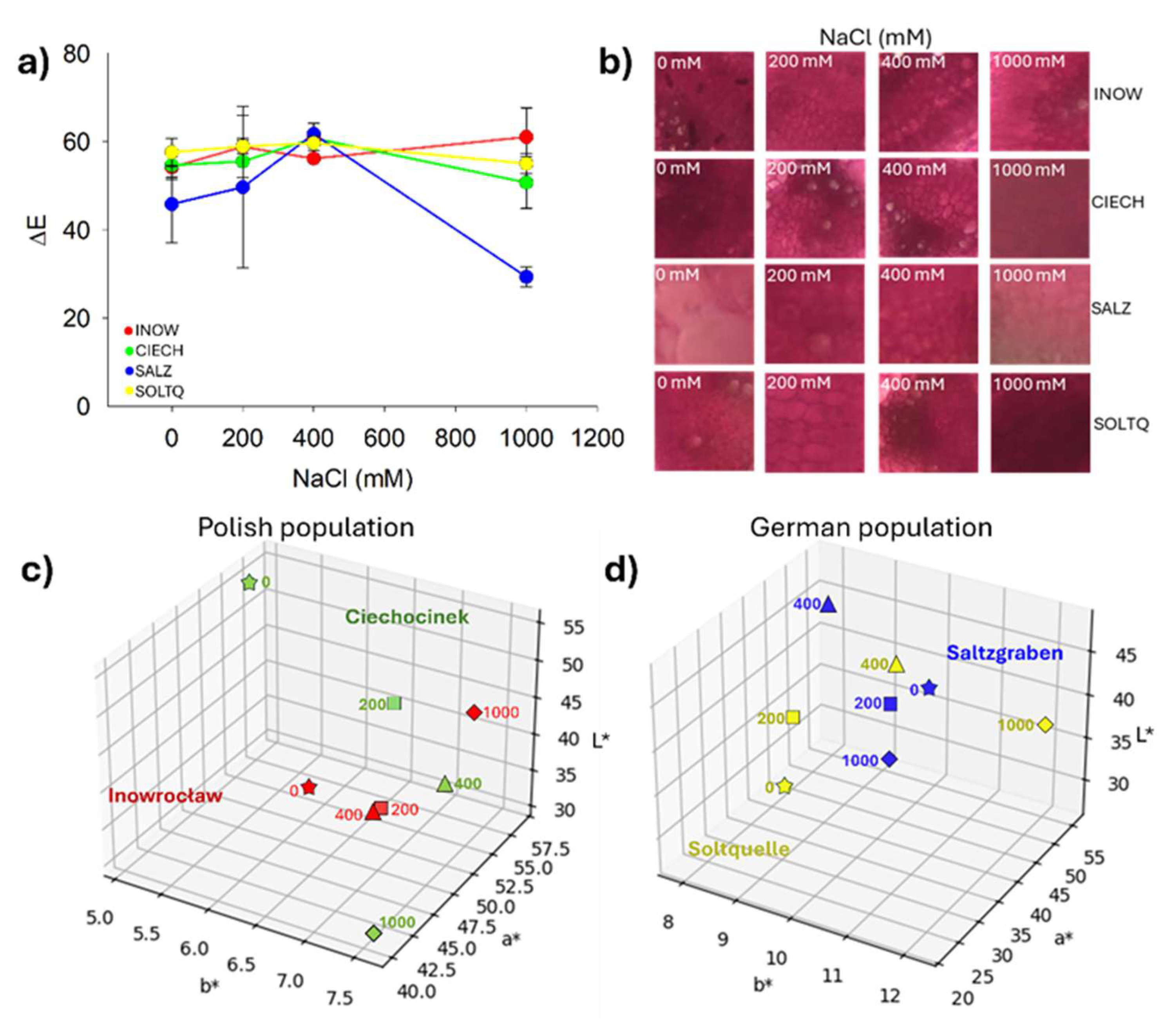
2.1.1. Shoot Colorimetric Parameters Through CIELab, Schiff’s/MDA
2.1.2. Root Colorimetric Parameters Through CIELab, Schiff’s/MDA
2.2. Hydrogen Peroxide Accumulation
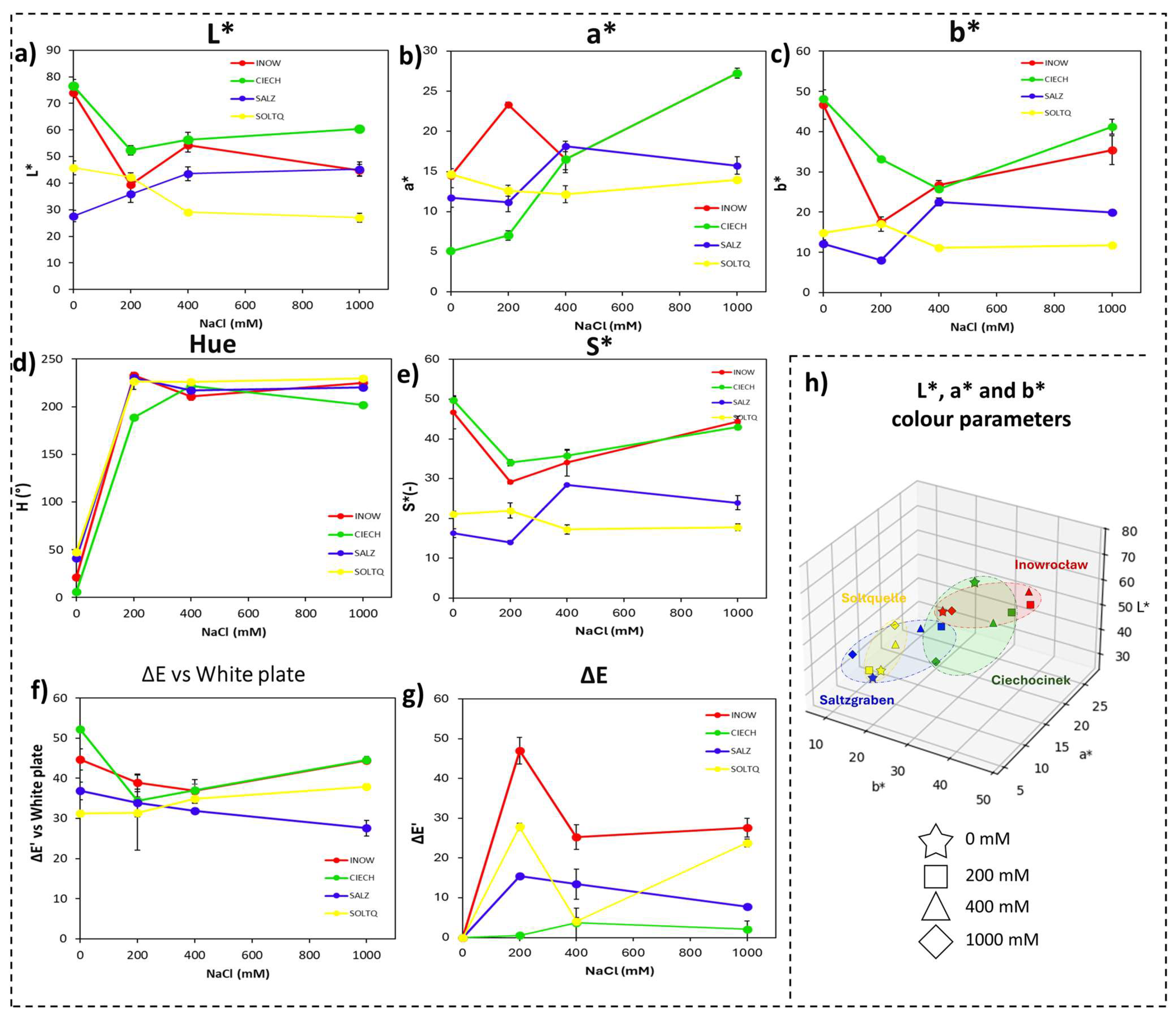
2.2.1. Shoot Colourimetric Parameters Through CIELab, DAB/H2O2
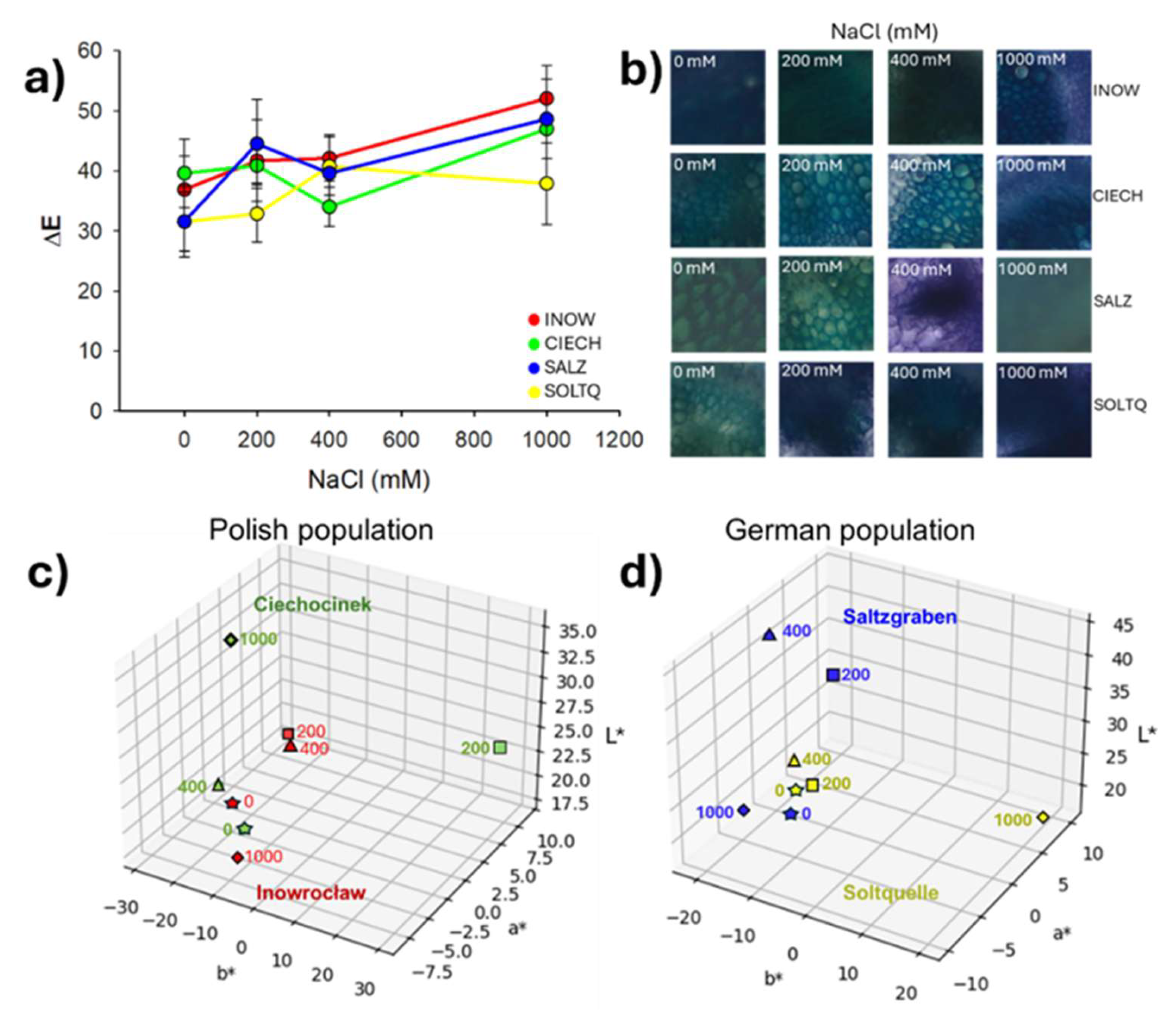
2.2.2. Root Colourimetric Parameters Through CIELab, DAB/H2O2
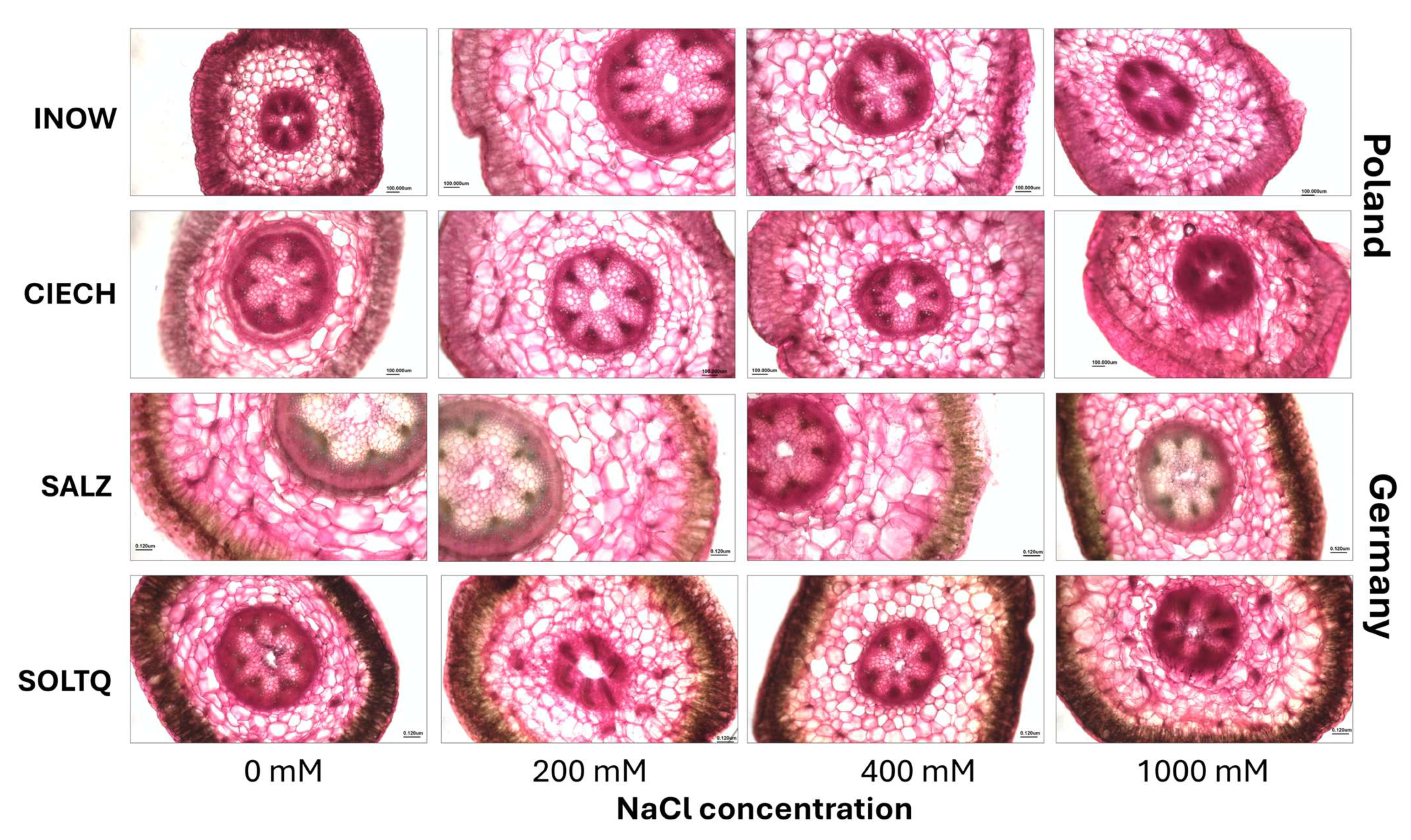
2.3. Histochemical Lignin Detection and Colourimetric Analysis
2.4. Pectin Distribution and Colourimetric Analysis
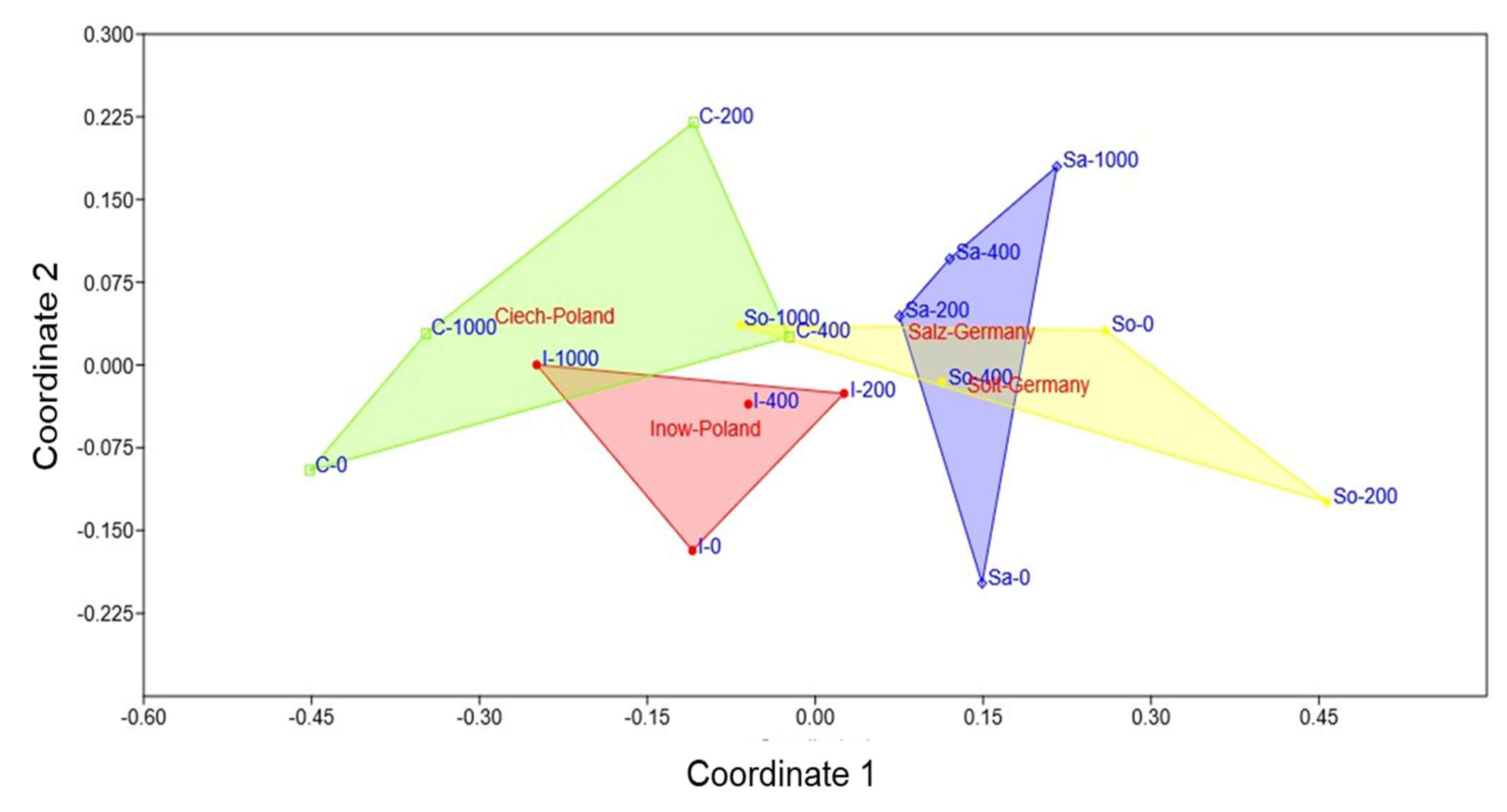
2.5. Population Variability Through Colourimetric Parameters
3. Discussion
4. Materials and Methods
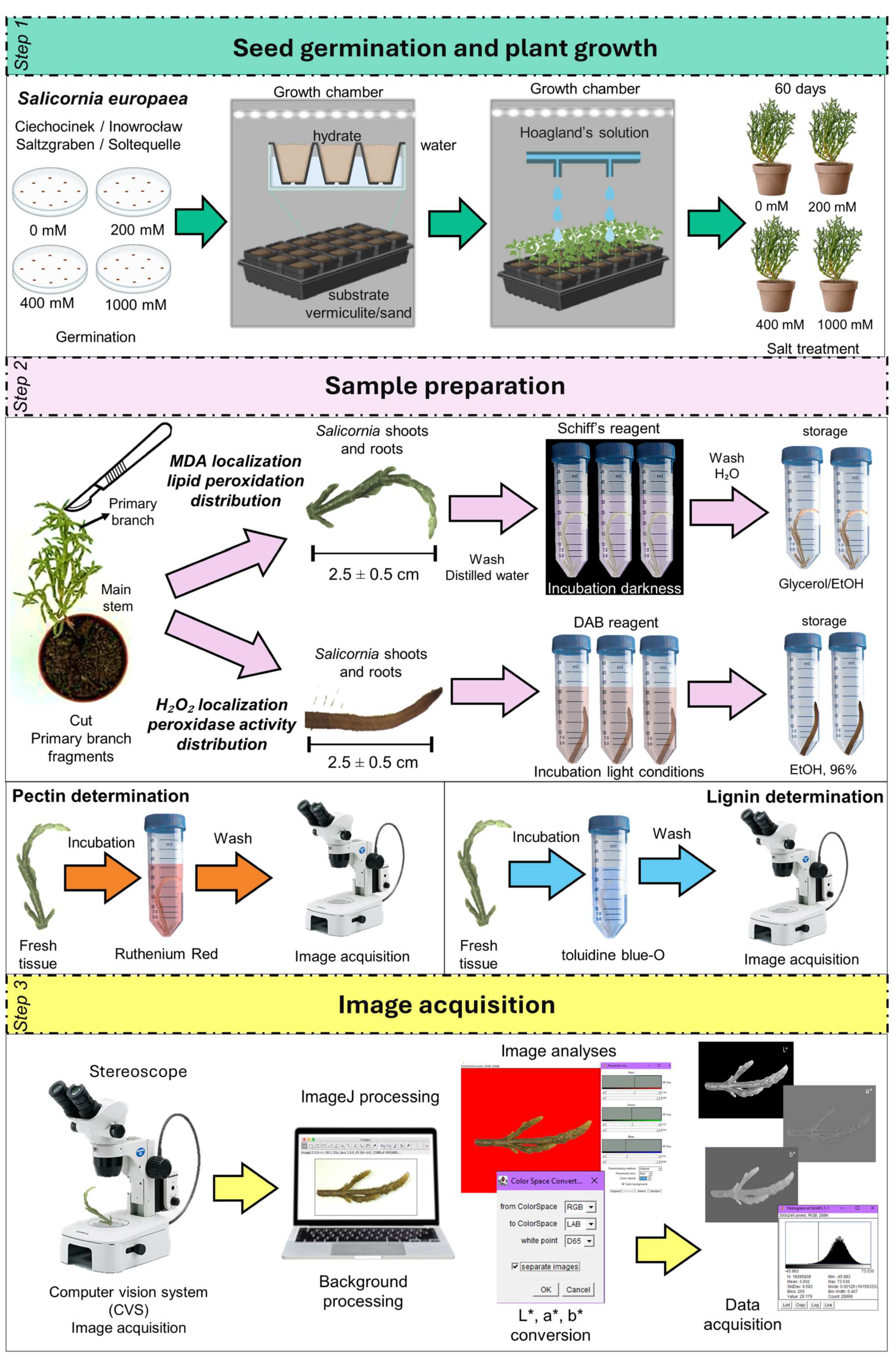
4.1. Seed Germination Conditions and Salinity Treatments
4.2. Histochemical Localization of MDA and H2O2
4.3. Pectin and Lignin Determination
4.4. Image and Colour Analysis
4.5. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MDA | Malondialdehyde |
| H2O2 | Hydrogen peroxide |
| DAB | 3,3′-diaminobenzidine |
| CVS | Computer vision system |
| NMDS | Non-metric multi-dimensional scaling |
| ROS | Reactive oxygen species |
| INOW | Inowrocław |
| CIECH | Ciechocinek |
| SALZ | Salzgraben Salzdahlum |
| SOLTQ | Soltauquelle |
| ΔE | Colour difference vs. white plate |
| ΔE′ | Intra-treatment colour difference |
References
- Rahman, M.; Mostofa, M.G.; Keya, S.S.; Siddiqui, N.; Ansary, M.U.; Das, A.K.; Rahman, A.; Tran, L.S.-P. Adaptive Mechanisms of Halophytes and Their Potential in Improving Salinity Tolerance in Plants. Int. J. Mol. Sci. 2021, 22, 10733. [Google Scholar] [CrossRef]
- Hnilickova, H.; Kraus, K.; Vachova, P.; Hnilicka, F. Salinity Stress Affects Photosynthesis, Malondialdehyde Formation, and Proline Content in Portulaca oleracea L. Plants 2021, 10, 845. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Ranjbar, G.; Pakniyat, H.; Emam, Y. Physiological mechanisms of salt stress tolerance in plants. In Plant-Environment Interaction; Wiley: Hoboken, NJ, USA, 2016; pp. 141–160. [Google Scholar] [CrossRef]
- Aghaleh, M.; Niknam, V.; Ebrahimzadeh, H.; Razavi, K. Effect of salt stress on physiological and antioxidative responses in two species of Salicornia (S. persica and S. europaea). Acta Physiol. Plant 2011, 33, 1261–1270. [Google Scholar] [CrossRef]
- Su, J.; Liu, Y.; Han, F.; Gao, F.; Gan, F.; Huang, K.; Li, Z. ROS, an Important Plant Growth Regulator in Root Growth and Development: Functional Genes and Mechanism. Biology 2024, 13, 1033. [Google Scholar] [CrossRef]
- Ksas, B.; Havaux, M. Determination of ROS-Induced Lipid Peroxidation by HPLC-Based Quantification of Hydroxy Polyunsaturated Fatty Acids. In Reactive Oxygen Species in Plants: Methods and Protocols; Mhamdi, A., Ed.; Springer: New York, NY, USA, 2022; pp. 181–189. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid Peroxidation as Measured by Chromatographic Determination of Malondialdehyde. Human Plasma Reference Values in Health and Disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, H.; Kumar, K.; Kaur, R.; Arora, A.; Kaur, N. Oxidative stress dynamics revealed the role of H2O2 in citrus rootstocks sensitivity to Phytophthora nicotianae. Physiol. Mol. Plant Pathol. 2024, 133, 102348. [Google Scholar] [CrossRef]
- Daudi, A.; O’Brien, J. Detection of Hydrogen Peroxide by DAB Staining in Arabidopsis Leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef]
- Jambunathan, N. Determination and Detection of Reactive Oxygen Species (ROS), Lipid Peroxidation, and Electrolyte Leakage in Plants. In Plant Stress Tolerance; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; Volume 639, pp. 291–297. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Piernik, A.; Ludwiczak, A.; Duszyn, M.; Szmidt-Jaworska, A.; Chanona-Pérez, J.J. Image and fractal analysis as a tool for evaluating salinity growth response between two Salicornia europaea populations. BMC Plant Biol. 2020, 20, 467. [Google Scholar] [CrossRef]
- Orzoł, A.; Głowacka, K.; Pätsch, R.; Piernik, A.; Gallegos-Cerda, S.D.; Cárdenas-Pérez, S. The local environment influences salt tolerance differently in four Salicornia europaea L. inland populations. Sci. Rep. 2025, 15, 13128. [Google Scholar] [CrossRef]
- Duan, H.; Tiika, R.J.; Tian, F.; Lu, Y.; Zhang, Q.; Hu, Y.; Cui, G.; Yang, H. Metabolomics Analysis Unveils Important Changes Involved in the Salt Tolerance of Salicornia europaea. Front. Plant Sci. 2023, 13, 1097076. [Google Scholar] [CrossRef]
- Cárdenas Pérez, S.; Niedojadło, K.; Mierek-Adamska, A.; Dąbrowska, G.B.; Piernik, A. Maternal salinity influences anatomical parameters, pectin content, biochemical and genetic modifications of two Salicornia europaea populations under salt stress. Sci. Rep. 2022, 12, 2968. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, J.P.; Saha, B.; Chowardhara, B.; Devi, S.S.; Borgohain, P.; Panda, S.K. Qualitative Analysis of Lipid Peroxidation in Plants under Multiple Stress Through Schiff’s Reagent: A Histochemical Approach. Bio-Protocol 2018, 8, e2807. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Levesque-Tremblay, G.; Pelloux, J.; Braybrook, S.A.; Müller, K. Tuning of pectin methylesterification: Consequences for cell wall biomechanics and development. Planta 2015, 242, 791–811. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246. [Google Scholar] [CrossRef]
- Moura, J.C.M.S.; Bonine, C.A.V.; de Oliveira Fernandes Viana, J.; Dornelas, M.C.; Mazzafera, P. Abiotic and biotic stresses and changes in the lignin content and composition in plants. J. Integr. Plant Biol. 2010, 52, 360–376. [Google Scholar] [CrossRef]
- Graña, E.; Sotelo, T.; Díaz-Tielas, C.; Araniti, F.; Krasuska, U.; Bogatek, R.; Reigosa, M.J.; Sánchez-Moreiras, A.M. Citral Induces Auxin and Ethylene-Mediated Malformations and Arrests Cell Division in Arabidopsis thaliana Roots. J. Chem. Ecol. 2013, 39, 271–282. [Google Scholar] [CrossRef]
- Pradhan Mitra, P.; Loqué, D. Histochemical Staining of Arabidopsis thaliana Secondary Cell Wall Elements. JoVE 2014, e51381. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Fujita, M. Plant Responses and Tolerance to Salt Stress: Physiological and Molecular Interventions. Int. J. Mol. Sci. 2022, 23, 4810. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- López-Hidalgo, C.; Meijón, M.; Lamelas, L.; Valledor, L. The rainbow protocol: A sequential method for quantifying pigments, sugars, free amino acids, phenolics, flavonoids and MDA from a small amount of sample. Plant Cell Environ. 2021, 44, 1977–1986. [Google Scholar] [CrossRef]
- Martins, M.D.C.d.C.e.; Oliveira, A.S.d.S.S.; da Silva, L.A.A.; Primo, M.G.S.; Lira, V.B.d.C. Biological Indicators of Oxidative Stress [Malondialdehyde, Catalase, Glutathione Peroxidase, and Superoxide Dismutase] and Their Application in Nutrition. In Biomarkers in Nutrition; Patel, V.B., Preedy, V.R., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–25. [Google Scholar] [CrossRef]
- Cárdenas Pérez, S.; Grigore, M.N.; Piernik, A. Prediction of Salicornia europaea L. biomass using a computer vision system to distinguish different salt-tolerant populations. BMC Plant Biol. 2024, 24, 1086. [Google Scholar] [CrossRef] [PubMed]
- Singkhonrat, J.; Sriprai, A.; Hirunwatthanakasem, S.; Angkuratipakorn, T.; Preechaburana, P. Digital image colorimetric analysis for evaluating lipid oxidation in oils and its emulsion. Food Chem. 2019, 286, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Bose, J.; Rodrigo-Moreno, A.; Shabala, S. ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 2014, 65, 1241–1257. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Das, A.B. Salt Tolerance and Salinity Effects on Plants: A Review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Mir, R.; Mircea, D.M.; Ruiz-González, M.X.; Brocal-Rubio, P.; Boscaiu, M.; Vicente, O. Cakile maritima: A Halophyte Model to Study Salt Tolerance Mechanisms and Potential Useful Crop for Sustainable Saline Agriculture in the Context of Climate Change. Plants 2024, 13, 2880. [Google Scholar] [CrossRef]
- Nisar, F.; Hameed, A.; Gul, B.; Aziz, I.; Nielsen, B.L. Insights into the salinity tolerance of the succulent halophyte Arthrocnemum macrostachyum: Comparative ecophysiology of plants from heteromorphic seeds. Front. Plant Sci. 2024, 15, 1–12. [Google Scholar] [CrossRef]
- Homayouni, H.; Razi, H.; Izadi, M.; Alemzadeh, A.; Kazemeini, S.A.; Niazi, A.; Vicente, O. Temporal Changes in Biochemical Responses to Salt Stress in Three Salicornia Species. Plants 2024, 13, 979. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative Stress and Antioxidant Defense Mechanisms in Plants Under Salt Stress. In Plant Abiotic Stress Tolerance: Agronomic, Molecular and Biotechnological Approaches; Hasanuzzaman, M., Hakeem, K.R., Nahar, K., Alharby, H.F., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 191–205. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas Pérez, S.; Rajabi Dehnavi, A.; Leszczyński, K.; Lubińska-Mielińska, S.; Ludwiczak, A.; Piernik, A. Salicornia europaea L. Functional Traits Indicate Its Optimum Growth. Plants 2022, 11, 1051. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; Van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef]
- Panda, A.; Rangani, J.; Kumar Parida, A. Cross talk between ROS homeostasis and antioxidative machinery contributes to salt tolerance of the xero-halophyte Haloxylon salicornicum. Environ. Exp. Bot. 2019, 166, 103799. [Google Scholar] [CrossRef]
- Hernández, J.A.; Ferrer, M.A.; Jiménez, A.; Barceló, A.R.; Sevilla, F. Antioxidant systems and O2· −/H2O2 production in the apoplast of pea leaves. Its relation with salt-induced necrotic lesions in minor veins. Plant Physiol. 2001, 127, 817–831. [Google Scholar] [CrossRef]
- Yan, H.; Chen, J.; Liu, J. The involvement of energy metabolism and Lipid peroxidation in lignin accumulation of postharvest Pumelos. Membranes 2020, 10, 269. [Google Scholar] [CrossRef]
- Hughes, J.; McCully, M.E. The Use of an Optical Brightener in the Study of Plant Structure. Stain. Technol. 1975, 50, 319–329. [Google Scholar] [CrossRef]
- Lionetti, V.; Fabri, E.; De Caroli, M.; Hansen, A.R.; Willats, W.G.; Piro, G.; Bellincampi, D. Three Pectin Methylesterase Inhibitors Protect Cell Wall Integrity for Arabidopsis Immunity to Botrytis. Plant Physiol. 2017, 173, 1844–1863. [Google Scholar] [CrossRef]
- Liu, J.; Otie, V.; Matsuura, A.; Junichi, K.; Irshad, M.; Zheng, Y.; Fujimaki, H.; An, P. Pectin Characteristics Affect Root Growth in Spinach under Salinity. Plants 2022, 11, 3130. [Google Scholar] [CrossRef]
- Wolf, S.; Mouille, G.; Pelloux, J. Homogalacturonan Methyl-Esterification and Plant Development. Mol. Plant 2009, 2, 851–860. [Google Scholar] [CrossRef]
- Rozema, J.; Schat, H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 2013, 92, 83–95. [Google Scholar] [CrossRef]
- Pérez, S.C.; Strzelecki, J.; Piernik, A.; Dehnavi, A.R.; Trzeciak, P.; Puchałka, R.; Mierek-Adamska, A.; Pérez, J.C.; Kačík, F.; Račko, V.; et al. Salinity-driven changes in Salicornia cell wall nanomechanics and lignin composition. Environ. Exp. Bot. 2024, 218, 105606. [Google Scholar] [CrossRef]
- Pompella, A.; Comporti, M. Histochemistrv The use of 3-hydroxy-2-naphthoic acid hydrazide and Fast Blue B for the histochemical detection of lipid peroxidation in animal tissues-a microphotometric study. Histochemistry 1991, 95, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Sekulska-Nalewajko, J.; Gocławski, J.; Chojak-Koźniewska, J.; Kuźniak, E. Automated image analysis for quantification of reactive oxygen species in plant leaves. Methods 2016, 109, 114–122. [Google Scholar] [CrossRef]
- Mohammadzadeh, Z.; Shojaeiyan, A.; Mahfeli, M.; Ayyari, M.; Tohidfar, M.; Mokhtassi-Bidgoli, A.; Atighi, M.R. Predictive Modeling of CIELAB Color Parameters in Okra Accessions Based on Phytochemical Composition and Antioxidant Activity: A Non-Destructive Imagej and RSM Approach. LWT 2025, 228, 118080. [Google Scholar] [CrossRef]
- Conesa, A.; Manera, F.C.; Brotons, J.M.; Fernandez-Zapata, J.C.; Simón, I.; Simón-Grao, S.; Alfosea-Simón, M.; Nicolás, J.M.; Valverde, J.M.; García-Sanchez, F. Changes in the Content of Chlorophylls and Carotenoids in the Rind of Fino 49 Lemons During Maturation and Their Relationship with Parameters from the CIELAB Color Space. Sci. Hortic. 2019, 243, 252–260. [Google Scholar] [CrossRef]




| Concentration | Population | L* | a* | b* | Hue | S* | ΔE′ | ΔE vs. White Plate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | ||
| 0 mM | INOW | 45.646 ± 1.58 c | 74.02 ± 1.99 a | 12.55 ± 0.34 b | 14.48 ± 0.34 a | 16.19 ± 0.53 b | 46.70 ± 3.66 a | 35.94 ± 2.42 b | 21.14 ± 1.96 c | 20.61 ± 0.34 b | 46.70 ± 4.13 b | 0.00 ± 0.00 | 0.00 ± 0.00 | 25.24 ± 0.49 c | 44.68 ± 2.68 b |
| CIECH | 75.53 ± 3.80 b | 76.59 ± 2.29 a | 13.65 ± 0.27 a | 5.08 ± 0.24 c | 11.18 ± 0.66 c | 48.11 ± 2.33 a | 52.20 ± 1.12 a | 6.09 ± 0.53 d | 17.62 ± 0.48 c | 49.71 ± 0.87 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 20.72 ± 1.35 d | 52.23 ± 0.24 a | |
| SALZ | 84.12 ± 3.92 a | 45.80 ± 2.60 b | 10.12 ± 0.52 c | 14.68 ± 1.22 a | 15.09 ± 0.52 b | 14.85 ± 0.58 b | 34.28 ± 1.32 b | 47.36 ± 0.20 a | 18.37 ± 0.31 c | 21.09 ± 0.65 c | 0.00 ± 0.00 | 0.00 ± 0.00 | 29.03 ± 1.70 b | 31.21 ± 0.82 d | |
| SOLTQ | 35.10 ± 2.14 d | 27.58 ± 2.11 c | 2.51 ± 0.40 d | 11.75 ± 0.66 b | 27.99 ± 0.86 a | 12.19 ± 0.98 b | 5.57 ± 0.30 c | 41.47 ± 0.86 b | 27.40 ± 1.46 a | 16.28 ± 1.11 d | 0.00 ± 0.00 | 0.00 ± 0.00 | 37.85 ± 1.00 a | 36.86 ± 2.21 c | |
| 200 mM | INOW | 83.53 ± 2.56 a | 39.41 ± 0.12 b | 8.20 ± 0.14 d | 23.34 ± 0.27 a | 9.56 ± 0.40 c | 17.44 ± 0.61 b | 220.51 ± 4.44 a | 233.09 ± 1.25 a | 12.15 ± 0.73 c | 29.14 ± 0.42 b | 38.54 ± 1.85 a | 47.01 ± 3.34 a | 26.34 ± 2.53 b | 38.87 ± 2.20 a |
| CIECH | 63.33 ± 1.91 b | 52.33 ± 1.80 a | 6.69 ± 0.23 c | 7.04 ± 0.60 c | 15.12 ± 1.19 b | 33.23 ± 0.03 a | 205.33 ± 3.76 c | 189.01 ± 1.49 c | 17.57 ± 0.20 b | 34.03 ± 0.80 a | 15.36 ± 2.55 c | 31.30 ± 0.51 b | 18.44 ± 0.59 c | 34.41 ± 0.95 b | |
| SALZ | 42.01 ± 0.21 c | 42.27 ± 1.61 b | 2.75 ± 0.50 b | 12.62 ± 1.19 b | 37.02 ± 5.09 a | 17.03 ± 1.81 b | 185.02 ± 1.64 d | 226.70 ± 8.44 b | 36.62 ± 0.85 a | 21.96 ± 1.94 c | 19.05 ± 1.43 b | 27.82 ± 0.95 c | 43.64 ± 2.44 a | 31.43 ± 9.37 b | |
| SOLTQ | 44.15 ± 1.63 c | 35.81 ± 3.11 c | 10.54 ± 0.42 a | 11.18 ± 0.68 b | 15.70 ± 1.06 b | 8.00 ± 0.63 c | 213.34 ± 2.24 b | 230.10 ± 5.95 ab | 18.27 ± 1.89 b | 13.99 ± 0.40 d | 36.87 ± 1.21 a | 15.47 ± 0.49 d | 26.20 ± 0.22 b | 33.86 ± 3.46 b | |
| 400 mM | INOW | 58.21 ± 0.88 b | 54.31 ± 2.68 a | 11.20 ± 0.10 a | 16.53 ± 1.68 c | 15.74 ± 1.44 b | 26.76 ± 1.06 a | 216.79 ± 3.36 a | 210.72 ± 4.14 c | 18.81 ± 1.49 b | 34.03 ± 3.36 a | 22.71 ± 1.64 b | 25.29 ± 3.09 b | 24.62 ± 1.40 b | 36.85 ± 2.83 a |
| CIECH | 87.06 ± 2.23 a | 56.32 ± 2.77 a | 4.86 ± 1.19 c | 22.93 ± 1.32 a | 8.39 ± 0.37 c | 25.79 ± 0.70 a | 214.65 ± 3.33 a | 222.13 ± 0.28 ab | 9.40 ± 0.17 d | 35.74 ± 1.37 a | 10.67 ± 0.38 d | 41.02 ± 3.70 a | 23.69 ± 2.93 b | 36.98 ± 1.49 a | |
| SALZ | 43.32 ± 1.03 d | 29.11 ± 0.23 c | 9.29 ± 0.14 b | 12.14 ± 0.65 d | 18.26 ± 0.09 a | 11.12 ± 0.62 c | 207.85 ± 5.49 b | 226.35 ± 1.12 a | 21.20 ± 0.69 a | 17.22 ± 1.19 c | 15.76 ± 0.79 c | 4.07 ± 0.99 d | 27.99 ± 0.83 a | 34.89 ± 1.14 a | |
| SOLTQ | 50.14 ± 1.65 c | 43.57 ± 2.58 b | 11.66 ± 1.39 a | 18.13 ± 1.06 b | 14.32 ± 1.30 b | 22.55 ± 0.94 b | 212.98 ± 4.78 a | 217.42 ± 2.18 b | 16.77 ± 0.76 c | 28.40 ± 0.09 b | 25.37 ± 2.28 a | 13.42 ± 3.81 c | 20.50 ± 1.31 c | 31.92 ± 0.24 b | |
| 1000 mM | INOW | 72.83 ± 2.37 b | 44.86 ± 2.11 b | 10.69 ± 0.43 a | 27.26 ± 0.26 a | 11.98 ± 0.54 d | 35.43 ± 3.61 b | 219.39 ± 3.31 a | 224.91 ± 3.83 b | 14.93 ± 0.71 b | 44.36 ± 1.36 a | 29.48 ± 0.44 b | 27.63 ± 2.39 a | 19.06 ± 0.65 b | 44.41 ± 0.16 a |
| CIECH | 76.59 ± 0.60 a | 60.46 ± 0.18 a | 10.62 ± 0.29 a | 16.51 ± 0.59 b | 17.47 ± 2.25 c | 41.22 ± 1.87 a | 215.67 ± 6.85 ab | 201.72 ± 1.74 d | 15.89 ± 0.75 b | 43.06 ± 0.79 a | 14.01 ± 1.56 c | 23.79 ± 2.06 b | 23.08 ± 0.94 a | 44.62 ± 0.86 a | |
| SALZ | 76.56 ± 4.24 a | 27.03 ± 1.63 c | 11.62 ± 0.47 a | 13.98 ± 1.09 c | 18.09 ± 1.41 b | 11.77 ± 0.38 d | 212.87 ± 1.52 b | 229.54 ± 2.34 a | 22.47 ± 1.07 a | 17.76 ± 0.87 c | 39.11 ± 2.19 a | 3.46 ± 0.99 d | 24.12 ± 2.05 a | 37.89 ± 0.51 b | |
| SOLTQ | 63.58 ± 2.61 c | 45.26 ± 2.69 b | 9.70 ± 0.99 b | 15.72 ± 0.32 b | 20.64 ± 0.27 a | 19.97 ± 0.58 c | 207.32 ± 3.73 c | 220.47 ± 1.89 c | 21.79 ± 0.85 a | 23.93 ± 1.74 b | 27.64 ± 2.10 b | 7.74 ± 0.23 c | 24.08 ± 0.47 a | 27.60 ± 1.94 c | |
| Concentration | Population | L* | a* | b* | Hue | S* | ΔE′ | ΔE vs. White Plate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | Schiff’s-MDA | DAB-H2O2 | ||
| 0 mM | INOW | 53.88 ± 2.49 b | 68.66 ± 0.79 a | −5.35 ± 0.81 a | 6.67 ± 0.29 d | 22.14 ± 0.17 b | 51.92 ± 1.07 a | −15.02 ± 0.62 a | 9.15 ± 1.84 d | 24.10 ± 2.08 a | 52.37 ± 1.34 b | 0.00 ± 0.00 | 0.00 ± 0.00 | 25.05 ± 1.54 a | 54.07 ± 0.40 c |
| CIECH | 56.55 ± 5.25 b | 55.13 ± 1.78 b | −4.45 ± 0.40 a | 14.54 ± 1.39 c | 25.51 ± 0.36 a | 52.15 ± 2.61 a | −9.55 ± 0.28 c | 17.30 ± 1.06 c | 24.60 ± 2.16 a | 53.85 ± 1.63 b | 0.00 ± 0.00 | 0.00 ± 0.00 | 25.22 ± 2.66 a | 54.47 ± 1.61 c | |
| SALZ | 62.13 ± 1.21 a | 45.92 ± 1.83 d | −5.39 ± 0.71 a | 21.99 ± 0.74 b | 20.10 ± 0.22 c | 52.25 ± 2.49 a | −15.18 ± 1.91 a | 22.21 ± 1.61 b | 22.06 ± 2.08 a | 56.45 ± 2.44 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 20.95 ± 0.02 b | 58.04 ± 1.89 b | |
| SOLTQ | 66.09 ± 0.34 a | 52.19 ± 1.69 c | −4.44 ± 0.37 a | 25.09 ± 1.39 a | 20.13 ± 0.78 c | 53.20 ± 1.48 a | −12.44 ± 0.92 b | 25.20 ± 1.63 a | 20.61 ± 0.80 b | 60.38 ± 3.85 a | 0.00 ± 0.00 | 0.00 ± 0.00 | 21.30 ± 0.80 b | 66.13 ± 2.24 a | |
| 200 mM | INOW | 60.58 ± 4.11 a | 54.92 ± 2.02 a | −3.32 ± 3.51 a | 24.01 ± 0.95 a | 9.89 ± 0.35 c | 16.77 ± 0.91 b | 217.18 ± 2.27 a | 233.09 ± 1.25 a | 12.15 ± 0.73 c | 25.81 ± 5.73 b | 38.54 ± 1.85 a | 47.01 ± 3.34 a | 26.34 ± 2.53 b | 38.87 ± 2.20 a |
| CIECH | 55.28 ± 2.55 b | 56.39 ± 2.52 a | −4.93 ± 1.07 a | 5.37 ± 0.03 c | 15.12 ± 1.19 b | 32.90 ± 0.54 a | 203.33 ± 0.89 c | 189.01 ± 1.49 c | 17.24 ± 0.73 b | 35.03 ± 2.53 a | 13.69 ± 3.65 b | 31.30 ± 0.51 b | 18.44 ± 0.59 c | 33.74 ± 2.09 b | |
| SALZ | 64.04 ± 0.61 a | 53.18 ± 2.53 a | −4.4 ± 0.34 a | 11.68 ± 1.29 b | 15.70 ± 1.06 b | 8.00 ± 0.63 c | 213.34 ± 2.24 b | 230.10 ± 5.95 a | 18.27 ± 1.89 b | 13.99 ± 0.40 d | 35.87 ± 1.09 a | 15.80 ± 1.05 c | 26.20 ± 0.22 b | 33.19 ± 2.35 b | |
| SOLTQ | 63.02 ± 2.14 a | 47.55 ± 0.31 b | −3.16 ± 1.51 a | 12.46 ± 1.43 b | 38.35 ± 3.38 a | 17.70 ± 2.81 b | 183.68 ± 1.35 d | 223.37 ± 2.66 b | 37.29 ± 1.22 a | 21.63 ± 2.21 c | 16.72 ± 5.47 b | 17.82 ± 0.95 c | 43.97 ± 2.46 a | 24.76 ± 2.35 c | |
| 400 mM | INOW | 62.56 ± 2.83 a | 64.48 ± 2.22 b | −6.62 ± 0.03 b | 10.97 ± 0.32 b | 27.38 ± 3.47 a | 53.07 ± 0.73 a | 162.59 ± 1.44 b | 192.32 ± 0.92 b | 24.81 ± 3.18 b | 52.96 ± 1.69 b | 7.41 ± 0.02 a | 4.73 ± 1.73 d | 24.54 ± 1.13 b | 54.23 ± 0.25 b |
| CIECH | 56.78 ± 2.00 b | 66.88 ± 2.99 a | −4.16 ± 0.39 c | 2.94 ± 0.80 c | 25.89 ± 0.83 a | 46.31 ± 1.09 c | 170.87 ± 0.77 a | 184.44 ± 2.17 c | 26.23 ± 0.85 a | 46.47 ± 1.11 c | 4.81 ± 1.34 b | 9.37 ± 0.20 c | 27.36 ± 1.96 a | 47.69 ± 0.63 c | |
| SALZ | 66.80 ± 2.73 a | 35.30 ± 0.73 d | −6.26 ± 0.21 a | 26.39 ± 0.83 a | 21.90 ± 1.24 b | 43.83 ± 1.26 d | 162.97 ± 1.61 b | 210.20 ± 2.03 a | 21.51 ± 1.93 c | 52.62 ± 3.78 b | 6.48 ± 1.56 a | 15.03 ± 0.91 b | 22.49 ± 1.75 c | 57.57 ± 1.60 a | |
| SOLTQ | 63.11 ± 0.53 a | 44.74 ± 0.11 c | −6.83 ± 0.13 a | 25.74 ± 1.80 a | 19.89 ± 0.68 b | 49.79 ± 0.22 b | 163.62 ± 2.37 b | 207.32 ± 1.74 a | 23.49 ± 5.03 b | 56.06 ± 0.64 a | 3.38 ± 0.17 c | 42.20 ± 1.03 a | 20.92 ± 0.71 d | 58.55 ± 0.64 a | |
| 1000 mM | INOW | 63.14 ± 1.78 a | 63.80 ± 2.30 b | −6.75 ± 0.17 a | 5.27 ± 0.58 b | 24.03 ± 2.17 b | 49.44 ± 3.54 a | 164.24 ± 1.19 c | 187.08 ± 2.94 c | 24.97 ± 2.11 b | 49.88 ± 3.89 b | 9.05 ± 0.29 a | 9.01 ± 2.15 c | 25.07 ± 2.14 a | 51.76 ± 1.79 b |
| CIECH | 55.78 ± 0.62 b | 67.78 ± 1.43 a | −4.81 ± 0.11 b | 6.23 ± 0.60 b | 25.74 ± 3.14 a | 45.26 ± 2.45 b | 173.05 ± 2.62 a | 184.70 ± 4.74 c | 26.05 ± 3.26 a | 45.53 ± 2.69 b | 5.40 ± 1.48 b | 18.09 ± 0.58 b | 25.15 ± 1.18 a | 47.08 ± 1.63 c | |
| SALZ | 63.90 ± 2.90 a | 44.56 ± 2.21 c | −4.80 ± 0.89 b | 23.64 ± 2.83 a | 21.27 ± 1.19 c | 50.57 ± 0.83 a | 167.50 ± 3.25 b | 205.94 ± 2.82 b | 22.48 ± 1.98 c | 54.06 ± 2.79 a | 3.46 ± 0.91 b | 7.76 ± 1.03 c | 21.84 ± 030 b | 56.94 ± 2.04 a | |
| SOLTQ | 64.53 ± 2.23 a | 31.49 ± 1.22 d | −6.39 ± 0.18 a | 22.70 ± 1.04 a | 18.72 ± 0.58 d | 36.87 ± 1.28 c | 162.98 ± 3.04 d | 213.16 ± 1.11 a | 19.60 ± 0.56 d | 46.29 ± 5.34 b | 4.03 ± 1.23 b | 57.87 ± 0.48 a | 20.04 ± 0.60 b | 53.28 ± 1.44 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallegos Cerda, S.D.; Orzoł, A.; Chanona Pérez, J.J.; Hernández Varela, J.D.; Piernik, A.; Cárdenas Pérez, S. Decoding Salinity Tolerance in Salicornia europaea L.: Image-Based Oxidative Phenotyping and Histochemical Mapping of Pectin and Lignin. Plants 2025, 14, 3055. https://doi.org/10.3390/plants14193055
Gallegos Cerda SD, Orzoł A, Chanona Pérez JJ, Hernández Varela JD, Piernik A, Cárdenas Pérez S. Decoding Salinity Tolerance in Salicornia europaea L.: Image-Based Oxidative Phenotyping and Histochemical Mapping of Pectin and Lignin. Plants. 2025; 14(19):3055. https://doi.org/10.3390/plants14193055
Chicago/Turabian StyleGallegos Cerda, Susana Dianey, Aleksandra Orzoł, José Jorge Chanona Pérez, Josué David Hernández Varela, Agnieszka Piernik, and Stefany Cárdenas Pérez. 2025. "Decoding Salinity Tolerance in Salicornia europaea L.: Image-Based Oxidative Phenotyping and Histochemical Mapping of Pectin and Lignin" Plants 14, no. 19: 3055. https://doi.org/10.3390/plants14193055
APA StyleGallegos Cerda, S. D., Orzoł, A., Chanona Pérez, J. J., Hernández Varela, J. D., Piernik, A., & Cárdenas Pérez, S. (2025). Decoding Salinity Tolerance in Salicornia europaea L.: Image-Based Oxidative Phenotyping and Histochemical Mapping of Pectin and Lignin. Plants, 14(19), 3055. https://doi.org/10.3390/plants14193055








