Advances in Ulva Linnaeus, 1753 Research: From Structural Diversity to Applied Utility
Abstract
1. Introduction
2. Morphological Variability and Environmental Plasticity in Ulva Species
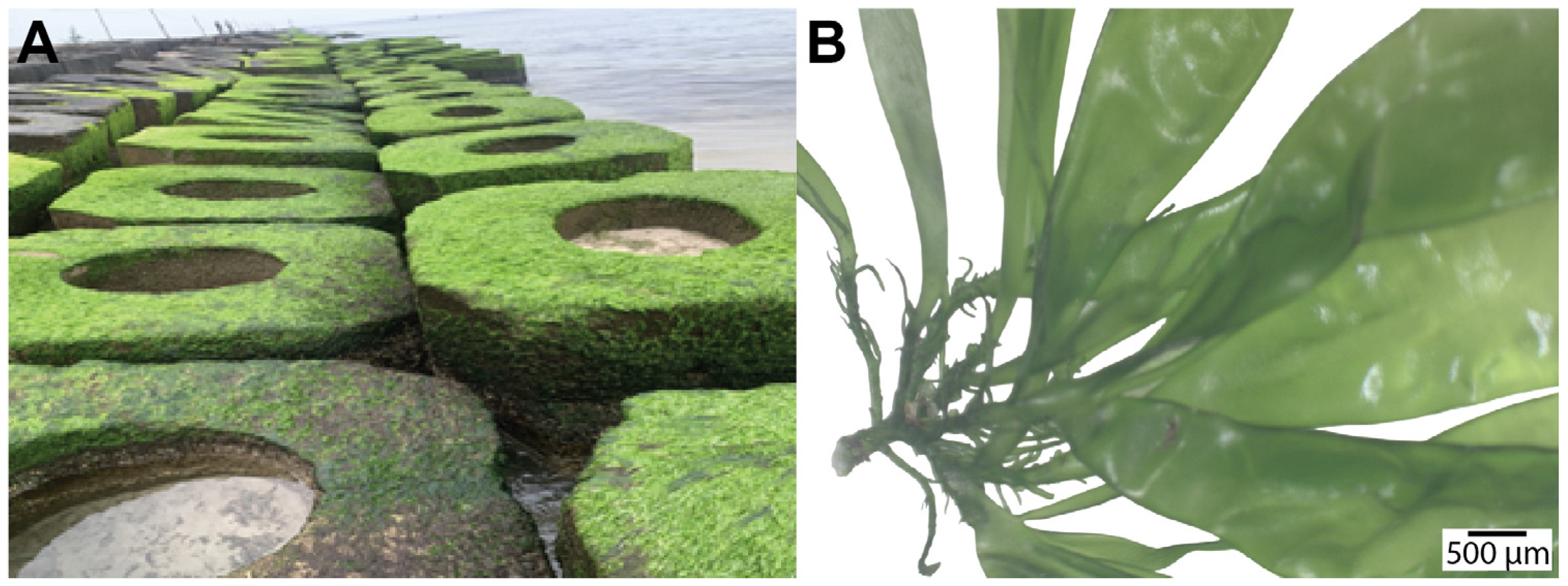
2.1. Morphological Diversity of Ulva Species
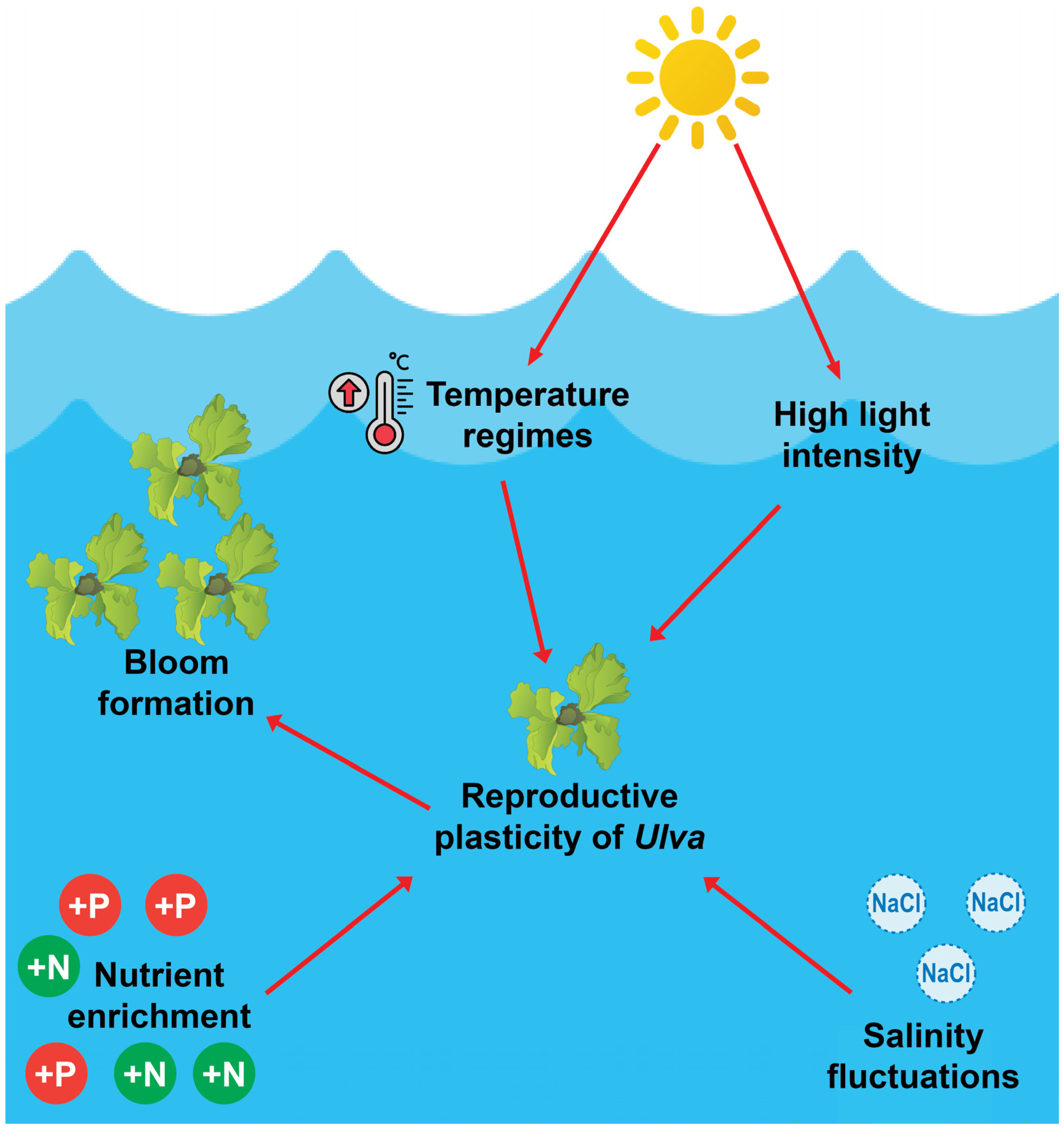
2.2. Effects of Salinity, Temperature, and Nutrient Availability on the Morphology of Ulva Species
2.3. Reproductive Strategies and Environmental Adaptability of Ulva Species
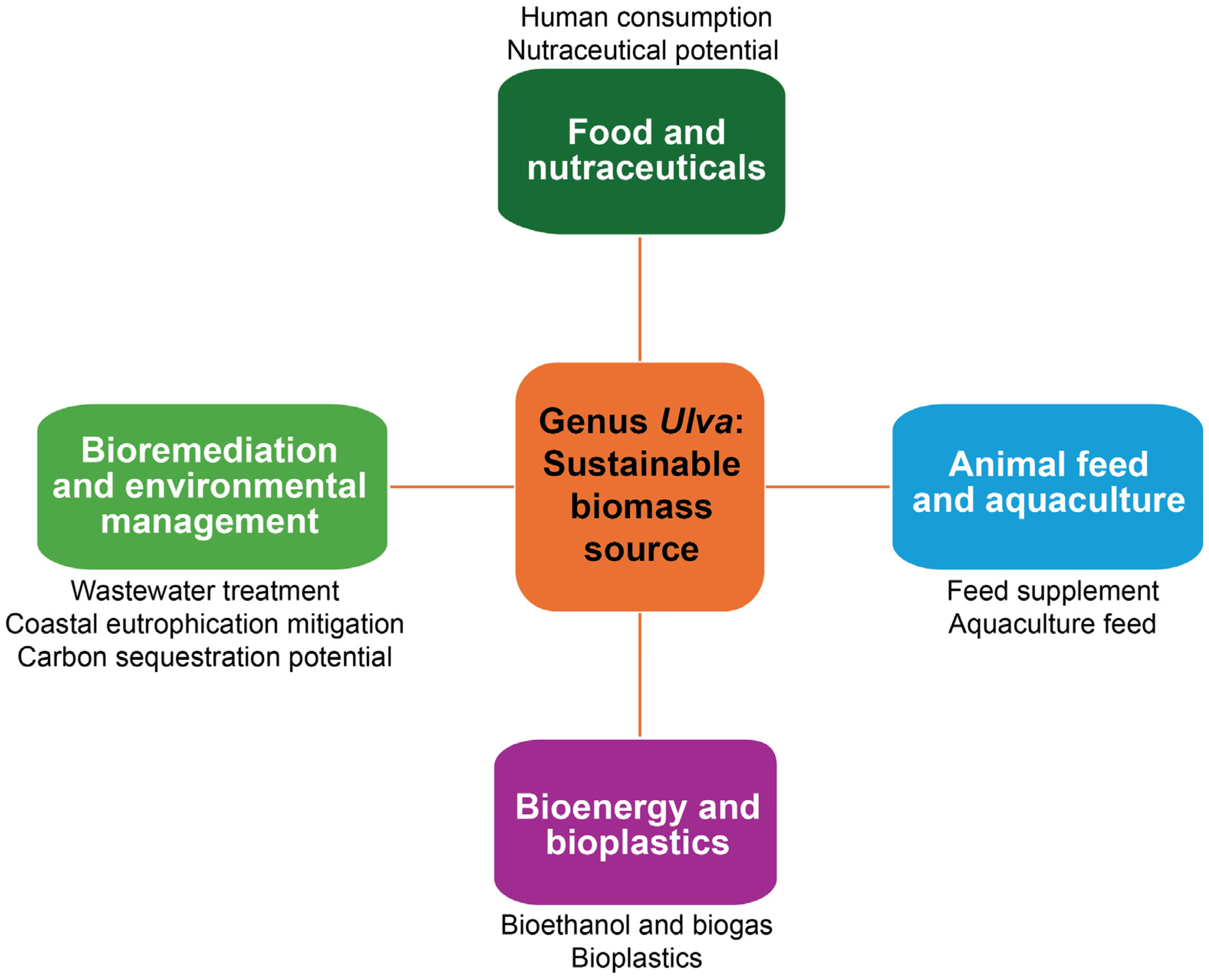
3. Economic Application of Ulva Species
4. Molecular Approaches in Ulva Taxonomy and Biodiversity Assessment
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Liao, W.; Liu, Y.; Guo, Y.; Jiang, S.; Zhao, C. An overview on the nutritional and bioactive components of green seaweeds. Food Prod. Process. Nutr. 2023, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Chelliapan, S.; Kamyab, H.; Thirugnana, S.; Othman, N.; Nasri, N.S. Treatment of wastewater using seaweed: A review. Int. J. Environ. Res. Public Health 2018, 15, 2851. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; McHale, M.; Sulpice, R. Applications of Ulva biomass and strategies to improve its yield and composition: A perspective for Ulva aquaculture. Biology 2022, 11, 1593. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, T.; Du, Y.; Jiang, L.; Yao, Z.; Ning, L.; Zhu, B. Ulvan and Ulva oligosaccharides: A systematic review of structure, preparation, biological activities and applications. Bioresour. Bioprocess. 2023, 10, 66. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Abdelghany, E.M.; Shabaka, S.; Ismail, M.; Shalaby, O.; Ismail, M. Morphological and molecular characterization of the green algae Ulva in the Mediterranean Coast of Egypt. Reg. Stud. Mar. Sci. 2024, 78, 103807. [Google Scholar] [CrossRef]
- Kang, J.H.; Jang, J.E.; Kim, J.H.; Byeon, S.Y.; Kim, S.; Choi, S.K.; Kang, Y.H.; Park, S.R.; Lee, H.J. Species composition, diversity, and distribution of the genus Ulva along the coast of Jeju Island, Korea based on molecular phylogenetic analysis. PLoS ONE 2019, 14, e0219958. [Google Scholar] [CrossRef]
- Leskinen, E.; Alstrom-Rapaport, C.; Pamilo, P. Phylogeographical structure, distribution and genetic variation of the green algae Ulva intestinalis and U. compressa (Chlorophyta) in the Baltic Sea area. Mol. Ecol. 2004, 13, 2257–2265. [Google Scholar] [CrossRef]
- Dominguez, H.; Loret, E.P. Ulva lactuca, A source of troubles and potential riches. Mar. Drugs 2019, 17, 357. [Google Scholar] [CrossRef]
- Bonanno, G.; Veneziano, V.; Piccione, V. The alga Ulva lactuca (Ulvaceae, Chlorophyta) as a bioindicator of trace element contamination along the coast of Sicily, Italy. Sci. Total Environ. 2020, 699, 134329. [Google Scholar] [CrossRef]
- Pari, R.F.; Uju, U.; Hardiningtyas, S.D.; Ramadhan, W.; Wakabayashi, R.; Goto, M.; Kamiya, N. Ulva seaweed-derived ulvan: A promising marine polysaccharide as a sustainable resource for biomaterial design. Mar. Drugs 2025, 23, 56. [Google Scholar] [CrossRef]
- Zeng, Y.; Yang, X.; Xia, Z.; Chen, R.; He, F.; Zhang, J.; He, P. Review of allelopathy in green tides: The case of Ulva prolifera in the South Yellow Sea. Biology 2024, 13, 456. [Google Scholar] [CrossRef]
- Mutizabal-Aros, J.; Ramirez, M.E.; Haye, P.A.; Meynard, A.; Pinilla-Rojas, B.; Nunez, A.; Latorre-Padilla, N.; Search, F.V.; Tapia, F.J.; Saldias, G.S.; et al. Morphological and molecular identification of Ulva spp. (Ulvophyceae; Chlorophyta) from Algarrobo Bay, Chile: Understanding the composition of green tides. Plants 2024, 13, 1258. [Google Scholar] [CrossRef]
- Wichard, T.; Charrier, B.; Mineur, F.; Bothwell, J.H.; Clerck, O.D.; Coates, J.C. The green seaweed Ulva: A model system to study morphogenesis. Front. Plant Sci. 2015, 6, 72. [Google Scholar] [CrossRef]
- Guan, C.; Zhao, X.; Qu, T.; Zhong, Y.; Hou, C.; Lin, Z.; Xu, J.; Tang, X.; Wang, Y. Physiological functional traits explain morphological variation of Ulva prolifera during the drifting of green tides. Ecol. Evol. 2022, 12, e8504. [Google Scholar] [CrossRef]
- Tan, I.H.; Blomster, J.; Hansen, G.; Leskinen, E.; Maggs, C.A.; Mann, D.G.; Sluiman, H.J.; Stanhope, M.J. Molecular phylogenetic evidence for a reversible morphogenetic switch controlling the gross morphology of two common genera of green seaweeds, Ulva and Enteromorpha. Mol. Biol. Evol. 1999, 16, 1011–1018. [Google Scholar] [CrossRef]
- Blomster, J.; Bäck, S.; Fewer, D.P.; Kiirikki, M.; Lehvo, A.; Maggs, C.A.; Stanhope, M.J. Novel morphology in Enteromorpha (Ulvophyceae) forming green tides. Am. J. Bot. 2002, 89, 1756–1763. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Zhang, X. A review of the green tides in the Yellow Sea, China. Mar. Environ. Res. 2016, 119, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Zhao, S.; Liu, J.; Tong, Y.; Xia, Z.; Xia, J.; Li, S.; Sun, Y.; Cao, J.; Zhang, J. The morphology, genetic diversity, and distribution of Ulva meridionalis (Ulvaceae, Chlorophyta) in Chinese seas. J. Mar. Sci. Eng. 2022, 10, 1873. [Google Scholar] [CrossRef]
- Spoerner, M.; Wichard, T.; Bachhuber, T.; Stratmann, J.; Oertel, W. Growth and thallus morphogenesis of Ulva mutabilis (Chlorophyta) depends on a combination of two bacterial species excreting regulatory factors. J. Phycol. 2012, 48, 1433–1447. [Google Scholar] [CrossRef]
- Fort, A.; Lebrault, M.; Allaire, M.; Esteves-Ferreira, A.A.; McHale, M.; Lopez, F.; Farinas-Franco, J.M.; Alseekh, S.; Fernie, A.R.; Sulpice, R. Extensive variations in diurnal growth patterns and metabolism among Ulva spp. strains. Plant Physiol. 2019, 180, 109–123. [Google Scholar] [CrossRef]
- Sonchaeng, U.; Wongphan, P.; Pan-Utai, W.; Paopun, Y.; Kansandee, W.; Satmalee, P.; Tamtin, M.; Kosawatpat, P.; Harnkarnsujarit, N. Preparation and characterization of novel green seaweed films from Ulva rigida. Polymers 2023, 15, 3342. [Google Scholar] [CrossRef]
- Dartois, M.; Pante, E.; Viricel, A.; Becquet, V.; Sauriau, P.G. Molecular genetic diversity of seaweeds morphologically related to Ulva rigida at three sites along the French Atlantic coast. PeerJ 2021, 9, e11966. [Google Scholar] [CrossRef]
- Carl, C.; de Nys, R.; Paul, N.A. The seeding and cultivation of a tropical species of filamentous Ulva for algal biomass production. PLoS ONE 2014, 9, e98700. [Google Scholar] [CrossRef] [PubMed]
- Bast, F.; John, A.A.; Bhushan, S. Strong endemism of bloom-forming tubular Ulva in Indian West Coast, with description of Ulva paschima Sp. Nov. (Ulvales, Chlorophyta). PLoS ONE 2014, 9, e109295. [Google Scholar] [CrossRef] [PubMed]
- Steinhagen, S.; Kramár, L.; Toth, G.B. The unheeded existence of the tubular greens: Molecular analyses reveal the distribution of a new Ulva species (Ulvophyceae, Chlorophyta), Ulva capillata sp. nov. in the Atlantic-Baltic Sea transect. J. Appl. Phycol. 2023, 35, 509–522. [Google Scholar] [CrossRef]
- Cai, C.; Wang, L.; Zhou, L.; He, P.; Jiao, B. Complete chloroplast genome of green tide algae Ulva flexuosa (Ulvophyceae, Chlorophyta) with comparative analysis. PLoS ONE 2017, 12, e0184196. [Google Scholar] [CrossRef] [PubMed]
- Imchen, T. Recruitment potential of a green alga Ulva flexuosa Wulfen dark preserved zoospore and its development. PLoS ONE 2012, 7, e32651. [Google Scholar] [CrossRef]
- Jiang, H.P.; Gao, B.B.; Li, W.H.; Zhu, M.; Zheng, C.F.; Zheng, Q.S.; Wang, C.H. Physiological and biochemical responses of Ulva prolifera and Ulva linza to cadmium stress. Sci. World J. 2013, 2013, 289537. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, R.-C.; Yan, T.; Zhang, Q.-C.; Zhou, M.-J. Laboratory study on the life history of bloom-forming Ulva prolifera in the Yellow Sea. Estuar. Coast. Shelf Sci. 2015, 163, 82–88. [Google Scholar] [CrossRef]
- Zhang, Y.; He, P.; Li, H.; Li, G.; Liu, J.; Jiao, F.; Zhang, J.; Huo, Y.; Shi, X.; Su, R.; et al. Ulva prolifera green-tide outbreaks and their environmental impact in the Yellow Sea, China. Natl. Sci. Rev. 2019, 6, 825–838. [Google Scholar] [CrossRef]
- Li, B.H.; Liu, C.Y.; Deng, X.; Wang, K.K.; Han, L.; Huang, Y.H.; Li, X.; Cai, W.J. Responses of the marine carbonate system to a green tide: A case study of an Ulva prolifera bloom in Qingdao coastal waters. Harmful Algae 2021, 110, 102133. [Google Scholar] [CrossRef]
- Zollmann, M.; Liberzon, A.; Palatnik, R.R.; Zilberman, D.; Golberg, A. Effects of season, depth and pre-cultivation fertilizing on Ulva growth dynamics offshore the Eastern Mediterranean Sea. Sci. Rep. 2023, 13, 14784. [Google Scholar] [CrossRef]
- Lawton, R.J.; Mata, L.; de Nys, R.; Paul, N.A. Algal bioremediation of waste waters from land-based aquaculture using Ulva: Selecting target species and strains. PLoS ONE 2013, 8, e77344. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.B.; Liu, F. Salinity-induced oxidative stress and regulation of antioxidant defense system in the marine macroalga Ulva prolifera. J. Exp. Mar. Biol. Ecol. 2011, 409, 223–228. [Google Scholar] [CrossRef]
- Gao, G.; Zhong, Z.; Zhou, X.; Xu, J. Changes in morphological plasticity of Ulva prolifera under different environmental conditions: A laboratory experiment. Harmful Algae 2016, 59, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Fort, A.; Jouanneau, D.; McHale, M.; Sulpice, R. Fast screening method to identify salinity tolerant strains of foliose Ulva species. Low salinity leads to increased organic matter of the biomass. J. Appl. Phycol. 2024, 36, 2161–2172. [Google Scholar] [CrossRef]
- Sato, Y.; Kinoshita, Y.; Mogamiya, M.; Inomata, E.; Hoshino, M.; Hiraoka, M. Different growth and sporulation responses to temperature gradient among obligate apomictic strains of Ulva prolifera. Plants 2021, 10, 2256. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, N.; Liang, C.; Mou, S.; Fan, X.; Xu, J.; Xu, D.; Zhuang, Z. De novo sequencing and analysis of the Ulva linza transcriptome to discover putative mechanisms associated with its successful colonization of coastal ecosystems. BMC Genom. 2012, 13, 565. [Google Scholar] [CrossRef]
- Van der Loos, L.M.; D’hondt, S.; Engelen, A.H.; Pavia, H.; Toth, G.B.; Willems, A.; Weinberger, F.; De Clerck, O.; Steinhagen, S. Salinity and host drive Ulva-associated bacterial communities across the Atlantic–Baltic Sea gradient. Mol. Ecol. 2023, 32, 6260–6277. [Google Scholar] [CrossRef]
- Rybak, A.S. Species of Ulva (Ulvophyceae, Chlorophyta) as indicators of salinity. Ecol. Indic. 2018, 85, 253–261. [Google Scholar] [CrossRef]
- Choi, T.-S.; Kang, E.-J.; Kim, J.-H.; Kim, K.-Y. Effect of salinity on growth and nutrient uptake of Ulva pertusa (Chlorophyta) from an eelgrass bed. Algae 2010, 25, 17–26. [Google Scholar] [CrossRef]
- Lartigue, J.; Neill, A.; Hayden, B.L.; Pulfer, J.; Cebrian, J. The impact of salinity fluctuations on net oxygen production and inorganic nitrogen uptake by Ulva lactuca (Chlorophyceae). Aquat. Bot. 2003, 75, 339–350. [Google Scholar] [CrossRef]
- Bews, E.; Booher, L.; Polizzi, T.; Long, C.; Kim, J.H.; Edwards, M.S. Effects of salinity and nutrients on metabolism and growth of Ulva lactuca: Implications for bioremediation of coastal watersheds. Mar. Pollut. Bull. 2021, 166, 112199. [Google Scholar] [CrossRef]
- Steinhagen, S.; Weinberger, F.; Karez, R. Molecular analysis of Ulva compressa (Chlorophyta, Ulvales) reveals its morphological plasticity, distribution and potential invasiveness on German North Sea and Baltic Sea coasts. Eur. J. Phycol. 2019, 54, 102–114. [Google Scholar] [CrossRef]
- Xia, J.; Li, Y.; Zou, D. Effects of salinity stress on PSII in Ulva lactuca as probed by chlorophyll fluorescence measurements. Aquat. Bot. 2004, 80, 129–137. [Google Scholar] [CrossRef]
- Rybak, A.S. Freshwater macroalga, Ulva pilifera (Ulvaceae, Chlorophyta) as an indicator of the trophic state of waters for small water bodies. Ecol. Indic. 2021, 121, 106951. [Google Scholar] [CrossRef]
- Bao, M.; Xing, Q.; Park, J.S.; He, P.; Zhang, J.; Yarish, C.; Kim, J.K. Temperature and high nutrients enhance hypo-salinity tolerance of the bloom forming green alga, Ulva prolifera. Harmful Algae 2023, 123, 102402. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Shi, X.; Chen, Y.; Tang, H.; Wang, L. Effects of temperature on the nitrate reductase activity and growth of Ulva prolifera. J. Phycol. 2021, 57, 955–966. [Google Scholar] [CrossRef]
- Liu, C.; Zou, D.; Yang, Y. Comparative physiological behaviors of Ulva lactuca and Gracilariopsis lemaneiformis in responses to elevated atmospheric CO2 and temperature. Environ. Sci. Pollut. Res. Int. 2018, 25, 27493–27502. [Google Scholar] [CrossRef]
- Gnayem, N.; Unis, R.; Gnaim, R.; Chemodanov, A.; Israel, Á.; Gnaim, J.; Golberg, A. Fatty acid content and profile in Ulva lactuca in response to exposure to variable growth conditions in indoor photobioreactors. Life 2025, 15, 57. [Google Scholar] [CrossRef]
- Figueroa, F.L.; Bonomi-Barufi, J.; Celis-Plá, P.S.M.; Nitschke, U.; Arenas, F.; Connan, S.; Abreu, M.H.; Malta, E.J.; Conde-Álvarez, R.; Chow, F.; et al. Short-term effects of increased CO2, nitrate and temperature on photosynthetic activity in Ulva rigida (Chlorophyta) estimated by different pulse amplitude modulated fluorometers and oxygen evolution. J. Exp. Bot. 2021, 72, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Paopun, Y.; Thanomchat, P.; Roopkham, C.; Umroong, P.; Pan-utai, W.; Satmalee, P.; Kosawatpat, P.; Thongdang, B.; Tamtin, M. Structural development of marine green alga (Ulva rigida C. Agardh, 1823) during cultivation. Trends Sci. 2023, 20, 6747. [Google Scholar] [CrossRef]
- Da Costa, E.; Ricardo, F.; Melo, T.; Mamede, R.; Abreu, M.H.; Domingues, P.; Domingues, M.R.; Calado, R. Site-specific lipidomic signatures of sea lettuce (Ulva spp., Chlorophyta) hold the potential to trace their geographic origin. Biomolecules 2020, 10, 489. [Google Scholar] [CrossRef]
- Reidenbach, L.B.; Fernandez, P.A.; Leal, P.P.; Noisette, F.; McGraw, C.M.; Revill, A.T.; Hurd, C.L.; Kübler, J.E. Growth, ammonium metabolism, and photosynthetic properties of Ulva australis (Chlorophyta) under decreasing pH and ammonium enrichment. PLoS ONE 2017, 12, e0188389. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Han, X.; Shi, X.; Rivkin, R.B.; Legendre, L. Growth responses of Ulva prolifera to inorganic and organic nutrients: Implications for macroalgal blooms in the southern Yellow Sea, China. Sci. Rep. 2016, 6, 26498. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, L.; Zhang, J.; Cai, C.; He, P. Complete mitochondrial genome of Ulva linza, one of the causal species of green macroalgal blooms in Yellow Sea, China. Mitochondrial DNA Part B 2016, 1, 31–33. [Google Scholar] [CrossRef]
- Vesty, E.F.; Kessler, R.W.; Wichard, T.; Coates, J.C. Regulation of gametogenesis and zoosporogenesis in Ulva linza (Chlorophyta): Comparison with Ulva mutabilis and potential for laboratory culture. Front. Plant Sci. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Pang, S.J.; Liu, F.; Shan, T.F.; Xu, N.; Zhang, Z.H.; Gao, S.Q.; Chopin, T.; Sun, S. Tracking the algal origin of the Ulva bloom in the Yellow Sea by a combination of molecular, morphological and physiological analyses. Mar. Environ. Res. 2010, 69, 207–215. [Google Scholar] [CrossRef]
- Huan, L.; Gu, W.; Wang, X.; Yan, Y.; Tang, Q.; Han, X.; Wang, Z.; Zhou, K.; Qiu, Q.; Xu, J.; et al. Reproductive traits of floating Ulva prolifera sporophytes and gametophytes and their contribution to the Yellow Sea green tide. Mar. Poll. Bull. 2025, 214, 117752. [Google Scholar] [CrossRef]
- Liao, J.; Wang, S.; Lin, K.; Huang, Y.; Chen, X.; Xin, R.; Guo, Y.; Xie, E. Taxonomic identification and life cycle comparison of two populations of the monostromatic green algae Monostroma nitidum. Ecol. Evol. 2024, 14, e11424. [Google Scholar] [CrossRef]
- Rybak, A.S.; Gąbka, M. The influence of abiotic factors on the bloom-forming alga Ulva flexuosa (Ulvaceae, Chlorophyta): Possibilities for the control of the green tides in freshwater ecosystems. J. Appl. Phycol. 2018, 30, 1405–1416. [Google Scholar] [CrossRef]
- Suzuki, S.; Yamaguchi, H.; Hiraoka, M.; Kawachi, M. Mitochondrial and chloroplast genome sequences of Ulva ohnoi, a green-tide-forming macroalga in the Southern coastal regions of Japan. Mitochondrial DNA Part B 2018, 3, 765–767. [Google Scholar] [CrossRef]
- Puppin-Gonçalves, C.T.; Medeiros, L.L.L.; Lopes, P.F.M.; Freire, F.A.M. Ulva lactuca blooms through the eyes of fishers: Threats to vulnerable coastal communities. Mar. Pollut. Bull. 2024, 208, 117038. [Google Scholar] [CrossRef]
- Ning, L.; Yao, Z.; Zhu, B. Ulva (Enteromorpha) polysaccharides and oligosaccharides: A potential functional food source from green-tide-forming macroalgae. Mar. Drugs 2022, 20, 202. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Lens, P.N.L. Sustainable biorefining and bioprocessing of green seaweed (Ulva spp.) for the production of edible (ulvan) and non-edible (polyhydroxyalkanoate) biopolymeric films. Microb. Cell Factories 2023, 22, 140. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, Z.; Yao, L.; Feng, T.; Song, S.; Sun, M. Insights into the edible and biodegradable Ulvan-based films and coatings for food packaging. Foods 2023, 12, 1622. [Google Scholar] [CrossRef] [PubMed]
- Premarathna, A.D.; Ahmed, T.A.E.; Humayun, S.; Rjabovs, V.; Darko, C.N.S.; Critchley, A.T.; Hincke, M.T.; Tuvikene, R. Ulvans, sulfated β-d-galactans and xyloarabinogalactan: A comparative approach to extraction, structural characterization and antioxidant activity assessment. Food Hydrocoll. 2024, 156, 110321. [Google Scholar] [CrossRef]
- Mutavski, Z.; Jerkovic, I.; Nikolic, N.C.; Radman, S.; Flanjak, I.; Aladic, K.; Subaric, D.; Vulic, J.; Jokic, S. Comprehensive phytochemical profiling of Ulva lactuca from the adriatic sea. Int. J. Mol. Sci. 2024, 25, 11711. [Google Scholar] [CrossRef]
- Taibi, M.; Aouji, M.; Razzak, S.; Benchehida, H.; Lrhorfi, L.A.; Bengueddour, R.; Hassani, O. Antioxidant activity, nutritional composition, and proximate analysis of the Moroccan alga Ulva lactuca from the Mehdia coast. EJABF 2024, 28, 107–135. [Google Scholar] [CrossRef]
- Stedt, K.; Gustavsson, O.; Kollander, B.; Undeland, I.; Toth, G.B.; Pavia, H. Cultivation of Ulva fenestrata using herring production process waters increases biomass yield and protein content. Front. Mar. Sci. 2022, 9, 988523. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Bruhn, A.; Rasmussen, M.B.; Olesen, B.; Larsen, M.M.; Møller, H.B. Cultivation of Ulva lactuca with manure for simultaneous bioremediation and biomass production. J. Appl. Phycol. 2012, 24, 449–458. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Qarabai, F.A.K.; Shahabuddin, F.S.; Al-Shaikh, T.M.; Makharita, R.R. Antibacterial activity of Ulva/nanocellulose and Ulva/Ag/cellulose nanocomposites and both blended with fluoride against bacteria causing dental decay. Polymers 2023, 15, 1047. [Google Scholar] [CrossRef]
- Korkmaz, N. Extract optimization of Ulva lactuca L. and biological activities of optimized extracts. BMC Biotechnol. 2025, 25, 21. [Google Scholar] [CrossRef]
- Thavasi Alagan, V.; Nakulan Vatsala, R.; Sagadevan, I.; Subbiah, V.; Ragothaman, V. Effect of dietary supplementation of seaweed (Ulva lactuca) and Azolla on growth performance, haematological and serum biochemical parameters of Aseel chicken. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 58. [Google Scholar] [CrossRef]
- Ardita, N.F.; Mithasari, L.; Untoro, D.; Salasia, S.I.O. Potential antimicrobial properties of the Ulva lactuca extract against methicillin-resistant Staphylococcus aureus-infected wounds: A review. Vet. World 2021, 14, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Antequera, F.P.; Martos-Sitcha, J.A.; Reyna, J.M.; Moyano, F.J. Evaluation of the inclusion of the green seaweed Ulva ohnoi as an ingredient in feeds for gilthead sea bream (Sparus aurata) and European sea bass (Dicentrarchus labrax). Animals 2021, 11, 1684. [Google Scholar] [CrossRef] [PubMed]
- Tennakoon, P.; Chandika, P.; Yi, M.; Jung, W.-K. Marine-derived biopolymers as potential bioplastics, an eco-friendly alternative. iScience 2023, 26, 106404. [Google Scholar] [CrossRef]
- Ghadiryanfar, M.; Rosentrater, K.A.; Keyhani, A.; Omid, M. A review of macroalgae production, with potential applications in biofuels and bioenergy. Renew. Sustain. Energy Rev. 2016, 54, 473–481. [Google Scholar] [CrossRef]
- Gengiah, K.; Rajendran, N.; Al-Ghanim, K.A.; Govindarajan, M.; Gurunathan, B. Process and technoeconomic analysis of bioethanol production from residual biomass of marine macroalgae Ulva lactuca. Sci. Total Environ. 2023, 868, 161661. [Google Scholar] [CrossRef]
- Gu, W.; Wang, G. Absorptive process and biological activity of Ulva prolifera and algal bioremediation of coking effluent. BioResources 2020, 15, 2605–2620. [Google Scholar] [CrossRef]
- Qiu, S.; Ge, S.; Champagne, P.; Robertson, R.M. Potential of Ulva lactuca for municipal wastewater bioremediation and fly food. Desalin Water Treat. 2017, 91, 23–30. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.D.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microbial. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Rahhou, A.; Layachi, M.; Akodad, M.; El Ouamari, N.; Rezzoum, N.E.; Skalli, A.; Oudra, B.; El Bakali, M.; Kolar, M.; Imperl, J.; et al. The bioremediation potential of Ulva lactuca (Chlorophyta) causing green tide in Marchica Lagoon (NE Morocco, Mediterranean Sea): Biomass, heavy metals, and health risk assessment. Water 2023, 15, 1310. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; De Saeger, J.; Depuydt, S.; Asselman, J.; Janssen, C.; Heynderickx, P.M.; Wu, D.; Ronsse, F.; Tack, F.M.G.; et al. Harnessing green tide Ulva biomass for carbon dioxide sequestration. Rev. Environ. Sci. Biotechnol. 2024, 23, 1041–1061. [Google Scholar] [CrossRef]
- Vargas-Murga, L.; Dürrani, Ö.; Adams, J.; Steinhagen, S.; Turan, G.; Aruçi, E.; Morrison, L.; Wichard, T.; Kraan, S.; Shpigel, M. Metal(oid)s in Ulva—Should we be worried? Food Chem. 2025, 493, 145941. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Lundebye, A.-K.; Li, N.; Ergon, Å.; Pang, S.; Jiang, Y.; Zhu, W.; Zhao, Y.; Li, X.; Yao, L.; et al. Comparative assessment of food safety regulations and standards for arsenic, cadmium, lead, mercury and iodine in macroalgae used as food and feed in China and Europe. Trends Food Sci. Technol. 2023, 141, 104204. [Google Scholar] [CrossRef]
- Antil, S.; Abraham, J.S.; Sripoorna, S.; Maurya, S.; Dagar, J.; Makhija, S.; Bhagat, P.; Gupta, R.; Sood, U.; Lal, R.; et al. DNA barcoding, an effective tool for species identification: A review. Mol. Biol. Rep. 2023, 50, 761–775. [Google Scholar] [CrossRef]
- Kress, W.J.; Erickson, D.L. DNA barcodes: Genes, genomics, and bioinformatics. Proc. Natl. Acad. Sci. USA 2008, 105, 2761–2772. [Google Scholar] [CrossRef]
- Armeli Minicante, S.; Melton, J.T.; Spagnuolo, D.; Manghisi, A.; Genovese, G.; Morabito, M.; Lopez-Bautista, J. A DNA barcode inventory of the genus Ulva (Chlorophyta) along two Italian regions: Updates and considerations. Bot. Mar. 2025, 68, 33–51. [Google Scholar] [CrossRef]
- Holzinger, A.; Herburger, K.; Kaplan, F.; Lewis, L.A. Desiccation tolerance in the chlorophyte green alga Ulva compressa: Does cell wall architecture contribute to ecological success? Planta 2015, 242, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.-A.T.; Vieira, C.; Steinhagen, S.; Maggs, C.A.; Hiraoka, M.; Shimada, S.; Van Nguyen, T.; De Clerck, O.; Leliaert, F. An appraisal of Ulva (Ulvophyceae, Chlorophyta) taxonomy. J. Appl. Phycol. 2022, 34, 2689–2703. [Google Scholar] [CrossRef]
- Liu, F.; Chen, N.; Wang, H.; Li, J.; Wang, J.; Qu, F. Novel insights into chloroplast genome evolution in the green macroalgal genus Ulva (Ulvophyceae, Chlorophyta). Front. Plant Sci. 2023, 14, 1126175. [Google Scholar] [CrossRef] [PubMed]
- Osorio, H.; Tapia-Reyes, P.; Espinoza, D.; Laporte, D.; Gonzalez, A.; Castro-Nallar, E.; Moenne, A. The genome of the marine alga Ulva compressa (Chlorophyta) reveals protein-coding genes with similarity to plants and green microalgae, but also to animal, bacterial, and fungal genes. Int. J. Mol. Sci. 2022, 23, 7279. [Google Scholar] [CrossRef]
- Liu, Y.; Cummins, S.F.; Zhao, M. A genomics resource for 12 edible seaweeds to predict seaweed-secreted peptides with potential anti-cancer function. Biology 2022, 11, 1458. [Google Scholar] [CrossRef]
- Edsinger, E.; Kieras, M.; Pirro, S. The genome sequences of 118 taxonomically diverse eukaryotes of the salish sea. Biodivers. Genomes 2024, 2024, 1–4. [Google Scholar] [CrossRef]
- Tamura, K.; Bono, H. Genome sequence of the edible green alga Ulva prolifera, originating from the Yoshinogawa River in Japan. Microbiol. Resour. Announc. 2022, 11, e0043022. [Google Scholar] [CrossRef]
- Puginier, C.; Libourel, C.; Otte, J.; Skaloud, P.; Haon, M.; Grisel, S.; Petersen, M.; Berrin, J.G.; Delaux, P.M.; Dal Grande, F.; et al. Phylogenomics reveals the evolutionary origins of lichenization in chlorophyte algae. Nat. Commun. 2024, 15, 4452. [Google Scholar] [CrossRef]
- Hu, Z.-M.; Shan, T.-F.; Zhang, Q.-S.; Liu, F.-L.; Jueterbock, A.; Wang, G.; Sun, Z.-M.; Wang, X.-Y.; Chen, W.-Z.; Critchley, A.T.; et al. Kelp breeding in China: Challenges and opportunities for solutions. Rev. Aquacult. 2024, 16, 855–871. [Google Scholar] [CrossRef]
- Lan, J.; Liu, P.; Hu, X.; Zhu, S. Harmful algal blooms in eutrophic marine environments: Causes, monitoring, and treatment. Water 2024, 16, 2525. [Google Scholar] [CrossRef]

| # | Application | Advantages | Disadvantages |
|---|---|---|---|
| 1 | Human food | Rich source of proteins, polysaccharides (ulvan), vitamins, and minerals; traditional use in coastal diets; bioactive compounds with antioxidant properties | Risk of heavy metal, microplastic, and pollutant accumulation; variable nutritional composition depending on environment; sensory acceptability issues |
| 2 | Animal feed | Enhances growth performance, antioxidant status, and immune response; reduces oxidative stress; potential to replace fishmeal in aquaculture | Contaminated thalli may transfer heavy metals to higher trophic levels; digestibility and palatability challenges at high inclusion rates |
| 3 | Bioremediation | Effective uptake of nitrogen and phosphorus; accumulation of heavy metals; potential for integration in aquaculture systems | Effective uptake of nitrogen and phosphorus; accumulation of heavy metals; potential for integration in aquaculture systems |
| 4 | Biofuel and bioenergy | High carbohydrate content suitable for bioethanol and biogas production; renewable and sustainable resource | Low lipid content limits biodiesel potential; preprocessing and conversion technologies are costly; economic viability not yet proven |
| 5 | Pharmaceuticals and nutraceuticals | Ulvan and other metabolites show antiviral, antibacterial, anticoagulant, and immunomodulatory activities; potential for functional food ingredients | Lack of standardized extraction and purification methods; variability in bioactivity between species and locations; limited clinical validation |
| # | Criteria | Traditional Taxonomy | Molecular Taxonomy |
|---|---|---|---|
| 1 | Diagnostic basis | External morphology (e.g., thallus shape, size, branching) | Genetic sequences (rbcL, ITS, tufA) |
| 2 | Accessibility | High, requires minimal equipment | Limited, requires laboratory infrastructure |
| 3 | Cost | Low | Moderate to high |
| 4 | Speed | Rapid in field settings | Slower due to DNA extraction, PCR, and sequencing |
| 5 | Resolution in cryptic species | Poor, often fails to separate genetically distinct species | High, reveals cryptic lineages within morphologically similar taxa |
| 6 | Sensitivity to environment | High, morphology varies with conditions | Low, genetic identity remains stable |
| 7 | Suitability for biodiversity surveys | Useful for rapid assessments | Necessary for precise species inventories |
| 8 | Examples of challenges | U. lactuca vs. U. fasciata; U. compressa vs. U. intestinalis | Resolution of U. ohnoi, U. paschima, and U. gigantea complexes |
| # | Genome Assembly | Accession | Species | Genome Size (Mb) | References |
|---|---|---|---|---|---|
| 1 | ASM2307855v1 | PRJNA705045 | U. prolifera isolate: Qingdaoensis | 88.9 | - |
| 2 | ASM2450001v1 | PRJNA824527 | U. compressa isolate: HO_PTR_2022v1 | 80.8 | [94] |
| 3 | Ulvmu_WT_fa | PRJEB25750 | U. mutabilis strain wild-type | 98.5 | [95] |
| 4 | ASM3236096v1 | PRJNA894635 | U. armoricana isolate: IRGN 2022FHL053 | 127.7 | [96] |
| 5 | U_prolifera_HU1.0 | PRJDB13471 | U. prolifera isolate: Hashirijima_01 | 103.8 | [97] |
| 6 | ASM413825v1 | PRJNA484545 | U. prolifera Isolate: YS-2018 | 87.9 | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duong, T.T.; Nguyen, H.T.T.; Nguyen, H.T.; Nguyen, Q.T.; Nguyen, B.D.; Chuong, N.N.; Chu, H.D.; Tran, L.-S.P. Advances in Ulva Linnaeus, 1753 Research: From Structural Diversity to Applied Utility. Plants 2025, 14, 3052. https://doi.org/10.3390/plants14193052
Duong TT, Nguyen HTT, Nguyen HT, Nguyen QT, Nguyen BD, Chuong NN, Chu HD, Tran L-SP. Advances in Ulva Linnaeus, 1753 Research: From Structural Diversity to Applied Utility. Plants. 2025; 14(19):3052. https://doi.org/10.3390/plants14193052
Chicago/Turabian StyleDuong, Thanh Thuy, Hang Thi Thuy Nguyen, Hoai Thi Nguyen, Quoc Trung Nguyen, Bach Duc Nguyen, Nguyen Nguyen Chuong, Ha Duc Chu, and Lam-Son Phan Tran. 2025. "Advances in Ulva Linnaeus, 1753 Research: From Structural Diversity to Applied Utility" Plants 14, no. 19: 3052. https://doi.org/10.3390/plants14193052
APA StyleDuong, T. T., Nguyen, H. T. T., Nguyen, H. T., Nguyen, Q. T., Nguyen, B. D., Chuong, N. N., Chu, H. D., & Tran, L.-S. P. (2025). Advances in Ulva Linnaeus, 1753 Research: From Structural Diversity to Applied Utility. Plants, 14(19), 3052. https://doi.org/10.3390/plants14193052







