Response Mechanism of Litter to Soil Water Conservation Functions Under the Density Gradient of Robinia pseudoacacia L. Forests in the Loess Plateau of the Western Shanxi Province
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Research Methods
2.2.1. Plot Setup and Investigation
2.2.2. Collection and Determination of Litter Samples
Canopy Interception Capacity
Water Holding Capacity of Litter
2.2.3. Soil Sample Collection and Determination
2.2.4. Determination of Soil Nutrient Indicators
2.3. Data Analysis and Processing
3. Results
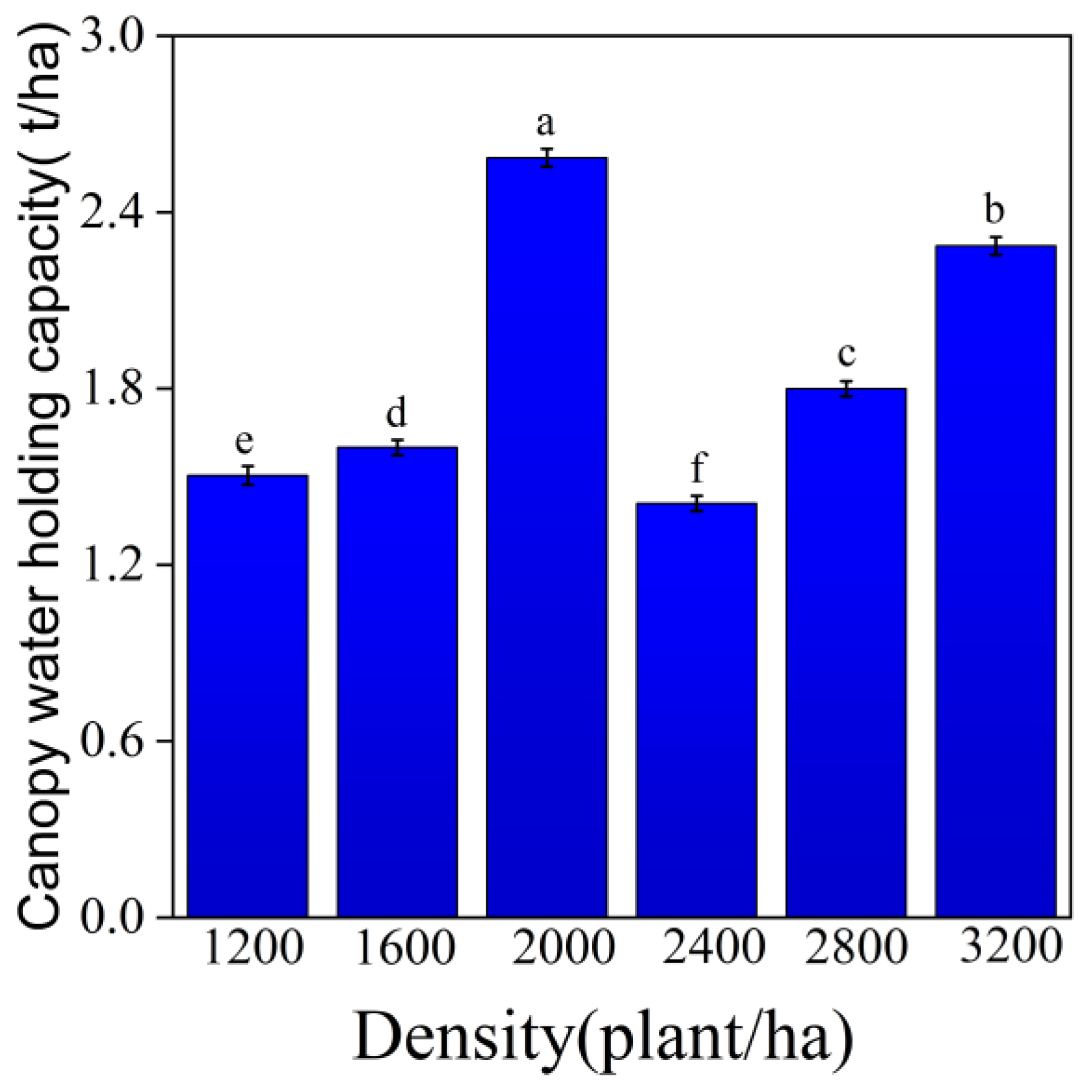
3.1. Water Conservation Function
3.1.1. Canopy Interception Capacity
3.1.2. Litter Water-Holding Capacity
3.1.3. Soil Layer Water-Holding Capacity
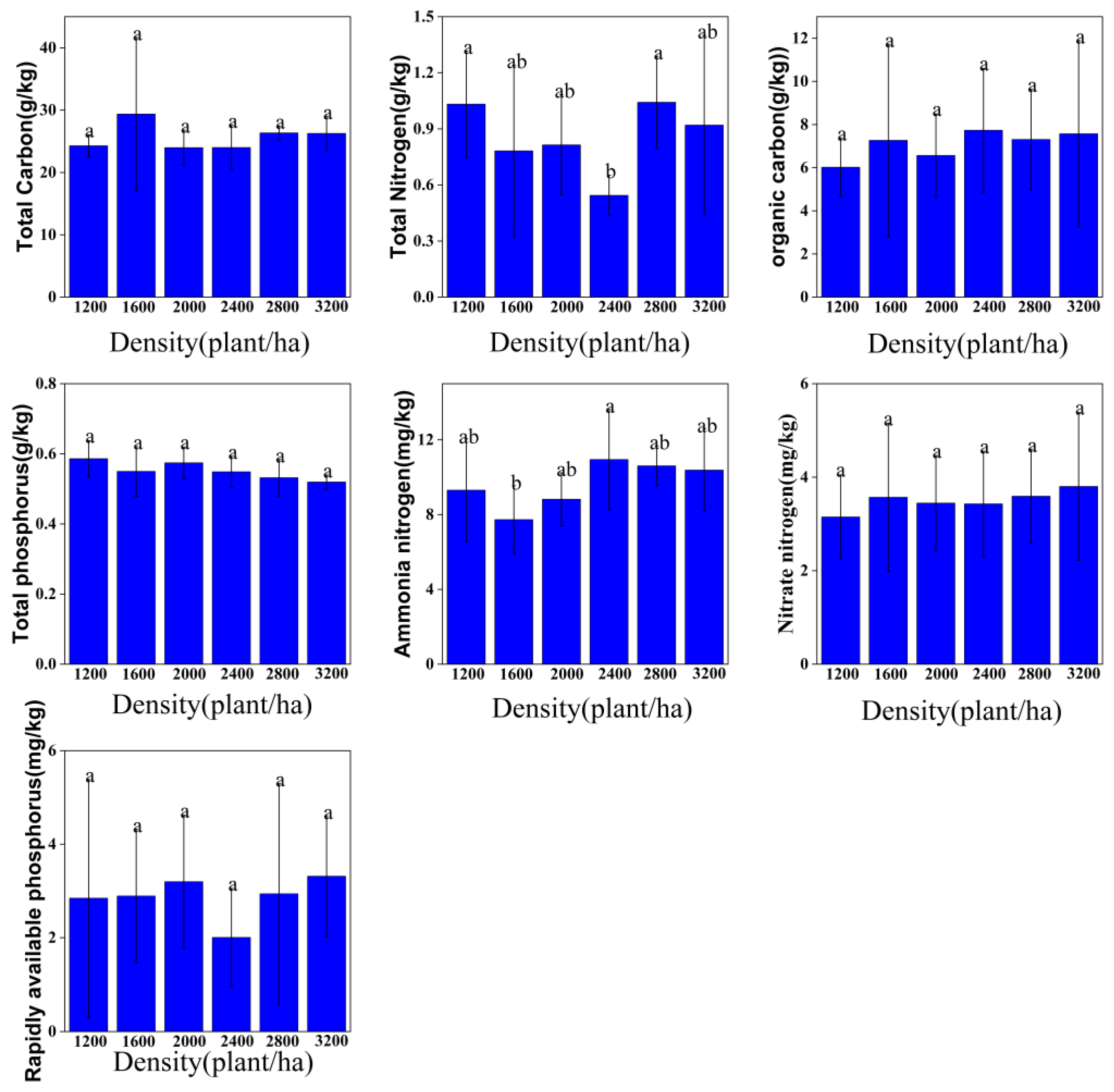
3.2. Soil Conservation Function
3.2.1. Soil Physical Properties
3.2.2. Soil Nutrient Indicators
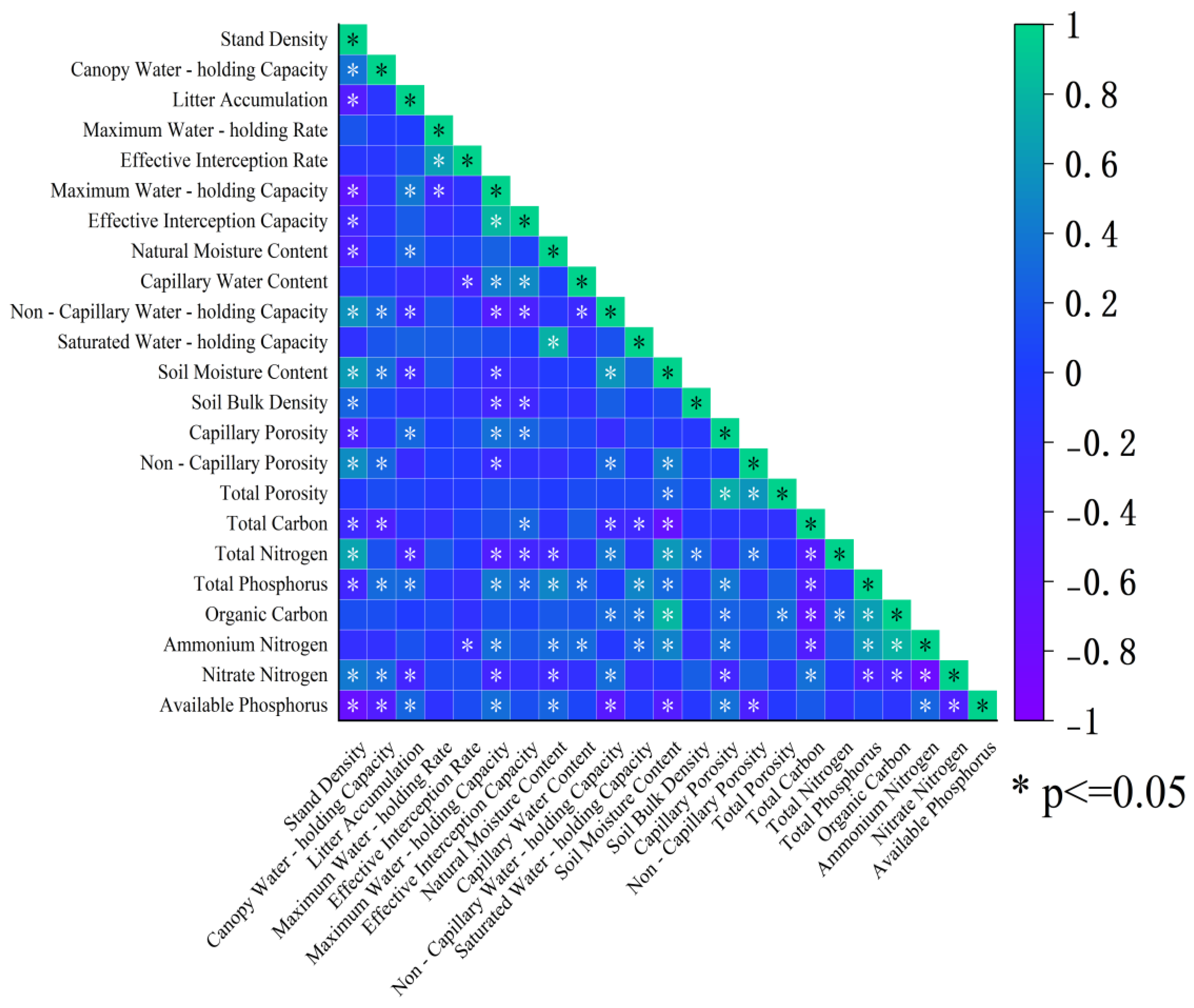
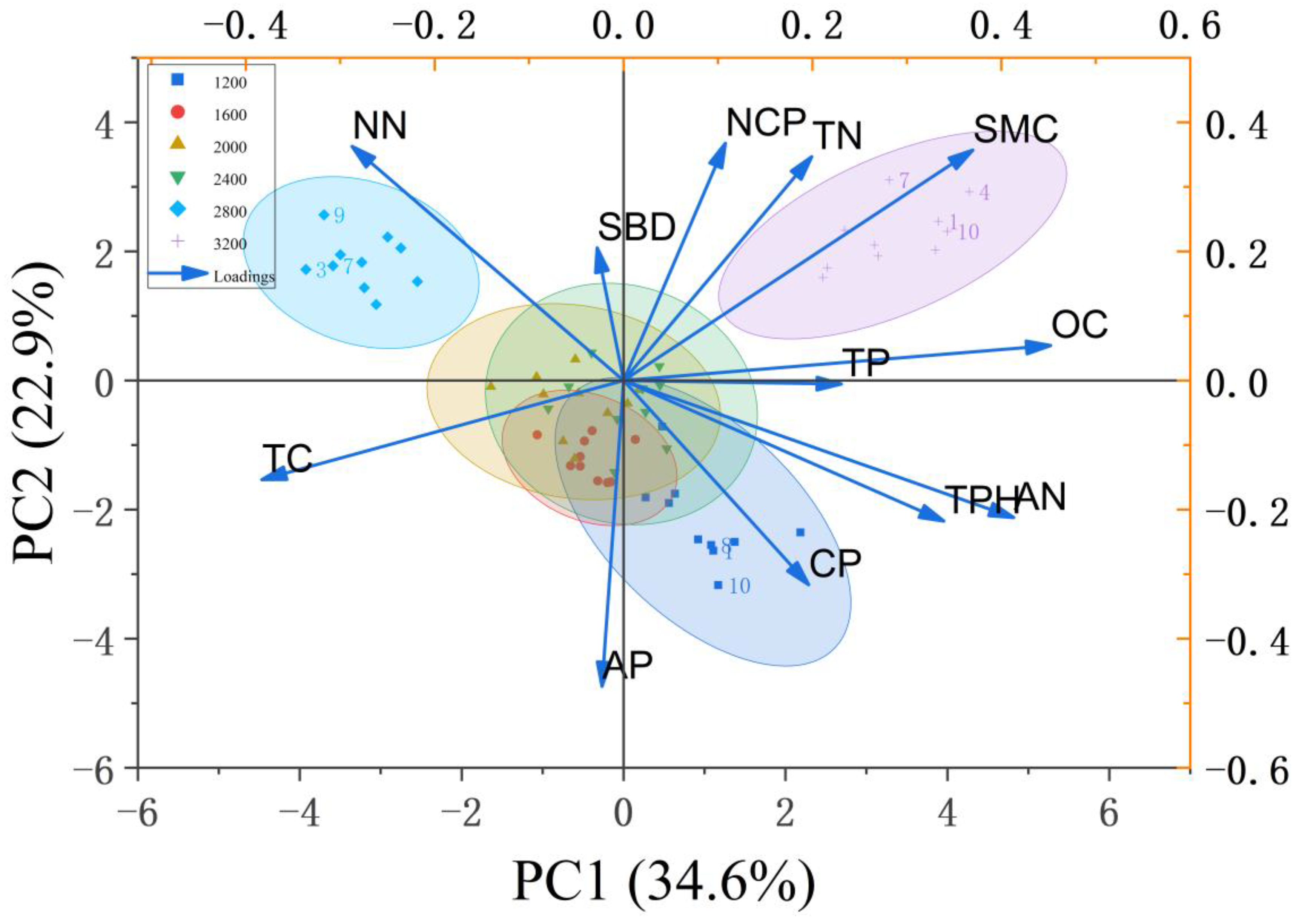
3.3. Correlation Analysis Between Litter Indicators and Soil Physicochemical Properties of Robinia pseudoacacia L. Forests
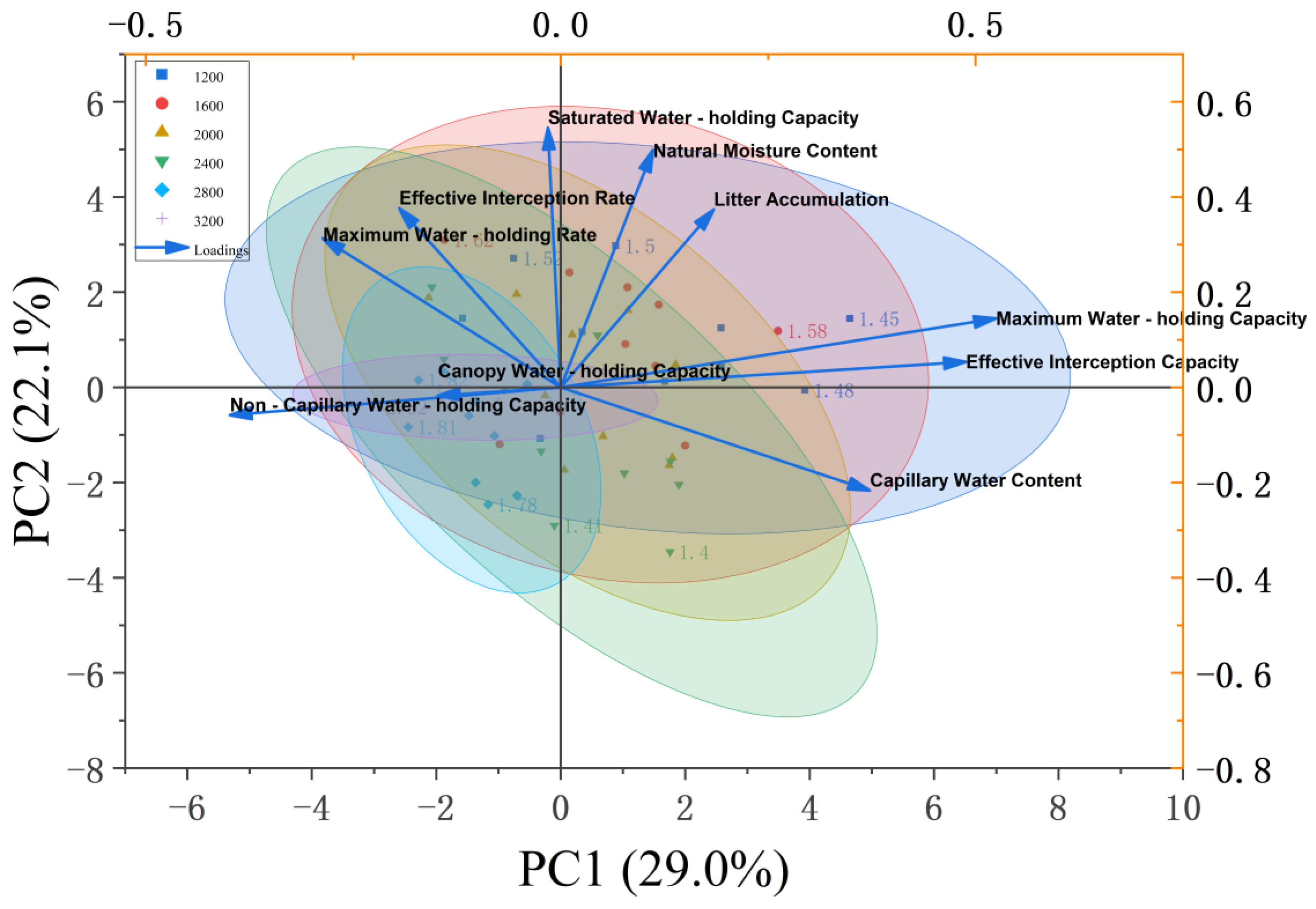
3.4. Principal Component Analysis of Water-Holding Capacity-Related Indicators of Litter
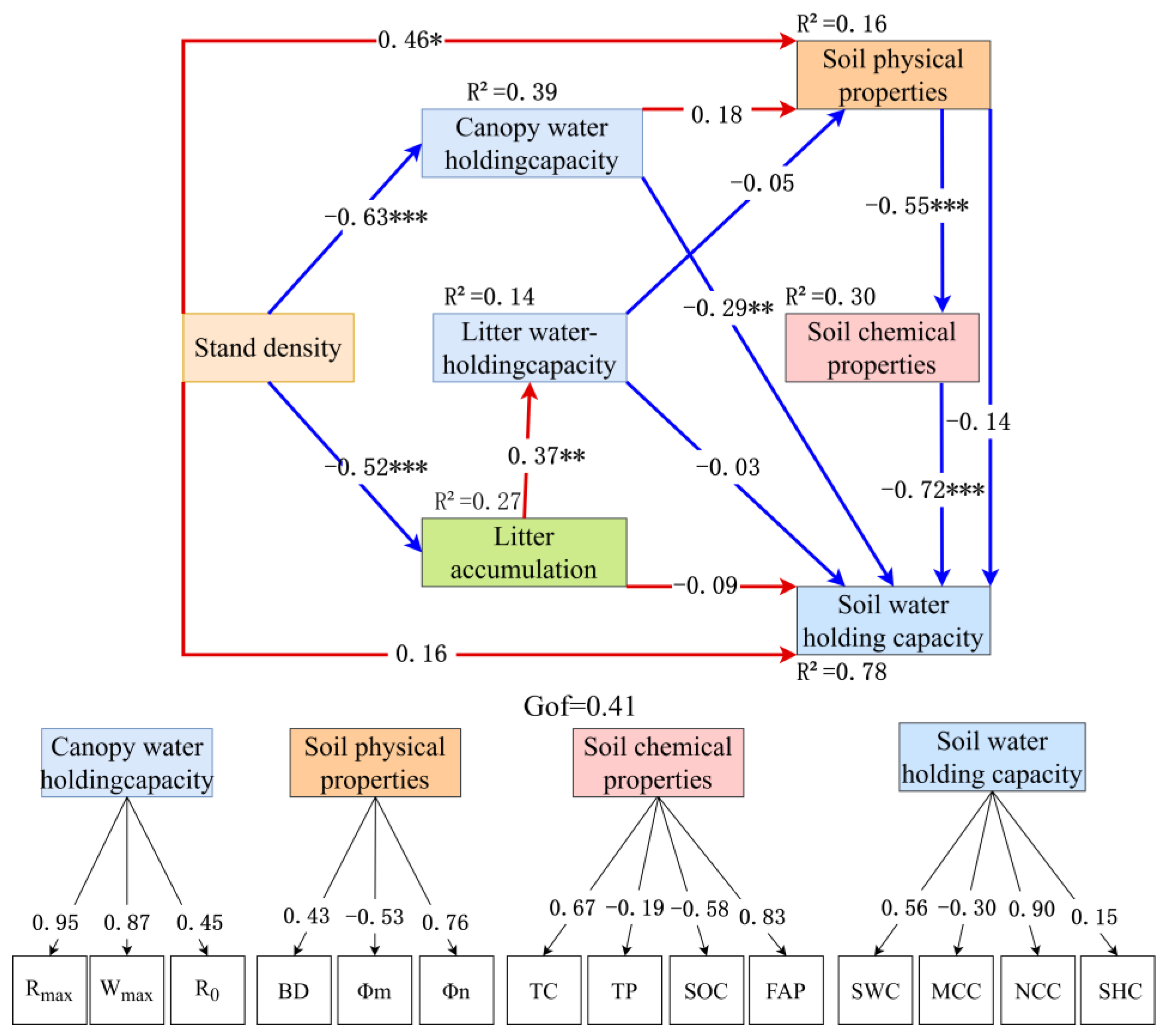
3.5. Structural Equation Model Between Canopy Interception Index, Litter Water Holding Index, Soil Physicochemical Properties Index, and Soil Nutrient Index
4. Discussion
4.1. Density Response Mechanism of Water Conservation Function in the Litter Layer
4.2. Response Law of Soil Physical and Chemical Properties to Density Gradient
4.3. Density Dependence and Driving Factors of Soil Nutrient Cycling
5. Conclusions
- (1)
- Litter hydrological function exhibits a critical density threshold: stands with a density of <1600 plants/ha maintain high litter accumulation (>6 t/ha) and water-holding capacity, forming a positive cycle of accumulation-decomposition-water retention. However, when density exceeds 2400 plants/ha, canopy shading reduces the litter decomposition rate by 56%, leading to a significant decline in hydrological regulation capacity.
- (2)
- Soil pore structure demonstrates density-dependent balance: low-density stands (≤2000 plants/ha) maintain capillary porosity >47% through litter-derived organic matter, ensuring continuous water transmission. In contrast, density >2400 plants/ha triggers a compensatory increase in non-capillary pores, disrupting the balance between water-holding and infiltration functions.
- (3)
- Nutrient cycling patterns shift with density: stands < 2000 plants/ha maintain efficient phosphorus release (68%) with a stable litter C/N ratio (25–30), while high-density stands (>2800 plants/ha) exhibit a “nitrogen accumulation-phosphorus limitation” pattern, with a C/N ratio > 30 and ammonification dominating (>60%).
- (4)
- Structural equation modeling confirms that litter accumulation mediates 68% of the density effect on soil water-holding capacity. The optimal density range (1200–1600 plants/ha) balances water conservation (capillary water-holding capacity 4788–4863 t/ha) and nutrient availability (available phosphorus > 2.1 mg/kg), providing a precise density regulation paradigm for the near-natural management of artificial forests on the Loess Plateau.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wang, J.; Li, Y.H.; Zhang, F. Regulation Mechanism of Forest and Grass Vegetation Structure Based on Water Conservation Function in the Loess Plateau. Chin. Soil Water Conserv. 2023, 37, 89–96. [Google Scholar] [CrossRef]
- Yu, B. Study on Density Regulation Model of Artificial Forests in Western Shanxi Based on Water Production Function. Ph.D. Dissertation, Beijing Forestry University, Beijing, China, 2010. [Google Scholar]
- Cai, L. Effect of Roots of Artificial Robinia pseudoacacia L. Forests on Soil Infiltration in the Loess Plateau. Master’s Thesis, Northwest A&F University, Yangling, China, 2024. [Google Scholar] [CrossRef]
- Han, A.L.; Ye, H.; Wang, H.J.; Liu, X.M. Effects of Interplanting Patterns under Robinia pseudoacacia L. Forests on Soil Physical and Chemical Properties and Nutrients. J. Henan For. Sci. Technol. 2025, 45, 14–18. [Google Scholar]
- Hou, G.R.; Bi, H.X.; Wei, X.; Li, Y.F. Litter and Soil Water Conservation Functions of Three Forest Types in the Loess Plateau Gully Region. J. Soil Water Conserv. 2018, 32, 357–363+371. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Z.; Zhu, B.; Sun, Y. Impacts of Mixed Litter Decomposition from Robinia pseudoacacia L. and Other Tree Species on C Loss and Nutrient Release in the Loess Plateau of China. J. For. Res. 2016, 27, 525–532. [Google Scholar] [CrossRef]
- Litt, G.F.; Ogden, F.L.; Mojica, A.; Hendrickx, J.M.H.; Kempema, E.W.; Gardner, C.B.; Bretfeld, M.; Regina, J.A.; Harrison, J.B.J.; Cheng, Y.; et al. Land Cover Effects on Soil Infiltration Capacity Measured Using Plot Scale Rainfall Simulation in Steep Tropical Lowlands of Central Panama. Hydrol. Process. 2020, 34, 878–897. [Google Scholar] [CrossRef]
- Liu, Y.P.; Wang, G.X.; Hu, Z.Y.; Guo, L.M. Litter Storage and Water-Holding Capacity of Typical Forests in Mountainous Area of Southwest China. Ying Yong Sheng Tai Xue Bao 2022, 33, 2113–2120. [Google Scholar] [CrossRef]
- Zhu, J.P.; Wang, F.; Gao, J.R. Water Conservation Functions of Different Forest Vegetations in Caijiachuan Watershed, Jixian County. Res. Soil Water Conserv. 2006, 13, 111–113+125. [Google Scholar]
- Xiang, Y.; Chen, H.; Feng, W.; Wen, Y.; Xie, Y.; Cheng, M.; Li, H. Nitrogen and Microelements Co-Drive the Decomposition of Typical Grass Litter in the Loess Plateau, China. Plants 2024, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.Y.; Maguire, D.A. Structural Equation Modeling: Theory and Applications in Forest Management. Int. J. For. Res. 2012, 2012, 263953. [Google Scholar] [CrossRef]
- Cheng, Z.; Yu, B.; Fu, S.; Liu, G. Comparative Evaluation of Three Infiltration Models for Runoff and Erosion Prediction in the Loess Plateau Region of China. Hydrol. Res. 2018, 49, 1484–1497. [Google Scholar] [CrossRef]
- Gao, Z.; Niu, F.; Wang, Y.; Lin, Z.; Luo, J.; Liu, M. Root-Induced Changes to Soil Water Retention in Permafrost Regions of the Qinghai-Tibet Plateau, China. J. Soils Sediments 2018, 18, 791–803. [Google Scholar] [CrossRef]
- Liang, H.; Meng, Z.; Li, Z.; Liu, G. The Effect of Robinia pseudoacacia L. Plantation on Soil Desiccation across Different Precipitation Zones of the Loess Plateau, China. Forests 2022, 13, 321. [Google Scholar] [CrossRef]
- Zhai, B.; Sun, M.; Shen, X.; Zhu, Y.; Li, G.; Du, S. Effects of Stand Density on Growth, Soil Water Content and Nutrients in Black Locust Plantations in the Semiarid Loess Hilly Region. Sustainability 2024, 16, 376. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Peng, X.; Wu, Q.; Peñuelas, J.; Peng, Y.; Li, Z.; Heděnec, P.; Yuan, C.; Wu, F.; et al. A Synthesis on the Spatial Patterns and Driving Factors of Water-Holding Capacity of Forest Litter Layer across China. J. Hydrol. 2025, 659, 133272. [Google Scholar] [CrossRef]
- Mallari, K.J.B.; Arguelles, A.C.C.; Kim, H.; Aksoy, H.; Kavvas, M.L.; Yoon, J. Comparative Analysis of Two Infiltration Models for Application in a Physically Based Overland Flow Model. Environ. Earth Sci. 2015, 74, 1579–1587. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Wang, Y.; Sun, Y.; Chen, Y. High Stand Density Promotes Soil Organic Carbon Sequestration in Robinia pseudoacacia L. Plantations in the Hilly and Gully Region of the Loess Plateau in China. Agric. Ecosyst. Environ. 2023, 343, 108256. [Google Scholar] [CrossRef]
- Ile, O.J.; Aguilos, M.; Morkoc, S.; Heitman, J.; King, J.S. Root Biomass Distribution and Soil Physical Properties of Short-Rotation Coppice American Sycamore (Platanus occidentalis L.) Grown at Different Planting Densities. Forests 2021, 12, 1806. [Google Scholar] [CrossRef]
- Wei, X.; Liang, W. Regulation of Stand Density Alters Forest Structure and Soil Moisture during Afforestation with Robinia pseudoacacia L. and Pinus tabulaeformis Carr. on the Loess Plateau. For. Ecol. Manag. 2021, 491, 119196. [Google Scholar] [CrossRef]
- Primo, A.A.; Araújo, M.D.M.; Silva, K.F.; Silva, L.A.; Pereira, G.d.A.C.; Fernandes, F.É.P.; Pompeu, R.C.F.F.; Natale, W.; de Souza, H.A. Litter Production and Nutrient Deposition from Native Woody Species in the Brazilian Semi-Arid Region. Agrofor. Syst. 2021, 95, 1459–1464. [Google Scholar] [CrossRef]
- Guo, J.J.; Zhang, L.T.; Che, J.F.; Jiao, H.H.; Ru, W.M.; Bai, Z.H. Community Structure of Forsythia suspensa and Its Influencing Factors in the Southern Taihang Mountains. Acta Ecol. Sin. 2021, 41, 8589–8601. [Google Scholar]
- Zhou, Q.Z.; Bi, H.X.; Kong, L.X.; Hou, G.R.; Wei, X.; Wei, X.Y. Hydrological and Ecological Functions of Litter Layers in Robinia pseudoacacia L. Forests with Different Densities in the Loess Region of Western Shanxi. J. Soil Water Conserv. 2018, 32, 115–121. [Google Scholar] [CrossRef]
- Qu, H.F.; Dong, X.B.; Zhang, T.; Tang, G.H.; Ma, X.B.; Guan, H.W.; Wang, Z.Y.; Yuan, J.F. Changes in Hydrological Effects of Litter after Supplementary Planting Improvement of Low-Quality Betula platyphylla Forests in the Greater Khingan Mountains. J. Northeast For. Univ. 2017, 45, 14–19. [Google Scholar] [CrossRef]
- GB/T 9837—1988; Standardization Administration of China (SAC). Soil—Determination of Total Phosphorus. China Standards Press: Beijing, China, 1988.
- GB/T 32737—2016; Standardization Administration of China (SAC). Soil—Determination of Nitrate Nitrogen—Ultraviolet Spectrophotometry Method. China Standards Press: Beijing, China, 2016.
- ISO 14255:1998; International Organization for Standardization (ISO). Soil Quality—Determination of Nitrate Nitrogen, Ammonium Nitrogen and Total Soluble Nitrogen in Air-Dry Soils Using Calcium Chloride Solution as Extractant. ISO: Geneva, Switzerland, 1998.
- Wang, S.Y.; Yu, X.X.; Jia, G.D.; Somg, S.M.; Xu, J.; Li, Q.Y. Hydrological Effects of Litter in Main Artificial Forests in the Mountainous Area of Beijing. Chin. J. Soil Water Conserv. Sci. 2011, 9, 42–47. [Google Scholar] [CrossRef]
- Geng, Q.; Wang, H.Y.; Zhang, M.N.; Zheng, Y.L. Research Progress and Trends in Factors Affecting Water-Holding Properties of Forest Litter. Ecol. Sci. 2020, 39, 220–226. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Lu, Y.; Wang, X.J.; Wei, B.; Liu, Y.; Jin, H.; Liu, F.; Wang, K.Y.; Wang, Y.N. Research Progress and Trends in Hydrological Effects of Forest Litter. Chin. For. By-Products Spec. 2021, 1, 113–114+116. [Google Scholar] [CrossRef]
- Zhu, Q. Decomposition Characteristics of Young Stand Litter Under Different Site Indexes and Densities of Cunninghamia lanceolata and Its Influencing Mechanisms. Master’s Thesis, Fujian Agriculture and Forestry University, Fuzhou, China, 2024. [Google Scholar] [CrossRef]
- Li, J.H. Effects of Different Vegetation Restoration Measures on Herbaceous Communities and Soil Carbon and Nitrogen in the Loess Plateau. Master’s Thesis, Northwest University, Xi’an, China, 2022. [Google Scholar]
- Hopmans, J.W. Soil Physical Properties, Processes, and Associated Root-Soil Interactions. In Dryland Ecohydrology; Springer: Dordrecht, The, Netherlands, 2019; pp. 49–69. [Google Scholar] [CrossRef]
- Bradford, M.A.; Wieder, W.R.; Bonan, G.B.; Fierer, N.; Raymond, P.A.; Crowther, T.W. Managing Uncertainty in Soil Carbon Feedbacks to Climate Change. Nat. Clim. Chang. 2016, 6, 751–758. [Google Scholar] [CrossRef]
- Qu, Y.Y. Effects of Vegetation Restoration on Soil Hydraulic Properties in Different Regions of the Loess Plateau. Master’s Thesis, Northwest A&F University, Yangling, China, 2024. [Google Scholar] [CrossRef]
- Guan, L.M.; Wu, Z.X.; Zhou, Z.D.; Xie, G.S.; Yang, C. Analysis of Soil Organic Carbon Pool and Its Influencing Factors in Rubber Plantations with Different Ages in Western Hainan. J. Anhui Agric. Sci. 2012, 40, 13437–13440. [Google Scholar] [CrossRef]
- Schimel, D.S. Terrestrial Ecosystems and the Carbon Cycle. Glob. Chang. Biol. 1995, 1, 77–91. [Google Scholar] [CrossRef]
- Yang, X. Study on the Relationship Between Stand Structure and Soil and Water Conservation Function Based on Structural Equation Model. Ph.D. Dissertation, Beijing Forestry University, Beijing, China, 2020. [Google Scholar] [CrossRef]
- Wang, C.; Xue, L.; Dong, Y.; Jiao, R. Effects of Stand Density on Soil Microbial Community Composition and Enzyme Activities in Subtropical Cunninghamia lanceolata (Lamb.) Hook Plantations. For. Ecol. Manag. 2021, 479, 118559. [Google Scholar] [CrossRef]
- Mooney, H.A.; Dunn, E.L. Convergent Evolution of Mediterranean-Climate Evergreen Sclerophyll Shrubs. Evolution 1970, 24, 292–303. [Google Scholar] [CrossRef]
- Vitousek, P. Nutrient Cycling and Nutrient Use Efficiency. Am. Nat. 1982, 119, 553–572. [Google Scholar] [CrossRef]
- Hou, G.; Bi, H.; Wang, N.; Cui, Y.; Ma, X.; Zhao, D.; Wang, S. Optimizing the Stand Density of Robinia pseudoacacia L. L. Forests of the Loess Plateau, China, Based on Response to Soil Water and Soil Nutrient. Forests 2019, 10, 663. [Google Scholar] [CrossRef]
- Wang, C.; Xue, L.; Dong, Y.; Hou, L.; Wei, Y.; Jiao, R. Responses of Soil Microbial Community Structure to Stand Densities of Chinese Fir Plantations. J. For. Res. 2019, 24, 162–167. [Google Scholar] [CrossRef]
- Bell, R.W. Land Restoration: Principles. In Encyclopedia of Soil Science; CRC Press: Boca Raton, FL, USA, 2006; pp. 978–981. [Google Scholar] [CrossRef]
- Amantai, N.R.; Meng, Y.Y.; Tang, Z.Y. Effects of Artificial Forest Planting and Growth on Ecosystem Carbon Sequestration and Hydrological Regulation Functions in the Loess Plateau—Evidence from Remote Sensing Time Series Analysis. Acta Ecol. Sin. 2024, 44, 7322–7333. [Google Scholar] [CrossRef]
| Plot Number | Density (stems/ha) | DBH (cm) | Tree Height (m) | Crown Width (m) | Altitude (m) | Slope Gradient (°) | Slope Aspect (°) | Aspect Index |
|---|---|---|---|---|---|---|---|---|
| 1 | 1200 | 13.59 | 11.88 | 4.19 | 1022.4 | 31 | 70 | 0.12 |
| 2 | 1200 | 13.72 | 12.05 | 4.24 | 1041.3 | 15 | 50 | 0.03 |
| 3 | 1200 | 9.55 | 9.64 | 3.57 | 1076 | 25 | 130 | 0.59 |
| 4 | 1200 | 11.02 | 9.21 | 3.62 | 1086.3 | 15 | 40 | 0.01 |
| 5 | 1200 | 8.43 | 8.13 | 2.96 | 1088.6 | 40 | 22 | 0 |
| 6 | 1600 | 10.67 | 11.28 | 3.95 | 1080 | 25 | 230 | 0.97 |
| 7 | 1600 | 12.26 | 12.7 | 4.11 | 1077.9 | 20 | 50 | 0.03 |
| 8 | 1600 | 13.71 | 12.05 | 4.39 | 1084.2 | 22.5 | 23 | 0 |
| 9 | 1600 | 7.76 | 8.21 | 3.69 | 1109.7 | 25 | 90 | 0.25 |
| 10 | 1600 | 9.87 | 8.51 | 4.38 | 1056 | 14 | 150 | 0.75 |
| 11 | 2000 | 8.71 | 8.39 | 3.57 | 1061.8 | 20 | 200 | 0.99 |
| 12 | 2000 | 9.92 | 9.74 | 3.83 | 1096.2 | 18 | 200 | 0.99 |
| 13 | 2000 | 11.33 | 11.24 | 4.1 | 1096 | 20 | 130 | 0.59 |
| 14 | 2000 | 11.06 | 8.7 | 3.93 | 1064.4 | 37 | 90 | 0.25 |
| 15 | 2000 | 7.89 | 6.72 | 3.06 | 1081.6 | 36 | 180 | 0.93 |
| 16 | 2400 | 10.2 | 10.19 | 4.47 | 1116.6 | 15 | 290 | 0.59 |
| 17 | 2400 | 10.26 | 11.46 | 4.18 | 1120 | 15 | 110 | 0.41 |
| 18 | 2400 | 10.52 | 9.97 | 4.19 | 1116 | 16 | 50 | 0.03 |
| 19 | 2400 | 12.58 | 12.74 | 4.68 | 1114 | 30 | 270 | 0.75 |
| 20 | 2400 | 11.2 | 11.53 | 4.64 | 1127.8 | 28 | 105 | 0.37 |
| 21 | 2800 | 9.54 | 6.36 | 3.36 | 1152.8 | 31 | 180 | 0.93 |
| 22 | 2800 | 11.14 | 9.61 | 4.41 | 1148 | 27 | 65 | 0.09 |
| 23 | 2800 | 10.99 | 11.02 | 4.02 | 1112 | 27 | 160 | 0.82 |
| 24 | 2800 | 8.3 | 7.03 | 3.22 | 1111.7 | 30 | 240 | 0.93 |
| 25 | 2800 | 10.05 | 8.22 | 3.54 | 1113 | 26 | 60 | 0.07 |
| 26 | 3200 | 9.57 | 8.7 | 3.73 | 1116.2 | 24 | 140 | 0.67 |
| 27 | 3200 | 10.71 | 11.11 | 3.93 | 1118.9 | 26 | 75 | 0.15 |
| 28 | 3200 | 11.28 | 10.92 | 3.64 | 1120 | 30 | 240 | 0.93 |
| 29 | 3200 | 9.96 | 9.46 | 3.66 | 1135.8 | 23 | 240 | 0.93 |
| 30 | 3200 | 11.71 | 8.36 | 3.8 | 1158.1 | 20 | 210 | 1 |
| Density (stems/ha) | Litter Stock (t/ha) | Maximum Water-Holding Capacity of Litter (%) | Effective Interception Rate of Litter (%) | Maximum Water-Holding Amount of Litter (t/ha) | Effective Interception Amount of Litter (t/ha) |
|---|---|---|---|---|---|
| 1200 | 6.25 ± 4.16 a | 356.34 ± 88.54 a | 158.71 ± 35.49 a | 20.58 ± 10.85 a | 10.12 ± 7.37 a |
| 1600 | 6.37 ± 4.09 a | 331.07 ± 62.36 a | 141.74 ± 50.08 a | 19.37 ± 9.58 a | 8.14 ± 4.42 a |
| 2000 | 6.08 ± 2.42 a | 317.90 ± 54.14 a | 138.90 ± 45.13 a | 18.70 ± 7.08 a | 8.09 ± 3.60 a |
| 2400 | 5.17 ± 2.24 a | 322.31 ± 84.48 a | 132.09 ± 49.89 a | 15.27 ± 4.65 a | 6.22 ± 2.49 a |
| 2800 | 2.93 ± 0.72 a | 362.49 ± 21.32 a | 167.43 ± 10.31 a | 10.57 ± 2.19 a | 4.96 ± 1.52 a |
| 3200 | 3.36 ± 0.72 a | 351.64 ± 6.24 a | 132.98 ± 15.55 a | 11.84 ± 2.77 a | 4.42 ± 0.45 a |
| Density/(stems/ha) | Capillary Water-Holding Capacity/(t/ha) | Non-Capillary Water-Holding Capacity/(t/ha) | Saturated Water-Holding Capacity/(t/ha) |
|---|---|---|---|
| 1200 | 4863.28 ± 360.36 a | 361.89 ± 104.71 a | 5225.17 ± 314.64 a |
| 1600 | 4788.87 ± 164.82 a | 480.86 ± 171.45 a | 5269.73 ± 154.78 a |
| 2000 | 4702.63 ± 187.00 a | 460.55 ± 136.43 a | 5163.18 ± 157.12 a |
| 2400 | 4654.73 ± 306.79 a | 437.88 ± 117.63 a | 5092.62 ± 299.71 a |
| 2800 | 4493.80 ± 188.65 a | 525.98 ± 31.84 a | 5019.78 ± 170.01 a |
| 3200 | 4649.67 ± 279.74 a | 621.01 ± 300.10 a | 5270.67 ± 20.36 a |
| Density/(stems/ha) | Soil Water Content/% | Soil Bulk Density/(g/cm3) |
|---|---|---|
| 1200 | 10.56 ± 0.22 b | 1.195 ± 0.03 a |
| 1600 | 10.31 ± 0.19 c | 1.195 ± 0.03 a |
| 2000 | 9.95 ± 0.15 d | 1.205 ± 0.03 a |
| 2400 | 10.56 ± 0.2 c | 1.215 ± 0.03 a |
| 2800 | 10.31 ± 0.1 b | 1.211 ± 0.02 a |
| 3200 | 15.2 ± 0.25 a | 1.213 ± 0.02 a |
| Density/(stems/ha) | Capillary Porosity/% | Non-Capillary Porosity/% | Total Porosity/% |
|---|---|---|---|
| 1200 | 48.63 ± 3.60 a | 3.62 ± 1.05 a | 52.25 ± 3.14 a |
| 1600 | 47.89 ± 1.65 a | 4.81 ± 1.71 a | 52.69 ± 1.54 a |
| 2000 | 47.03 ± 1.87 a | 4.61 ± 1.36 a | 51.63 ± 1.57 a |
| 2400 | 46.55 ± 3.07 a | 4.38 ± 1.18 a | 50.92 ± 2.99 a |
| 2800 | 44.94 ± 1.89 a | 5.26 ± 0.32 a | 50.19 ± 1.70 a |
| 3200 | 46.50 ± 2.80 a | 6.21 ± 3.00 a | 52.70 ± 0.20 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Yang, J.; Zhang, J.; Zhang, B. Response Mechanism of Litter to Soil Water Conservation Functions Under the Density Gradient of Robinia pseudoacacia L. Forests in the Loess Plateau of the Western Shanxi Province. Plants 2025, 14, 3042. https://doi.org/10.3390/plants14193042
Zhang Y, Yang J, Zhang J, Zhang B. Response Mechanism of Litter to Soil Water Conservation Functions Under the Density Gradient of Robinia pseudoacacia L. Forests in the Loess Plateau of the Western Shanxi Province. Plants. 2025; 14(19):3042. https://doi.org/10.3390/plants14193042
Chicago/Turabian StyleZhang, Yunchen, Jianying Yang, Jianjun Zhang, and Ben Zhang. 2025. "Response Mechanism of Litter to Soil Water Conservation Functions Under the Density Gradient of Robinia pseudoacacia L. Forests in the Loess Plateau of the Western Shanxi Province" Plants 14, no. 19: 3042. https://doi.org/10.3390/plants14193042
APA StyleZhang, Y., Yang, J., Zhang, J., & Zhang, B. (2025). Response Mechanism of Litter to Soil Water Conservation Functions Under the Density Gradient of Robinia pseudoacacia L. Forests in the Loess Plateau of the Western Shanxi Province. Plants, 14(19), 3042. https://doi.org/10.3390/plants14193042





