Synergistic Lanthanum-Cysteine Chelate and Corn Steep Liquor Mitigate Cadmium Toxicity in Chinese Cabbage via Physiological–Microbial Coordination
Abstract
1. Introduction
2. Results and Discussion
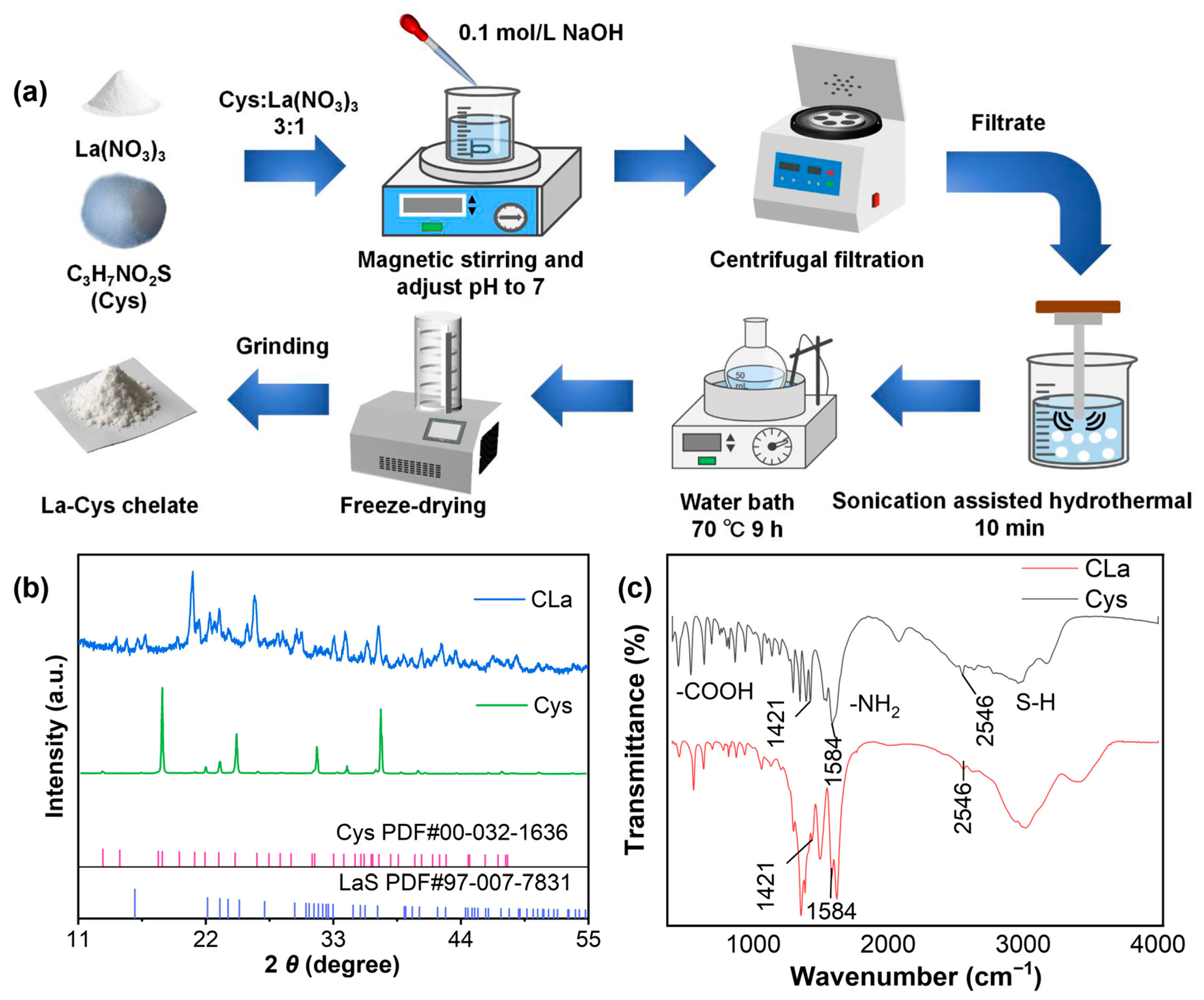
2.1. Characterization of CLa
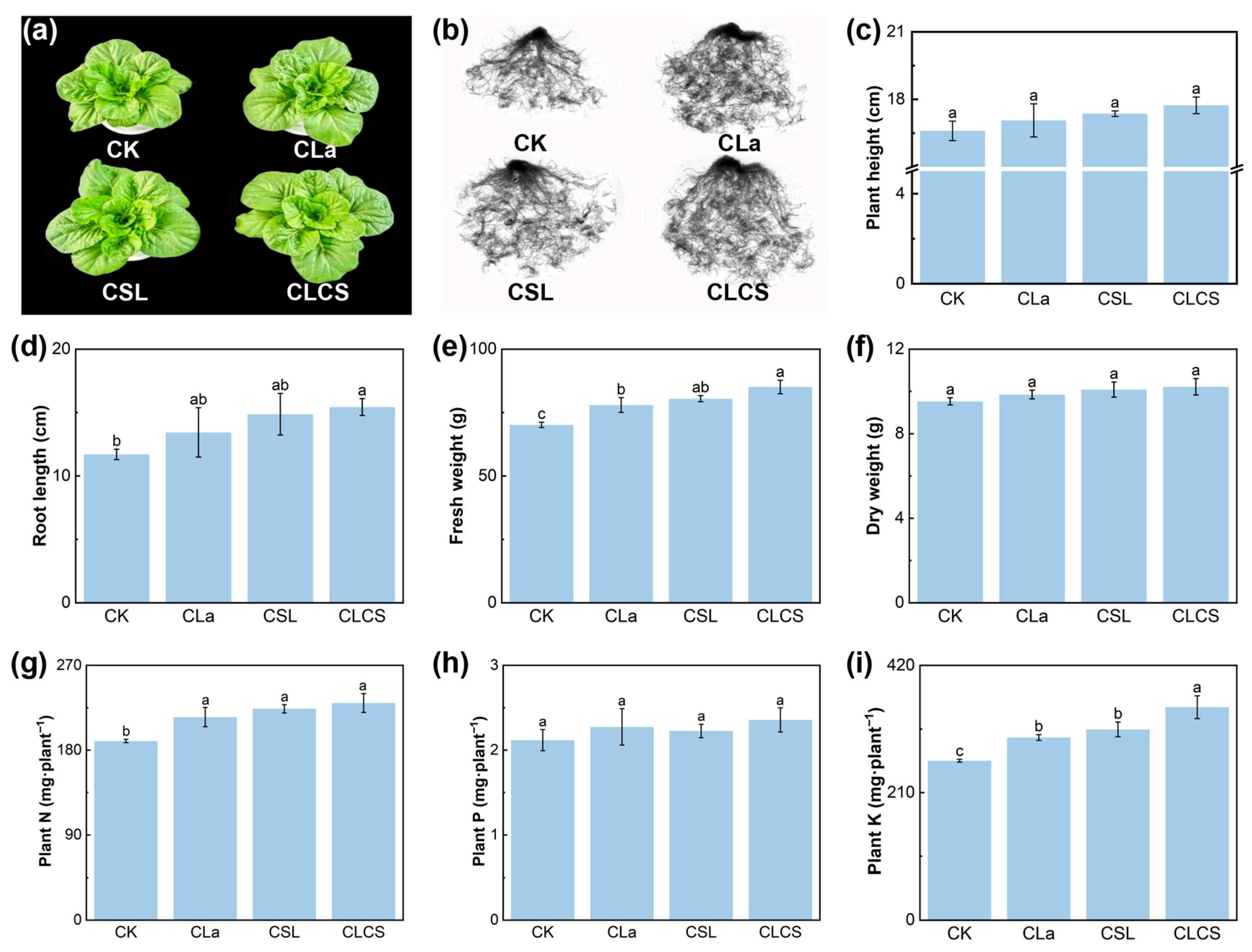
2.2. Growth Responses to Cd Stress and CLCS Treatment
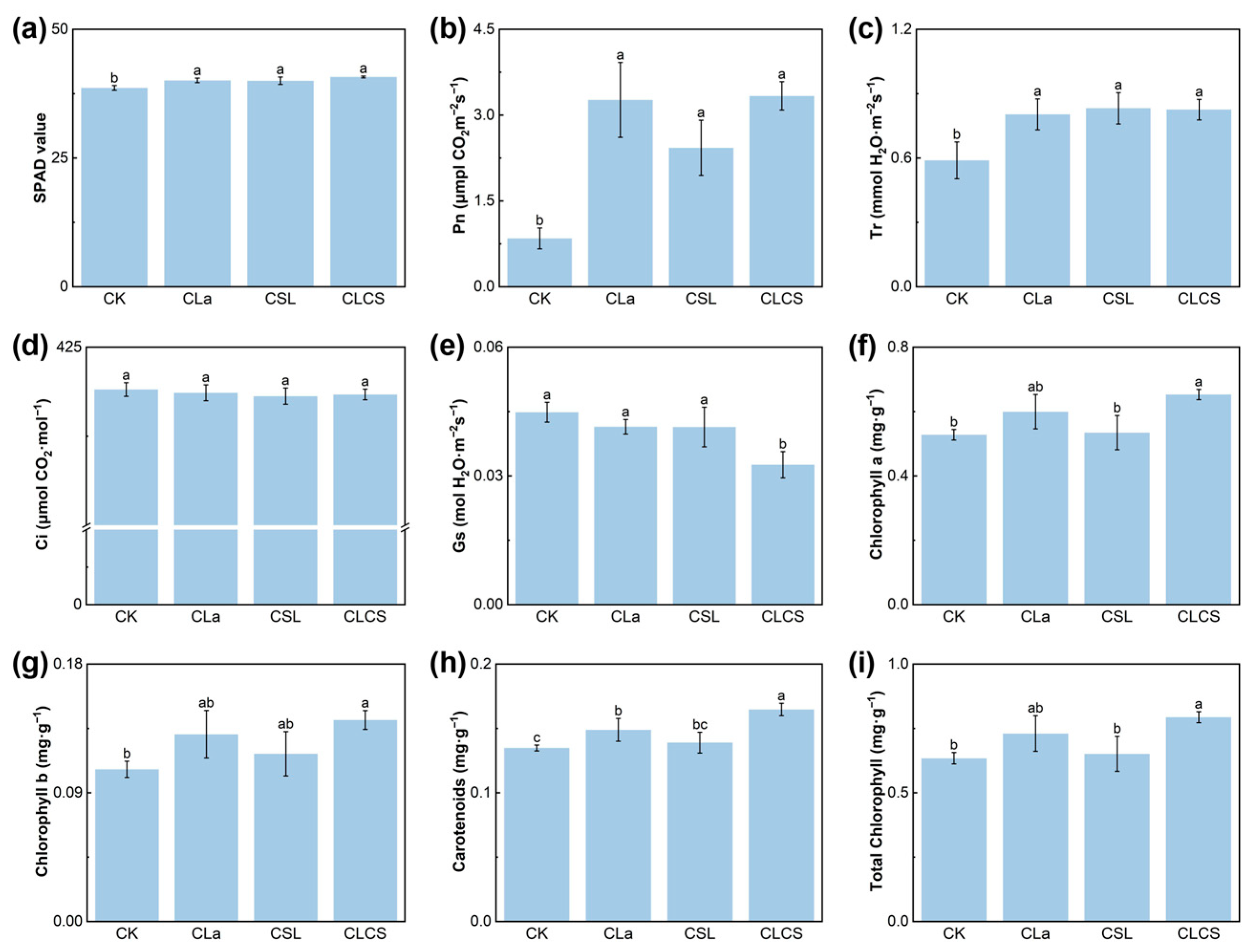
2.3. Mechanisms Underlying Cd Detoxification
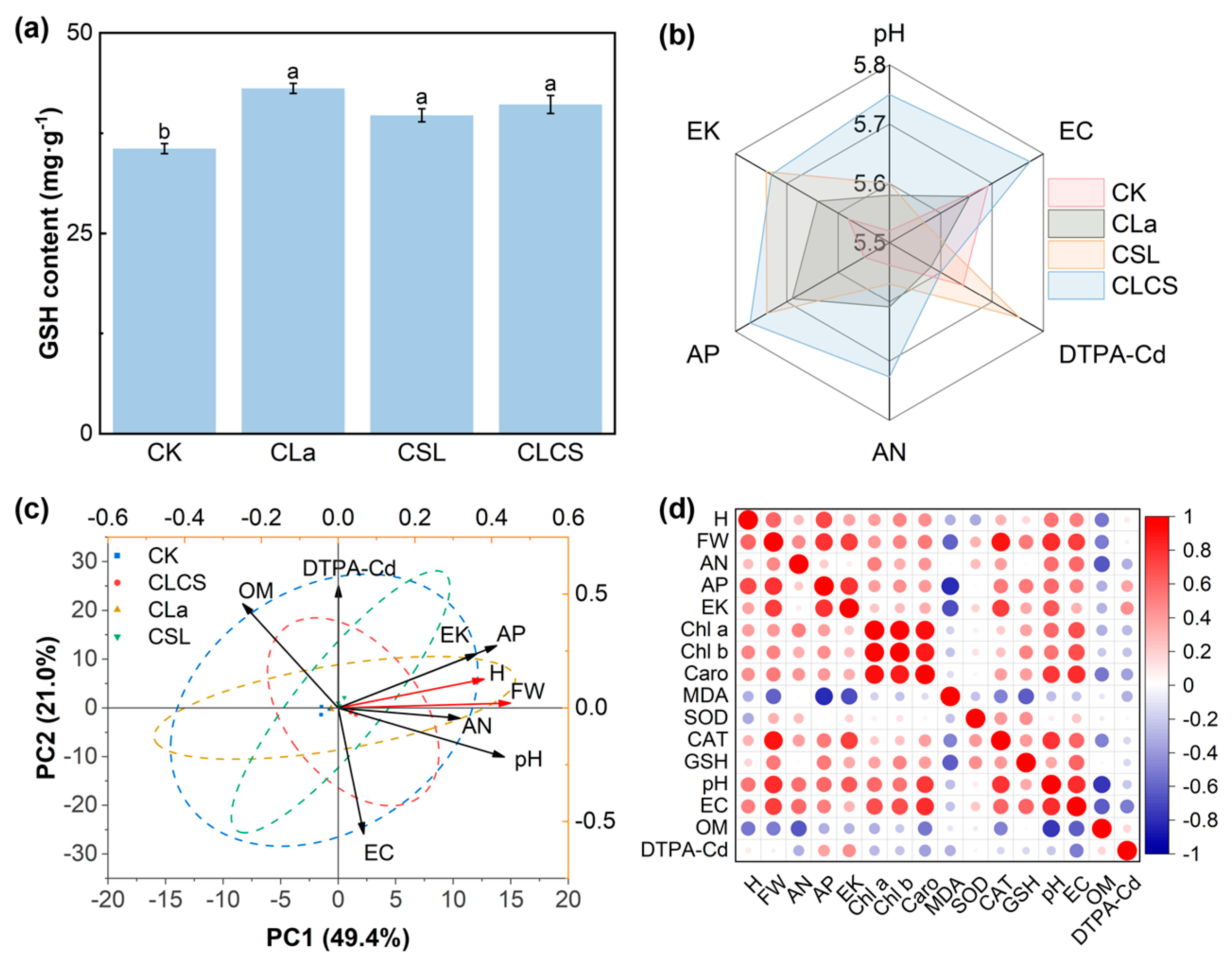
2.4. Integrated Insights into CLCS-Mediated Cd Remediation Efficacy
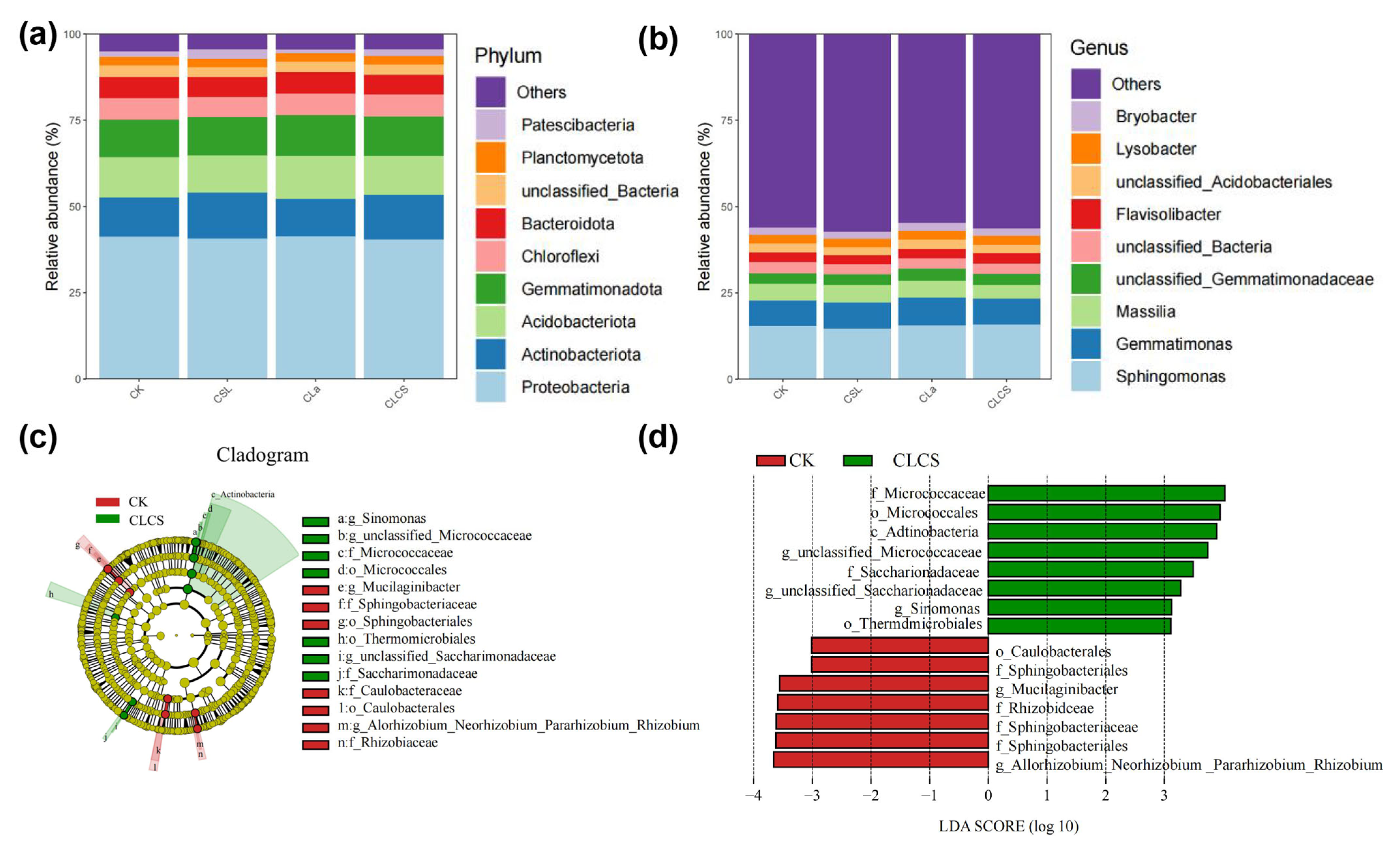
2.5. CLCS-Induced Modulation of Rhizosphere Microbiota and Associated Functional Implications
3. Materials and Methods
3.1. Material Synthesis and Characterization
3.2. Experimental Setup
3.3. Plant Growth and Physiological Analyses
3.3.1. Plant Growth Parameters
3.3.2. Assessment of Oxidative Stress Markers in Plants
3.3.3. Analysis of Antioxidant Enzyme Activities
3.4. Cadmium Analysis
3.5. Microbial High-Throughput Sequencing
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, D.; Jia, X.; Wang, L.; McGrath, S.P.; Zhu, Y.G.; Hu, Q.; Zhao, F.J.; Bank, M.S.; O’Connor, D.; Nriagu, J. Global soil pollution by toxic metals threatens agriculture and human health. Science 2025, 388, 316–321. [Google Scholar] [CrossRef]
- Huang, X.; Li, X.; Zheng, L.; Zhang, Y.; Sun, L.; Feng, Y.; Du, J.; Lu, X.; Wang, G. Comprehensive assessment of health and ecological risk of cadmium in agricultural soils across China: A tiered framework. J. Hazard. Mater. 2024, 465, 133111. [Google Scholar] [CrossRef]
- Ministry of Environmental Protection of the People’s Republic of China. National Soil Contamination Survey Report; Ministry of Environmental Protection: Beijing, China, 2014.
- Wang, M.; Chen, W.; Peng, C. Risk assessment of Cd polluted paddy soils in the industrial and township areas in Hunan, Southern China. Chemosphere 2016, 144, 346–351. [Google Scholar] [CrossRef]
- Fang, H.; Li, W.; Tu, S.; Ding, Y.; Wang, R.; Rensing, C.; Li, Y.; Feng, R. Differences in cadmium absorption by 71 leaf vegetable varieties from different families and genera and their health risk assessment. Ecotoxicol. Environ. Saf. 2019, 184, 109593. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, L.; Wang, L.; Zhou, C.; Shangguan, Y.; Yang, Y. Antioxidative enzymes activity and thiol metabolism in three leafy vegetables under Cd stress. Ecotoxicol. Environ. Saf. 2019, 173, 214–224. [Google Scholar] [CrossRef]
- Zheng, G.; Lv, H.P.; Gao, S.; Wang, S.R. Effects of cadmium on growth and antioxidant responses in Glycyrrhiza uralensis seedlings. Plant Soil Environ. 2010, 56, 508–515. [Google Scholar] [CrossRef]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 2006, 100, 241–254. [Google Scholar] [CrossRef]
- Fan, P.; Wu, L.; Wang, Q.; Wang, Y.; Luo, H.; Song, J.; Yang, M.; Yao, H.; Chen, S. Physiological and molecular mechanisms of medicinal plants in response to cadmium stress: Current status and future perspective. J. Hazard. Mater. 2023, 450, 131008. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.Y.; Ahammed, G.J.; Qi, Z.Y. Responses of Plant Proteins to Heavy Metal Stress-A Review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Luo, X.; Tian, Y.; Xie, Y.; Wang, S.; Li, Y.; Tian, L.; Wang, X. Biphasic effects of lanthanum on Vicia faba L. seedlings under cadmium stress, implicating finite antioxidation and potential ecological risk. Chemosphere 2012, 86, 530–537. [Google Scholar] [CrossRef]
- He, E.; Peijnenburg, W.; Qiu, H. Photosynthetic, antioxidative, and metabolic adjustments of a crop plant to elevated levels of La and Ce exposure. Ecotoxicol. Environ. Saf. 2022, 242, 113922. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Kamran, M.; Song, Q.; Zuo, B.; Jia, Z.; Han, Q. Lanthanum chloride improves maize grain yield by promoting photosynthetic characteristics, antioxidants enzymes and endogenous hormone at reproductive stages. J. Rare Earths 2019, 37, 781–790. [Google Scholar] [CrossRef]
- He, Q.; Zhao, H.; Teng, Z.; Wang, Y.; Li, M.; Hoffmann, M.R. Phosphate removal and recovery by lanthanum-based adsorbents: A review for current advances. Chemosphere 2022, 303, 134987. [Google Scholar] [CrossRef]
- Persy, V.P.; Behets, G.J.; Bervoets, A.R.; De Broe, M.E.; D’Haese, P.C. Lanthanum: A safe phosphate binder. Semin. Dial. 2006, 19, 195–199. [Google Scholar] [CrossRef]
- Chen, J.; Zhong, Y. Lanthanum(III)-amino acid chelate mitigates copper(II) stress in rice (Oryza sativa). Sci. Rep. 2024, 14, 22315. [Google Scholar] [CrossRef]
- Wu, J.L.; Wei, Z.G.; Zhao, H.Y.; Li, H.X.; Hu, F. The role of amino acids in the long-distance transport of La and Y in the xylem sap of tomato. Biol. Trace Elem. Res. 2009, 129, 239–250. [Google Scholar] [CrossRef]
- Wu, J.; Chen, A.; Peng, S.; Wei, Z.; Liu, G. Identification and application of amino acids as chelators in phytoremediation of rare earth elements lanthanum and yttrium. Plant Soil 2013, 373, 329–338. [Google Scholar] [CrossRef]
- Roblin, G.; Octave, S.; Faucher, M.; Fleurat-Lessard, P.; Berjeaud, J.M. Cysteine: A multifaceted amino acid involved in signaling, plant resistance and antifungal development. Plant Physiol. Biochem. 2018, 129, 77–89. [Google Scholar] [CrossRef]
- Genisel, M.; Erdal, S.; Kizilkaya, M. The mitigating effect of cysteine on growth inhibition in salt-stressed barley seeds is related to its own reducing capacity rather than its effects on antioxidant system. Plant Growth Regul. 2014, 75, 187–197. [Google Scholar] [CrossRef]
- Sadak, M.S.; Abd El-Hameid, A.R.; Zaki, F.S.A.; Dawood, M.G.; El-Awadi, M.E. Physiological and biochemical responses of soybean (Glycine max L.) to cysteine application under sea salt stress. Bull. Natl. Res. Cent. 2019, 44, 1. [Google Scholar] [CrossRef]
- Sarkodie, E.K.; Jiang, L.; Li, K.; Guo, Z.; Yang, J.; Shi, J.; Peng, Y.; Wu, X.; Huang, S.; Deng, Y.; et al. The influence of cysteine in transformation of Cd fractionation and microbial community structure and functional profile in contaminated paddy soil. Sci. Total Environ. 2024, 906, 167535. [Google Scholar] [CrossRef]
- Zhou, K.; Yu, J.; Ma, Y.; Cai, L.; Zheng, L.; Gong, W.; Liu, Q.A. Corn Steep Liquor: Green Biological Resources for Bioindustry. Appl. Biochem. Biotechnol. 2022, 194, 3280–3295. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, X.; Wang, C.; Yu, Q.; Yang, H.; Xu, W.; Wang, T.; Gao, L.; Meng, X.; Luo, S.; et al. Combined application of myo-inositol and corn steep liquor enhances seedling growth and cold tolerance in cucumber and tomato. Physiol. Plant. 2024, 176, e14422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, X.; Zhang, W.; Chen, Q. Combined Application of Myo-Inositol and Corn Steep Liquor from Agricultural Waste Alleviate Salt Stress in Brassica rapa. Plants 2023, 12, 4110. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Zhang, X.; Li, L.; Qi, Q.; Cui, W.; Chen, Q.; Mu, K. Enhancing Growth and Nutrient Uptake in Chinese Cabbage Through the Application of Corn Steep Liquor and Myo-Inositol in Salt-Stressed Soils. Agronomy 2025, 15, 1196. [Google Scholar] [CrossRef]
- Pang, X.; Li, D.; Peng, A. Application of rare-earth elements in the agriculture of China and its environmental behavior in soil. Environ. Sci. Pollut. Res. Int. 2002, 9, 143–148. [Google Scholar] [CrossRef]
- Hu, Z.; Richter, H.; Sparovek, G.; Schnug, E. Physiological and Biochemical Effects of Rare Earth Elements on Plants and Their Agricultural Significance: A Review. J. Plant Nutr. 2004, 27, 183–220. [Google Scholar] [CrossRef]
- Amiri, M.; Eynaki, H.; Mansoori, Y. Cysteine-anchored receptor on carbon nanoparticles for dopamine sensing. Electrochimica Acta 2014, 123, 362–368. [Google Scholar] [CrossRef]
- Sanga, T.R.; Mwakalapa, E.B.; Mng’ong’o, M.; Kalugendo, K.; Ponraj, M.; Maseka, K.K. Effects of cadmium uptake on growth and productivity of Chinese cabbage (Brassica rapa subsp. pekinensis) and pumpkin (Cucurbita moschata Duchesne) vegetables. J. Agric. Food Res. 2023, 14, 100744. [Google Scholar] [CrossRef]
- Yu, Y.; Alseekh, S.; Zhu, Z.; Zhou, K.; Fernie, A.R. Multiomics and biotechnologies for understanding and influencing cadmium accumulation and stress response in plants. Plant Biotechnol. J. 2024, 22, 2641–2659. [Google Scholar] [CrossRef]
- Azhar, M.; Zia Ur Rehman, M.; Ali, S.; Qayyum, M.F.; Naeem, A.; Ayub, M.A.; Anwar Ul Haq, M.; Iqbal, A.; Rizwan, M. Comparative effectiveness of different biochars and conventional organic materials on growth, photosynthesis and cadmium accumulation in cereals. Chemosphere 2019, 227, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Aiken, R.M.; Smucker, A.J. Root system regulation of whole plant growth. Annu. Rev. Phytopathol. 1996, 34, 325–346. [Google Scholar] [CrossRef]
- Wang, X.; Below, F.E. Cytokinins in Enhanced Growth and Tillering of Wheat Induced by Mixed Nitrogen Source. Crop Sci. 1996, 36, 121–126. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, Q.; Liu, L.; Pang, J.; Wang, X.; Zhou, Q.; Wang, L.; Huang, X. Endocytosis of root cells induced by low-dose lanthanum(III) can promote seedling photomorphogenesis and leaf photosynthesis. Plant Soil 2023, 488, 637–651. [Google Scholar] [CrossRef]
- Eckardt, N.A.; Allahverdiyeva, Y.; Alvarez, C.E.; Buchel, C.; Burlacot, A.; Cardona, T.; Chaloner, E.; Engel, B.D.; Grossman, A.R.; Harris, D.; et al. Lighting the way: Compelling open questions in photosynthesis research. Plant Cell 2024, 36, 3914–3943. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Zhou, Q.; Huang, X. Combined effects of lanthanum (III) chloride and acid rain on photosynthetic parameters in rice. Chemosphere 2014, 112, 355–361. [Google Scholar] [CrossRef]
- Wen, K.; Liang, C.; Wang, L.; Hu, G.; Zhou, Q. Combined effects of lanthanum ion and acid rain on growth, photosynthesis and chloroplast ultrastructure in soybean seedlings. Chemosphere 2011, 84, 601–608. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, B.; Zhang, Y.; Li, Y.; Yu, C.; Peng, Z.; Wang, K.; Liu, Z.; Huang, J.-a.; Xiong, L.; et al. Transcriptomic and biochemical analysis reveal differential regulatory mechanisms of photosynthetic pigment and characteristic secondary metabolites between high amino acids green-leaf and albino tea cultivars. Sci. Hortic. 2022, 295, 110823. [Google Scholar] [CrossRef]
- Bakhoum, G.S.; Badr, E.A.E.; Sadak, M.S.; Kabesh, M.O.; Amin, G.A. Improving Growth, Some Biochemical Aspects and Yield of Three Cultivars of Soybean Plant by Methionine Treatment Under Sandy Soil Condition. Int. J. Environ. Res. 2018, 13, 35–43. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J. Lanthanum Promotes Bahiagrass (Paspalum notatum) Roots Growth by Improving Root Activity, Photosynthesis and Respiration. Plants 2022, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Q.; Xu, X.J.; Wang, S.; Shan, C.J. Lanthanum improves salt tolerance of maize seedlings. Photosynthetica 2016, 54, 148–151. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sugano, S.S.; Shimada, T.; Hara-Nishimura, I. Enhancement of leaf photosynthetic capacity through increased stomatal density in Arabidopsis. New Phytol. 2013, 198, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jin, L.; Wang, X. Cadmium absorption and transportation pathways in plants. Int. J. Phytorem. 2017, 19, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhao, X.; Sun, X.; Tan, Q.; Tang, Y.; Nie, Z.; Qu, C.; Chen, Z.; Hu, C. Antioxidant enzyme systems and the ascorbate-glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere 2015, 138, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, J.; Xiao, C.; Long, J.; Li, S.; Zhang, S.; Xiong, Z. Lanthanum supplementation at the heading stage efficiently reduces cadmium content in rice by regulating key genes involved in cadmium uptake, translocation, and redistribution pathways. Environ. Pollut. 2025, 376, 126393. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Liu, R.; Xiong, Z. Lanthanum reduces the cadmium accumulation by suppressing expression of transporter genes involved in cadmium uptake and translocation in wheat. Plant Soil 2019, 441, 235–252. [Google Scholar] [CrossRef]
- Xiao, C.; Yang, H.; Chen, X.; Li, J.; Cai, X.; Long, J. Application of Lanthanum at the Heading Stage Effectively Suppresses Cadmium Accumulation in Wheat Grains by Downregulating the Expression of TaZIP7 to Increase Cadmium Retention in Nodes. Plants 2024, 13, 2921. [Google Scholar] [CrossRef]
- Haghighi, M.; Kafi, M.; Fang, P.; Gui-Xiao, L. Humic Acid Decreased Hazardous of Cadmium Toxicity on Lettuce (Lactuca sativa L.). J. Fruit Ornam. Plant Res. 2010, 72, 49–61. [Google Scholar] [CrossRef]
- Kou, B.; Yu, T.; Tang, J.; Zhu, X.; Yuan, Y.; Tan, W. Kitchen compost-derived humic acid application promotes ryegrass growth and enhances the accumulation of Cd: An analysis of the soil microenvironment and rhizosphere functional microbes. Sci. Total Environ. 2024, 919, 170879. [Google Scholar] [CrossRef]
- Sen Raychaudhuri, S.; Deng, X.W. The role of superoxide dismutase in combating oxidative stress in higher plants. Bot. Rev. 2000, 66, 89–98. [Google Scholar] [CrossRef]
- Zeng, C.Q.; Liu, W.X.; Hao, J.Y.; Fan, D.N.; Chen, L.M.; Xu, H.N.; Li, K.Z. Measuring the expression and activity of the CAT enzyme to determine Al resistance in soybean. Plant Physiol. Biochem. 2019, 144, 254–263. [Google Scholar] [CrossRef]
- Veitch, N.C. Structural determinants of plant peroxidase function. Phytochem. Rev. 2004, 3, 3–18. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef]
- Maslennikova, D.; Ivanov, S.; Petrova, S.; Burkhanova, G.; Maksimov, I.; Lastochkina, O. Components of the Phenylpropanoid Pathway in the Implementation of the Protective Effect of Sodium Nitroprusside on Wheat under Salinity. Plants 2023, 12, 2123. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Shan, C.; Zhao, H.; Jia, G.; Chen, D. Lanthanum improves the cadmium tolerance of Zea mays seedlings by the regulation of ascorbate and glutathione metabolism. Biol. Plant. 2017, 61, 551–556. [Google Scholar] [CrossRef]
- Li, L.; Wang, C.; Wang, W.; Zhou, L.; Zhang, D.; Liao, H.; Wang, Z.; Li, B.; Peng, Y.; Xu, Y.; et al. Uncovering the mechanisms of how corn steep liquor and microbial communities minimize cadmium translocation in Chinese cabbage. Environ. Sci. Pollut. Res. Int. 2024, 31, 22576–22587. [Google Scholar] [CrossRef]
- Mishra, J.; Singh, R.; Arora, N.K. Alleviation of Heavy Metal Stress in Plants and Remediation of Soil by Rhizosphere Microorganisms. Front. Microbiol. 2017, 8, 1706. [Google Scholar] [CrossRef]
- Mao, Y.; Tan, H.; Wang, M.; Jiang, T.; Wei, H.; Xu, W.; Jiang, Q.; Bao, H.; Ding, Y.; Wang, F.; et al. Research Progress of Soil Microorganisms in Response to Heavy Metals in Rice. J. Agric. Food Chem. 2022, 70, 8513–8522. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhou, J.; Xu, X.; Na, M.; Xu, S.; Huang, Y.; Zhang, J.; Li, X.; Zheng, X. Inoculation of cadmium-tolerant bacteria to regulate microbial activity and key bacterial population in cadmium-contaminated soils during bioremediation. Ecotoxicol. Environ. Saf. 2024, 271, 115957. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Yu, L.; Sun, X.; Qin, L.; Liu, J.; Han, Y.; Chen, S. Integrating rhizosphere bacterial structure and metabolites with soil Cd availability in different parent paddy soils. Sci. Total Environ. 2024, 955, 177096. [Google Scholar] [CrossRef]
- Liu, Z.; Li, S.; Liu, N.; Huang, G.; Zhou, Q. Soil Microbial Community Driven by Soil Moisture and Nitrogen in Milk Vetch (Astragalus sinicus L.)–Rapeseed (Brassica napus L.) Intercropping. Agriculture 2022, 12, 1538. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Xiao, A.; Li, C.; Li, Y. Regulation of soil properties by amendments and their impact on Cd fractions and bacterial community structure: Exploring the mechanism of inhibition on Cd phytoavailability. Ecotoxicol. Environ. Saf. 2025, 294, 118033. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, O.S.; Fernandes, A.S.; Tupy, S.M.; Ferreira, T.G.; Almeida, L.N.; Creevey, C.J.; Santana, M.F. Insights into plant interactions and the biogeochemical role of the globally widespread Acidobacteriota phylum. Soil Biol. Biochem. 2024, 192, 109369. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Bai, E. Decreasing soil microbial diversity is associated with decreasing microbial biomass under nitrogen addition. Soil Biol. Biochem. 2018, 120, 126–133. [Google Scholar] [CrossRef]
- Sangthong, C.; Setkit, K.; Prapagdee, B. Improvement of cadmium phytoremediation after soil inoculation with a cadmium-resistant Micrococcus sp. Environ. Sci. Pollut. Res. 2015, 23, 756–764. [Google Scholar] [CrossRef]
- Badawy, I.H.; Hmed, A.A.; Sofy, M.R.; Al-Mokadem, A.Z. Alleviation of Cadmium and Nickel Toxicity and Phyto-Stimulation of Tomato Plant L. by Endophytic Micrococcus luteus and Enterobacter cloacae. Plants 2022, 11, 2018. [Google Scholar] [CrossRef]
- Duncan, D.R.; Widholm, J.M. Osmotic induced stimulation of the reduction of the viability dye 2,3,5-triphenyltetrazolium chloride by maize roots and callus cultures. J. Plant Physiol. 2004, 161, 397–403. [Google Scholar] [CrossRef]
- Cambrollé, J.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Luque, T.; Figueroa, M.E. Growth, reproductive and photosynthetic responses to copper in the yellow-horned poppy, Glaucium flavum Crantz. Environ. Exp. Bot. 2011, 71, 57–64. [Google Scholar] [CrossRef]
- Faizan, M.; Bhat, J.A.; Hessini, K.; Yu, F.; Ahmad, P. Zinc oxide nanoparticles alleviates the adverse effects of cadmium stress on Oryza sativa via modulation of the photosynthesis and antioxidant defense system. Ecotoxicol. Environ. Saf. 2021, 220, 112401. [Google Scholar] [CrossRef]
- Cui, J.; Jiang, R. Experimental Course of Soil, Plant, and Environmental Analysis; China Agricultural University Press: Beijing, China, 2020. [Google Scholar]
- Jambunathan, N. Determination and Detection of Reactive Oxygen Species (ROS), Lipid Peroxidation, and Electrolyte Leakage in Plants. In Plant Stress Tolerance: Methods and Protocols; Sunkar, R., Ed.; Humana Press: Totowa, NJ, USA, 2010; pp. 291–297. [Google Scholar] [CrossRef]
- Hernández, J.A.; Díaz-Vivancos, P.; Rubio, M.; Olmos, E.; Ros-Barceló, A.; Martínez-Gómez, P. Long-term plum pox virus infection produces an oxidative stress in a susceptible apricot, Prunus armeniaca, cultivar but not in a resistant cultivar. Physiol. Plant. 2005, 126, 140–152. [Google Scholar] [CrossRef]
- Babitha, M.P.; Prakash, H.S.; Shekar shetty, H. Purification and partial characterization of manganese superoxide dismutase from downy mildew resistant pearl millet seedlings inoculated with Sclerospora graminicola. Plant Sci. 2002, 163, 917–924. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, M.; Amaresan, N.; Sankaranarayanan, A. Estimation of Peroxidase (POD). In Plant-Microbe Interactions: Laboratory Techniques; Springer: New York, NY, USA, 2021; pp. 123–125. [Google Scholar] [CrossRef]
- Xiao, Z.; Zou, D.; Zeng, X.; Zhang, L.; Liu, F.; Wang, A.; Zeng, Q.; Zhang, G.; Li, L. Cadmium accumulation in oilseed rape is promoted by intercropping with faba bean and ryegrass. Ecotoxicol. Environ. Saf. 2020, 205, 111162. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zhao, Y.; Li, Y.; Wang, Y.; Pei, J.; Xu, H. Cadmium induced changes in rhizosphere microecology to enhance Cd intake by Ligusticum sinense cv. Chuanxiong. J. Hazard. Mater. 2024, 468, 133851. [Google Scholar] [CrossRef] [PubMed]

| Treatment | Surface Root Area (cm2) | Root Diameter (mm) | Root Volume (cm3) | Root Tips | Root Forks | Root Crossing | Root Activity (μg g−1 h−1) |
|---|---|---|---|---|---|---|---|
| CK | 147 b | 0.41 b | 1.54 b | 4230 b | 13,359 b | 4002 b | 256.44 c |
| CLa | 215 a | 0.43 ab | 2.10 b | 4952 ab | 17,941 ab | 5368 ab | 292 b |
| CSL | 223 a | 0.40 b | 2.25 b | 5764 a | 20,484 a | 6269 a | 260.89 bc |
| CLCS | 262 a | 0.45 a | 3.09 a | 5998 a | 22,477 a | 6610 a | 358.67 a |
| Treatment | CLa (mg kg−1) | CSL (g kg−1) |
|---|---|---|
| CK | 0 | 0 |
| CLa | 2.5 | 0 |
| CSL | 0 | 1.14 |
| CLCS | 2.5 | 1.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Wang, Z.; Wang, W.; Wang, X.; Ma, X.; Zhang, X.; Liu, Y.; Chen, Q.; Mu, K. Synergistic Lanthanum-Cysteine Chelate and Corn Steep Liquor Mitigate Cadmium Toxicity in Chinese Cabbage via Physiological–Microbial Coordination. Plants 2025, 14, 3040. https://doi.org/10.3390/plants14193040
Ma F, Wang Z, Wang W, Wang X, Ma X, Zhang X, Liu Y, Chen Q, Mu K. Synergistic Lanthanum-Cysteine Chelate and Corn Steep Liquor Mitigate Cadmium Toxicity in Chinese Cabbage via Physiological–Microbial Coordination. Plants. 2025; 14(19):3040. https://doi.org/10.3390/plants14193040
Chicago/Turabian StyleMa, Fengbo, Zihao Wang, Wenhao Wang, Xian Wang, Xiaojing Ma, Xinjun Zhang, Yanli Liu, Qing Chen, and Kangguo Mu. 2025. "Synergistic Lanthanum-Cysteine Chelate and Corn Steep Liquor Mitigate Cadmium Toxicity in Chinese Cabbage via Physiological–Microbial Coordination" Plants 14, no. 19: 3040. https://doi.org/10.3390/plants14193040
APA StyleMa, F., Wang, Z., Wang, W., Wang, X., Ma, X., Zhang, X., Liu, Y., Chen, Q., & Mu, K. (2025). Synergistic Lanthanum-Cysteine Chelate and Corn Steep Liquor Mitigate Cadmium Toxicity in Chinese Cabbage via Physiological–Microbial Coordination. Plants, 14(19), 3040. https://doi.org/10.3390/plants14193040







