Palynological Characteristics of Neogene Deposits from Bełchatów Lignite Mine (Central Poland)
Abstract
1. Introduction

Geological Setting
2. Materials and Methods
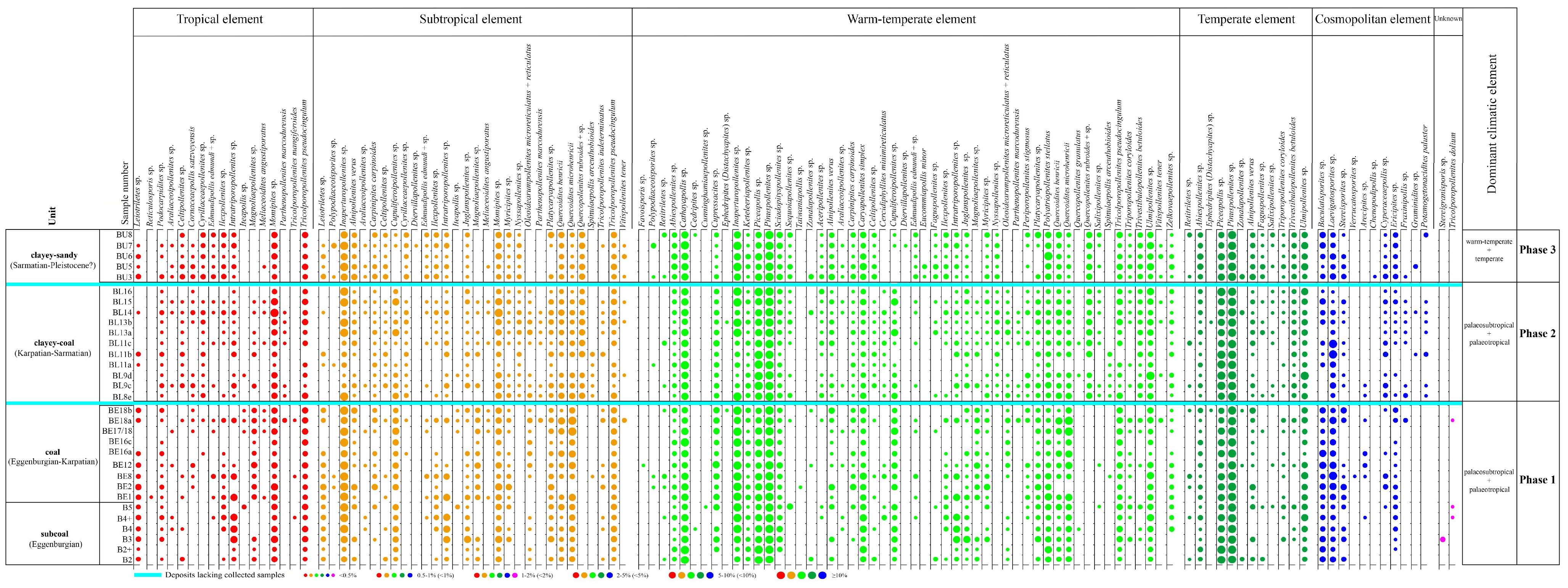
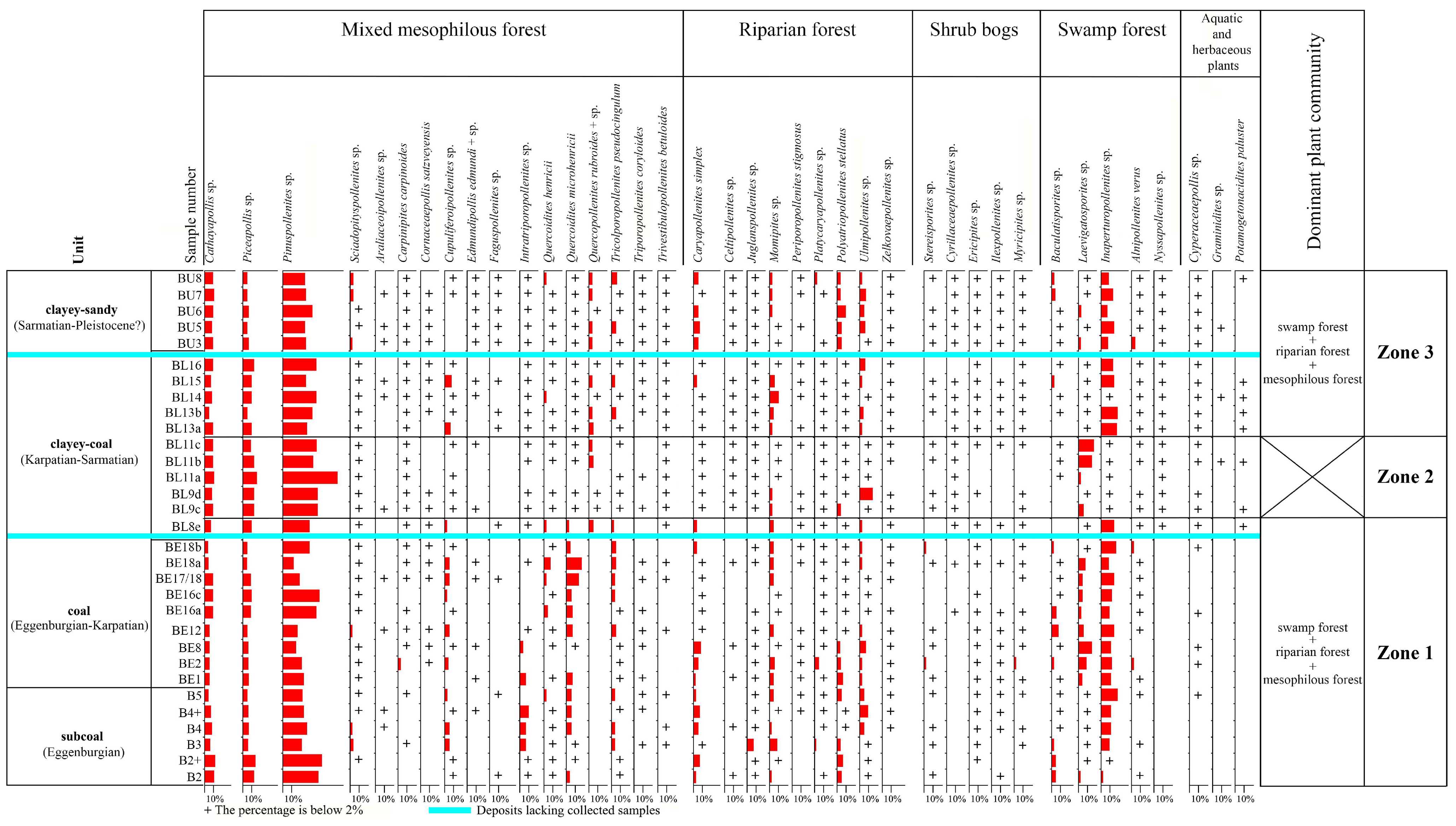
3. Results
4. Discussion
4.1. Palaeovegetation and Palaeoenvironment
4.2. Palaeoclimate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviation
| BLM | Bełchatów Lignite Mine |
References
- Bruch, A.A.; Utescher, T.; Mosbrugger, V.; Gabrielyan, I.; Ivanov, D.A. Late Miocene Climate in the Circum-Alpine Realm—A Quantitative Analysis of Terrestrial Palaeofloras. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 238, 270–280. [Google Scholar] [CrossRef]
- Kvaček, Z.; Kováč, M.; Kovar-Eder, J.; Doláková, N.; Jechorek, H.; Parashiv, V.; Kováčová, M.; Sliva, U. Miocene Evolution of Landscape and Vegetation in the Central Paratethys. Geol. Carpathica 2006, 57, 295–310. [Google Scholar]
- Widera, M.; Bechtel, A.; Chomiak, L.; Maciaszek, P.; Słodkowska, B.; Wachocki, R.; Worobiec, E.; Worobiec, G.; Zieliński, T. Palaeoenvironmental Reconstruction of the Konin Basin (Central Poland) during Lignite Accumulation Linked to the Mid-Miocene Climate Optimum. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 568, 110307. [Google Scholar] [CrossRef]
- Scheiner, F.; Havelcová, M.; Holcová, K.; Doláková, N.; Nehyba, S.; Ackerman, L.; Trubač, J.; Hladilová, S.; Rejšek, J.; Utescher, T. Evolution of Palaeoclimate, Palaeoenvironment and Vegetation in Central Europe during the Miocene Climate Optimum. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2023, 611, 111364. [Google Scholar] [CrossRef]
- Stuchlik, L.; Szynkiewicz, A.; Łańcucka-Środoniowa, M.; Zastawniak, E. Results of the Hitherto Palaeobotanical Investigations of the Tertiary Brown Coal Bed “Bełchatów” (Central Poland). Acta Palaeobot. 1990, 30, 259–305. [Google Scholar]
- Wójcicki, J.J.; Zastawniak, E. Trapa Srodoniana, a New Fossil Species from the Pliocene of Bełchatów (Middle Poland). Acta Palaeobot. 1998, 38, 167–174. [Google Scholar]
- Worobiec, G.; Lesiak, M.A. Plant Megafossils from the Neogene Deposits of Stawek-1A (Bełchatów, Middle Poland). Rev. Palaeobot. Palynol. 1998, 101, 179–208. [Google Scholar] [CrossRef]
- Otto, A.; Simoneit, B.R.T.; Lesiak, M.; Wilde, V.; Worobiec, G. Resin and Wax Biomarkers Preserved in Miocene Cupressaceae s. l. from Bełchatów and Lipnica Wielka, Poland. Acta Palaeobot. 2001, 41, 195–206. [Google Scholar]
- Worobiec, G. New Fossil Floras from Neogene Deposits in the Bełchatów Lignite Mine. Acta Palaeobot. 2003, (Suppl. S3), 1–133. [Google Scholar]
- Worobiec, G. Laurus Abchasica (Kolakovsky & Shakryl) Ferguson from the Neogene of the Bełchatów Lignite Mine (Central Poland). Acta Palaeobot. 2007, 47, 203–215. [Google Scholar]
- Worobiec, G. Late Neogene Leaf Assemblage from Bełchatów Lignite Mine (Central Poland). Acta Palaeobot. 2014, 54, 249–277. [Google Scholar] [CrossRef][Green Version]
- Worobiec, E.; Worobiec, G. Leaves and Pollen of Bamboos from the Polish Neogene. Rev. Palaeobot. Palynol. 2005, 133, 39–50. [Google Scholar] [CrossRef]
- Worobiec, G.; Szynkiewicz, A. Betulaceae Leaves in Miocene Deposits of the Bełchatów Lignite Mine (Central Poland). Rev. Palaeobot. Palynol. 2007, 147, 28–59. [Google Scholar] [CrossRef]
- Worobiec, G.; Szynkiewicz, A. Neogene Wetland Vegetation Based on a Leaf Assemblage from the Bełchatów Lignite Mine (Central Poland). Acta Palaeobot. 2016, 56, 441–497. [Google Scholar] [CrossRef]
- Worobiec, G.; Worobiec, E.; Szynkiewicz, A. Plant Assemblage from the Upper Miocene Deposits of the Bełchatów Lignite Mine (Central Poland). Acta Palaeobot. 2012, 52, 369–413. [Google Scholar]
- Worobiec, G.; Worobiec, E. Wetland Vegetation from the Miocene Deposits of the Bełchatów Lignite Mine (Central Poland). Palaeontol. Electron. 2019, 22, 1–38. [Google Scholar] [CrossRef]
- Worobiec, G.; Worobiec, E. The Whole Plant Concept of Reevesia from the Neogene of the Bełchatów Lignite Mine (Central Poland). Rev. Palaeobot. Palynol. 2020, 273, 104145. [Google Scholar] [CrossRef]
- Worobiec, E.; Worobiec, G. Fossil zygospores of Zygnemataceae algae (Chlorophyta) from the Upper Miocene of the Bełchatów Lignite Mine. Przegląd Geol. 2008, 56, 1000–1004. [Google Scholar]
- Worobiec, G.; Worobiec, E. Epiphyllous Fungi from Miocene Deposits of the Bełchatów Lignite Mine (Central Poland). Mycosphere 2017, 8, 1003–1013. [Google Scholar] [CrossRef]
- Worobiec, G.; Worobiec, E.; Erdei, B. Fossil Callimothalloid Fungi: Revised Taxonomy, Modern Equivalents and Palaeoecology. Fungal Biol. 2020, 124, 835–844. [Google Scholar] [CrossRef]
- Dumont, H.J.; Pociecha, A.; Zawisza, E.; Szeroczyńska, K.; Worobiec, E.; Worobiec, G. Miocene Cladocera from Poland. Sci. Rep. 2020, 10, 12107. [Google Scholar] [CrossRef]
- Wegierek, P. Two new locations of Tertiary insects in Poland. Przegląd Geol. 1995, 43, 660–661. [Google Scholar]
- Stworzewicz, E. Palaeobiogeographical Characteristics of the Miocene Land Snail Fauna of Poland. Scr. Geol. 1993, 2, 397–406. [Google Scholar]
- Stworzewicz, E. Miocene Land Snails from Bełchatów (Central Poland). IV: Pupilloidea (Gastropoda Pulmonata). Systematic, Biostratigraphic and Palaeoecological Studies. Folia Malacol. 1999, 7, 133–170. [Google Scholar] [CrossRef][Green Version]
- Jerzmańska, A.; Hałuszczak, A. A new locality of Tertiary fresh-water fish fauna (Teleostei) in Poland. Przegląd Geol. 1986, 34, 25–27. [Google Scholar][Green Version]
- Kovalchuk, O.; Nadachowski, A.; Świdnicka, E.; Stefaniak, K. Fishes from the Miocene Lacustrine Sequence of Bełchatów (Poland). Hist. Biol. 2020, 32, 1011–1018. [Google Scholar] [CrossRef]
- Kowalski, K. Neocometes Schaub and Zapfe, 1953 (Rodentia, Mammalia) from the Miocene of Belchatów (Poland). Acta Zool. Cracoviensia 1993, 36, 259–265. [Google Scholar]
- Rzebik-Kowalska, B.; Kowalski, K. The Northernmost Fossil Locality of Fruit Bats (Megachiroptera, Mammalia) in the Miocene of Bełchatów (Poland). Acta Zool. Cracoviensa 2001, 44, 59–63. [Google Scholar]
- Garapich, A. An Overview of Miocene Rodents from Bełchatów (Poland). Folia Zool. 2002, 51, 59–66. [Google Scholar]
- Kowalski, K.; Rzebik-Kowalska, B. Paleoecology of the Miocene Fossil Mammal Fauna from Bełchatów (Poland). Acta Theriol. 2002, 47, 115–126. [Google Scholar] [CrossRef]
- Fostowicz-Frelik, Ł.; Nadachowski, A.; Kowalewska-Groszkowska, M. New Data on the Miocene Stem Lagomorph Eurolagus Fontannesi, and Its Northernmost Record. Acta Palaeontol. Pol. 2012, 57, 1–20. [Google Scholar] [CrossRef][Green Version]
- Ziembińska-Tworzydło, M. Stratigraphy of Tertiary sediments of the “Bełchatów” bed on the basis of sporo-pollen analysis. Geol. Q. 1966, 10, 1117–1118. [Google Scholar][Green Version]
- Worobiec, E.; Worobiec, G. Miocene Palynoflora from the KRAM-P 218 Leaf Assemblage from the Bełchatów Lignite Mine (Central Poland). Acta Palaeobot. 2016, 56, 499–517. [Google Scholar] [CrossRef]
- Worobiec, E.; Worobiec, G. Palynoflora and Palaeoenvironment of the Early Miocene Palaeolake from the Bełchatów Mine, Central Poland. Geol. Q. 2022, 66, 32. [Google Scholar] [CrossRef]
- Czarnecki, L.; Frankowski, R.; Kuszneruk, J. Synthetic lithostratigraphic profile of the Tertiary formations of the “Bełchatów” deposit. In Proceedings of the 15th Symposium Geology of Coal-Bearing Formations in Poland, Kraków, Poland, 18–19 September; 1992; pp. 19–23. [Google Scholar]
- Matl, K. Selected elements of geological structure of the Bełchatów deposit. In Petrological Studies and Sedimentological Conditions of Select Lithologic Series in Miocene from Bełchatów Lignite Deposit, Poland; Commission of Geological Sciences of the Polish Academy of Sciences, Kraków Branch, Geological Works: Warsaw, Poland, 2000; pp. 11–19. [Google Scholar]
- Burchart, J.; Kasza, L.; Lorenc, S. Fission-Track Zircon Dating of Tuffitic Intercalations (Tonstein) in the Brown-Coal Mine “Bełchatów”. Bull. Pol. Acad. Sci. Earth Sci. 1988, 36, 281–286. [Google Scholar]
- Piwocki, M.; Ziembińska-Tworzydło, M. Neogene of the Polish Lowlands—Litostratigraphy and Pollen-Spore Zones. Geol. Q. 1997, 41, 21–40. [Google Scholar]
- Peryt, M.; Piwocki, M. Geological Structure of Poland, Volume I, Stratigraphy, Part 3a Cenozoic, Paleogene and Neogene; Polish Geological Institute: Warsaw, Poland, 2004; ISBN 83-7372-736-1.
- Erdtman, G. The Acetolysis Method-A Revised Description. Sven. Bot. Tidskr. 1960, 54, 561–564. [Google Scholar]
- Stuchlik, L.; Ziembińska-Tworzydło, M.; Kohlman-Adamska, A.; Grabowska, I.; Ważyńska, H.; Słodkowska, B.; Sadowska, A. Atlas of Pollen and Spores of the Polish Neogene. Volume 1—Spores; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2001. [Google Scholar]
- Stuchlik, L.; Ziembińska-Tworzydło, M.; Kohlman-Adamska, A.; Grabowska, I.; Ważyńska, H.; Sadowska, A. Atlas of Pollen and Spores of the Polish Neogene. Volume 2—Gymnosperms; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002. [Google Scholar]
- Stuchlik, L.; Ziembińska-Tworzydło, M.; Kohlman-Adamska, A.; Grabowska, I.; Słodkowska, B.; Ważyńska, H.; Sadowska, A. Atlas of Pollen and Spores of the Polish Neogene. Volume 3—Angiosperms (1); W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2009. [Google Scholar]
- Stuchlik, L.; Ziembińska-Tworzydło, M.; Kohlman-Adamska, A.; Słodkowska, B.; Worobiec, E.; Durska, E. Atlas of Pollen and Spores of the Polish Neogene. Volume 4—Angiosperms (2); W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2014. [Google Scholar]
- Słodkowska, B.; Ziembińska-Tworzydło, M. Pollen Morphotype Edmundi and Its Significance for Palaeoclimate Reconstructions of the Neogene. Geol. Q. 2017, 61, 267–275. [Google Scholar] [CrossRef][Green Version]
- Mai, D.H. Development and Climatic Differenciation of the Broad-Leaved Forests of Middle Europe in the Tertiary Period. Flora 1981, 171, 525–582. [Google Scholar] [CrossRef]
- Mai, D.H. Tertiäre Vegetationsgeschichte Europas. Methoden Und Ergebnisse. 691 S., 257 Abb., 14 Taf., 23 Tab. Gustav Fischer Verlag, Jena, Stuttgart, New York, 1995. ISBN 3-334-60456-X. Preis: DM 238,–. Feddes Repert. 1995, 106, 331. [Google Scholar] [CrossRef]
- Worobiec, E. Middle Miocene Palynoflora of the Legnica Lignite Deposit Complex, Lower Silesia, Poland. Acta Palaeobot. 2009, 49, 5–133. [Google Scholar]
- Sadowska, A. Vegetation and stratigraphy of Upper Miocene coal seam of the South-Western Poland. Acta Palaeobot. 1977, 18, 87–122. [Google Scholar]
- Mosbrugger, V.; Gee, C.T.; Belz, G.; Ashraf, A.R. Three-Dimensional Reconstruction of an in-Situ Miocene Peat Forest from the Lower Rhine Embayment, Northwestern Germany—New Methods in Palaeovegetation Analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1994, 110, 295–317. [Google Scholar] [CrossRef]
- Figueiral, I.; Mosbrugger, V.; Rowe, N.P.; Ashraf, A.R.; Utescher, T.; Jones, T.P. The Miocene Peat-Forming Vegetation of Northwestern Germany: An Analysis of Wood Remains and Comparison with Previous Palynological Interpretations. Rev. Palaeobot. Palynol. 1999, 104, 239–266. [Google Scholar] [CrossRef]
- Ziembińska-Tworzydło, M. Palynological Characteristics of the Neogene of Western Poland. Acta Palaeontol. Pol. 1974, 19, 309–432. [Google Scholar]
- Skłodowska, B.; Widera, M. Vegetation Response to Environmental Changes Based on Palynological Research on the Middle Miocene Lignite at the Jóźwin IIB Open-Cast Mine (Konin Region, Central Poland). Ann. Soc. Geol. Pol. 2021, 91, 149–166. [Google Scholar] [CrossRef]
- Zachos, J.; Pagani, M.; Sloan, L.; Thomas, E.; Billups, K. Trends, Rhythms, and Aberrations in Global Climate 65 Ma to Present. Science 2001, 292, 686–693. [Google Scholar] [CrossRef]
- Mosbrugger, V.; Utescher, T.; Dilcher, D.L. Cenozoic Continental Climatic Evolution of Central Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 14964–14969. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Do, T.V.; Durska, E. Palynological Characteristics of Neogene Deposits from Bełchatów Lignite Mine (Central Poland). Plants 2025, 14, 3034. https://doi.org/10.3390/plants14193034
Do TV, Durska E. Palynological Characteristics of Neogene Deposits from Bełchatów Lignite Mine (Central Poland). Plants. 2025; 14(19):3034. https://doi.org/10.3390/plants14193034
Chicago/Turabian StyleDo, Thang Van, and Ewa Durska. 2025. "Palynological Characteristics of Neogene Deposits from Bełchatów Lignite Mine (Central Poland)" Plants 14, no. 19: 3034. https://doi.org/10.3390/plants14193034
APA StyleDo, T. V., & Durska, E. (2025). Palynological Characteristics of Neogene Deposits from Bełchatów Lignite Mine (Central Poland). Plants, 14(19), 3034. https://doi.org/10.3390/plants14193034





